Abstract
Background
A parastomal hernia is defined as an incisional hernia related to a stoma, and belongs to the most common stoma‐related complications. Many factors, which are considered to influence the incidence of parastomal herniation, have been investigated. However, it remains unclear whether the enterostomy should be placed through, or lateral to the rectus abdominis muscle, in order to prevent parastomal herniation and other important stoma complications.
Objectives
To assess if there is a difference regarding the incidence of parastomal herniation and other stoma complications, such as ileus and stenosis, in lateral pararectal versus transrectal stoma placement in people undergoing elective or emergency abdominal wall enterostomy.
Search methods
For this update, we searched for all types of published and unpublished randomized and non‐randomized studies in four medical databases: CENTRAL, PubMed, LILACS, Science Ciation Index, and two trials registers: ICTRP Search Portal and ClinicalTrials.gov to 9 November 2018. We applied no language restrictions.
Selection criteria
Randomized and non‐randomized studies comparing lateral pararectal versus transrectal stoma placement with regard to parastomal herniation and other stoma‐related complications.
Data collection and analysis
Two authors independently assessed study quality and extracted data. We conducted data analyses according to the recommendations of Cochrane and the Cochrane Colorectal Cancer Group (CCCG). We rated quality of evidence according to the GRADE approach.
Main results
Randomized controlled trials (RCT)
Only one RCT met the inclusion criteria. The participants underwent enterostomy placement in the frame of an operation for: rectal cancer (37/60), ulcerative colitis (14/60), familial adenomatous polyposis (7/60), and other (2/60).
The results between the lateral pararectal and the transrectal approach groups were inconclusive for the incidence of parastomal herniation (risk ratio (RR) 1.34, 95% confidence interval (CI) 0.40 to 4.48; low‐quality evidence); development of ileus or stenosis (RR 2.0, 95% CI 0.19 to 20.9; low‐quality evidence); or skin irritation (RR 0.67, 95% CI 0.21 to 2.13; moderate‐quality evidence). The results were also inconclusive for the subgroup analysis in which we compared the effect of ileostomy versus colostomy on parastomal herniation. The study did not measured other stoma‐related morbidities, or stoma‐related mortality, but did measure quality of life, which was not one of our outcomes of interest.
Non‐randomized studies (NRS)
Ten retrospective cohort studies, with a total of 864 participants, met the inclusion criteria. The indications for enterostomy placement and the baseline characteristics of the participants (age, co‐morbidities, disease‐severity) varied between studies. All included studies reported results for the primary outcome (parastomal herniation) and one study also reported data on one of the secondary outcomes (stomal prolapse).
The effects of different surgical approaches on parastomal herniation (RR 1.22, 95% CI 0.84 to 1.75; 10 studies, 864 participants; very low‐quality evidence) and the occurrence of stomal prolapse (RR 1.23, 95% CI 0.39 to 3.85; 1 study, 145 participants; very low‐quality evidence) are uncertain.
None of the included studies measured other stoma‐related morbidity or stoma‐related mortality.
Authors' conclusions
The present systematic review of randomized and non‐randomized studies found inconsistent results between the two compared interventions regarding their potential to prevent parastomal herniation.
In conclusion, there is still a lack of high‐quality evidence to support the ideal surgical technique of stoma formation. The available moderate‐, low‐, and very low‐quality evidence, does not support or refute the superiority of one of the studied stoma formation techniques over the other.
Plain language summary
Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation
We asked
Is there a difference in the rate of parastomal herniation, and other stoma‐related complications in people undergoing abdominal wall enterostomy, when comparing two stoma formation techniques: lateral pararectal, in which the stoma is located beside the rectus abdominis muscle, one of the muscles of the abdominal wall, versus transrectal, in which the stoma is pulled through the rectus abdominis muscle?
Background
A stoma is an opening in the abdomen, which is surgically created to divert the flow of urine or feces; an enterostomy is a stoma that starts in the bowel. A parastomal hernia is an incisional hernia, through which abdominal contents protrude through a defect in the abdominal wall at the site of previous surgery. It is related to a stoma, and is one of the most common stoma‐related complications. Many factors that potentially influence the occurrence of parastomal herniation have been investigated. However, it remains unclear whether the enterostomy should be placed through or beside the rectus abdominis muscle in order to prevent parastomal herniation.
Study characteristics
The evidence is current to 9 November 2018. In this update, we included 10 retrospective cohort studies with a total of 864 participants, and one randomized controlled trial (RCT: a study in which participants are randomly allocated to the treatment groups), including 60 participants. The target population was individuals, regardless of age, who received a temporary or permanent enterostomy for any reason in either the elective (planned) or the emergency setting.
Key results
The results found inconclusive results between the two techniques for the risk of parastomal herniation (11 studies, 924 participants), stomal prolapse (1 study, 145 participants), ileus or stenosis (1 study, 60 participants), and skin irritation (1 study, 60 participants).
Neither technique was found to be better than the other for any of the stoma‐related outcomes of interest.
None of the studies measured other stoma‐related problems, or death.
Quality of the evidence
We downgraded the quality of the evidence to moderate, low, or very low, because of high risk of bias, small sample sizes, few events, and diversity across studies.
Conclusion
Based on the current knowledge presented in this review, there is no evidence to support the use of one stoma formation technique over the other. Further research is likely to have an important impact on our confidence in the estimate of effect.
Summary of findings
Summary of findings for the main comparison. Lateral pararectal versus transrectal enterostomy placement for prevention of parastomal herniation.
| Lateral pararectal versus transrectal enterostomy placement for prevention of parastomal herniation | ||||||
| Patient or population: people undergoing enterostomy placement for any reason Settings: elective or emergency settings in university and community hospitals Intervention: lateral pararectal enterostomy placement Comparison: transrectal enterostomy placement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transrectal enterostomy placement | Lateral pararectal enterostomy placement | |||||
|
parastomal hernia (NRS) clinical examination, CT scan, or botha Follow‐up: 2 to 240 months |
Study population | RR 1.22 (0.84 to 1.75) | 864 (10 studies) | ⊕⊝⊝⊝ very lowb,c,d,e | ||
| 295 per 1000 | 360 per 1000 (248 to 517) | |||||
| Moderate | ||||||
| 348 per 1000 | 425 per 1000 (292 to 609) | |||||
|
parastomal hernia (RCT) clinical exam, sonography, intraoperatively, or combination, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) |
Study population | RR 1.34 (0.4 to 4.48) | 56 (1 study) | ⊕⊕⊝⊝ lowf | ||
| 138 per 1000 | 185 per 1000 (55 to 618) | |||||
| Moderate | ||||||
| 138 per 1000 | 185 per 1000 (55 to 618) | |||||
|
stomal prolapse clinical exam, documented on a standard pro forma Follow‐up: mean 110.4 months |
Study population | RR 1.23 (0.39 to 3.85) | 145 (1 study) | ⊕⊝⊝⊝ very lowb,d | ||
| 78 per 1000 | 96 per 1000 (30 to 299) | |||||
| Moderate | ||||||
| 78 per 1000 | 96 per 1000 (30 to 300) | |||||
|
ileus or stenosis clinical exam, sonography Follow‐up: 0.5 to 21.4 months (15 to 642 days) |
Study population | RR 2 (0.19 to 20.9) | 60 (1 study) | ⊕⊕⊝⊝ lowf | ||
| 33 per 1000 | 67 per 1000 (6 to 697) | |||||
| Moderate | ||||||
| 33 per 1000 | 66 per 1000 (6 to 690) | |||||
|
skin irritation clinical exam, intraoperative exam, or both, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) |
Study population | RR 0.67 (0.21 to 2.13) | 60 (1 study) | ⊕⊕⊕⊝ moderated | ||
| 200 per 1000 | 134 per 1000 (42 to 426) | |||||
| Moderate | ||||||
| 200 per 1000 | 134 per 1000 (42 to 426) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aIn four studies the presence of parastomal hernia was assessed by clinical examination and CT scan, or CT scan alone. In the remaining studies, participants were clinically examined, e.g. in stoma therapy or outpatient clinic at follow‐up visits, and the findings were documented in the patient chart. bWe downgraded by 1 level because of a substantial risk of bias in the included studies. cThere are widely differing estimates of the intervention effect (i.e. heterogeneity in results) across studies, but we failed to identify a plausible explanation. Thus, we downgraded by 1 level for inconsistency. dWe downgraded by 1 level for imprecision because of small sample sizes and few events (< 300), wide confidence intervals that included both no effect and appreciable benefit or appreciable harm. eThe funnel plot test did not suggest asymmetry. Since there was no evidence of publication bias, we did not downgraded further. fWe downgraded by 2 levels for substantial imprecision, because very small number of events, and a very wide confidence interval.
Background
Description of the condition
A parastomal hernia is an incisional hernia related to a stoma (Pearl 1989). Parastomal herniation is one of the most common stoma‐related complications (Carne 2003). On physical examination, a parastomal hernia is diagnosed if bulging restricted to the peristomal area occurs during the Valsalva maneuver, and a fascial defect can be palpated (Cingi 2006; Sjodahl 1988). On a computed tomography (CT) scan, a parastomal hernia can be described as 'a loop of intestine or any abdominal organ, as well as preperitoneal fat, protruding through the defect alongside the ostomy' (Cingi 2006).
The incidence of parastomal hernias varies greatly between studies, but most likely ranges from 30% to 50%, depending on the length of follow‐up and on the type of enterostomy (Israelsson 2008). The consequences and implications of parastomal hernias are highly relevant for people. Many people complain of pain, suffer from an impaired body image, and have increasing difficulties in applying the stoma bag properly, leading to leakage and skin irritation (Martin 1996; Ripoche 2011). In rare cases, bowel incarceration occurs, requiring emergency surgery (Cuthbertson 1977). In addition, stomal herniation necessitating reoperation and hospitalization has a negative socioeconomic impact, especially since recurrence rates after surgical correction are high (Israelsson 2008; Tekkis 1999), ranging up to 50% within an average of six months after operative repair (Ripoche 2011). With regard to the etiology of parastomal herniation, one has to differentiate between patient‐related and surgical or technical risk factors. Ripoche 2011 studied several patient‐related potential risk factors, such as age, gender, past medical history (ventral hernia, diabetes, corticosteroid therapy), and diagnosed condition requiring stoma formation (for example malignancy, inflammatory bowel disease, diverticulosis). The only patient factor that was significantly associated with parastomal herniation in a multivariate analysis was age > 60 years at the time of stoma placement (Ripoche 2011), which is supported by data from other studies investigating long‐term hernia rate and risk factors (Hong 2013; Mylonakis 2001; Pilgrim 2010). The influence of operative factors is also much debated. Trephine size, closure of the lateral space, stomal fixation to the fascia, intra‐ versus extraperitoneal route, and location in relation to the rectus abdominis muscle may have an influence on the occurrence of parastomal herniation (Carne 2003; Hotouras 2013). Furthermore, type of enterostomy (ileostomy versus colostomy, end versus loop ostomy), operative setting (emergency versus elective surgery), and whether a stoma therapist marked the site of the stoma preoperatively, seem to have an impact on the risk of parastomal herniation.
Description of the intervention
Two interventions were compared; placement of the stoma lateral to versus through the rectus abdominis muscle (lateral pararectal versus transrectal).
How the intervention might work
Lateral pararectal stoma placement may have a different incidence of parastomal herniation in comparison to transrectal stoma formation. Since the transrectal stoma is surrounded by the thick muscle layers of the rectus abdominis muscle, one could expect a tighter fit and a reduced incidence of parastomal herniation due to the muscle contractions. On the other hand, one could argue that preserving the integrity of the rectus abdominis muscle and its sheath, as is the case in lateral pararectal stomas, minimizes anterior abdominal wall disruption, and consequently reduces the risk of parastomal hernia (Evans 2011; Stephenson 2010).
Why it is important to do this review
Parastomal herniation is one of the most common complications after stoma placement. Since parastomal hernias often have a negative impact on health and quality of life, the operative techniques should be optimized in order to prevent this postoperative complication. The need for prevention becomes even more relevant in the light of the high recurrence rates after operative correction of parastomal hernia, ranging from 50% to 76% after aponeurotic repair and from 24% to 86% after relocation (Israelsson 2008). People with a permanent stoma, for example after abdominoperineal resection for low rectal cancer, especially suffer from the morbidity caused by parastomal herniation. Many surgical approaches have been attempted and propagated.
Two studies showed a significant reduction in the incidence of parastomal herniation if the stoma was situated through the rectus abdominis muscle versus a location lateral to the muscle (Eldrup 1982; Sjodahl 1988). One prospective uncontrolled cohort study, which included 72 consecutive, unselected patients, described a novel anatomical approach to stoma formation, the lateral rectus abdominis positioned stoma (LRAPS), which involves minimal anterior abdominal wall disruption. The authors presented parastomal herniation rates of 5% (3/62) at one‐year follow‐up and 10% (4/41) after two years (Evans 2011; Stephenson 2010), which is consistent with a relevant reduction in parastomal hernia rate when compared to data from the literature (Robertson 2005).
When we conducted the first systematic review, there was no clinical evidence from randomized controlled trials (RCT) on the best stoma location in relation to the rectus abdominis muscle (Hardt 2013). At that time, we assumed clinical equipoise in regard to the review question, and designed the PATRASTOM trial, a phase 1 single center RCT, which assessed the safety and efficacy of the LRAPS in reducing the incidence of parastomal herniation in patients with temporary loop ileostomies, as a pilot trial, in preparation for a prospective multicenter RCT, comparing lateral pararectal to transrectal positioning of a permanent stoma, in regard to parastomal herniation (Hardt 2016).
The current update of our systematic review was considered justified, since the first RCT addressing the review's research question was recently published. The aim of the update was to integrate the newly identified randomized and non‐randomized evidence into the existing body of evidence, in order to synthesize and summarize the current state of knowledge in regard to the review objective.
Objectives
To assess if there is a difference regarding the incidence of parastomal herniation and other stoma complications, such as ileus and stenosis, in lateral pararectal versus transrectal stoma placement in people undergoing elective or emergency abdominal wall enterostomy.
Methods
Criteria for considering studies for this review
Types of studies
Randomized (RCT) and non‐randomized studies (NRS) were eligible. The initial rationale for including non‐randomized studies in the first version of this Cochrane Review was the fact that our preliminary search did not identify any randomized trials that compared the two interventions of interest. Therefore, we conducted a systematic review of non‐randomized studies. Since the only randomized trial, which was eligible for inclusion in the current, updated review version, has a relatively small sample size and short follow‐up, we decided to present results from both randomized and non‐randomized studies regarding the review objective, in order to depict the complete body of evidence. We included non‐randomized studies if they met the inclusion criteria, and compared a lateral pararectal versus a transrectal enterostomy placement. We excluded studies that only reported data on transrectal (for example Carlstedt 1987; Leenen 1989) or lateral pararectal enterostomies (for example Stephenson 2010).
Types of participants
We included trials with individuals who received a temporary or permanent abdominal wall enterostomy, for any reason, in either the elective or the emergency setting, with no regard for the underlying disease.
Types of interventions
Two interventions were compared: lateral pararectal versus transrectal stoma placement.
Types of outcome measures
Primary outcomes
Incidence of parastomal herniation.
Secondary outcomes
Stoma‐related morbidity, especially stenosis, obstruction, prolapse, necrosis, retraction, fistulization, and skin irritation;
Stoma‐related mortality.
Search methods for identification of studies
In November 2018, we searched for all types of published and unpublished RCTs and NRS with no restriction on language or country. We undertook a broad search approach, due to the scarcity of RCTs regarding the review objective. Therefore, we did not apply search filters for study designs on any of our searches.
Electronic searches
For this update, we identified studies by searching the following databases and trials registers:
Cochrane Central Register of Controlled Trials (CENTRAL; Cochrane Register of Studies Online; searched 9 November 2018), Appendix 1.
PubMed (searched 9 November 2018), Appendix 2.
Science Citation Index Expanded Web of Science (searched 9 November 2018), Appendix 3.
LILACS (Latin American and Caribbean Health Science Information database; searched 9 November 2018), Appendix 4.
ClinicalTrials.gov (searched 9 November 2018), Appendix 5.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/; (searched 9 November 2018), Appendix 6.
For this search update, we did not include Embase, as it was no longer available at our institutions. However, RCTs from Embase are now prospectively added to CENTRAL via a highly sensitive screening process (CENTRAL creation details). In addition, all of the included retrospective cohort studies in the previous review version were indexed in MEDLINE, and the new included NRS for this update was also indexed in MEDLINE.
Searching other resources
We endeavored to identify other potentially eligible studies or ancillary publications by screening the reference lists of included studies, systematic reviews, and meta‐analyses. In addition, we contacted the authors of the included studies if we required additional information on their studies.
Data collection and analysis
Selection of studies
Two authors (JH and FH) independently screened all titles and abstracts of papers identified by the search strategies for relevance. They only excluded clearly irrelevant reports at this stage. We obtained full copies of all potentially relevant papers. Afterwards, the two review authors (JH and FH) independently screened the full texts, identified relevant studies, and assessed eligibility of studies for inclusion. They resolved disagreements on the eligibility of studies by discussion and consensus, or if necessary, by consulting a third review author (JM or PK). We excluded all irrelevant records, and recorded the reasons for exclusion.
Data extraction and management
JH and FH independently extracted the data for the review. In addition to the outcomes, they extracted data on population characteristics (such as sex, age, underlying disease, obesity (BMI > 30 kg/m²)), and type of stoma (ileostomy versus colostomy, end ostomy versus loop ostomy), if available. We carefully customized data collection forms to the review question being investigated. During the review process, we repeatedly revised the data collection forms to enable us to handle the different kinds of information about study findings.
Assessment of risk of bias in included studies
Assessment of risk of bias in RCT
We applied the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We assessed risk of bias in the included RCT in the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, reporting bias, and other bias.
Assessment of risk of bias in NRS
As recommended by Reeves and colleagues in Chapter 13 in the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2011), we applied the Newcastle‐Ottawa Scale (NOS) to assess the methodological quality of the included cohort studies in the following domains: selection of cohorts, comparability of cohorts, and assessment of outcome (Wells 2011). We also assessed reporting bias and other bias which are not included in the NOS. In addition, we attempted the use of the Downs and Black Checklist for 'Risk of bias' assessment, which is applicable to both RCTs and NRS, and is also recommended by Reeves and colleagues, but we had to conclude that the NOS was easier to use with the included cohort studies (Downs 1998).
For all included studies, JH and FH independently assessed the risk of bias.
Measures of treatment effect
We treated the presence of parastomal herniation, as well as the presence of other stoma complications, such as stomal prolapse, ileus or stenosis, and skin irritation, as dichotomous variables. For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI).
Unit of analysis issues
The unit of analysis was each participant recruited into the trials.
Dealing with missing data
We carefully assessed the risk of incomplete outcome data using the NOS and the Cochrane tool for assessing risk of bias. Since all included NRS were retrospective cohort studies, with the majority published more than 20 years ago, we refrained from contacting the authors for missing data on losses to follow‐up and their reasons. However, we successfully contacted the authors of Ho 2018 for missing data on parastomal hernia rates, and discussed the potential impact of missing outcome data (for example due to losses of follow‐up or exclusions from analysis) in the discussion section (Appendix 7).
Assessment of heterogeneity
We used the Cochrane Q test to assess for statistical heterogeneity. We considered a P value of 0.10 to be statistically significant. Even more relevant than the assessment of heterogeneity, is the quantification of its impact on the meta‐analysis. Thus, we applied the I² value to quantify the inconsistency of the included NRS (Deeks 2011). An I² value less than 40% might not be important; greater values may represent moderate (< 60%), substantial, or even considerable (> 75%) heterogeneity. Nonetheless, we were aware of the fact that there is much uncertainty in measures, such as the I² statistic, when there are few studies.
Assessment of reporting biases
Reporting biases occur if the dissemination of research findings is influenced by the nature and direction of trial results. There are several types of reporting bias: publication bias, multiple publication bias, time lag bias, location bias, citation bias, language bias, and outcome reporting bias (Sterne 2011).
We used a comprehensive search strategy, including the search of two different trials registers, in order to minimize the likelihood of publication bias (see Electronic searches), but we were aware of the problem of inconsistent indexing in trials registers, especially with NRS. Furthermore, we searched LILACS, the most important and comprehensive index of scientific and technical Latin‐American and Caribbean literature, to diminish the risk of language bias.
To assess publication bias in our review, we used a funnel plot (see Figure 1). Publication bias is considered minimum if the plot resembles a funnel with the base down. The funnel plot test by Harbord did not suggest asymmetry in the funnel plot (P = 0.6667 (Harbord 2006)). However, we were aware of the fact that asymmetry is difficult to detect with such a small number of included studies (Sterne 2011).
1.
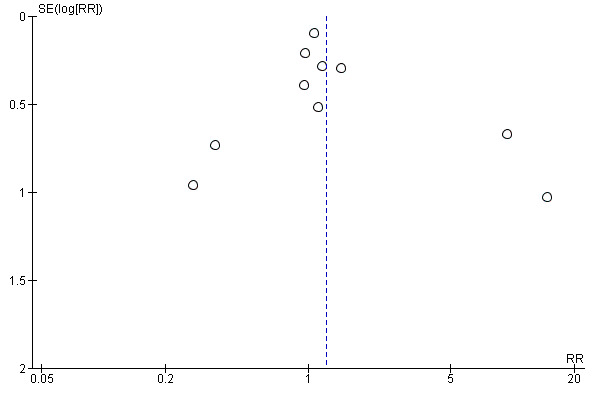
Funnel plot of comparison 1. Lateral pararectal versus transrectal enterostomy placement, outcome 1.1. parastomal hernia. Only non‐randomized studies
Data synthesis
From the analysis options in Review Manager 5.3, we applied the DerSimonian and Laird random‐effects model, assuming that the effects being estimated in the different studies were not identical but followed some distribution (DerSimonian 1986). The random‐effects model estimates the extent of variation among the intervention effects of the included studies (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analysis:
ileostomy versus colostomy.
For the following intended subgroup analyses, the included studies reported the required data insufficiently or not at all:
obesity (BMI > 30 kg/m²), overweight (BMI > 25 < 30 kg/m²) versus normal weight (BMI > 20 < 25 kg/m²);
end versus loop ostomy.
Sensitivity analysis
Sensitivity analysis investigates how the variation in the output of a statistical model depends on the different variations in the inputs of the model. We could not perform either of the two planned sensitivity analyses because of the following reasons:
Sensitivity analysis based on assessment of standardization of stoma surgery was not performed, since there was insufficient evidence of standardization of the surgical interventions in the included studies.
Sensitivity analysis based on assessment of risk of bias was not performed, since the majority of included studies were at high risk of bias in at least one of the assessed domains, and showed very similar risk of bias profiles (see Figure 2; Figure 3; Table 2).
2.
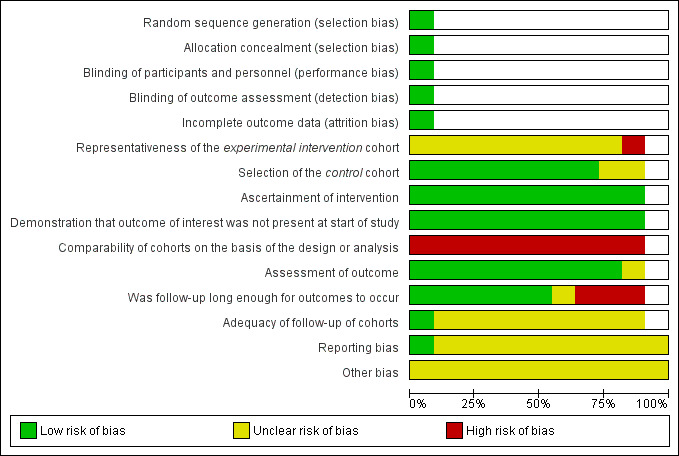
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
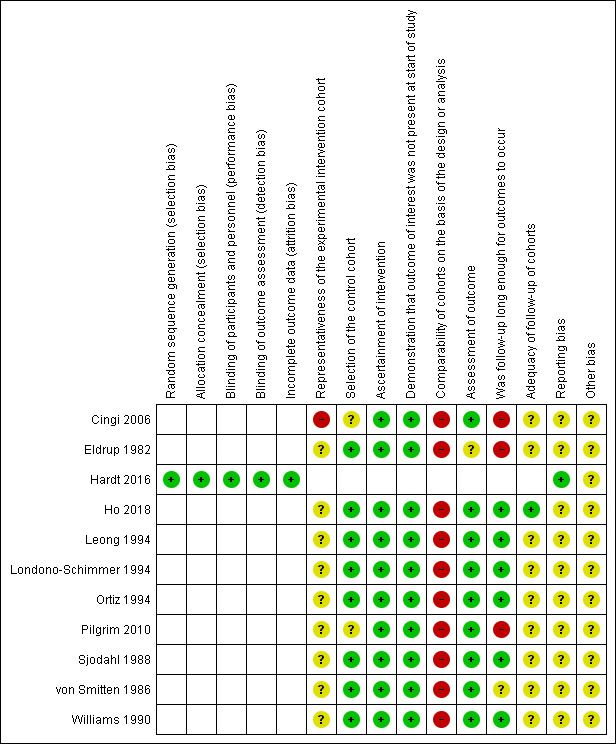
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
1. Risk of bias summary: review authors' judgements about each quality item using the NOS criteria.
| Representativeness of the experimental intervention cohort | Selection of the control cohort | Ascertainment of intervention | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow‐up of cohorts | |
| Cingi 2006 | ★ | ★ | ★ | |||||
| Eldrup 1982 | ★ | ★ | ★ | ★ | ||||
| Ho 2018 | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Leong 1994 | ★ | ★ | ★ | ★ | ★ | |||
| Londono‐Schimmer 1994 | ★ | ★ | ★ | ★ | ★ | |||
| Ortiz 1994 | ★ | ★ | ★ | ★ | ★ | |||
| Pilgrim 2010 | ★ | ★ | ★ | |||||
| Sjodahl 1988 | ★ | ★ | ★ | ★ | ★ | |||
| von Smitten 1986 | ★ | ★ | ★ | ★ | ||||
| Williams 1990 | ★ | ★ | ★ | ★ | ★ |
A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. A maximum of nine stars can be awarded overall.
Results
Description of studies
See: Characteristics of included studies
Results of the search
As recommended by the PRISMA statement, a study flow diagram summarizes the study selection process for the review update (Liberati 2009; Figure 4). The flow diagram presenting the study selection process of the original version of the review is available in Hardt 2013.
4.
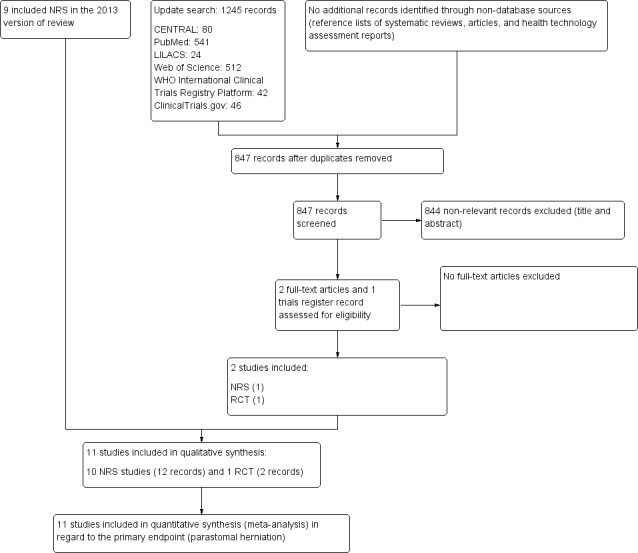
Trial flow diagram
RCT: randomized controlled trial; NRS: non‐randomized study
The electronic searches, run on 9 November 2018, retrieved a total of 1245 references; The Cochrane Library (CENTRAL 80), PubMed (541), Web of Science (512), LILACS (24), ClinicalTrials.gov (46) and ICTRP (42). We excluded 398 duplicates and 844 clearly irrelevant references, identified by reading titles and abstracts. We retrieved the three remaining references for further assessment. All three references, relating to one RCT and one NRS, fulfilled our inclusion criteria (see: Included studies).
Included studies
One randomized controlled trial (RCT) and ten retrospective non‐randomized studies (NRS) met the inclusion criteria. In order to determine the type of NRS and distinguish between case series and cohort studies, we applied the criteria outlined by Dekkers and colleagues (Dekkers 2012). According to their proposal, a cohort study is defined by the following main features:
sampling is based on exposure;
the occurrence of outcomes is assessed during a specified follow‐up period;
exposure can be a risk factor, a disease, or an intervention;
absolute risk (or rate) calculation is possible;
if a comparison group is included, relative risk can be calculated.
All included NRS showed these features, and we consequently classified them as cohort studies.
For more details on the included studies, see the 'Characteristics of included studies' tables.
Excluded studies
We excluded 11 studies due to various reasons, as presented in the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
Assessment of risk of bias in RCT
Figure 2 and Figure 3 contain the risk of bias assessments for both the RCT and the NRS. Since not all risk of bias domains apply to both study types, some lines and cells of the two figures remain blank.
Assessment of risk of bias in NRS
Reeves and colleagues hypothesized that the risk of bias in NRS depends on the specific study features rather than on the design labels (such as cohort or case‐control study (Reeves 2011)). Thus, we attempted to assess the study features that had a major impact on the risk of bias using the Newcastle‐Ottawa Scale (NOS) for cohort studies (Wells 2011). See Figure 2; Figure 3; Table 2.
Selection bias
RCT
In the PATRASTOM trial (Hardt 2016), the only included RCT, successful randomization, including adequate sequence generation and allocation sequence concealment prevented selection bias. Thus, we considered the PATRASTOM trial at low risk of selection bias.
NRS
In NRS, groups are unlikely to be comparable due to the lack of concealed randomization within the allocation process. Consequently, NRS are more susceptible to selection bias compared to RCTs, which is regarded as the main difference between RCTs and NRS (Reeves 2011). We checked the representativeness of the intervention cohort (lateral pararectal stoma placement) and the comparability of the intervention (lateral pararectal) and control (transrectal) cohorts. The majority of the included studies were at unclear risk of selection bias since the authors did not sufficiently describe the derivation of the intervention cohort, and whether the intervention and control cohorts were drawn from the same community.
Performance bias
RCT
We judged the risk of performance bias as low in the PATRASTOM trial because participants were blinded to the intervention they received. As stated in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, blinding is not always possible (Higgins 2011). This was the case with the operating surgeon who could not be blinded to the intervention he performed. Nonetheless, it is unlikely that the lack of blinding of the surgeon influenced the outcome.
NRS
We considered the risk of performance bias to be relatively low in the included studies because the surgical procedure (lateral pararectal versus transrectal stoma placement) was ascertained reliably either by reviewing secure records, such as operative reports and patient charts, or by computerized tomography (CT), or clinical examination, or both. In two radiological studies, the position of the stoma in relation to the rectus abdominis muscle was assessed by CT scanning (Cingi 2006; Williams 1990). In the most recently included study, the type of intervention was ascertained both by database review and by CT scans (Ho 2018). In another study, the intervention was reassessed by CT examination if it remained unclear after clinical evaluation (Ortiz 1994). In one study, the stomal site in relation to the rectus muscle was determined by clinical examination, by a surgeon and a staff nurse at the stoma therapy service (Sjodahl 1988). In the remaining studies, the intervention must have been ascertained by chart review (though this was not always explicitly stated (Eldrup 1982; Pilgrim 2010; von Smitten 1986)), or review of a standard operative 'pro forma' (Leong 1994; Londono‐Schimmer 1994).
Detection bias
RCT
There were no systematic differences between groups in how outcomes were determined in the PATRASTOM trial. Although the outcome assessor (i.e. the surgeon who performed the stoma reversal operation) was not blinded to the previously performed study intervention, it is unlikely that this influenced the outcome assessment results.
NRS
We also estimated the risk of detection bias to be low in most of the included studies, as the outcome (parastomal herniation) was assessed by either clinical examination, or CT scanning, or both, or by reviewing reliable patient records. In four studies, the outcome was assessed by physical examination and CT scanning (Cingi 2006; Ortiz 1994; Williams 1990) or by CT scanning alone (Ho 2018). In two Scandinavian studies from the 1980s, parastomal herniation was assessed only by clinical examination (Sjodahl 1988; von Smitten 1986). In another study, the outcome was ascertained by physical examination including 'digital examination through the stomal opening' by enterostomal nurse specialists (Pilgrim 2010). In both studies from St. Mark's Hospital in London, UK, the outcomes (parastomal herniation and stomal prolapse) were assessed by reviewing standard operative pro formas (Leong 1994; Londono‐Schimmer 1994). In the oldest of the included studies, a Danish study including patients from two Copenhagen county hospitals, all eligible living participants were screened for parastomal hernia by physical examination, whereas all eligible dead patients were assessed for the outcome by chart review (Eldrup 1982).
Incomplete outcome data
RCT
Outcome data were not available in 4 of the 60 randomized patients (Hardt 2016), but the reasons for missing outcome data were similar across the intervention groups and unlikely to be related to the true outcome. Therefore, the risk of attrition bias is judged as low in this study.
NRS
We found the majority of included NRS at high risk of presenting incomplete outcome data (attrition bias). Applying the NOS, we identified that five of the ten included studies showed follow‐up rates < 80% (Cingi 2006; Eldrup 1982; Leong 1994; Londono‐Schimmer 1994; Williams 1990). Four studies either did not present the number of people examined for eligibility or lost to follow‐up (Ortiz 1994; Pilgrim 2010; Sjodahl 1988), or the numbers given seemed unreliable from a clinician's standpoint (von Smitten 1986). Only the most current included NRS described the exact number of people examined for eligibility and the reasons for exclusion (Ho 2018). Since 103 of 112 patients were confirmed eligible, the follow‐up rate was about 92%. Accordingly, we considered the risk for attrition bias in this study as low.
Selective outcome reporting bias
RCT
Reporting bias due to selective outcome reporting seemed unlikely because the PATRASTOM trial was registered prospectively in the German Clinical Trials Register, and all of the study’s prespecified outcomes were reported in the prespecified way (see Appendix 8).
NRS
The common lack of a study protocol for NRS leads to a higher risk of potential selective outcome reporting bias for NRS compared to RCTs (Reeves 2011). Features of RCTs that prevent reporting bias (for example a prespecified protocol, ethical approval, progress and final reports, and the CONSORT statement) were missing in all included NRS. Comparing the information given in the methods and results sections regarding outcomes to be assessed and the outcomes assessed, we did not discover any discrepancies. Hence, we concluded that the likelihood of selective outcome reporting remained unclear. We considered the risk of reporting bias in the most recently included NRS was lower than in the remaining nine NRS because this study was reported in accordance with the STROBE guidelines (Elm 2014) and the European Hernia Society (EHS) recommendations on the reporting of hernia surgery (Muysoms 2013). Nonetheless, in the absence of a study protocol, it remains unclear whether selective (or incomplete) outcome reporting was present (Ho 2018).
Other potential sources of bias
One study was supported by a grant, but no further information was provided (Ortiz 1994). This could potentially introduce additional bias.
Effects of interventions
See: Table 1
The only identified RCT reported data on the primary endpoint (parastomal herniation), as well as on the secondary outcomes ileus or stenosis and stoma‐related skin irritation (Hardt 2016). They also assessed quality of life before and after ileostomy placement, using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires QLQ‐CR29 and QLQ‐C30.
All NRS included in the meta‐analysis reported results for the primary outcome of parastomal herniation, and one study also reported data on one of the secondary outcomes, stomal prolapse (Leong 1994). None of the included studies compared the two interventions with regard to other secondary outcomes.
The results from one RCT (N = 56) were inconclusive between the lateral pararectal (5 of 27) and the transrectal groups (4 of 29) for the incidence of parastomal herniation (risk ratio (RR) 1.34, 95% confidence interval (CI) 0.40 to 4.48; P = 0.63; Analysis 1.1); development of ileus or stenosis (RR 2.0, 95% CI 0.19 to 20.90; P = 0.56; Analysis 1.4); stoma‐related skin irritation (RR 0.67, 95% CI 0.21 to 2.13; P = 0.49; Analysis 1.5), and quality of life.
1.1. Analysis.
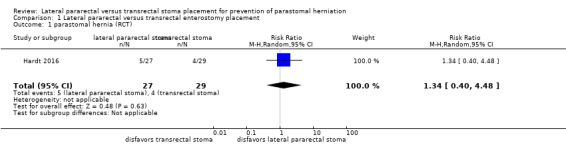
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 1 parastomal hernia (RCT).
1.4. Analysis.
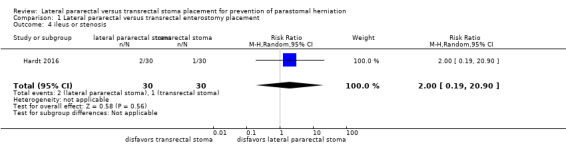
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 4 ileus or stenosis.
1.5. Analysis.
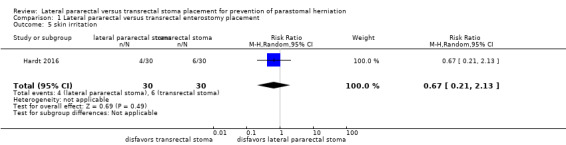
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 5 skin irritation.
The results from the NRS were also inconclusive for parastomal herniation rates (80/258 in the lateral pararectal, 179/606 in the transrectal group; RR 1.22, 95% CI 0.84 to 1.75; P = 0.29; 10 NRS, 864 participants; Analysis 1.2), and the development of stomal prolapse (RR 1.23, 95% CI 0.39 to 3.85; P = 0.73; 1 NRS, 145 participants; Analysis 1.3).
1.2. Analysis.
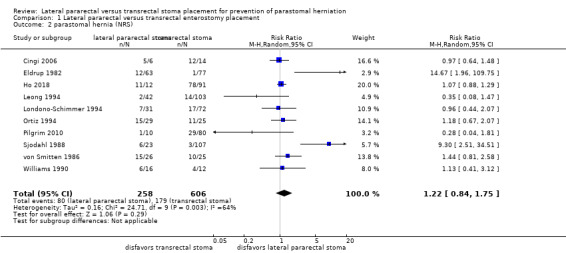
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 2 parastomal hernia (NRS).
1.3. Analysis.
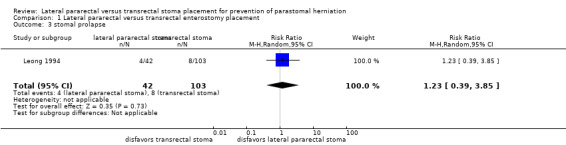
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 3 stomal prolapse.
The subgroup analysis found inconclusive results for the incidence of parastomal herniation between participants who received an ileostomy and those who received a colostomy (RR 1.16, 95% CI 0.81 to 1.65; 7 NRS, 624 participants; Analysis 2.1). The results were also inconclusive in both the ileostomy subgroup (RR 0.70, 95% CI 0.21 to 2.29; 2 NRS, 173 participants; Analysis 2.1) and the colostomy subgroup (RR 1.30, 95% CI 0.80 to 2.11; 5 NRS, 451 participants; Analysis 2.1).
2.1. Analysis.
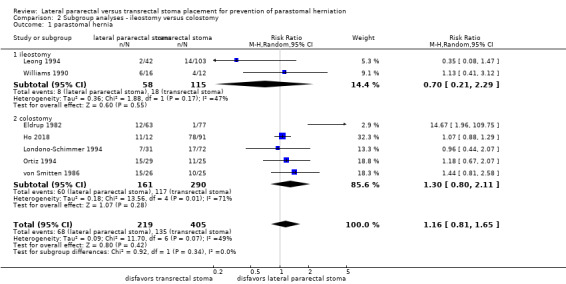
Comparison 2 Subgroup analyses ‐ ileostomy versus colostomy, Outcome 1 parastomal hernia.
Discussion
Parastomal herniation is one of the most common stoma complications and causes significant stoma‐related morbidity, especially in people with permanent enterostomies, for example people with low rectal cancer who had to undergo abdominoperineal resection. The patient relevance of this complication is high because parastomal herniation often results in further morbidity, such as skin irritation and maceration due to leakage. Moreover, it can necessitate emergency surgery for strangulated bowel. A recent retrospective study from France, which included 782 people with a stoma, showed that after a mean follow‐up of 10.5 years, the prevalence of parastomal herniation was 25.6% (n = 202). Of the 202 participants with parastomal hernia, more than one third (35%) complained of pain and almost one third (28%) suffered from leakage due to difficulties in fitting the stomal appliance. More than half of the people (n = 114) underwent operative repair, but again, in half of these (n = 57) parastomal herniation recurred within six months (Ripoche 2011). The high recurrence and morbidity rates after hernia repair make the situation of the affected people even more precarious and underline the need for effective preventive measures.
Therefore, and on the premise that surgical prevention of parastomal herniation without the use of mesh is worthy of consideration, we aimed to collect all existing evidence regarding the question of whether a stoma should be placed through or lateral to the rectus abdominis muscle. The main results of our reviewing process are summarized in the Summary of main results.
Summary of main results
Currently, the evidence for our review objectives consists of a small single center pilot randomized controlled trial (RCT) and ten retrospective controlled cohort studies (NRS). Due to the lack of further RCT evidence, we still included NRS in this updated version of our Cochrane Review. All included NRS reported the primary outcome (parastomal hernia), and one study (145 participants), also reported one of the secondary outcomes (stomal prolapse (Leong 1994)). Eight of the ten included cohort studies (594 participants), did not find a statistically significant difference in parastomal herniation rates after lateral pararectal compared to transrectal stoma formation. In contrast, the remaining two studies found a significant reduction of parastomal herniation if the stoma was pulled out through the belly of the rectus abdominis muscle (Eldrup 1982; Sjodahl 1988).
Pooling the data of all included NRS did not provide conclusive results. Reasons for the widely differing estimates of the intervention effect (heterogeneity) across studies could be due to the lack of standardization of the surgical procedure, as well as the absence of a uniform definition and detection method for parastomal hernia. An I² value of 64% indicated substantial statistical heterogeneity in the meta‐analysis (Analysis 1.2). The I² statistic describes the percentage of total variation across studies that is caused by heterogeneity rather than by chance (Deeks 2011). In Analysis 1.2, the calculated P value was interpreted as a finding of uncertainty. The wide confidence interval could be the result of the small number of studies combined in the meta‐analysis, as well as the substantial heterogeneity.
The wide confidence intervals in the other analyses were likely caused by the small sample size of the only included RCT, the PATRASTOM trial, which found inconclusive results between lateral pararectal and transrectal stoma placement for the incidence of parastomal herniation, other stoma‐related complications, and quality of life (Hardt 2016). However, due to the limited sample size and the wide confidence intervals, a small and even a moderately sized difference in favor of one of the two stoma placement techniques cannot be entirely ruled out.
In conclusion, there is no evidence supporting the surgical dogma that the transrectal route of stoma formation should be preferred in order to prevent parastomal herniation.
Overall completeness and applicability of evidence
The present review aimed to compare lateral pararectal to transrectal stoma placement for prevention of parastomal herniation and other patient‐relevant stoma‐related complications. This was only partly accomplished. All included studies reported parastomal herniation, the primary outcome, but only one of the NRS and the RCT presented data on stomal prolapse. Only the RCT, but none of the NRS, reported any other secondary outcomes. Furthermore, we could only conduct one of the planned subgroup analyses and none of the sensitivity analyses because the required data were not reported in the included studies. Thus, the evidence with regard to the review objective is very limited, and the identified studies could not address all of the objectives of the review. The NRS identified are all retrospective cohort studies and at high risk of bias, especially with regard to selection bias and incomplete outcome data (attrition bias). Since the participants were not randomly assigned to the two intervention groups, the risk of selection bias is high. Moreover, it mostly remained unclear whether the individual participants were representative of the population. Hence, external validity and generalizability of the results from the meta‐analysis of NRS remain unclear. Although overall risk of bias is considered moderate to low in the included RCT, there are some obvious limitations (small sample size, short follow‐up, non‐blinding of the surgeons assessing the primary endpoint), which restrict the applicability of the study results.
Quality of the evidence
We included one RCT and ten retrospective cohort studies in this systematic review. For key methodological features and limitations of the studies, please see Characteristics of included studies. Confidence in the estimates of the treatment effect is limited because of risk of bias, publication bias, imprecision, inconsistency, and indirectness. All these issues are included in the GRADE system and are summarized in the Table 1. Overall risk of bias in the included observational studies is high, especially due to possible selection bias (no randomization, unclear representativeness and comparability of the two cohorts), and high risk of attrition bias. It remains unclear whether publication bias affected the estimates of treatment effect, but it cannot be excluded, because observational studies reporting higher rates of parastomal herniation and other stoma‐related complications might be less likely to be published than studies presenting lower complication rates. This may be a particular concern in surgery. Since the 95% confidence interval around the estimate of effect includes both no effect and appreciable benefit or appreciable harm, and because the total number of events is less than 300, we assume that there is considerable imprecision. Moreover, the quality of evidence is diminished due to inconsistency, because of the widely differing estimates of the intervention effect (heterogeneity) across studies, for which we failed to identify a plausible explanation. However, we did not find any evidence of indirectness with respect to the target population, intervention, or outcome of interest.
Hence, we downgraded the quality of the evidence from NRS for the primary endpoint (parastomal herniation) by one level, from low to very low, due to high risk of bias, imprecision, and inconsistency.
In summary, the body of evidence identified does not allow a robust conclusion regarding the objectives of the review.
Potential biases in the review process
Although we followed a strict protocol consistent with the new version of the MECIR, published in 2016 (Higgins 2016), and the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) statements (Stroup 2000), and applied a comprehensive search strategy, this review is still prone to bias. For example, publication bias cannot be excluded in our review because NRS are not consistently indexed in trials registers (Reeves 2011). Consequently, it must be taken into consideration that we may have missed completed or ongoing NRS. Moreover, we did not screen conference proceedings, which could potentially lead to further publication bias.
As also stated in the "Declarations of interest" paragraph, four of the review authors (JH, FH, PK, SP) conducted one of the included studies, the PATRASTOM trial. In order to prevent confounding and to guarantee transparency and objectivity, an external clinician not involved in the trial checked data extraction, risk of bias assessment and interpretation of the PATRASTOM trial.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only systematic review addressing this particular review question. However, several narrative reviews have discussed the objective of our review (Carne 2003; Hotouras 2013; Israelsson 2008; Martin 1996; Pearl 1989).
Regardless of the fact that until recently, when the results of the PATRASTOM trial were published, there was no evidence from randomized trials, surgical dogma has emerged that a stoma should be placed through the rectus abdominis rather than lateral to the muscle, in order to prevent parastomal herniation. This credo has been persistently repeated in the surgical literature, usually without any scientific evidence (Park 1999; Pearl 1985). But how could this surgical dictum arise? And why does it still persist, despite the lack of evidence? In the mid 1930s, Gabriel and Lloyd‐Davies recommended transrectal colostomy formation to prevent parastomal herniation, which was approved by Turnbull 20 years later (Gabriel 1935; Turnbull 1958). In the mid‐1970s, a study from St. Mark's Hospital in London, UK, was published, reporting data on 227 people undergoing surgery for rectal adenocarcinoma. In 35 of the included participants, the colostomy was created transrectally, and after a follow‐up of one to six years, six of the 35 participants were diagnosed with a parastomal hernia (Marks 1975). Thus, on the basis of their data, the authors concluded that the transrectal route could not generally be recommended. One explanation of why surgeons continued to adhere to the thesis that the stomata should be placed transrectally could be the assumption that pulling the stoma through the rectus abdominis muscle provided a more snug fit, since the muscle contracted around the stomal aperture. On the other hand, the same thinking could lead to the concern that there was a higher risk of stoma‐related stenosis and ileus, due to tight muscle contractions around the enterostomy.
In 2010, Stephenson described a novel approach to stoma formation, the lateral rectus abdominis positioned stoma (LRAPS), being convinced that preservation of the rectus abdominis muscle and its sheath could prevent parastomal herniation (Stephenson 2010). The new technique implied that the anterior and posterior rectus sheath were only divided horizontally (not vertically) and that the rectus muscle was not incised, but separated from its sheath, by sharp and blunt dissection and was then retracted medially. After the bowel was delivered, any defect of the medial margins of the incisions in the anterior and posterior sheath was closed with interrupted absorbable sutures. Stephenson presented data from 29 consecutive, unselected people who received a LRAPS. After a mean follow‐up of 13 months (range 7 to 18), no parastomal hernia had been detected. In 2011, Evans published a further follow‐up of the LRAPS; at one year of follow‐up, the authors observed parastomal hernias in 5% of the participants with a LRAPS (3/62), and at two years, the parastomal hernia rate had increased to 10% (4/41 (Evans 2011)). Although the follow‐up duration was still rather short, the reported parastomal herniation rates were lower than what was usually reported in the literature (Israelsson 2008; Robertson 2005).
Another emerging surgical trend is the use of prophylactic mesh for prevention of parastomal herniation. In the last decade, multiple systematic reviews of randomized trials addressed the question of whether prophylactic implantation of prosthetic mesh could reduce the rate of parastomal herniation. The most recently published systematic reviews pooled the data of six to ten RCTs; meta‐analyses showed that mesh reinforcement at the time of stoma formation significantly reduced the incidence of clinically‐ and radiologically‐detected parastomal herniation without increasing stoma‐ or mesh‐related morbidity and other surgical complications (Chapman 2017; Cornille 2017; Cross 2017; López‐Cano 2017; Patel 2017; Pianka 2017; Wang 2016). However, these results should be interpreted with caution, due to the heterogeneity among the included studies, the high risk of bias, and the small sample sizes of the individual studies (the median sample size of the RCTs included in the systematic review by Chapman was 54 (range 34 to 113)). Because of these severe limitations, some of the authors of the most recent systematic reviews call for larger, higher‐quality RCTs, which also assessed different mesh types, locations, and techniques, as well as long‐term cost‐effectiveness (Chapman 2017; Cornille 2017; Patel 2017; Wang 2016). Interestingly, the very recently published high‐quality multicenter double‐blinded RCT from Sweden, STOMAMESH, with a remarkably larger sample size than all previously conducted RCTs (211 analyzed participants), could not confirm the findings of the above‐cited meta‐analyses: Odensten and colleagues described that mesh reinforcement around the stoma did not alter the rate of parastomal herniation at one‐year follow‐up (Odensten 2019). In summary, there is still contradictory evidence for the effect of prophylactic mesh placement at the time of stoma formation, and at least on the basis of the results of the current large RCT, the standard use of prophylactic mesh reinforcement cannot be recommended.
Authors' conclusions
Implications for practice.
The analyses of the included studies showed inconsistent differences between the two interventions in their potential to prevent parastomal herniation. Therefore, people undergoing stoma surgery and clinicians should be aware of the considerable uncertainty as to which treatment is preferable, lateral pararectal or transrectal stoma formation. Since the currently available evidence neither supports nor refutes the advantage of one technique over the other, a clinical and pragmatic interpretation of the evidence suggests that surgeons may apply a transrectal or pararectal stoma formation technique at their discretion, and without concern for causing more harm by one or the other technique.
Implications for research.
Although the body of evidence has been extended by another cohort study and even by one RCT since the first version of this review was published in 2013, the research question still cannot be answered with certainty. The synopsis of the current limited evidence suggests, on the one hand, that lateral pararectal stoma formation does not alter the rate of parastomal herniation compared to the transrectal position (evidence of no difference). On the other hand, since the evidence is of only moderate to (very) low quality due to considerable risk of bias in most of the included studies, one may question the claimed absence of a difference on the grounds that the evidence base is inadequate to draw such a final conclusion. If the current evidence is judged as insufficient, further RCTs and especially large high‐quality multicenter RCTs are required to conclusively and reliably determine the effect of the compared interventions in regard to the primary (parastomal herniation) and other highly patient‐relevant (secondary) outcomes on the long‐term.
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2019 | New citation required but conclusions have not changed | |
| 5 January 2019 | New search has been performed | The present Cochrane Review update was planned and initiated (latest literature search run November 9, 2018) after the publication of the first randomized trial addressing the review objective. In addition to the only randomized study comparing lateral pararectal to transrectal stoma formation, the literature search and screening revealed one further cohort study which met the inclusion criteria. Despite the addition of two studies, the currently available evidence still remains of moderate to very low quality. |
Acknowledgements
Thanks to:
Gerta Rücker, Institute of Medical Biometry and Medical Informatics of the University Medical Center Freiburg, Freiburg i. Br., Germany, for statistical advice and methodological support;
Lasse T. Krogsbøll, Nordic Cochrane Center, Rigshospitalet, Copenhagen, Denmark, for the translation of Eldrup 1982 and the valuable discussion on how to review observational studies;
the Cochrane Colorectal Cancer Group (CCCG), Copenhagen, Denmark, especially to Henning K. Andersen and Marija Barbateskovic;
Gerd Antes and the team at the German Cochrane Center, Freiburg i. Br., Germany.
Appendices
Appendix 1. CENTRAL search strategy (via Cochrane Register of Studies Online)
| No. | Search |
| #1 | (stoma OR stomas OR stomata OR stomal OR parastom*):TI,AB,KY |
| #2 | (enterostom* OR colostom* OR cecostom* OR duodenostom* OR ileostom* OR jejunostom*):TI,AB,KY |
| #3 | ("anus praeter" OR "preternatural anus" OR "artificial anus"):TI,AB,KY |
| #4 | MESH DESCRIPTOR Surgical Stomas EXPLODE ALL TREES |
| #5 | MESH DESCRIPTOR Enterostomy EXPLODE ALL TREES |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | herni*:TI,AB,KY |
| #8 | MESH DESCRIPTOR Hernia |
| #9 | MESH DESCRIPTOR Hernia, Abdominal |
| #10 | 7 OR #8 OR #9 |
| #11 | #6 AND #10 |
| #12 | 2012 TO 2018:YR |
| #13 | #11 AND #12 |
Appendix 2. PubMed search strategy
| No. | Search |
| #1 | stoma [tiab] OR stomas [tiab] OR stomata [tiab] OR stomal [tiab] OR parastom* [tiab] |
| #2 | enterostom* [tiab] OR colostom* [tiab] OR cecostom* [tiab] OR duodenostom* [tiab] OR ileostom* [tiab] OR jejunostom* [tiab] |
| #3 | "anus praeter" [tiab] OR "preternatural anus" [tiab] OR "artificial anus" [tiab] |
| #4 | Surgical Stomas [mh] OR Enterostomy [mh] |
| #5 | #1 OR #2 OR #3 OR #4 |
| #6 | herni* [tiab] |
| #7 | hernia [Mesh:NoExp] OR hernia, abdominal [Mesh:NoExp] |
| #8 | #6 OR #7 |
| #9 | #5 AND #8 |
| #10 | animals [mh] NOT humans [mh] |
| #11 | #9 NOT #10 |
| #12 | 2012/10:2018/11 [crdt] |
| #13 | #11 AND #12 |
Appendix 3. Science Citation Index Expanded search strategy (via Web of Science)
| No. | Search |
| #1 | Topic=(stoma OR stomas OR stomata OR stomal OR parastom*) |
| #2 | Topic=(enterostom* OR colostom* OR cecostom* OR duodenostom* OR ileostom* OR jejunostom*) |
| #3 | Topic=("anus praeter" OR "preternatural anus" OR "artificial anus") |
| #4 | #3 OR #2 OR #1 |
| #5 | Topic=(herni*) |
| #6 | #5 AND #4 Timespan=2012‐2018 |
Appendix 4. LILACS search strategy (via iAHx)
| Search |
| (TW:stoma OR stomas OR stomata OR stomal OR estoma OR estomas OR parastom$ OR paraestom$ OR "surgical stomas" OR "estomas quirúrgicos" OR "estomas cirúrgicos" OR "anus praeter" OR "preternatural anus" OR "artificial anus" OR "anal artificial" OR enterostom$ OR colostom$ OR cecostom$ OR duodenostom$ OR ileostom$ OR jejunostom$ OR yeyunostom$) AND (TW:herni$) AND DA:201$ |
Appendix 5. ClinicalTrials.gov search strategy (via expert search)
| Search |
| (stoma OR stomas OR stomata OR stomal OR parastomal OR enterostomy OR enterostomies OR colostomy OR colostomies OR cecostomy OR cecostomies OR duodenostomy OR duodenostomies OR ileostomy OR ileostomies OR jejunostomy OR jejunostomies OR (anus AND praeter) OR (preternatural AND anus) OR (artificial AND anus)) AND (hernia OR hernias OR herniae OR herniation OR herniations OR hernial OR herniated) |
Appendix 6. ICTRP Search Platform search strategy (via standard search)
| Search |
| stoma AND herni* OR stomas AND herni* OR stomata AND herni* OR stomal AND herni* OR parastom* AND herni* OR enterostom* AND herni* OR colostom* AND herni* OR cecostom* AND herni* OR duodenostom* AND herni* OR ileostom* AND herni* OR jejunostom* AND herni* OR anus praeter AND herni* OR preternatural anus AND herni* OR artificial anus AND herni* |
Appendix 7. Survey of study investigators
| For the update of this Cochrane Review, we contacted the authors of the newly included NRS (Ho 2018). Since the authors of this Cochrane Review are identical to the investigators who conducted Hardt 2016, we did not need a survey of these investigators. | ||||
| Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) | |
| Ho 2018 | 28 June 2017 | 13 July 2017 | On 17 September 2017, the authors sent us their manuscript, which was at that time not yet published, but had already been submitted to a journal (until then we only had the information given in a study abstract published in the journal Gut in 2015). On 19 September 2017, we requested data that was not presented in the manuscript, to enable us to include their study in this Cochrane Review. | On 20 September 2017, the authors sent us a table with the requested information (the rates of parastomal herniation in both groups). |
Appendix 8. Matrix of RCT endpoints
| The only included study for which a study protocol was available and which had been registered prospectively in a trials register was Hardt 2016. | ||||
| Endpoints quoted in trial document(s)a | Trial results available in trial register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Hardt 2016 |
Sources: ICTRP register record and DRKS00003534 Primary outcome measure(s): parastomal herniation |
Yes (last verified: 23 December 208) |
Primary outcome measure(s): All of the study’s prespecified outcomes were reported in the prespecified way, in both the publication and the abstract. |
Primary outcome measure(s): All of the study’s prespecified outcomes were reported in the prespecified way, in both the publication and the abstract. |
Secondary outcome measure(s):
|
Secondary outcome measure(s): See above |
Secondary outcome measure(s): See above |
||
|
aTrial document(s) refers to trial information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers)
bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents, or multiple reports of a primary trial)
cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures EMA: European Medicines Agency; FDA: Food and Drug Administration (US). | ||||
Data and analyses
Comparison 1. Lateral pararectal versus transrectal enterostomy placement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 parastomal hernia (RCT) | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.40, 4.48] |
| 2 parastomal hernia (NRS) | 10 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.75] |
| 3 stomal prolapse | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.39, 3.85] |
| 4 ileus or stenosis | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.90] |
| 5 skin irritation | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
Comparison 2. Subgroup analyses ‐ ileostomy versus colostomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 parastomal hernia | 7 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.81, 1.65] |
| 1.1 ileostomy | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.21, 2.29] |
| 1.2 colostomy | 5 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.80, 2.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cingi 2006.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 46 participants operated on between January 2000 and January 2005 at Marmara University Hospital in Istanbul, Turkey:
|
|
| Interventions |
Standardization of the surgical procedure: unclear |
|
| Outcomes | Incidence of parastomal hernia:
Incidence of closed ostomy site incisional hernia
|
|
| Notes | Definitions:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | High risk | No detailed description of derivation of the cohorts. All people who underwent any kind of enterostomy for any underlying disease from January 2000 to January 2005 at Marmara University Hospital were contacted and invited for interview, physical examination, and CT evaluation. |
| Selection of the control cohort | Unclear risk | It seems likely that both intervention groups (lateral pararectal versus transrectal stoma placement) were drawn from the same, or at least a similar, community. |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was ascertained by CT scanning. |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (parastomal hernia) was assessed by physical examination and CT scanning. |
| Was follow‐up long enough for outcomes to occur | High risk | Median follow‐up was only 15 months (range 2 to 63), which is rather short with regard to the likelihood of occurrence of parastomal hernia. |
| Adequacy of follow‐up of cohorts | Unclear risk | The authors identified 161 eligible people, 58 of whom had already died. Of the remaining 103, only 46 accepted the invitation for interview and re‐examination. 23 of the 46 participants still had an ostomy, whereas the other half had already undergone ostomy reversal. 3 of the 23 participants with ostomy refused to undergo CT evaluation. Follow‐up rate < 80%. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Eldrup 1982.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 140 participants with colorectal (N = 131), or gynecological malignancies (N = 3), or with benign colorectal disease (N = 6) were operated on at two different Danish county hospitals, one hospital in Herlev and one in Gentofte, between June 1976 and July 1980. | |
| Interventions | 63 participants underwent placement of a lateral pararectal end sigmoidostomy versus 77 participants who underwent placement of a transrectal end sigmoidostomy Standardization of the surgical procedure: unclear |
|
| Outcomes | Incidence of paracolostomy hernia | |
| Notes | No definition of paracolostomy hernia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | The cohorts were derived from two Copenhagen county hospitals, one hospital in Herlev and one in Gentofte. It remains unclear whether cohorts were representative of the average, elderly, community‐dwelling resident. |
| Selection of the control cohort | Low risk | It seems likely that both intervention groups (lateral pararectal versus transrectal stoma placement) were drawn from the same, or at least a similar, community. |
| Ascertainment of intervention | Low risk | Type of intervention was ascertained by patient record (site of the stoma depended on the hospital where it was placed: in one hospital – it was not mentioned if this was the one in Herlev or the one in Gentofte – only transrectal stomas were constructed, in the other one, only lateral pararectal stomas). |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Unclear risk | Outcome (paracolostomy hernia) was assessed by physical examination (in the 93 participants who were still alive and agreed to re‐examination), or patient records were screened for the occurrence of paracolostomy hernia if participants had died (n = 47 participants). Risk of detection bias remains unclear in the 47 cases where participants had already died, since the following source of bias could not be excluded: It was unclear how much attention was paid during follow‐up clinic visits to the presence or absence of parastomal herniation, and it was also unclear whether this was checked at all during routine follow‐up visits if there was no standard pro forma or standardized checklist for clinic follow‐up visits. Even if participants were examined for parastomal herniation, it was possible that examination findings were not recorded, since they were not routinely assessed. |
| Was follow‐up long enough for outcomes to occur | High risk | Only 143 of 261 people, who had received a permanent end sigmoidostomy during the 4‐year inclusion period, had a minimum follow‐up of 6 months, which was one of the inclusion criteria of the study (follow‐up rate < 80%). 3 of the 96 eligible people who were still alive were not assessed for parastomal herniation by clinical re‐examination. Reasons for the 3 dropouts were not stated. |
| Adequacy of follow‐up of cohorts | Unclear risk | See above |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Hardt 2016.
| Methods | A pilot single center randomized trial with two parallel study groups | |
| Participants | Participants needing a temporary loop ileostomy (recruitment period: April 2012 to April 2014) Key inclusion criteria:
Key exclusion criteria:
|
|
| Interventions | Lateral pararectal versus transrectal (control intervention) enterostomy placement Standardization of the surgical procedure: yes (the applied technique for creation of a lateral pararectal enterostomy was a slightly modified version of the procedure described in detail by Stephenson 2010; all operating surgeons received detailed written instructions on both stoma formation techniques) |
|
| Outcomes | Primary endpoint: incidence of parastomal hernias, defined by any of the following events:
Secondary endpoints:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "were allocated randomly (1:1) following a computer‐generated list of random numbers during the operation, to receive either a lateral pararectal or a transrectal loop ileostomy." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was concealed from researchers enrolling, by use of sequentially numbered, opaque and sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants "were blinded to the randomization result, whereas blinding of the operating surgeons was not feasible." However, the outcome was not likely to be influenced by lack of blinding of the surgeon. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the outcome assessment was unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were not available in 4 cases (3 participants in the lateral pararectal group and one participant with a transrectal stoma) because of the following reasons: "One patient from overseas was lost to follow‐up. Another patient who had been randomized died due to tumor progression before the ileostomy could be taken down, and two patients did not have their ileostomies reversed until our database closed". However, reasons for missing outcome data were similar across the intervention groups and unlikely to be related to the true outcome. Furthermore, the proportion of missing outcomes compared to the observed event risk was not enough to have a relevant impact on the intervention effect estimate. |
| Reporting bias | Low risk | Reporting bias due to selective outcome reporting seemed unlikely because the trial was registered prospectively in the German Clinical Trials Register (registration number DRKS00003534) and all of the study’s prespecified outcomes were reported in the prespecified way. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Ho 2018.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 103 participants operated on at Royal Devon & Exeter Hospital in Exeter, UK, between January 2006 and November 2014 for anorectal malignancy or dysplasia (adenocarcinoma of the rectum: N = 100; anal squamous cell carcinoma: N = 1; adenoma: N = 2) | |
| Interventions | Abdominoperineal excision (N = 103) with placement of lateral pararectal (N = 12) versus transrectal (N = 91) permanent end colostomy. 24 cases (23%) were performed laparoscopically, in the remaining 79 cases (77%) an open surgical approach was used Standardization of the surgical procedure: unclear (the authors only stated that all operations were performed by three consultant surgeons) |
|
| Outcomes | Progression of the colostomy trephine size (defined as the "rate of change of the axial and sagittal trephine diameters, the trephine area, and the ratio of the trephine over time"):
Incidence of parastomal hernia (in relation to, or being potentially influenced by: the trephine diameters, size and position relative to the rectus abdominis muscle (RAM), shape of the incision, age, and sex)
|
|
| Notes | 1. After being asked via email, the authors provided the incidences of parastomal herniation in each intervention group (these numbers were not mentioned in the publication, which was currently under review). 2. Classifications of parastomal hernias used by Ho and colleagues:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants had undergone abdominoperineal excision with end colostomy placement between 2006 and 2014 at the same hospital in Exeter, UK. All patients suffered from anorectal malignancies (or dysplasia). Therefore, one could deduce that both intervention groups (lateral pararectal versus transrectal end colostomy) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was ascertained by database review: "...colostomy position relative to the RAM and shape of incision (...) were recorded and inserted into a Microsoft Excel Database...". Furthermore, the siting of the colostomy in relation to the RAM was "assessed via CT scans". |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcomes (progression of the colostomy trephine size and incidence of parastomal herniation) were assessed by CT scanning. |
| Was follow‐up long enough for outcomes to occur | Low risk | The median CT imaging follow‐up was 24 months (0 to 92). |
| Adequacy of follow‐up of cohorts | Low risk | 112 people had undergone elective abdominoperineal excision between 2006 and 2014 at the Royal Devon & Exeter Hospital in Exeter, UK, and were thus examined for eligibility. 9 people were excluded from the study because 8 of them had no postoperative CT scan(s) and one had total abdominal wall failure. The remaining 103 were confirmed eligible. Thus, the follow‐up rate was about 92%. |
| Reporting bias | Unclear risk | The risk of reporting bias was considered lower than in the 9 other included cohort studies because this study was reported in accordance with the STROBE guidelines, and the European Hernia Society (EHS) recommendations on the reporting of hernia surgery (Elm 2014; Muysoms 2013). However, since there was no study protocol (or information on prespecified outcomes) available, it remained unclear whether selective (or incomplete) outcome reporting was present in this study. |
| Other bias | Unclear risk | There was insufficient information to assess whether other sources of bias were present. |
Leong 1994.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 150 participants operated on at St Mark's Hospital in London, UK, during the decade January 1971 to December 1980, for the following underlying disease: ulcerative colitis (N = 71), ulcerative colitis and carcinoma (N = 13), Crohn’s colitis (N = 59), indeterminate colitis (N = 5), familial adenomatous polyposis (FAP) with malignant change (N = 2) | |
| Interventions | Proctocolectomy (N = 82) or colectomy (N = 68), with placement of lateral pararectal (N = 42) versus transrectal (N = 103) permanent end ileostomy (site of stoma unclear in 5 patients) Standardization of the surgical procedure: unclear |
|
| Outcomes |
|
|
| Notes | All presented data on participants, interventions, and outcomes were extracted from a "standard pro forma" which was used for all people undergoing surgery for inflammatory bowel disease at the hospital where the study was conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants had undergone (procto)colectomy with ileostomy placement for inflammatory bowel disease between 1971 and 1980 at the same hospital in London, UK. Consequently, one could deduce that both intervention groups (lateral pararectal versus transrectal ileostomy) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was ascertained by chart review: "A standard pro forma is used at St. Mark's for patients undergoing surgery for inflammatory bowel disease. From this were extracted the following details: (...) and siting of the stoma with regard to abdominal wall musculature (through or lateral to the rectus abdominis)." |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcomes (para‐ileostomy hernia, prolapse, and other stomal complications) were assessed by chart review: "A standard pro forma is used at St. Mark's for patients undergoing surgery for inflammatory bowel disease. From this were extracted the following details: (...). Stomal complications evaluated were: (...); prolapse; (...); parastomal herniation; (...)". |
| Was follow‐up long enough for outcomes to occur | Low risk | Mean duration of follow‐up was 9.2 years (range 0.3 to 20.0) |
| Adequacy of follow‐up of cohorts | Unclear risk | The authors did not state how many people were potentially eligible or examined for eligibility. There was also no information on participants lost to follow‐up, or dropouts. The authors only stated that reasons for termination of follow‐up were return to residence abroad, inability to travel due to poor health, and death. 150 participants were included in the analysis. It seems likely that follow‐up rate was < 80%. All participants were operated on 14 to 23 years before the study was published. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Londono‐Schimmer 1994.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 203 participants operated on at St Mark's Hospital in London, UK, during the decade January 1971 to December 1980: 184 with rectal cancer, 14 with squamous cell anal cancer, 1 with rectal leiomyosarcoma, 4 who had to undergo Hartmann’s procedure (diagnoses not stated) | |
| Interventions | Abdomino‐perineal resection (N = 199), or Hartmann's procedure (N = 4), with placement of a lateral pararectal (N = 31) versus transrectal (N = 72) end sigmoid colostomy Standardization of the surgical procedure: unclear |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants had undergone abdominoperineal resection or Hartmann's procedure with sigmoidostomy placement between 1971 and 1980 at the same hospital in London, UK. The majority of patients suffered from colorectal cancer. Consequently, one could deduce that both intervention groups (lateral pararectal versus transrectal sigmoidostomy) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was ascertained by chart review: "There is a standard operative pro forma at St. Mark's for patients undergoing surgery for colorectal cancer. From this pro forma, the following details were extracted: (...) and the siting of the stoma with regard to abdominal wall musculature (through or lateral to the rectus abdominis)." |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcomes (para‐colostomy hernia, other stomal complications) were assessed by chart review: "There is a standard operative pro forma at St. Mark's for patients undergoing surgery for colorectal cancer. From this pro forma, the following details were extracted: (...). The stomal complications evaluated were (...), parastomal herniation, (...)". |
| Was follow‐up long enough for outcomes to occur | Low risk | Participants were followed up for at least 13 years after the intervention. |
| Adequacy of follow‐up of cohorts | Unclear risk | 289 people had received a permanent end colostomy during the decade from 1971 to 1980 and were thus examined for eligibility. 203 of these were confirmed eligible. Reasons for exclusion in the remaining 86 were: 5 died early in the postoperative period, 11 were followed up at other hospitals, 27 lived abroad, 36 had no follow‐up recorded, and in 7 cases files could not be found. Hence, the follow‐up rate was about 70%. But in only 103 of 203 included participants was the exact siting of the stoma with regard to the rectus abdominis muscle known to the authors. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Ortiz 1994.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 54 participants with rectal cancer operated on at Virgen del Camino Hospital, Pamplona, Spain | |
| Interventions | Abdomino‐perineal resection with placement of a lateral pararectal (N = 29) versus transrectal (N = 25) colostomy Standardization of the surgical procedure: unclear |
|
| Outcomes | Incidence of paracolostomy hernia (assessed by clinical examination, and if unclear, by CT scan) | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants had undergone abdominoperineal excision with colostomy for rectal cancer at the same hospital in Pamplona, Spain, five years before the study was conducted. The authors stated that all operations were performed by the same team of surgeons. Consequently, one could deduce that both intervention groups (lateral pararectal versus transrectal sigmoidostomy) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was determined by clinical examination (N = 45), or if still doubtful, by CT scanning (N = 9). |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (parastomal hernia) was determined by clinical examination. 9 participants also underwent CT scanning, because the anatomical position of the stoma still remained in doubt after physical examination. |
| Was follow‐up long enough for outcomes to occur | Low risk | The authors only stated that they included "54 consecutive patients who had previously undergone an abdominoperineal excision for rectal cancer five years before" (follow‐up = 5 years). There were no data reported on the number of people who had been examined for eligibility. It was also not stated how many participants had been lost to follow‐up. |
| Adequacy of follow‐up of cohorts | Unclear risk | See above |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | The study was supported by a grant FIss‐90/0725, but no further information was provided. This could be a potential source of bias. |
Pilgrim 2010.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 90 "unselected consecutive patients attending routine outpatient clinic visits" at Alfred Hospital, Melbourne, Australia, between August 2004 and July 2006 with the following underlying diseases: cancer (N = 40), inflammatory bowel disease (N = 26), 4 requiring urinary diversion, diverticular disease (N = 6), anastomotic breakdown (N = 2), slow transit constipation (N = 2), bowel ischemia (N = 4), fecal incontinence (N = 2), perianal sepsis (N = 2), bowel perforation (N = 2) | |
| Interventions | Lateral pararectal (N = 10) versus transrectal (N = 80) stoma placement Types of surgical procedures not reported. All participants underwent stoma formation and the following types of ostomies were created: 37 sigmoidostomies, 6 transversostomies, 41 ileostomies (of which 24 were loop ileostomies), 5 ileal conduits, 1 cecostomy Standardization of the surgical procedure: unclear |
|
| Outcomes | Prevalence of parastomal hernia (assessed by physical examination including digital examination through the stomal opening) | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants attended the outpatient stomal therapy service at the same hospital in Melbourne, Australia, during the two‐year study inclusion period from August 2004 until July 2006. Underlying diseases and surgical therapies varied, but the majority of participants required stoma surgery due to cancer (N = 40) or inflammatory bowel disease (N = 26). It remained unclear whether both intervention groups (lateral pararectal versus transrectal stoma placement) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Unclear risk | See above |
| Ascertainment of intervention | Low risk | Though it was not stated explicitly, one must assume that the type of intervention was ascertained by operative report, or patient chart, or both. |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (parastomal hernia) was ascertained by physical examination including "digital examination through the stomal opening ... by enterostomal nurse specialists". |
| Was follow‐up long enough for outcomes to occur | High risk | Median length of follow‐up was 14.1 months. Follow‐up rates could not be calculated. |
| Adequacy of follow‐up of cohorts | Unclear risk | The authors only stated that they "prospectively audited" 90 "unselected consecutive participants attending routine outpatient clinic visits". |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Sjodahl 1988.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 130 participants attending the stoma therapy service at the university hospital in Linköping, Sweden, with the following underlying diseases: rectal cancer (N = 54), ulcerative colitis (N = 28), Crohn’s disease (N = 22), fecal incontinence (N = 14), gynecological disease (N = 6), diverticulitis (N = 3), colon cancer (N = 1), anal cancer (N = 1), pseudo‐obstruction (N = 1) | |
| Interventions | Placement of a lateral pararectal (N = 23) versus a transrectal (N = 107) enterostomy (79 sigmoidostomies, 45 end ileostomies, 3 loop ileostomies, 2 end transversostomies, 1 loop transversostomy) Standardization of the surgical procedure: unclear |
|
| Outcomes | Prevalence of parastomal hernia (assessed by physical examination by a surgeon and a staff nurse at the stoma therapy service) | |
| Notes | Definition of parastomal hernia on physical examination ("the patient was examined in both supine and standing position"): "a bulge restricted to that area and caused by protrusion of abdominal contents. Subcutaneous prolapse or general muscular weakness with bulging of the lower part of the abdomen were thus excluded from the definition". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants attended the stoma therapy service at the Swedish university hospital where the study was conducted. Underlying diseases and indications for stoma placement varied widely, but the majority of participants suffered either from rectal cancer (54/130) or from inflammatory bowel disease (50/130). There was no information on socioeconomic or educational background of the participants. Nonetheless, it seemed likely that both intervention groups (lateral pararectal versus transrectal stoma placement) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was determined by clinical examination "performed by the surgeon and staff nurse at the stomatherapy service". |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (parastomal hernia) was ascertained by clinical examination "performed by the surgeon and staff nurse at the stomatherapy service". A clear definition of parastomal hernia was given. |
| Was follow‐up long enough for outcomes to occur | Low risk | Mean follow up was 84 ± 80.4 months (± SD); range 12 to 432 months (1 to 36 years). |
| Adequacy of follow‐up of cohorts | Unclear risk | The authors stated that they included "65 men and 65 women attending the stomatherapy service" at the hospital where the study was conducted. There were no data reported with on the number of people who had been examined for eligibility, or the number lost to follow‐up. The authors only explained that they excluded people "whose general health was too poor to permit attendance at the clinic, e.g. with end‐stage cancer or senility". Again, no number was given. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
von Smitten 1986.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 54 participants with rectal carcinoma (N = 50), anal carcinoma (N = 3), or anorectal melanoma (N = 1) operated on at Helsinki University Central Hospital, Helsinki, Finland, between 1973 and 1980 | |
| Interventions | Abdominoperineal excision (N = 53) or Hartmann's procedure (N = 1), with placement of a lateral pararectal (N = 26) versus transrectal (N = 25) end sigmoidostomy (for 3 participants, the stomal site in relation to the rectus abdominis muscle is not reported) Standardization of the surgical procedure: unclear |
|
| Outcomes | Late complications of end sigmoidostomy (assessed by interviewing the patient, and clinical examination):
|
|
| Notes | Parastomal hernias with a diameter ≥10 cm were classified as large | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants underwent surgery for anorectal malignancies at the Finnish university hospital where the study was conducted. The authors claimed that there were only 58 people who received a permanent end sigmoidostomy in the management of anorectal malignancy within a time period of 7 years. Considering the fact that this number is very small for such a time period in combination with the setting of a university hospital, one could assume that selection bias probably occurred. There was no information on socioeconomic or educational background of the participants. Hence, it remained unclear whether the participants were somewhat representative of the average, elderly, community‐dwelling resident. Nonetheless, it seemed likely that both intervention groups (lateral pararectal versus transrectal stoma placement) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Though it was not stated explicitly, one must assume that the type of intervention was ascertained by the operative report, or patient chart, or both. |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (parastomal hernia) was ascertained by interviewing the participants, and clinical examination. |
| Was follow‐up long enough for outcomes to occur | Unclear risk | Length of follow up ranged from 12 to 96 months. |
| Adequacy of follow‐up of cohorts | Unclear risk | No data presented on how many people were examined for eligibility. The authors claimed that there were only 58 people who received a permanent end sigmoidostomy in the management of anorectal malignancy within a time period of 7 years. 54 of these could be included in the analysis. It was not stated why the remaining 4 were excluded. Since it was likely that important data were missing, no reliable follow‐up rates could be calculated. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Williams 1990.
| Methods | A retrospective cohort study (according to the definition of a cohort study by Dekkers 2012) | |
| Participants | 46 participants with inflammatory bowel disease (13 with Crohn's disease, 33 with ulcerative colitis) operated on at the Department of Surgery, University of Wales College of Medicine, Heath Park, Cardiff, UK, between 1972 and 1987 | |
| Interventions | 16 participants underwent colectomy with placement of a lateral pararectal end ileostomy versus 12 who underwent colectomy with placement of a transrectal end ileostomy (only 28/46 participants agreed to undergo CT scanning; for the remaining participants, the stomal site in relation to the rectus abdominis muscle could not be assessed by CT scan, and was thus, not reported). Standardization of the surgical procedure: unclear |
|
| Outcomes |
|
|
| Notes | Definitions:
All CT scans were reviewed independently by two radiologists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the experimental intervention cohort | Unclear risk | All participants had undergone colectomy with ileostomy placement for inflammatory bowel disease under the care of one surgeon between 1972 and 1987. The authors claimed that there were only 60 eligible people within a time period of 15 years. Considering the fact that this number was very small for such a time period in combination with the setting of a university hospital, one could assume that selection bias probably occurred. There was no information on socioeconomic or educational background of the participants. Hence, it remained unclear whether the included patients were somewhat representative of the average, elderly, community‐dwelling resident. Nonetheless, one could deduce that both intervention groups (lateral pararectal versus transrectal ileostomy) were drawn from the same, or at least a similar, community. |
| Selection of the control cohort | Low risk | See above |
| Ascertainment of intervention | Low risk | Type of intervention (lateral pararectal versus transrectal stoma placement) was ascertained by CT scanning. |
| Demonstration that outcome of interest was not present at start of study | Low risk | In the absence of an enterostomy, parastomal herniation and other stoma‐related complications could not have been present. |
| Comparability of cohorts on the basis of the design or analysis | High risk | The experimental intervention group and the control group were not matched in design, nor were confounders adjusted for in the analysis. |
| Assessment of outcome | Low risk | Outcome (para‐ileostomy hernia) was assessed by physical examination and CT scanning. The CT scans were reviewed independently by two radiologists. |
| Was follow‐up long enough for outcomes to occur | Low risk | Median length of follow‐up was 78 months (6.5 years). |
| Adequacy of follow‐up of cohorts | Unclear risk | The authors claimed that there were only 60 potentially eligible people. After examination for eligibility, there were only 46 participants available for review. The remaining 14 had either died (N = 7), were lost to follow‐up (N = 5; reasons not stated), or had undergone ileorectal anastomosis (N = 2). Only 28 of the 46 participants agreed to be assessed for para‐ileostomy hernia by CT scanning. Rates for clinical follow‐up were 77%, radiological (CT scan) follow‐up rates were 47%. |
| Reporting bias | Unclear risk | Unclear whether selective outcome reporting was present. |
| Other bias | Unclear risk | Unclear whether other bias was present. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caricato 2007 | No information on stomal site in relation to the rectus abdominis muscle. Follow‐up was rather short, with a mean follow‐up of 4 months (range: 3 to 23). |
| Carlstedt 1987 | Only transrectal stomas. Parastomal hernia not defined as an outcome measure, thus not assessed. Aim of the study was to investigate the need for surgical ileostomy revision. |
| Green 1966 | No information on stomal site in relation to the rectus abdominis muscle. Parastomal hernia not defined as an outcome measure, thus not assessed. |
| Hallböök 2002 | No information on stomal site in relation to the rectus abdominis muscle. Parastomal hernia not defined as an outcome measure, thus not assessed. |
| Hoffman 1992 | No information on stomal site in relation to the rectus abdominis muscle. |
| Leenen 1989 | Only transrectal stomas. No comparison drawn (not even with a historical control or data from literature) between transrectal and lateral pararectal stoma placement. |
| Miles 1983 | No information on stomal site in relation to the rectus abdominis muscle. |
| Park 1999 | No information on stomal site in relation to the rectus abdominis muscle in the Methods and Results sections. In the Discussion, the authors state that, at their hospital, surgeons are taught to place all stomas through the rectus abdominis muscle, claiming that this bears a lower risk for parastomal herniation as compared to lateral pararectal stoma siting. |
| Pearl 1985 | No information on stomal site in relation to the rectus abdominis muscle. In the Discussion section, the authors recommend placing all stomas through the rectus abdominis muscle without providing any data to support this recommendation. |
| Stephenson 2010 | Only lateral pararectal enterostomies. There was no internal transrectal control group. Though the authors of this prospective uncontrolled cohort study provided detailed information on the technique for lateral pararectal stoma formation, as well as valuable data on the incidence of parastomal herniation after lateral pararectal stoma placement at a follow‐up of up to two years, we decided to exclude the study because of the absence of a control arm. |
| Zanolla 1979 | The study compared complications of median (the stoma is pulled through the median explorative wound) versus lateral colostomy. No information on stomal site in relation to the rectus abdominis muscle. |
Differences between protocol and review
We concretised the inclusion criteria for studies. Studies had to compare lateral pararectal versus transrectal enterostomy placement with regard to the incidence of parastomal herniation.
Only one of the planned subgroup analyses could be conducted (see Subgroup analysis and investigation of heterogeneity and Analysis 2.1). None of the planned sensitivity analyses were conducted (see Sensitivity analysis).
Embase was not searched for this update of the review because of the following reasons and based on the following rationale:
1. Embase was no longer available to the author team. Since we still included Web of Science, another large biomedical database, in addition to PubMed and CENTRAL (searches in both databases are mandatory according to MECIR C24, whereas the recommendation to search Embase is facultative), our electronic searches are still in compliance with the MECIR standards (Higgins 2016).
2. RCTs from Embase are now regularly and prospectively included in CENTRAL, approximately four weeks after publication in Embase (see: www.cochranelibrary.com/help/central‐creation‐details.html), so we are confident that we did not miss a RCT that was only indexed in Embase.
3. All of the included studies in the previous and the present review version were indexed in MEDLINE.
Taking these aspects into account, it is very unlikely that searching Embase would have identified further studies for inclusion, especially further RCTs.
Contributions of authors
Design, data extraction, analysis, interpretation, and drafting of review: JH, FH, MIM.
Design, resolution of discrepancies, interpretation, drafting, and supervision of review: JM, PK, SP.
Design and execution of search strategies, search documentation: MIM.
Sources of support
Internal sources
-
University Medicine Mannheim, Germany.
The authors received their regular salaries while they worked on the review update.
-
Institute for Evidence in Medicine (for Cochrane Germany Foundation), Germany.
The authors received their regular salaries while they worked on the review update.
-
Cochrane Metabolic and Endocrine Disorders Group, Institute of General Practice, Medical Faculty of the Heinrich‐Heine‐University Düsseldorf, Germany.
The authors received their regular salaries while they worked on the review update.
External sources
There was no external funding available., Other.
Declarations of interest
The PATRASTOM trial was undertaken by some of the authors of this review (JH, FH, PK, SP). In order to prevent confounding and to guarantee transparency and objectivity in regard to the evaluation of the PATRASTOM trial, the data extraction and risk of bias assessment of this trial was checked by an external clinician not involved in the trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cingi 2006 {published data only}
- Cingi A, Cakir T, Sever A, Aktan AO. Enterostomy site hernias: a clinical and computerized tomographic evaluation. Diseases of the Colon and Rectum 2006;49(10):1559‐63. [DOI] [PubMed] [Google Scholar]
Eldrup 1982 {published data only}
- Eldrup J, Wied U, Bischoff N, Pedersen VM. Post‐colostomy hernias. Incidence and relation to placing of the stoma. Ugeskrift for Læger 1982;144(50):3742‐3. [PubMed] [Google Scholar]
Hardt 2016 {published data only}
- DRKS00003534. Incidence of parastomal hernias after lateral pararectal versus transrectal stoma placement. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00003534 (first registered 14 February 2012).
- Hardt J, Seyfried S, Weiß C, Post S, Kienle P, Herrle F. A pilot single‐centre randomized trial assessing the safety and efficacy of lateral pararectus abdominis compared with transrectus abdominis muscle stoma placement in patients with temporary loop ileostomies: the PATRASTOM trial. Colorectal Disease 2016;18(2):O81–90. [DOI: 10.1111/codi.13251] [DOI] [PubMed] [Google Scholar]
Ho 2018 {published and unpublished data}
- Ho KK, Economou T, Smart NJ, Daniels IR. Radiological progression of end colostomy trephine diameter and area. BJS Open 24 October 2018;3(1):112‐8. [DOI: 10.1002/bjs5.50109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KK, Economou T, White ES, Smart NJ, Daniels IR. The colostomy location and radiological progression of trephine size over time: a clue to the development of parastomal hernia?. Gut 2015;64(Suppl 1):A534‐5. [Google Scholar]
Leong 1994 {published data only}
- Leong AP, Londono‐Schimmer EE, Phillips RK. Life‐table analysis of stomal complications following ileostomy. British Journal of Surgery 1994;81(5):727‐9. [DOI] [PubMed] [Google Scholar]
Londono‐Schimmer 1994 {published data only}
- Londono‐Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Diseases of the Colon and Rectum 1994;37(9):916‐20. [DOI] [PubMed] [Google Scholar]
Ortiz 1994 {published data only}
- Ortiz H, Sara MJ, Armendariz P, Miguel M, Marti J, Chocarro C. Does the frequency of paracolostomy hernias depend on the position of the colostomy in the abdominal wall?. International Journal of Colorectal Diseases 1994;9(2):65‐7. [DOI] [PubMed] [Google Scholar]
Pilgrim 2010 {published data only}
- Pilgrim CH, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated comorbidities. Diseases of the Colon and Rectum 2010;53:71‐6. [DOI] [PubMed] [Google Scholar]
Sjodahl 1988 {published data only}
- Sjodahl R, Anderberg B, Bolin T. Parastomal hernia in relation to site of the abdominal stoma. British Journal of Surgery 1988;75(4):339‐41. [DOI] [PubMed] [Google Scholar]
von Smitten 1986 {published data only}
- Smitten K, Husa A, Kyllonen L. Long‐term results of sigmoidostomy in patients with anorectal malignancy. Acta Chirurgica Scandinavica 1986;152:211‐3. [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Etherington RJ, Williams JG, Hayward MW, Hughes LE. Demonstration of para‐ileostomy herniation using computed tomography. Clinical Radiology 1990;41:333‐6. [DOI] [PubMed] [Google Scholar]
- Williams JG, Etherington R, Hayward MW, Hughes LE. Paraileostomy hernia: a clinical and radiological study. British Journal of Surgery 1990;77(12):1355‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Caricato 2007 {published data only}
- Caricato M, Ausania F, Ripetti V, Bartolozzi F, Campoli G, Coppola R. Retrospective analysis of long‐term defunctioning stoma complications after colorectal surgery. Colorectal Diseases 2007;9:559‐61. [DOI] [PubMed] [Google Scholar]
Carlstedt 1987 {published data only}
- Carlstedt A, Fasth S, Hultén L, Nordgren S, Palselius I. Long‐term ileostomy complications in patients with ulcerative colitis and Crohn's disease. International Journal of Colorectal Disease 1987;2:22‐5. [DOI] [PubMed] [Google Scholar]
Green 1966 {published data only}
- Green EW. Colostomies and their complications. Surgery, Gynecology & Obstetrics 1966;122:1230‐2. [PubMed] [Google Scholar]
Hallböök 2002 {published data only}
- Hallböök O, Matthiessen P, Leinsköld T, Nyström PO, Sjödahl R. Safety of the temporary loop ileostomy. Colorectal Disease 2002;4:361‐4. [DOI] [PubMed] [Google Scholar]
Hoffman 1992 {published data only}
- Hoffman MS, Barton DP, Gates J, Roberts WS, Fiorica JV, Finan MA, et al. Complications of colostomy performed on gynecologic cancer patients. Gynecological Oncology 1992;44:231‐4. [DOI] [PubMed] [Google Scholar]
Leenen 1989 {published data only}
- Leenen LP, Kuypers JH. Some factors influencing the outcome of stoma surgery. Diseases of the Colon and Rectum 1989;32:500‐4. [DOI] [PubMed] [Google Scholar]
Miles 1983 {published data only}
- Miles RM, Greene RS. Review of colostomy in a community hospital. The American Surgeon 1983;49:182‐6. [PubMed] [Google Scholar]
Park 1999 {published data only}
- Park JJ, Pino A, Orsay CP, Nelson RL, Pearl RK, Cintron JR, et al. Stoma complications ‐ the Cook County Hospital experience. Diseases of the Colon and Rectum 1999;42:1575‐80. [DOI] [PubMed] [Google Scholar]
Pearl 1985 {published data only}
- Pearl RK, Prasad ML, Orsay CP, Abcarian H, Tan AB, Melzl MT. Early local complications from intestinal stomas. Archives of Surgery 1985;120:1145‐7. [DOI] [PubMed] [Google Scholar]
Stephenson 2010 {published data only}
- Evans MD, Thomas C, Beaton C, Williams GL, McKain ES, Stephenson BM. Lowering the incidence of stomal herniation: further follow up of the lateral rectus abdominis positioned stoma. Colorectal Diseases 2011;13(6):716‐7. [DOI] [PubMed] [Google Scholar]
- Stephenson BM, Evans MD, Hilton J, McKain ES, Williams GL. Minimal anatomical disruption in stoma formation: the lateral rectus abdominis positioned stoma (LRAPS). Colorectal Diseases 2010;12(10):1049‐52. [DOI] [PubMed] [Google Scholar]
Zanolla 1979 {published data only}
- Zanolla R, Bozzetti F, Vecchio M, Ventafridda V. Complications of median versus lateral colostomy. Diseases of the Colon and Rectum 1979;22(6):387‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Carne 2003
- Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. British Journal of Surgery 2003;90(7):784‐93. [DOI] [PubMed] [Google Scholar]
CENTRAL creation details
- CENTRAL creation details. www.cochranelibrary.com/central/central‐creation (accessed 21 August 2018).
Chapman 2017
- Chapman SJ, Wood B, Drake TM, Young N, Jayne DG. Systematic review and meta‐analysis of prophylactic mesh during primary stoma formation to prevent parastomal hernia. Diseases of the Colon and Rectum 2017;60:107‐15. [DOI] [PubMed] [Google Scholar]
Cornille 2017
- Cornille JB, Pathak S, Daniels IR, Smart NJ. Prophylactic mesh use during primary stoma formation to prevent parastomal hernia. Annals of the Royal College of Surgeons of England 2017;99:2‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cross 2017
- Cross AJ, Buchwald PL, Frizelle FA, Eglinton TW. Meta‐analysis of prophylactic mesh to prevent parastomal hernia. British Journal of Surgery 2017;104:179‐86. [DOI] [PubMed] [Google Scholar]
Cuthbertson 1977
- Cuthbertson AM, Collins JP. Strangulated para‐ileostomy hernia. The Australian and New Zealand Journal of Surgery 1977;47(1):86‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. in Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dekkers 2012
- Dekkers OM, Egger M, Altman DG, Vandenbroucke JP. Distinguishing case series from cohort studies. Annals of Internal Medicine 2012;156:37‐40. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiological Community Health 1998;52:377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elm 2014
- Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of Internal Medicine 2007;147(8):573‐7. [DOI] [PubMed] [Google Scholar]
Evans 2011
- Evans MD, Thomas C, Beaton C, Williams GL, McKain ES, Stephenson BM. Lowering the incidence of stomal herniation: further follow‐up of the lateral rectus abdominis positioned stoma. Colorectal Diseases 2011;13(6):716‐7. [DOI] [PubMed] [Google Scholar]
Gabriel 1935
- Gabriel WB, Lloyd‐Davies OV. Colostomy. British Journal of Surgery 1935;22:520‐38. [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological expectations of Cochrane Intervention Reviews. community.cochrane.org/mecir‐manual (last accessed 15 November 2017).
Hong 2013
- Hong SY, Oh SY, Lee JH, Kim DY, Suh KW. Risk factors for parastomal hernia: based on radiological definition. Journal of the Korean Surgical Society 2013;84:43‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotouras 2013
Israelsson 2008
- Israelsson LA. Parastomal hernias. The Surgical Clinics of North America 2008;88(1):113‐25, ix. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
López‐Cano 2017
- López‐Cano M, Brandsma HT, Bury K, Hansson B, Kyle‐Leinhase I, Alamino JG, et al. Prophylactic mesh to prevent parastomal hernia after end colostomy: a meta‐analysis and trial sequential analysis. Hernia 2017;21:177‐89. [DOI] [PubMed] [Google Scholar]
Marks 1975
- Marks CG, Ritchie JK. The complications of synchronous combined excision for adenocarcinoma of the rectum at St Mark's Hospital. British Journal of Surgery 1975;62:901‐5. [DOI] [PubMed] [Google Scholar]
Martin 1996
- Martin L, Foster G. Parastomal hernia. Annals of the Royal College of Surgeons in England 1996;78(2):81‐4. [PMC free article] [PubMed] [Google Scholar]
Moreno‐Matias 2009
- Moreno‐Matias J, Serra‐Aracil X, Darnell‐Martin A, Bombardo‐Junca J, Mora‐Lopez L, Alcantara‐Moral M, et al. The prevalence of parastomal hernia after formation of an end colostomy: a new clinico‐radiological classification. Colorectal Disease 2009;11(2):173‐7. [DOI] [PubMed] [Google Scholar]
Muysoms 2013
- Muysoms FE, Deerenberg EB, Peeters E, Agresta F, Berrevoet F, Campanelli G, et al. Recommendations for reporting outcome results in abdominal wall repair: results of a consensus meeting in Palermo, Italy, 28‐30 June 2012. Hernia 2013;17(4):423‐33. [DOI] [PubMed] [Google Scholar]
Mylonakis 2001
- Mylonakis E, Scarpa M, Barollo M, Yarnoz C, Keighley MR. Life table analysis of hernia following end colostomy construction. Colorectal Diseases 2001;3:334‐7. [DOI] [PubMed] [Google Scholar]
Odensten 2019
- Odensten C, Strigård K, Rutegård J, Dahlberg M, Ståhle U, Gunnarsson U, et al. Use of prophylactic mesh when creating a colostomy does not prevent parastomal hernia: a randomized controlled trial – STOMAMESH. Annals of Surgery 2019;269(3):427‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2017
- Patel SV, Zhang L, Chadi SA, Wexner SD. Prophylactic mesh to prevent parastomal hernia: a meta‐analysis of randomized controlled studies. Techniques in Coloproctology 2017;21:5‐13. [DOI] [PubMed] [Google Scholar]
Pearl 1989
- Pearl RK. Parastomal hernias. World Journal of Surgery 1989;13:569‐72. [DOI] [PubMed] [Google Scholar]
Pianka 2017
- Pianka F, Probst P, Keller AV, Saure D, Grummich K, Büchler MW, et al. Prophylactic mesh placement for the PREvention of paraSTOmal hernias: the PRESTO systematic review and meta‐analysis. PLOS ONE 2017;12(2):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ripoche 2011
- Ripoche J, Basurko C, Fabbro‐Perray P, Prudhomme M. Parastomal hernia. A study of the French federation of ostomy patients. Journal of Visceral Surgery 2011;148(6):e435‐41. [DOI] [PubMed] [Google Scholar]
Robertson 2005
- Robertson I, Leung E, Hughes D, Spiers M, Donelly L, Mackenzie I, et al. Prospective analysis of stoma‐related complications. Colorectal Diseases 2005;7(3):279‐85. [DOI] [PubMed] [Google Scholar]
Smietanski 2014
- Śmietański M, Szczepkowski M, Alexandre JA, Berger D, Bury K, Conze J, et al. European Hernia Society classification of parastomal hernias. Hernia 2014;18(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stroup 2000
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology. JAMA 2000;283:2008‐12. [DOI] [PubMed] [Google Scholar]
Tekkis 1999
- Tekkis PP, Kocher HM, Payne JG. Parastomal hernia repair: modified thorlakson technique, reinforced by polypropylene mesh. Diseases of the Colon and Rectum 1999;42(11):1505‐8. [DOI] [PubMed] [Google Scholar]
Turnbull 1958
- Turnbull RB. Intestinal stomas. The Surgical Clinics of North America 1958;38:1361‐72. [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang S, Wang W, Zhu B, Song G, Jiang C. Efficacy of prophylactic mesh in end‐colostomy construction: a systematic review and meta‐analysis of randomized controlled trials. World Journal of Surgery 2016;40:2528‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2011
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 27 September 2011).
References to other published versions of this review
Hardt 2013
- Hardt J, Meerpohl JJ, Metzendorf MI, Kienle P, Post S, Herrle F. Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD009487.pub2] [DOI] [PubMed] [Google Scholar]


