Abstract
Background
Previously, we reported that transcripts of immunoglobulins were increased in coronary arteries dissected from cardiac transplants with arteriopathy, but the prevelance and patterns of B cell and plasma cell infiltration in cardiac allografts has not been documented.
Methods
In this study, we documented the frequency and distribution of B cells and plasma cells in 16 cardiac transplants with advanced chronic rejection that were explanted during a second transplant procedure. Coronary arteries with pathologically confirmed allograft vasculopathy (AV) and controls with native atherosclerosis were immunohistologically stained for markers of T cells, B cells, plasma cells, IgG subclasses, C4d, CD21, and CXCL13.
Results
We found that B cells and plasma cells were prevalent in most of the samples analyzed (14/16) and were distributed in three patterns: adventitial nodules, diffuse adventitial infiltrates, and neointimal infiltrates. These cells were found most frequently in nodules, some of which had distinct compartmentalization and granular C4d deposits on follicular dendritic cells (FDCs) that typify tertiary lymphoid nodules. FDCs also stained for CD21 and CXCL13. Diffuse infiltrates of B cells and plasma cells were found in fibrotic areas of the neointima and adventitia. Only a minority of control coronaries with atherosclerosis contained B cells.
Conclusions
B cells and plasma cell infiltrates are consistent findings in and around coronary arteries with AV and are significantly more frequent than in coronaries with native atherosclerosis. The presence of C4d on FDCs in the tertiary lymphoid nodules suggests active antigen presentation.
Keywords: Chronic Allograft Vasculopathy, B Cells, Plasma Cells
Introduction
Advances in immunosupression have decreased the incidence of acute cell-mediated rejection of organ transplants, but the survival of cardiac and kidney allografts continues to be limited by chronic rejection (1). In cardiac transplantation, chronic rejection is caused by the development of vasculopathy in the coronary arteries that is characterized by a diffuse concentric intimal proliferation and adventitial sclerosis (2–4). Endomyocardial biopsies, which are the standard method for assessing acute cardiac transplant rejection (5), are not useful in evaluating the pathological changes in epicardial coronary arteries. Chronic rejection is usually detected by surveillance angiographic or intravenous ultrasound studies and pathologically assessed upon autopsy. Allografts examined at autopsy introduce a number of confounding variables such as comorbidities (cancer, infections, sepsis, etc.) as well as postmortem cell autolysis. In this study, we have circumvented this problem by studying allografts removed because of chronic rejection at the time of retransplantation.
T cells have been studied extensively in cardiac allograft rejection because they are a major component of most acute rejection episodes. Increasingly sophisticated immunosuppressive regimens directed at T cell responses have decreased graft loss due to acute rejection. Unfortunately, the rate of chronic rejection has not been significantly abated. In contrast to T cells, which act locally within the graft, most B cells reside within lymphoid organs. Although B cells constitute a small portion of the circulating lymphocytes, substantial numbers of B cells and plasma cells have been demonstrated within transplanted organs, often in nodules or sometimes forming tertiary lymphoid structures with distinct T and B cell compartments (6–8).
B cell infiltrates have been described in renal transplants as features of both acute and chronic rejection. However, the significance and function of B cell infiltrates is not fully understood. Some groups have reported that CD20+ B cells in renal allograft biopsies (9, 10) as well as explanted renal grafts (11–13) are associated with more severe rejection, but other groups have found that B cells do not correlate with decreased graft survival (14–16). Although the presence of intra-graft B cells has not been found to correlate with C4d deposition or donor specific antibody (DSA) in clinical studies, experimental models have demonstrated that tertiary lymphoid nodules can support the generation of memory T cells in skin grafts (17) and the production of DSA in segmental aortic grafts (7).
Considerably less data are available on B cells and plasma cells in cardiac transplants. The majority of reports on B cells in cardiac allografts are confined to endomyocardial mononuclear cell infiltrates, known as the Quilty effect, which have been described in as many as 50%–70% of all cardiac transplant recipients (18–22). Quilty lesions are characterized by nodular infiltrates that may contain compartmentalized B and T cell populations surrounding high-endothelial venules on the endomyocardial surface.
Previously, we reported gene microarray profiles of coronary arteries dissected from 24 human heart explants recovered in the operating room at the time of transplantation including 6 hearts with dilated cardiomyopathy without coronary lesions, 6 hearts with native atherosclerosis, and 12 cardiac transplants that were replaced because of transplant vasculopathy (8). Genes for immunoglobulins (heavy and light chains) as well as receptors (CR2; CD21) that are expressed by B lymphocytes were upregulated in 11 of 12 coronaries with vasculopathy compared with native atherosclerosis or no lesions. In 5 of these samples, these probes were increased in the range of 5- to 25-fold. The presence of B cells and plasma cells was confirmed by preliminary immunohistology on 8 of these hearts.
The almost universal expression of B cell genes in coronary arteries with vasculopathy and the paucity of B cells in atherosclerosis led us to more thoroughly evaluate the prevalence, location and clonality of the B cells and plasma cells in transplant vasculopathy.
Materials and Methods
Tissue Samples
Between 1989 and 2008, a total of 26 cardiac transplants with chronic rejection were explanted during a second transplant procedure at The Johns Hopkins Hospital. Tissue samples from 16 of these explants contained intact coronary arteries and were used in this study. This retrospective study was approved by the Johns Hopkins Medical Institutional Review Board.
All tissues were operating room specimens and were immediately placed in formalin and processed in a timely matter for diagnostic purposes. The number of tissue blocks and vessels available for analysis from each patient was variable with an average of 3 tissue blocks containing coronary vessels per patient. Approximately 3 vessels per block were present in each patient.
Immunohistology
Five micron sections were deparaffinized and antigen retrieval was performed by immersing slides in two changes of Trilogy EDTA (pH 8.0; Cell Marque, Hot Springs, AR) in a steamer for a total of 60 minutes. Slides were rinsed and cooled in H2O for 5 minutes. Endogenous peroxidase activity was blocked by incubation in 0.3% H2O2 in methanol. Non-specific protein activity was blocked with a serum-free protein block (DAKO Corporation, Carpinteria, CA). For IgG1 staining, slides were incubated with an anti-human IgG1 (AbD Serotec, Oxford, UK) for 60 minutes. Secondary labeling and visualization of these slides was performed using the SuperPicture HRP conjugated polymer DAB system from Zymed Laboratories. (San Francisco, Ca.) Additional primary antibodies used in staining were CD3 and syndecan-1 (Abcam, Cambridge, MA), CD20cy (Dako, Carpinteria, CA), IgG2, IgG3 and IgG4 (Zymed, San Francisco, CA), Kappa and Lambda (Diagnostic Biosystems, Pleasanton, CA), C4d (American Research Products, Belmont, MA), CXCL13 (R&D Systems, Minneapolis, MN. Sections were incubated with biotinylated anti-rabbit IgG or anti-mouse IgG (Jackson Labs, West Grove, PA) depending on the primary antibody, for 30 minutes at room temperature. Immunoperoxidase staining was performed using Vectastain ABC Elite (Vector Labs, Burlingame,CA). The avidinbiotin complex was visualized using a 3, 3′ diaminobenzidine (DAB) peroxidase substrate (Vector Labs). Sections were counterstained in hematoxylin (Richard-Allen, Kalamazoo, MI).
Histopathologic Scoring
B cell and plasma cell infiltrates were evaluated in a blinded fashion on tissue samples that contained vessels with AV. B cell nodules were considered to be associated with a vessel if they were within one 20X power field of the smooth muscle cell layer of the affected vessel. Nodular B cell infiltrates were categorized into groups of 0 nodules, 1–3 nodules, or greater than 3 nodules. Adventitial plasma cells clustering around B cell nodules were evaluated on the percent of the nodule encircled by plasma cells: 3+ = >75% of the nodule; 2+ = >50–75% of the nodule; 1+ = 25–50% of the B cell nodule; 0 = <25% of the nodule. T and B cell compartmentalization was noted when there was a clear separation of T and B cells. Histological scoring of diffuse B cell and plasma infiltrates as well as neointimal infiltrates was based on the degree the vessel encircled by B cell infiltrate; 3+ = >75% of vessel; 2+ = >50–75% of vessel; 1+ = 25–50% of vessel; 0 = <25% of vessel.
Assays for antibodies to HLA
Lymphocyte crossmatch tests were performed by complement-dependent cytotoxicity (AHG-enhanced for T cells and one-wash for B cells) and by flow cytometry. Tests of HLA-specific antibodies were by solid-phase immunoassay on the ELISA (Quik-ID class I and class II, GTI, Brookfield, WI) and/or Luminex platforms (Lifematch ID and LifeScreen kits, Tepnel LifeCodes, Stamford, CT; Single Antigen Bead kits, One Lambda).
Statistics
Statistical differences in the presence or absence of B cells between AV and native atherosclerosis were evaluated using a Chi-square test.
Results
Overall prevalence of B cell and plasma cell infiltrates associated with AV
In agreement with our microarray data reported previously, B cells and plasma cells were a consistent feature of AV. In fact, all 16 of this expanded group of patients had infiltrates of B cells (14/16), plasma cells (15/16) or both (13/16).
Patterns of B cell infiltrates associated with AV lesions
B cell infiltrates associated with AV lesions displayed three distinct patterns: adventitial nodules, diffuse adventitial infiltration and neointimal infiltration (Table 1). To compensate for the variable number of blocks and vessels per patient, we averaged the scores in all vessels for each patient for each of the infiltrate patterns. The most common finding was B cell nodules in the adventitia of arteries with AV (Figure 1A–C). Thirteen of sixteen patients (81%) analyzed demonstrated B cell nodules associated with affected arteries. Nodular adventitial infiltrates were compartmentalized into B and T cell regions as shown in Figure 2 in 7 of 13 patients (44%). The second most common pattern of B cell infiltrates was diffuse cellular infiltrates in adventitial tissues (Figure 1B), which occurred in 10 of the 16 patients (63%) and were accompanied by T cell infiltrates. Of note was the finding that these B cell infiltrates were frequently surrounded by dense fibrosis in the adventitia. Within the vessel, B cells infiltrated the neointima in 7 of the 16 patients evaluated (44%) (Figure 1A, inset).
Table 1.
Clinical and histological characteristics of explanted hearts from patients with AV.
| Patient | Gender | Age (years) | Graft Survival (years) | B Cells | Plasma Cells | Quilty Lesion | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodules | Diffuse | Neointima | Surrounding B Cell Nodule | Diffuse | Neointima | |||||
| 1 | M | 60 | 1.4 | 0 | 0 | 0 | 0 | 1+ | 2+ | N |
| 2 | M | 44 | 6.0 | 0 | 0 | 0 | 0 | 1+ | 1+ | N |
| 3 | F | 51 | 13.4 | 0 | 1+ | 0 | 0 | 2+ | 0 | N |
| 4 | M | 60 | 11.1 | 1–3 | 0 | 0 | 1+ | 0 | 0 | Y |
| 5 | F | 29 | 2.2 | 1–3 | 0 | 0 | 1+ | 0 | 0 | Y |
| 6 | F | 64 | 1.8 | 1–3* | 0 | 0 | 1+ | 2+ | 0 | Y |
| 7 | M | 31 | 5.6 | 1–3 | 0 | 0 | 3+ | 3+ | 0 | N |
| 8 | F | 19 | 2.7 | 1–3 | 1+ | 0 | 0 | 0 | 0 | Y |
| 9 | F | 43 | 8.1 | 1–3 | 1+ | 0 | 1+ | 2+ | 0 | N |
| 10 | M | 43 | 14.6 | 1–3* | 1+ | 1+ | 2+ | 1+ | 0 | Y |
| 11 | M | 56 | 12.7 | 1–3 | 1+ | 2+ | 1+ | 1+ | 0 | N |
| 12 | F | 46 | 3.6 | 1–3* | 1+ | 1+ | 1+ | 1+ | 1+ | Y |
| 13 | M | 27 | 10.8 | 1–3* | 1+ | 1+ | 2+ | 1+ | 1+ | N |
| 14 | F | 48 | 12.3 | 1–3* | 1+ | 1+ | 2+ | 1+ | 1+ | N |
| 15 | M | 58 | 5.2 | >3* | 1+ | 1+ | 2+ | 2+ | 1+ | Y |
| 16 | M | 57 | 6.7 | >3* | 1+ | 1+ | 1+ | 2+ | 1+ | Y |
| Totals | 7F:9M | 46.0 ± 13.5 | 7.4 ± 4.5 | 13/16 positive |
10/16 positive |
7/16 positive |
12/16 positive |
13/16 positive |
7/16 positive |
8Y:8N |
represents compartmentalization of nodular adventitial T and B cell infiltrates.
Figure 1.
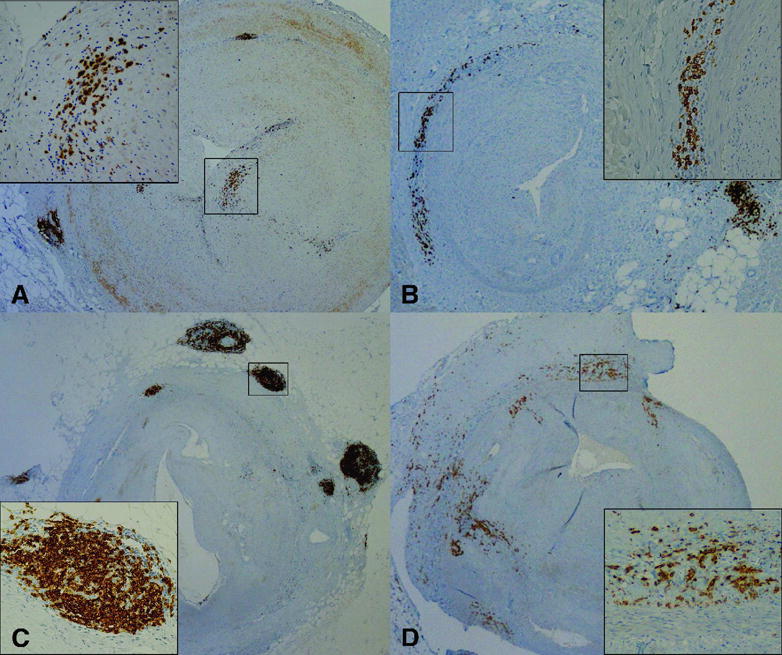
Epicardial coronary arteries with AV showing distinct patterns of B cell and plasma cell infiltrates. A–C; Immunohistochemistry staining with CD20 showing B cell infiltrates as adventitial nodules (A–C), diffuse adventitial infiltrates (B), or neointimal infiltrates (A). D; Immunohistochemistry staining with syndecan-1 showing plasma cell infiltrates in the sclerotic adventitia. A–D; 2X. Insets; 20X.
Figure 2.
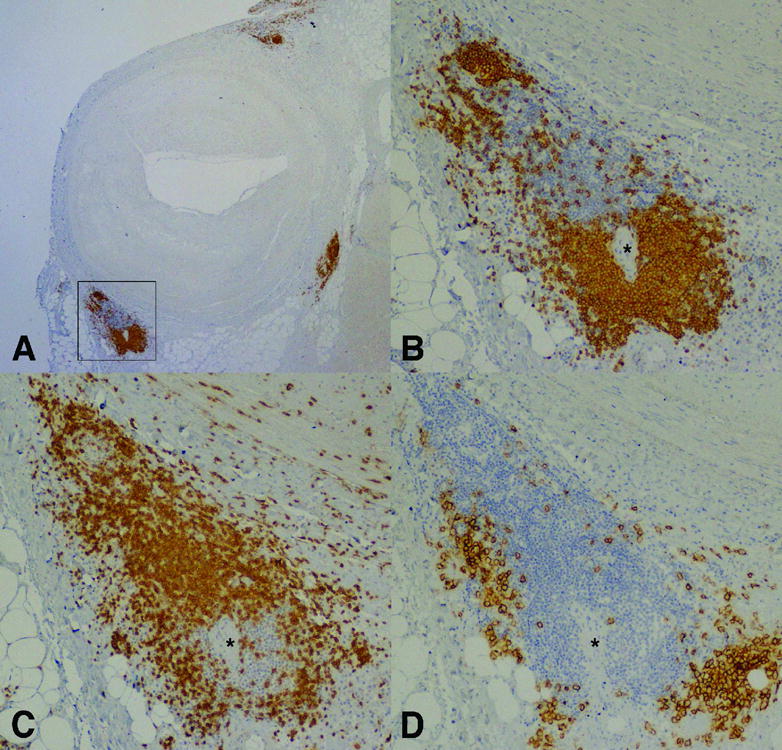
Epicardial coronary artery with AV and adventitial nodular mononuclear cell infiltrates showing compartmentalization of B and T lymphocytes as well as plasma cells. Serial sections are stained by immunoperoxidase for B lymphocytes (CD20; A&B), T lymphocytes (CD3; C) and plasma cells (syndecan-1; D). (Magnification: A; 2X and B–D; 20X) * = arteriole.
Many patients had more than one pattern of B cell infiltrate. All but one of the patients with diffuse infiltrates also had adventitial nodules, but 4 of 13 patients with B cell nodules had only nodular infiltrate. Seven patients had vessels sampled at multiple sites ranging from proximal, middle and distal regions. B cells were located in all regions, although the pattern of distribution as well as the extent of infiltrating B cells throughout the length of each vessel was variable (data not shown).
Quilty lesions are not associated with patterns of B cell infiltrates
We reviewed all patient endomyocardial biopsies taken prior to explantation to look for a possible association of endomyocardial Quilty lesions and epicardial B cell infiltrates in patients with chronic rejection. Half of the patients (8/16) had a Quilty lesion present in at least one of their previous endomyocardial biopsies. Of the 8 patients with Quilty lesions, 6 patients had 1–3 adventitial B cell nodules while the remaining two patients had greater than 3 adventitial B cell nodules. Diffuse B cell infiltrates were present in 5 of the 8 patients with Quilty lesions. Only 4 of the 8 patients with Quilty lesions had infiltrating B cells in the neointima. Therefore, while all patients with Quilty lesions had epicardial B cell infiltrates, the presence of Quilty lesions on the endocardium was not predictive of the extent or pattern of B cell infiltrate in the epicardium surrounding coronary arteries with AV.
Compartmentalized lymphoid nodules exhibit evidence of functional antigen presenting units
The formation of compartmentalized tertiary lymphoid nodules suggested a functional role for these cells. Critical for the formation of a functional tertiary lymphoid nodule is a central FDC network. FDCs are known to secrete CXCL13, a B cell chemoattractant, and also have complement receptors which facilitate in antigen presentation. Immunohistochemical staining using CD21 for FDCs revealed that compartmentalized lymphoid aggregates, but not diffuse infiltrates, contained a FDC network (data not shown). C4d staining was seen on FDCs in these nodules (Figure 3C). Additionally, CXCL13 staining was more extensive in nodules than in diffuse infiltrates (Figure 3D).
Figure 3.
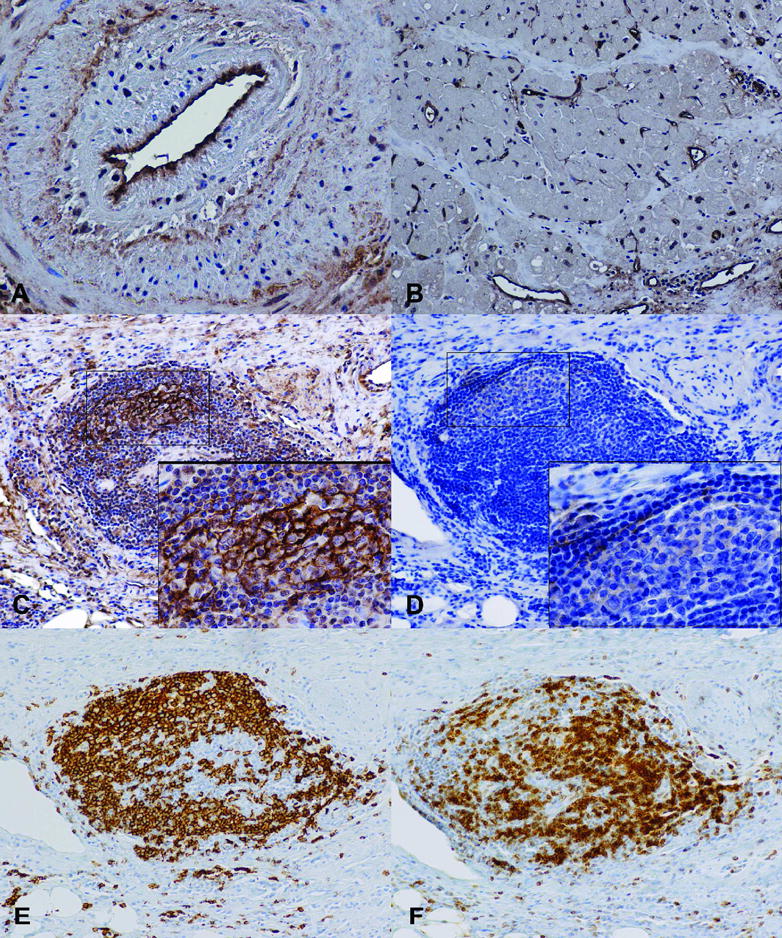
Immunohistochemistry stains of C4d deposition and CXCL13 in patients with AV. C4d deposits are shown on the endothelium of a coronary artery with AV (A) and capillaries of adjacent myocardium (B). Lymphoid nodules stained for C4d (C), CXCL13 (D), B cells (CD20; E), and T cells (CD3; F). (Magnification: A −40X; B–D −20X; Insets − 60X)
Plasma cell infiltrates are associated with AV lesions
Similar to B cell infiltrates, plasma cell infiltrates were seen in three patterns: clusters around B cell nodules, diffuse adventitial infiltrates and neointimal infiltrates (Table 1). Almost 70% of the patients (12/16) analyzed had plasma cells in clusters around B cell nodules (Figure 2D) while over 80% of patients (13/16) had diffuse plasma cell infiltrates in the adventitia (Figure 1D). Plasma cell infiltrates were also present in the neointima of seven patients (44%) with AV lesions. All but one patient had plasma cell infiltrates. Moreover, the extent of plasma cell infiltrate was not dependent on the extent or location of B cell infiltration.
Plasma cells in coronaries with AV are heterogeneous
Compact collections of plasma cells in the neointima, adventitia and epicardium raised the question of the clonality of plasma cells in these regions. Patients with sufficient tissue and plasma cells were evaluated for cytoplasmic content of all four IgG subclasses by immunohistochemistry. In 10 of 12 patients (83%) with sufficient tissue, all four IgG subclasses were present within plasma cell infiltrates. The remaining two patients showed the presence of only IgG2 and IgG3. However, in these samples the quality of IgG1 staining was inadequate. In addition, plasma cells in nodules were heterogeneous for kappa and lambda light chains indicating that the plasma cell infiltrates were polyclonal (Supplemental Digital Content, Figure S1).
B cell and plasma cell infiltrates encompass all patient groups
To explore the relationship between B cell and plasma cell infiltrates with length of graft survival, we divided the patient population into short (<3 years), intermediate (3–12 years), and long (>12 years) graft survival groups (Table 2). In both the short and long graft survival groups, 3 of 4 grafts had 1–3 adventitial B cell nodules and one heart had no B cell nodules. The two patients with greater than 3 adventitial B cell nodules were in the intermediate graft survival group. Diffuse adventitial B cell infiltrates were only present in 1 of 4 hearts in the short graft survival group and no neointimal B cell infiltrates were seen in this group. However, the long graft survival group had diffuse B cell infiltrates in all of the hearts and neointimal B cell infiltrates in 3 of 4 of the hearts.
Table 2.
B cell and plasma cell infiltrates by length of graft survival.
| Graft Survival Group | Patient | Graft Survival (years) | B Cells | Plasma Cells | Donor Specific Antibody | C4d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodules | Diffuse | Neointima | B Cell Nodule | Diffuse | Neointima | |||||
| Short (<3 years) | 2 | 1.4 | 0 | 0 | 0 | 0 | 1+ | 2+ | NS | − |
| 13 | 1.8 | 1–3 | 0 | 0 | 1+ | 2+ | 0 | No DSA | + | |
| 15 | 2.2 | 1–3 | 0 | 0 | 1+ | 0 | 0 | NS | − | |
| 9 | 2.7 | 1–3 | 1+ | 0 | 0 | 0 | 0 | No DSA | NM | |
| Intermediate (3–12 years) | 10 | 3.6 | 1–3 | 1+ | 1+ | 1+ | 1+ | 1+ | No DSA* | − |
| 6 | 5.2 | >3 | 1+ | 1+ | 2+ | 2+ | 1+ | No DSA* | − | |
| 1 | 5.6 | 1–3 | 0 | 0 | 3+ | 3+ | 0 | NS | + | |
| 3 | 6 | 0 | 0 | 0 | 0 | 1+ | 1+ | No DSA | − | |
| 12 | 6.7 | >3 | 1+ | 1+ | 1+ | 2+ | 1+ | No DSA | − | |
| 7 | 8.1 | 1–3 | 1+ | 0 | 1+ | 2+ | 0 | NS | + | |
| 5 | 10.8 | 1–3 | 1+ | 1+ | 2+ | 1+ | 1+ | No DSA | − | |
| 14 | 11.1 | 1–3 | 0 | 0 | 1+ | 0 | 0 | No DSA | − | |
| Long (>12 years) | 11 | 12.3 | 1–3 | 1+ | 1+ | 2+ | 1+ | 1+ | NS | − |
| 4 | 12.7 | 1–3 | 1+ | 2+ | 1+ | 1+ | 0 | No DSA | − | |
| 8 | 13.4 | 0 | 1+ | 0 | 0 | 2+ | 0 | No DSA | NM | |
| 16 | 14.6 | 1–3 | 1+ | 1+ | 2+ | 1+ | 0 | No DSA | − | |
NS = No serum available
NM = No myocardium available for analysis
DSA = Donor Specific Antibody
= Low levels of non-donor MHC Class II antibodies present
= positive diffuse capillary C4d staining
Similarly, we examined the presence of plasma cells in relation to length of graft survival. The presence of plasma cells surrounding B cell nodular infiltrates was slightly higher (3/4) in the long graft survival group than the short survival group (2/4). Likewise, the presence of diffuse adventitial plasma cells were slightly higher in the long graft survival group (4/4) compared to the short survival group (2/4). The presence of neointimal plasma cells between these two groups was the same (1/4). Overall there was only a slight increase in B cell or plasma cell infiltrates with length of graft survival. In addition, we saw no correlation between the gender nor age of patient with the presence or pattern of B cell or plasma cell infiltrates (Table 1).
Circulating donor specific antibodies were not found at the time of explantation
The presence of B cells and plasma cells along with compartmentalized infiltrate nodules suggested a local immune response to the transplant. Therefore, we looked for donor specific antibodies in patient serum at the time of explantation. Serum samples were available for the 10 patients who were retransplanted after 1998. Donor specific antibodies were not detected in the serum from any of these 10 patients. Two patients had low levels of antibodies to MHC Class II antigens that were not present on the donor heart.
C4d deposition in coronary arteries and capillaries
Tissue sections with sufficient myocardium adjacent to coronaries were stained with C4d to assess complement deposition. Two of 14 patients showed strong diffuse capillary C4d staining and one patient demonstrated a weak diffuse capillary C4d staining in the myocardium (Figure 3B). In these three patients, the endothelium of main coronary arteries were not well enough preserved for definitive evaluation of C4d, however, medium sized branching arteries showed C4d deposits (Figure 3A). There was no correlation between C4d positivity and the length of graft survival or B cell and plasma cell infiltrates (Table 2).
Studies of B cell and plasma cell infiltrates in control patients with open heart surgery and native atherosclerosis
In order to eliminate the possibility that B cell and plasma cell infiltrates were a nonspecific inflammatory response to open heart surgery, we examined native hearts from patients who had undergone open heart surgery 7 to 10 years prior to explantation. The indication for open heart surgery included coronary artery bypass grafts, stent placement, and valve replacement. To maximize the possibility of finding surgically related inflammatory responses, we examined the surgical site as indicated by suture sites. Control coronary arteries revealed minimal inflammatory infiltrates and specifically no epicardial lymphoid nodules surrounding the repaired coronary arteries.
Hearts with native atherosclerosis that were removed from nine patients who received primary cardiac transplants were analyzed for the presence of B cell, plasma cell and T cell infiltrates (Table 3). Two patients had one small mixed B cell and T cell nodule adjacent to the affected coronary artery and there were no diffuse or neointimal B cell infiltrates (Figure S2). Only one patient demonstrated minimal diffuse plasma cell infiltrates. T cells were scattered diffusely within the atherosclerotic lesions.
Table 3.
Clinical and histological characteristics of hearts from patients with native atherosclerosis.
| Patient | Gender | Age (years) | B Cells | Plasma Cells | ||||
|---|---|---|---|---|---|---|---|---|
| Nodule | Diffuse | Neointima | Surrounding B Cell Nodule | Diffuse | Neointima | |||
| 1 | M | 53 | 1–3 | 0 | 0 | 0 | 0 | 0 |
| 2 | F | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | M | 46 | 1–3 | 0 | 0 | 0 | 1+ | 0 |
| 4 | M | 70 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | M | 59 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | M | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | M | 55 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | M | 44 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | M | 65 | 0 | 0 | 0 | 0 | 0 | 0 |
| Totals | 1F:8M | 57.6 ± 8.8 | 1/9* positive |
0/9* positive |
0/9* positive |
0/9* positive |
1/9* positive |
0/9* positive |
significance of p<0.001 by Chi-square test when compared with corresponding categories from patients with AV
Discussion
This study demonstrates that B cells or plasma cells are a consistent component of AV. In fact, B cells or plasma cells were found in coronary arteries from all 16 patients. In contrast, only a minority of vessels with native atherosclerosis (2/9 patients) had clusters of B cells, which is consistent with the existing concepts of a prominent role for T cells and macrophages in the pathogenesis of atheroscerosis (23, 24). However, prominent B cell infiltrates have been described in atherosclerotic lesions of patients with rheumatoid arthritis (25). These data substantiate our previously reported data from microarrays, in which transcripts from immunoglobulin genes were consistently elevated in 12 coronary arteries with AV, but not in 6 coronary arteries with native atherosclerosis (8).
We have characterized three main patterns of B cell and plasma cell infiltration. Most frequently, B cells and plasma cells formed nodules in the adventitia of coronary arteries with AV; 13 of the 16 hearts had nodules of B cells, 12 of which contained nodules of both B cells and plasma cells. Nodules of B cells have been reported in renal transplants, but this finding is confined to only a subset of patients. In endomyocardial biopsies from cardiac transplants, B cells can be a prominent feature of the Quilty effect. In the current diagnostic formulation (26), the Quilty effect is defined as nodular endocardial infiltrates that are distinguished from acute rejection by the presence of B lymphocytes and plasma cells in a background of fibrosis and prominent vascularity. Although several recent publications have emphasized the B cell content of these lesions and have reported correlations with acute rejection (20, 21, 27), the relationship of Quilty lesions and chronic rejection remains unclear. We reviewed all endomyocardial biopsies from the hearts in our study before explantation and identified the Quilty effect in one or more biopsies from only 8 of the 16 patients. Therefore, nodular infiltrates of B cells and plasma cells were more frequently found in the epicardium adjacent to coronaries with AV than in the endocardium. While some characteristics of Quilty lesions are present in the nodules described here, the epicardial location of the nodules excludes them from being conventional Quilty lesions. In addition, the presence of Quilty lesions was not indicative of the extent or pattern of epicardial B cell infiltrates.
In 7 of 13 patients, adventitial nodules contained compartmentalized B and T cell zones characteristic of tertiary lymphoid neogenesis. This phenomenon has been studied extensively in chronic inflammatory diseases such as rheumatoid arthritis. In patients with rheumatoid arthritis, B cell nodules have been described in coronary arteries as well as the synovium (25, 28). There are reports (6, 29) of tertiary lymphoid follicles in renal transplants and a few reports in experimental (7, 30) and clinical heart transplants (7, 8). The organization of lymphoid tissue at the site of immune response allows a continued reaction to the immune stimulus. The fact that these nodules contained FDCs that stained positively for C4d provides evidence that they are functional. Complement split products are known to localize antigen to the complement receptors on FDCs (31, 32). Dendritic cells in these nodules also expressed CXCL13 in a similar pattern to the staining reported for lymphoid neogenesis in rheumatoid arthritis (28), suggesting a mechanism for the recruitment of B cells.
In addition to B cell and plasma cell nodules, diffuse infiltrates were found in the adventitia surrounding vessels with AV. All but 2 of the hearts had diffuse infiltrates of B cells or plasma cells in the adventia and half of the hearts had diffuse infiltrates of both B cells and plasma cells in the adventia. Over half (9/16) of the hearts had infiltrates of B cells or plasma cells in the neointimal while almost a third of the hearts (5/16) had B cell and plasma cell infiltrates in the neointima of vessels with AV. These neointimal and advential infiltrates were often embedded in fibrosis. Mengel and colleagues (33) have reported that transcripts associated with B cells and plasma cells are a signature of scarring in renal transplants. Recent data from experimental models indicate that B cells can promote fibrosis (36, 37). Adventitial fibrosis has functional consequence because it prevents compensatory outward remodeling of coronary arteries in response to AV.
The finding that nodules of plasma cell infiltrates stained heterogeneously for heavy and light chains indicated that the nodules did not result from expansions of single clones of B cells. This heterogeneity also decreases the likelihood that the nodules are related to post-transplant lymphoproliferative disorder.
The availability of multiple segments from several individual coronary arteries allowed us to determine whether infiltrates were continuous or focal. We found that B cells and plasma cells were prevalent throughout vessels and were not confined to one region or single coronary vessel. However, the composition and degree of infiltration varied along the length of the vessels.
With the pervasiveness of B cells and especially plasma cells with demonstrable cytoplasmic immunoglobulins in this group of patients, we tested the possibility that there may be a high incidence of DSA in these patients. However, we found no evidence of DSA at the time of explantation. The absence of antibodies to HLA in the circulation does not exclude the possibility that antibodies to HLA were absorbed to the transplant or that the plasma cells produced tissue specific antibodies or autoantibodies, both of which have been reported in clinical and experimental cardiac transplants (34). In fact, 3 of 14 transplants had diffuse C4d deposits in capillaries in myocardium adjacent to the coronary arteries, which are consistent with complement activation by donor reactive antibodies.
In summary, B cells and plasma cells are consistent findings in and around coronary arteries with AV. These cells were found most frequently in nodules, some of which had distinct compartmentalization that typifies tertiary lymphoid nodules. In addition, diffuse infiltrates of B cells and plasma cells were found in fibrotic areas of the neointima and adventitia. The frequency of B cells and plasma cells within and surrounding vessels with AV was significantly higher than in coronaries with native atherosclerosis or surgical procedures.
Supplementary Material
Figure S1. Plasma cell populations surrounding epicardial coronary arteries with AV show both kappa (A; 2X, inset 20X) and lambda (B; 2X, inset 20X) light chain positive plasma cells.
Figure S2. Coronary arteries with native atherosclerosis show minimal B cell infiltrates. A; H&E of a coronary artery with native atherosclerosis (2X). B; CD20 stain for B cells reveals minor B cell infiltrates (10X).
Acknowledgments
We would like to thank Rene Rodriguez and Carmela Tan for their advice in selecting a reagent for human C4d. This work was supported by funding from the National Institute of Health grants R01-AI42387 and P01-HL56091.
Abbreviations
- AV
allograft vasculopathy
- DSA
donor specific antibody
- FDC
follicular dendritic cell
References
- 1.Taylor DO, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report–2008. J Heart Lung Transplant. 2008;27(9):943. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Billingham ME. Pathology and etiology of chronic rejection of the heart. Clin Transplant. 1994;8(3 Pt 2):289. [PubMed] [Google Scholar]
- 3.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 4.Paul LC, Fellstrom B. Chronic vascular rejection of the heart and the kidney–have rational treatment options emerged? Transplantation. 1992;53(6):1169. doi: 10.1097/00007890-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Wong BW, Rahmani M, Rezai N, McManus BM. Progress in heart transplantation. Cardiovasc Pathol. 2005;14(4):176. doi: 10.1016/j.carpath.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Segerer S, Schlondorff D. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int. 2008;73(5):533. doi: 10.1038/sj.ki.5002734. [DOI] [PubMed] [Google Scholar]
- 7.Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102(41):14723. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., 3rd Antibody and complement in transplant vasculopathy. Circ Res. 2007;100(2):191. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 9.Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB. CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation. 2006;82(12):1769. doi: 10.1097/01.tp.0000250572.46679.45. [DOI] [PubMed] [Google Scholar]
- 10.Martins HL, Silva C, Martini D, Noronha IL. Detection of B lymphocytes (CD20+) in renal allograft biopsy specimens. Transplant Proc. 2007;39(2):432. doi: 10.1016/j.transproceed.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation. 2008;85(12):1705. doi: 10.1097/TP.0b013e318177793e. [DOI] [PubMed] [Google Scholar]
- 12.Zarkhin V, Kambham N, Li L, et al. Characterization of intragraft B cells during renal allograft rejection. Kidney Int. 2008;74(5):664. doi: 10.1038/ki.2008.249. [DOI] [PubMed] [Google Scholar]
- 13.Hippen BE, DeMattos A, Cook WJ, Kew CE, 2nd, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5(9):2248. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Bagnasco SM, Tsai W, Rahman MH, et al. CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant. 2007;7(8):1968. doi: 10.1111/j.1600-6143.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 15.Kayler LK, Lakkis FG, Morgan C, et al. Acute cellular rejection with CD20-positive lymphoid clusters in kidney transplant patients following lymphocyte depletion. Am J Transplant. 2007;7(4):949. doi: 10.1111/j.1600-6143.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheepstra C, Bemelman FJ, van der Loos C, et al. B cells in cluster or in a scattered pattern do not correlate with clinical outcome of renal allograft rejection. Transplantation. 2008;86(6):772. doi: 10.1097/TP.0b013e3181860a74. [DOI] [PubMed] [Google Scholar]
- 17.Nasr IW, Wang Y, Gao G, et al. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005;174(10):6161. doi: 10.4049/jimmunol.174.10.6161. [DOI] [PubMed] [Google Scholar]
- 18.Kottke-Marchant K, Ratliff NB. Endomyocardial lymphocytic infiltrates in cardiac transplant recipients. Incidence and characterization. Arch Pathol Lab Med. 1989;113(6):690. [PubMed] [Google Scholar]
- 19.Radio SJ, McManus BM, Winters GL, et al. Preferential endocardial residence of B-cells in the “Quilty effect” of human heart allografts: immunohistochemical distinction from rejection. Mod Pathol. 1991;4(5):654. [PubMed] [Google Scholar]
- 20.Zakliczynski M, Nozynski J, Konecka-Mrowka D, et al. Quilty effect correlates with biopsy-proven acute cellular rejection but does not predict transplanted heart coronary artery vasculopathy. J Heart Lung Transplant. 2009;28(3):255. doi: 10.1016/j.healun.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Hiemann NE, Knosalla C, Wellnhofer E, Lehmkuhl HB, Hetzer R, Meyer R. Quilty in biopsy is associated with poor prognosis after heart transplantation. Transpl Immunol. 2008;19(3–4):209. doi: 10.1016/j.trim.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Chu KE, Ho EK, de la Torre L, Vasilescu ER, Marboe CC. The relationship of nodular endocardial infiltrates (Quilty lesions) to survival, patient age, anti-HLA antibodies, and coronary artery disease following heart transplantation. Cardiovasc Pathol. 2005;14(4):219. doi: 10.1016/j.carpath.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 24.Ovchinnikova O, Robertson AK, Wagsater D, et al. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe(−/−) mice. Am J Pathol. 2009;174(2):693. doi: 10.2353/ajpath.2009.080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubry MC, Riehle DL, Edwards WD, et al. B-Lymphocytes in plaque and adventitia of coronary arteries in two patients with rheumatoid arthritis and coronary atherosclerosis: preliminary observations. Cardiovasc Pathol. 2004;13(4):233. doi: 10.1016/j.carpath.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Di Carlo E, D’Antuono T, Contento S, Di Nicola M, Ballone E, Sorrentino C. Quilty effect has the features of lymphoid neogenesis and shares CXCL13-CXCR5 pathway with recurrent acute cardiac rejections. Am J Transplant. 2007;7(1):201. doi: 10.1111/j.1600-6143.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- 28.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 29.Thaunat O, Patey N, Gautreau C, et al. B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation. 2008;85(11):1648. doi: 10.1097/TP.0b013e3181735723. [DOI] [PubMed] [Google Scholar]
- 30.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5(3):510. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 31.Zwirner J, Felber E, Schmidt P, Riethmuller G, Feucht HE. Complement activation in human lymphoid germinal centres. Immunology. 1989;66(2):270. [PMC free article] [PubMed] [Google Scholar]
- 32.Bu X, Zheng Z, Wang C, Yu Y. Significance of C4d deposition in the follicular lymphoma and MALT lymphoma and their relationship with follicular dendritic cells. Pathol Res Pract. 2007;203(3):163. doi: 10.1016/j.prp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Mengel M, Reeve J, Bunnag S, et al. Molecular correlates of scarring in kidney transplants: the emergence of mast cell transcripts. Am J Transplant. 2009;9(1):169. doi: 10.1111/j.1600-6143.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- 34.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170(4):1415. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plasma cell populations surrounding epicardial coronary arteries with AV show both kappa (A; 2X, inset 20X) and lambda (B; 2X, inset 20X) light chain positive plasma cells.
Figure S2. Coronary arteries with native atherosclerosis show minimal B cell infiltrates. A; H&E of a coronary artery with native atherosclerosis (2X). B; CD20 stain for B cells reveals minor B cell infiltrates (10X).


