Abstract
Novel silver nanoparticles from Gleichenia Pectinata (Willd.) C. Presl. was synthesized. A combination of spectroscopic and microscopic techniques were utilized to characterize the newly synthesized Gleichenia Pectinata Silver Nanoparticles (GPAgNPs) vis-à-vis UV-Vis Spectroscopy, Fourier Transform Infra-Red (FTIR) Spectroscopy, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive X-ray Spectroscopy (EDX) and X-Ray Diffraction (XRD) Analyses. Significant absorption was observed at 460 nm resulting from the surface Plasmon resonance (SPR). A rapid rate of synthesis was observed and the best surface plasmon resonance was obtained at 105 minutes contact time. SEM and TEM showed an spherical shape of GPAgNPs with an average size of 7.51 nm. The XRD result revealed a crystalline and polydispersed GPAgNPs. GPAgNPs were effective against four antibiotic resistant pathogens and they exhibited excellent antimicrobial activity against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Candida albicans. GPAgNPs favorably competed with standard antibiotics. This therefore enlisted GPAgNPs as potential antimicrobial and therapeutic agents against multidrug resistant micro-organisms (MDRM).
Keywords: Materials chemistry, Nanotechnology
1. Introduction
Nanotechnology has emerged as an intriguing area of study due to its vast application in diverse fields such as adsorption, optical sensor, catalysis, water treatment, drug delivery and nanomedicine (Sadeghi and Gholamhoseinpoor, 2015). Nanoparticles are organic or inorganic materials that can be synthesized through various physical, chemical and biological methods, and they possess particle sizes in the range of 1–100 nm with large surface area to volume ratio which makes them extraordinarily significant (Seil and Webster, 2012; Iravani et al., 2014).
It is worthy of note that both physical and biological methods which involve the use of expensive, toxic and non-biodegradable materials which pose a great risk to humans, animals and the environment (Bagheri and Banihashemi, 2015). However, those which involve the use of plant extracts (green synthesis) are eco-friendly, cost effective, sustainable, easy to synthesize. The form products formed are stable, well dispersed with limited aggregation and afford better size control with no sludge generation. Recently, various plant parts such as leaves, stem bark, roots as well as their extracts are now being used to synthesize nanoparticles because they are greener and can also serve as stabilizing and reducing agents for the synthesis (Dada et al., 2018a,b; Song and Kim, 2009).
Antimicrobial resistance is a global threat to drug development due to the ability of microorganisms to adapt to the drugs designed to kill them, rendering them less effective. There is therefore a need for continuous discovery of potential antimicrobial agents that have the potent enough to end the menace of bacterial resistance (WHO, 2015). Green synthesis using plant extracts has proven to be an efficient method for the preparation of silver nanoparticles that could be useful to suppress the challenges surrounding antimicrobial resistance (Haase et al., 2015).
Silver nanoparticles are among the most commercialized nanoparticles worldwide which have found useful application in wound dressing, coating of working surfaces or surgical instruments and prostheses, textile and cosmetics (Haase et al., 2015). Silver nanoparticles synthesized using green techniques have also been reported to exhibit different biological activities such as anti-diabetic, anti-inflammatory, anticancer, antifungal, antiplasmodial and antimicrobial activity against various bacterial strains because of the secondary metabolites present in them (Maiti et al., 2014; Haase et al., 2015; Kuppusamy et al., 2016; Femi-Adepoju et al., 2018).
Previous studies have focused on the great activity of silver nanoparticles against a wide spectrum of Gram-positive bacteria, Gram-negative bacteria and fungi using higher plants as biological sources (Paulkumar et al., 2013; Kang et al., 2014). The following are some of the extracts previously explored for the green synthesis of silver nanoparticles: Clerodendrum inerme (Arshad et al., 2010), Cardiospermum helicacabum (Martinez-Castanon et al., 2008), Acanthephylum bracteatum (Mehrdad and Khalil, 2010), Thevetia peruviana (Oluwaniyi et al., 2016) and Elettaria cardamomom (GnanaDhas et al., 2012). Unfortunately, the potentials of lower terrestrial plants to synthesize silver nanoparticles which could in turn serve as new biological sources for the process has not been explored except for the recent work of Femi-Adepoju et al. (2018) using the extract of Phymatode scolopendria (a fern).
Consequently, in this study, the use of a neglected plant known as Gleichenia pectinata was employed for the green synthesis of silver nanoparticles. The nanoparticles were in turn tested for their antimicrobial potency on the growth of some antibiotic resistant gram negative microorganisms. The microorganisms involved in this study were: Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Candida albicans. The synthesized GPAgNPs were tested against three Gram negative bacteria and one fungus. These Gram-negative microorganisms and the fungus are classified as multi-drug resistant (MDR), deadly pathogens which are highly infectious, creating prevailing diseases. Diseases commonly caused by the invasion of these MDR organisms range from pneumonia, urinary tract catherization, hemorrhage and necrosis, cystic fibrosis, hemolytic-uremic syndrome, candidiasis, and induction of soft rot in plants (Pappas et al., 2016; Kirienko et al., 2015; Tenaillon et al., 2010; Itah and Essien, 2005).
Moreover, the Gleichenia pectinata employed for the synthesis of GPAgNPs in this study is a terrestrial fern with creeping rhizomes, scabrous, light brown and deciduous articulate hairs (Acevedo-Rodríguez, 2005). In Nigeria, it is not widely distributed, but rather abundant only in the Western part of Nigeria. To the best of our knowledge, there is no report on the synthesis of silver nanoparticles using the extract of Gleichenia pectinata. Therefore, in this study, novel silver nanoparticles synthesized from Gleichenia pectinata extracts were characterized by spectroscopic and microscopic techniques such as UV-Vis Spectroscopy, FTIR, SEM/EDX, TEM and XRD. The surface plasmon resonance of the synthesized nanoparticles on the rate of formation was determined. The GPAgNPs were also tested against four antibiotic resistant pathogenic microorganisms to determine the therapeutic potentials of GPAgNPs against multi-drug resistant microorganisms.
2. Materials and methods
2.1. Plant material and preparation of the extract
The Gleichenia pectinata (Willd.) C. Presl. plant sample (Fig. 1) was collected from Ifetedo (7o, 10.848′ N 4o 41.857′ E; 933ft), a small town in Osun State, Southwestern Nigeria. The plant was authenticated at University of Ilorin Herbarium and voucher number UILH/001/1263 was assigned to it. The plant material was subjected to air drying at room temperature to avoid the denaturing of some active principles. After drying, it was grinded to increase the surface area. The powdered Gleichenia pectinata (Gp) plant sample (Fig. 1) was thereafter soaked in methanol at plant material to solvent ratio of 1:10 w/v for 72 h at room temperature with frequent manual agitation. The filtrate of the extract was dried in a laboratory oven at 40 °C. The dry extract was re-suspended in distilled water to prepare desired concentrations in mg mL−1for further experiments.
Fig. 1.
A typical Terrestrial fern: Gleichenia pectinata (G.p).
2.2. Green synthesis of Gleichenia Pectinata Silver Nanoparticles (GPAgNPs)
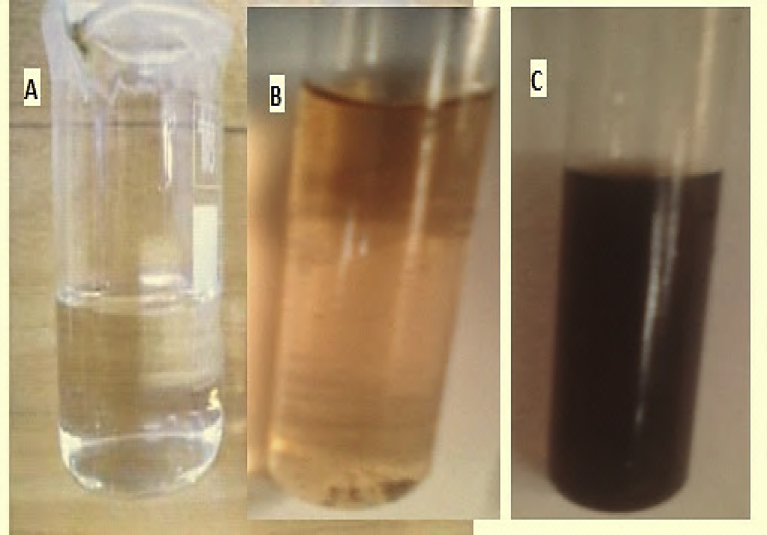
The green synthesis of silver nanoparticles using the extract of Gp plant (Fig. 1) was carried out by a bottom up approach and reduction technique in a single pot system improving the procedure by Dada et al. (2018b). The synthesis was investigated at initial silver ion concentration of 5 mM. In a typical experiment, 50 mL of 5 mM silver nitrate (Fig. 2A) was reacted with 10 mL of GP extract (Fig. 2B). Thereafter, a rapid appearance of reddish brown coloration (Fig. 2C) which indicates the formation of Ag nanoparticles was observed within a contact time of 30 mins. The formation of Gleichenia pectinata silver nanoparticles (GPAgNPs) was monitored with a Double beam Biochrom Libra PCB 1500 UV-Vis spectrophotometer. An aliquot was withdrawn at a 15 mins interval for 2 h.
Fig. 2.
(A) Ag+ solution (B) GP aqueous leaf extract (C) GPAgNPs.
2.3. Surface plasmon resonance on the rate of biosynthesis of GPAgNPs
The rate of biosynthesis and the quantitative formation of Gleichenia pectinata silver nanoparticles (GPAgNPs) were studied by monitoring the contact time interval during the GPAgNPs formation. This was carried out using a plant extract with 5 mM concentration of AgNO3. The GPAgNPs formation was monitored using the Double beam Biochrom Libra PCB 1500 UV-Vis spectrophotometer at different time intervals of 15 mins for 2 h. The progress of the AgNO3 solution bio-reduction by the GP extract and formation of GPAgNPs were studied at a resolution of 1 nm from 340 to 650 nm. The synthesized GPAgNPs were purified through centrifugation at 4000 rpm for 15 mins and the dispersion of pellet in Milli-Q water was done purposely to remove unwanted materials. The GPAgNPs were dried in the oven at 80 °C to remove the moisture content before further characterization (Gozdziewska et al., 2015; Oluwaniyi et al., 2016).
2.4. Characterization of GPAgNPs
The FTIR study of the GPAgNPs palletized with KBr was carried out by means of a Shimadzu 8400S (Japan) in the spectral region of 4000–400 cm−1 using a resolution of 4 cm−1 to determine the functional groups of the available biomolecules in the plant extract. The morphology, sizes and atomic weight composition of the obtained GPAgNPs were examined using Scanning Electron Microscope (SEM) integrated with Energy Dispersive X-ray (EDX) analyser. A TESCAN Vega TS 5136LM typically at 20 kV at a working distance of 20 mm, and a Tecnai F20 Transmission Electron Microscope at 80kV voltage were used. X-ray powder diffraction (XRD) patterns were obtained with Cu-Kα radiation using a Shimadzu XRD model 6000 diffractometer (Japan) equipped with a graphite monochromator. XRD measurements on a film of biologically synthesized GPAgNPs were performed using a step-scanning program with 0.02◦ per step and acquisition time of 5 seconds per step at 2-theta. The XRD data were analyzed using JCPDS -International Center for Diffraction Data for the identification of the crystalline phases.
2.5. Antimicrobial assay
The clinical strains of the microorganisms were obtained from the University of Ilorin Teaching Hospital (UITH), Ilorin, Kwara State, Nigeria. These were re-cultured to prepare the pure culture of the microbial strains. The antimicrobial activity of the synthesized GPAgNPs was studied as follows: 20 mL of prepared agar solution was poured into each of the Petri-dish aseptically. Mueller-Hinton agar was used for both bacterial and fungal strains due to its universality for the growth of both. A loop was used to spread each of the microorganism strain on the entire surface of separate Petri-dishes containing the solidified agars in a sequential order for each of the microorganism and considering the molar concentration of the AgNO3 solution to be introduced. The method used for the determination of the antimicrobial activity was the disc diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS, 2002). Special precaution was taken to avoid mix-up and the experiment was performed under strict aseptic conditions. The plates were inoculated with the different microorganism strains accordingly. 6 mm discs were incubated with 50 μL each of the nanoparticle solutions and mounted unto the solidified agar surface. Already prepared discs of streptomycin and nystatin were used as positive control standards for bacterial and fungal strains respectively while AgNO3 solutions and the extract were used as negative control standards. The plates were incubated appropriately and zones of inhibition were recorded.
3. Results and discussion
3.1. Surface plasmon resonance and UV-Vis spectra analysis
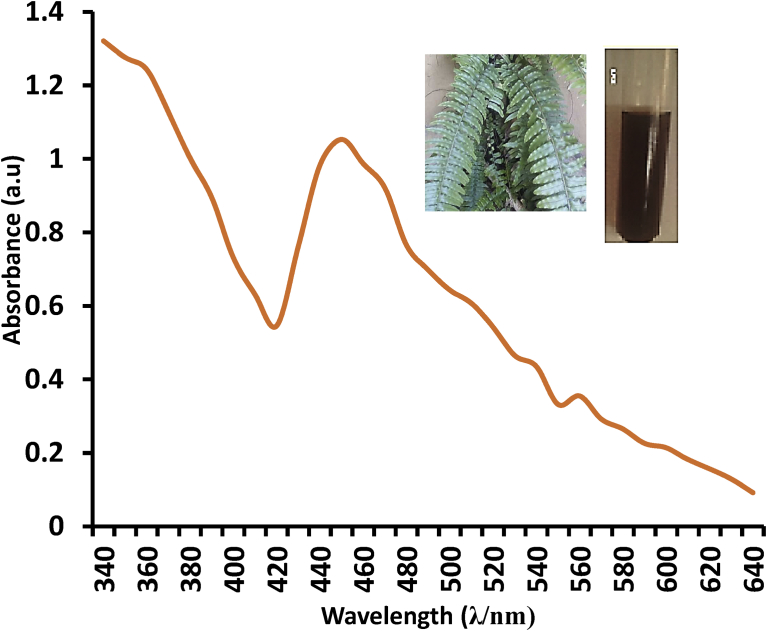
A very important characterization procedure in nanoparticle biosynthesis is UV-Vis spectroscopy. Fig. 3 shows the Surface Plasmon Resonance (SPR) of Gleichenia pectinata silver nanoparticles (GPAgNPs) obtained from the UV-visible spectrum. It was evident from the results obtained that the extract of G. pectinata reduced Ag+ which afforded the formation of GPAgNPs. The Gaussian-shape of the SPR peak at 460 nm suggests the presence of uniform spheres of the individual silver nanoparticles. This was confirmed by a color change in the reaction mixture to reddish brown (Fig. 2 A – C) due to the surface plasmon resonance (SPR). The SPR is affected by three factors namely; the particle size, dielectric medium and chemical neighborhood surroundings (Wani et al., 2010; Brajesh et al., 2014; Dada et al., 2017a). The formation of silver nanoparticles was monitored by using the UV-Vis Spectrophotometer at a wavelength range of 340–650 nm. A single, strong and broad SPR peak in the UV-Vis spectrum was observed at 460 nm (Fig. 3) which is an indication of the polydispersity nature of GPAgNPs (Wang et al., 2007; Vinod et al., 2011; Dada et al., 2018a,b). As observed in GPAgNPs, previous studies too have confirmed that SPR peak located within 410–460 nm region is attributed to silver nanoparticles (Megiel, 2017).
Fig. 3.
UV-visible spectrum of GPAgNPs.
3.2. Rate of synthesis of GPAgNPs
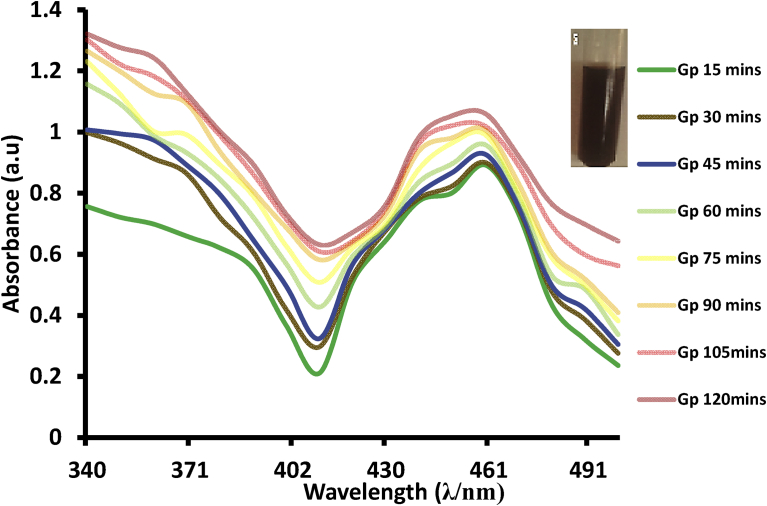
The kinetics of GPAgNPs formation was studied by investigating the rate of GPAgNPs synthesis at 5 mM concentration and contact time intervals of 15 mins for 2 h (Fig. 4). It was discovered that the colour of the solution changed from light yellow to reddish brown within 30 mins. This is a confirmation that nanoparticles have been formed since there is usually the excitation of surface plasmon vibrations which results into the colour change as observed (Donda et al., 2013). The intensity of the absorbance increased with an increase in the reaction time, while the maximum absorbance was recorded at 105 mins. The maximum absorbance intensity of the GPAgNPs indicated that it takes only 105 mins for the completion of bioreduction process initiated by biomolecules present in GP extract serving as capping, stabilizing and reducing agents (Kumar et al., 2017).
Fig. 4.
Surface Plasmon Resonance of formation of GPAgNPs monitored at different contact time.
3.3. FTIR analysis
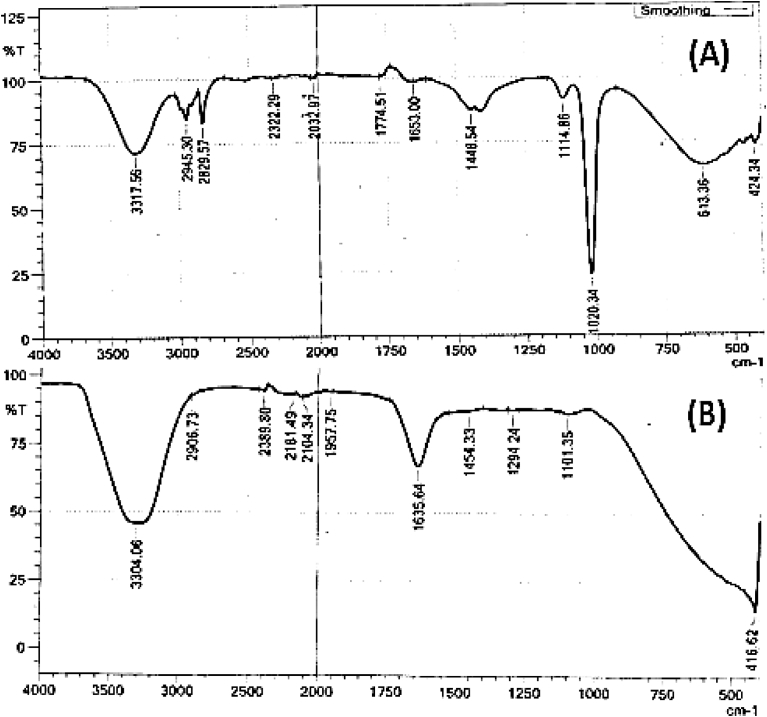
FTIR has become an important tool in identifying the functional groups present in the interaction between metal particles and biomolecules which are responsible for the bioreduction of Ag+ and also functioning as capping and stabilizing agents (Dada et al., 2017a; Dada et al., 2016). Fig. 5(A) shows the FTIR spectrum of G. pectinata extract before the synthesis of AgNPs while Fig. 5(B) shows the FTIR Spectrum of GPAgNPs after synthesis. The relevant peaks observed from the extract of G. pectinata (Fig. 5A) are 3317 cm−1 corresponding to O-H stretching vibration which indicates the presence of alcohol (Dada et al., 2017b), 2945 cm−1 C-H stretching of aromatic compound, 1797 cm−1 which attributes to C=O stretching for carbonyl compound, 1658 cm−1 corresponding to C-C and C-N stretching and 1448 cm−1 corresponding to N-H stretch vibration present in amide links serving as the stabilizing and capping agents as reported in many studies (Jyoti et al., 2016). Whereas, the intense absorption peak at 1020 cm−1 is attributed to C–O–C and secondary –OH groups and 613 cm−1 is assigned to C≡C bending of alkyne (Kumar et al., 2017; Dada et al., 2016). However, after the synthesis of GPAgNPs, the disappearance of some vibration bands such as those at 2945, 2829, 1448 cm−1 and the intense peak at 1020 cm−1 (Fig. 5B) is a confirmation that the different functional groups have participated in the bioreduction mechanism leading to the biosynthesis of GPAgNPs. The analysis of the FTIR result indicates that silver nanoparticles (AgNPs) were surrounded by terpenoid, alcohol, and carbonyl group serving as strong binding sites for AgNPs. This finding is supported by Tran et al. (2013) and Dada et al. (2018a,b).
Fig. 5.
(A): FTIR spectrum of G. pectinata extract (B) FTIR Spectrum of GPAgNPs.
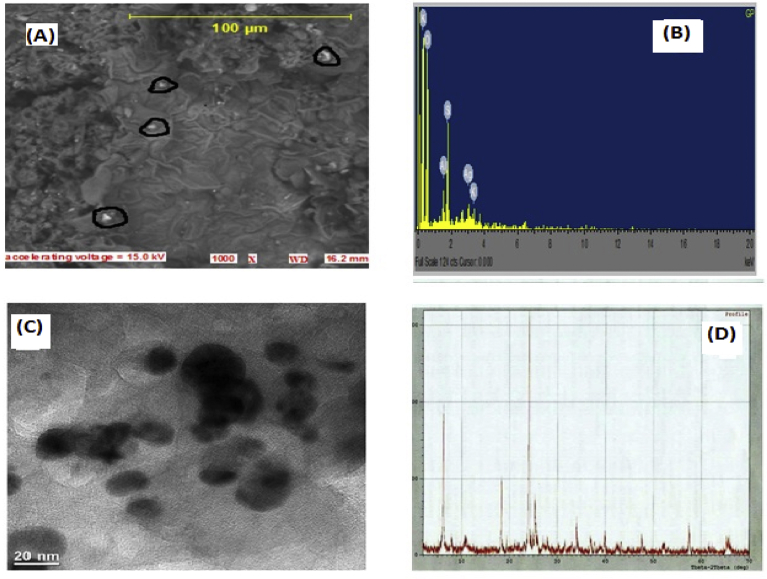
3.4. SEM/EDX and TEM analyses
The surface morphology and elemental composition were determined using SEM/EDX (Dada et al., 2017c; Dada et al., 2016). Fig. 6A shows the morphology of Gleichenia pectinata silver nanoparticles (GPAgNPs) synthesized as aggregates of spherical shape. The elemental constituents and relative abundance of the biosynthesized GPAgNPs were obtained from Energy Dispersive X-ray (EDX) as presented in Fig. 6B. The EDX spectrum (Fig. 6B) reveals the purity and the complete chemical composition of GPAgNPs. The percentage of Ag metal found in occurrence with other chemical elements was found to be appreciable. The reduced silver nanoparticles were subjected to EDX analysis with an optical absorption characteristic peak at 3 keV. The EDX analysis showed percentage relative composition of elements such as Oxygen (O) 68%, Silicon (Si) 12%, Aluminium (Al) 5%, Potassium (K) 4% and Silver (Ag) 16%. The other elements served as capping organic agents bound to the surface of the silver nanoparticles (Dada et al., 2017d). The TEM Micrograph revealed the size, shape and general morphology of the nanoparticles (Dada et al., 2018a). The TEM image (Fig. 6C) further confirms the formation of spherical and polydispersed GPAgNPs. Specifically, GPAgNPs have an average mean size of 7.51 ± 2.88 nm. The small average size of the GPAgNPs has a role to play in its antimicrobial activity. Thus, the smaller the nanoparticles, the greater the antimicrobial activity (Nayak et al., 2016).
Fig. 6.
(A) SEM Micrograph (B) EDX spectrum (C) TEM image (D) XRD pattern for GPAgNPs.
3.5. XRD analysis
In this study also, the XRD patterns of GPAgNPs (Fig. 6D) revealed sharp and clear peaks of Ag nanoparticles indicating the crystalline nature of the biosynthesized silver nanoparticles as confirmed by the corresponding peaks with respect to Bragg's model of diffraction given by the Joint Committee on Powder Diffraction Standards (JCPDS). The obtained diffraction silver nano peaks at 31.65o, 38.28o, 45.01o and 65.28o are respectively assigned to (101), (111), (200) and (220) planes. This corresponds to the JCPDS- International Center for Diffraction Data No. 65-2871. The sharpening of the peaks clearly indicates that the particles are in the regime of nanoparticles (Bar et al., 2009; Ashok et al., 2010). The intensity of silver nanoparticles is an indication of high degree of crystallinity as corroborated by findings from other researchers in literature (Dada et al., 2018a, 2018b; Jassal et al., 2016; Wani et al., 2011).
3.6. Antimicrobial studies
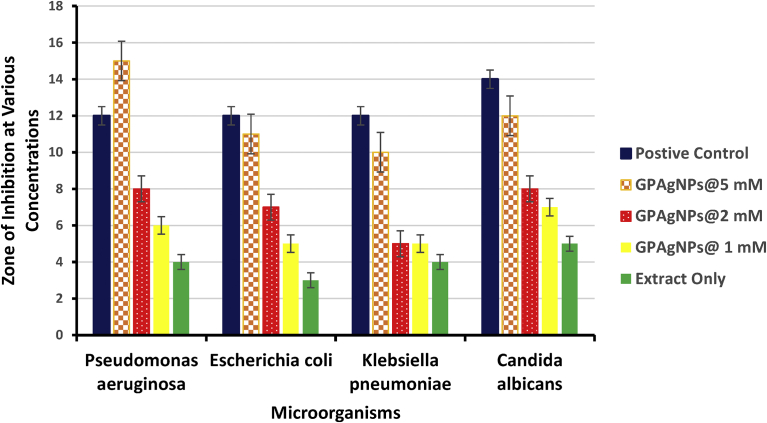
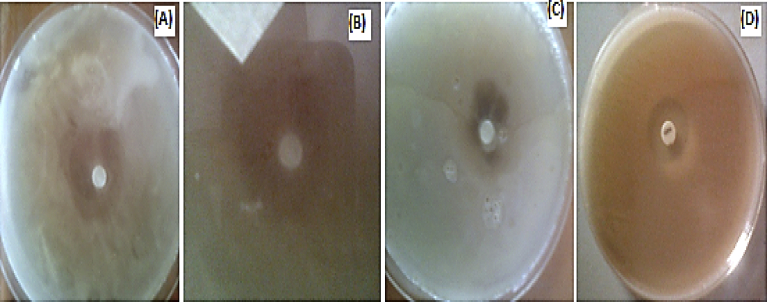
Fig. 7 and Table 1 show the antimicrobial activity of the Gleichenia pectinata silver nanoparticles (GPAgNPs) against different pathogenic microorganisms at various investigated concentrations. The zones of inhibition at various concentrations of GPAgNPs in comparison with both positive and negative controls were presented. Streptomycin and nystatin were used as positive control drugs against bacteria and fungi respectively while the negative control was sterile water. Both the extract and sterile water did not show significant inhibition. Fig. 8 shows the various plates indicating the antimicrobial activities of biosynthesized GPAgNPs against: (A) C. albicans (B) P. aeruginosa (C) K. pneumonia (D) E. coli. The results in Fig. 7 and Table 1 shows that GPAgNPs at Minimum Inhibitory Concentration (MIC) of 5 mM competed significantly with streptomycin used as positive control against the activity of P. aeruginosa, E. coli, K. pneumonia and nystatin against the activity of C. albicans. At 1 mM and 2 mM, the GPAgNPs showed inhibitory activities against the life cycle of all the microbes. This study is in agreement with reports given in literature (Prasad and Elumalai, 2011). The antimicrobial potency of GPAgNPs is size and dosage dependent. This study shows that the synthesized GPAgNPs exhibited good antimicrobial activity against multi-drug resistant microbial pathogens: Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Candida albicans. The activity of the GPAgNPs at the highest investigated concentration was found to be significantly the same with that of the tested antibiotic drugs against P. aeruginosa, E. coli and C. albicans (Sathishkumar et al., 2009; Singhal et al., 2011; Wani et al., 2013). The result shows that GPAgNPs can favorably compete with the already established drugs in combating both the bacterial and fungal infections of the investigated pathogenic microorganisms.
Fig. 7.
Zone of Inhibition at Various GPAgNPs Concentrations against different Pathogenic Microorganisms.
Table 1.
The zone of inhibition of GPAgNPs against some multi-drug resistant microorganisms at various concentrations.
| Microorganisms | Positive Control | Zone of Inhibition measured in mm |
Extra Only | ||
|---|---|---|---|---|---|
| GPAgNPs (5 mM) | GPAgNPs (2 mM) | GPAgNPs (1 mM) | |||
| Pseudomonas aeruginosa | 12 | 15 | 8 | 6 | 4 |
| Escherichia coli | 12 | 11 | 7 | 5 | 3 |
| Klebsiella pneumoniae | 12 | 10 | 4 | 5 | 4 |
| Candida albicans | 14 | 13 | 8 | 7 | 5 |
Positive Control = Streptomycin/nystatin.
Fig. 8.
Plates showing the antimicrobial activities of biosynthesized GPAgNPs against: (A) C. albicans (B) P. aeruginosa (C) K. pneumonia (D) E. coli.
4. Conclusion
GPAgNPs were successfully synthesized using extract of medicinal Gleichenia pectinata (Willd.) C. Presl. plant. This low cost and environmentally benign extract of G. pectinata served as a potential stabilizing and reducing agent. The formation of GPAgNPs was first confirmed by a reddish brown color change due to SPR peak in the UV-Vis spectrum observed at 460 nm. The resulting GPAgNPs was characterized by FTIR, SEM, EDX, TEM, and XRD. FTIR spectroscopy confirmed the presence of functional biomolecules. The SEM revealed spherical shape morphology corroborated by the TEM result. A mean size of 7.51 ± 2.88 nm of GPAgNPS was estimated from TEM. The elemental composition of GPAgNPs with an intense signal of Ag at 3.0 keV was confirmed by EDX analysis while the crystallinity nature of GPAgNPs was determined by XRD. GPAgNPs showed an effective antimicrobial activity against four opportunist and multidrug resistant pathogens. GPAgNPs antimicrobial potency favourably competed with some standard antibiotic drugs. This study therefore confirmed that the synthesized silver nanoparticles (GPAgNPs) are good antimicrobial agents. GPAgNP is therefore recommended as product of value in the field of nanobiotechnology and nanomedicine.
Declarations
Author contribution statement
Abioloa G. Femi-Adepoju: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Adewumi O. Dada: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Opeyemi K. Otun: Performed the experiments; Analyzed and interpreted the data.
Adeyinka O. Adepoju: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ojo P. Fatoba: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This work was supported by Tertiary Education Trust Fund, Abuja, Nigeria.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Acevedo-Rodríguez P. Vol. 51. Smithsonian Institution; Washington, D.C: 2005. Vines and Climbing Plants of Puerto Rico and the Virgin Islands; pp. 1–483. [Google Scholar]
- Arshad F., Prakash S.C., Praveen K., Jameel S. Extraction of silver nanoparticles from the leaf extracts of Clerodendruminerme. Digest J. Nanomater. Biostruct. 2010;5(1):43–49. (2010) [Google Scholar]
- Ashok B., Bhagyashree J., Ameeta R.K., Smita Z. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf. B. 2010;80:45–50. doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Bagheri H., Banihashemi S. Sol-gel-based silver nanoparticles-doped silica–polydiphenylaminenanocomposite for micro-solid-phase extraction. Anal. Chim. Acta. 2015;886:56–65. doi: 10.1016/j.aca.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Bar H., Bhui D.K., Sahoo G.P., Sarkar P., Pyne S., Misra A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloid. Surf. Physicochem. Eng. Asp. 2009;348:212–216. [Google Scholar]
- Brajesh K., Kumari S., Luis C., Alexis D., Ravinandan N.P. Sonochemical synthesis of silver nanoparticles using starch: a comparison. Bioinorgan. Chem. Appl. 2014;2014:784268. doi: 10.1155/2014/784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada A.O., Adekola F.A., Adeyemi O.S., Bello M.O., Adetunji C.O., Awakan O.J., Femi-Adepoju G.A. Silver Nanoparticles - Fabrication, Characterization and Applications. 2018. Characterization and effect of operational factors imperative to the synthesis of silver nanoparticles. [Google Scholar]
- Dada A.O., Inyinbor A.A., Idu I.E., Bello O.M., Oluyori A.P., Adelani-Akande T.A., Okunola A.A., Dada O. Effect of operational parameters, characterization and antibacterial studies of green synthesis of Silver Nanoparticles, using Tithonia diversifolia. PeerJ. 2018;6:e5865. doi: 10.7717/peerj.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada A.O., Ojediran O.J., Dada F.E., Olalekan A.P., Awakan O.J. Green synthesis and characterization of silver nanoparticles using Calotropis Procera extract. J. Appl. Chem. Sci. Int. 2017;8(4):137–143. [Google Scholar]
- Dada A.O., Adekola F.A., Odebunmi E.O. Liquid phase scavenging of Cd(II) and Cu(II) ions onto novel nanoscale zerovalent manganese (nZVMn): equilibrium, kinetic and thermodynamic studies. Environ. Nanotechnol. Monit. Manag. 2017;8:63–72. [Google Scholar]
- Dada A.O., Adekola F.A., Odebunmi E.O. Kinetics, mechanism, isotherm and thermodynamic studies of liquid phase adsorption of Pb2+ onto wood activated carbon supported zerovalent iron (WAC-ZVI) nanocomposite. Cogent Chem. 2017;3(1):1–20. [Google Scholar]
- Dada A.O., Adekola F.A., Odebunmi E.O. A novel zerovalent manganese for removal of copper ions: synthesis, characterization and adsorption studies. Appl. Water Sci. 2017;7(3):1409–1427. [Google Scholar]
- Dada A.O., Adekola F.A., Odebunmi E.O. Kinetics and equilibrium models for sorption of Cu(II) onto a novel manganese nano-adsorbent. J. Dispersion Sci. Technol. 2016;37(1):119–133. [Google Scholar]
- Donda M.R., Karunakar R.K., Jahnavi A., Anila M., Sreedhar B., Pratap R.P. Synthesis of silver nanoparticles using extracts of Securinegaleucopyrusand evaluation of its antibacterial activity. Int. J. Curr. Sci. 2013;7:1–8. [Google Scholar]
- Femi-Adepoju A.G., Adepoju A.O., Fatoba P.O., Olayemi V.T. Biosynthesis, characterization and antimicrobial potency of silver nanoparticles fabricated from Phymatode scolopendria (Burm. F.) Ching. Int. J. Curr. Res. 2018;10(12):76229–76233. [Google Scholar]
- GnanaDhas G., Gurusamy A., Chellapandian K. Green synthesis of silver nanoparticles using Elettaria cardamomomand assessment of its antimicrobial activity. Int. J. Pharm. Sci. Res. 2012;3(3):323–330. [Google Scholar]
- Gozdziewska M., Cichowicz G., Markowska K., Zawadac K., Megiel E. Nitroxide-coated silver nanoparticles: synthesis, surface physicochemistry and antibacterial activity. RSC Adv. 2015;5(72):58403–58415. [Google Scholar]
- Haase A., Mantion A., Graf P., Plendi J., Thuenemann A., Meier W., Taubert A., Luch A. A novel type of silver nanoparticles and their advantages in toxicity testing in cell culture systems. Arch. Toxicol. 2015;86:1089–1098. doi: 10.1007/s00204-012-0836-0. [DOI] [PubMed] [Google Scholar]
- Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- Itah A., Essien J. Growth Profile and hydrocarbonoclastic potential of microorganisms isolated from tarballs in the bight of bonny, Nigeria. World J. Microbiol. Biotechnol. 2005;21(6–7):1317–1322. [Google Scholar]
- Jassal V., Shanker U., Gahlot S., Kaith B.S., uddin Kamal, Iqubal M.A., Samuel P. Sapindus mukorossi mediated green synthesis of some manganese oxide nanoparticles interaction with aromatic amines. Appl. Phys. A. 2016;122:271. [Google Scholar]
- Jyoti M., Baunthiyal M., Singh A. Characterization of silver nanoparticles synthesized using Urticadioica Linn. leaves and their synergistic effects with antibiotics. J. Radiation Res. and Appl. Sci. 2016;9:217–227. [Google Scholar]
- Kang Y.O., Lee T.S., Park W.H. Green synthesis and antimicrobial activity of silver chloride nanoparticles stabilized with chitosan oligomer. J. Mater. Sci. Mater. Med. 2014;25:2629–2638. doi: 10.1007/s10856-014-5294-1. [DOI] [PubMed] [Google Scholar]
- Kirienko N.V., Ausubel F.M., Ruvkun G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 2015;112(6):1821–1826. doi: 10.1073/pnas.1424954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Smita K., Cumbal L., Debut A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017;24:45–50. doi: 10.1016/j.sjbs.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy P., Yusoff M.M., Maniam G.P., Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – an updated report. Saudi Pharmaceut. J. 2016;24:473–484. doi: 10.1016/j.jsps.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S., Krishnan D., Barman G., Ghosh K.S., Jayasree Konar Laha J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J. Anal. Sci. Technol. 2014;5:40. 2014. [Google Scholar]
- Martinez-Castanon G.A., Nino-Martinez N., Martines-Gutierrez F., Martinez-Mendoza J.R., Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008;10(8):1343–1348. [Google Scholar]
- Megiel E. Surface modification using TEMPO and its derivatives. Adv. Colloid Interface Sci. 2017;250:158–184. doi: 10.1016/j.cis.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Mehrdad F., Khalil F. Biological and green synthesis of silver nanoparticles. Turk. J. Eng. Environ. Sci. 2010;34:281–287. [Google Scholar]
- Nayak D., Ashe S., Rauta P.R., Kumari M., Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:44–52. doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards) 2002. Approved Standard. NCCLS M38-A. Villanova, PA, USA. [Google Scholar]
- Oluwaniyi O.O., Adegoke H.I., Adesuji E.T., Aderemi B.A., Sunday O.B., Ayomide H.L., Charles O.O. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruvianaJuss and its antimicrobial activities. Appl. Nanosci. 2016;6:903–912. [Google Scholar]
- Pappas P.G., Kauffman C.A., Andes D.R. Clinical Practice guideline for the management of candidiasis: 2016. Update by the infectious diseases society of America. Clin. Infect. Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulkumar K., Rajeshkumar S., Gnanajobitha G., Vanaja M., Malarkodi C., Annadurai G. Eco-friendlysynthesis of silver chloride nanoparticles using Klebsiella planticola (MTCC 2277) Int. J. Green Chem. Bioprocess. 2013;3:12–16. [Google Scholar]
- Prasad T.N., Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011;2011:439–442. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi B., Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphoratenuior (Zt) extract at room temperature Spectrochimica. Acta Part A. Mol. Biomol. Spectrosc. 2015;134 doi: 10.1016/j.saa.2014.06.046. [DOI] [PubMed] [Google Scholar]
- Sathishkumar M., Sneha K., Won S.W., Cho C.W., Kim S., Yun Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surfaces B Biointerfaces. 2009;73:332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomed. 2012;7:2767. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G., Bhavesh R., Kasariya K., Sharma A.R., Singh R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart Res. 2011;13:2981–2988. [Google Scholar]
- Song Y.J., Kim B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioproc. Biosyst. Eng. 2009;32(1):79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- Tenaillon O., Skumik D., Picard B., Denamur E. The population genetics of commensal Escherichia Coli. Nat. Rev. Microbiol. 2010;8(3):207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- Tran T.T.T., Vu T.T.H., Nguyen T.H. Biosynthesis of silver nanoparticles using Tithonia diversifolia leaf extract and their antimicrobial activity. Mater. Lett. 2013;105:220–222. [Google Scholar]
- Vinod V.T.P., Saravanan P., Sreedhar B., Keerthi D.D., Sashidhar R.B. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermumgossypium) Colloids Surfaces B Biointerfaces. 2011;83:291–298. doi: 10.1016/j.colsurfb.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Wang G., Shi C., Zhao N., Du X. Synthesis and characterization of nanoparticles assembled in ordered array pores of porous anodic alumina by chemical deposition. Mater. Lett. 2007;61:3795–3797. [Google Scholar]
- Wani I.A., Khatoon S., Ganguly A., Ahmed J., Ahmad T. Structural characterization and antimicrobialproperties ofsilvernanoparticlespreparedbyinversemicroemulsion method. Colloids Surfaces B Biointerfaces. 2013:243–250. doi: 10.1016/j.colsurfb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Wani I.A., Ganguly A., Ahmed J., Ahmad T. Silver nanoparticles: ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater. Lett. 2011;65:520–522. [Google Scholar]
- Wani A.I., Khatoon S., Ganguly A., Ahmed J., Ganguli A.K., Ahmad T. Silver nanoparticles: large scale solvothermal synthesis and optical properties. Mater. Res. Bull. 2010;45:1033–1038. [Google Scholar]
- WHO . 2015. Antimicrobial Resistance; p. 7. Fact sheet N°194. [Google Scholar]