Graphical abstract
Keywords: Patent ductus arteriosus, Full-term infant, Indomethacin, Prostaglandin
Highlights
-
•
Full-term infants have mature DA closure mechanisms.
-
•
Premature infants show delayed lung metabolism of prostaglandin.
-
•
IND may be a useful therapy for PDA.
Introduction
Patent ductus arteriosus (PDA) accounts for approximately 5% to 10% of all congenital heart diseases. Most cases of PDA are diagnosed and treated from infancy through childhood, but those clinically diagnosed in adulthood also are not rare. Basically, the presence of a heart murmur and symptomatic cases are considered to be indications for PDA treatment. If medical management does not lead to PDA closure, then indomethacin (IND) is administered intravenously, mainly in preterm infants.1, 2 However, the effectiveness of IND injection in full-term infants with PDA remains undetermined. We herein report our experience with a 12-day-old, full-term infant with symptomatic PDA successfully treated with IND therapy.
Case Presentation
A 12-day-old girl was referred to our hospital because of tachypnea and retractive breathing. The patient was born via elective cesarean section at 37 weeks and 0 days of gestation, weighing 2,955 g, with Apgar scores of 6/9. On day 6 after birth, a heart murmur was noted, and echocardiographic results led to the diagnosis of PDA. Tachypnea, retractive breathing, and tachycardia were observed, which were indicative of symptomatic PDA. Considering the potential need for surgical treatment, the patient was transferred to our hospital by neonatal transport on day 12 after birth. Her weight was 3,015 g, body temperature was 37.3°C, blood pressure was 87/43 mm Hg, heart rate was 155 beats/min and regular, respiratory rate was 67 breaths/min, and blood oxygen saturation (lower extremity) was 96% (room air). A continuous murmur was heard at the left upper sternal border. Respiratory sounds were clear, and tachycardia and retractive breathing were observed. Blood tests revealed no abnormalities. Chest radiography showed that the cardiothoracic ratio was 67%, indicating cardiomegaly, and lung permeability was reduced bilaterally (Figure 1). Twelve-lead electrocardiography showed that the heart rate was 150 beats/min and regular, with right-axis deviation and left atrial overload with the downward deflection of the P wave in lead V1.
Figure 1.
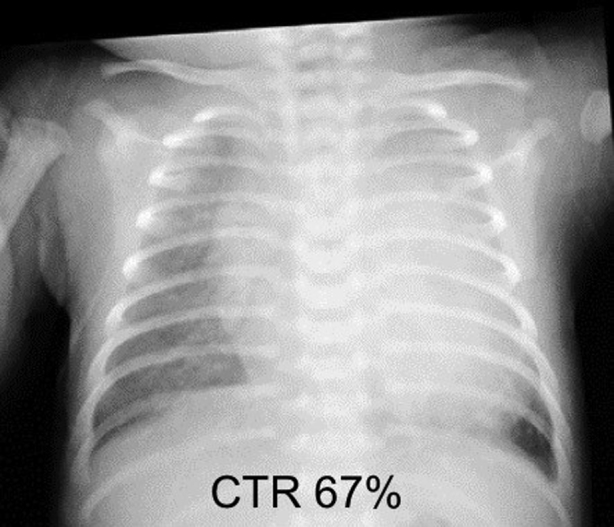
Chest radiography on admission demonstrates a cardiothoracic ratio (CTR) of was 67%, indicating cardiomegaly, and reduced lung permeability.
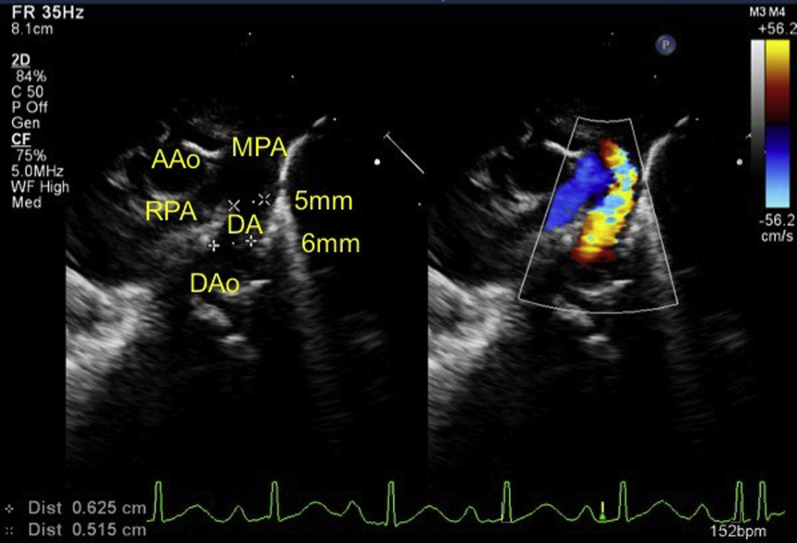
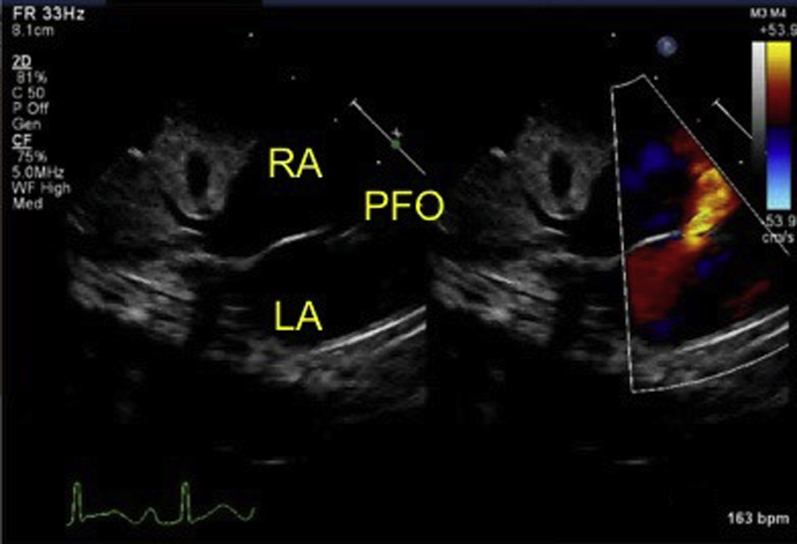
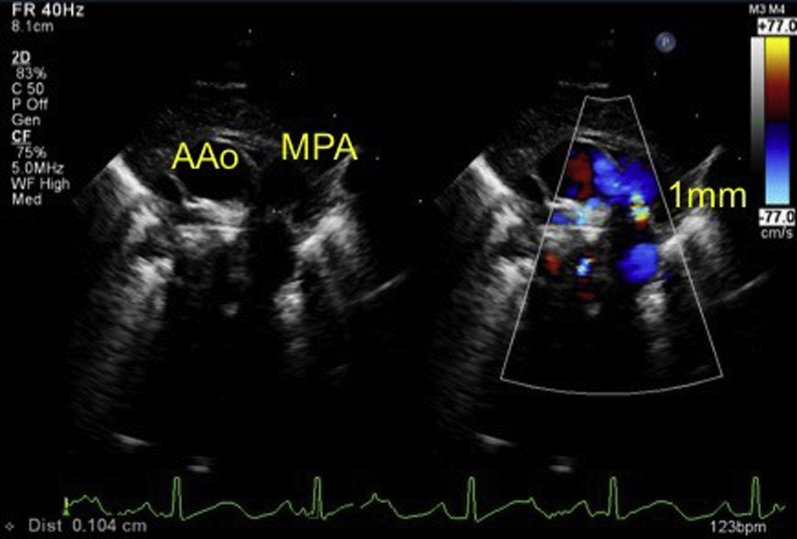
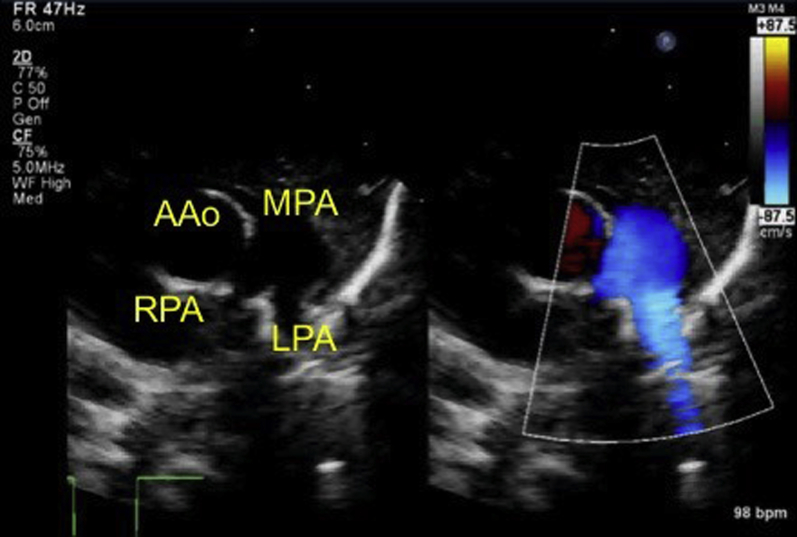
Initial echocardiography on admission demonstrated the presence of a large PDA measuring 6 mm on the aortic side and 5 mm on the pulmonary arterial side (Krichenko type A), and a left-to-right shunt of blood flow was shown by color Doppler (Figure 2). A patent foramen ovale (PFO) was also present, with left-to-right shunt flow (Figure 3). The right and left ventricles were almost the same size (left ventricular end-diastolic dimension 98% of normal predictive value; Figure 4), indicating the presence of volume overload in the left heart due to the PDA and in the right heart due to the PFO. The treatment strategy was to administer three doses of IND with a 12-hour interval between doses (first course, 0.2 mg/kg; second and third courses, 0.25 mg/kg); if no effects were obtained, we would perform surgery (PDA clipping). Thirty-six hours after first IND administration, no continuous murmur was heard with a stethoscope. Echocardiography revealed a reduction in PDA diameter to approximately 1 mm and rapid systolic left-to-right shunt flow (Figure 5). Improvements were also noted in the heart rate (from 150–170 beats/min to 130 beats/min), as well as in the respiratory rate (from 70 to 40 breaths/min). Retractive breathing also showed resolution. No side effects were observed, such as decreased urine volume, hypoglycemia, or bleeding. The condition had improved at least to the point at which emergency surgery was not necessary. As the patient showed a favorable response to IND without side effects, the second course of IND therapy was initiated after a 1-day washout. Closure of the PDA was confirmed after the fourth dose of IND, and the treatment was therefore terminated. The fluid restriction was lifted, and the absence of PDA recanalization was confirmed. The patient remained in good systemic condition and was discharged from the hospital on day 20 after birth. Her 1-month checkup showed a substantial improvement in the cardiothoracic ratio (51%; Figure 6), and there were no signs of PDA recanalization (Figure 7).
Figure 2.
Echocardiography on admission demonstrates the presence of a large PDA measuring 6 mm on the aortic side and 5 mm on the pulmonary arterial side, and a left-to-right shunt of blood flow is shown by color Doppler. AAo, Ascending aorta; DAo, descending aorta; MPA, main pulmonary artery; RPA, right pulmonary artery.
Figure 3.
A PFO was also present, with left-to-right shunt flow. LA, Left atrium; RA, right atrium.
Figure 4.
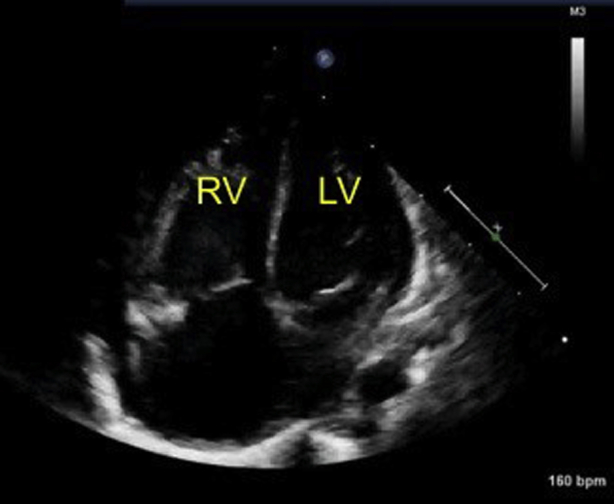
Apical four-chamber view shows that the right ventricle (RV) and left ventricle (LV) are almost the same size, indicating the presence of volume overload.
Figure 5.
After IND treatment, echocardiography reveals a reduction in PDA diameter to approximately 1 mm. AAo, Ascending aorta; MPA, main pulmonary artery.
Figure 6.
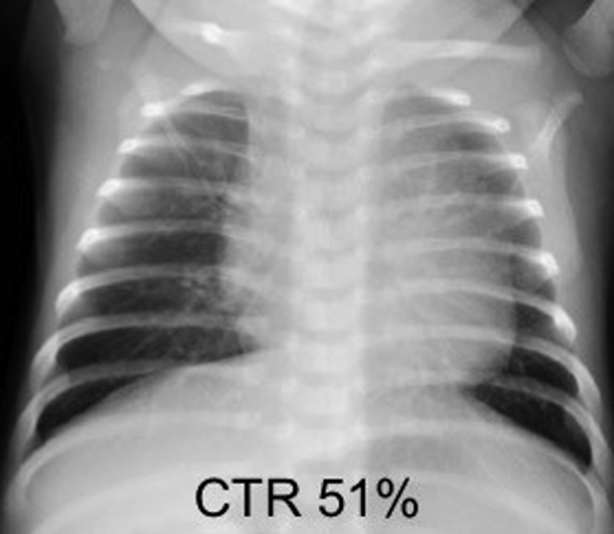
Chest radiography at 1-month checkup shows a substantial improvement in the cardiothoracic ratio (CTR).
Figure 7.
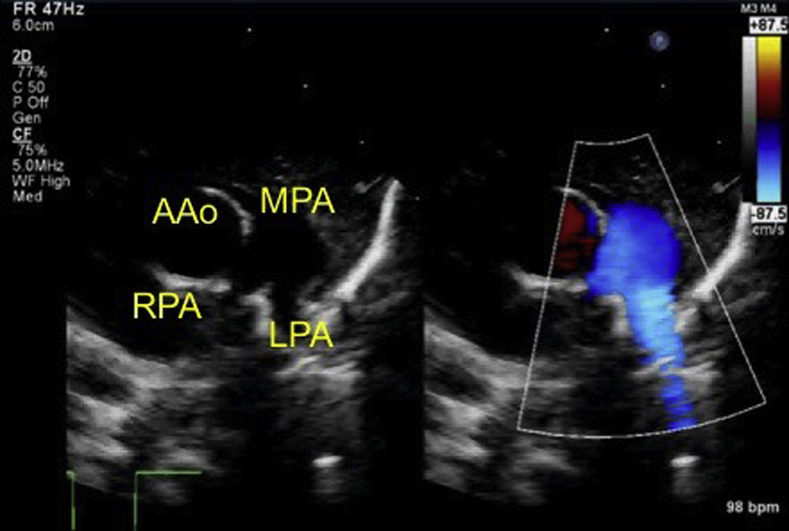
Echocardiography at 1-month checkup shows no signs of PDA recanalization. AAo, Ascending aorta; LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery.
Discussion
Mechanisms for ductus arteriosus (DA) closure are roughly classified into two types. One is the constriction of the DA itself following the start of pulmonary respiration at birth, induced by oxygen stimulation resulting from increased partial pressure of oxygen in blood and the disappearance of vasodilators due to decreased production of prostaglandin E2 (PGE2) and reduced PGE2 receptor (EP4).3, 4 The other is attributable to the histologic structure of the DA. During the fetal period, PGE2 is produced mainly in the placenta and DA; PGE2 stimulation increases the production of hyaluronic acid, which induces the migration of DA smooth muscle cells, causing intimal thickening of the DA.Additionally, one of the characteristics of the histologic structure of the DA is poor formation of elastic fibers. Unlike the great arteries, a vessel without elastic fibers is unlikely to be restored to its original size after intense vasoconstriction.5, 6, 7 There is also a relatively old report suggesting that elastic fibers were abnormally distributed in the vascular wall of the DA (particularly elastic fibers under endothelial cells) in infants ≥4 months of age.8 Therefore, it is reasonable that persistently PDA until adulthood may have a different histologic structure.
Because a full-term infant has a mature DA closure mechanism and is highly reactive to oxygen, functional closure of the DA generally occurs within a few days after birth. Following vascular remodeling, the anatomic closure of the DA is completed in several days to a few months. In a premature infant, however, the DA is poorly responsive to oxygen, and premature infants with compromised or immature respiratory function show a delay in lung metabolism of prostaglandin, resulting in delayed or failed closure of the PDA.9
Symptomatic premature infants with PDA are managed through fluid restriction, correction of anemia, use of diuretics, and other appropriate interventions. If the DA remains patent, the prostaglandin synthesis inhibitor IND is injected intravenously. IND can affect the smooth muscle tone of the DA and promote ductal constriction. The PDA closure rate in premature infants on IND is said to be 70% to 90%.10 In a study that examined IND therapy for PDA in full-term infants, the response rate was 61%.11 There was no significant difference between responders and nonresponders of IND in gestational age, body weight, Apgar score at 1 min, minimum diameter of the DA before treatment, age at the initiation of treatment, or DA flow pattern. If full-term infants have unstable perinatal conditions such as hypoxia or acidosis, it may lead to delayed DA closure and subsequent heart failure. Delayed ductal closure often perpetuates hypoxia in infants and vice versa. Apgar scores can provide essential information on the perinatal condition, which plays an important role in the development of PDA.12 The present patient was a full-term infant with a mature DA closure mechanism. However, her Apgar scores at 1 min were low (6/9), indicating that she had some form of pulmonary prematurity. Lung metabolism of PGE2 was thereby delayed, resulting in persistent and symptomatic PDA. This patient had a PFO, and left-to-right shunt flow was observed on day 12 after birth, but the possibility of right-to-left shunt of PFO leading to systemic hypoxemia at birth cannot be denied. However, the patient had a good response to IND therapy. Still, as shown by the present case, there is a possibility that IND may be a useful therapy for PDA without side effects and that it would, therefore, be worthwhile to consider the use of IND therapy before performing surgery.
Conclusion
We experienced a full-term infant with symptomatic PDA successfully treated with IND administration. Even in full-term infants, unstable perinatal conditions such as hypoxia or acidosis may lead to delayed prostaglandin metabolism and DA closure. Thus, IND administration might be a useful medical treatment option before considering surgery for PDA in full-term infants.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
References
- 1.Anilkumar M. Patent ductus arteriosus. Cardiol Clin. 2013;31:417–430. doi: 10.1016/j.ccl.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Weber S.C., Weiss K., Buhrer C., Hansmann G., Koehne P., Sallmon H. Natural history of patent ductus arteriosus in very low birth weight infants after discharge. J Pediatr. 2015;167:1149–1151. doi: 10.1016/j.jpeds.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama U., Minamisawa S., Quan H., Ghatak S., Akaike T., Segi-Nishida E. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama U., Minamisawa S., Ishikawa Y. Regulation of vascular tone and remodeling of the ductus arteriosus. J Smooth Muscle Res. 2010;46:77–87. doi: 10.1540/jsmr.46.77. [DOI] [PubMed] [Google Scholar]
- 5.Segi E., Sugimoto Y., Yamasaki A., Aze Y., Oida H., Nishimura T. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen M., Camenisch T., Snouwaert J.N., Hicks E., Coffman T.M., Anderson P.A. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama U., Minamisawa S., Shioda A., Ishiwata R., Jin M.H., Masuda M. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation. 2014;129:487–496. doi: 10.1161/CIRCULATIONAHA.113.004726. [DOI] [PubMed] [Google Scholar]
- 8.Gitttenberger-de Groot A.C. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977;39:610–618. doi: 10.1136/hrt.39.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurphy D.M., Heymann M.A., Rudolph A.M., Melmon K.L. Developmental changes in constriction of the ductus arteriosus: responses to oxygen and vasoactive agents in the isolated ductus arteriosus of the fetal lamb. Pediatr Res. 1972;6:231–238. doi: 10.1203/00006450-197204000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Mitra S., Florez I.D., Tamayo M.E., Mbuagbaw L., Vanniyasingam T., Veroniki A.A. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. 2018;319:1221–1238. doi: 10.1001/jama.2018.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takami T., Yoda H., Kawakami T., Yamamura H., Nakanishi T., Nakazawa T. Usefulness of indomethacin for patent ductus arteriosus in full-term infants. Pediatr Cardiol. 2007;28:46–50. doi: 10.1007/s00246-006-1426-9. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y.C., Huang H.R., Lien R., Yang P.H., Su W.J., Chung H.T. Management of patent ductus arteriosus in term or near-term neonates with respiratory distress. Pediatr Neonatol. 2010;51:160–165. doi: 10.1016/S1875-9572(10)60030-7. [DOI] [PubMed] [Google Scholar]