Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine tumor that is locally aggressive and frequently associated with metastatic disease. Our literature review found that MCC may be associated with paraneoplastic neurological syndromes (PNS) more frequently than other skin cancers. We hypothesize that the neuroendocrine nature of MCC may be a predisposing factor for such an increase in associated PNS. Moreover, monitoring the status of neurologic symptoms may be an important mechanism for assessing primary disease response to treatment, as illustrated by our case of a 55-year-old man who had cerebellar ataxia and was found to have metastatic MCC of unknown primary (UPMCC). We conclude that neurologic findings without clear cause warrant a workup for cancer.
Case report
A 55-year-old white man with an 8-month history of progressive cerebellar ataxia and a recent lymph node biopsy consistent with UPMCC, presented to our clinic for further evaluation. Approximately 8 months prior, the patient had new-onset dizziness, gait instability, and lower extremity weakness. He subsequently had multiple admissions to an outside hospital for progressive neurologic symptoms, including diplopia, erectile dysfunction, and confusion. Extensive medical workup including imaging was unremarkable except cerebrospinal fluid analysis showed mild elevations in calcium channel binding antibody P/Q type (0.08 nmol/L; normal, <0.02 nmol/L), glutamic acid decarboxylase antibody (0.14 nmol/L; normal, <0.02 nmol/L), and angiotensin-converting enzyme (89 U/L; normal, 9-67 U/L), and marked elevations in thyroid peroxidase antibody (64.4 IU/mL; normal, <5.6 IU/mL) and thyroglobulin antibody (30.7 IU/mL; normal, <4.1). Cerebrospinal fluid findings were suggestive of an autoimmune cerebellar process, so the patient was started on intravenous immunoglobulin; however, his symptoms continued to worsen.
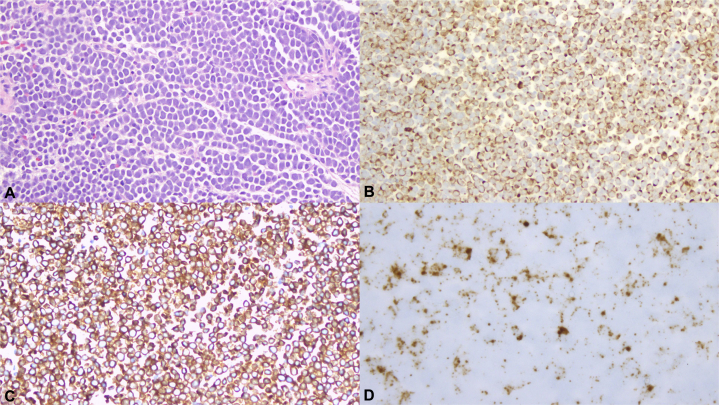
Repeat positron emission tomography/computed tomography 1 month before presentation was notable for significant left inguinal lymphadenopathy, with the largest node measuring 2.1 × 0.6 cm (standardized uptake value, 19.1). A left inguinal nodal biopsy showed nests of basophilic tumor cells with scant cytoplasm and round-to-ovoid nuclei with evenly distributed chromatin and nuclear molding (Fig 1, A). The immunohistochemical stain for cytokeratin 20, an epithelial marker, showed a characteristic dot-like, membranous pattern (Fig 1, B). These tumor cells also express neuroendocrine markers such as chromogranin A and synaptophysin (Fig 1, C), rendering the diagnosis of MCC. Furthermore, in situ hybridization detected the presence of polyoma virus RNA within these tumor cells (Fig 1, D).
Fig 1.
Merkel cell carcinoma. A, Nests of basophilic tumor cells displaying round nuclei, powdery chromatin, scant cytoplasm, and nuclear molding. B, Tumor cells stained with CK20 antibody show a membranous and dot-like paranuclear pattern. C, Immunohistochemical stain for synaptophysin shows diffuse cytoplasmic positivity. D, In situ hybridization of the Merkel cell polyomavirus RNA shows positive nuclear staining. (A, Hematoxylin-eosin stain; B, CK20; C, synaptophysin; D, Merkel cell polyomavirus RNA ISH; original magnifications: A-C, ×200; D, ×400.)
In light of his diagnosis of at least stage III UPMCC with likely paraneoplastic cerebellar ataxia, he was referred to our comprehensive skin cancer clinic for cutaneous evaluation. On examination, there were no cutaneous findings suspicious for a primary lesion. Intravenous immunoglobulin was discontinued, and the patient was scheduled for complete left inguinal lymph node dissection. After surgery, the patient noted an immediate improvement in his neurologic symptoms with significant resolution of his gait stability and lower extremity weakness. Additionally, his diplopia, erectile dysfunction, and mental clarity all improved. Follow-up imaging after lymph node dissection showed interval decreased left inguinal lymphadenopathy and fluorodeoxyglucose uptake.
Unfortunately, over the subsequent weeks, he reported slowly progressive deterioration with return of gait ataxia, diplopia, and lower extremity weakness, suggesting residual microscopic disease. Two months later, he was locally treated with adjuvant radiotherapy, and after treatment, again reported improvement in his neurologic status. On follow-up, the patient has continued to report durable and significant recovery, although he has not completely returned to baseline.
Discussion
MCC is a rare and aggressive cutaneous neuroendocrine tumor arising from Merkel cells, tactile epithelial mechanoreceptors found in the basal layer of the epidermis. Positive staining for CK20 is a reliable, specific marker for disease.1 Mortality for MCC is high; 5-year survival rates for patients with local, nodal, and metastatic disease are 64%, 39%, and 18%, respectively.2 Surgery often with adjuvant radiation therapy is the mainstay of treatment and may be curative, but relapses are common. Distant metastatic disease is often treated with chemotherapy, but long-term efficacy is questionable.3
Paraneoplastic neurologic syndromes (PNS) associated with MCC may occur as a response to either ectopic hormone secretion or an autoimmune response to tumor antigens.4 Interestingly, MCC shares a neuroendocrine origin and similar histology with small cell lung cancer, which is associated with PNS in 20% of cases.4 PNS in these cases are being investigated for use in early detection and markers for relapse. Likewise, our patient's paraneoplastic symptoms allowed for detection of underlying UPMCC and mirrored his treatment course.
A review of the published literature found 25 cases linking MCC to PNS with diverse manifestations including paraneoplastic encephalomyelitis, paraneoplastic cerebellar degeneration, Lambert-Eaton syndrome, necrotizing myopathy, and paraneoplastic polyarthritis.5, 6, 7, 8 It is thought that neuronal nuclear antibody (Hu) and voltage-activated Ca2+ channels may be neuronal antigens causing PNS in MCC.9 Indeed, 6 months before diagnosis, our patient was noted to have mildly elevated levels of calcium channel P/Q Ab (0.08 nmol/L; normal, 0.02 nmol/L) and at the time of diagnosis was noted to have further elevation to 0.12 nmol/L. Anti-Hu was within normal limits. Antibody levels were not checked again postsurgery.
It is very unusual for MCCs to present first with lymph node metastasis and have no identifiable primary tumor (UPMCC). However, these patients generally have improved outcomes compared with those who have MCCs with known primary.10 UPMCCs may possibly arise owing to an immune response that causes spontaneous regression of the primary tumor. Coincidentally, there is also an elevated incidence of PNS in UPMCC compared with MCC with known primary.5 We posit that the same immune response that destroyed the primary tumor may have been misdirected toward self-antigens, explaining the elevated incidence of PNS in UPMCC patients.
In our patient, the paraneoplastic nature of his neurologic symptoms was highlighted by the dramatic and immediate improvement of his neurologic symptoms with treatment of the underlying malignancy. Likewise, the progression of his symptoms when off treatment may have reflected residual microscopic disease. This case adds to the growing body of literature associating MCC with PNS, emphasizing the importance of searching for an underlying malignancy and monitoring the patient's neurologic symptoms as an indicator for primary tumor status.
Acknowledgments
We acknowledge Dr John P. Crapanzano and Dr Richard D. Carvajal.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Munde P.B., Khandekar S.P., Dive A.M., Sharma A. Pathophysiology of Merkel cell. J Oral Maxillofac Pathol. 2013;17(3):408–412. doi: 10.4103/0973-029X.125208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemos B.D., Storer B.E., Iyer J.G. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinowits G. Is this the end of cytotoxic chemotherapy in Merkel cell carcinoma? Onco Targets Ther. 2017;10:4803–4807. doi: 10.2147/OTT.S126640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi L., Johnson B.E. Paraneoplastic syndromes associated with small cell lung cancer. J Natl Compr Canc Netw. 2006;4(6):631–638. doi: 10.6004/jnccn.2006.0052. Review. [DOI] [PubMed] [Google Scholar]
- 5.Pavolucci L., Giannini G., Giannoccaro M.P. Paraneoplastic cerebellar degeneration and Lambert-Eaton myasthenia in a patient with Merkel cell carcinoma and voltage-gated calcium channel antibodies. Muscle Nerve. 2017;56(5):998–1000. doi: 10.1002/mus.25530. [DOI] [PubMed] [Google Scholar]
- 6.Iyer J.G., Parvathaneni K., Bhatia S. Paraneoplastic syndromes (PNS) associated with Merkel cell carcinoma (MCC): A case series of 8 patients highlighting different clinical manifestations. J Am Acad Dermatol. 2016;75(3):541–547. doi: 10.1016/j.jaad.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharobeam A., Ray J., Dong J., Chong V. subacute cerebellar degeneration due to a paraneoplastic phenomenon associated with metastatic Merkel cell carcinoma: a case report. Case Rep Oncol. 2017;10(2):764–768. doi: 10.1159/000479731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santomasso B., D'Angelo S. Anti-CRMP5-associated paraneoplastic neurologic syndrome developing in a patient with metastatic Merkel cell carcinoma during immune checkpoint inhibitor treatment. Neurology. 2018;90(15 Supplement):P5.409. [Google Scholar]
- 9.Balegno S., Ceroni M., Corato M. Antibodies to cerebellar nerve fibres in two patients with paraneoplastic cerebellar ataxia. Anticancer Res. 2005;25:3211–3214. [PubMed] [Google Scholar]
- 10.Tarantola T.I., Vallow L.A., Halyard M.Y. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol. 2013;68:433–440. doi: 10.1016/j.jaad.2012.07.035. [DOI] [PubMed] [Google Scholar]