Graphical abstract
Keywords: Double-chambered left ventricle, Pseudoaneurysm, Ventricular tachycardia
Highlights
-
•
DCLV is a rare congenital defect.
-
•
Echocardiographic findings include a muscular ridge in the left ventricle.
-
•
Cardiac MRI can differentiate DCLV from pseudoaneurysm or aneurysm.
-
•
The structural abnormality in DCLV can act as substrate for ventricular tachycardia.
Introduction
Left-sided double ventricle or double-chambered left ventricle (DCLV) is a rare congenital defect that generally remains asymptomatic without complications1, 2 but can present with life-threatening arrhythmias.3, 4
The differentiation between this rare congenital abnormality and acquired conditions such as postinfarction or posttraumatic pseudoaneurysm can pose a diagnostic challenge. The distinction is important because the presence of pseudoaneurysm typically requires surgical management.5
We report the case of a man who presented with monomorphic ventricular tachycardia and was ultimately diagnosed with DCLV. We also review previously reported cases in which patients were incidentally discovered to have DCLV and various imaging studies used to diagnose this condition.
Case Presentation
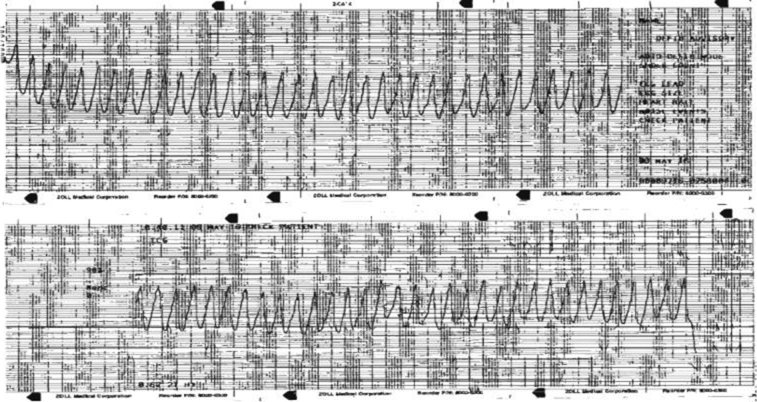
A 49-year-old man with a medical history of alcohol abuse and type 1 diabetes mellitus on insulin was admitted to the hospital with sudden onset of profound palpitations and chest discomfort while exercising. The initial rhythm upon arrival was monomorphic ventricular tachycardia at a rate of 280 beats/min (Figure 1). He was successfully converted to sinus rhythm with an amiodarone bolus and was brought to the hospital.
Figure 1.
Monomorphic ventricular tachycardia at the time of presentation (emergency medical services strip).
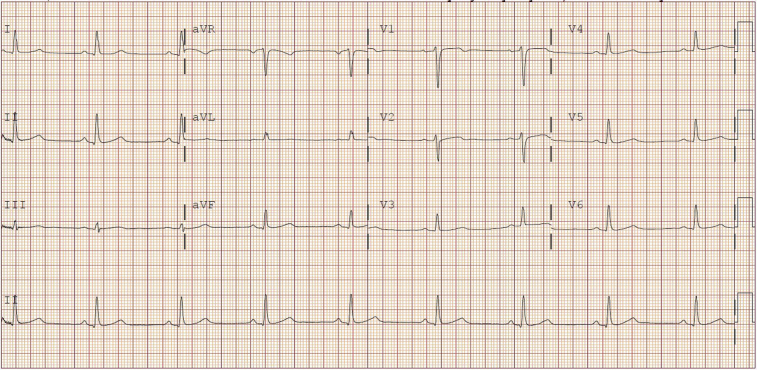
He had no known history of myocardial infarction, cardiac trauma, illicit drug use, or infection. There was no family history of sudden cardiac death. Physical examination was unremarkable and subsequent electrocardiographic findings were normal (Figure 2).
Figure 2.
Admission 12-lead electrocardiogram shows normal sinus rhythm and is within normal limits, without ST-segment elevations or Q waves.
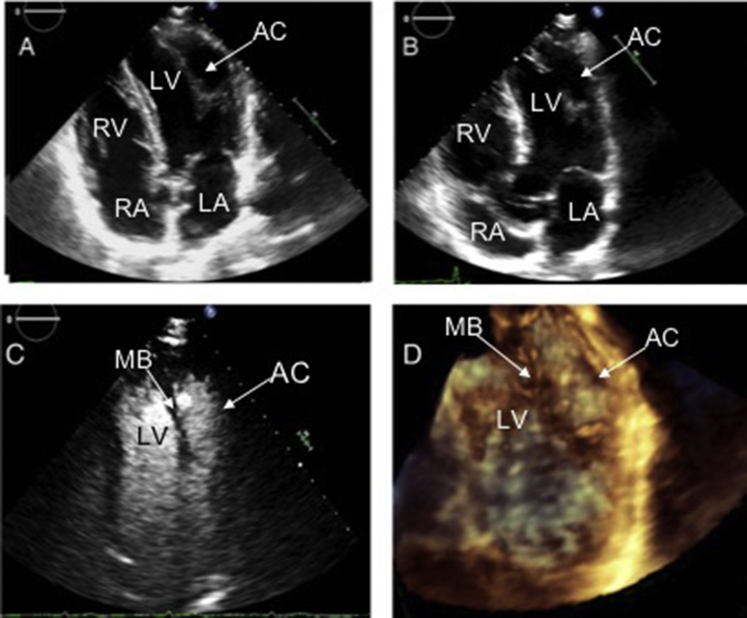
Transthoracic echocardiography showed an unusual appearance to the left ventricle. The lateral aspect of the apex was thinned and akinetic to mildly dyskinetic with a suspicious structure or attachment originating from or residing in the apex (Figure 3, Video 1). There were no other wall motion abnormalities identified, and global left ventricular (LV) systolic function was mildly reduced to an estimated ejection fraction of 45%. An enhancing ultrasound agent was introduced for further clarification. Definity (perflutren lipid microspheres) is useful for chamber opacification, delineation of tissue-blood interface, and better definition of trabeculations and other intracardiac structures.6 It was injected and yielded an appreciation of a primary LV cavity and a questionable accessory chamber and ruled out a questionable apical thrombus (Figure 1C, Video 2). It appeared that the LV cavity was subdivided by a vertical tissue ridge in the center of the left ventricle, but this disappeared at times while viewing during the cardiac cycle. This suspected cavity had a similar thickness and appearance to myocardial tissue and contained a large visible gap through which the primary LV cavity communicated with a questionable accessory chamber. There was no evidence of structural narrowing at the site of communication by two-dimensional and color flow Doppler to suggest an increased pressure gradient (Video 1). Three-dimensional echocardiography also demonstrated the possible muscular ridge between the primary LV cavity and an accessory chamber (Figure 1D, Video 3). The mitral valve was structurally normal, with very mild regurgitation.
Figure 3.
Transthoracic echocardiogram showed unusual double-chambered appearance of the left ventricle with thinned and akinetic lateral aspect of the apex on two-dimensional echocardiography (A, B). Double-chambered appearance of left ventricle seen with contrast study on two-dimensional echocardiography (C) and three-dimensional echocardiography (D). AC, Accessory chamber; LA, left atrium; MB, muscle band; RA, right atrium; LV, left ventricle; RV, right ventricle.
The echocardiographic findings seemed consistent with DCLV, but the differential diagnosis also included a pseudoaneurysm at a prior silent myocardial rupture site, as well as a true aneurysm, which was least likely given the narrow neck appearance created by the gap in the tissue ridge relative to the size of the accessory chamber (Video 4).
Parachute mitral valve can also have a similar appearance, but two distinct papillary muscles were identified that received chordae tendineae that appeared to be of equal lengths from both leaflets (Videos 4 and 5). The mitral valve opened normally in a symmetric manner, without any stenosis and with minimal regurgitation (Video 1). However, there was distinct asymmetry of the anterolateral papillary muscle, which appeared to be attached to the questionable muscular ridge. The anterolateral papillary muscle was significantly larger than the posteromedial papillary muscle (Figure 4). Thus, this anatomy likely falls within the wide spectrum of parachute-like mitral valve variants without the typical valvular dysfunction that is typically associated with a parachute mitral valve.
Figure 4.
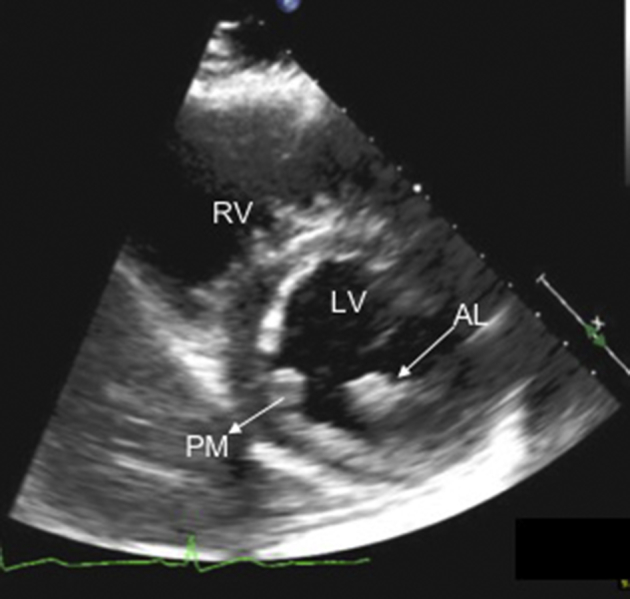
A parasternal short-axis image showing two distinct papillary muscles. The anterolateral papillary muscle is dominant, as it appears larger than the posteromedial papillary muscle. AL, Anterolateral papillary muscle; LV, left ventricle; PM, posteromedial papillary muscle; RV, right ventricle.
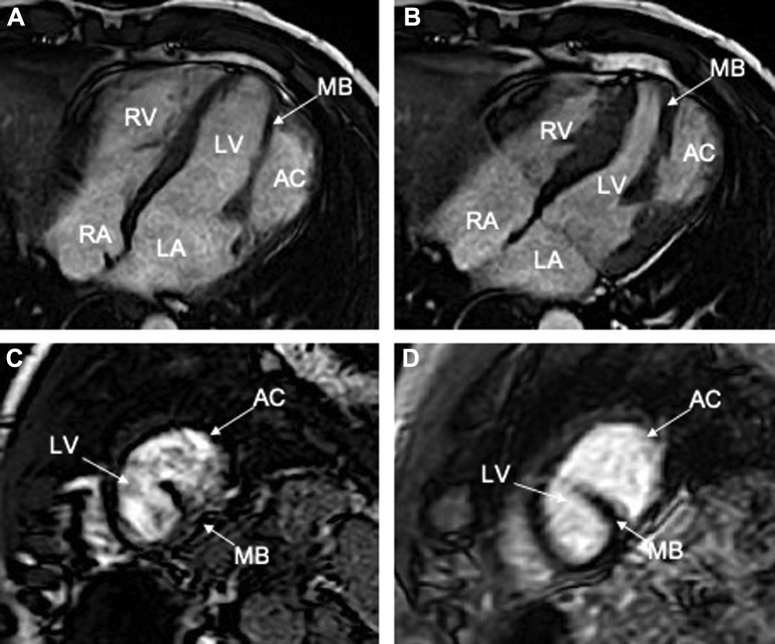
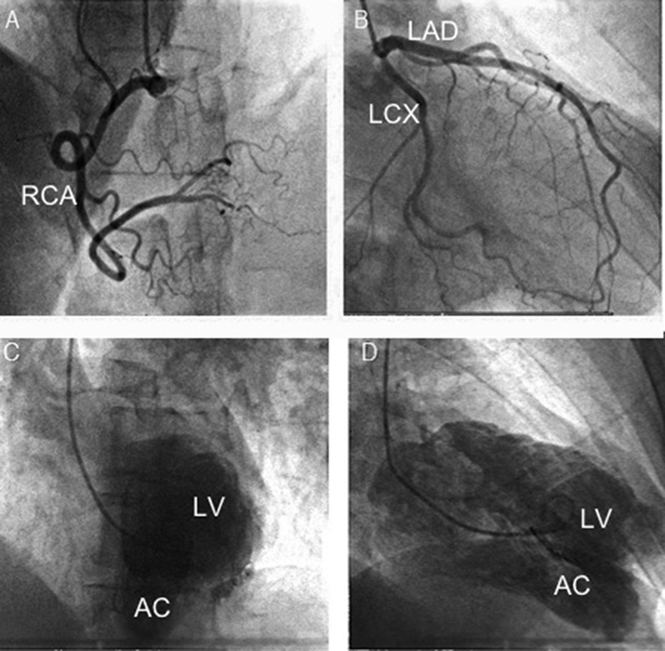
Subsequent coronary angiography failed to demonstrate obstructive coronary disease. However, left ventriculography did reveal a large LV outpouching lateral to the LV apex, consistent with the presence of an accessory chamber (Figure 5). Cardiac magnetic resonance imaging (MRI) was obtained to further define the anatomy and to define tissue characteristics of the suspected accessory chamber wall. Cardiac MRI demonstrated an akinetic lateral wall consisting of myocardial tissue. There was a prominent muscular band separating the left ventricle into two chambers. Importantly, there was no late gadolinium enhancement to suggest scar from prior ischemic injury (Figure 6, Video 6). The calculated ejection fraction by MRI was 42%. These findings were diagnostic of congenital DCLV rather than an aneurysm or a pseudoaneurysm. Cardiothoracic surgery was consulted to address the potentially fatal ventricular arrhythmias related to this extra ridge of tissue. Surgical resection was deferred given the close proximity of the defect to the papillary muscle and submitral apparatus, thereby increasing the risk for a complication that could potentially lead to mitral valve replacement in a young patient who otherwise had a fairly normal mitral valve. Because the etiology of the ventricular tachycardia was thought to be likely due to the dyskinetic aneurysmal accessory chamber wall acting as a substrate for reentry (Videos 3 and 4), the possibility of ablation was also discussed with electrophysiology. Given the episode of high-risk ventricular tachycardia, the final decision was made to implant an implantable cardioverter-defibrillator (ICD) for secondary prevention of sudden cardiac death. The patient had a recurrence of the rapid ventricular tachycardia approximately 1 year after the implantation, which was successfully aborted by the implanted device.
Figure 5.
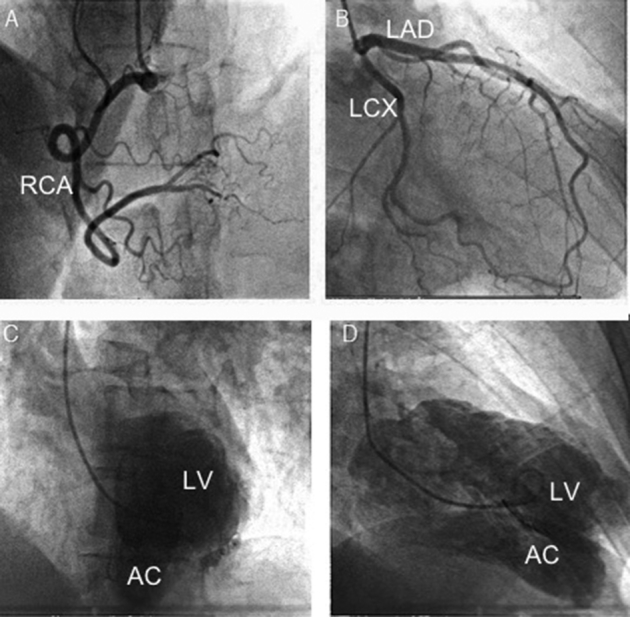
Coronary angiogram did not demonstrate obstructive coronary disease in the right (A) or left (B) system. Left ventriculography showed a large posterolateral LV chamber in addition to main left ventricle in left anterior oblique cranial view (C) and right anterior oblique cranial view (D). AC, Accessory chamber; LAD, left anterior descending artery, LCX, left circumflex artery; LV, left ventricle; RCA, right coronary artery.
Figure 6.
Cardiac MRI demonstrated that the akinetic lateral wall consisted of myocardial tissue, and there was a prominent anomalous muscular band that appeared to separate the left ventricle into two chambers in long axis (A, B) and short-axis (C). The muscle band shortens and thickens with systole (B) compared with diastole (A). There was no late gadolinium enhancement (D) to suggest scar from prior ischemic injury. AC, Accessory chamber; LA, left atrium; LV, left ventricle; MB, muscle band; RA, right atrium; RV, right ventricle.
Discussion
DCLV is considered to result from hypoplasia of regional myocardial intratrabecular sinusoids or from intramyocardial aneurysm that develops during embryogenesis.7 DCLV is characterized by division of LV chamber into two chambers by abnormal muscular tissue. This can be mistaken for aneurysm or pseudoaneurysm of the left ventricle. Pseudoaneurysms do not contain all three layers of cardiac tissue, have a narrow neck, and may exhibit paradoxical (dyskinetic) movement during systole. Both DCLV and LV diverticulum contain all layers of cardiac tissue that typically contract synchronously with the rest of the ventricle.7, 8 Diverticulum, however, differs from DCLV in having a narrow neck connecting the diverticulum to the left ventricle.9, 10 DCLV is generally suspected when an abnormally configured left ventricle with two distinct contracting chambers is seen by echocardiography,7, 9 but the accessory chamber is not necessarily contractile in all patients with this condition and can also exhibit akinetic to dyskinetic contractility in some patients, as illustrated in our case (Videos 1–4).
The exact prevalence of congenital DCLV is not known, although studies have reported prevalence of 0.04% to 0.42%.11, 12 Review of the literature reveals that five overlapping and poorly defined terms are used in reporting this rare entity: LV accessory chamber, LV aneurysm, LV diverticulum, DCLV, and accessory left ventricle, which confounds the prevalence reported.
Patients with DCLV generally remain asymptomatic, and the abnormality is typically incidentally discovered during unrelated cardiovascular evaluation.1, 7, 8 There are only a few case reports regarding ventricular arrhythmia associated with DCLV, including a 22-year-old man who presented with nonsustained ventricular tachycardia13 and a 68-year-old man who was admitted with ventricular fibrillation and found to have DCLV.3 There is only one large case series report in the literature, in which 250 patients with congenital LV aneurysms and diverticula were reviewed, including 30 patients who presented with ventricular arrhythmias, only nine of whom had ventricular tachycardia. However, that study included patients with LV aneurysm or LV diverticulum rather than focusing solely on DCLV and did not describe a specific structural abnormality on echocardiography as the substrate for ventricular arrhythmia.4
In our patient, echocardiography and left ventriculography suggested the presence of DCLV, but cardiac MRI was diagnostic. The absence of late gadolinium enhancement on MRI was indicative of the lack of a scar and thereby confirmed that the patient had not experienced a prior silent myocardial infarction. Additionally, there were no signs of fibrosis, which excluded the possibility that the double-chambered appearance was formed as a result of trauma or infarction. MRI showed that the thinned wall consisted of myocardial tissue, which excluded the possibility of a pseudoaneurysm. Although echocardiography and computed tomography can suggest a diagnosis of DCLV, cardiac MRI was confirmatory. MRI allows better delineation of this condition because of its higher spatial resolution and the ability for tissue characterization, especially differentiating fibrosis from normal myocardium.2, 14
Our patient presented with ventricular tachycardia and received an ICD for secondary prevention. However, there is no evidence to indicate that an ICD should be placed for primary prevention if DCLV is discovered incidentally in an asymptomatic patient.
Whether certain imaging criteria can be used to help predict future occurrences of ventricular arrhythmias in asymptomatic patients with DCLV and thereby guide ICD placement for primary prevention in not known.
Most previously reported cases of DCLV in asymptomatic individuals have normal accessory chamber wall thickness. The thin dyskinetic appearance described in this case is highly unusual, confounding echocardiographic interpretation, and likely contributed to the propensity to develop sustained ventricular tachycardia.
Conclusion
DCLV should be suspected by echocardiography in the presence of an unusual aneurysmal appearance and subdivision of the left ventricle and by an anomalous muscular ridge. The diagnosis is aided by the use of ultrasound-enhancing agents and three-dimensional imaging. Cardiac MRI is a valuable tool to further characterize the division of the left ventricle, define the structure of the accessory chamber wall, and exclude the presence of fibrosis, to confirm the diagnosis of a DCLV. This structural abnormality can serve as substrate for the development of rapid sustained monomorphic ventricular tachycardia.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.11.008.
Supplementary Data
Apical four-chamber view with color Doppler. The lateral aspect of the apex is thinned and akinetic to mildly dyskinetic. Color Doppler demonstrates the absence of flow acceleration between the primary LV cavity and the accessory chamber. It also shows a normally function mitral valve without stenosis or regurgitation.
Ultrasound enhancing agent (perflutren lipid microspheres [Definity]), which defines the primary LV cavity and the accessory chamber being subdivided by an anomalous muscular ridge that originates from the left ventricle near its apex. It also shows the free unobstructed communication between the primary LV cavity and the accessory chamber.
Three-dimensional transthoracic imaging with multiplanar reconstruction, which further defines the underlying anatomy. The dyskinetic lateral wall of the accessory chamber is noted in the left upper image. The papillary muscles are visualized in the upper right image. The lower left image is a cross section near the LV apex showing the right ventricle and subdivision of the left ventricle by a muscular ridge into a primary cavity and an accessory chamber.
An apical long-axis view that shows the subdivision of the left ventricle by an anomalous muscular ridge with a visible gap that forms the entry point to the accessory chamber. This entry point is significantly narrower than the size of the accessory chamber.
Parasternal long-axis view showing two papillary muscles and a structurally normal mitral valve. Contractility of the interventricular septum and inferolateral walls are normal.
Cardiac MRI steady-state free precession long-axis cine imaging demonstrating the presence of a large muscle band that subdivides the left ventricle into a primary and an accessory chamber. The wall of the accessory chamber is thin and akinetic.
References
- 1.Masci P.G., Pucci A., Fontanive P., Coceani M., Marraccini P., Lombardi M. Double-chambered left ventricle in an asymptomatic adult patient. Eur Heart J Cardiovasc Imaging. 2012;13:E1–E3. doi: 10.1093/ejechocard/jer242. [DOI] [PubMed] [Google Scholar]
- 2.Breithardt O.A., Ropers D., Seelinger T., Schmid A., von Erffa J., Garlich C. A heart within the heart: double-chambered left ventricle. Eur J Echocardiogr. 2008;9:739–741. doi: 10.1093/ejechocard/jen158. [DOI] [PubMed] [Google Scholar]
- 3.Kato M., Sasaki S., Dote K. Double-chambered left ventricle with ventricular fibrillation. Intern Med. 2012;51:2245–2246. doi: 10.2169/internalmedicine.51.7972. [DOI] [PubMed] [Google Scholar]
- 4.Haegeli L.M., Ercin E., Wolber T., Brunckhorst C., Tanner F.C., Jenni R. Arrhythmic manifestations in patients with congenital left ventricular aneurysms and diverticula. Am J Cardiol. 2011;108:1826–1830. doi: 10.1016/j.amjcard.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Sherif H., Maniar H., Spades N., Marge M., Banbury M.K. Left ventricular diverticulum mimicking Ventricular pseudoaneurysm in an adult. Texas Heart Inst J. 2010;37:584–586. [PMC free article] [PubMed] [Google Scholar]
- 6.Porter T.R., Mulvagh S.L., Abdelmoneim S.S., Becher H., Belcik J.T., Bierig M. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31:241–274. doi: 10.1016/j.echo.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X.Y., Cao T.S., Yuan L.J. Double-chambered left ventricle in echocardiography. Echocardiography. 2012;29:E67–E68. doi: 10.1111/j.1540-8175.2011.01569.x. [DOI] [PubMed] [Google Scholar]
- 8.Mordi I., Carrick D., Tzemos N. Diagnosis of double-chambered left ventricle using advanced cardiovascular imaging. Echocardiography. 2013;30:E206–E208. doi: 10.1111/echo.12244. [DOI] [PubMed] [Google Scholar]
- 9.Bilici M., Demir F., Akm A., Guzel A., Akdeniz O., Tan I. Echocardiographic diagnosis of double-chambered left ventricle. J Echocardiogr. 2016;14:176–178. doi: 10.1007/s12574-016-0297-1. [DOI] [PubMed] [Google Scholar]
- 10.Sanz J., Rius T., Kuschnir P., Macaluso F., Fuster V., Poon M. Double-chambered left ventricle: complete characterization by cardiac magnetic resonance and multidetector-row computed tomography. Circulation. 2004;110:e502–e503. doi: 10.1161/01.CIR.0000147273.67609.7D. [DOI] [PubMed] [Google Scholar]
- 11.Skapinker S. Diverticulum of the left ventricle of the heart; review of literature and report of a successful removal of the diverticulum. AMA Arch Surg. 1951;63:629–634. doi: 10.1001/archsurg.1951.01250040643009. [DOI] [PubMed] [Google Scholar]
- 12.Ohlow M.A., Secknus M.A., Geller J.C., Vonkorn H., Lauer B. Prevalence and outcome of congenital left ventricular aneurysms and diverticula in an adult population. Cardiology. 2009;112:287–293. doi: 10.1159/000159122. [DOI] [PubMed] [Google Scholar]
- 13.Koz C., Yokusoglu M., Uzun M., Baysan O., Bulakbasi N. Double-chambered left ventricle with nonsustained ventricular tachycardia. Anatol J Cardiol. 2009;93:5005. [PubMed] [Google Scholar]
- 14.Nacif M.S., Mello R.A., Lacerda O.O., Sibley C.T., Machado R., Marchiori E. Double-chambered left ventricle in an adult: diagnosis by CMRI. Clinics. 2010;65:1393–1395. doi: 10.1590/S1807-59322010001200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber view with color Doppler. The lateral aspect of the apex is thinned and akinetic to mildly dyskinetic. Color Doppler demonstrates the absence of flow acceleration between the primary LV cavity and the accessory chamber. It also shows a normally function mitral valve without stenosis or regurgitation.
Ultrasound enhancing agent (perflutren lipid microspheres [Definity]), which defines the primary LV cavity and the accessory chamber being subdivided by an anomalous muscular ridge that originates from the left ventricle near its apex. It also shows the free unobstructed communication between the primary LV cavity and the accessory chamber.
Three-dimensional transthoracic imaging with multiplanar reconstruction, which further defines the underlying anatomy. The dyskinetic lateral wall of the accessory chamber is noted in the left upper image. The papillary muscles are visualized in the upper right image. The lower left image is a cross section near the LV apex showing the right ventricle and subdivision of the left ventricle by a muscular ridge into a primary cavity and an accessory chamber.
An apical long-axis view that shows the subdivision of the left ventricle by an anomalous muscular ridge with a visible gap that forms the entry point to the accessory chamber. This entry point is significantly narrower than the size of the accessory chamber.
Parasternal long-axis view showing two papillary muscles and a structurally normal mitral valve. Contractility of the interventricular septum and inferolateral walls are normal.
Cardiac MRI steady-state free precession long-axis cine imaging demonstrating the presence of a large muscle band that subdivides the left ventricle into a primary and an accessory chamber. The wall of the accessory chamber is thin and akinetic.