Introduction
Hidradenitis suppurativa (HS) is a chronic relapsing inflammatory skin disease, characterized by recurrent and painful nodules, abscesses, and sinus tracts involving the hair follicle.1 HS has been related to various cutaneous and systemic complications, such as chronic pain, anemia, lymphedema, and amyloid A (AA) amyloidosis, with a significant reduction in the patient's quality of life.1
AA amyloidosis in HS has been rarely reported2, 3, 4, 5, 6, 7, 8, 9; however, AA amyloidosis related to other chronic inflammatory diseases has been well described. The deposition of AA may induce damage to viscera, connective tissue, and blood vessel walls, with the kidney as a major target. AA amyloidosis has been detected in a subclinical phase in some reports. An early detection of AA fibrils and a prompt systemic treatment of inflammation can stop the long-term deposition of AA and prevent related complications.10
Case report
We assessed a 73-year-old man presenting with Hurley II HS. He had a history of well-controlled type 2 diabetes, hypothyroidism, and acute myocardial infarction. The disease duration was 51 years, with a worsening of clinical features over the last 3 years. The patient had been using short-term antibiotic therapies.
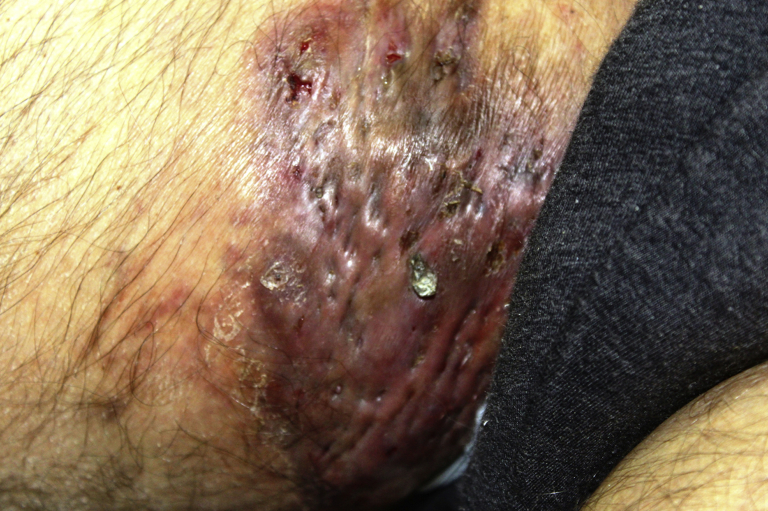
Physical examination found nodules and abscesses in axillae, inguinal areas (Fig 1), perineal region, back, and chest. Laboratory tests found a high serum level (168 μg/mL) of acute-phase reactant serum amyloid A (SAA) compared with normal range from 0 to 6.4 μg/mL, normal creatinine clearance (63 mL/min), no urinary proteinuria (<150 mg/24 h), glycated hemoglobin (<7%), and microalbuminuria (280 mg/24 h).
Fig 1.
In a patient with HS, infiltrated inflammatory plaque affecting inguinal area.
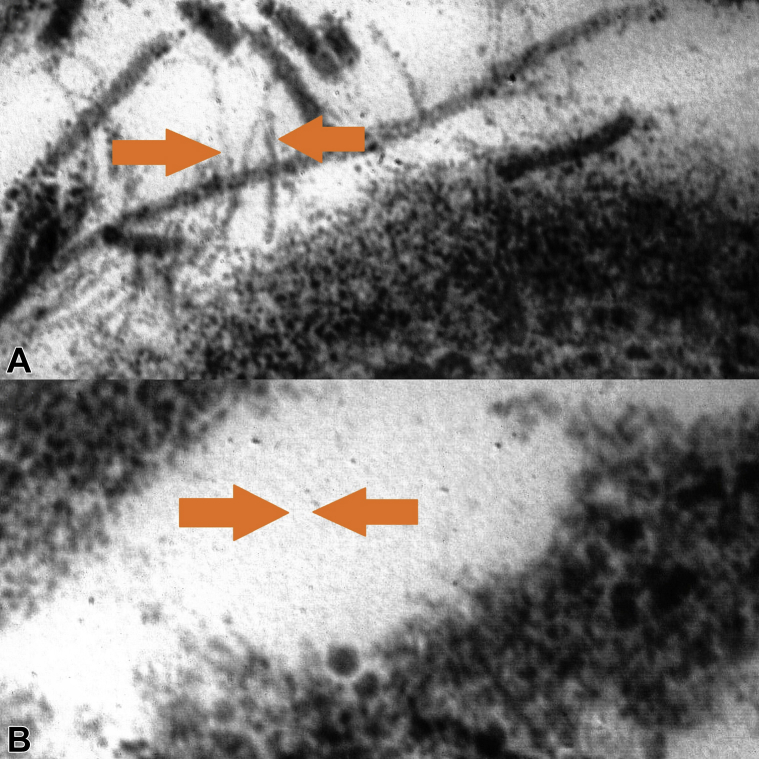
Considering the very high serum level of SAA as an inflammatory marker, we decided to investigate by performing a biopsy from abdominal adipose tissue stained with Congo-red–based light microscopy, which yielded a negative result. We used immunoelectron microscopy of abdominal fat, which showed perivascular fibril deposits, marked as AA fibrils using gold-labeled Immunogold secondary antibodies (Fig 2, A). This was a case of subclinical AA amyloidosis, with false-negative response to Congo-red–based light microscopy caused by scanty and unevenly distributed amyloid deposits. The detection of a high serum level of SAA was key in the decision to obtain a prompt diagnosis and start systemic treatment that could provide a rapid improvement.
Fig 2.
A, Chains, not branched, marked by gold-labeled Immunogold secondary antibodies on abdominal fat biopsy with amyloid ultrastructure in an HS patient before treatment. B, Absence of amyloid A chain on abdominal fat biopsy in the patient after treatment. (Electron microscopy; original magnifications: A, ×70; B, ×100.)
We treated the patient with clindamycin, 600 mg/d, and rifampicin, 600 mg/d, for 12 weeks. We achieved a good response with improvement in skin lesions, reduction of SAA (8 μg/mL), and reduction in AA fibrils in abdominal fat (Fig 2, B). We repeated SAA after 3 months (last follow-up) when it was in the normal range (5.3 μg/mL). Clinical follow-up showed a disease-remission phase with cordlike scars, bridging scars, hyperpigmented atrophic scars, fibrotic plaques, and one isolated active nodule.
Because systemic diseases are known to be associated with elevated SSA, we evaluated all the comorbidities of the patient's medical history, but the episode of acute myocardial infarction occurred more than 5 years before SAA elevation without dysfunctional complications. Diabetes was well under control as shown by the glycated hemoglobin values, so the only disease in acute phase at the time of our evaluation was HS, and the reduction in SAA after treatment reinforced our hypothesis that the elevated SAA is related to hidradenitis and not to the patient's comorbidities.
Discussion
We found eight cases reported in the literature on amyloidosis and HS in the last decades2, 3, 4, 5, 6, 7, 8, 9 (Table I); none of the patients had presented with subclinical amyloidosis. All patients were between 39 and 62 years and had chronic HS of variable duration, from 3 to 25 years. Previous treatments were also very heterogeneous. Clinical presentation of AA amyloidosis was characterized by nephrotic proteinuria or nephritic syndrome. In all 8 cases, AA deposits were confirmed by kidney or colon biopsies.
Table I.
Literature data report on HS and amyloidosis
| Study | Age of patient (y) | HS duration (y) | Previous therapies | Comorbidities | Localization | Clinical amyloidosis presentation | Detection of amyloid deposits | Assigned therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Titze et al2 | 62 | 25 | NR | Coronary heart disease, hypertension, hypercholesterolemia | Axillae, inguinal areas, perineal area | Nephrotic syndrome, diarrhea | Colon biopsy | Ramipril, diuretics, cefuroxime |
Kidney failure |
| Caliskan et al3 | 44 | NR | None | None | Axillae, inguinal areas, perianal area | Nephrotic proteinuria | Kidney biopsy | Ramipril, colchicine | Kidney failure |
| Montes-Romero et al4 | 39 | 9 | Antiandrogens, topic and systemic antibiotics, isotretinoin | Hypothyroidism | Axillae, perineal area, scrotum, Perianal area |
Nephrotic proteinuria | Kidney biopsy | Infliximab | Improvement in 14 wks |
| Girouard et al5 | 39 | 21 | Infliximab, surgery Systemic tetracycline, linezolid and clindamycin |
NR | NR | Nephrotic proteinuria | Kidney biopsy | Adalimumab and fludrocortisone | Improvement |
| Ilgen et al6 | 42 | 3 | Topical steroids, topical keratolytics, systemic roxithromycin, rifampicin and clindamycin | None | Axillae, inguinal areas, perineal area, perianal area | Nephrotic syndrome | Perineal skin biopsy Kidney biopsy |
Colchicine | Kidney failure |
| Utrera-Busquets et al7 | 37 | 16 | Systemic antibiotics, antiandrogens, dapsone, prednisone, infliximab, methotrexate, adalimumab | Graves disease, obesity | Axillae, inguinal areas, gluteal area | Nephrotic syndrome | Renal biopsy | Allopurinol, furosemide, sodium bicarbonate, lanthanum carbonate, systemic prednisone and clindamycin, surgery | Improvement in 24 wks |
| Schandorff et al8 | 54 | 8 | Long-term systemic tetracycline, systemic rifampicin and clindamycin | None | Anogenital region, inguinal areas | Kidney failure | Renal biopsy | Acitretin, prednisolone, adalimumab, anakinra, surgery | Kidney failure |
| Özer et al9 | 43 | 11 | Systemic antibiotics and steroid injections | None | Axillae, inguinal areas | Nephrotic proteinuria | Renal biopsy | Infliximab | Improved in 4 wks |
NR, Data not reported.
Assigned therapies were very heterogeneous. Two patients showed an improvement in renal function in 4 to 14 weeks of treatment with infliximab (5 mg/kg)4, 9; one patient showed an improvement in renal function after 24 weeks of clindamycin, 600 mg/d, and prednisone, 50 mg/d,7 another patient showed a systemic improvement after treatment with adalimumab and fludrocortisone.5 All the other patients had kidney failure.2, 3, 4, 5, 6, 7, 8
A literature review on AA amyloidosis found that more effective therapeutic control of inflammatory activity in patients with subclinical amyloidosis could prevent evolution of disease. No precise data are available on the role of SAA in subclinical amyloidosis, but, in patients with symptomatic AA amyloidosis, outcome is favorable when the SAA concentration is maintained below 10 mg/L.10
HS should be managed as a chronic inflammatory systemic disease. As in several chronic inflammatory diseases, there may be damage to internal organs from the aggregation and deposition of fibrils originating from proteolytic fragments of SAA, produced by the liver in response to chronic inflammation. To avoid severe complications, we recommend a careful survey of adult patients with moderate-to-severe HS and clinical duration of greater than 3 years including workup to detect serum levels of SAA. We also recommend more effective anti-inflammatory treatment in patients with elevated SAA, maintaining SAA levels less than 10 mg/L.
Since high serum levels of SAA could be predictive of a severe complication such as AA amyloidosis and no studies are available to date, clinicians should consider further research to establish whether this association can be verified at the population level and what its clinical significance is in hidradenitis patients.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Oranges T., Dini V., Chiricozzi A., Panduri S., Romanelli M. Complications. In: John Wiley and Sons, editor. Hidradenitis suppurativa: a diagnostic atlas. Wiley; Hoboken, NY: 2017. pp. 65–68. [Google Scholar]
- 2.Titze J., Schneider M., Krause H. Diarrhea, nephrotic syndrome and hidradenitis suppurativa: an unusual case. Nephrol Dial Transplant. 2003;18:192–194. doi: 10.1093/ndt/18.1.192. [DOI] [PubMed] [Google Scholar]
- 3.Caliskan Y., Yazici H., Kucuk M. Nephrotic syndrome associated with hidradenitis suppurativa. Clin Nephrol. 2005;63:171–172. doi: 10.5414/cnp63171. [DOI] [PubMed] [Google Scholar]
- 4.Montes-Romero J.A., Callejas-Rubio J.L., Sánchez-Cano D. Amyloidosis secondary to hidradenitis suppurativa. Exceptional response to infliximab. Eur J Intern Med. 2008;19:32–33. doi: 10.1016/j.ejim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Girouard S.D., Falk R.H., Rennke H.G. Hidradenitis suppurativa resulting in systemic amyloid A amyloidosis: a case report and review of the literature. Dermatol Online J. 2012;18(1):2. [PubMed] [Google Scholar]
- 6.Ilgen U., Çelebi Z.K., Kuzu I. Renal amyloidosis secondary to hidradenitis suppurativa. Clin Kidney J. 2013;6:667–668. doi: 10.1093/ckj/sft132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utrera-Busquets M., Romero-Maté A., Castaño Á. Severe hidradenitis suppurativa complicated by renal AA amyloidosis. Clin Exp Dermatol. 2016;41:287–289. doi: 10.1111/ced.12731. [DOI] [PubMed] [Google Scholar]
- 8.Schandorff K.D., Miller I.M., Krustrup D. Renal amyloid A amyloidosis as a complication of hidradenitis suppurativa. Clin Nephrol. 2016;86:51–54. doi: 10.5414/cn108787. [DOI] [PubMed] [Google Scholar]
- 9.Özer İ., Karaçin C., Adışen E. Two diseases one remedy? Systemic amyloidosis secondary to hidradenitis suppurativa: Treatment with infliximab. Dermatol Ther. 2017;30(2) doi: 10.1111/dth.12445. [DOI] [PubMed] [Google Scholar]
- 10.Obici L., Merlini G. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss Med Wkly. 2012;142:w13580. doi: 10.4414/smw.2012.13580. [DOI] [PubMed] [Google Scholar]