Abstract
Background
It has been suggested that in comparison with open radical cystectomy, robotic‐assisted radical cystectomy results in less blood loss, shorter convalescence, and fewer complications with equivalent short‐term oncological and functional outcomes; however, uncertainty remains as to the magnitude of these benefits.
Objectives
To assess the effects of robotic‐assisted radical cystectomy versus open radical cystectomy in adults with bladder cancer.
Search methods
Review authors conducted a comprehensive search with no restrictions on language of publication or publication status for studies comparing open radical cystectomy and robotic‐assisted radical cystectomy. The date of the last search was 1 July 2018 for the Cochrane Central Register of Controlled Trials, MEDLINE (1999 to July 2018), PubMed Embase (1999 to July 2018), Web of Science (1999 to July 2018), Cancer Research UK (www.cancerresearchuk.org/), and the Institute of Cancer Research (www.icr.ac.uk/). We searched the following trials registers: ClinicalTrials.gov (clinicaltrials.gov/), BioMed Central International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com), and the World Health Organization International Clinical Trials Registry Platform.
Selection criteria
We searched for randomised controlled trials that compared robotic‐assisted radical cystectomy (RARC) with open radical cystectomy (ORC).
Data collection and analysis
This study was based on a published protocol. Primary outcomes of the review were recurrence‐free survival and major postoperative complications (class III to V). Secondary outcomes were minor postoperative complications (class I and II), transfusion requirement, length of hospital stay (days), quality of life, and positive margins (%). Three review authors independently assessed relevant titles and abstracts of records identified by the literature search to determine which studies should be assessed further. Two review authors assessed risk of bias using the Cochrane risk of bias tool and rated the quality of evidence according to GRADE. We used Review Manager 5 to analyse the data.
Main results
We included in the review five randomised controlled trials comprising a total of 541 participants. Total numbers of participants included in the ORC and RARC cohorts were 270 and 271, respectively.
Primary outomes
Time‐to‐recurrence: Robotic cystectomy and open cystectomy may result in a similar time to recurrence (hazard ratio (HR) 1.05, 95% confidence interval (CI) 0.77 to 1.43); 2 trials; low‐certainty evidence). In absolute terms at 5 years of follow‐up, this corresponds to 16 more recurrences per 1000 participants (95% CI 79 fewer to 123 more) with 431 recurrences per 1000 participants for ORC. We downgraded the certainty of evidence for study limitations and imprecision.
Major complications (Clavien grades 3 to 5): Robotic cystectomy and open cystectomy may result in similar rates of major complications (risk ratio (RR) 1.06, 95% CI 0.76 to 1.48); 5 trials; low‐certainty evidence). This corresponds to 11 more major complications per 1000 participants (95% CI 44 fewer to 89 more). We downgraded the certainty of evidence for study limitations and imprecision.
Secondary outcomes
Minor complications (Clavien grades 1 and 2): We are very uncertain whether robotic cystectomy may reduce minor complications (very low‐certainty evidence). We downgraded the certainty of evidence for study limitations and for very serious imprecision.
Transfusion rate: Robotic cystectomy probably results in substantially fewer transfusions than open cystectomy (RR 0.58, 95% CI 0.43 to 0.80; 2 trials; moderate‐certainty evidence). This corresponds to 193 fewer transfusions per 1000 participants (95% CI 262 fewer to 92 fewer) based on 460 transfusion per 1000 participants for ORC. We downgraded the certainty of evidence for study limitations.
Hospital stay: Robotic cystectomy may result in a slightly shorter hospital stay than open cystectomy (mean difference (MD) ‐0.67, 95% CI ‐1.22 to ‐0.12); 5 trials; low‐certainty evidence). We downgraded the certainty of evidence for study limitations and imprecision.
Quality of life: Robotic cystectomy and open cystectomy may result in a similar quality of life (standard mean difference (SMD) 0.08, 95% CI 0.32 lower to 0.16 higher; 3 trials; low‐certainty evidence). We downgraded the certainty of evidence for study limitations and imprecision.
Positive margin rates: Robotic cystectomy and open cystectomy may result in similar positive margin rates (RR 1.16, 95% CI 0.56 to 2.40; 5 trials; low‐certainty evidence). This corresponds to 8 more (95% CI 21 fewer to 67 more) positive margins per 1000 participants based on 48 positive margins per 1000 participants for ORC. We downgraded the certainty of evidence for study limitations and imprecision.
Authors' conclusions
Robotic cystectomy and open cystectomy may have similar outcomes with regard to time to recurrence, rates of major complications, quality of life, and positive margin rates (all low‐certainty evidence). We are very uncertain whether the robotic approach reduces rates of minor complications (very low‐certainty evidence), although it probably reduces the risk of blood transfusions substantially (moderate‐certainty evidence) and may reduce hospital stay slightly (low‐certainty evidence). We were unable to conduct any of the preplanned subgroup analyses to assess the impact of patient age, pathological stage, body habitus, or surgeon expertise on outcomes. This review did not address issues of cost‐effectiveness.
Plain language summary
Robotic versus open radical cystectomy for bladder cancer in adults
Review question
For patients with bladder cancer that involves the deep muscle wall, does use of a robotic device lead to better or worse outcomes than open surgery?
Background
Patients with bladder cancer that involves the deep muscle wall are best treated by an operation that removes the entire bladder and creates an artificial bladder or channel from the bowel to allow urine to drain to the outside world. This has been done traditionally through open surgery using one large incision. Recently, this operation has been performed with robotic assistance using several small incisions. It is uncertain which approach is better.
Study characteristics
We performed a comprehensive literature search until 1 July 2018. We found five trials comparing robotic assisted versus open surgery. The total number of participants in these trials was 541. Four studies were conducted in the USA and one in the UK.
Key results
There may be little to no difference in the time to recurrence, the rate of major complications or minor complications, quality of life, and rates of positive margins (signalling that cancer may have been left behind). Robotic surgery probably results in fewer blood transfusions and may lead to a slightly shorter hospital stay when compared with open surgery.
Certainty of evidence
Reviewers rated the certainty of evidence as low for most outcomes, except for minor complications (very low) and transfusions (moderate). This means that the true results for these outcomes could be quite different.
Summary of findings
Summary of findings for the main comparison. Robotic‐assisted laparoscopic vs open radical cystectomy for bladder cancer in adults.
| Robotic‐assisted laparoscopic vs open radical cystectomy for bladder cancer in adults | |||||
| Patient or population: bladder cancer in adults Setting: tertiary care centres in the United States and the United Kingdom Intervention: robotic‐assisted laparoscopic cystectomy Comparison: open radical cystectomy | |||||
| Outcomes | No. of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with open radical cystectomy | Risk difference with robotic‐assisted laparoscopic cystectomy | ||||
| Time to recurrence (here: recurrence rate at 5 years)1 assessed with clinical examination and imaging | 277 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | HR 1.05 (0.77 to 1.43) | Study population | |
| 431 per 1000 | 16 more per 1000 (79 fewer to 123 more) | ||||
| Major postoperative complications assessed with Clavien‐Dindo system (rated grade 3 to 5) | 541 (5 RCTs) | ⊕⊕⊝⊝ LOWb,c | RR 1.06 (0.76 to 1.48) | Study population | |
| 185 per 1000 | 11 more per 1000 (44 fewer to 89 more) | ||||
| Minor postoperative complications assessed with Clavien‐Dindo system (rated grade 1 or 2) | 423 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | RR 0.82 (0.58 to 1.17) | Study population | |
| 443 per 1000 | 80 fewer per 1000 (186 fewer to 75 more) | ||||
| Transfusion rate assessed with transfused units of packed red blood cells | 326 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | RR 0.58 (0.43 to 0.80) | Study population | |
| 460 per 1000 | 193 fewer per 1000 (262 fewer to 92 fewer) | ||||
| Hospital stay assessed in days | 541 (5 RCTs) | ⊕⊕⊝⊝ LOWb,c | ‐ | Mean hospital stay ranged from 5.1 to 11.9 days | MD 0.67 days lower (1.22 lower to 0.12 lower) |
| Quality of life (higher scores indicate better quality of life) assessed with SMD calculated from various validated quality of life instruments Scale from 0 to 1 | 270 (3 RCTs) | ⊕⊕⊝⊝ LOWc,e | ‐ | Mean quality of life (higher scores indicate better quality of life) was 0 SD | SMD 0.08 SD lower (0.32 lower to 0.16 higher) |
| Positive margins assessed through pathological evaluation of cystectomy specimen | 541 (5 RCTs) | ⊕⊕⊝⊝ LOWb,c | RR 1.16 (0.56 to 2.40) | Study population | |
| 48 per 1000 | 8 more per 1000 (21 fewer to 67 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1The control event rate at 5 years was based on an overall recurrence rate of 25/58 (43.1%) in the ORC arm reported in Bochner 2015
aDowngraded by one level for study limitations; risk of performance, detection, and attrition bias.
bDowngraded by one level for imprecision: wide confidence intervals consistent with both no effect and clinically important benefit or harm.
cDowngraded by one level for study limitations; risk of performance and detection bias.
dDowngraded by two levels for very serious imprecision: wide confidence interval consistent with small benefit, no effect, and small harm.
eDowngraded by one level for imprecision: wide confidence intervals consistent with both no effect and clinically important reduction in quality of life, assuming SMD of 0.2.
Background
Description of the condition
Over 400,000 new cases of bladder cancer are diagnosed annually, accounting for 3% of all cancers (Ferlay 2013; Ferlay 2015; Ploeg 2009). Radical cystectomy (RC) with pelvic lymph node dissection (PLND) and urinary diversion is the gold standard surgical treatment for muscle‐invasive bladder cancer (MIBC) (Hayn 2010; Jonsson 2011; Lee 2011; Redorta 2010; Smith 2011; Witjes 2014). Other indications for RC include high‐risk non‐muscle‐invasive bladder cancer (NMIBC) and recurrent multifocal superficial disease (Hayn 2010; Jonsson 2011; Lee 2011; Redorta 2010; Smith 2011; Witjes 2014). The procedure has traditionally been performed using an open approach. Morbidity with open radical cystectomy (ORC) is high. In a retrospective review of a prospectively maintained database of 1142 patients who underwent ORC/urinary diversion by high‐volume fellowship‐trained urological oncologists, the reported 90‐day overall complication rate and the 30‐day mortality rate were 64% and 1.5%, respectively (Shabsigh 2009).
Description of the intervention
A significant interest in minimally invasive surgery (MIS) has arisen in the last two decades in an attempt to reduce morbidity, expedite recovery, and decrease hospital stay (Hu 2009; Schwenk 2005; Wright 2013). MIS approaches, both conventional laparoscopy and robotic‐assisted approaches, have replaced a significant number of open surgical techniques (Hu 2009; Schwenk 2005; Wright 2013). The uptake of conventional laparoscopic radical cystectomy has been impeded by technical challenges associated with the procedure, in particular the reconstructive aspects of the procedure (Aboumarzouk 2012; Aboumarzouk 2013; Castillo 2006; Castillo 2009; Cathelineau 2005; Haber 2008; Hosseini 2011; Huang 2008; Huang 2010; Jonsson 2011; Khan 2011; Sighinolfi 2007; Smith 2011). Robotic‐assisted radical cystectomy (RARC)—which offers such advantages as increased manoeuvrability, superior magnification, enhanced EndoWrist® dexterity, and tremor elimination—has been suggested as an alternative to overcome issues associated with the conventional laparoscopic approach (Ishii 2014).
How the intervention might work
Adoption of the robotic approach has been swift in contemporary urological practice, with widespread application of robotic‐assisted radical prostatectomy and robotic‐assisted partial nephrectomy in Europe and the USA leading to favourable perioperative outcomes in comparison with open and laparoscopic counterparts (Novara 2012). Three systematic reviews of randomised and nonrandomised controlled trials suggested shorter operative time and less blood loss for ORC when compared with RARC (Ishii 2014; Novara 2015; Tang 2014). These reviews also demonstrated reduced Clavien grade 3 complications for RARC. Two comparative studies have suggested similar survival outcomes between ORC and RARC (Khan 2012; Nepple 2013).
Why it is important to do this review
Although over 2000 procedures have been reported to the International Robotic Cystectomy Consortium from 37 centres worldwide, well‐conducted studies comparing RARCs to ORCs are lacking (Raza 2015). Randomised controlled trials are necessary to establish how RARC compares to ORC. We performed a systematic review to summarise and critically appraise the body of evidence comparing these two approaches to inform clinical decision‐making as well as health policy.
Objectives
To assess the effects of robotic‐assisted radical cystectomy versus open radical cystectomy in adults with bladder cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs of individual participants comparing ORC and RARC. We did not include cluster‐randomised trials. We considered all studies regardless of their publication status and language of publication.
Types of participants
We included adult participants with a diagnosis of bladder malignancy who were undergoing radical cystectomy as part of their treatment for pathologically proven MIBC or high‐grade NMIBC (T1‐4/carcinoma in situ (CIS), N0M0). We included participants irrespective of histological bladder cancer type (i.e. we included those with urothelial cell carcinoma, squamous cell carcinoma, or adenocarcinoma). We included participants receiving neoadjuvant or adjuvant chemotherapy. We excluded studies of participants with prior radiotherapy in which cystectomy was performed as a salvage procedure.
Types of interventions
We investigated the following comparison of experimental intervention versus comparator intervention. We included trials independent of the urinary diversion method employed. We analysed data by intention‐to‐treat analysis.
Experimental intervention
Robotic‐assisted radical cystectomy.
Comparator intervention
Open radical cystectomy.
Types of outcome measures
Primary outcomes
Time to recurrence
Major postoperative complications (class III to V) (Dindo 2004)
Secondary outcomes
Minor postoperative complications (class I and II) (Dindo 2004)
Transfusion requirement
Length of hospital stay (days)
Quality of life as evaluated via validated participant‐reported questionnaire scores or domains reflecting overall or global health of the participant
Positive margins (%)
Search methods for identification of studies
Electronic searches
We performed a comprehensive search with no restrictions on language of publication nor publication status. We searched the following electronic databases (date of last search was 1 July 2018):
Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) in the Cochrane library via Wiley
MEDLINE (1999 to July 2018); PubMed search. We used these terms and medical subject heading (MeSH) phrases: (cystectomy [MeSH terms] AND robotic AND open) AND "surgery" [MeSH subheading]
EMBASE (1999 to July 2018); Ovid search using the terms cystectomy, open, and robotic
Web of Science (1999 to July 2018)
Cancer Research UK (www.cancerresearchuk.org/)
Institute of Cancer Research (www.icr.ac.uk/)
We searched the following trials registers:
ClinicalTrials.gov (clinicaltrials.gov/)
BioMed Central ISRCTN registry (www.isrctn.com)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/)
See Appendix 1 for search terms used in strategies for this review. We limited back‐searching from 1999 onward because the earliest da Vinci robotic‐assisted device was not introduced until 1999 (Ballantyne 2003).
Searching other resources
We further evaluated the reference lists of included studies and of relevant review articles identified by the search. To identify unpublished studies, we searched the online conference proceedings of annual meetings of the American Urological Association (www.auanet.org) and the European Association of Urology (http://uroweb.org) from 2012 to July 2018.
Data collection and analysis
Selection of studies
Three review authors (BR, OMA, JB) independently assessed relevant titles and abstracts of records identified by the literature search to determine which studies should be assessed further. Three review authors (BR, OMA, JB) investigated all potentially relevant records as full text, mapped records to unique studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion and consensus or by recourse to a fourth review author (KG). We adapted a PRISMA flow diagram to demonstrate the process of study selection (Liberati 2009).
Data extraction and management
For studies that fulfil inclusion criteria, three review authors (BR, OMA, JB) independently extracted the following information, which is provided in the Characteristics of included studies tables:
Study design (e.g. parallel‐group randomised trial)
Study dates (if dates were not available, this was reported)
Study settings and country
Participant inclusion and exclusion criteria
Participant details and baseline demographics, such as age and sex
Numbers of participants by study and by study arm
Details of relevant experimental and comparator interventions and conversion rates from robotic to open
Definitions of relevant outcomes and methods and timing of outcome measurement, as well as any relevant subgroups
Study funding sources
Declarations of interest by primary investigators
For dichotomous outcomes, we attempted to obtain numbers of events and totals for populations on a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information. For time‐to‐event outcomes, we attempted to obtain hazard ratios (HRs) with corresponding measures of variance or data necessary to calculate this information.
We resolved all disagreements by consensus.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximised the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (BR, OMA) independently assessed the risk of bias of each included study and resolved all disagreements by consensus.
We assessed risk of bias using Cochrane’s ‘Risk of bias’ assessment tool for the following domains (Higgins 2011):
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
For detection bias, we evaluated the risk of bias separately for each outcome. We regarded outcomes such as transfusion requirement and hospital stay as objective, and, if reported, we judged these studies as low risk. If studies did not report these outcomes, we judged them as unclear risk.
For attrition bias, we evaluated risk of bias separately for quality of life. We combined the outcomes major and minor postoperative complications, hospital stay, transfusion requirement, and positive margin rates into a single group for attrition bias.
Measures of treatment effect
We used Review Manager 5 (RevMan 5) (RevMan) to analyse the data. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). For time‐to‐event outcomes, we calculated the hazard ratio with 95% CI. We expressed continuous data as mean differences (MDs) or standardised mean differences (SMDs) (if the same outcome was evaluated by different tools) with 95% CIs.
Unit of analysis issues
Parallel‐group designs were to be analysed. The unit of analysis was the individual participant. In the event we identified trials with more than two intervention groups for inclusion in the review, we handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
When data were missing, we contacted corresponding authors of the trials (Bochner 2015; Nix 2010; Parekh 2013; Parekh 2018). We had received no response from the corresponding authors of individual trials at the time of submission of this review. We imputed missing standard deviations in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We imputed means and standard deviations from median and range in accordance with guidance provided in Hozo 2005.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity by using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 for statistical significance, and using the I² statistic (Higgins 2003). We interpreted the I² statistic as follows:
0% to 40%: may not be important
30% to 60%: may indicate moderate heterogeneity
50% to 90%: may indicate substantial heterogeneity
75% to 100%: indicates considerable heterogeneity
Assessment of reporting biases
We planned to obtain study protocols to evaluate studies for reporting bias. We did not formally perform funnel plot analysis, as the review included only five trials.
Data synthesis
We summarised data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method; and for time‐to‐event outcomes, we used the generic inverse variance method. We used RevMan software to perform analyses.
GRADE and ‘Summary of findings’ table
We presented the overall quality of evidence for each outcome according to the GRADE approach, which takes into account five criteria related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity, such as directness of results (Guyatt 2008). Two review authors (BR, OMA) independently rated the quality of evidence for each outcome as ‘high’, ‘moderate’, ‘low’, or ‘very low’, using GRADEpro GDT. We resolved any discrepancies by consensus. We presented a summary of evidence for the main outcomes in a ‘Summary of findings’ table, which provides key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of overall confidence in effect estimates for each outcome (Guyatt 2011).
Main outcomes for ‘Summary of findings’ table
We have presented a ‘Summary of findings’ table to report the following outcomes listed according to priority:
Time to recurrence
Major postoperative complications (class III to V) (Dindo 2004)
Minor postoperative complications (class I and II) (Dindo 2004)
Length of hospital stay (days)
Quality of life
Positive margins (%)
Subgroup analysis and investigation of heterogeneity
We attempted to perform subgroup analyses to explore possible sources of heterogeneity. We considered the following subgroups:
Participant age (younger than 60 years vs 60 years of age and older)
Participant body mass index (< 30 kg/m² vs ≥ 30 kg/m²)
Pathological stage (≤ pT2 disease vs pT3 disease)
Surgeon’s level of experience (less than expert vs expert, as defined by trial authors)
We planned to test for subgroup differences using RevMan 5 to compare subgroup analyses if we found sufficient studies (RevMan). We could not do this with the information provided in the included studies.
Results
Description of studies
We identified 332 references through electronic searches of the different databases.
We retrieved a total of 32 references for further detailed assessment. We excluded 26 references for the reasons listed in the Characteristics of excluded studies table. We found that seven references on five randomised controlled trials (RCTs) fulfilled the review inclusion criteria (see Characteristics of included studies). Two trials published outcomes in two separate publications (Bochner 2015; Parekh 2013). We have presented the reference flow in Figure 1 .
1.
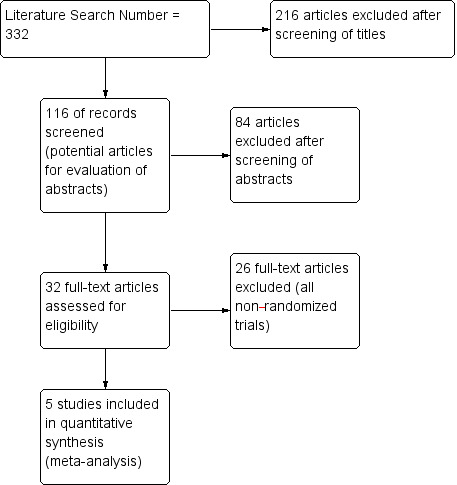
Study flow diagram.
Included studies
Study design and setting
Five trials were published between 2010 and 2018:
Nix 2010 reported the first trial of RARC versus ORC. Researchers conducted this study at the University of North Carolina in the USA and randomised 21 participants to an open approach and 20 to a robotic‐assisted laparoscopic approach. The study had a noninferiority design, and its primary outcome was lymph node yield.
The RAZOR trial (a prospective, multicentre, randomised trial of open vs robotic radical cystectomy) was the largest and most recently published trial (Parekh 2018). It was conducted at 15 academic centres in the USA and randomised 159 and 153 participants to RARC and ORC cohorts, respectively (Parekh 2018). After exclusions, 150 participants were included in the RARC cohort and 152 participants in the ORC cohort, in the per‐protocol analysis set (Parekh 2018). The study used a noninferiority design and included a primary outcome of progression‐free survival at two years.
Parekh 2013 reported the results of a preceding pilot trial leading up to the RAZOR trial that was conducted at the University of Texas at San Antonio in the USA. Study authors randomised 20 participants each to RARC and ORC and reported oncological outcomes and quality of life outcomes ‐ in two separate publications (Parekh 2013). This study had no specific primary endpoint aside from establishing randomisation.
Bochner 2015 reported the results of a single‐institution, randomised trial conducted at Memorial Sloan Kettering Cancer Center in the USA. Investigators randomised 60 and 58 participants to RARC and ORC cohorts, respectively. The study was described as an expertise‐based trial. Study authors reported oncological outcomes in a second publication.
Khan 2016 reported the results of a single‐institution, three‐armed, randomised trial conducted at Guy’s Hospital, in London, United Kingdom, that randomised 20 participants each to RARC, ORC, and (pure) laparoscopic cystectomy. This study was described as an expertise‐based trial.
Participants
The total numbers of participants included in the ORC and RARC cohorts were 270 and 271, respectively. Most participants in both the ORC (221; 82%) and RARC (226; 83.4%) groups were men. Three studies reported demographic data using the median (Bochner 2015; Parekh 2013; Parekh 2018) and two using the mean (Khan 2016; Nix 2010). The mean age of participants in the ORC cohort ranged between 66.6 years and 69.2 years. The mean age of participants in the RARC cohort ranged between 67.4 years and 68.6 years. The median age of participants in the ORC cohort ranged between 64.5 years and 65 years. The median age of participants in the RARC cohort ranged between 66 years and 69.5 years. The mean body mass index (BMI) (in kg/m²) of participants in the ORC cohort ranged between 27.4 and 28.4. The mean BMI (kg/m²) of participants in the RARC cohort ranged were similar at 27.5. The median BMI (kg/m²) of participants in the ORC cohort ranged between 24.9 and 31.7, and the median BMI (kg/m²) of participants in the RARC cohort ranged between 25 and 30.8.
Interventions and comparators
All five studies compared ORC to RARC (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018); one trial included an arm of laparoscopic radical cystectomy (Khan 2016). Four studies performed urinary diversion extracorporeally (Bochner 2015; Khan 2016; Nix 2010; Parekh 2018). One study performed urinary diversion at the discretion of the surgeon and did not explicitly report the type (Parekh 2013). In the ORC cohort, urinary diversion was ileal conduit, neobladder, and continent cutaneous type in 194 (72%), 73 (27%), and 3 (1%) participants, respectively. In the RARC cohort, urinary diversion was ileal conduit, neobladder, and continent cutaneous type in 191 (70.6%), 79 (29%), and 1 (0.4%) participant, respectively. All five trials performed a pelvic lymph node dissection (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018). We have summarised the inclusion criteria for each study in the Characteristics of included studies table.
Outcomes
Bochner 2015 reported on patient‐reported outcomes (PROs) of quality of life (QoL) using the validated European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ‐C30) survey. Khan 2016 evaluated QoL using the validated Functional Assessment of Cancer Therapy –Bladder (FACT‐Bl) scale v4 questionnaire. Parekh 2013 and Parekh 2018 evaluated QoL using the validated Functional Assessment of Cancer Therapy –Vanderbilt Cystectomy Index (FACT–VCI) questionnaire.
Funding
Parekh 2018 was funded by the National Institutes of Health National Cancer Institute.
Bochner 2015 was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael and Zena Wienerfor Therapeutics Program in Bladder Cancer. Study sponsors were involved in the design and conduct of the study; in collection, analysis, management, and interpretation of the data; and in preparation, review, and approval of the manuscript.
Khan 2016 was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. Study authors acknowledge support from the NIHR Biomedical Research Centre, the Medical Research Council Centre for Transplantation, King’s Health Partners, Guy’s and St. Thomas’ Charity, the School of Surgery, the London Deanery, the Royal College of Surgeons of England, Intuitive Surgical, the Urology Foundation, Olympus, EU‐FP7, ProstateCancer UK, the Technology Strategy Board, and the Vattikuti Foundation.
The remaining two studies did not report funding (Nix 2010; Parekh 2013).
Excluded studies
We excluded 26 of these publications. All of these studies were nonrandomised comparative studies comparing ORC and RARC (Excluded studies). We have documented further details of individual studies in the Characteristics of excluded studies table.
Risk of bias in included studies
We have summarised the methodology and risk of bias of individual trials in the Characteristics of included studies table.
We have summarised the risk of bias for individual trials in Figure 2.
2.

Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged all five trials to have low risk of bias for random sequence allocation (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018).
Allocation concealment
Nix 2010 performed a randomisation schema with five sequential participants undergoing one approach before alternating with another approach. Their concealment would have to be deemed inadequate and hence judged to be at high risk of bias. The remaining trials had low risk of bias in their allocation concealment strategy (Bochner 2015; Khan 2016; Parekh 2013; Parekh 2018).
Blinding
Performance bias
Due to the nature of the intervention (RARC vs ORC), it is considered unlikely that participants or personnel were blinded for any of the review outcomes. We therefore judged all included studies to be at high risk of performance bias.
Detection bias
Time to recurrence
Two trials reported on recurrence‐free survival (Bochner 2015; Parekh 2018). Due to the lack of blinding of outcome assessors, we judged Parekh 2018 to be at high risk of detection bias for recurrence‐free survival (Parekh 2018). We judged one trial as having unclear risk of detection bias for recurrence‐free survival, as trial authors did not explicitly state who assessed this outcome (Bochner 2015).
Complications (all grades)
Three included studies were unblinded; we therefore judged them to be at high risk of detection bias for complications (Bochner 2015; Khan 2016; Parekh 2018). Two studies did not report who the assessors were and whether blinding had taken place; hence we judged them to be at unclear risk of detection bias for complications (Nix 2010; Parekh 2013).
Quality of life
In all four included studies, participants were not blinded; we therefore judged these trials to be at high risk of detection bias for the self‐assessed outcome of quality of life survey (Bochner 2015; Khan 2016; Parekh 2013; Parekh 2018).
One study did not report quality of life data (Nix 2010).
Positive margin rates, hospital stay, and transfusion rates
The review authors opined that positive margin rates, hospital stay, and transfusion rates were unlikely to be affected by the blinding status of outcome assessors in these trials. We therefore judged all five studies to be at low risk of detection bias for positive surgical margin rates and hospital stay (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018).
Nix 2010, Bochner 2015, and Khan 2016 did not report on transfusion rates. We judged Parekh 2018 and Parekh 2013 to be at low risk of detection bias for transfusion rates.
Incomplete outcome data
Quality of life
We judged four studies to be at high risk of attrition bias for quality of life survey results, given that a large proportion of participants (> 20%) failed to provide information (Bochner 2015; Khan 2016; Parekh 2013; Parekh 2018).
Major and minor postoperative complications, transfusion requirements, hospital stay, and positive margins
We rated all studies as having low risk of attrition bias with near complete inclusion of randomised participants in analyses for these outcomes (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018).
Time to recurrrence
We rated Bochner 2015 as having low risk of attrition bias, with all randomised participants included in the analysis. We rated Parekh 2018 as having unclear risk of attrition bias.
Selective reporting
Four studies had protocols registered in a trials registry (Bochner 2015; Khan 2016; Parekh 2013; Parekh 2018). We noted no obvious selective reporting for the outcomes of this review in these studies, and hence we judged them as having low risk of reporting bias. We were unable to find a protocol for the Nix 2010 trial. Therefore, we judged this trial as having an unclear risk of reporting bias.
Other potential sources of bias
We identified no other biases in any of the other included trials (Bochner 2015; Khan 2016; Nix 2010; Parekh 2013; Parekh 2018).
Effects of interventions
See: Table 1
Primary outcomes
Time to recurrence
RARC may result in a similar time to recurrence as ORC (hazard ratio (HR) 1.05, 95% confidence interval (CI) 0.77 to 1.43); 2 trials; low‐certainty evidence) (Figure 3). We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.1;Table 1). In absolute terms, this corresponds to 16 more recurrences per 1000 participants (95% CI 79 fewer to 123 more). The control event rate at 5 years was based on an overall recurrence rate of 25/58 (43.1%) in the ORC arm reported in Bochner 2015.
3.
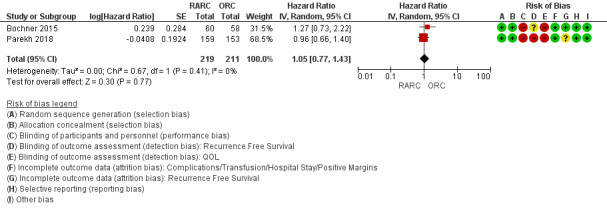
Forest plot of comparison: 1 Primary Outcome, outcome: 1.1 Recurrence‐Free Survival.
1.1. Analysis.
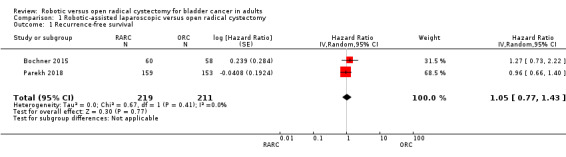
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 1 Recurrence‐free survival.
Major complications (Clavien grades 3 to 5)
RARC may result in similar rates of major complications as ORC (risk ratio (RR) 1.06, 95% CI 0.76 to 1.48); 5 trials; low‐certainty evidence) (Figure 4). This corresponds to 11 more major complications per 1000 participants (95% CI 44 fewer to 89 more). We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.2; Table 1).
4.
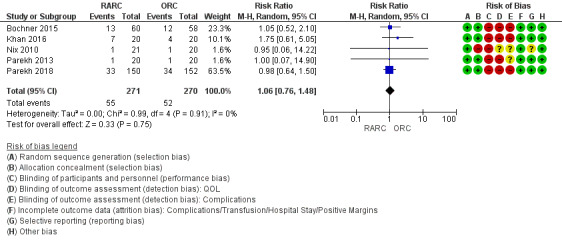
Forest plot of comparison: 1 Primary outcome, outcome: 1.1 Major postoperative complication rates (Clavien 3 to 5).
1.2. Analysis.
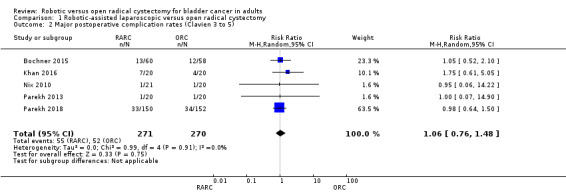
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 2 Major postoperative complication rates (Clavien 3 to 5).
Five trials reported on complications. Three studies reported the total number of Clavien grade 3 to 5 complications (Bochner 2015; Khan 2016; Parekh 2018). The other two studies reported specific complications (Nix 2010; Parekh 2013), based on which the review authors were able to classify complications by adopting the Clavien‐Dindo grading system (Dindo 2004).
Secondary outcomes
Minor complications (Clavien grades 1 and 2)
We are very uncertain whether RARC results in fewer minor complications than ORC (RR 0.82, 95% CI 0.58 to 1.17; 4 trials; very low‐certainty evidence). This corresponds to 80 fewer minor complications per 1000 participants (95% CI 186 fewer to 75 more). We downgraded the certainty of evidence for serious study limitations and very serious imprecision (Analysis 1.3; Table 1).
1.3. Analysis.
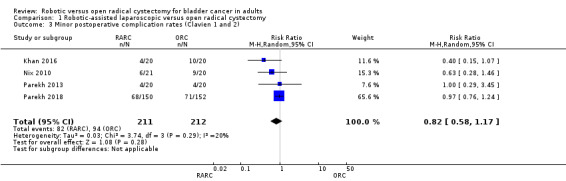
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 3 Minor postoperative complication rates (Clavien 1 and 2).
Transfusion rate
RARC probably results in fewer transfusions than ORC (RR 0.58, 95% CI 0.43 to 0.80; 2 trials; moderate‐certainty evidence). This corresponds to 193 fewer transfusions per 1000 participants (95% CI 262 fewer to 92 fewer). We downgraded the certainty of evidence for study limitations (Analysis 1.4; Table 1). Only two studies reported on transfusion rates (Parekh 2013; Parekh 2018).
1.4. Analysis.
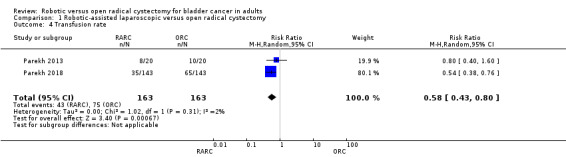
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 4 Transfusion rate.
Length of hospital stay (days)
All five trials provided information on hospital stay. One trial reported similar mean hospital stays of 5.1 days and 6 days in the RARC and ORC cohorts but did not report a standard deviation (Nix 2010). We therefore imputed the standard deviation. Two trials reported hospital stay in median and range values (Parekh 2013; Parekh 2018). We therefore imputed the mean and standard deviation for these trials. Two studies provided explicit data on mean hospital stay for meta‐analysis (Bochner 2015; Khan 2016).
Overall, we found that RARC may reduce mean hospital stay slightly (mean difference (MD) ‐0.67, 95% CI ‐1.22 to ‐0.12; 5 trials; low‐certainty evidence). We downgraded the quality of evidence for study limitations and imprecision (Analysis 1.5; Table 1).
1.5. Analysis.
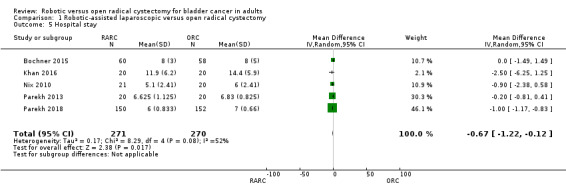
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 5 Hospital stay.
Quality of life
RARC may result in similar quality of life when compared with ORC (standard mean difference (SMD) 0.08, 95% CI: 0.32 lower to 0.16 higher; 3 trials; low‐certainty evidence). We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.6; Table 1).
1.6. Analysis.
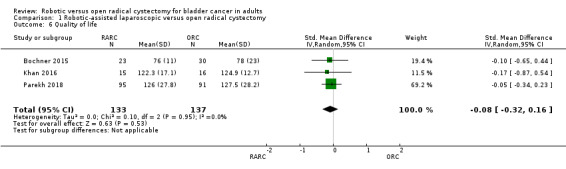
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 6 Quality of life.
Four studies reported on quality of life (QoL) outcomes (Bochner 2015; Khan 2016; Parekh 2013; Parekh 2018). One trial used the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ‐C30) survey (Bochner 2015). In this trial, data from the Global Health status domain were used for analysis, as this information reflected overall health status. One trial used the Functional Assessment of Cancer Therapy –Bladder (FACT‐Bl) scale v4 and covered physical, emotional, and social well‐being, as well as questions specific to bladder cancer (Khan 2016). Two trials used the Functional Assessment of Cancer Therapy –Vanderbilt Cystectomy Index (FACT–VCI) questionnaire (Parekh 2013; Parekh 2018). The standardised mean difference was used in view of the different QoL assessment tools used. One study reported QoL in median and range values (Parekh 2013). We therefore imputed mean and standard deviation for this study.
Positive margin rates
RARC may result in similar positive margin rates when compared to ORC (RR 1.16, 95% CI 0.56 to 2.40; 5 trials; low‐certainty evidence). This corresponds to eight more positive margins per 1000 participants (95% CI 21 fewer to 67 more).
We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.7; Table 1).
1.7. Analysis.
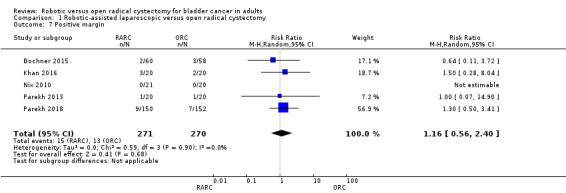
Comparison 1 Robotic‐assisted laparoscopic versus open radical cystectomy, Outcome 7 Positive margin.
We were unable to conduct any of the preplanned secondary analyses due to lack of suitable data.
Discussion
Summary of main results
There may be little to no difference in time to recurrence and in risk of major complications between the two surgical approaches to treat muscle‐invasive bladder cancer. We are very uncertain whether RARC reduces the rate of minor complications. There may be little to no difference in quality of life and positive margin rates. RARC probably reduces transfusions substantially and may reduce length of stay slightly.
Overall completeness and applicability of evidence
Follow‐up of the included trials is generally limited; only one trial has reported longer‐term follow‐up for the outcome of recurrence‐free survival at a median follow‐up of 4.9 years (Bochner 2015). Another trial reported on progression‐free survival at two years (Parekh 2018). Review authors judged this trial to have high risk of performance and detection bias for recurrence‐free survival. One small trial provided data on recurrence rates and overall and disease‐specific mortality at 12 months (Khan 2016). We judged this trial to have high risk of performance, detection, attrition, and reporting bias. Follow‐up was very short, at 12 months, further emphasising the lack of vital long‐term oncological data derived from randomised controlled trials (RCTs) comparing open radical cystectomy (ORC) and robotic‐assisted radical cystectomy (RARC).
All studies reported on complication rates. However, two studies did not demonstrate clear categorisation into minor and major complications using the Clavien‐Dindo grading system, as suggested by this review (Nix 2010; Parekh 2013). We therefore examined individual complications reported by these trials and classified them using the Clavien‐Dindo grading system. Although data show no obvious differences between ORC and RARC for major complications, the outcomes again were of low quality, suggesting significant uncertainty of the results, and hence must be viewed with caution.
For the outcomes "hospital stay" and "quality of life", three studies reported unfavourable metrics and statistical methods (e.g. median, no standard deviations reported for means) for meta‐analysis (Nix 2010; Parekh 2013, Parekh 2018). The review authors therefore imputed these data.
Quality of the evidence
We rated the certainty of evidence as low for all outcomes, except transfusion rates and hospital stay. We consistently downgraded evidence for a combination of study limitations, most often performance bias (lack of blinding of participants and personnel) and detection bias (lack of blinding of outcome assessors). We also frequently downgraded evidence for imprecision due to wide confidence intervals that indicated no effect but also included the possibility of clinically relevant benefit or harm.
Potential biases in the review process
We performed this systematic review in accordance with current Cochrane standards. The review nevertheless has the following limitations:
The review authors cannot be absolutely certain if we missed identifying any other potential randomised trials comparing ORC and RARC in our search, although we think this is unlikely.
We excluded from the meta‐analysis some of the data reported by individual studies due to lack of appropriate data points. We contacted the authors of these individual studies but were not successful in obtaining additional data. We chose to impute data in accordance with the editorial policy of Cochrane standards.
Agreements and disagreements with other studies or reviews
We identified five relevant systematic reviews of randomised and nonrandomised controlled trials comparing robotic and open radical cystectomy (Ishii 2014; Novara 2015; Tang 2014; Yuh 2015; Sathianathen 2018). These reviews used pooled data derived across all study designs, and none considered evaluation of the quality of evidence as defined by GRADE.
Yuh 2015 performed a cumulative analysis of oncological and functional outcomes of robotic‐assisted radical cystectomy (RARC). This review identified 65 surgical series and 22 comparative studies reporting on pathological, oncological, and functional outcomes of RARC. Two trials in the review were randomised trials (Nix 2010; Parekh 2013). We included both of these studies in our review. A majority of the studies included in this review were retrospective studies. No certainty of evidence was assessed. The review identified two nonrandomised comparative studies that reported similar survival outcomes between ORC and RARC (Khan 2012; Nepple 2013). Review authors suggested caution when interpreting these results due to short follow‐up, small series, and study limitations.
Novara 2015 performed a cumulative analysis of perioperative outcomes and postoperative complications of RARC. This review identified 70 surgical series and 23 comparative studies. Three trials included in the review were randomised trials (Bochner 2015; Nix 2010; Parekh 2013). We have included these three studies in our review. A majority of studies included in the Novara review were retrospective studies. Review authors categorised individual studies to the 2011 level of evidence and IDEAL recommendations and scrutinised the quality of reporting of complications of individual studies using the Martin criteria (Martin 2002). They performed no other quality assessment of individual studies. These review authors reported 90‐day complication rates of any grade and found that 90‐day grade 3 complication rates were lower for RARC, whereas high‐grade complication and mortality rates were similar. It is unclear from the review how the review authors differentiated between grade 3 complications and high‐grade complications. The analysis for grade 3 complications did not include any of the RCTs. The analysis for high‐grade complications included one RCT (Bochner 2015). The RCT included in this analysis contributed 19.3% to the study weight.
Tang 2014 performed a systematic review that included 13 studies comparing RARC and ORC. One trial in the review was a randomised trial (Nix 2010). We have included this study in our review. These review authors reported perioperative and pathological outcomes and complications. Review authors pooled data across all study designs. They rated the level of evidence (LOE) of included studies according to criteria provided by the Centre for Evidence‐Based Medicine in Oxford, UK. They assessed risk of bias of the RCT using the Jadad scale and of observational studies using the Newcastle–Ottawa scale. Pooled analysis favoured the RARC cohort for overall complication rate. Nix 2010, the only RCT included in the analysis, contributed only 5.5% to the study weight.
Ishii 2014 performed a systematic review that included seven studies comparing RARC and ORC. Two trials in the review were randomised trials (Nix 2010; Parekh 2013). We have included both of these studies in our review. Review authors assessed the methodological quality of these included studies in line with the Cochrane Handbook for Systematic Reviews of Interventions. The primary outcome of this study was complication rates. Pooled analysis favoured the RARC cohort for major complication rates. Analysis for major complications included one RCT (Parekh 2013), which contributed to 6.7% to the study weight.
Sathianathen 2018 has published the most recent and highest‐quality review to date. Methodolgical hallmarks include an a priori registered protocol with predefined primary outcomes, a comprehensive search of multiple data sources, and study inclusion irrespective of language of publication status and use of GRADE to assess the quality of evidence on a per‐outcome basis. Instead of recurrence‐free survival as a time‐to‐event outcome used in our review, these review authors analysed risk of recurrence as a dichotomous outcome. They rated findings as moderate‐quality evidence, which is more optimistic than our rating of low‐quality evidence, while qualifying that there is little to no difference between the two approaches. What our review further adds is a summary of findings table (Table 1) with corresponding absolute effect size estimates.
Authors' conclusions
Implications for practice.
Based on the findings of this review, oncological outcomes and rates of major complications may be similar for both approaches. Robotic‐assisted cystectomy probably reduces transfusion needs substantially and may slightly reduce length of hospital stay. We are uncertain whether minor complications are also reduced. We were unable to address how patients’ and surgeons’ characteristics may affect these outcomes. Furthermore, this review was not designed to address resource utilisation or cost‐effectiveness.
Implications for research.
This review is based on five relatively small trials with methodological limitations that provided low‐quality evidence for most outcomes. Only one trial has provided long‐term oncological outcomes (Bochner 2015). We see the following research needs:
Investigators of existing trials should report longer‐term results for longer‐term oncological outcomes.
Researchers should assess the influence of patient factors such as pathological stage and body habitus.
Studies should establish the impact of surgeon factors such as skills and experience on outcomes.
Most instances of urinary diversion reported in included trials were performed through an extracorporeal approach. Future trials should evaluate outcomes between open radical cystectomy and robotic‐assisted radical cystectomy performed through intracorporeal urinary diversions.
Any future trial should apply widely accepted methodological safeguards against bias and should transparently report them.
Notes
We have based parts of the Methods section of the protocol for this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group that has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We would like to thank the editors of Cochrane Urology for the support they provided.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | #1 MeSH descriptor: [Robotics] explode all trees #2 (robot*):kw,ti,ab near/3 (surgery* or assist):kw,ti,ab #3 #1 or #2 #4 MeSH descriptor: [Cystectomy] explode all trees #5 (surg* or excis*):kw,ti,ab near/3 (urin* or bladder*):kw,ti,ab #6 #4 or #5 #7 #3 and #6 #8 (robot*):kw,ti,ab near/3 (cystectom*):kw,ti,ab #9 #7 or #8 |
| MEDLINE (PubMed) | 1 Robotics 2 Robot 3 “Robotics”[MeSH] 4 1 OR 2 OR 3 5 Cystectomy 6 “Cystectomy” [MeSH] 7 5 OR 6 8 Randomized control trial[tw] 9 Controlled clinical trial[tw] 10 Randomized[tw] 11 Randomly[tw] 12 Trial[tw] 13 “Randomized Controlled Trial”[publication Type] 14 8 OR 9 OR 10 OR 11 OR 12 OR 13 15 4 AND 7 AND 14 |
| Embase (Ovid) | 1. (cystectomy and controlled clinical trial and robotic).af. 2. (randomised controlled trial and robotic and cystectomy).af. 3. (cystectomy and robotic).af. |
| Web of Science | 1. (TOPIC:(cystostomy) AND TOPIC: (robotic)) 2. ((TOPIC:(cystostomy) AND TOPIC: (robotic))AND TOPIC: (randomizedcontrolled trial)) 3. TITLE: (cystectomy) ANDTITLE: (robotic) ANDTITLE: (randomized controlled trial) 4. TITLE: (cystectomy) ANDTITLE: (randomized controlled trial) |
| Cancer Research UK | 1. Bladder cancer trials 2. Robotic cystectomy |
| Institute of Cancer Research | 1. Bladder cancer trials 2. Robotic cystectomy |
| ClinicalTrials.gov | 1. robotic | bladder cancer | cystectomy | Child, Adult, Senior 2. robotic | Open Studies | bladder cancer | cystectomy |
| BioMed Central ISRCTN | 1. robotic Remove filter within Condition: bladder cancer Remove filterInterventions: cystectomy Remove filter |
| WHO ICTRP | 1. bladder cancer AND cystectomy AND Robot 2. bladder cancer AND cystectomy AND Robot AND trial |
| Terms used | robotic cystectomy robot cystectomy robot assisted cystectomy robotic radical cystectomy |
Data and analyses
Comparison 1. Robotic‐assisted laparoscopic versus open radical cystectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence‐free survival | 2 | 430 | Hazard Ratio (Random, 95% CI) | 1.05 [0.77, 1.43] |
| 2 Major postoperative complication rates (Clavien 3 to 5) | 5 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.76, 1.48] |
| 3 Minor postoperative complication rates (Clavien 1 and 2) | 4 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.17] |
| 4 Transfusion rate | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.43, 0.80] |
| 5 Hospital stay | 5 | 541 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.22, ‐0.12] |
| 6 Quality of life | 3 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.32, 0.16] |
| 7 Positive margin | 5 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.56, 2.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bochner 2015.
| Methods |
|
|
| Participants |
Adults undergoing radical cystectomy (n = 118) Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Demographic data: RARC vs ORC Median age years (IQR) = 66 (60 to 71) vs 65 (58 to 69) Male sex, n (%) = 51 (85) vs 42 (72) Body mass index, kg/m², median (IQR) = 27.9 (24.7 to 31.0) vs 29.0 (26.3 to 33.7) |
|
| Interventions | Cohort 1 = ORC with urinary diversion and PLND (n = 58) Cohort 2 = RARC with extracorporeal urinary diversion and PLND (n = 60)
|
|
| Outcomes |
|
|
| Funding sources | This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael and Zena Wienerfor Therapeutics Program in Bladder Cancer. Study sponsors were involved in the design and conduct of the study; in collection, analysis, management, and interpretation of the data; and in preparation, review, and approval of the manuscript. | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Consenting patients were stratified by age (64 vs 65 yr) and American Society of Anesthesiologists score (1–2 vs 3–4), then randomly assigned 1:1 to undergo RARC or ORC using randomly permuted blocks of random length." Comment: adequate random sequence generation performed |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomization was conducted by an independent office, where allocation concealment was ensured by a password‐protected database, such that the randomization group could not be predicted prior to receiving group assignment and group could not be changed after randomization." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Recurrence Free Survival | Unclear risk | Comment: Trial does not explicitly state who collected these data. |
| Blinding of outcome assessment (detection bias) QOL | High risk | Comment: participant‐reported outcomes; participants not blinded |
| Blinding of outcome assessment (detection bias) Complications | High risk |
Quote from publication: "All complications were graded on the MSKCC modified Clavien grading scale. Complications data were collected prospectively by unblinded MSKCC research study staff at the initial postoperative, 3‐mo, and 6‐mo follow‐up visits using the institution’s standard reporting method for postoperative complications." Comment: assessor unblinded |
| Blinding of outcome assessment (detection bias) Transfusion Rates | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Hospital Stay | Low risk | Comment: unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Positive Margin Rates | Low risk |
Quote from publication: "All pathologic specimens were reviewed blinded to surgical technique." Comment: adequate blinding; additionally, regardless of blinding, low risk of detection bias |
| Incomplete outcome data (attrition bias) Complications/Transfusion/Hospital Stay/Positive Margins | Low risk | Comment: All randomised participants were included in the analysis for these outcomes. |
| Incomplete outcome data (attrition bias) QOL | High risk |
Quote from publication: "Fifty‐eight patients returned evaluable baseline surveys and 53 returned follow‐up surveys at 3 and 6 mo." Comment: In the RARC group, 60 participants were randomised, and 30 (50%) participants returned surveys at 6 months. In the ORC group, 58 participants were randomised, and 22 (38%) participants returned surveys at 6 months. |
| Incomplete outcome data (attrition bias) Recurrence Free Survival | Low risk | Comment: All randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported for both groups in the time period suggested. |
| Other bias | Low risk | Comment: not detected |
Khan 2016.
| Methods |
|
|
| Participants |
Adults undergoing radical cystectomy (n = 60) Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Demographic data: RARC vs ORC Mean age years (SD) = 68.6 (6.8) vs 66.6 (8.8) Male sex, n (%) = 17 (85) vs 18 (90) Body mass index, kg/m², mean (SD) = 27.5 (4.2) vs 27.4 (3.9) |
|
| Interventions | Cohort 1 = ORC with urinary diversion and PLND (n = 20) Cohort 2 = RARC with extracorporeal urinary diversion and PLND (n = 20) Cohort 3 = LRC with extracorporeal urinary diversion and PLND (n = 20)
|
|
| Outcomes |
|
|
| Funding sources | The research, including statistical support (Jennifer A. Summers and Janet L. Peacock), was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre, based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. Prokar Dasgupta and Kamran Ahmed acknowledge support from the NIHR Biomedical Research Centre, Medical Research Council Centre for Transplantation, King’s Health Partners, Guy’s and St. Thomas’ Charity, School of Surgery, London Deanery, Royal College of Surgeons of England, Intuitive Surgical, The Urology Foundation, Olympus, EU‐FP7, ProstateCancer UK, Technology Strategy Board, and The Vattikuti Foundation. | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomisation was undertaken by the trial nurse (J.W.) using identical sealed opaque envelopes, each containing a piece of paper designating the surgical modality (ORC, LRC, or RARC). Simple randomisation was performed in two groups of 30. In each group, each modality was allocated 10 envelopes. These were shuffled and then numbered 1–30. Patients received the next envelope in numerical order." Comment: random sequence generation adequate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Envelopes were kept in a locked room, accessed only by the trial nurse to minimise opportunities for tampering, and they were opened by the patient in the presence of three members of the research team to ensure that no changes were made to allocation." Comment: allocation concealment adequate |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) QOL | High risk |
Quote from publication: "This study was nonblinded because the different incisions would be difficult to camouflage." Comment: participant‐reported outcomes; participants not blinded |
| Blinding of outcome assessment (detection bias) Complications | High risk |
Quote from publication: "This study was nonblinded because the different incisions would be difficult to camouflage." Comment: no outcome assessor blinding; study does not report the assessor |
| Blinding of outcome assessment (detection bias) Transfusion Rates | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Hospital Stay | Low risk | Comment: unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Positive Margin Rates | Low risk | Comment: unlikely to be affected by nonblinding |
| Incomplete outcome data (attrition bias) Complications/Transfusion/Hospital Stay/Positive Margins | Low risk | Comment: All randomised participants were included in the analysis for these outcomes. |
| Incomplete outcome data (attrition bias) QOL | High risk |
Quote from publication: "Overall, 53 patients completed the QoL questionnaire. One questionnaire was analysed per patient (average 8 mo postoperatively). Incomplete questionnaires were excluded." Comment: In the RARC group, 20 participants were randomised, and 15 (75%) participants returned surveys. In the ORC group, 20 participants were randomised, and 16 (80%) participants returned surveys. |
| Incomplete outcome data (attrition bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Low risk | Comment: predefined outcomes reported for both groups in the time period suggested |
| Other bias | Low risk | Comment: not detected |
Nix 2010.
| Methods |
|
|
| Participants |
Adults undergoing radical cystectomy (n = 41) Diagnostic and inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Cohort 1 = ORC with urinary diversion and PLND (n = 20) Cohort 2 = RARC with extracorporeal urinary diversion and PLND (n = 21)
Demographic data: RARC vs ORC Mean age years = 67.4 vs 69.2 Male:Female = 14:7 vs 17:3 Body mass index, kg/m², mean = 27.5 vs 28.4 |
|
| Outcomes |
|
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The randomizations schema was performed with five sequential patients undergoing an approach before alternating surgical modality." Comment: random sequence generation adequate |
| Allocation concealment (selection bias) | High risk |
Quote from publication: "The randomization schema was performed with five sequential patients undergoing an approach before alternating surgical modality. This scheme was chosen, as opposed to randomizing each sequential patient, for the purpose of resident education. We believed that alternating each sequential surgery as to approach would make it significantly more difficult for residents to progress through their knowledge and acquisition of proficiency in each of the individual procedures." Comment: allocation concealment inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) QOL | Unclear risk | Comment: not applicable as not reported |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Comment: no report on who collected outcomes and if they were blinded to the procedure |
| Blinding of outcome assessment (detection bias) Transfusion Rates | Unclear risk | Comment: not applicable as not reported |
| Blinding of outcome assessment (detection bias) Hospital Stay | Low risk | Comment: unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Positive Margin Rates | Low risk | Comment: unlikely to be affected by nonblinding |
| Incomplete outcome data (attrition bias) Complications/Transfusion/Hospital Stay/Positive Margins | Low risk | Comment: all randomised participants included in analysis for these outcomes |
| Incomplete outcome data (attrition bias) QOL | Unclear risk | Comment: not applicable as not reported |
| Incomplete outcome data (attrition bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
| Other bias | Low risk | None |
Parekh 2013.
| Methods |
|
|
| Participants |
Adults undergoing radical cystectomy (n = 40) Diagnostic criteria:
Inclusion criteria:
Exclusion criteria:
Demographic data: RARC vs ORC Median age years (IQR) = 69.5 (62.3 to 74) vs 64.5 (59.8 to 72.3) Male:Female = 18:2 vs 16:4 Body mass index, kg/m², median (IQR) = 27.6 (24.2 to 29.9) vs 28.3 (26.1 to 32.3) |
|
| Interventions | Cohort 1 = ORC with urinary diversion and PLND (n = 20) Cohort 2 = RARC with open urinary diversion and PLND (n = 20)
|
|
| Outcomes |
|
|
| Funding sources | Not reported in the study | |
| Declarations of interest | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Patients who met the inclusion criteria were randomized to open or robotic radical cystectomy at their preoperative clinic visit using a computerized randomization program (www.randomization.com) (see figure). This program generated a list of surgical slots numbered 1 through 60, and randomly assigned open or robotic assisted cystectomy to each slot (30 slots for each procedure)." Comment: random sequence generation adequate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Each assignment was placed in a sealed envelope with the corresponding slot number written on the outside. At the time of consent the lowest numbered envelope remaining was opened and the patient was assigned to the surgical procedure listed on the piece of paper inside the envelope." Comment: allocation concealment adequate |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) QOL | High risk |
Quote from publication: "The surgical team and the patient were then made aware of the type of surgery." Comment: Participants were aware of the approach they had; hence there would have been an expectation bias when they completed their QoL questionnaires. Participant‐reported outcomes. Participants not blinded. |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Comment: The study does not state who the outcome assessor was; it is unclear if the assessor was blinded. |
| Blinding of outcome assessment (detection bias) Transfusion Rates | Low risk | Comment: unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Hospital Stay | Low risk | Comment: unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Positive Margin Rates | Low risk | Comment: unlikely to be affected by nonblinding |
| Incomplete outcome data (attrition bias) Complications/Transfusion/Hospital Stay/Positive Margins | Low risk | Comment: Most randomised participants were included in the analysis for these outcomes. |
| Incomplete outcome data (attrition bias) QOL | High risk |
Quote from second publication: "The study is limited by the response rate to the questionnaires, with sampling at only 50% for some time periods, which only underscores the difficulty of obtaining prospective data with regard to HRQoL." Comment: Reported in the second publication. In the RARC group, 20 participants were randomised, and 12 (60%) participants returned surveys at 12 months. In the ORC group, 20 participants were randomised, and 13 (65%) participants returned surveys at 12 months. |
| Incomplete outcome data (attrition bias) Recurrence Free Survival | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes measured |
| Other bias | Low risk | None |
Parekh 2018.
| Methods |
|
|
| Participants |
Adults undergoing radical cystectomy Diagnostic criteria and inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intention‐to‐treat analysis Cohort 1 = ORC with urinary diversion and PLND (n = 159) Cohort 2 = RARC with open urinary diversion and PLND (n = 153)
|
|
| Outcomes |
Primary outcome
Quote from publication: "Disease progression was determined on the basis of radiographical or pathological evidence of disease, or death from disease according to Response Evaluation Criteria in Solid Tumours criteria version 1.1." Secondary outcomes
Exploratory end points
|
|
| Funding sources | National Institutes of Health National Cancer Institute | |
| Declarations of interest | Quote from publication: "EPC reports personal fees from Intuitive Surgical outside the submitted work. MEW reports grants from the National Institutes of Health (NIH) during the study, and outside the submitted work. AZW reports grants from the University of Michigan during the study. BRK reports grants from the National Cancer Institute during the study; and grants from Photocure, Roche‐Genentech, Genomic Health, Myriad Genetics, Spectrum, and FKD Therapies, outside the submitted work. MT reports grants from the NIH during the study. TLK reports grants from the NIH during the study. DAB reports grants from the NIH, during the study; and personal fees from AstraZeneca, Tolmar Pharmaceuticals, and Janssen, outside the submitted work. ASK reports advisory board fees from Profound, Sanofi‐Aventis, and Janssen, outside the submitted work. CJW reports grants from Myriad Genetics and personal fees from Abbott Molecular, outside the submitted work. MSC has served on advisory boards for Astellas Pharma US, MDxHealth, Janssen, Bayer Healthcare, CicloMed, Abbott Laboratories, Tolmar Pharmaceuticals, Genomic Health, Altor Bioscience, Photocure, and Takeda Pharmaceutical; and reports consultancy fees from Myovant Sciences, TesoRx Pharma, and Pacific Edge Diagnostics. All other authors declare no competing interests." | |
| Notes | Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from study: "By use of a dynamic balancing algorithm, patients were centrally randomly assigned (1:1) via a web‐based system, to receive open cystectomy or robotic cystectomy. Using each institution as a block, the dynamic allocation procedure allocated an approximately equal number of patients to treatment groups to minimise imbalance between groups, stratified by type of urinary diversion (incontinent or continent), clinical T stage (carcinoma in situ, T1–T2, or T3–T4), and Eastern Cooperative Oncology Group (ECOG) performance status (0–1, or =2). On accrual a hierarchical decision‐rule was applied, and the allocation was deterministic if certain predefined limits were exceeded, and random otherwise." |
| Allocation concealment (selection bias) | Low risk | Quote from study: "By use of a dynamic balancing algorithm, patients were centrally randomly assigned (1:1) via a web‐based system, to receive open cystectomy or robotic cystectomy. Using each institution as a block, the dynamic allocation procedure allocated an approximately equal number of patients to treatment groups to minimise imbalance between groups, stratified by type of urinary diversion (incontinent or continent), clinical T stage (carcinoma in situ, T1–T2, or T3–T4), and Eastern Cooperative Oncology Group (ECOG) performance status (0–1, or =2). On accrual a hierarchical decision‐rule was applied, and the allocation was deterministic if certain predefined limits were exceeded, and random otherwise." |
| Blinding of participants and personnel (performance bias) | High risk |
Comment: in view of the nature of the study, unlikely to be blinded Quote from study: "Treatment allocation was only masked from pathologists, who analysed the cystectomy specimens." |
| Blinding of outcome assessment (detection bias) Recurrence Free Survival | High risk | Quote from study: "Treatment allocation was only masked from pathologists, who analysed the cystectomy specimens." |
| Blinding of outcome assessment (detection bias) QOL | High risk | Quote from study: "Treatment allocation was only masked from pathologists, who analysed the cystectomy specimens." |
| Blinding of outcome assessment (detection bias) Complications | High risk | Quote from study: "Treatment allocation was only masked from pathologists, who analysed the cystectomy specimens." |
| Blinding of outcome assessment (detection bias) Transfusion Rates | Low risk | Unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Hospital Stay | Low risk | Unlikely to be affected by nonblinding |
| Blinding of outcome assessment (detection bias) Positive Margin Rates | Low risk |
Quote from study: "Treatment allocation was only masked from pathologists, who analysed the cystectomy specimens." Comment: Unlikely to be affected by nonblinding |
| Incomplete outcome data (attrition bias) Complications/Transfusion/Hospital Stay/Positive Margins | Low risk | Comment: Most randomised participants were included in analysis for these outcomes. |
| Incomplete outcome data (attrition bias) QOL | High risk | Comment: At 6 months, QoL data were available for only 198 participants (63.4%). |
| Incomplete outcome data (attrition bias) Recurrence Free Survival | Unclear risk | Quote from study: "Between July 1, 2011, and Nov 18, 2014, 350 patients were randomly assigned to treatment: 176 to the robotic cystectomy group and 174 to the open cystectomy group. Of the 176 patients who were randomly assigned to receive robotic cystectomy, 17 (10%) patients did not have surgery and nine (5%) patients had a different surgery to that they were assigned. Of the 174 patients assigned to receive open cystectomy, 21 (12%) patients did not have surgery and one (1%) patient had robotic cystectomy instead of open cystectomy." |
| Selective reporting (reporting bias) | Low risk | Comment: all predefined outcomes measured |
| Other bias | Low risk | Comment: nil |
CI: confidence interval; EBL: estimated blood loss; FACT‐VCI: Functional Assessment of Cancer Therapy –Vanderbilt Cystectomy Index; HRQoL: health‐related quality of life; IQR: interquartile range; ITT: intention‐to‐treat; LN: lymph node; LOS: length of (hospital) stay; LRC: laparoscopic radical cystectomy; MIBC: muscle‐invasive bladder cancer; NMIBC: non‐muscle‐invasive bladder cancer; ORC: open radical cystectomy; PLND: pelvic lymph node dissection; QoL: quality of life; RARC: robotic‐assisted radical cystectomy; RC: radical cystectomy; RCT: randomised controlled clinical trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2013 | Not a randomised controlled study. In this study, benign ureteroenteric anastomotic stricture rates of open and robot assisted laparoscopic radical cystectomy were compared using a prospectively maintained database. |
| Atmaca 2015 | Not a randomised controlled study. Retrospective comparison of open vs totally intracorporeal robotic‐assisted radical cystectomy, bilateral extended pelvic lymph node dissection, and Studer urinary diversion. Researchers evaluated operative and postoperative parameters, pathological parameters, complications, and functional outcomes. |
| Bak 2016 | Although not clearly specified, this appears to be a nonrandomised retrospective observational study. |
| Borza 2017 | Not a randomised controlled study. Participants were identified from International Classification of Diseases 9th edition codes and administrative claims from a large, national US health insurer. This study compared readmission rates for ORC and RARC. |
| Cusano 2016 | Not a randomised controlled study. Retrospective comparison of preliminary oncological outcomes for ORC and RARC. |
| Galich 2006 | Not a randomised controlled study. This study compared early perioperative outcomes following radical cystectomy by the robotic method vs the conventional open method using a prospectively maintained database. |
| Gandaglia 2016 | Not a randomised controlled study. Retrospective comparison of perioperative and oncological outcomes of open (ORC) and robotic‐assisted radical cystectomy (RARC) between 2 large‐volume European centres. |
| Ginot 2016 | Not a randomised controlled study. Comparison of robotic‐assisted cystectomy vs open cystectomy, with urinary diversion by bladder substitution. |
| Gondo 2012 | Not a randomised controlled study. This study compared early perioperative outcomes following radical cystectomy by the robotic method vs the conventional open method using a prospectively maintained database in a Japanese population. |
| Khan 2012 | Not a randomised controlled study. This prospective study compared perioperative outcomes of ORC, RARC, and LRC. |
| Koupparis 2015 | Not a randomised controlled study. This study compared the impact of ERAS between prospective RARC data and retrospectively maintained ORC data. |
| Lee 2011 | Not a randomised controlled study. This prospective comparative study compared the economic burden of ORC vs RARC. |
| Li 2016 | Not a randomised controlled study. This retrospective study compared health‐related quality of life (HRQoL) and short‐term convalescence among bladder cancer patients who underwent ORC and RARC. |
| Martin 2011 | Not a randomised controlled study. This prospective comparative study performed cost analysis of ORC and RARC. |
| Matulewicz 2016 | Not a randomised controlled study. Researchers used the National Cancer Data Base to compare oncological quality indicators between open and robotic‐assisted radical cystectomy. |
| Musch 2014 | Not a randomised controlled study. This prospective comparative study compared early postoperative morbidity of ORC and RARC. |
| Nepple 2013 | Not a randomised controlled study. This retrospective non‐matched study compared pathology, recurrence, and survival between ORC and RARC. |
| Ng 2010 | Not a randomised controlled study. This prospective study compared complications between ORC and RARC. |
| Nguyen 2015 | Not a randomised controlled study. This retrospective study compared RFS and recurrence patterns between ORC and RARC. |
| Rhee 2006 | Not a randomised controlled study. This prospective comparative study compared estimated blood loss (EBL), transfusion requirements, operative duration, hospital stay, and body mass index (BMI) between ORC and RARC. |
| Satkunasivam 2016 | Not a randomised controlled study. This retrospective study compared urodynamic features of intracorporeal orthotopic neobladder and bladder cancer‐specific and general health‐related quality of life between RARC and ORC. |
| Sharma 2017 | Not a randomised controlled study. This retrospective study compared pathological and postoperative outcomes of RARC vs open radical cystectomy (ORC) with high‐risk disease (pT3/T4). |
| Styn 2012 | Not a randomised controlled study. This matched‐pair analysis compared ORC and RARC. Researchers match‐paired age, sex, urinary diversion, and clinical stage, and they compared perioperative complications and pathological outcomes. |
| Tan 2016 | Not a randomised controlled study. Investigators compared early oncological outcomes and cancer recurrence sites among patients undergoing ORC and RARC with intracorporeal urinary diversion (iRARC). |
| Wang 2008 | Not a randomised controlled study. This prospective study compared estimated blood loss (EBL), transfusion requirement, operative duration, time to resumption of regular diet, hospital stay, complication rates, and pathological outcomes between ORC and RARC. |
| Winters 2016 | Not a randomised controlled study. This study compared perioperative surgical outcomes among elderly patients. |
BMI: body mass index; EBL: estimated blood loss; ERAS: Enhanced recovery after syrgery; HRQoL: health‐related quality of life; iRARC: intracorporeal urinary diversion; LRC: laparoscopic radical cystectomy; ORC: open radical cystectomy; RARC: robotic‐assisted radical cystectomy; RFS: Recurrence‐free survival.
Characteristics of ongoing studies [ordered by study ID]
Kelly , Catto 2017.
| Trial name or title | IROC (Trial to Compare Robotically Assisted Radical Cystectomy With Open Radical Cystectomy) |
| Methods | Phase III multicentre randomised controlled trial |
| Participants | Patients with non‐muscle‐invasive bladder cancer (NMIBC) or muscle‐invasive bladder cancer (MIBC) who had selected radical cystectomy for treatment of bladder cancer; planned accrual of 320 participants |
| Interventions | Robotically assisted radical cystectomy and intracorporeal urinary diversion vs open radical cystectomy |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 01/03/2017 (estimated study completion date: 15 February 2020) |
| Contact information | Chris Brew‐Graves; 0207 679 9280; situ.iroc@ucl.ac.uk |
| Notes | ClinicalTrials.gov identifier (NCT number): NCT03049410 |
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EQ‐5D‐5L: EuroQol 5 Domains 5 Levels; MIBC: muscle‐invasive bladder cancer; NMIBC: non‐muscle‐invasive bladder cancer; WHODAS: World Health Organization Disability Assessment Schedule.
Differences between protocol and review
None
Contributions of authors
BR: wrote the review and contributed to data extraction and analysis, concept, and data interpretation.
JB: contributed to data extraction and analysis and drafting work.
NV: contributed to text of review and to data analysis, concept, and data interpretation.
JA: contributed to text of review and to data analysis, concept, and data interpretation.
TL: contributed to text of review and to data analysis, concept, and data interpretation.
KA: contributed to text of review and to concept.
MSK: contributed to text of review, concept, and data interpretation.
PD: contributed to text of review, concept, and data interpretation.
KG: contributed to text of review and to concept and data interpretation.
PLC: contributed to text of review and to interpretation of data and concept.
OMA: wrote the review and contributed to data extraction and analysis, concept, and data interpretation.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
BR: none known.
JB: none known.
NV: none known.
JA: none known.
TL: none known.
KA: none known.
MSK: none known.
PD: none known.
KG: none known.
PLC: none known.
OMA: none known.
New
References
References to studies included in this review
Bochner 2015 {published data only}
- Bochner BH, Dalbagni G, Marzouk KH, Sjoberg DD, Lee J, Donat SM, et al. Randomized trial comparing open radical cystectomy and robot‐assisted laparoscopic radical cystectomy: oncologic outcomes. European Urology 2018;74(4):465‐71. [PUBMED: 29784190] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot‐assisted laparoscopic radical cystectomy: a randomized clinical trial. European Urology 2015;67(6):1042‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2016 {published data only}
- Khan MS, Gan C, Ahmed K, Ismail AF, Watkins J, Summers JA, et al. A single‐centre early phase randomised controlled three‐arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). European Urology 2016;69(4):613‐21. [DOI] [PubMed] [Google Scholar]
Nix 2010 {published data only}
- Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. European Urology 2010;57(2):196‐201. [DOI] [PubMed] [Google Scholar]
Parekh 2013 {published data only}
- Messer JC, Punnen S, Fitzgerald J, Svatek R, Parekh DJ. Health‐related quality of life from a prospective randomised clinical trial of robot‐assisted laparoscopic versus open radical cystectomy. BJU International 2014;114(6):896‐902. [DOI] [PubMed] [Google Scholar]
- Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. Journal of Urology 2013;189(2):474‐9. [DOI] [PubMed] [Google Scholar]
Parekh 2018 {published data only}
- Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot‐assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open‐label, randomised, phase 3, non‐inferiority trial. Lancet 2018;391:2525‐36. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2013 {published data only}
- Anderson CB, Morgan TM, Kappa S, Moore D, Clark PE, Davis R, et al. Ureteroenteric anastomotic strictures after radical cystectomy ‐ does operative approach matter?. Journal of Urology 2013;189(2):541‐7. [DOI] [PubMed] [Google Scholar]
Atmaca 2015 {published data only}
- Atmaca AF, Canda AE, Gok B, Akbulut Z, Altinova S, Balbay MD. Open versus robotic radical cystectomy with intracorporeal Studer diversion. Journal of the Society of Laparoendoscopic Surgeons 2015;19(1):e2014.00193. [PUBMED: 25848187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bak 2016 {published data only}
- Bak DJ, Lee YJ, Woo MJ, Chung JW, Ha YS, Kim HT, et al. Complications and oncologic outcomes following robot‐assisted radical cystectomy: what is the real benefit?. Investigative and Clinical Urology 2016;57(4):260‐7. [PUBMED: 27437535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borza 2017 {published data only}
- Borza T, Jacobs BL, Montgomery JS, Weizer AZ, Morgan TM, Hafez KS, et al. No differences in population‐based readmissions after open and robotic‐assisted radical cystectomy: implications for post‐discharge care. Urology 2017;104:77‐83. [PUBMED: 28267606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cusano 2016 {published data only}
- Cusano A, Haddock P Jr, Jackson M, Staff I, Wagner J, Meraney A. A comparison of preliminary oncologic outcome and postoperative complications between patients undergoing either open or robotic radical cystectomy. International Brazilian Journal of Urology 2016;42(4):663‐70. [PUBMED: 27564275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galich 2006 {published data only}
- Galich A, Sterrett S, Nazemi T, Pohlman G, Smith L, Balaji KC. Comparative analysis of early perioperative outcomes following radical cystectomy by either the robotic or open method. Journal of the Society of Laparoendoscopic Surgeons 2006;10(2):145‐50. [PMC free article] [PubMed] [Google Scholar]
Gandaglia 2016 {published data only}
- Gandaglia G, Karl A, Novara G, Groote R, Buchner A, D'Hondt F, et al. Perioperative and oncologic outcomes of robot‐assisted versus open radical cystectomy in bladder cancer patients: a comparison of two high‐volume referral centers. European Journal of Surgical Oncology 2016;42(11):1736‐43. [PUBMED: 27032295] [DOI] [PubMed] [Google Scholar]
Ginot 2016 {published data only}
- Ginot R, Rouget B, Bensadoun H, Pasticier G, Bernhard JC, Capon G, et al. Radical cystectomy with orthotopic neobladder replacement: comparison of robotic assisted and open surgical route [Cystectomie totale avec remplacement vesical orthotopique: comparaison des resultats des patients operes par voie ouverte et par voie coelioscopique robot‐assistee.]. Progres en Urologie 2016;26(8):457‐63. [PUBMED: 27460787] [DOI] [PubMed] [Google Scholar]
Gondo 2012 {published data only}
- Gondo T, Yoshioka K, Nakagami Y, Okubo H, Hashimoto T, Satake N, et al. Robotic versus open radical cystectomy: prospective comparison of perioperative and pathologic outcomes in Japan. Japanese Journal of Clinical Oncology 2012;42(7):625‐31. [DOI] [PubMed] [Google Scholar]
Khan 2012 {published data only}
- Khan MS, Challacombe B, Elhage O, Rimington P, Coker B, Murphy D, et al. A dual‐centre, cohort comparison of open, laparoscopic and robotic‐assisted radical cystectomy. International Journal of Clinical Practice 2012;66(7):656‐62. [DOI] [PubMed] [Google Scholar]
Koupparis 2015 {published data only}
- Koupparis A, Villeda‐Sandoval C, Weale N, El‐Mahdy M, Gillatt D, Rowe E. Robot‐assisted radical cystectomy with intracorporeal urinary diversion: impact on an established enhanced recovery protocol. BJU International 2015;116(6):924‐31. [PUBMED: 25943158] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee R, Ng CK, Shariat SF, Borkina A, Guimento R, Brumit KF, et al. The economics of robotic cystectomy: cost comparison of open versus robotic cystectomy. BJU International 2011;108(11):1886‐92. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li AY, Filson CP, Hollingsworth JM, He C, Weizer AZ, Hollenbeck BK, et al. Patient‐reported convalescence and quality of life recovery: a comparison of open and robotic‐assisted radical cystectomy. Surgical Innovation 2016;23(6):598‐605. [PUBMED: 27354552] [DOI] [PubMed] [Google Scholar]
Martin 2011 {published data only}
- Martin AD, Nunez RN, Castle EP. Robot‐assisted radical cystectomy versus open radical cystectomy: a complete cost analysis. Urology 2011;77(3):621‐5. [DOI] [PubMed] [Google Scholar]
Matulewicz 2016 {published data only}
- Matulewicz RS, DeLancey JO, Manjunath A, Tse J, Kundu SD, Meeks JJ. National comparison of oncologic quality indicators between open and robotic‐assisted radical cystectomy. Urologic Oncology 2016;34(10):431.e9‐431.e15. [PUBMED: 27264169] [DOI] [PubMed] [Google Scholar]
Musch 2014 {published data only}
- Musch M, Janowski M, Steves A, Roggenbuck U, Boergers A, Davoudi Y, et al. Comparison of early postoperative morbidity after robot‐assisted and open radical cystectomy: results of a prospective observational study. BJU international 2014;113(3):458‐67. [DOI] [PubMed] [Google Scholar]
Nepple 2013 {published data only}
- Nepple KG, Strope SA, Grubb RL 3rd, Kibel AS. Early oncologic outcomes of robotic versus open radical cystectomy for urothelial cancer. Urologic Oncology 2013;31(6):894‐8. [PUBMED: 21803615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2010 {published data only}
- Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. A comparison of postoperative complications in open versus robotic cystectomy. European Urology 2010;57(2):274‐82. [DOI] [PubMed] [Google Scholar]
Nguyen 2015 {published data only}
- Nguyen DP, Al Hussein Al Awamlh B, Wu X, O'Malley P, Inoyatov IM, Ayangbesan A, et al. Recurrence patterns after open and robot‐assisted radical cystectomy for bladder cancer. European Urology 2015;68(3):399‐405. [PUBMED: 25709026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhee 2006 {published data only}
- Rhee JJ, Lebeau S, Smolkin M, Theodorescu D. Radical cystectomy with ileal conduit diversion: early prospective evaluation of the impact of robotic assistance. BJU International 2006;98(5):1059‐63. [DOI] [PubMed] [Google Scholar]
Satkunasivam 2016 {published data only}
- Satkunasivam R, Santomauro M, Chopra S, Plotner E, Cai J, Miranda G, et al. Robotic intracorporeal orthotopic neobladder: urodynamic outcomes, urinary function, and health‐related quality of life. European Urology 2016;69(2):247‐53. [PUBMED: 26164417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2017 {published data only}
- Sharma P, Zargar‐Shoshtari K, Poch MA, Pow‐Sang JM, Sexton WJ, Spiess PE, et al. Surgical control and margin status after robotic and open cystectomy in high‐risk cases: caution or equivalence?. World Journal of Urology 2017;35(4):657‐63. [PUBMED: 27495912] [DOI] [PubMed] [Google Scholar]
Styn 2012 {published data only}
- Styn NR, Montgomery JS, Wood DP, Hafez KS, Lee CT, Tallman C, et al. Matched comparison of robotic‐assisted and open radical cystectomy. Urology 2012;79(6):1303‐9. [DOI] [PubMed] [Google Scholar]
Tan 2016 {published data only}
- Tan WS, Sridhar A, Ellis G, Lamb B, Goldstraw M, Nathan S, et al. Analysis of open and intracorporeal robotic assisted radical cystectomy shows no significant difference in recurrence patterns and oncological outcomes. Urologic Oncology 2016;34(6):257.e1‐9. [PUBMED: 26968561] [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU International 2008;101(1):89‐93. [DOI] [PubMed] [Google Scholar]
Winters 2016 {published data only}
- Winters BR, Bremjit PJ, Gore JL, Lin DW, Ellis WJ, Dalkin BL, et al. Preliminary comparative effectiveness of robotic versus open radical cystectomy in elderly patients. Journal of Endourology 2016;30(2):212‐7. [PUBMED: 26414964] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Kelly , Catto 2017 {published data only}
- Kelly J, Catto J. Trial to compare robotically assisted radical cystectomy with open radical cystectomy (iROC). https://clinicaltrials.gov/ct2/show/NCT03049410 (accessed 7 June 2017).
Additional references
Aboumarzouk 2012
- Aboumarzouk OM, Drewa T, Olejniczak P, Chlosta PL. Laparoscopic radical cystectomy: a 5‐year review of a single institute’s operative data and complications and a systematic review of the literature. International Brazilian Journal of Urology 2012;38(3):330‐40. [DOI] [PubMed] [Google Scholar]
Aboumarzouk 2013
- Aboumarzouk OM, Hughes O, Narahari K, Drewa T, Chlosta PL, Kynaston H. Safety and feasibility of laparoscopic radical cystectomy for the treatment of bladder cancer. Journal of Endourology / Endourological Society 2013;27(9):1083‐95. [DOI] [PubMed] [Google Scholar]
Ballantyne 2003
- Ballantyne GH, Moll F. The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surgical Clinics of North America 2003;83(6):1293‐304, vii. [DOI] [PubMed] [Google Scholar]
Castillo 2006
- Castillo OA, Abreu SC, Mariano MB, Tefilli MV, Hoyos J, Pinto I, et al. Complications in laparoscopic radical cystectomy: the South American experience with 59 cases. International Brazilian Journal of Urology 2006;32(3):300‐5. [DOI] [PubMed] [Google Scholar]
Castillo 2009
- Castillo OA, Vitagliano G, Vidal‐Mora I. Laparoscopic radical cystectomy: the new gold standard for bladder carcinoma?. Archivos Espanoles de Urologia 2009;62(9):737‐44. [DOI] [PubMed] [Google Scholar]
Cathelineau 2005
- Cathelineau X, Arroyo C, Rozet F, Barret E, Vallancien G. Laparoscopic assisted radical cystectomy: the Montsouris experience after 84 cases. European Urology 2005;47(6):780‐4. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. globocan.iarc.fr (accessed 5 June 2015).
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Hamilton (ON): McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Haber 2008
- Haber GP, Crouzet S, Gill IS. Laparoscopic and robotic assisted radical cystectomy for bladder cancer: a critical analysis. European Urology 2008;54(1):54‐62. [DOI] [PubMed] [Google Scholar]
Hayn 2010
- Hayn MH, Hussain A, Mansour AM, Andrews PE, Carpentier P, Castle E, et al. The learning curve of robot‐assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European Urology 2010;58(2):197‐202. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hosseini 2011
- Hosseini A, Adding C, Nilsson A, Jonsson MN, Wiklund NP. Robotic cystectomy: surgical technique. BJU International 2011;108(6b):962‐8. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2009
- Hu JC, Gu X, Lipsitz SR, Barry MJ, D'Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive versus open radical prostatectomy. JAMA 2009;302(14):1557‐64. [PUBMED: 19826025] [DOI] [PubMed] [Google Scholar]
Huang 2008
- Huang J, Lin T, Xu K, Huang H, Jiang C, Han J, et al. Laparoscopic radical cystectomy with orthotopic ileal neobladder: a report of 85 cases. Journal of Endourology / Endourological Society 2008;22(5):939‐46. [DOI] [PubMed] [Google Scholar]
Huang 2010
- Huang J, Lin T, Liu H, Xu K, Zhang C, Jiang C, et al. Laparoscopic radical cystectomy with orthotopic ileal neobladder for bladder cancer: oncologic results of 171 cases with a median 3‐year follow‐up. European Urology 2010;58(3):442‐9. [DOI] [PubMed] [Google Scholar]
Ishii 2014
- Ishii H, Rai BP, Stolzenburg JU, Bose P, Chlosta PL, Somani BK, et al. Robotic or open radical cystectomy, which is safer? A systematic review and meta‐analysis of comparative studies. Journal of Endourology / Endourological Society 2014;28(10):1215‐23. [PUBMED: 25000311] [DOI] [PubMed] [Google Scholar]
Jonsson 2011
- Jonsson MN, Adding LC, Hosseini A, Schumacher MC, Volz D, Nilsson A, et al. Robot‐assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. European Urology 2011;60(5):1066‐73. [DOI] [PubMed] [Google Scholar]
Khan 2011
- Khan MS, Elhage O, Challacombe B, Rimington P, Murphy D, Dasgupta P. Analysis of early complications of robotic‐assisted radical cystectomy using a standardized reporting system. Urology 2011;77(2):357‐62. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2002
- Martin RC 2nd, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Annals of Surgery 2002;235(6):803‐13. [PUBMED: 12035036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Novara 2012
- Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, et al. Systematic review and meta‐analysis of perioperative outcomes and complications after robot‐assisted radical prostatectomy. European Urology 2012;62(3):431‐52. [DOI] [PubMed] [Google Scholar]
Novara 2015
- Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot‐assisted radical cystectomy. European Urology 2015;67(3):376‐401. [DOI] [PubMed] [Google Scholar]
Ploeg 2009
- Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World Journal of Urology 2009;27(3):289‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raza 2015
- Raza SJ, Wilson T, Peabody JO, Wiklund P, Scherr DS, Al‐Daghmin A, et al. Long‐term oncologic outcomes following robot‐assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European Urology 2015;68(4):721‐8. [PUBMED: 25985883] [DOI] [PubMed] [Google Scholar]
Redorta 2010
- Redorta JP, Gaya JM, Breda A, Gausa L, Rodríguez O, Villavicencio H. Robotic cystectomy versus open cystectomy: are we there yet?. European Urology Supplements 2010;9(3):433‐7. [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sathianathen 2018
- Sathianathen NJ, Kalapara A, Frydenberg M, Lawrentschuk N, Weight, C, Parek D, Konety BR. Robotic‐assisted radical cystectomy vs open radical cystectomy: systematic review and meta‐analysis. Journal of Urology 2018/10/16;201(4):715‐720. [PUBMED: 30321551] [DOI] [PubMed] [Google Scholar]
Schwenk 2005
- Schwenk W, Haase O, Neudecker J, Muller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database of Systematic Reviews 2005;3:CD003145. [PUBMED: 16034888] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shabsigh 2009
- Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. European urology 2009;55(1):164‐74. [PUBMED: 18675501] [DOI] [PubMed] [Google Scholar]
Sighinolfi 2007
- Sighinolfi MC, Micali S, Celia A, DeStefani S, Grande M, Rivalta M, et al. Laparoscopic radical cystectomy: an Italian survey. Surgical Endoscopy 2007;21(8):1308‐11. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith AB, Raynor MC, Pruthi RS. Peri‐ and postoperative outcomes of robot‐assisted radical cystectomy (RARC). BJU International 2011;108(6 Pt 2):969‐75. [DOI] [PubMed] [Google Scholar]
Tang 2014
- Tang K, Xia D, Li H, Guan W, Guo X, Hu Z, et al. Robotic vs. open radical cystectomy in bladder cancer: a systematic review and meta‐analysis. European Journal of Surgical Oncology 2014;40(11):1399‐411. [PUBMED: 24767803] [DOI] [PubMed] [Google Scholar]
Witjes 2014
- Witjes JA, Comperat E, Cowan NC, Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle‐invasive and metastatic bladder cancer: summary of the 2013 guidelines. European Urology 2014;65(4):778‐92. [PUBMED: 24373477] [DOI] [PubMed] [Google Scholar]
Wright 2013
- Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted versus laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 2013;309(7):689‐98. [PUBMED: 23423414] [DOI] [PubMed] [Google Scholar]
Yuh 2015
- Yuh B, Wilson T, Bochner B, Chan K, Palou J, Stenzl A, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot‐assisted radical cystectomy. European Urology 2015;67(3):402‐22. [PUBMED: 25560797] [DOI] [PubMed] [Google Scholar]


