Abstract
Background
Neuroblastoma is a rare malignant disease that primarily affects children. The tumours mainly develop in the adrenal medullary tissue, and an abdominal mass is the most common presentation. High‐risk disease is characterised by metastasis and other primary tumour characteristics resulting in increased risk for an adverse outcome. The GD2 carbohydrate antigen is expressed on the cell surface of neuroblastoma tumour cells and is thus a promising target for anti‐GD2 antibody‐containing immunotherapy.
Objectives
To assess the efficacy of anti‐GD2 antibody‐containing postconsolidation immunotherapy after high‐dose chemotherapy (HDCT) and autologous haematopoietic stem cell transplantation (HSCT) compared to standard therapy after HDCT and autologous HSCT in people with high‐risk neuroblastoma. Our primary outcomes were overall survival and treatment‐related mortality. Our secondary outcomes were progression‐free survival, event‐free survival, early toxicity, late non‐haematological toxicity, and health‐related quality of life.
Search methods
We searched the electronic databases CENTRAL (2018, Issue 9), MEDLINE (PubMed), and Embase (Ovid) on 20 September 2018. We searched trial registries and conference proceedings on 28 October 2018. Further searches included reference lists of recent reviews and relevant articles as well as contacting experts in the field. There were no limits on publication year or language.
Selection criteria
Randomised controlled trials evaluating anti‐GD2 antibody‐containing immunotherapy after HDCT and autologous HSCT in people with high‐risk neuroblastoma.
Data collection and analysis
Two review authors independently performed study selection, abstracted data on study and participant characteristics, and assessed risk of bias and GRADE. Any differences were resolved by discussion, with third‐party arbitration unnecessary. We performed analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions. We used the five GRADE considerations, that is study limitations, consistency of effect, imprecision, indirectness, and publication bias, to judge the quality of the evidence.
Main results
We identified one randomised controlled trial that included 226 people with high‐risk neuroblastoma who were pre‐treated with autologous HSCT. The study randomised 113 participants to receive immunotherapy including isotretinoin, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interleukin‐2, and ch14.18, a type of anti‐GD2 antibody also known as dinutuximab. The study randomised another 113 participants to receive standard therapy including isotretinoin.
The results on overall survival favoured the dinutuximab‐containing immunotherapy group (hazard ratio (HR) 0.50, 95% confidence interval (CI) 0.31 to 0.80; P = 0.004). The results on event‐free survival also favoured the dinutuximab‐containing immunotherapy group (HR 0.61, 95% CI 0.41 to 0.92; P = 0.020). Randomised data on adverse events were not reported separately. The study did not report progression‐free survival, late non‐haematological toxicity, and health‐related quality of life as separate endpoints. We graded the quality of the evidence as moderate.
Authors' conclusions
The evidence base favours dinutuximab‐containing immunotherapy compared to standard therapy concerning overall survival and event‐free survival in people with high‐risk neuroblastoma pre‐treated with autologous HSCT. Randomised data on adverse events are lacking, therefore more research is needed before definitive conclusions can be made regarding this outcome.
Plain language summary
Anti‐GD2 antibody‐containing immunotherapy for people with high‐risk neuroblastoma treated with autologous haematopoietic stem cell transplantation
Review question
We searched for studies of people with high‐risk neuroblastoma who received high‐dose chemotherapy (which kills stem cells) followed by autologous (from the same person) haematopoietic stem cell (blood cell precursors) transplantation (to rescue and replace the killed stem cells). We reviewed the evidence concerning the effect of anti‐GD2 antibody‐containing immunotherapy compared to standard therapy in such people on overall survival, treatment‐related death, progression‐free survival, event‐free survival, early toxicity, late non‐haematological toxicity, and health‐related quality of life.
Background
Neuroblastoma is a rare form of cancer that primarily affects children. High‐risk disease is characterised by metastasis at diagnosis and other primary tumour characteristics resulting in increased risk for an poor outcome. The GD2 carbohydrate antigen is expressed on the cell surface of neuroblastoma tumour cells and is thus a promising target for anti‐GD2 antibody‐containing immunotherapy.
Study characteristics
The evidence is current to 20 September 2018. We included a single randomised trial (a type of study in which participants are assigned to one of two or more treatment groups using a random method) with 113 people allocated to immunotherapy including isotretinoin, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interleukin‐2, and ch14.18, a distinct type of anti‐GD2 antibody also known as dinutuximab. Another 113 people were allocated to receive standard therapy including isotretinoin.
Key results
The results on overall survival and event‐free survival favoured the dinutuximab‐containing immunotherapy group. Other outcomes including those on adverse events were not adequately reported; more research is needed before definitive conclusions can be made regarding these outcomes.
Quality of the evidence
We assessed the quality of the evidence as moderate.
Summary of findings
Summary of findings for the main comparison. Dinutuximab‐containing immunotherapy compared to standard therapy for people with high‐risk neuroblastoma pre‐treated with autologous hematopoietic stem cell transplantation.
| Dinutuximab‐containing immunotherapy compared to standard therapy for people with high‐risk neuroblastoma pre‐treated with autologous haematopoietic stem cell transplantation | ||||||
| Patient or population: People with high‐risk neuroblastoma pre‐treated with autologous haematopoietic stem cell transplantation Setting: Paediatric oncology and haematology, specialised centres Intervention: Dinutuximab‐containing immunotherapy Comparison: Standard therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard therapy | Risk with dinutuximab‐containing immunotherapy | |||||
| Overall survival (reported as mortality) | 549 per 10001 | 328 per 1000 (219 to 471) | HR 0.50 (0.31 to 0.80) | 226 (1 RCT) | ⊕⊕⊕⊖ MODERATE2,3 | The follow‐up time regarding all randomised participants is unclear. Median follow‐up after randomisation in participants alive without an event was 2.0 years (5 days to 6.5 years) and 2.1 years (4 days to 6.9 years) for the dinutuximab‐containing immunotherapy arm and the standard therapy arm, respectively. |
| Treatment‐related mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided. Randomised data were mixed with non‐randomised data, and the randomised data were not analysed separately, so this outcome could not be assessed. |
| Progression‐free survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided. |
| Event‐free survival (reported as relapse, progressive disease, secondary cancer, and mortality) | 717 per 10001 | 537 per 1000 (404 to 687) | HR 0.61 (0.41 to 0.92) | 226 (1 RCT) | ⊕⊕⊕⊖ MODERATE3,4 | The follow‐up time regarding all randomised participants is unclear. Median follow‐up after randomisation in participants alive without an event was 2.0 years (5 days to 6.5 years) and 2.1 years (4 days to 6.9 years) for the dinutuximab‐containing immunotherapy arm and the standard therapy arm, respectively. |
| Early toxicity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided. Randomised data were mixed with non‐randomised data, and the randomised data were not analysed separately, so this outcome could not be assessed. |
| Late non‐haematological toxicity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided. |
| Health‐related quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The assumed risk is based on the number of events in the control group at the final time point of the survival curve presented in the included study. 2The presence of selection bias, performance bias, attrition bias, and other bias is unclear; there is a low risk of detection bias and reporting bias; we downgraded one level for study limitations. 3We did not downgrade for imprecision. The study is small but the effect is large and the confidence interval is below no effect. 4The presence of selection bias, performance bias, detection bias, attrition bias, and other bias is unclear; there is a low risk of reporting bias; we downgraded one level for study limitations.
Background
Description of the condition
Neuroblastoma is a rare malignant disease that primarily affects children (GARD 2016; Park 2013; Pinto 2015). Tumours develop in the sympathetic nervous system (e.g. in the adrenal medullary tissue or paraspinal ganglia) and may be localised or metastatic at diagnosis (Bagatell 2016; Cole 2012; Matthay 2016). The median age at diagnosis is 17 months, and the incidence rate of neuroblastoma is age‐dependent: 64 per million children in the first year of life, falling to 29 per million children in the second year of life (Goodman 1999). The incidence rate in adults is less than one per million per year, but adults have a considerably worse prognosis (Esiashvili 2007). The International Neuroblastoma Risk Group (INRG) classification system, proposed by Cohn 2009, is shown in Table 2. The authors of Cohn 2009 have estimated the event‐free survival for each of the four risk groups and tested the clinical importance of 13 potential prognostic factors. The Children's Oncology Group (COG) assignment to low‐, intermediate‐, and high‐risk groups, published by the National Cancer Institute (NCI) at the National Institutes of Health (NIH) (NCI PDQ 2018), is shown in Table 3.
1. The International Neuroblastoma Risk Group (INRG) consensus pre‐treatment classification schema.
| INRG stage | Age (months) | Histologic category | Grade of tumour differentiation | MYCN | 11q aberration | Ploidy | Pre‐treatment risk group | |
| Code | Interpretation | |||||||
| L1/L2 | ‐ | Ganglioneuroma maturing; ganglioneuroblastoma intermixed | ‐ | ‐ | ‐ | ‐ | A | Very low |
| L1 | ‐ | Any, except ganglioneuroma or ganglioneuroblastoma | ‐ | Not amplified | ‐ | ‐ | B | Very low |
| Amplified | ‐ | ‐ | K | High | ||||
| L2 | < 18 | Any, except ganglioneuroma or ganglioneuroblastoma | ‐ | Not amplified | No | ‐ | D | Low |
| Yes | ‐ | G | Intermediate | |||||
| ≥ 18 | Ganglioneuroblastoma nodular; neuroblastoma | Differentiating | Not amplified | No | ‐ | E | Low | |
| Yes | ‐ | H | Intermediate | |||||
| Poorly differentiated or undifferentiated | Not amplified | ‐ | ‐ | H | Intermediate | |||
| ‐ | Amplified | ‐ | ‐ | N | High | |||
| M | < 18 | ‐ | ‐ | Not amplified | ‐ | Hyperdiploid | F | Low |
| < 12 | ‐ | ‐ | Not amplified | ‐ | Diploid | I | Intermediate | |
| 12 to < 18 | ‐ | ‐ | Not amplified | ‐ | Diploid | J | Intermediate | |
| < 18 | ‐ | ‐ | Amplified | ‐ | ‐ | O | High | |
| ≥ 18 | ‐ | ‐ | ‐ | ‐ | ‐ | P | High | |
| MS | < 18 | ‐ | ‐ | Not amplified | No | ‐ | C | Very low |
| Yes | ‐ | Q | High | |||||
| Amplified | ‐ | ‐ | R | High | ||||
Reference: Cohn 2009
The International Neuroblastoma Risk Group (INRG) consensus classification schema includes the criteria for INRG stage, age, histologic category, grade of tumour differentiation, MYCN status, presence/absence of 11q aberrations, and tumour cell ploidy. Sixteen statistically and/or clinically different pre‐treatment groups of patients (lettered A through R) have been identified using these criteria. The categories are designated as very low (A, B, C), low (D, E, F), intermediate (G, H, I, J), or high (K, N, O, P, Q, R) pre‐treatment risk subsets.
Abbreviations: MYCN: the official gene symbol approved by the Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC), which is a short abbreviated form of the gene name 'v‐myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog'.
2. Children's Oncology Group (COG) assignment to low‐, intermediate‐, and high‐risk group.
| INSS stage | Age | MYCN | INPC classification | DNA index | Risk group |
| 1 | 0 to 21 y | Any | Any | Any | Low |
| 2A/2B | < 365 d | Any | Any | Any | Low |
| ≥ 365 d to 21 y | Non‐amplified | Any | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Favourable | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Unfavourable | ‐ | High | |
| 3 | < 365 d | Non‐amplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 365 d to 21 y | Non‐amplified | Favourable | ‐ | Intermediate | |
| ≥ 365 d to 21 y | Non‐amplified | Unfavourable | ‐ | High | |
| ≥ 365 d to 21 y | Amplified | Any | ‐ | High | |
| 4 | < 548 d | Non‐amplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 548 d to 21 y | Any | Any | ‐ | High | |
| 4S | < 365 d | Non‐amplified | Favourable | > 1 | Low |
| < 365 d | Non‐amplified | Any | = 1 | Intermediate | |
| < 365 d | Non‐amplified | Unfavourable | Any | Intermediate | |
| < 365 d | Amplified | Any | Any | High |
Reference: NCI PDQ 2018
DNA index: favourable > 1 (hyperdiploid) or < 1 (hypodiploid); unfavourable = 1 (diploid)
Abbreviations: d: days; INPC: International Neuroblastoma Pathology Committee (also called Shimada system); INSS: The International Neuroblastoma Staging System; MYCN: the official gene symbol approved by the Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC), which is a short abbreviated form of the gene name 'v‐myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog'; y: years
Neuroblastoma originates mostly in the abdomen, in the adrenal medulla, or along the sympathetic nervous system side chain. As approximately 70% of children with neuroblastoma have metastatic disease at diagnosis, organ‐specific symptoms may be caused by the local presence of metastases, such as eye problems associated with retrobulbar tumours, pancytopenia associated with bone marrow infiltration, abdominal distension and respiratory problems associated with liver enlargement, and paralysis and Horner syndrome associated with ganglion involvement (Maris 2010; Matthay 2016; NCI PDQ 2018). Some neuroblastomas regress spontaneously without therapy, whereas others progress and have a fatal outcome despite therapy (NCI PDQ 2018). A tumour mass and the presence of metastases may be confirmed on ultrasound, X‐ray, computed tomography, scintigraphy using metaiodobenzylguanidine (MIBG scan), or magnetic resonance imaging. Guidelines for using imaging methods have been developed in response to the increased importance of image‐defined factors in staging and risk assessment (Bleeker 2015; Brisse 2011). The International Neuroblastoma Staging System (INSS) for neuroblastoma is shown in Table 4; INSS definitions of treatment response are shown in Table 5 (Brodeur 1993).
3. The International Neuroblastoma Staging System (INSS).
| Stage | Definition |
| 1 | Localised tumour with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph nodes negative for tumour microscopically (nodes attached to and removed with the primary tumour may be positive) |
| 2A | Localised tumour with incomplete gross excision; representative ipsilateral non‐adherent lymph nodes negative for tumour microscopically |
| 2B | Localised tumour with or without complete gross excision, with ipsilateral non‐adherent lymph nodes positive for tumour. Enlarged contralateral lymph nodes must be negative microscopically. |
| 3 | Unresectable unilateral tumour infiltrating across the midline,1 with or without regional lymph node involvement; or localised unilateral tumour with contralateral regional lymph node involvement; or midline tumour with bilateral extension by infiltration (unresectable) or by lymph node involvement |
| 4 | Any primary tumour with dissemination to distant lymph nodes, bone, bone marrow, liver, skin, and/or other organs (except as defined for stage 4S) |
| 4S | Localised primary tumour (as defined for stages 1, 2A, or 2B), with dissemination limited to skin, liver, and/or bone marrow2 (limited to infants < 1 year of age) |
Reference: Brodeur 1993
Note: Multifocal primary tumours (e.g. bilateral adrenal primary tumours) should be staged according to the greatest extent of disease, as defined above, and followed by a subscript letter 'M' (e.g. 3M). 1The midline is defined as the vertebral column. Tumours originating on one side and crossing the midline must infiltrate to or beyond the opposite side of the vertebral column. 2Marrow involvement in stage 4S should be minimal (i.e. < 10% of total nucleated cells identified as malignant on bone marrow biopsy or on marrow aspirate). More extensive marrow involvement would be considered to be stage 4. An MIBG (metaiodobenzylguanidine) scan (if performed) should be negative in the marrow.
4. Response to treatment.
| Response | Primary tumour | Metastatic sites |
| Complete response | No tumour | No tumour; catecholamines normal |
| Very good partial response | Decreased by 90% to 99% | No tumour; catecholamines normal; residual 99Tc bone changes allowed |
| Partial response | Decreased by more than 50% | All measurable sites decreased by greater than 50%. Bones and bone marrow: number of positive bone sites decreased by greater than 50%; no more than one positive bone marrow site allowed |
| Minimal response | No new lesions; more than 50% reduction in any measurable lesion (primary or metastases) with less than 50% reduction in any other; less than 25% increase in any existing lesion | |
| No response | No new lesions; less than 50% reduction but less than 25% increase in any existing lesion | |
| Progressive disease | Any new lesion; greater than 25% increase in any measurable lesion; previous negative marrow positive for tumour | |
Reference: Brodeur 1993
The American Cancer Society has reported the five‐year survival rates for neuroblastoma based on the COG risk groups: low risk (95%), intermediate risk (90% to 95%), and high risk (40% to 50%) (American Cancer Society 2016; Park 2013). It was stated: "The 5‐year survival rate refers to the percentage of children who live at least 5 years after their cancer is diagnosed." It should be noted that historical survival data should be used cautiously, as medical advancement has achieved more favourable outcomes.
Description of the intervention
According to Pinto 2015, the current standard‐of‐care treatment strategy for high‐risk neuroblastoma consists of three treatment blocks: induction (chemotherapy and primary tumour resection), consolidation (high‐dose chemotherapy with autologous stem cell rescue and external‐beam radiotherapy), and postconsolidation (anti‐GD2 immunotherapy with cytokines and cis‐retinoic acid). Rapid COJEC (cisplatin, vincristine, carboplatin, etoposide, and cyclophosphamide) induction chemotherapy was evaluated by Peinemann 2015b; high‐dose chemotherapy with autologous stem cell rescue by Yalcin 2015; anti‐GD2 antibody ch14.18 combined with cytokines by Yu 2010; and retinoic acid by Peinemann 2015c.
A small number of tumour cells may survive and cause regrowth and new metastasis. This so‐called minimal residual disease is associated with relapse and eventual cancer treatment failure (Kushner 2015). Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti‐GD2 immunotherapy (Cheung 2015; Stutterheim 2011; Viprey 2014). As the majority of patients relapse even after intense therapy, research tries to identify alternative treatment modalities that hopefully can attain an improved outcome (Cheung 2015). Immunotherapy is one of various alternatives, and it has shown promising effects not only with neuroblastoma but also with other diseases such as metastasising renal cell carcinoma (Unverzagt 2017). Ploessl 2016 stated: "For patients achieving clinical remission, limited treatments exist for preventing relapse." The ganglioside GD2 is a T‐cell independent carbohydrate antigen that is expressed on the cell surface during foetal development (Perez‐Horta 2016). In healthy people, it is restricted mainly to neurons, melanocytes, and pain fibres (Cheung 2013). However, it is highly expressed on neuroectoderm‐derived tumours including neuroblastoma (Ahmed 2014). GD2 is thus a promising target for antibody‐based immunotherapy (Cheung 2013). It is believed that anti‐GD2 antibody therapy can achieve continual remission (Cheung 2013); may improve relapses (Matthay 2012); and enable long‐term progression‐free survival (Kushner 2015). Anti‐GD2 antibodies may be applied either as monotherapy or as part of an immunotherapy that combines anti‐GD2 antibodies with interleukin‐2 and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) (Kushner 2015).
'Anti‐GD2 antibodies' is an umbrella term for various types of anti‐GD2 antibodies for GD2‐expressing neuroblastoma, for example murine antibodies, chimeric antibody, and humanised antibodies (Cheung 2012; Cheung 2014; Navid 2010). This Cochrane Review includes various possible anti‐GD2 monoclonal antibodies. When anti‐GD2 antibodies are mentioned, they are identified appropriately, such as 14G2a, ch14.18, ch14.18beta, 3F8, hu3F8, or hu14.18.
Concerning the dose of anti‐GD2, Simon 2011a applied 20 mg/m2/day on five subsequent days, and ANBL0032 applied 25 mg/m2/day for four subsequent days. According to Navid 2014, "The most common non–dose‐limiting grade 3 or 4 toxicities during course one were pain in 68% (26 of 38) patients and fever in 21% (8 of 38) patients." Pain management may include paracetamol (acetaminophen), ibuprofen, morphine, fentanyl, gabapentin, and lidocaine (Ploessl 2016). Other reported toxicities include anaphylactoid reactions, posterior reversible encephalopathy syndrome (Kushner 2016), as well as capillary leak syndrome and hypotension (Ploessl 2016). The application of further toxic drugs is thus required to keep adverse events manageable.
Why it is important to do this review
According to the follow‐up analysis of a cohort study, Simon 2011a reported that anti‐GD2 antibody ch14.18 provided a better nine‐year overall survival compared to no additional therapy. Simon 2011a also suggested that anti‐GD2 antibody ch14.18 may prevent late relapses. This would be a vital improvement because it is known that recurrent disease can occur in any patient including those who have achieved complete remission (Berthold 2018). Based on a randomised study, Yu 2010 concluded that immunotherapy with ch14.18, GM‐CSF, and interleukin‐2 was associated with a significantly improved outcome as compared with standard therapy in people with high‐risk neuroblastoma. It is important to evaluate if such a beneficial outcome can be substantiated with available study data. It is also important to evaluate potential treatment‐related complications and to search for ongoing trials that might influence the conclusion in timely updates.
Objectives
To assess efficacy of anti‐GD2 antibody‐containing postconsolidation immunotherapy after high‐dose chemotherapy (HDCT) and autologous haematopoietic stem cell transplantation (HSCT) compared to standard therapy after HDCT and autologous HSCT in people with high‐risk neuroblastoma. Our primary outcomes were overall survival and treatment‐related mortality. Our secondary outcomes were progression‐free survival, event‐free survival, early toxicity, late non‐haematological toxicity, and health‐related quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials to evaluate efficacy.
Types of participants
People with high‐risk neuroblastoma according to the INRG or the COG classification of risk groups shown in Table 2 and Table 3. We planned that if not all participants were eligible for inclusion, we would include such studies when information on eligible participants was presented separately.
Types of interventions
Intervention
Anti‐GD2 antibody‐containing immunotherapy as part of a postconsolidation therapy after high‐dose chemotherapy followed by autologous haematopoietic stem cell transplantation.
Comparator
Standard therapy after high‐dose chemotherapy followed by autologous haematopoietic stem cell transplantation.
Types of outcome measures
The outcomes listed here are not used as criteria for including studies, but are the outcomes of interest within studies identified for inclusion.
Primary outcomes
Overall survival (as defined in the original studies).
Treatment‐related mortality: incidence of deaths that were classified as treatment related or the participants died of treatment complications.
Secondary outcomes
Progression‐free survival (as defined in the original studies).
Event‐free survival (as defined in the original studies).
Early toxicity: adverse events within 90 days of the therapy; incidence of all reported adverse events and severe (grade 3 and 4) events; and incidence of toxicity‐related discontinuations from treatment. Examples of potentially used classification systems: National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 2018); World Health Organization (WHO) toxicity grading scale for determining the severity of adverse events (ICSSC 2003).
Late non‐haematological toxicity such as organ toxicity and secondary malignancy.
Health‐related quality of life measured by validated questionnaires.
Search methods for identification of studies
We used search methods as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011) and by Cochrane Childhood Cancer (Kremer 2018). We planned to update the search every two years. We did not apply any publication year or language restrictions. Cochrane Childhood Cancer ran the searches in CENTRAL, MEDLINE, and Embase; the review authors ran all other searches.
Electronic searches
We conducted an electronic literature database search in the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library) on 20 September 2018 in Issue 9 of 2018 (Appendix 1). We searched in MEDLINE (PubMed format) on 20 September 2018 for articles published from 1946 onwards using the search strategy shown in Appendix 2, and we searched in Embase (Ovid format) on 20 September 2018 for articles published from 1980 onwards (Appendix 3). We tailored the terms and syntax used for the search in MEDLINE to the requirements of the other two databases.
We scanned the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) for ongoing trials on 28 October 2018 using the term 'neuroblastoma' in the field condition and 'anti gd2' in the field intervention. We also scanned the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) for ongoing trials on 28 October 2018 using the term 'neuroblastoma AND anti gd2' in the search field.
Searching other resources
We searched for information about trials not registered in electronic databases, either published or unpublished, in the reference lists of relevant articles and review articles. We asked experts in the field for missing information on potentially eligible studies.
We searched for abstracts presented at the annual meetings of the American Society of Clinical Oncology (ASCO), the American Society of Pediatric Hematology/Oncology (ASPHO), the International Society of Paediatric Oncology (SIOP), and the Advances in Neuroblastoma Research (ANR) Association using the terms 'neuroblastoma' and 'anti‐gd2'. We planned to search the abstracts of the last five consecutive annual meetings. We searched the abstracts in the congress proceedings of the following ANR meetings, which took place every other year: ANR Meeting 2012; ANR Meeting 2014; ANR Meeting 2016; ANR Meeting 2018. We searched the abstracts in the congress proceedings of SIOP Meeting 2017 and SIOP Meeting 2018, and we searched the online meeting archive of ASCO Meeting 2018. We did not search abstracts of meetings before 2012. The abstracts of the following meetings were covered by the search results from the Embase database: ASCO meetings from 2012 to 2017, ASPHO meetings from 2012 to 2018, and SIOP meetings from 2012 to 2016; we therefore did not double search the congress proceedings of those.
Data collection and analysis
Selection of studies
While preparing this systematic review, we endorsed the PRISMA statement, adhered to its principles, and conformed to its checklist (Moher 2009). We downloaded all titles and abstracts retrieved by electronic searching to an Excel spreadsheet and removed any duplicates (Microsoft Corp 2011). Two review authors independently examined any remaining references. We included a study selection flow chart in the review (Figure 1).
1.
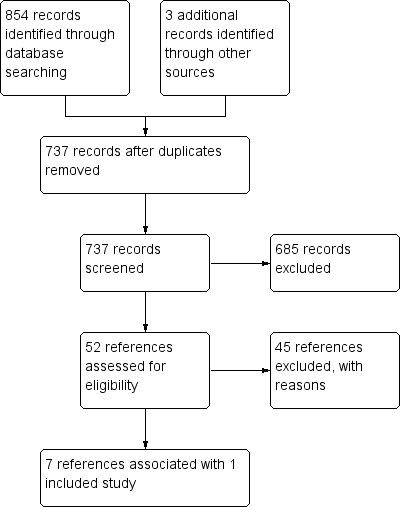
Study flow diagram.
We excluded those studies that clearly did not meet our inclusion criteria and obtained full‐text copies of potentially relevant references. Two review authors independently assessed the eligibility of retrieved papers. Any disagreements were resolved by discussion between the two review authors, with no third‐party arbitration needed. We documented reasons for exclusion. If we identified multiple reports of one study, we used the most‐up‐to‐date full‐text results. We checked the multiple reports for possible duplicate data, addressed the issue, and did not include duplicate data in the analysis.
Data extraction and management
Two review authors independently extracted from the included studies data on characteristics of participants (inclusion criteria, age, stage, comorbidity, previous treatment, number enrolled in each arm) and interventions (type of anti‐GD2 antibody‐containing immunotherapy and standard induction therapy, dose applied, duration of therapy, control treatment), risk of bias, duration of follow‐up, outcomes, funding source, conflicts of interest of primary investigators, and deviations from protocol into the review. We noted the time points at which outcomes were collected and reported. Any differences between review authors were resolved by discussion with no third‐party arbitration needed.
Assessment of risk of bias in included studies
Two review authors independently appraised the risk of bias in the included studies. Any differences between review authors were resolved by discussion with no third‐party arbitration needed. We used the items listed in the module of Cochrane Childhood Cancer (Kremer 2018), which is based on Cochrane's tool for assessing risk of bias (Higgins 2011a):
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants (performance bias);
blinding of personnel (performance bias);
blinding of outcome assessment (detection bias) for each outcome separately if appropriate;
incomplete outcome data such as missing data (attrition bias) for each outcome separately;
selective reporting, such as not reporting prespecified outcomes (reporting bias); and
other sources of bias, such as bias related to the specific study design (other bias).
We applied Cochrane's criteria for judging risk of bias (Higgins 2011a). In general, a 'low risk' of bias was attributed if plausible bias was unlikely to have a marked effect on the results; for example, participants and investigators enrolling participants could not have foreseen assignment. A 'high risk' of bias was attributed if plausible bias seriously weakened confidence in the results; for example, participants or investigators enrolling participants could possibly have foreseen assignments. An 'unclear' risk of bias was attributed if plausible bias raised some doubts about the results; for example, the method of concealment was not described or not described in sufficient detail to permit a definitive judgement. In addition to a 'Risk of bias' table, we included a methodological quality summary figure. We took into account the results of the 'Risk of bias' assessment when interpreting the results of the review.
Measures of treatment effect
For time‐to‐event data, such as survival, we extracted the hazard ratio (HR) and its standard error or confidence interval (CI) from trial reports; if these were not reported, then we attempted to estimate the log (HR) and its standard error using the methods of Parmar 1998 and Tierney 2007.
For dichotomous outcomes (e.g. response, adverse events, and treatment‐related mortality), we planned to extract the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR). This was not applicable, as no such outcomes were addressed in the single included study.
For continuous outcomes (e.g. quality of life measures), we planned to extract the final value or change from baseline and corresponding standard deviation of the outcome of interest, and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up. We planned to analyse and present continuous data as mean difference (MD) provided all the results were measured on the same scale (e.g. length of hospital stay). If this was not the case (e.g. pain or quality of life), we planned to use the standardised mean difference (SMD). This was not applicable as no such outcomes were addressed in the single included study.
Dealing with missing data
We conformed to Cochrane's principal options for dealing with missing data (Higgins 2011b). If data were missing, or if only imputed data were reported, we planned to contact trial authors to request data on the outcomes for participants who were assessed. The single included study did not report the randomised toxicity data separately from the non‐randomised toxicity data (ANBL0032), therefore we could not include the pooled data in the review. If the study included an analysis of the randomised toxicity data only, then we could include the results in our review. We thus emailed Alice Yu, the principal investigator of the study, asking if she could send us the results of the separate analysis.
When relevant data regarding study selection, data extraction, and 'Risk of bias' assessment were missing, we attempted to contact the study authors to retrieve the missing data. The study titled 'High risk neuroblastoma study 1.7 of SIOP‐Europe (SIOPEN)', which is registered at ClinicalTrials.gov (clinicaltrials.gov/ct2/show/NCT01704716), includes four different randomisation procedures. The so‐called R4 randomisation includes retinoic acid and anti‐GD2 antibody and is described at ClinicalTrials.gov as "isotretinoin and ch14.18/CHO, with or without aldesleukin (IL‐2)". We were unsure of the exact study design. We emailed Ruth Ladenstein, principal investigator of the study, to clarify eligibility. She promptly responded that participants in both arms received the anti‐GD2 antibody "ch14.18/CHO". Because there was no arm without anti‐GD2 antibody, we excluded references associated with this study.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which we analysed all participants in the groups to which they had been assigned. If this was not possible, we would report this. The single included study conducted an intention‐to‐treat analysis.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage of heterogeneity between trials that cannot be ascribed to sampling variation (I2 statistic) (Higgins 2003), and, if possible, by sensitivity analyses. If there was evidence of substantial heterogeneity, we planned to investigate and report the possible reasons for it. We considered an I2 statistic greater than 50% as indicative of substantial heterogeneity. This was not applicable as only one study was included in the review.
Assessment of reporting biases
In addition to the evaluation of reporting bias described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias (such as publication bias, time‐lag bias, multiple (duplicate) publication bias, location bias, citation bias, language bias) by constructing a funnel plot when there was a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis), because otherwise the power of the tests would be too low to distinguish chance from real asymmetry (Sterne 2011). This was not applicable as only one study was included in the review.
Data synthesis
We analysed data using Review Manager 5 (RevMan 2014). This was done by one review author and checked by another review author. If sufficient, clinically similar studies were available, we planned to pool their results in meta‐analyses if comparable outcome definitions were used. Otherwise, we planned to present the results descriptively. If any trials had multiple treatment groups, we planned to divide the 'shared' comparison group into the number of treatment groups, and treat comparisons between each treatment group and the split comparison group as independent comparisons. Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986). Not all planned methods were applicable as only one study was included in the review. In cases with zero events in one group, we calculated P values using Fisher's exact test (Social Science Statistics 2018).
For each comparison, we used GRADEpro GDT software to prepare a 'Summary of findings' table in which we presented the following outcomes: overall survival, treatment‐related mortality, progression‐free survival, event‐free survival, early toxicity, late non‐haematological toxicity, and health‐related quality of life (GRADEpro GDT 2015). For each outcome, two review authors independently assessed the quality of the evidence by using the five GRADE considerations, that is study limitations, inconsistency, indirectness, imprecision, and publication bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Sensitivity analysis
For all outcomes for which pooling was possible, we planned to perform sensitivity analyses for all 'Risk of bias' criteria separately. We planned to exclude studies with a high risk of bias and studies for which the risk of bias was unclear in sensitivity analyses, and compare the results of studies with a low risk of bias with the results of all available studies. We planned to perform sensitivity analyses only when at least two studies with a low risk of bias remained in the analyses. This was not applicable as only one study was included in the review.
Results
Description of studies
Results of the search
Running the searches in the electronic databases CENTRAL, MEDLINE, and Embase yielded a total of 734 distinct references. We identified one reference through searching the ongoing trials registries and two references through searching the Advances in Neuroblastoma Research (ANR) congress proceedings (Figure 1). Scanning the reference lists of relevant studies and asking experts in the field did not result in any additional references.
Following initial screening of the titles, abstracts, or both, we excluded 685 references that clearly did not meet our inclusion criteria. We assessed the 52 remaining references in full, of which seven fulfilled our inclusion criteria and were thus eligible for inclusion in the review, and 45 were excluded with reasons (Characteristics of excluded studies). One full‐text article was associated with one included study (ANBL0032). Five conference proceedings and one ClinicalTrials.gov record were also associated with this study (ANBL0032); however, as it is understood that information provided in conference proceedings often differs substantially from information provided in subsequent full‐text publications (Yoon 2012), we did not include these results in our analyses. An overview of reference and study selection is provided in Figure 1.
Included studies
Details of the characteristics of the single included study, ANBL0032, are provided in the Characteristics of included studies table.
Design
We judged the single included study, ANBL0032, to be a randomised, prospective, parallel, controlled clinical trial. The study enrolled participants from 2001 to 2009.
Sample sizes
ANBL0032 randomised 226 participants with high‐risk neuroblastoma after high‐dose chemotherapy (HDCT) followed by autologous haematopoietic stem cell transplantation (HSCT) to dinutuximab‐containing immunotherapy (N = 113) or standard therapy (N = 113).
Setting
ANBL0032 was conducted as a multicentre study in 166 centres mainly in the USA, some in Canada, and a few in Australia.
Participants
ANBL0032 included people with high‐risk neuroblastoma who had a complete, very good, or partial response after HDCT followed by autologous HSCT. Subsequently, patients with progressive disease were not included. The majority of participants were classified as stage 4 of the International Neuroblastoma Staging System (INSS). The Characteristics of included studies table shows a considerable clinical heterogeneity among the participants of the study with respect to response before autologous HSCT, INSS stage, and MYCN status. A proportion of 23% (52 of 226) had partial response before autologous HSCT; 20% (45 of 226) had INSS stage 2, 3, 4S, or unknown; and 54% (123 of 226) had tumour MYCN status amplified or unknown.
Interventions
ANBL0032 randomised 113 participants to the dinutuximab‐containing immunotherapy group. The treatment of participants in the dinutuximab‐containing immunotherapy group consisted of isotretinoin + dinutuximab (a distinct type of anti‐GD2 antibody) + granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) + interleukin‐2 scheduled in 6 cycles with a total duration of 24 weeks (see Characteristics of included studies). ANBL0032 randomised 113 participants to the standard therapy group. The treatment of participants in the standard therapy group consisted of isotretinoin scheduled in 6 cycles with a total duration of 24 weeks (see Characteristics of included studies).
Primary outcome
ANBL0032 reported the following outcomes regarded as primary outcomes by the present Cochrane Review and compliant with the inclusion criteria of the present review.
Overall survival
Secondary outcomes
ANBL0032 reported the following outcomes regarded as secondary outcomes by the present Cochrane Review and compliant with the inclusion criteria of the present review.
Event‐free survival
ANBL0032 did not report progression‐free survival, late non‐haematological toxicity, or health‐related quality of life.
Excluded studies
We excluded 45 of 52 evaluated full‐text articles (Figure 1). We determined the reasons for exclusion as follows.
Not population of interest: high‐risk neuroblastoma (N = 0)
Not intervention of interest: anti‐GD2 antibody‐containing immunotherapy (N = 4)
Not comparator of interest: standard therapy (N = 22)
Not outcome of interest (N = 0)
Not study design of interest (N = 19)
The excluded studies are described in the Characteristics of excluded studies table.
Risk of bias in included studies
Details of each item of the 'Risk of bias' tool for the included study are provided in the the 'Risk of bias' table in Characteristics of included studies. An overview is provided in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of random sequence generation appeared to be conditional. We do not know if the condition applied and had any effect. We judged ANBL0032 as having an unclear risk of bias for random sequence generation. We assumed that the allocation concealment was provided by the remote system. We judged this study as having a low risk of bias for allocation concealment. Overall we judged ANBL0032 as having an unclear risk of selection bias.
Blinding
Blinding of participants and physicians and nurses was not reported. We judged ANBL0032 as having an unclear risk of performance bias. Blinding of outcome assessors was not reported, but that is not relevant for the outcome overall survival. We judged the outcome overall survival as having a low risk of detection bias. However, blinding of outcome assessors may be relevant for the outcome event‐free survival. We judged the outcome event‐free survival as having an unclear risk of detection bias.
Incomplete outcome data
In the dinutuximab‐containing immunotherapy arm, 5% (6 of 113) of randomised participants did not receive the assigned intervention, and 31% (35 of 113) did not complete the entire assigned intervention. In the standard therapy arm, 6% (7 of 113) of randomised participants did not receive the assigned intervention, and 27% (30 of 113) did not complete the entire assigned intervention. The authors stated that they performed intention‐to‐treat analyses, but provided no additional information. We judged this study as having an unclear risk of attrition bias.
Selective reporting
We did not identify signs of selective reporting when comparing the outcomes and methods of the publication with those of published protocol items at ClinicalTrials.gov. We judged ANBL0032 as having a low risk of reporting bias.
Other potential sources of bias
We judged ANBL0032 as having an unclear risk of other bias. A full explanation can be found in the 'Risk of bias' table in Characteristics of included studies.
Effects of interventions
See: Table 1
Primary outcomes
Overall survival
In the study ANBL0032, the results on overall survival favoured the dinutuximab‐containing immunotherapy group. We estimated a hazard ratio (HR) of 0.50 (95% confidence interval (CI) 0.31 to 0.80; P = 0.004) (Analysis 1.1; Figure 3) by replicating the survival functions shown in Figure 2B of the article Yu 2010using all available data. There were 113 participants in the dinutuximab‐containing immunotherapy group and 113 participants in the standard therapy group. The study authors limited the statistical testing at the time point of 2 years and estimated an overall survival of 86% (standard error +/‐ 4) in the dinutuximab‐containing immunotherapy group and an overall survival of 75% (standard error +/‐ 5) in the standard therapy group, resulting in a P value of 0.02. We judged the quality of the evidence to be moderate (see the Table 1).
1.1. Analysis.
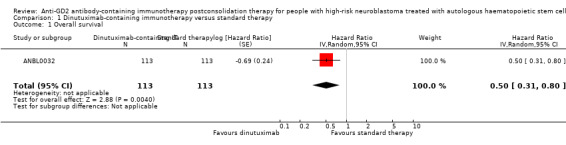
Comparison 1 Dinutuximab‐containing immunotherapy versus standard therapy, Outcome 1 Overall survival.
3.
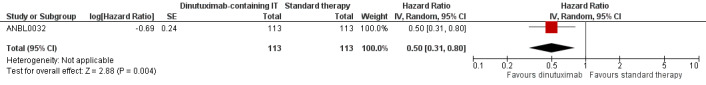
Forest plot of comparison: 1 Intervention (dinutuximab‐containing immunotherapy) versus comparator (standard therapy), outcome: 1.1 Overall survival.
CI: confidence interval; dinutuximab: dinutuximab‐containing immunotherapy; IV: inverse variance (statistical method); log: logarithm; P: P value of overall effect; Random: random‐effects (analysis method); SE: standard error (standard deviation of the sampling distribution of a statistic)
Treatment‐related mortality
In the study ANBL0032, data from randomised participants were mixed with data from non‐randomised participants. Separate data from randomised participants were not available, so this outcome could not be assessed.
Secondary outcomes
Progression‐free survival
In the study ANBL0032, progression‐free survival was not reported as a separate endpoint.
Event‐free survival
In the study ANBL0032, the results on event‐free survival also favoured the dinutuximab‐containing immunotherapy group. We estimated an HR of 0.61 (95% CI 0.41 to 0.92; P = 0.020) (Analysis 1.2; Figure 4) by replicating the survival functions shown in Figure 2A of the article Yu 2010using all available data. There were 113 participants in the dinutuximab‐containing immunotherapy group and 113 participants in the standard therapy group. The study authors limited the statistical testing at the time point of 2 years and estimated an event‐free survival of 66% (standard error +/‐ 5) in the dinutuximab‐containing immunotherapy arm and an event‐free survival of 46% (standard error +/‐ 5) in the standard therapy group, resulting in a P value of 0.01. We judged the quality of the evidence to be moderate (see the Table 1).
1.2. Analysis.
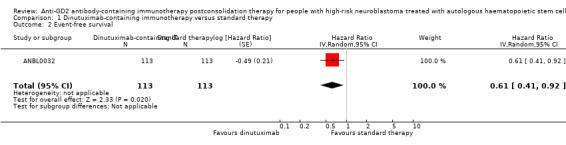
Comparison 1 Dinutuximab‐containing immunotherapy versus standard therapy, Outcome 2 Event‐free survival.
4.
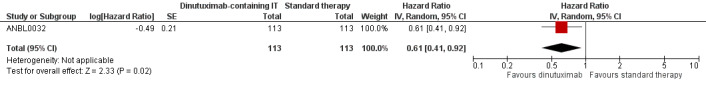
Forest plot of comparison: 1 Intervention (dinutuximab‐containing immunotherapy) versus comparator (standard therapy), outcome: 1.2 Event‐free survival.
CI: confidence interval; dinutuximab: dinutuximab‐containing immunotherapy; IV: inverse variance (statistical method); log: logarithm; P: P value of overall effect; Random: random‐effects (analysis method); SE: standard error (standard deviation of the sampling distribution of a statistic)
Early toxicity
In the study ANBL0032, data from randomised participants were mixed with data from non‐randomised participants. Separate data from randomised participants were not available, so this outcome could not be assessed.
Late non‐haematological toxicity
In the study ANBL0032, late non‐haematological toxicity was not reported as a separate endpoint.
Health‐related quality of life
In the study ANBL0032, health‐related quality of life was not reported as a separate endpoint.
Discussion
Summary of main results
The present Cochrane Review evaluated the current state of evidence on the efficacy of anti‐GD2 antibody‐containing immunotherapy versus standard therapy in people diagnosed with high‐risk neuroblastoma and pre‐treated with HDCT followed by autologous HSCT (see Table 1).
We identified a single randomised controlled trial that included 226 participants with high‐risk neuroblastoma who were randomised to receive either dinutuximab‐containing immunotherapy (N = 113) or standard therapy (N = 113) after pre‐treatment with HDCT followed by autologous HSCT (ANBL0032). The primary objective of ANBL0032 "was an intention‐to‐treat comparison of event‐free survival in the two treatment groups".
The results for our primary outcome of overall survival favoured the dinutuximab‐containing immunotherapy group (HR 0.50, 95% CI 0.31 to 0.80; P = 0.004; moderate‐quality evidence). The results for our secondary outcome of event‐free survival also favoured the dinutuximab‐containing immunotherapy group (HR 0.61, 95% CI 0.41 to 0.92; P = 0.020; moderate‐quality evidence). We calculated the HRs using the complete follow‐up period of the trial by replicating the survival functions shown in the relevant figures of the article by Yu 2010.
The results on treatment‐related mortality and early toxicity were not reported separately for randomised participants and thus were not included in the present review. No data were available for progression‐free survival, late non‐haematological toxicity, and health‐related quality of life.
Overall completeness and applicability of evidence
The inclusion of a single study, ANBL0032, in the present review limits the inferences we can make from the extracted data.
Participants were treated in the time period from 2001 to 2009 (ANBL0032). The applicability of these data to current clinical practice might be partially restricted, as medical knowledge and terms of health care have progressed and changed since then.
We could not extract and include data on treatment‐related mortality, progression‐free survival, early toxicity, late non‐haematological toxicity, and health‐related quality of life. As a result, we could not make any conclusions regarding those outcomes, which are important for clinical practice.
We only included randomised controlled trials, since it is widely recognised that this is the only study design that can be used to obtain unbiased evidence on the use of different treatment options, provided that the design and execution of the randomised controlled trials are adequate. However, even though randomised controlled trials are the highest level of evidence, it should be noted that data from non‐randomised studies are available and can be helpful in evaluating adverse events (Peinemann 2015a).
Regarding the evaluation of treatment‐related mortality and early toxicity, the authors of ANBL0032 did not report randomised and non‐randomised data separately. We asked Alice Yu of the Children's Oncology Group, the principal investigator of the included study, for the provision of separate data from randomised participants. In a kind reply Alice Yu told us that the policy of the Children's Oncology Group is that sharing of unpublished data is not permitted. We therefore could not include the data in the results section of the present review, but available data on randomised and non‐randomised participants combined are presented in Table 6 (data from 137 people in the dinutuximab‐containing immunotherapy group: 113 randomised and 25 non‐randomised, data of one person either randomised or non‐randomised could not be evaluated, and data from 108 of 113 randomised people in the control group). Alice Yu also commented "[...]that there appear to be no significant difference in toxicities between those in CR (complete response) and those with biopsy proven small residual disease". The possible risk of bias from the non‐randomised data should be kept in mind.
5. Adverse events including non‐randomised participants.
| Adverse event | Immunotherapy (N = 137) | Standard therapy (N = 108) | Risk ratio1 (95% CI) | Fisher's exact test2 |
| Adverse events statistically significantly more often in the dinutuximab‐containing immunotherapy group than in the standard therapy group | ||||
| Any toxic effect of grade 3 or 43 | 94% (129 of 137) | 63% (68 of 108) | 1.50 (1.29, 1.74) | ‐ |
| Neuropathic pain | 52% (71 of 137) | 6% (6 of 108) | 9.33 (4.22, 20.64) | ‐ |
| Hypotension | 18% (24 of 137) | 0 | ‐ | P < 0.001 |
| Hypoxia | 13% (18 of 137) | 2% (2 of 108) | 7.09 (1.68, 29.91) | ‐ |
| Fever without neutropenia | 39% (53 of 137) | 6% (6 of 108) | 6.96 (3.11, 15.59) | ‐ |
| Acute capillary leak syndrome | 23% (31 of 137) | 0 | ‐ | P < 0.001 |
| Hypersensitivity reaction | 25% (34 of 137) | 1% (1 of 108) | 26.80 (3.73, 192.68) | ‐ |
| Urticaria | 13% (18 of 137) | 0 | ‐ | P = 0 |
| Infection (any) | 39% (54 of 137) | 22% (24 of 108) | 1.77 (1.18, 2.67) | ‐ |
| Diarrhoea | 13% (18 of 137) | 1% (1 of 108) | 14.19 (1.92, 104.62) | ‐ |
| Hyponatraemia | 23% (31 of 137) | 4% (4 of 108) | 6.11 (2.22, 16.78) | ‐ |
| Hypokalaemia | 35% (48 of 137) | 2% (2 of 108) | 18.92 (4.70, 76.10) | ‐ |
| Abnormal ALT4 | 31% (31 of 137) | 3% (3 of 108) | 8.15 (2.56, 25.93) | ‐ |
| Abnormal AST4 | 10% (14 of 137) | 0 | ‐ | P < 0.001 |
| Difference in adverse events between dinutuximab‐containing immunotherapy group and standard therapy group not statistically significant | ||||
| Hypercalcaemia | 5% (7 of 137) | 6% (6 of 108) | 0.92 (0.32, 2.66) | ‐ |
| Serum sickness | 1% (1 of 137) | 0 | ‐ | P = 1 |
| Ocular symptoms | 0 | 1% (1 of 108) | ‐ | P = 0.4408 |
| Seizure | 1% (1 of 137) | 1% (1 of 108) | 1.00 (0.06, 15.83) | ‐ |
| CNS cortical symptom5 | 4% (5 of 137) | 0 | ‐ | P = 0.0687 |
| Infection, catheter related | 13% (18 of 137) | 7% (7 of 108) | 2.03 (0.88, 4.68) | ‐ |
| Nausea | 3% (4 of 137) | 1% (1 of 108) | 3.15 (0.36, 27.80) | ‐ |
| Vomiting | 6% (8 of 137) | 3% (3 of 108) | 2.10 (0.57, 7.73) | ‐ |
| Treatment‐related mortality6 | 1% (1 of 137) | 0 | ‐ | P = 1 |
1We calculated risk ratios using Review Manager 5 software (RevMan 2014). 2In cases with zero events in one group, we calculated P values using Fisher's exact test (Social Science Statistics 2018).
3Toxic effects of grade 3 or 4 according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (CTCAE V3) (NCI CTCAE 2018). 4Grade 3 elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were defined as levels that were 5 to 20 times the upper limit of the normal range, and grade 4 elevations were levels that were more than 20 times the upper limit of the normal range. 5Central nervous system (CNS) cortical symptoms were encephalopathy, confusion, and psychosis. 6Treatment‐related mortality: one event due to an interleukin‐2 overdose.
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; CI: confidence interval; CNS: central nervous system; N: number of participants
Bold typeface indicates that the result of the statistical test showed the adverse events were statistically significantly more often in the dinutuximab‐containing immunotherapy group than in the standard therapy group.
Eligibility requirements for the included study were high‐risk neuroblastoma, achievement of at least a partial response at the time of evaluation before autologous stem‐cell transplantation, autologous stem‐cell transplantation performed within 9 months after the initiation of induction therapy, enrolment between day 50 and day 100 after the final autologous stem‐cell transplantation, and absence of progressive disease. This means that a considerable number of patients with high‐risk neuroblastoma were not treated with dinutuximab‐containing immunotherapy, and for those patients the effect of anti‐GD2 antibody‐containing immunotherapy remains unclear.
For example, about 10% (25 of 251) of high‐risk neuroblastoma patients with residual disease were excluded from the randomisation and assigned non‐randomly to receive dinutuximab‐containing immunotherapy. Studies applying iodine‐123 metaiodobenzylguanidine (MIBG) scintigraphy as diagnostic have shown that patients with residual disease are associated with a poorer survival than those without residual disease (Decarolis 2013; Ladenstein 2018). It would thus have been nice to also have had separate results on adverse events for randomised and non‐randomised participants. Another example is the requirement for patients to be in complete remission, very good partial remission, or partial remission before transplantation. The presence of these different options in both treatment groups is comparable, but the prognosis is different: the authors estimated a 2‐year overall survival of 67% in the partial‐response group, 83% in the very good partial‐response group, and 86% in the complete‐response group. It would thus also have been nice to have had separate data for these different groups.
With regard to overall survival and event‐free survival, we stated in the Effects of interventions section that the study authors limited the statistical testing at the time point of two years. We think that it is appropriate to consider all information provided by Kaplan‐Meier survival functions (Liu 2007; Logan 2008). We therefore believe that the estimation of a hazard ratio and the application of a log‐rank test meet this presumption. A statistical test at a fixed point in time does not take into account all available survival data. As the authors did not provide hazard ratios, we re‐enacted the survival functions and complemented an estimate of the concerning hazard ratio.
Our search of the conference proceedings identified an abstract, Yu 2014, associated with ANBL0032. This abstract provided updated data on event‐free survival, and as opposed to the original publication the difference between the treatment groups was no longer statistically significant: “The updated EFS (event‐free survival) (± standard error) for immunotherapy was 67±4%(2‐year) and 59±5%(4‐year) versus 51±5%(2‐year) and 48±5%(4‐year) for isotretinoin alone (p= 0.11).” We did not include the data in the results section of the present review as we did not identify a corresponding full‐text publication. It is understood that information provided in conference proceedings often differs substantially from that provided in subsequent full‐text articles (Yoon 2012). If a full‐text article is published, we will consider it in a future update of the present review.
Quality of the evidence
We judged there to be a low risk of detection bias for overall survival and of reporting bias and did not make any judgements of high risk of bias. Nevertheless, the risk of bias for most outcomes of the single included study was difficult to assess, in part due to lack of reporting. We judged there to be an unclear risk of selection bias, performance bias, detection bias, attrition bias (for outcomes other than overall survival), and other bias. We therefore could not rule out those potential biases. However, this is currently the best available evidence from randomised controlled trials comparing the efficacy of dinutuximab versus standard therapy in people with high‐risk neuroblastoma pre‐treated with HDCT followed by autologous HSCT.
The unclear risk of other bias resulted from the following inconsistencies: "The study was designed to enroll 386 randomly assigned patients, for a statistical power of 80% with a two‐sided log‐rank test at a level of 0.05 (or a one‐sided test at a level of 0.025) to detect an absolute difference of 15 percentage points between the two groups in the 3‐year estimate of event‐free survival (50% in the standard‐therapy group vs. 65% in the immunotherapy group). Early stopping was considered if a significant difference between the two groups was found or if the conditional power fell below 20%. The relative risk of an event was calculated for standard therapy as compared with immunotherapy on the basis of the 3‐year estimate of event‐free survival. The COG data and safety monitoring committee determined that the study met the criteria for early stopping of the randomization, on the basis of the superiority of immunotherapy over standard therapy with regard to event‐free survival." At the time of early stopping, about 59% (226 of 386) of planned eligible patients were enrolled and randomly assigned to a treatment group, and about 61% (83 of 137) of the expected events were documented. The question might arise what would be the result if the study had included all planned people. It also may be questioned if the characteristics of people who were enrolled early and were included in the study differed from the characteristics of people who were planned to be enrolled later and were not included due to the early stopping.
Our GRADE assessment of the quality of the evidence based on the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) judged the quality of the evidence for the outcomes for which data were available as moderate. We downgraded for study limitations, as risk of bias could not be ruled out. We did not downgrade for imprecision. The evidence is based on a single study, but the measured effect on survival was large and the confidence interval below no effect (see Table 1).
We want to acknowledge the tremendous achievement of all contributors to the included randomised controlled trial in view of the many challenges associated with the realisation of the study, such as the rarity of the disease and the length of time for enrolment.
Potential biases in the review process
One of the strengths of this systematic review is the broadness of the search strategy such that study retrieval bias is very unlikely. Nevertheless, there remains a slight possibility that an unknown number of studies were not registered and not published. Duplicate publication bias is very unlikely because we searched for follow‐up papers of a single study in order to ensure that we included the updated version, and we excluded secondary analyses of registers or databases, which may use data that have been published previously by individual contributing study centres. Overall, the possibility that reporting bias is present appears to be small.
Authors' conclusions
Implications for practice.
We identified a single randomised controlled trial (RCT) that evaluated dinutuximab‐containing immunotherapy versus standard therapy in people with high‐risk neuroblastoma pre‐treated with high‐dose chemotherapy followed by autologous haematopoietic stem cell transplantation. The difference in overall survival and event‐free survival was statistically significant in favour of the dinutuximab‐containing immunotherapy group. We judged the quality of the evidence to be moderate. We could not extract and report randomised data on adverse events, progression‐free survival, late non‐haematological toxicity, and health‐related quality of life. More research concerning those outcomes, especially adverse effects, is needed.
Implications for research.
Future trials on the use of anti‐GD2 antibody‐containing immunotherapy for people with high‐risk neuroblastoma should be RCTs focusing on overall survival, early adverse events, and quality of life. Randomised data should be reported separately. Randomised controlled trials should be performed in homogeneous study populations (e.g. stage of disease) and have a long‐term follow‐up. The number of included participants should be sufficient to obtain the power needed for the results to be reliable. Different risk groups, using the most recent definitions, should be taken into account. For example, potential subgroups of patients may benefit from anti‐GD2 antibody‐containing immunotherapy. Various types of antibodies different from dinutuximab may be investigated in RCTs. Furthermore, long‐term follow‐up studies investigating late adverse events should be performed. However, we are aware that the international neuroblastoma community has decided that a new RCT comparing postconsolidation immunotherapy with and without anti‐GD2 antibodies is not required (Elliott 2017; Park 2013; Simon 2017; Yu 2010).
Matthay 2016 reviewed advances in therapy for patients with high‐risk disease and indicated that new approaches including targeting the noradrenaline transporter and others may be candidates for future improvements in survival and long‐term quality of life. Bosse 2016 reviewed neuroblastoma genetics and genomics and its possibilities in improved prognostication and potential therapeutic opportunities. Morgenstern 2016 evaluated prognostic factors in patients with stage 4 neuroblastoma that may be especially important in the context of new therapies.
Acknowledgements
We thank FS van Etten‐Jamaludin (clinical librarian at the Medical Library of the Academic Medical Center, Amsterdam, the Netherlands) for running a small update of the searches in CENTRAL, MEDLINE, and Embase in September 2018. We would like to thank the Editorial Base of Cochrane Childhood Cancer for their advice and support. The Editorial Base of Cochrane Childhood Cancer has been funded by KIKA and is located in the Prinsess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands. We also thank 'Stichting Kinderen Kankervrij' (KiKa), the Netherlands, for the financial support that made it possible to perform this systematic review. We thank the two peer reviewers, Nai‐Kong V Cheung and an undisclosed person, for their helpful comments on the protocol. We thank Frank Berthold for his work on the protocol. We would like to thank Alice Yu for providing additional information on ANBL0032, the single included study. We thank the four peer reviewers, Alice Yu and three undisclosed people, for their helpful comments on the review.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. For Anti‐GD2 we used the following text words:
monoclonal antibodies or ganglioside or ganglioside* or immunotherapy or anti gd2 or ch14 18 or dinutuximab or unituxin or 3f8
2. For Neuroblastoma we used the following text words:
neuroblastoma or neuroblastomas or neuroblast* or ganglioneuroblastoma or ganglioneuroblastomas or ganglioneuroblast* or neuroepithelioma or neuroepitheliomas or neuroepitheliom* or esthesioneuroblastoma or esthesioneuroblastomas or esthesioneuroblastom* or schwannian
Final search 1 and 2
[*=zero or many characters]
We performed the search in title, abstract or keywords.
Appendix 2. Search strategy for MEDLINE (PubMed)
1. For Anti‐GD2 we used the following MeSH headings and text words:
“antibodies, monoclonal/therapeutic use”[mh] OR “gangliosides/therapeutic use”[mh] OR immunotherapy/methods[mh] OR anti gd2[tiab] OR ch14 18 OR ganglioside* OR dinutuximab OR unituxin OR 3f8
2. For Neuroblastoma we used the following MeSH headings and text words:
neuroblastoma OR neuroblastomas OR neuroblast* OR ganglioneuroblastoma OR ganglioneuroblastomas OR ganglioneuroblast* OR neuroepithelioma OR neuroepitheliomas OR neuroepitheliom* OR esthesioneuroblastoma OR esthesioneuroblastomas OR esthesioneuroblastom* OR schwannian
3. For RCTs/CCTs we used the following MeSH headings and text words according to Lefebvre 2011:
(Randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals [mh] NOT humans [mh])
Final search 1 and 2 and 3
[*=zero or more characters; mh=MeSH term; tiab=title or abstract; sh=subheading; pt=publication type; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 3. Search strategy for Embase (Ovid)
1. For Anti‐GD2 we used the following Emtree terms and text words:
1. exp monoclonal antibody/ 2. exp ganglioside/ 3. exp immunotherapy/ or exp cancer immunotherapy/ 4. anti gd2.mp. or exp ganglioside antibody/ 5. ch14 18.mp. or exp dinutuximab/ or 3F8.mp. 6. (ganglioside$ or dinutuximab or unituxin).mp. 7. or/1‐6
2. For Neuroblastoma we used the following Emtree terms and text words:
1 exp neuroblastoma/ 2 (neuroblastoma or neuroblastomas or neuroblast$).mp. 3 (ganglioneuroblastoma or ganglioneuroblastomas or ganglioneuroblast$).mp. 4 (neuroepithelioma or neuroepitheliomas or neuroepitheliom$).mp. 5 exp esthesioneuroblastoma/ 6 (esthesioneuroblastoma or esthesioneuroblastomas or esthesioneuroblastoma$).mp. 7 schwannian.mp. 8 or/1‐7
3. For RCTs/CCTs we used the following Emtree terms and text words:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. (randomized or randomised).ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab.7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. animals/ not human/ 11. 9 not 10
Final search 1 and 2 and 3
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; sh = subject heading; ti,ab = title, abstract; / = Emtree term; $=zero or many characters ; RCT = randomized controlled trial; CCT = controlled clinical trial]
Data and analyses
Comparison 1. Dinutuximab‐containing immunotherapy versus standard therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | 226 | Hazard Ratio (Random, 95% CI) | 0.50 [0.31, 0.80] |
| 2 Event‐free survival | 1 | 226 | Hazard Ratio (Random, 95% CI) | 0.61 [0.41, 0.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ANBL0032.
| Methods | Setting
Duration of enrolment
Randomisation
Follow‐up time
|
|
| Participants | Eligibility criteria
"An additional eligibility criterion enforced early on in the study was the requirement for enrollment in the COG biology study (ANBL00B1)." Number of participants enrolled in each group:
Age, dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Stage: International Neuroblastoma Staging System (INSS); dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Comorbidity: the authors reported important findings associated with neuroblastoma, such as tumour MYCN status, tumour histologic features, and tumour ploidy; other disease entities were not reported. Tumour MYCN status; dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Tumour histologic features; dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Tumour ploidy; dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Previous treatment; treatment prior to enrolling in this study (as described in the Supplemental Explanatory Material of the paper):
Response before ASCT; dinutuximab‐containing immunotherapy group versus standard therapy group (N):
Number of ASCTs; dinutuximab‐containing immunotherapy group versus standard therapy group (N):
|
|
| Interventions | The cumulative total dose participants received was not mentioned. We calculated these figures by using the treatment schedule information reported in the article. Treatment:
Dinutuximab‐containing immunotherapy group: 6 cycles * 28 days = 168 days
Standard therapy group: 6 cycles * 28 days = 168 days
The following information describes deviations from the study protocol:
|
|
| Outcomes | We included only the outcomes for which results were available from randomised data. The study reported adverse events results from mixed populations including both randomised and non‐randomised participants. As separate randomised data were not available, we did not report these outcomes. Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Stratified permuted blocks were used for randomization. Procedurally this was accomplished by the COG Remote Data Entry (RDE1) system. The treatment group was assigned in real‐time based on the balance existing at that time within “blocks”, where blocks in this case were the study strata. The block size, or “margin” was set (margin=2 within each stratum) prior to the activation of the study. In this RDE approach, the treatment group assignment is random until such time as a margin within a stratum is exceeded, and only then does the method become deterministic. Once a randomized treatment group assignment was made for a given patient, that patient’s treatment group was never changed for any reason." The method of randomisation appeared conditional. We do not know if the condition applied and had any effect. We judged risk of bias to be unclear. |
| Allocation concealment (selection bias) | Low risk | "Procedurally this was accomplished by the COG Remote Data Entry (RDE1) system." We assumed that the allocation concealment was provided by the remote system. We judged risk of bias to be low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and physicians and nurses was not reported. We judged risk of bias to be unclear. |
| Blinding of outcome assessment (detection bias) Overall survival Overall survival | Low risk | Blinding of outcome assessors was not reported, but this is not relevant to the outcome overall survival. We judged risk of bias to be low. |
| Blinding of outcome assessment (detection bias) Event‐free survival All outcomes | Unclear risk | Blinding of outcome assessors was not reported, and this may be relevant to the outcome event‐free survival. We judged this outcome as having an unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
We judged risk of bias to be unclear. |
| Selective reporting (reporting bias) | Low risk | We did not identify any signs of selective reporting when comparing the outcomes and methods of the publication with those of published protocol items at ClinicalTrials.gov. We judged risk of bias to be low. |
| Other bias | Unclear risk | Interim analysis:
We did not identify any baseline imbalances with respect to age, INSS stage, tumour MYCN status, tumour histologic features, tumour ploidy, and response before transplantation. We judged risk of bias to be unclear. |
ASCT: autologous stem‐cell transplantation COG: Children's Oncology Group GM‐CSF: granulocyte‐macrophage colony‐stimulating factor IU: international units
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berthold 2018 | Not study design of interest |
| Cheung 1991 | Not comparator of interest |
| Cheung 1998 | Not comparator of interest |
| Cheung 2000 | Not intervention of interest |
| Cheung 2012 | Not study design of interest |
| Cheung 2014 | Not comparator of interest |
| Chi 2009 | Not comparator of interest |
| Diccianni 2018 | Not study design of interest |
| Erbe 2017 | Not study design of interest |
| Erbe 2018 | Not study design of interest |
| Federico 2014 | Not comparator of interest |
| Hoy 2016 | Not study design of interest |
| Kushner 2015 | Not comparator of interest |
| Kushner 2016 | Not comparator of interest |
| Ladenstein 2011 | Not comparator of interest |
| Ladenstein 2012 | Not comparator of interest |
| Ladenstein 2013 | Not comparator of interest |
| Ladenstein 2014a | Not comparator of interest |
| Ladenstein 2014b | Not comparator of interest |
| Ladenstein 2014c | Not comparator of interest |
| Ladenstein 2014d | Not comparator of interest |
| Ladenstein 2015 | Not comparator of interest |
| Ladenstein 2016a | Not comparator of interest |
| Ladenstein 2016b | Not comparator of interest |
| Ladenstein 2016c | Not comparator of interest |
| Ladenstein 2016d | Not comparator of interest |
| London 2009 | Not study design of interest |
| Marachelian 2016 | Not comparator of interest |
| Mody 2017 | Not intervention of interest |
| Morgenstern 2018 | Not intervention of interest |
| Ozkaynak 2014 | Not comparator of interest |
| Ozkaynak 2018a | Not study design of interest |
| Ozkaynak 2018b | Not study design of interest |
| Ploessl 2016 | Not study design of interest |
| Seif 2013 | Not intervention of interest |
| Simon 2004 | Not study design of interest |
| Simon 2005 | Not study design of interest |
| Simon 2009 | Not study design of interest |
| Simon 2011a | Not study design of interest |
| Simon 2011b | Not study design of interest |
| Simon 2016 | Not study design of interest |
| Sondel 2017 | Not study design of interest |
| Talleur 2017 | Not study design of interest |
| Yang 2017 | Not study design of interest |
| Yu 1998 | Not comparator of interest |
Differences between protocol and review
We included the outcomes overall survival and event‐free survival data in the review, although the definitions for these outcomes differed from the ones in our protocol. Otherwise important information could have been missed. We did not use a data extraction form specifically designed for the review.
We clarified the description of the types of interventions. The intervention group is now presented as 'anti‐GD2 antibody‐containing immunotherapy' (formerly 'addition of anti‐GD2'). We specified the comparator group as 'standard therapy' (formerly 'the same treatment but no addition of anti‐GD2').
Contributions of authors
All authors approved the final version of the review.
FP: concept, selecting studies, extracting and analysing data, interpretation of results, GRADE assessment, and primary manuscript preparation.
ECVD: extracting and analysing data, interpretation of results, GRADE assessment, and manuscript review.
HE: selecting studies, extraction of toxicity data, interpretation of results, and manuscript review.
GAMT: providing a clinical perspective, interpretation of results, and manuscript review.
Sources of support
Internal sources
-
University of Cologne, Germany.
Provision of the full texts of articles
External sources
-
Stichting Kinderen Kankervrij (KiKa), Netherlands.
The salary of ECVD is paid by KiKa; KiKa was not involved in the design and execution of this Cochrane Review
Declarations of interest
FP: no conflicts of interest
ECVD: no conflicts of interest
HE: no conflicts of interest
GAMT: no conflicts of interest
New
References
References to studies included in this review
ANBL0032 {published data only}
- Erbe AK, Wang W, Carmichael L, Kim KM, Mendonca EA, Song Y, et al. Impact of KIR/KIR ligand genotype for neuroblastoma patients in a phase III COG immunotherapy trial (Abstract). Journal of Clinical Oncology 2016;34(Suppl):e14014. [Google Scholar]
- NCT00026312. Isotretinoin with or without dinutuximab, aldesleukin, and sargramostim following stem cell transplant in treating patients with neuroblastoma. clinicaltrials.gov/ct2/show/NCT00026312 (first received 27 January 2003).
- Ozkaynak MF, Gilman AL, Yu AL, London WB, Sondel PM, Smith MA, et al. A comprehensive safety trial of chimeric antibody 14.18 (ch14.18) with GM‐CSF, IL‐2, and isotretinoin in high‐risk neuroblastoma patients following myeloablative therapy: a Children's Oncology Group study. Journal of Clinical Oncology 2014;32(15 Suppl 1):[Abstract]. [Google Scholar]
- Yu AL. GD2‐targeted immunotherapy of high risk neuroblastoma. Advances in Neuroblastoma Research (ANR) Congress; 2012 Jun 18‐21; Toronto, Canada. anrmeeting.org/meetingabstracts.php, 2012:82. [PL15]
- Yu AL, Batova A, Gribi R, Dicciani M, Bridgeman L, Geske D, et al. Antibody‐dependent cellular cytotoxicity (ADCC) in COG ANBL0032: a phase III randomized trial of chimeric anti‐GD2 and GM‐CSF/IL2 in high risk neuroblastoma following myeloablative therapy and autologous stem cell transplant (ASCT). Journal of Clinical Oncology 2004;22(14s):183 [Abstract No. 2582]. [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak FM, Sondel PM, London WB, Cretella S, et al. Update of outcome for high‐risk neuroblastoma treated on a randomized trial of chimeric anti‐GD2 antibody (ch14.18) + GM‐CSF / IL2 immunotherapy in 1st response: A Children's Oncology Group Study. Advances in Neuroblastoma Research (ANR) Congress; 2014 May 13‐16; Cologne, Germany. anrmeeting.org/meetingabstracts.php, 2014:108. [PL013]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti‐GD2 antibody with GM‐CSF, interleukin‐2, and isotretinoin for neuroblastoma. New England Journal of Medicine 2010;363(14):1324‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Berthold 2018 {published data only}
- Berthold F, Ernst A, Hero B, Klingebiel T, Kremens B, Schilling FH, et al. Long‐term outcomes of the GPOH NB97 trial for children with high‐risk neuroblastoma comparing high‐dose chemotherapy with autologous stem cell transplantation and oral chemotherapy as consolidation. British Journal of Cancer 2018;119(3):282‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 1991 {published data only}
- Cheung NK, Burch L, Kushner BH, Munn DH. Monoclonal antibody 3F8 can effect durable remissions in neuroblastoma patients refractory to chemotherapy: a phase II trial. Progress in Clinical and Biological Research 1991;366:395‐400. [PubMed] [Google Scholar]
Cheung 1998 {published data only}
- Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, et al. Anti‐G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. Journal of Clinical Oncology 1998;16(9):3053‐60. [DOI] [PubMed] [Google Scholar]
Cheung 2000 {published data only}
- Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3' antibody was associated with long‐term survival after anti‐G(D2) antibody therapy of stage 4 neuroblastoma. Clinical Cancer Research 2000;6(7):2653‐60. [PubMed] [Google Scholar]
Cheung 2012 {published data only}
- Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti‐GD2 monoclonal antibody 3F8 combined with granulocyte‐macrophage colony‐stimulating factor and 13‐cis‐retinoic acid in high‐risk patients with stage 4 neuroblastoma in first remission. Journal of Clinical Oncology 2012;30(26):3264‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2014 {published data only}
- Cheung NK, Cheung IY, Kramer K, Modak S, Kuk D, Pandit‐Taskar N, et al. Key role for myeloid cells: phase II results of anti‐G(D2) antibody 3F8 plus granulocyte‐macrophage colony‐stimulating factor for chemoresistant osteomedullary neuroblastoma. International Journal of Cancer 2014;135(9):2199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2009 {published data only}
- Chi F, Godfrey C, Matthew MKS, Chung Wing L, Anselm CWL, Siu Cheung Alvin L. Treatment outcome of metastatic neuroblastoma with antiganglioside 2 monoclonal antibody (3f8): the Hong Kong experience. 41th Annual Conference of the International Society of Paediatric Oncology, SIOP, Sao Paulo, Brazil. Pediatric Blood & Cancer 2009;53:731 [Abstract O.069]. [Google Scholar]
Diccianni 2018 {published data only}
- Diccianni MB, Mielke J, Williams R, Messer K, Ozkaynak F, Gilman AL, et al. Natural antibodies to non‐human glycans Neu5Gc and alpha‐gal correlate with outcome of high‐risk neuroblastoma patients treated with dinutuximab on COG ANBL0032 and ANBL0931. Cancer Research 2018;78(13 Suppl):LB‐300 [Abstract]. [Google Scholar]
Erbe 2017 {published data only}
- Erbe AK, Wang W, Carmichael L, Kim K, Reville PK, London WB, et al. Impact of KIR/KIR ligand genotype for neuroblastoma patients in a Phase 3 COG immunotherapy trial. Cancer Research 2017;77(13 Suppl):LB‐048 [Abstract]. [Google Scholar]
Erbe 2018 {published data only}
- Erbe AK, Wang W, Carmichael L, Kim K, Mendonca EA, Song Y, et al. Neuroblastoma patients' KIR and KIR‐ligand genotypes influence clinical outcome for dinutuximab‐based immunotherapy: a report from the Children's Oncology Group. Clinical Cancer Research 2018;24(1):189‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Federico 2014 {published data only}
- Federico S, Leung W, Pappo AS, Navid F, Santana VM, Wu J, et al. Humanized anti‐GD2 antibody (HU14.18K322A) given with chemotherapy +/‐ parental natural killer (NK) cells in children with recurrent or refractory neuroblastoma. Pediatric Blood & Cancer 2014;61:S123 [Abstract]. [Google Scholar]
Hoy 2016 {published data only}
- Hoy SM. Dinutuximab: a review in high‐risk neuroblastoma. Targeted Oncology 2016;11(2):247‐53. [DOI] [PubMed] [Google Scholar]
Kushner 2015 {published data only}
- Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Kramer K, Modak S, et al. Prolonged progression‐free survival after consolidating second or later remissions of neuroblastoma with anti‐GD2 immunotherapy and isotretinoin: a prospective Phase II study. Oncoimmunology 2015;4(7):e1016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kushner 2016 {published data only}
- Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Modak S, Kramer K, et al. Lack of survival advantage with autologous stem‐cell transplantation in high‐risk neuroblastoma consolidated by anti‐GD2 immunotherapy and isotretinoin. Oncotarget 2016;7(4):4155‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladenstein 2011 {published data only}
- Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Early high‐dose treatment: SCT results from the European High Risk Neuroblastoma study. Bone Marrow Transplantation 2011;46:S17 [Abstract]. [Google Scholar]
Ladenstein 2012 {published data only}
- Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Major results from the HR‐NBl1/Siopen trial for high risk neuroblastoma. Pediatric Blood & Cancer 2012;59(6):988‐9. [Google Scholar]
Ladenstein 2013 {published data only}
- Ladenstein R, Weixler S, Baykan B, Bleeke M, Kunert R, Katinger D, et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: a SIOPEN Phase 1 study. mAbs 2013;5(5):801‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladenstein 2014a {published data only}
- Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Risk factors within the European High Risk Neuroblastoma HR‐NBL1/SIOPEN trial. Bone Marrow Transplantation 2014;49:S65‐6 [Abstract]. [Google Scholar]
Ladenstein 2014b {published data only}
- Ladenstein R, Poetschger U, Luksch R, Elliot M, Castel V, Yaniv I, et al. Myeloablative therapy (MAT) and immunotherapy (IT) with CH14.18/CHO for high risk neuroblastoma: update and news of randomised results from the HR‐NBL1/SIOPEN trial. Pediatric Blood & Cancer 2014;61:S122‐3 [Abstract]. [Google Scholar]
Ladenstein 2014c {published data only}
- Ladenstein R, Poetschger U, Luksch R, Elliot M, Castel V, Yaniv I, et al. Risk factors within the European high risk neuroblastoma HR‐NBL1/SIOPEN trial. Pediatric Blood & Cancer 2014;61:S119 [Abstract]. [Google Scholar]
Ladenstein 2014d {published data only}
- Ladenstein RL, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Immunotherapy (IT) with ch14.18/CHO for high‐risk neuroblastoma: first results from the randomised HR‐NBL1/SIOPEN trial. Journal of Clinical Oncology 2014;32(15 Suppl 1):no pagination. [Google Scholar]
Ladenstein 2015 {published data only}
- Ladenstein R, Poetschger U, Gray J, Valteau Couanet D, Luksch R, Castel V, et al. Short (STI) and long term infusion (LTI) of ch14.18/cho immunotherapy: toxicity profiles and outcomes in 530 high risk neuroblastoma (HR‐NBL) patients in two Siopen trials. Pediatric Blood & Cancer 2015;62:S145 [Abstract]. [Google Scholar]
Ladenstein 2016a {published data only}
- Ladenstein R, Poetschger U, Elliot M, Luksch R, Castel V, Yaniv I, et al. Risk factors in stage 4 neuroblastoma patients treated with Busulphan‐Melphalan. Report from the European high risk neuroblastoma HR‐NBL1/SIOPEN TRIAL. Bone Marrow Transplantation 2016;51:S46 [Abstract]. [Google Scholar]
Ladenstein 2016b {published data only}
- Ladenstein R, Poetschger U, Elliott M, Luksch R, Castel V, Yaniv I, et al. Prognostic factors in stage 4 neuroblastoma treated with busulphan‐melphalan: report from the European HR‐NBL1/Siopen trial. Journal of Clinical Oncology 2016;34:no pagination [Abstract]. [Google Scholar]
Ladenstein 2016c {published data only}
- Ladenstein R, Poetschger U, Gray J, Valteau‐Couanet D, Luksch R, Castel V, et al. Final results of 406 patients with short‐term infusion ch14.18/CHO randomised for subcutaneous aldesleukin and results in 161 confirmatory patients: the current Siopen HR‐NBL1 front‐line experience. Pediatric Blood & Cancer 2016;63:S38 [Abstract]. [Google Scholar]
Ladenstein 2016d {published data only}
- Ladenstein R, Poetschger U, Gray J, Valteau‐Couanet D, Luksch R, Castel V, et al. Toxicity and outcome of anti‐GD2 antibody ch14.18/CHO in front‐line, high‐risk patients with neuroblastoma: final results of the phase III immunotherapy randomisation (HR‐NBL1/SIOPEN trial). Journal of Clinical Oncology 2016;34:no pagination [Abstract]. [Google Scholar]
London 2009 {published data only}
- London WB, Sondel P, Gilman AL, Ozkaynak F, Kreissman S, Buxton A, et al. Success of chimeric anti‐GD2 antibody + GM‐CSF + IL2 immunotherapy in high‐risk neuroblastoma (NB) in first response: a critical appraisal. Pediatric Blood & Cancer 2009;53(5):708 [Abstract]. [Google Scholar]
Marachelian 2016 {published data only}
- Marachelian A, Desai A, Balis F, Katzenstein H, Qayed M, Armstrong M, et al. Comparative pharmacokinetics, safety, and tolerability of two sources of ch14.18 in pediatric patients with high‐risk neuroblastoma following myeloablative therapy. Cancer Chemotherapy and Pharmacology 2016;77(2):405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mody 2017 {published data only}
- Mody R, Naranjo A, Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan‐temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open‐label, randomised, phase 2 trial. Lancet Oncology 2017;18(7):946‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgenstern 2018 {published data only}
- Morgenstern DA, Potschger U, Moreno L, Papadakis V, Owens C, Ash S, et al. Risk stratification of high‐risk metastatic neuroblastoma: a report from the HR‐NBL‐1/SIOPEN study. Pediatric Blood & Cancer 2018;65(11):e27363. [DOI] [PubMed] [Google Scholar]
Ozkaynak 2014 {published data only}
- Ozkaynak MF, Gilman AL, Yu AL, London WB, Sondel PM, Smith MA, et al. A comprehensive safety trial of chimeric antibody 14.18 (ch14.18) with GM‐CSF, IL‐2, and isotretinoin in high‐risk neuroblastoma patients following myeloablative therapy: a Children's Oncology Group study. Journal of Clinical Oncology 2014;32(15 Suppl):10044 [Abstract]. [Google Scholar]
Ozkaynak 2018a {published data only}
- Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. A comprehensive safety trial of chimeric antibody 14.18 with GM‐CSF, IL‐2, and isotretinoin in high‐risk neuroblastoma patients following myeloablative therapy: Children's Oncology Group Study ANBL0931. Frontiers in Immunology 2018;9:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozkaynak 2018b {published data only}
- Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. Corrigendum: a comprehensive safety trial of chimeric antibody 14.18 with GM‐CSF, IL‐2, and isotretinoin in high‐risk neuroblastoma patients following myeloablative therapy: Children's Oncology Group Study ANBL0931. Frontiers in Immunology 2018;9:1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ploessl 2016 {published data only}
- Ploessl C, Pan A, Maples KT, Lowe DK. Dinutuximab: an anti‐GD2 monoclonal antibody for high‐risk neuroblastoma. Annals of Pharmacotherapy 2016;50(5):416‐22. [DOI] [PubMed] [Google Scholar]
Seif 2013 {published data only}
- Seif AE, Naranjo A, Baker DL, Bunin NJ, Kletzel M, Kretschmar CS, et al. A pilot study of tandem high‐dose chemotherapy with stem cell rescue as consolidation for high‐risk neuroblastoma: Children's Oncology Group study ANBL00P1. Bone Marrow Transplantation 2013;48(7):947‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2004 {published data only}
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Consolidation treatment with chimeric anti‐GD2‐antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. Journal of Clinical Oncology 2004;22(17):3549‐57. [DOI] [PubMed] [Google Scholar]
Simon 2005 {published data only}
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Infants with stage 4 neuroblastoma: the impact of the chimeric anti‐GD2‐antibody ch14.18 consolidation therapy [Säuglinge mit neuroblastom stadium 4: konsolidierungsbehandlung mit chimärem anti‐GD2‐antikörper ch14.18]. Klinische Pädiatrie 2005;217(3):147‐52. [DOI] [PubMed] [Google Scholar]
Simon 2009 {published data only}
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Berthold F. No success of anti GD2 treatment in high‐risk neuroblastoma? A critical appraisal. Pediatric Blood & Cancer 2009;53(5):708‐9. [Google Scholar]
Simon 2011a {published data only}
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, et al. Long term outcome of high‐risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2011b {published data only}
- Simon T, Hero B, Handgretinger R, Schrappe M, Klingebiel T, Fruehwald M, et al. Anti‐GD2‐antibody CH14.18 or retinoic acid as consolidation therapy in high‐risk neuroblastoma. Pediatric Blood & Cancer 2011;57(5):789 [Abstract]. [Google Scholar]
Simon 2016 {published data only}
- Simon T, Hero B, Ruth V, Angelika E, Frank B. Improved long‐term survival after myeloablative chemotherapy with autologous stem cell transplantation in high‐risk neuroblastoma patients. Results of the NB97 trial. Pediatric Blood & Cancer 2016;63:S39 [Abstract]. [Google Scholar]
Sondel 2017 {published data only}
- Sondel PM, Erbe AK, Wang W, Carmichael L, Hoefges A, Kim KM, et al. Certain KIR/KIR ligand genotypes influence patient response to immunotherapy in neuroblastoma patient. Abstract P26 on page 29 of the 32nd Annual Meeting and Pre‐Conference Programs of the Society for Immunotherapy of Cancer (SITC 2017): Part One. Journal for ImmunoTherapy of Cancer 2017;5(Suppl 2):86 [Abstract]. [Google Scholar]
Talleur 2017 {published data only}
- Talleur AC, Triplett BM, Federico S, Mamcarz E, Janssen W, Wu J, et al. Consolidation therapy for newly diagnosed pediatric patients with high‐risk neuroblastoma using busulfan/melphalan, autologous hematopoietic cell transplantation, anti‐GD2 antibody, granulocyte‐macrophage colony‐stimulating factor, interleukin‐2, and haploidentical natural killer cells. Biology of Blood and Marrow Transplantation 2017;23(11):1910‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2017 {published data only}
- Yang L, Ma X, Liu YC, Zhao W, Yu L, Qin M, et al. Chimeric antigen receptor 4SCAR‐GD2‐modified T cells targeting high‐risk and recurrent neuroblastoma: a phase II multi‐center trial in China. 59th Annual Meeting of the American Society of Hematology, ASH. Blood 2017;130:3335 [Abstract]. [Google Scholar]
Yu 1998 {published data only}
- Yu AL, Uttenreuther‐Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human‐mouse chimeric anti‐disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. Journal of Clinical Oncology 1998;16(6):2169‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 2014
- Ahmed M, Cheung NK. Engineering anti‐GD2 monoclonal antibodies for cancer immunotherapy. FEBS Letters 2014;588(2):288‐97. [DOI] [PubMed] [Google Scholar]
American Cancer Society 2016
- American Cancer Society. Survival rates for neuroblastoma based on risk groups. www.cancer.org/cancer/neuroblastoma/detailedguide/neuroblastoma‐survival‐rates (accessed 2 June 2016).
ANR Meeting 2012
- Advances in Neuroblastoma Research (ANR) Association. ANR Meeting Abstracts 2012. www.anrmeeting.org/meetingabstracts.php (accessed 26 December 2016).
ANR Meeting 2014
- Advances in Neuroblastoma Research (ANR) Association. ANR Meeting Abstracts 2014. www.anrmeeting.org/meetingabstracts.php (accessed 26 December 2016).
ANR Meeting 2016
- Advances in Neuroblastoma Research (ANR) Association. ANR Meeting Abstracts 2016. www.anrmeeting.org/meetingabstracts.php (accessed 26 December 2016).
ANR Meeting 2018
- Advances in Neuroblastoma Research (ANR) Association. ANR Meeting Abstracts 2018. www.anrmeeting.org/anr2018‐abstracts/meetings‐2018_abstracts.php (accessed 28 October 2018).
ASCO Meeting 2018
- American Society of Clinical Oncology (ASCO). 2018 ASCO Annual Meeting. meetinglibrary.asco.org (accessed 28 October 2018).
Bagatell 2016
- Bagatell R, Cohn SL. Genetic discoveries and treatment advances in neuroblastoma. Current Opinion in Pediatrics 2016;28(1):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleeker 2015
- Bleeker G, Tytgat GAM, Adam JA, Caron HN, Kremer LCM, Hooft L, et al. 123I‐MIBG scintigraphy and 18F‐FDG‐PET imaging for diagnosing neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD009263.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosse 2016
- Bosse KR, Maris JM. Advances in the translational genomics of neuroblastoma: from improving risk stratification and revealing novel biology to identifying actionable genomic alterations. Cancer 2016;122(1):20‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brisse 2011
- Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton‐Gorges SL, Kanegawa K, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011;261(1):243‐57. [DOI] [PubMed] [Google Scholar]
Brodeur 1993
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of Clinical Oncology 1993;11(8):1466‐77. [DOI] [PubMed] [Google Scholar]
Cheung 2013
- Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nature Reviews Cancer 2013;13(6):397‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2015
- Cheung NK, Ostrovnaya I, Kuk D, Cheung IY. Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti‐GD2 immunotherapy. Journal of Clinical Oncology 2015;33(7):755‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohn 2009
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of Clinical Oncology 2009;27(2):289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2012
- Cole KA, Maris JM. New strategies in refractory and recurrent neuroblastoma: translational opportunities to impact patient outcome. Clinical Cancer Research 2012;18(9):2423‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decarolis 2013
- Decarolis B, Schneider C, Hero B, Simon T, Volland R, Roels F, et al. Iodine‐123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study. Journal of Clinical Oncology 2013;31(7):944‐51. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Elliott 2017
- Elliott M, Gray J, Tweddle D, Gaze M, Ramanujachar R, Wheeler K, et al. Treatment and management of newly diagnosed patients with high‐risk neuroblastoma (not patients currently registered on HR‐NBL‐1): statement from the Children's Cancer and Leukaemia Group neuroblastoma special interest group, June 2017. www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/Treatment_and_management_of_newly_diagnosed_HRNB_Jun_17.pdf (accessed 11 August 2018).
Esiashvili 2007
- Esiashvili N, Goodman M, Ward K, Marcus RB Jr, Johnstone PA. Neuroblastoma in adults: incidence and survival analysis based on SEER data. Pediatric Blood & Cancer 2007;49(1):41‐6. [DOI] [PubMed] [Google Scholar]
GARD 2016
- Genetic and Rare Diseases Information Center (GARD). Neuroblastoma. rarediseases.info.nih.gov/gard/7185/neuroblastoma/resources/1 (accessed 2 June 2016).
Goodman 1999
- Goodman MT, Gurney JG, Smith MA, Olshan AF. Chapter IV Sympathetic nervous system tumors. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al. editor(s). Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975‐1995. Bethesda: National Cancer Institute, 1999. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 26 December 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICSSC 2003
- International Clinical Studies Support Center (ICSSC). WHO toxicity grading scale for determining the severity of adverse events. www.icssc.org/Documents/Resources/AEManual2003AppendicesFebruary_06_2003%20final.pdf (accessed 2 June 2016).
Kremer 2018
- Kremer LCM, Leclercq E, Noorman JK, Jellema P, Dalen EC. Module Cochrane Childhood Cancer. childhoodcancer.cochrane.org/sites/childhoodcancer.cochrane.org/files/public/uploads/cochrane_childhood_cancer_module.pdf (accessed 5 March 2019).
Ladenstein 2018
- Ladenstein R, Lambert B, Pötschger U, Castellani MR, Lewington V, Bar‐Sever Z, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high‐risk neuroblastoma populations: the SIOPEN/HR‐NBL1 and COG‐A3973 trials. European Journal of Nuclear Medicine and Molecular Imaging 2018;45(2):292‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2007
- Liu K, Qiu P, Sheng J. Comparing two crossing hazard rates by Cox proportional hazards modelling. Statistics in Medicine 2007;26(2):375‐91. [DOI] [PubMed] [Google Scholar]
Logan 2008
- Logan BR, Klein JP, Zhang MJ. Comparing treatments in the presence of crossing survival curves: an application to bone marrow transplantation. Biometrics 2008;64(3):733‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maris 2007
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007;369(9579):2106‐20. [DOI] [PubMed] [Google Scholar]
Maris 2010
- Maris JM. Recent advances in neuroblastoma. New England Journal of Medicine 2010;362(23):2202‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthay 2012
- Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clinical Cancer Research 2012;18(10):2740‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthay 2016
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. Neuroblastoma. Nature Reviews Disease Primers 2016;2:16078. [DOI] [PubMed] [Google Scholar]
Microsoft Corp 2011 [Computer program]
- Microsoft Corporation. Excel. Redmond: Microsoft Corporation, 2011.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgenstern 2016
- Morgenstern DA, London WB, Stephens D, Volchenboum SL, Simon T, Nakagawara A, et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: a study from the International Neuroblastoma Risk Group database. European Journal of Cancer 2016;65:1‐10. [DOI] [PubMed] [Google Scholar]
Navid 2010
- Navid F, Santana VM, Barfield RC. Anti‐GD2 antibody therapy for GD2‐expressing tumors. Current Cancer Drug Targets 2010;10(2):2000‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navid 2014
- Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I trial of a novel anti‐GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. Journal of Clinical Oncology 2014;32(14):1445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCI CTCAE 2018
- National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC) (last updated: 1 March 2018). ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 28 May 2018).
NCI PDQ 2018
- National Cancer Institute (NCI) at the National Institutes of Health (NIH), Physician Data Query (PDQ). Neuroblastoma treatment (updated: 28 September 2017). www.cancer.gov/cancertopics/pdq/treatment/neuroblastoma/HealthProfessional (accessed 28 May 2018).
Park 2013
- Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KK, et al. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatric Blood & Cancer 2013;60(6):985‐93. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Peinemann 2015a
- Peinemann F, Kleijnen J. Development of an algorithm to provide awareness in choosing study designs for inclusion in systematic reviews of healthcare interventions: a method study. BMJ Open 2015;5(8):e007540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peinemann 2015b
- Peinemann F, Tushabe DA, Dalen EC, Berthold F. Rapid COJEC versus standard induction therapies for high‐risk neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010774.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peinemann 2015c
- Peinemann F, Dalen EC, Tushabe DA, Berthold F. Retinoic acid post consolidation therapy for high‐risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010685.pub2] [DOI] [PubMed] [Google Scholar]
Perez‐Horta 2016
- Perez‐Horta Z, Goldberg JL, Sondel PM. Anti‐GD2 mAbs and next‐generation mAb‐based agents for cancer therapy. Immunotherapy 2016;8(9):1097‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2015
- Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology 2015;33(27):3008‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simon 2017
- Simon T, Hero B, Schulte JH, Deubzer H, Hundsdoerfer P, Schweinitz D, et al. 2017 GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klinische Pädiatrie 2017;229(3):147‐67. [DOI] [PubMed] [Google Scholar]
SIOP Meeting 2017
- Societe Internationale d'Oncologie Pediatrique (SIOP). Abstracts from the 49th Congress of the International Society of Paediatric Oncology (SIOP) Washington, DC, USA, October 12‐15, 2017. Pediatric Blood & Cancer 2017;64(S3):e26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIOP Meeting 2018
- Societe Internationale d'Oncologie Pediatrique (SIOP). Abstracts from the 50th Congress of the International Society of Paediatric Oncology (SIOP) Kyoto, Japan, November 16‐19, 2018. Pediatric Blood & Cancer 2018;65(S2):e27455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Social Science Statistics 2018
- Stangroom J. Fisher Exact Test Calculator for 2 x 2 Contingency Table. www.socscistatistics.com/tests/Default.aspx (accessed 29 June 2018).
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stutterheim 2011
- Stutterheim J, Zappeij‐Kannegieter L, Versteeg R, Caron HN, Schoot CE, Tytgat GA. The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. European Journal of Cancer 211;47(8):1193‐202. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unverzagt 2017
- Unverzagt S, Moldenhauer I, Nothacker M, Rossmeissl D, Hadjinicolaou AV, Peinemann F, et al. Immunotherapy for metastatic renal cell carcinoma. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011673.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viprey 2014
- Viprey VF, Gregory WM, Corrias MV, Tchirkov A, Swerts K, Vicha A, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: a European HR‐NBL1/SIOPEN study. Journal of Clinical Oncology 2014;32(10):1074‐83. [DOI] [PubMed] [Google Scholar]
Yalcin 2015
- Yalcin B, Kremer LCM, Dalen EC. High‐dose chemotherapy and autologous haematopoietic stem cell rescue for children with high‐risk neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD006301.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2012
- Yoon U, Knobloch K. Assessment of reporting quality of conference abstracts in sports injury prevention according to CONSORT and STROBE criteria and their subsequent publication rate as full papers. BMC Medical Research Methodology 2012;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2010
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti‐GD2 antibody with GM‐CSF, interleukin‐2, and isotretinoin for neuroblastoma. New England Journal of Medicine 2010;363(14):1324‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Peinemann 2016
- Peinemann F, Dalen EC, Berthold F. Anti‐GD2 antibody‐containing immunotherapy post‐consolidation therapy for people with high‐risk neuroblastoma treated with autologous hematopoietic stem cell transplantation. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012442] [DOI] [PMC free article] [PubMed] [Google Scholar]


