Abstract
Purpose
We aimed to examine associations between right bundle branch block (RBBB) following heart transplantation (HT) and graft rejection.
Materials and Methods
We investigated 51 patients who underwent endomyocardial biopsies, electrocardiogram, right-side cardiac catheterization, and echocardiography at 1 month and 1 year after HT. We classified patients into four groups according to the development of RBBB, based on electrocardiogram at 1 month and 1 year: 1) sustained RBBB, 2) disappeared RBBB, 3) newly developed RBBB, and 4) sustained non-RBBB. The RBBB was defined as an RSR' pattern in V1 with a QRS duration ≥100 ms on electrocardiogram.
Results
The newly developed RBBB group (n=13, 25.5%) had a higher rate of new onset graft rejection (from grade 0 to grade ≥1R, 30.8% vs. 10.0% vs. 21.4%, p=0.042) at 1 year, compared with sustained RBBB (n=10, 19.6%) and sustained non-RBBB group (n=28, 54.9%). In contrast, the incidence of resolved graft rejection (from grade ≥1R to grade 0) was higher in the sustained RBBB group than the newly developed RBBB and sustained non-RBBB groups (70.0% vs. 7.7% vs. 25.0%, p=0.042). Left atrial volume index was significantly higher in the newly developed RBBB group than the sustained RBBB and sustained non-RBBB groups (60.6±25.9 mL/m2 vs. 36.0±11.0 mL/m2 vs. 38.4±18.1 mL/m2, p=0.003).
Conclusion
Close monitoring for new development of RBBB at 1 year after HT, which was associated with a higher incidence of new onset graft rejection, may be helpful to identify high risk patients for graft rejection.
Keywords: Right bundle branch block, heart transplantation, rejection
INTRODUCTION
A right bundle branch block (RBBB) is a common electrocardiographic abnormality in patients with heart transplantation (HT), with a prevalence rate of 12–79%.1,2,3,4 Although limited studies have reported conflicting clinical outcomes, the occurrence of RBBB after HT appears to be associated with poor clinical outcomes, including increased mortality, especially in patients with progressive RBBB.4,5,6 Small studies have described inflammatory infiltration related with graft rejection in the conduction system, as well as in the myocardium. Foerster7 reported that the conduction system was involved in 8 cases among 11 allografts with acute graft rejection, and Calzolari, et al.8 reported that complete or incomplete RBBB was founded in 11 cases out of 12 transplanted hearts with acute or chronic rejection in necropsy histologic study. Chan, et al.9 indicated that conduction abnormalities including RBBB could be associated with rejection, which might result in worse clinical outcomes.
However, there were not enough data about the patterns of development of RBBB and its relation with graft rejection and hemodynamic parameters following HT. Therefore, we sought to investigate the time-course development of RBBB on electrocardiogram (ECG) and its association with graft rejection, echocardiographic parameters, and invasive hemodynamic parameters in HT patients.
MATERIALS AND METHODS
Study population
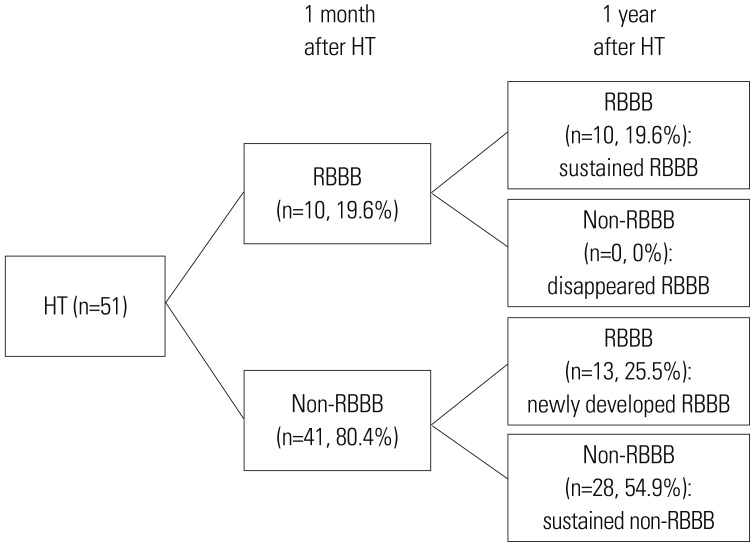
We performed a retrospective analysis of 79 patients who underwent bicaval orthotopic HT at Severance Cardiovascular Hospital from 2011 to 2015. The study protocol was approved by the Institutional Review Board of Severance Hospital (No. 4-2013-0665). All patients received basiliximab induction therapy, followed by triple immunosuppressive therapy (tacrolimus, mycophenolate mofetil, prednisolone). We excluded patients (n=17) who died due to fatal infection or multi-organ failure within 1 month and patients (n=11) who did not undergo either endomyocardial biopsy, echocardiography, or right-side cardiac catheterization at 1 month and 1 year after HT. Finally, we analyzed 51 patients who underwent all of previously mentioned exams at 1 month and 1 year after HT (Fig. 1).
Fig. 1. Flow diagram of each group according to the development of RBBB after HT. HT, heart transplantation; RBBB, right bundle branch block.
Based on ECG at 1 month and 1 year following HT, four groups of patients were identified: 1) sustained RBBB group (RBBB at 1 month and remaining RBBB at 1 year), 2) disappeared RBBB group (RBBB at 1 month and ECG at 1 year showed non-RBBB), 3) newly developed RBBB group (ECG at 1 month was non-RBBB, but ECG at 1 year showed RBBB), and 4) sustained non-RBBB group (ECG at 1 month was non-RBBB and remaining non-RBBB at 1 year).
Electrocardiography and endomyocardial biopsy
Standard 12-lead ECGs were obtained in the hospital after HT and at the outpatient clinic during follow-up. The RBBB was defined as an RSR' pattern in V1 with an R' amplitude of at least 0.2 mV with a QRS duration ≥100 ms on ECG.6 PR interval was defined as the interval from the onset of the P wave to the end of the PR segment, and was measured from the limb lead in which the interval was longest.10 The diagnosis of acute graft rejection was made by endomyocardial biopsy according to the International Society for Heart and Lung Transplantation (ISHLT) 2004 grading system: Grade 0, no rejection; Grade 1R, mild; Grade 2R, moderate; or Grade 3R, severe.11
Echocardiography and right-side cardiac catheterization
Two-dimensional echocardiography was performed to measure the dimensions and volume of the left ventricle (LV) and left atrium (LA), and LV ejection fraction was measured by the Simpson method as recommended.12 Right-side cardiac catheterization was performed to acquire hemodynamic data simultaneously during endomyocardial biopsy, and the following variables were measured: systolic, diastolic, and mean pressure of the pulmonary artery (PA) and right ventricle (RV) and pulmonary capillary wedge pressure.
Statistical analysis
Continuous variables including hemodynamic and echocardiographic parameters are expressed as mean±SD, and they were compared using Student's t test. Discrete variables are expressed as percentages and were compared using the chi-square test and Fisher exact test for small groups. All statistical analyses were conducted using SPSS statistical software (ver. 21.0; IBM Corp., Armonk, NY, USA). A two-sided p value of <0.05 was considered as statistically significant.
RESULTS
Patient clinical characteristics
The mean age of all donors was 33.6±11.0 years, and 64.7% were male. The mean age of all recipients was 41.7±15.1 years, and 58.8% were male. Table 1 shows the patient clinical characteristics of each group. Total ischemic time and immunosuppressive drug regimens during follow up were not different among each group. The prevalence of RBBB in our study was 19.6% (n=10) at 1 month and 45.1% (n=23) at 1 year after HT. There were 10 patients (19.6%) with sustained RBBB, 0 patients (0%) with disappeared RBBB, 13 patients (25.5%) with newly developed RBBB, and 28 patients (54.9%) with sustained non-RBBB.
Table 1. Patient Clinical Characteristics.
| Sustained RBBB (n=10, 19.6%) | Newly developed RBBB (n=13, 25.5%) | Sustained non-RBBB (n=28, 54.9%) | p value | |
|---|---|---|---|---|
| Donor data | ||||
| Male sex | 9 (90) | 7 (53.8) | 17 (60.7) | 0.147 |
| Age (yr) | 30.4±9.5 | 38.0±10.5 | 32.7±11.5 | 0.223 |
| Body surface area (m2) | 1.76±0.17 | 1.74±0.17 | 1.76±0.17 | 0.690 |
| Preoperative LVEF (%) | 64.9±6.9 | 59.7±6.1 | 62.7±6.7 | 0.180 |
| Cause of death on donor | 0.117 | |||
| Hypoxic brain damage | 2 (20.0) | 3 (23.1) | 9 (32.1) | |
| Non-traumatic brain hemorrhage | 6 (60.0) | 2 (15.4) | 4 (14.3) | |
| Traumatic brain hemorrhage | 2 (20.0) | 7 (53.8) | 14 (50.0) | |
| Others | 0 (0.0) | 1 (7.7) | 1 (3.6) | |
| Recipient data | ||||
| Male sex | 5 (50.0) | 9 (69.2) | 16 (57.1) | 0.596 |
| Age (yr) | 38.3±16.0 | 44.9±14.5 | 41.5±15.1 | 0.583 |
| Body surface area (m2) | 1.65±0.25 | 1.71±0.23 | 1.68±0.19 | 0.470 |
| Preoperative LVEF (%) | 23.9±9.9 | 25.6±9.0 | 22.2±14.0 | 0.694 |
| Total ischemic time (min) | 163.7±53.6 | 159.0±36.9 | 145.7±36.5 | 0.401 |
| Previous open-heart surgery history | 3 (30.0) | 7 (53.8) | 6 (21.4) | 0.125 |
LVEF, left ventricular ejection fraction; RBBB, right bundle branch block.
Values are presented as n (%) or mean±SD.
Echocardiographic and invasive hemodynamic parameters according to the development of RBBB
Details on RBBB development on ECG, right-side cardiac catheterization, echocardiography, and endomyocardial biopsy in each group are listed in Table 2. The newly developed RBBB group had significantly longer PR interval at 1 year than the sustained RBBB group and sustained non-RBBB group (164.8±13.7 ms vs. 148.6±25.7 ms vs. 149.2±18.3 ms, respectively, p=0.044). However, no invasive hemodynamic parameters measured by right-side cardiac catheterization were different between each group at 1 month and 1 year following HT. LA volume index was significantly higher in the newly developed RBBB group than the sustained RBBB and sustained non-RBBB groups (60.6±25.9 mL/m2 vs. 36.0±11.0 mL/m2 vs. 38.4±18.1 mL/m2, respectively, p=0.003). LV ejection fraction and parameters related with LV diastolic function were not significantly different among the groups.
Table 2. Electrocardiographic, Echocardiographic, and Invasive Hemodynamic Parameters and Endomyocardial Biopsy Results in Each Group.
| Sustained RBBB (n=10) | Newly developed RBBB (n=13) | Sustained non-RBBB (n=28) | ||||
|---|---|---|---|---|---|---|
| 1 month | 1 year | 1 month | 1 year | 1 month | 1 year | |
| Electrocardiogram | ||||||
| QRS width (ms) | 109.6±11.1* | 112.6±8.8 | 87.0±10.4 | 110.6±12.1 | 82.0±8.7 | 90.1±10.1∥ |
| PR interval (ms) | 133.4±13.0 | 148.6±25.7 | 143.8±14.6 | 164.8±13.7§ | 145.4±16.5 | 149.2±18.3 |
| QTc (ms) | 456.8±27.0 | 466.5±22.2 | 446.0±25.8 | 441.9±23.0 | 446.0±25.8 | 441.9±23.0 |
| Right heart catheterization | ||||||
| RVSP (mm Hg) | 30.2±7.9 | 28.3±7.4 | 31.1±6.6 | 26.3±6.8 | 30.3±6.6 | 28.4±7.8 |
| RVDP (mm Hg) | 4.5±7.2 | 1.3±4.0 | 2.6±2.4 | 0.1±3.5 | 3.2±3.3 | 1.6±2.7 |
| RVMP (mm Hg) | 16.0±7.2 | 12.4±2.7 | 14.8±4.7 | 11.0±5.1 | 15.0±3.8 | 14.4±5.1 |
| PASP (mm Hg) | 27.6±8.5 | 26.5±5.7 | 29.1±6.2 | 24.8±7.5 | 29.0±7.7 | 27.4±6.5 |
| PADP (mm Hg) | 10.7±2.9 | 11.3±2.8 | 13.4±4.3 | 9.9±4.4 | 12.3±4.6 | 12.8±4.7 |
| PAMP (mm Hg) | 18.3±3.2 | 18.2±3.9 | 18.0±5.7 | 17.0±5.7 | 19.7±5.8 | 19.7±5.4 |
| PCWP (mm Hg) | 8.7±4.3 | 9.6±2.8 | 12.8±4.5 | 9.2±3.8 | 10.7±3.3 | 12.1±6.1 |
| Echocardiographic data | ||||||
| LVEF (%) | 68.2±6.3 | 65.2±6.8 | 66.0±7.2 | 68.2±4.3 | 67.5±5.5 | 65.0±7.9 |
| LVMI (g/m2) | 94.1±21.5 | 84.5±13.1 | 106.9±36.7 | 93.9±28.6 | 94.2±24.9 | 81.7±17.3 |
| LAVI (mL/m2) | 38.6±12.8 | 36.0±11.0 | 63.2±24.1† | 60.6±25.9‡ | 39.2±13.9 | 38.4±18.1 |
| E velocity (m/s) | 0.71±0.12 | 0.70±0.21 | 0.83±0.27 | 0.79±0.23 | 0.73±0.21 | 0.75±0.17 |
| E′ velocity (cm/s) | 6.3±2.4 | 8.0±2.8 | 5.7±2.2 | 7.1±2.7 | 6.2±1.8 | 7.7±2.0 |
| E/E′ | 12.4±3.7 | 9.3±3.1 | 15.2±5.8 | 11.8±4.1 | 12.7±4.5 | 10.2±2.8 |
| RVSP (mm Hg) | 33.8±9.1 | 29.2±8.4 | 35.1±11.6 | 26.0±8.7 | 34.6±9.3 | 29.5±5.1 |
| TAPSE (mm) | 14.0±3.9 | 16.8±0.6 | 15.6±3.4 | 16.0±2.6 | 15.7±2.9 | 15.9±2.2 |
| TV TDI S' (cm/s) | 9.1±1.8 | 10.2±2.0 | 8.6±3.2 | 10.0±2.2 | 8.6±2.4 | 9.3±2.6 |
| Histology | ||||||
| Graft rejection (≥1) | 7 (70.0) | 1 (10.0) | 6 (46.2) | 9 (69.2) | 14 (50.0) | 13 (46.4) |
| Immunosuppressant level | ||||||
| Tacrolimus level (ng/mL) | 10.9±5.8 | 9.5±3.8 | 8.9±2.1 | 7.9±2.2 | 9.5±3.5 | 9.8±3.1 |
LAVI, left atrial volume index; LVMI, left ventricular mass index; PCWP, pulmonary capillary wedge pressure; RBBB, right bundle branch block; RVSP, right ventricular systolic pressure; RVDP, right ventricular diastolic pressure; RVMP, right ventricular mean pressure; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; PAMP, pulmonary artery mean pressure; PCWP, pulmonary capillary wedge pressure; TAPSE, tricuspid annular plane systolic excursion; TV TDI S', tricuspid valve tissue Doppler imaging S'.
Values are expressed as mean±SD or number (percentage).
p<0.05, by ANOVA with post hoc test using Bonferroni technique (*sustained RBBB group vs. newly developed RBBB group and sustained non-RBBB group at 1 month following heart transplantation; †newly developed RBBB group vs. sustained RBBB group and sustained non-RBBB group at 1 month following heart transplantation; ‡newly developed RBBB group vs. sustained RBBB group and sustained non-RBBB group at 1 year following heart transplantation; §newly developed RBBB group vs. sustained non-RBBB group at 1 year following heart transplantation; ∥sustained non-RBBB group vs. sustained RBBB group and newly developed RBBB group at 1 year following heart transplantation).
Graft rejection after HT
As shown in Table 3, we identified 4 cases (30.8%) of new onset graft rejection (from grade 0 to grade ≥1R) in the newly developed RBBB group, which was higher than that in the other groups (1 case, 10.0% in sustained RBBB vs. 6 cases, 21.4% in sustained non-RBBB group). Interestingly, 7 patients (70.0%) in the sustained RBBB group experienced resolved histologic finding (from grade ≥1R to grade 0) over time, compared to other groups (7.7% in newly developed RBBB vs. 25.0% in sustained non-RBBB group). Among three patients with graft rejection Grade 2R at 1 year, two patients were in the newly developed RBBB group; one patient was in the sustained non-RBBB group.
Table 3. Graft Rejection after HT in Each Group.
| No change of GR (n=12, 23.5%) | No evidence of GR (n=13, 25.5%) | New onset of GR (n=11, 21.6%) | Resolving of GR (n=15, 29.4%) | p value | |
|---|---|---|---|---|---|
| Sustained RBBB (n=10) | 0 (0.0) | 2 (20.0) | 1 (10.0) | 7 (70.0) | 0.042 |
| Newly developed RBBB (n=13) | 5 (38.5) | 3 (23.1) | 4 (30.8) | 1 (7.7) | |
| Sustained non-RBBB (n=28) | 7 (25.0) | 8 (28.6) | 6 (21.4) | 7 (25.0) |
HT, heart transplantation; GR, graft rejection; RBBB, right bundle branch block.
Values are presented as n (%). p value by chi-square test.
In our HT patients, the PR interval (142.6±15.6 ms vs. 152.7±19.9 ms, p=0.002) and QRS duration (87.2±13.3 ms vs. 97.0±14.8 ms, p<0.001) increased significantly from 1 month to 1 year after HT. Furthermore, 9 patients (81.8%) with new onset graft rejection (from grade 0 to grade ≥1R) had a significantly longer PR (≥153 ms) interval at 1 year than other groups (58.3% in no change of GR vs. 38.5% in no evidence of GR vs. 26.7% in resolving of GR) (Table 4).
Table 4. Graft Rejection according to PR Interval at 1 Year after HT.
| The change of graft rejection from 1 month to 1 year following HT | |||||
|---|---|---|---|---|---|
| No change in GR | No evidence of GR | New onset of GR | Resolving of GR | p value | |
| PR interval (<153 ms*) | 5 (41.7) | 8 (61.5) | 2 (18.2) | 11 (73.3) | 0.033 |
| PR interval (≥153 ms) | 7 (58.3) | 5 (38.5) | 9 (81.8) | 4 (26.7) | |
HT, heart transplantation; GR, graft rejection; RBBB, right bundle branch block.
Values are presented as n (%). p value by chi-square test.
*153 ms: median value of PR interval in overall study population at 1 year following HT.
DISCUSSION
The principal findings of this study are that 1) newly developed RBBB was associated with higher new onset graft rejection, higher LA volume index, and longer PR interval at 1 year and that 2) individuals with sustained RBBB experienced a significantly higher incidence of resolved graft rejection at 1 year, compared with newly developed RBBB and sustained non-RBBB.
The occurrence of conduction abnormalities after HT is well known, and the most common of these is RBBB. The prevalences of RBBB in the present study population were 15.9% at 1 month and 36.5% at 1 year, similar to previous studies (12% to 79%). Most previous studies reported that the development of RBBB and QRS width increased over the time after HT.4,13,14,15 Meanwhile, several studies indicated that the occurrence of conduction disorders after HT was related with longer ischemic time, extracorporeal circulation time, surgery-related myocardial damage, and graft rejection.3,4,16 Among conduction disorders, the occurrence of RBBB immediately within 1 month after HT could pose different clinical implications from those with the occurrence of RBBB later. Since the right bundle branch has a unique anatomical structure, it might be susceptible to damage during HT: much of its septal course is near the endocardium and consists of narrow cylindrical tubes.17 Also, biatrial anastomosis and clockwise rotation of a transplanted heart, as a result of the surgical technique, could be other causative factors for RBBB.1,15,18,19 Considering these factors, our study indicated that the occurrence of RBBB early within 1 month following HT was less likely to be related with early graft rejection.
Our study showed that the newly developed RBBB after 1 month later following HT could be related with graft rejection. As in our findings, Osa, et al.6 showed that progressive RBBB, during which QRS width increases more than 0.5 mm during follow-up, was associated with a poorer long-term prognosis than non-progressive RBBB and related to a greater number of treatable rejections. Also, patients with gradually worsening conduction abnormalities exhibited significant differences in the number of rejections to treatment, suggesting a correlation between the progression of conduction abnormalities and poor clinical outcomes, as described in the study of Leonelli, et al.3,20 On the other hand, the sustained RBBB group in our study had a relatively low rejection rate and a higher resolution of histologic findings during follow up, compared with the newly developed RBBB group. The absence of changes in ECG during follow up might be more histologically stable, although further research is needed to evaluate and clarify this.
Mild graft rejection, such as Grade 1R on the revised ISHLT grading system, was previously found to be the strongest predictor for severe graft rejection of more than Grade 2R, suggesting short-term follow up seems to be warranted.21 Furthermore, mild graft rejection at the conduction system, including the atrioventricular (AV) node and right bundle branch, could be associated with the development of RBBB, PR interval prolongation, and AV block.8 Prolonged PR interval, another type of conduction abnormality, was linked to conduction system fibrosis, and graft rejection.10,22 Some studies reported AV block was associated with a higher risk of graft rejection, which resulted from lymphocyte infiltration, inflammation, myocyte necrosis or fibrosis at the conduction system.23,24,25,26 Furthermore, Calzolari, et al.8 reported that appearance of first-degree AV block in a HT recipient may be suggestive of involvement of the conduction system with impending third-degree block. Also, Chan, et al.9 reported early signs of first degree AV block after HT may portend aggravated conduction abnormalities with occurrence of subclinical, isolated rejection of the conduction system. In our study, at 1 year after HT, newly developed RBBB posed a significantly longer PR interval, and PR interval in patients who had new onset graft rejection at 1 year was significantly longer than those in patients without new onset graft rejection (161.5±12.2 ms vs. 150.8±20.9 ms, p=0.041) (detailed in Supplementary Table 1, only online). Accordingly, we suggest that newly developed RBBB with a relatively longer PR interval at 1 year following HT might be strongly associated with graft rejection involving conduction system.
Our study had several limitations. First, this study was a retrospective single-center study, even though the data were collected in a prospective manner. Second, our study population was too small, and the follow-up period was too short, only up to 1 year. Third, endomyocardial biopsies were just performed two times at 1 month and at 1 year following HT. It remains still unknown what kind of histologic changes have occurred in relation to ECG between 1 month and 1 year after HT. However, a recent study showed that morbidity and mortality of lower frequency routine surveillance EMB were comparable with data from the ISHLT registry.27 Fourth, we did not analyze these new parameters for graft rejection in all the HT recipients. Considering the prognostic importance of antibody mediated rejection and donor specific antibody in HT recipients, further study of the relationship between RBBB and these parameters is warranted. Fifth, we did not measure rejection-related biomarkers, such as natriuretic peptide, CK-MB, and troponin, serially in all HT recipients. Sixth, we could not obtain and analyze the ECG of every donor heart in this study. Finally, clinical outcomes, such as sudden cardiac death or occurrence of heart failure, related primarily to conduction disturbance or acute graft rejection were not analyzed. Further larger long-term follow-up study is warranted to confirm our hypothesis-generating findings. In conclusion, close monitoring for newly developed RBBB and a relatively longer PR interval at 1 year following HT may help to identify patients at high risk for graft rejection.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jaewon Oh, Seok-Min Kang.
- Data curation: Min Ji Kim.
- Formal analysis: In-Cheol Kim.
- Methodology: Jin-Ho Kim.
- Supervision: Hui-Nam Pak.
- Validation: Jae-Sun Uhm.
- Visualization: Jin-Ho Kim.
- Writing—original draft: Jin-Ho Kim.
- Writing—review & editing: Jin-Ho Kim, Jaewon Oh, Seok-Min Kang.
SUPPLEMENTARY MATERIAL
PR Interval and QRS Duration at 1 Year according to Change in Graft Rejection Following HT
References
- 1.Sandhu JS, Curtiss EI, Follansbee WP, Zerbe TR, Kormos RL. The scalar electrocardiogram of the orthotopic heart transplant recipient. Am Heart J. 1990;119:917–923. doi: 10.1016/s0002-8703(05)80332-1. [DOI] [PubMed] [Google Scholar]
- 2.Gao SZ, Hunt SA, Wiederhold V, Schroeder JS. Characteristics of serial electrocardiograms in heart transplant recipients. Am Heart J. 1991;122(3 Pt 1):771–774. doi: 10.1016/0002-8703(91)90524-l. [DOI] [PubMed] [Google Scholar]
- 3.Leonelli FM, Pacifico A, Young JB. Frequency and significance of conduction defects early after orthotopic heart transplantation. Am J Cardiol. 1994;73:175–179. doi: 10.1016/0002-9149(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 4.Jessen ME, Olivari MT, Wait MA, Meyer DM, Yancey CW, Jr, Ring WS. Frequency and significance of right bundle branch block after cardiac transplantation. Am J Cardiol. 1994;73:1009–1011. doi: 10.1016/0002-9149(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 5.Golshayan D, Seydoux C, Berguer DG, Stumpe F, Hurni M, Ruchat P, et al. Incidence and prognostic value of electrocardiographic abnormalities after heart transplantation. Clin Cardiol. 1998;21:680–684. doi: 10.1002/clc.4960210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osa A, Almenar L, Arnau MA, Martínez-Dolz L, Rueda J, Morillas P, et al. Is the prognosis poorer in heart transplanted patients who develop a right bundle branch block? J Heart Lung Transplant. 2000;19:207–214. doi: 10.1016/s1053-2498(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Foerster A. The conduction system in human cardiac allografts. A histological and immunopathological study. Pathol Res Pract. 1992;188:783–790. doi: 10.1016/S0344-0338(11)80178-0. [DOI] [PubMed] [Google Scholar]
- 8.Calzolari V, Angelini A, Basso C, Livi U, Rossi L, Thiene G. Histologic findings in the conduction system after cardiac transplantation and correlation with electrocardiographic findings. Am J Cardiol. 1999;84:756–759. doi: 10.1016/s0002-9149(99)00431-2. [DOI] [PubMed] [Google Scholar]
- 9.Chan JB, Levi DS, Lai CK, Alejos JC, Fishbein MC. Cellular rejection of the conduction system after orthotopic heart transplantation for congenital atrioventricular block. J Heart Lung Transplant. 2006;25:1371–1375. doi: 10.1016/j.healun.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, et al. Prognostic significance of prolonged PR interval in the general population. Eur Heart J. 2014;35:123–129. doi: 10.1093/eurheartj/eht176. [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Marcus GM, Hoang KL, Hunt SA, Chun SH, Lee BK. Prevalence, patterns of development, and prognosis of right bundle branch block in heart transplant recipients. Am J Cardiol. 2006;98:1288–1290. doi: 10.1016/j.amjcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Villa AE, de Marchena EJ, Myerburg RJ, Castellanos A. Comparisons of paired orthotopic cardiac transplant donor and recipient electrocardiograms. Am Heart J. 1994;127:70–74. doi: 10.1016/0002-8703(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferretto S, Tafciu E, Giuliani I, Feltrin G, Bottio T, Gambino A, et al. Interventricular conduction disorders after orthotopic heart transplantation: risk factors and clinical relevance. Ann Noninvasive Electrocardiol. 2017;22:e12402. doi: 10.1111/anec.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martí S, Almenar L, Luis Vicente J, Torregrosa S, Roldán I, Moreno MT, et al. [Which variables predict the appearance of right bundle-branch block and its course after heart transplantation?] Rev Esp Cardiol. 1996;49:328–333. [PubMed] [Google Scholar]
- 17.Thalen HJTh, Meere CC. Fundamentals of cardiac pacing. 1st ed. The Hague: Springer; 1979. p. 44. [Google Scholar]
- 18.Butman SM, Phibbs B, Wild J, Copeland JG. One heart, two bodies: insight from the transplanted heart and its new electrocardiogram. Am J Cardiol. 1990;66:632–635. doi: 10.1016/0002-9149(90)90493-k. [DOI] [PubMed] [Google Scholar]
- 19.Alderman EL, Wexler L. Angiographic implications of cardiac transplantation. Am J Cardiol. 1989;64:16E–21E. doi: 10.1016/0002-9149(89)90729-7. [DOI] [PubMed] [Google Scholar]
- 20.Leonelli FM, Dunn JK, Young JB, Pacifico A. Natural history, determinants, and clinical relevance of conduction abnormalities following orthotopic heart transplantation. Am J Cardiol. 1996;77:47–51. doi: 10.1016/s0002-9149(97)89133-3. [DOI] [PubMed] [Google Scholar]
- 21.Brunner-La Rocca HP, Sütsch G, Schneider J, Follath F, Kiowski W. Natural course of moderate cardiac allograft rejection (International Society for Heart Transplantation grade 2) early and late after transplantation. Circulation. 1996;94:1334–1338. doi: 10.1161/01.cir.94.6.1334. [DOI] [PubMed] [Google Scholar]
- 22.Kwok CS, Rashid M, Beynon R, Barker D, Patwala A, Morley-Davies A, et al. Prolonged PR interval, first-degree heart block and adverse cardiovascular outcomes: a systematic review and meta-analysis. Heart. 2016;102:672–680. doi: 10.1136/heartjnl-2015-308956. [DOI] [PubMed] [Google Scholar]
- 23.Payne ME, Murray KD, Watson KM, Galbraith TA, Horwanitz EP, Starling RC, et al. Permanent pacing in heart transplant recipients: underlying causes and long-term results. J Heart Lung Transplant. 1991;10(5 Pt 1):738–742. [PubMed] [Google Scholar]
- 24.Collins KK, Thiagarajan RR, Chin C, Dubin AM, Van Hare GF, Robbins RC, et al. Atrial tachyarrhythmias and permanent pacing after pediatric heart transplantation. J Heart Lung Transplant. 2003;22:1126–1133. doi: 10.1016/s1053-2498(02)01193-2. [DOI] [PubMed] [Google Scholar]
- 25.Cannon BC, Denfield SW, Friedman RA, Fenrich AL, Dreyer WJ, Towbin JA, et al. Late pacemaker requirement after pediatric orthotopic heart transplantation may predict the presence of transplant coronary artery disease. J Heart Lung Transplant. 2004;23:67–71. doi: 10.1016/s1053-2498(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 26.Grutter G, Alfieri S, Calcagni G, Castelluzzo MA, Silvetti MS, Parisi F, et al. Paroxysmal atrioventricular block after heart transplantation in children: an early sign of rejection? Pediatr Transplant. 2016;20:1164–1167. doi: 10.1111/petr.12832. [DOI] [PubMed] [Google Scholar]
- 27.Weckbach LT, Maurer U, Schramm R, Huber BC, Lackermair K, Weiss M, et al. Lower frequency routine surveillance endomyocardial biopsies after heart transplantation. PLoS One. 2017;12:e0182880. doi: 10.1371/journal.pone.0182880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PR Interval and QRS Duration at 1 Year according to Change in Graft Rejection Following HT