Abstract
Adequate preservation of biospecimens has been proven to be critical to obtain reliable and reproducible results in genomics, transcriptomics, proteomics, and many other assays. Most biological assays can be performed on specimens preserved in −80°C ultra-low freezers, but their quality can be influenced by temperature variability within storage units. Thus, regulatory standards such as those from the College of American Pathologists (CAP), the federal Clinical Laboratory Improvement Amendments, and standards from the Food and Drug Administration require temperature mapping, a standard quality assessment for accreditation when using ultra-low freezers for long-term biospecimen storage. The current mapping methods, providing annual/periodic data, may not be adequate indicators of temperature stability within the different zones of the freezers. In addition, they frequently require manual handling of biospecimens periodically, as they require freezers to be emptied or rearranged temporarily for the installation of temperature probes, risking the integrity of biospecimen quality. In this article, we describe a novel monitoring methodology based on real-time temperature reading of multiple zones by permanently installing thermocouples. An online cloud-based application records temperature variations within 1 minute intervals, and its 24/7 alert system triggers text alarm messages to a predefined set of users when temperature values increase above preset defaults. This provides an opportunity to take remedial action and to obtain a better-quality assessment. Our results indicate that real-time temperature monitoring at multiple zones of a freezer with a 1 minute resolution is a stable and sustainable methodology and, most importantly, lowers the risk of compromising the quality of the biospecimen. The design and use of the real-time monitoring system for ultra-low freezers is one of the acceptable methods by CAP to ensure the stability of biospecimen quality during long-term storage.
Keywords: storage, biorepository, CAP accreditation, real-time temperature, ultra-low freezer
Introduction
High-quality human biospecimens that are adequately processed and stored at a well-maintained and monitored temperature are crucial for many studies such as genomics, transcriptomics, and proteomics. As previously reported, the preservation of DNA, RNA, and proteins in biological specimens is essential for initial diagnosis, subsequent verification and comparison, as well as for archival retention of pathological materials in modern molecular diagnostics and precision medicine.1 To ensure high-quality results, it is essential after biospecimen collection and processing to have a reliable method to assure appropriate storage temperature, as the temperature of the biospecimen itself drives its integrity. To preserve genome, transcriptome, and proteome integrity, the acceptable temperature range for blood derivatives is below −70°C.1,2 Maintenance of ultra-low temperature throughout the freezer and the ability for real-time monitoring are important for the preservation of high-quality biospecimens. In the process of developing a standard method of freezer mapping for biobanks, we conducted an extensive search in PubMED and Google using search strings such as freezer mapping, biospecimen, ultra-low freezers, biorepository, and biobanking quality assurance. However, the search turned up a limited amount of information regarding guidelines for mapping requirements and their implications. Temperature mapping validation is mandatory for the manufacturing of regulatory controlled products, food, and pharmaceuticals when thermal processing or storage is involved (heating or cooling). The U.S. Code of Federal Regulations (CFR)3 and the International Society for Pharmaceutical Engineering (ISPE) provide some guidance.4 The College of American Pathologists (CAP) is the main regulatory/accreditation agency for biorepositories/biobanks in the United States. As per their guidelines, the best practice is to perform and record temperature mapping for each new freezer before deployment, and periodically for freezers currently in service. The director/designee determines the frequency of mapping and data review. These guidelines are subjective and provided us with a challenge to generate baseline mapping for existing freezers with specimens. Baseline mapping and periodic validation pose critical risks to the specimens of being exposed to temperatures higher than those within the acceptable range, at the time of baseline mapping and at periodic revalidation. In light of a lack of specifics, there is an evident need for an improved method of freezer mapping. We therefore developed a real-time continuous temperature mapping method of multiple zones of ultra-low freezers.
We deployed a method that provides real-time temperature monitoring with 1 minute resolution on each shelf for a better-quality assessment of long-term storage. The ISPE4 recommends nine sensors for spaces less than 2 m3 in volume. Our ultra-low freezer space is ∼0.75 m3, and we used one sensor per shelf and placed them staggered in left/right/front/back of the shelf to achieve comprehensive temperature recording and monitoring within the unit.
Materials and Methods
To validate real-time temperature mapping, three ultra-low freezers with multi-zonal temperature sensors (TSs) were used. The pre-calibrated resistance temperature detector (RTD) TSs were provided by and installed by Klatu (100 Ohm Platinum 3-wire; TC = 3850 ppm/K). The freezers were plugged only into special red outlets that are part of the emergency electrical backup system at the Moores Cancer Center. The online monitoring system allows for real-time 24/7 monitoring with 1 minute resolution. When the temperature rose above a preset critical value, all users at the biorepository automatically receive a notification through a phone messaging system and via email. After receiving the notification, appropriate actions can be taken following the CAP compliance risk management standard operating procedures (SOPs).
Baseline ultra-low freezer mapping
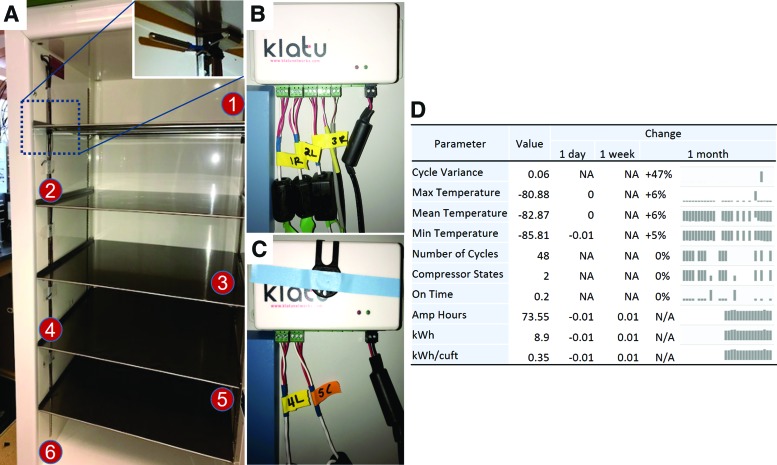
Before powering the ultra-low temperature freezer, precalibrated TSs were installed in an alternating order—bottom sixth shelf sensor on the left front, fifth shelf sensor on the right back, fourth shelf sensor left front, third shelf sensor right back, and second shelf left front. The first shelf sensor is factory-installed with retrievable data from a touchscreen interface. The TSs were connected to a cloud-based online real-time tracking system (TRAXX; Klatu Networks, Inc.), and the data were recorded with 1 minute resolution (Fig. 1A). Door sensors were also installed, and opening and closing data were recorded with cloud-based online monitoring. TRAXX energy consumption, ambient temperature, and compressor performance data were also recorded in real time. Validation of freezer temperature uniformity was performed with an online monitoring system under four different conditions: empty freezer, freezer filled with empty racks, freezer filled to capacity, and a door opening test at 60- and 90-second intervals. The tests were performed in empty racks, as it was considered that this would lead to more extreme temperature variations, thus increasing the stringency of the test. A freezer that is full may hold less exchangeable air that an empty one; so there is less warm air inside it to cool down when the door is closed.
FIG. 1.
TS locations; the TS in shelf 1 is factory-installed; shelves 2, 4, 6—TSs are installed at the front of the freezer; and shelves 3–5—TSs are installed at the back of the freezer (A–C). Data of six performance parameters were exported using TRAXX online monitoring system in real time (Klatu Networks, Inc.) (D). TS, temperature sensors. Color images are available online.
Baseline ultra-low freezer mapping for freezers with specimens
Biospecimens from −80°C ultra-low freezers were transferred to standing −80°C ultra-low units to comply with CAP guidelines. In brief, both recipient and donor freezers were moved to close proximity, then all the racks were transferred shelf by shelf in a time-efficient manner; the doors of the freezers were opened as required. The empty freezers were thawed before installation of TSs. Baseline freezer mapping was performed as described above. Data was recorded for 72 hours in 1 minute resolution and then compared with factory-installed temperature data. Currently, continuous temperature data recording of ultra-low freezers with full capacity for 5 + 1 zones is in progress for all mapped freezers, and remedial actions are being performed as needed.
To validate real-time mapping, we performed (1) baseline temperature mapping on empty freezers, (2) temperature mapping with empty racks and boxes, (3) temperature mapping with freezers filled with capacity (full specimen load), and (4) door opening testing (with empty racks).
Statistical analysis was done using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA; www.graphpad.com).
Cost analysis was performed by requesting quotes from multiple vendors (Sharpe Refrigeration, Inc., Cascade Thermal Solutions and Omega, Inc.) for ultra-low freezer mapping.
Results
Multiple sensors were successfully installed in the various zones of ultra-low freezers and connected to the cloud-based monitoring system (Fig. 1A, D, C). Continuous transmission of temperature values with 1 minute resolution was recorded in real time, along with six performance parameters—compressor performance, energy consumption, door position, room temperature, freezer temperature, and multi-zone temperature (Fig. 1D), providing the overall ultra-low freezer health status in real time. Temperature uniformity among various conditions of freezer storage was successfully monitored (Fig. 2). To establish baseline mapping, an empty ultra-low freezer was set up with multiple sensors, and data were recorded in 1 minute intervals. Data analysis was performed in 1 hour intervals, which resulted in temperatures within the acceptable range (greater than −70°C). Temperature variation coefficients between the factory-default TS and shelves 2–6 were well within acceptable values (range −79.1 to −83.17; no significant differences). Similar outcomes were recorded when comparing racks filled with empty boxes or racks filled to capacity (Fig. 2). This demonstrates the ability to monitor an entire freezer's temperature status in real time, and therefore validate the temperature uniformity among shelves in real time. Furthermore, we were able to monitor door opening/closing in real time and its temperature increase and recovery time (Fig. 3). Door opening for 60 and 90 seconds resulted in a temperature drop that was recorded in real time along with recovery time. Sensors installed at the back of the freezer showed less temperature fluctuation when doors were open for 60/90 seconds (range −80°C to −70°C), while sensors at the front showed greater temperature fluctuation (range −80°C to −54°C). This captures temperature variance among all areas of the freezer (Fig. 3).
FIG. 2.
Real-time monitoring system validation was performed by plotting the temperature (in Celsius) on the y-axis over minute-by-minute recording on the x-axis to illustrate the real-time monitoring system in the top graph. The data were exported using TRAXX online monitoring system by Klatu Networks, Inc. The bottom graph shows temperature variations in three freezers under different conditions over 72 hours. The average temperature for each freezer in Celsius was plotted on the y-axis, and each condition (freezer filled to capacity, freezer with empty racks, empty freezer) on the x-axis at 2-hour intervals. Each data point represents an average freezer temperature. Color images are available online.
FIG. 3.
Real-time monitoring of temperature opening recovery with empty racks. The doors were opened for 60 and 90 seconds. Color images are available online.
A cost analysis from estimates obtained from multiple vendors showed that expenditures per time for each ultra-low freezer mapping ranged from $1200 to $1800 annually/biannually as per regulatory agencies' guidelines. Moreover, paying for mapping materials and installation procedures costed about $3500–$5000 and required about 20–30 work-hours to complete the mapping process per freezer at each mapping time (annually/biannually). Using our methodology, the one-time cost of a continuous monitoring of multiple zones was $700 and $45 annual online monitoring fee per freezer.
Discussion
This study presents a novel approach for continuous temperature monitoring in real time for various zones on ultra-low freezers, which provides a tool for maintaining the temperature chain of custody in real time and enabling remedial action shelf-by-shelf in real time. The overall health of ultra-low freezers is continuously monitored by recording compressor performance, energy consumption, door position, room temperature, freezer temperature, and multi-zone temperature in real time. Thus, real-time mapping improves the overall maintenance of high-quality biospecimens that meets the requirements of many regulatory agencies, including CAP. This study confirms the suitability of using multi-probe real-time monitoring software to achieve regulatory compliance at lower cost, avoiding almost entirely the manual and invasive handling of specimens during accreditation and recertification processes.
A one-time investment of installing multi-probe real-time monitoring is cost-effective in comparison with per-time/periodical mapping. More importantly, this improved methodology provides minute-by-minute continuous freezer multi-zonal heath monitoring versus one-time/periodic mapping. Other aspects of this system provide biorepositories with new tools to predict and mitigate cold storage equipment failure risks on a shelf-by-shelf basis before they result in degradation or loss of specimens. This design and use of real-time monitoring system for ultra-low freezers is an accepted method by CAP.
Acknowledgments
We would like to thank Mason Kyle for help in the installation of temperature probes and critical comments on the article. We thank Donna Peterson and Pete Larsson form Klatu Networks, Inc. (TRAXX) for Technical Support. This work was supported by CCSG grant P30CA23100.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1. Fabre AL, Luis A, Colotte M, et al. High DNA stability in white blood cells and buffy coat lysates stored at ambient temperature under anoxic and anhydrous atmosphere. PLoS One 2017;12:e0188547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shabihkhani M, Lucey GM, Wei B, et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem 2014;47:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. CFR. PART 211.142 Current Good Manufacturing Practice for Finished Pharmaceuticals. In: 21 T, (ed). Office of the Federal Register; Silver Spring, MD: FDA; 2017. https://www.fda.gov/drugs/developmentapprovalprocess/manufacturing/ucm090016.htm [Google Scholar]
- 4. ISPE. Good Practice Guide: Cold Chain Management. https://ispe.org/publications/guidance-documents/good-practice-guide-cold-chain-management 2011:140 (accessed September10, 2018)