Introduction
We present a case of recalcitrant, localized patch disease in a patient with stage IB (T2, N0, M0) mycosis fungoides (MF) that clinically responded to treatment with topical gentian violet (GV) after undergoing several other therapies, including narrowband ultraviolet B (UVB) therapy and topical clobetasol, bexarotene, and mechlorethamine. A reduction of erythema of her treated patches was noted within 2 months of once-daily application of topical GV, and there was no disease progression. However, subsequent biopsy of the most prominent patch found the presence of active disease despite reduction of erythema. There was a partial clinical response to topical GV, and this report is among the first to describe the safety and efficacy of GV in MF.
Case report
A 52-year-old woman with a 7-year history of stage IB (T2, N0, M0) MF presented to the clinic for follow-up. Many of her lesions had resolved with narrowband UVB therapy 2 years prior, but there were localized recurrent/persistent patches. She had approximately 2% body surface area involvement including several patches on the abdomen, with the most prominent patch on the right thigh (Fig 1) of at least 2 years' duration. A biopsy found atypical lymphocytes with irregular nuclear contours and halos within the epidermis singly and in small collections, confirming the diagnosis of MF (Fig 2).
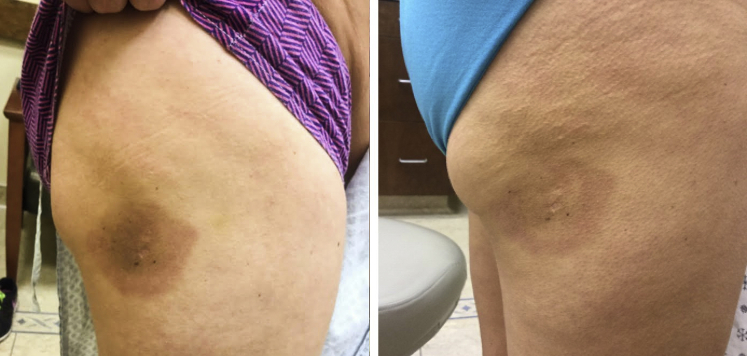
Fig 1.
Active patch of MF before (left) and after (right) 2 months of once-daily treatment with topical GV.
Fig 2.
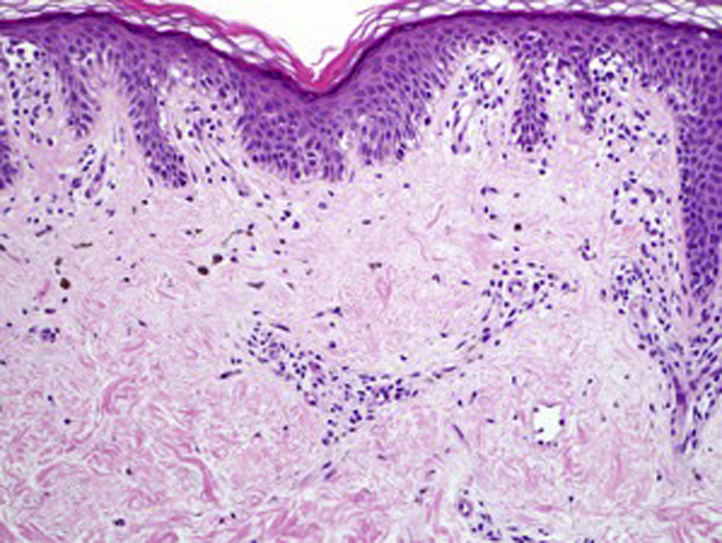
Histopathology of patch shown in Fig 1 shows atypical lymphocytes with irregular nuclear contours and halos within the epidermis, confirming the diagnosis of MF in this patch. (Hematoxylin-eosin stain; original magnification: ×200.)
This patient did not respond or had a partial response to several therapies due to disease persistence and adverse effects. She was previously treated with total-body narrowband UVB therapy with a partial response but ceased treatment because of inconvenience and intolerance, including burning of her normal skin despite dose reductions. Some of her residual disease was also refractory to topical bexarotene and clobetasol, with no improvement after months on each of these treatments. She was prescribed topical mechlorethamine, which resulted in a dermatitis complicated by erysipelas, leading to discontinuation of therapy. Several patches persisted.
After multiple failed treatments, a trial of topical GV (1% solution) was attempted. After 2 months of once-daily application to active (erythematous) patches, clinical regression was noted, with subtle persistent erythema of some patches, whereas others seemed to resolve with postinflammatory hyperpigmentation (Figs 1 and 3). The patient denied having any adverse effects (staining properties of the solution were discussed prior to initiation of treatment). After a 2-month period of observation, a biopsy was performed, which found the presence of histologically active disease. However, there was no progression of disease at this or other sites during this period, and other treated areas on the trunk showed resolving macular hyperpigmentation without recurrence of erythema (Fig 3), confirming a partial clinical response to topical GV.
Fig 3.
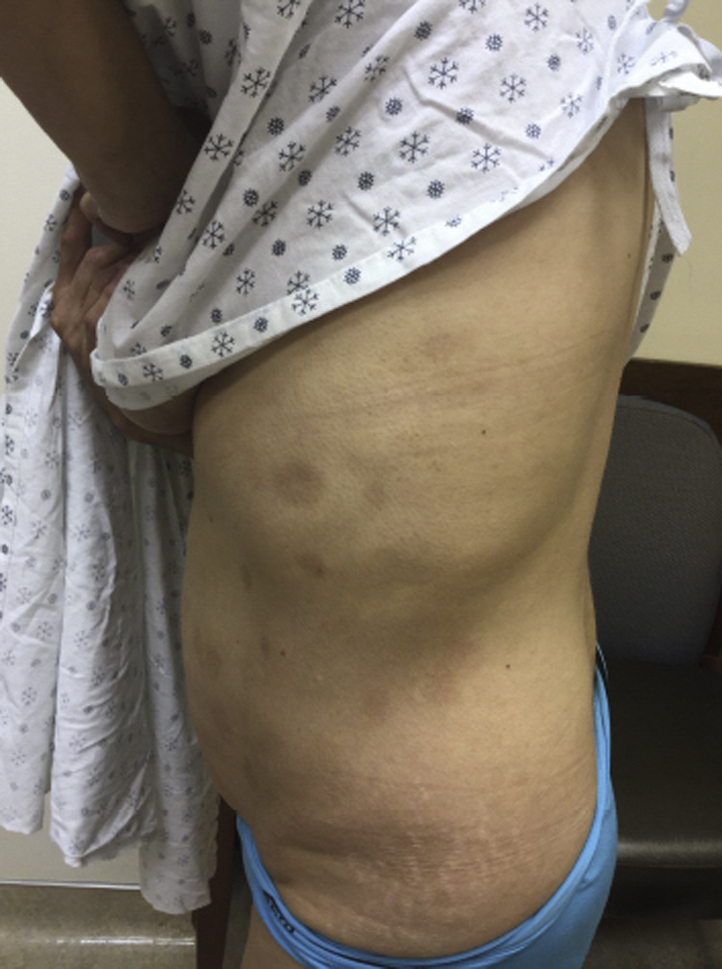
Previously active truncal macules and patches of MF show postinflammatory hyperpigmentation with only focal areas of erythema after 2 months of once-daily treatment with topical GV.
Discussion
MF is the most common subtype of cutaneous T-cell lymphoma (CTCL). Although it is usually an indolent form of malignancy in early stages, its chronic course and tendency to recur present a challenge for many patients and physicians who treat this disease. Effective therapies for early-stage disease are desired, as members of the treated population have a favorable prognosis, although morbidity and mortality rates increase with progressive disease.1 First-line therapies for early (stage IA-IIA) disease include ultraviolet light and topical corticosteroids, mechlorethamine, and bexarotene.2 Although many patients tolerate and respond to these therapies, others fail to respond or have significant financial burden from many first- and second-line agents, creating a need for other options.
Gentian violet, a triarylmethane dye, has classically been used as an antimicrobial, and has been reported as having potential antitumor activity.3 The mechanism of this antitumor activity is not fully understood, although GV has been shown to inhibit nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which decreases local reactive oxygen species and contributes to antiangiogenesis and might inhibit certain tumor suppressor pathways.3, 4 Regression of primary cutaneous diffuse B-cell lymphoma after injection of intralesional GV has been reported,5 and abrogation of NADPH oxidase–mediated tumor promotion in addition to facilitating antitumor activity have been proposed as mechanisms by these investigators. GV also inhibits NF-kB, also possibly through NADPH oxidase inhibition, which has been implicated in proliferation of CTCL tumor cells. A recent report by Wu and Wood6 found GV to enhance Fas and TRAIL pathway-dependent apoptosis in CTCL cell lines and Sezary syndrome blood samples.6 Speculation exists regarding a theoretical carcinogenesis risk associated with GV, although this has not be found to exist in clinical practice of the author or in the literature.7
Bacterial infections and colonization have been identified as a possible driving factor in MF, and clinical improvement has been noted with antibiotic therapy.8 However, this patient did not show clinical or histopathologic signs of impetiginization (Figs 1 and 2), and GV was not used as an antimicrobial in this case. Rather, the intent was to use the antitumor activities that have been proposed and observed in the literature.3, 6 To our knowledge, this is the first reported case of treatment of MF with GV. GV was associated with a partial clinical response, without progression of disease, and it was a safe and affordable therapy for this patient. Further studies are warranted to investigate the mechanisms that might predict a clinical response and to determine the overall effectiveness and safety in larger patient populations. Moreover, the confirmation of this patient's histologically active disease despite a clinical improvement highlights the importance of histologic confirmation in the evaluation of clinical response of MF to treatment modalities such as GV. Future clinical trials evaluating the treatment of MF should consider the importance of posttreatment biopsies in light of this observation.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Talpur R., Singh L., Daulat S. Long-term outcomes of 1,263 patients with mycosis fungoides and sézary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18(18):5051–5060. doi: 10.1158/1078-0432.CCR-12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawed S.I., Myskowski P.L., Horwitz S., Moskowitz A., Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70(2):223.e1–223.e17. doi: 10.1016/j.jaad.2013.08.033. quiz 240-2. [DOI] [PubMed] [Google Scholar]
- 3.Maley A.M., Arbiser J.L. Gentian violet: A 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22(12):775–780. doi: 10.1111/exd.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry B.N., Govindarajan B., Bhandarkar S.S. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126(10):2316–2322. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 5.Rao S., Morris R., Rice Z.P., Arbiser J.L. Regression of diffuse B-cell lymphoma of the leg with intralesional gentian violet. Exp Dermatol. 2018;27(1):93–95. doi: 10.1111/exd.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Wood G.S. Analysis of the effect of gentian violet on apoptosis and proliferation in cutaneous T-cell lymphoma in an in vitro study. JAMA Dermatol. 2018;154(10):1191–1198. doi: 10.1001/jamadermatol.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbiser J.L. Gentian violet is safe. J Am Acad Dermatol. 2009;61(2):359. doi: 10.1016/j.jaad.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Jawed S.I., Myskowski P.L., Horwitz S., Moskowitz A., Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70(2):205.e1–205.e16. doi: 10.1016/j.jaad.2013.07.049. quiz 221-222. [DOI] [PubMed] [Google Scholar]