Abstract
The goal of this study was to demonstrate that a novel resting state BOLD ALFF (amplitude of low frequency fluctuations)-based correction method can substantially enhance the detectability of motor task activation in the presence of tumor-induced neurovascular uncoupling (NVU). Twelve de novo brain tumor patients who underwent comprehensive clinical BOLD fMRI exams including task fMRI and resting state fMRI (rsfMRI) were evaluated. Each patient displayed decreased/absent task fMRI activation in the ipsilesional primary motor cortex in the absence of corresponding motor deficit or suboptimal task performance, consistent with NVU. Z-score maps for the motor tasks were obtained from general linear model (GLM) analysis (reflecting motor activation vs. rest). ALFF maps were calculated from rsfMRI data. Precentral and postcentral gyri in contralesional (CL) and ipsilesional (IL) hemispheres were parcellated using an Automated Anatomical Labeling (AAL) template for each patient. A novel ALFF-based correction method was used to identify the NVU affected voxels in the ipsilesional primary motor cortex (PMC), and a correction factor was applied to normalize the baseline Z-scores for these voxels. In all cases, substantially greater activation was seen on post-ALFF correction motor activation maps within the ipsilesional precentral gyri than in the pre-ALFF correction activation maps. We have demonstrated the feasibility of a new resting state ALFF-based technique for effective correction of brain tumor-related NVU in the primary motor cortex.
Keywords: frequency domain metrics ALFF (amplitude of low frequency fluctuations), motor activation, neurovascular uncoupling, presurgical mapping
Introduction
Neurovascular uncoupling (NVU) is a major problem in the interpretation of task-based BOLD fMRI (blood oxygen level-dependent functional magnetic resonance imaging) activation maps in the setting of brain tumors or other focal brain lesions because it may lead to spuriously decreased or even absent activation in electrically active, essential functional (i.e., eloquent) cortex (DeYoe and Ulmer, 2008; Holodny et al., 2000; Hou et al., 2006). BOLD fMRI detects alterations in deoxyhemoglobin concentration influenced by hemodynamic factors that occur in response to neuronal activity. Brain activation mapping using BOLD fMRI relies on the tight coupling between hemodynamic changes and neuronal activity (Attwell et al., 2010; Villringer and Dirnagl, 1995).
The mechanisms underlying brain tumor-induced NVU are not yet completely understood, but preclinical studies have suggested that tumors induce a variety of physiologic changes in the microenvironment that may contribute to this phenomenon (Lee et al., 2009; Pak et al., 2017). For example, tumors may induce remodeling of the extracellular matrix, changes in endothelial cells, and changes in endothelial-associated astrocytes. Previous studies (Jiang et al., 2010; Pillai and Zaca, 2011, 2012; Ulmer et al., 2003; Zaca et al., 2011, 2014) have demonstrated prevalence of NVU in patients with brain tumors. Recent studies (Agarwal et al., 2016a,b, 2017b; Mallela et al., 2016) have demonstrated that brain tumor-induced NVU can cause impaired resting-state BOLD fMRI (rsfMRI) signals similar to the findings in task fMRI activations (Zaca et al., 2014).
The low-frequency fluctuations (<0.1 Hz) in rsfMRI contain information about cortical brain activity of the subject, scanned while at rest (Biswal et al., 1995). Biswal and colleagues (1995) noticed that amplitude of low frequency fluctuations (ALFF) was higher in gray matter than in white matter. This finding indicated that the relative magnitude of these low-frequency fluctuations in brain regions can be used as a marker of regional difference or dysfunction. Zang and associates (2007) proposed ALFF (0.01–0.08 Hz) and used it to investigate baseline brain function in patients with attention-deficit/hyperactivity disorder.
We have recently shown that brain tumor-induced NVU can be detected on ALFF and fractional amplitude of low frequency fluctuations (fALFF) maps (Agarwal et al., 2017a). Therefore, in this study, we propose a novel correction method based on the resting-state frequency domain metric ALFF to enhance detectability of ipsilesional (IL) impaired task-based activation in the setting of brain tumor-induced NVU. To our knowledge, no study has been published to date describing methods for correction of impaired BOLD signal caused by brain tumor-induced NVU. Here, we evaluated a cohort of 12 de novo (i.e., newly diagnosed and before any surgical resection or chemoradiation therapy) brain tumor patients with both resting-state fMRI mapping and clinical sensorimotor cortical mapping with task-based BOLD fMRI acquisitions.
We demonstrate the feasibility of correcting for brain tumor-induced NVU at a single subject level for sensorimotor activation, and show how this approach enhances the detectability of motor task-based activation.
Materials and Methods
Patients
Twelve de novo (treatment naive) brain tumor patients referred for routine clinical presurgical motor mapping with BOLD fMRI were included in this study, which was approved by our institutional review board. These patients demonstrated evidence of potential NVU based on results of their clinical task fMRI scans, as described in previous publications (Zaca et al., 2014). In every patient, the decreased/absent task fMRI activation in the primary IL sensorimotor cortex without associated clinical deficits precluding adequate task performance was highly suggestive of NVU. These patients all presented with primary perirolandic gliomas with NVU affecting the face or hand representation area (RA) of the primary motor cortex (PMC).
In Table 1, a description of tumor location, histopathology, World Health Organization (WHO) grade, and fMRI tasks performed is provided for all patients in our study. In addition, for each patient, note was made regarding the presence or absence of intraoperative mapping confirmation of PMC localization. Evoked potential cortical mapping was performed for identification of the central sulcus. Evoked potentials from the contralesional (CL) median nerve were obtained with recordings made from an eight-contact strip electrode placed directly on the cortex. A moderately well-defined phase reversal was suggestive of the location of central sulcus. Every patient demonstrated evidence of sensorimotor NVU affecting the face or hand RA of the PMC on the initial fMRI examination.
Table 1.
Tumor Location, Histology, and Tumor Grade As Well As Task Description for the Patients Included in This Study
| Patient No. | Motor tasks demonstrating NVU | Tumor location | Histopathology | WHO tumor grade | Intraoperative confirmation of PMC localization, Yes (Y)/No (N) |
|---|---|---|---|---|---|
| 1 | TM | R Frontal perirolandic | Anaplastic astrocytoma | III | Y |
| 2 | TM | L Frontal lobe | Oligoastrocytoma | II | N |
| 3 | TM | R Perirolandic | Oligoastrocytoma | II | Y |
| 4 | FINGM | L Frontoparietal | Anaplastic oligoastrocytoma | III | Y |
| 5 | TM | L Hemispheric | Oligoastrocytoma | II | Y |
| 6 | TM | L Hemispheric | Glioma with necrosis | IV | Y |
| 7 | TM | R Perirolandic frontoparietal operculum | Oligodendroglioma | II | Y |
| 8 | FINGM | L Perirolandic | Glioblastoma | IV | Y |
| 9 | FINGM | R Frontal | Oligodendroglioma | II | Y |
| 10 | TM | R Frontoparietal operculum | Oligodendroglioma | II | N |
| 11 | FINGM | L Frontal lobe | Oligodendroglioma | II | N |
| 12 | FINGM | R Parietal | Oligodendroglioma | II | Y |
For each case, presence or absence of intraoperative mapping confirmation of PMC localization was also noted. Note that all patients demonstrated abnormally decreased task activation in the face or hand representation area of the PMC as a manifestation of NVU in the absence of corresponding motor deficit or suboptimal task performance.
FINGM, bilateral simultaneous sequential finger tapping; L, left; NVU, neurovascular uncoupling; PMC, primary motor cortex; R, right; TM, vertical tongue movement; WHO, World Health Organization.
MRI acquisition
Scanning was performed using our standard clinical sequences for fMRI studies on a 3.0 Tesla (T) Siemens Trio MRI system (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12-channel head matrix coil.
The imaging protocol included a three-dimensional T1-weighted imaging sequence (TR = 2300 ms, TI = 900 ms, TE = 3.5 ms, flip angle = 9°, field of view = 24 cm, acquisition matrix = 256 × 256 × 176, slice thickness = 1 mm) as well as an axial two-dimensional (2D) T2 fluid-attenuated inversion recovery imaging sequence (TR = 9000 ms, TI = 2500 ms, TE = 116 ms, flip angle = 141°, field of view = 17.2 × 23 cm, acquisition matrix = 240 × 320 × 53, slice thickness = 3 mm with 3 mm interslice gap) for structural imaging and multiple 2D gradient echo echo planar imaging T2*-weighted BOLD sequences for functional imaging (TR = 2000 ms, TE = 30 ms, flip angle = 90°, field of view = 24 cm, acquisition matrix = 64 × 64 × 33, slice thickness = 4 mm with 1 mm gap between slices, interleaved acquisition) run while patients were performing a motor paradigm, as described in more detail in the following subsection.
For resting-state fMRI, 180 volumes were acquired using the same functional imaging protocol. For the resting-state fMRI acquisition, each patient was instructed to remain still with eyes closed without falling asleep during the scanning period of 6 min. For breath-hold cerebrovascular reactivity (CVR) mapping, the method described by Pillai and Mikulis (2015) has been used. Please refer to this reference for details of image acquisition.
Motor paradigm
All patients performed one or more motor tasks for sensorimotor activation mapping during scanning. To map the face RA of the PMC, a 3-min-long tongue movement task was used, consisting of three cycles of 30-sec blocks of rest alternating with 30-sec blocks of repetitive vertical tongue movement. The hand RA was mapped using a 3-min duration finger tapping task, consisting of three cycles of 30-sec blocks of rest alternating with 30-sec blocks of bilateral simultaneous sequential finger tapping.
Instructions for all tasks were visually cued. A comprehensive prescan training session outside the MRI scanner ensured full patient understanding of task instructions and confirmed their ability to adequately perform each of the tasks. Patient task performance was monitored during the scan via use of both an LCD monitor in the scan suite and real-time fMRI for patient observation and assessment of activation, respectively.
Data analysis
SPM12 software was used for preprocessing of both task fMRI and rsfMRI data. As preprocessing steps, fMRI raw data were slice-time corrected, spatially realigned to correct for head motion, normalized to MNI space at 2 mm voxel resolution, and spatially smoothed using a 6 mm isotropic full-width at half-maximum Gaussian filter.
For the motor task, a voxel-wise general linear model (GLM) analysis was performed with the expected BOLD response modeled by convolving the stimulus corresponding to each paradigm with a theoretical hemodynamic response function (HRF). We used a standard SPM canonical gamma HRF for the motor tasks. Z-score maps for the motor tasks were obtained reflecting motor activation versus rest. For the breath-hold task, a similar GLM analysis was performed to analyze the contrast between the hypercapnia and normocapnia conditions (Pillai and Mikulis, 2015; Pillai and Zaca, 2011, 2012; Zaca et al., 2011, 2014).
Preprocessed rsfMRI data were analyzed using the REST (version 1.8) (Song et al., 2011) toolkit. After detrending for removal of systematic linear trend and low-frequency (0.01–0.08 Hz) bandpass filtering, ALFF maps were calculated from rsfMRI data.
For region of interest (ROI) selection, precentral and postcentral gyri were automatically parcellated using an Automated Anatomical Labeling template (Smith, 2002; Tzourio-Mazoyer et al., 2002) for each patient. CL and IL ROIs circumscribing the combination of precentral and postcentral gyri were obtained for each slice. Consecutive axial sections were evaluated along the z-axis along the craniocaudal length of the tumors. ROIs were then visually inspected to ensure that they encompassed the precentral and postcentral gyri even in the presence of tumor-induced regional mass effects and gyral expansion. Identical ROIs were used for analysis of both maps (task fMRI activation maps and ALFF maps).
The Z-score thresholds used to generate sensorimotor activation maps were determined using an operator-independent method called activation mapping as a percentage of local excitation (AMPLE) (Voyvodic et al., 2009). The maximum Z-score in the CL ROI was determined, and the Z-score threshold for all voxels in both the CL and IL ROIs was set to 50% of this maximum CL ROI Z-score.
NVU correction technique
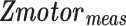 represents the standard Z-score of a voxel in an fMRI motor activation map.
represents the standard Z-score of a voxel in an fMRI motor activation map. 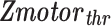 represents the threshold used for generating the baseline motor activation map and was obtained using AMPLE thresholding of 50% (i.e., Z-score at 50% of a local cluster Z-score maximum). Only voxels in the IL ROI that demonstrated subthreshold activation (
represents the threshold used for generating the baseline motor activation map and was obtained using AMPLE thresholding of 50% (i.e., Z-score at 50% of a local cluster Z-score maximum). Only voxels in the IL ROI that demonstrated subthreshold activation (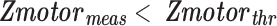 ) were considered for application of the NVU correction technique since suprathreshold activation by definition is seen in voxels not affected by NVU.
) were considered for application of the NVU correction technique since suprathreshold activation by definition is seen in voxels not affected by NVU.
 represents Z-scores of those voxels that fall below a 25% AMPLE threshold (i.e., Z-score below 25% of a local cluster Z-score maximum) and thus considered noise-affected voxels. Only voxels in the IL ROI that demonstrated Z-scores between 25% and 50% of a local cluster Z-score maximum (
represents Z-scores of those voxels that fall below a 25% AMPLE threshold (i.e., Z-score below 25% of a local cluster Z-score maximum) and thus considered noise-affected voxels. Only voxels in the IL ROI that demonstrated Z-scores between 25% and 50% of a local cluster Z-score maximum (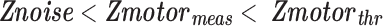 ) were candidate voxels for application of this NVU correction method.
) were candidate voxels for application of this NVU correction method.
To enhance the detectability of BOLD signal in candidate voxels, a correction factor was used. Mean of ALFF of all voxels in the normal CL ROI was calculated and designated as mALFF. We used the ratio of mALFF to the abnormally decreased individual voxel ALFF in the IL ROI (iALFF) as the correction factor since the contralateral mean ALFF is more representative of normal cortical ALFF.
The overall NVU calibration factor and resultant individual voxel Z-value correction are given by the following equations:
 |
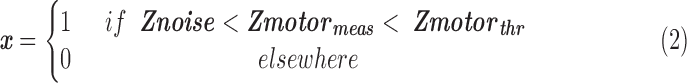 |
where
Zmotorcorr = ALFF-based corrected Z-score in motor task activation map;
mALFF = average voxel ALFF value in contralesional ROI;
iALFF = ALFF value of individual NVU-affected ipsilesional voxel
Results
Qualitative analysis of baseline and post-ALFF correction motor activation maps was performed individually for each patient. We found that there was an increase in the number of active voxels in the eloquent motor cortex affected by NVU in the post-ALFF correction motor maps compared with the pre-ALFF correction (baseline) motor maps in all patients. The numbers of active voxels in the eloquent motor cortex affected by NVU in the pre- and post-ALFF correction motor maps for all 12 patients are summarized in Table 2.
Table 2.
Number of Active Suprathreshold Voxels in Ipsilesional Sensorimotor Cortex Before and After Application of the Neurovascular Uncoupling Correction Method in 12 Patients
| Patient No. | Number of active voxels pre-ALFF correction | Number of active voxels post-ALFF correction |
|---|---|---|
| 1 | 89 | 269 |
| 2 | 784 | 1206 |
| 3 | 1102 | 2110 |
| 4 | 1041 | 1800 |
| 5 | 731 | 1169 |
| 6 | 711 | 1146 |
| 7 | 1230 | 1999 |
| 8 | 205 | 1242 |
| 9 | 365 | 1478 |
| 10 | 1316 | 2678 |
| 11 | 1188 | 1861 |
| 12 | 631 | 1157 |
The first column describes individual patient number, while the second column describes number of active voxels pre-ALFF correction (thresholded at 50% AMPLE). The third column lists the number of active voxels present following correction.
ALFF, amplitude of low frequency fluctuations; AMPLE, activation mapping as a percentage of local excitation.
Figure 1 displays a case of left hemispheric glioblastoma demonstrating severe NVU affecting the sensorimotor network (Patient No. 8 in Table 1). Suprathreshold voxels in the expected hand RA of the PMC are highlighted in the pre-ALFF correction map on two axial slices. Postcorrection and difference maps resulting from subtraction of the pre-ALFF correction map from the post-ALFF correction map are also plotted. The difference map shows the newly detectable BOLD activation in NVU-affected primary sensorimotor cortex following application of the correction. Two more examples of pre- and post-ALFF correction sensorimotor activation maps for individual patients with moderate NVU and mild NVU are provided in Figure 1 (Patients No. 11 and 6, respectively). The difference map shows the newly detectable BOLD activation in NVU-affected primary sensorimotor cortex following application of the correction/normalization algorithm.
FIG. 1.
Patient 1 with a left hemispheric WHO grade IV glioblastoma (Patient 8 in Table 1) performed bilateral simultaneous sequential finger tapping task. This case displays severe NVU affecting the sensorimotor network. Suprathreshold voxels (Z-score >2.5) in the expected hand representation area of the primary motor cortex are highlighted in the pre-correction map on one axial slice. Post-correction and difference maps resulting from subtraction of the pre-correction map from the post-correction map are also displayed. The difference map shows the newly detectable BOLD activation (arrow) in the NVU-affected primary sensorimotor cortex following application of the correction algorithm. Patient 2 with a grade II oligodendroglioma performed bilateral simultaneous sequential finger tapping task and Patient 3 with grade IV glioma performed tongue movement task (Patient No. 11 and 6 in Table 1, respectively). Patient 2 is a moderate NVU case, and Patient 3 is a mild NVU case. Pre- and post-correction motor maps for each patient are provided. Suprathreshold voxels (Z-score >2.5) in the primary motor cortex are highlighted in the pre-correction map. Difference maps resulting from subtraction of pre-correction maps from post-correction maps are also displayed. The difference map shows the newly detectable BOLD activation (arrow) in the NVU-affected primary sensorimotor cortex following application of the correction. In addition, BH CVR maps are also displayed for each case in the last column on the right, overlaid on T1-weighted anatomic images. Statistical thresholds used for the severe, moderate, and mild NVU cases are 0.30, 0.40, and 0.20 BOLD percentage signal changes, respectively. Note the IL reduction in CVR compared with the CL hemisphere; in the setting of preserved motor function, these findings are indicative of lesion-induced NVU. BH CVR, breath-hold cerebrovascular reactivity; BOLD, blood oxygen level dependent; CL, contralesional; IL, ipsilesional; NVU, neurovascular uncoupling; WHO, World Health Organization. Color images are available online.
Figure 2 provides the average fMRI time courses of sensorimotor cortex in CL and IL hemispheres in the three cases displaying severe NVU, moderate NVU, and mild NVU, respectively. The time courses of “CL” represent an average time course of all of the activated, that is, suprathreshold, voxels in the CL sensorimotor cortex, and “IL Difference” represents an average time course of all the newly detected voxels in IL sensorimotor cortex obtained following application of the ALFF correction algorithm. Note the similarity of the “CL” and “IL Difference” time courses in terms of periodicity in each patient, as well as the relatively low amplitude of IL BOLD signal compared with CL signal in all cases, reflecting the effects of NVU on the voxels that were selected for correction. An upward signal drift was noted for the case of severe NVU, so the designated baseline was adjusted accordingly.
FIG. 2.
The average fMRI time courses of sensorimotor cortex in CL and IL hemispheres in three cases displaying severe NVU, moderate NVU, and mild NVU, respectively, are plotted. “Amplitude” refers to the intensity of the BOLD signal (in a.u.) extracted from the IL and CL ROIs. The time course of “CL” represents an average time course of all of the activated, that is, suprathreshold voxels in the CL sensorimotor cortex and “IL Difference” represents an average time course of all newly detected “active” voxels in the IL sensorimotor cortex obtained following application of the ALFF correction algorithm. The bold black vertical dashed lines in the “CL” and “IL Difference” plots separate successive cycles of task and rest epochs in the fMRI paradigm. Note that the task fMRI paradigm includes three cycles. Light gray dotted lines highlight the baseline and maximal BOLD signals in each time course. The difference between maximal signal and baseline in each time course is designated as the BOLD amplitude (A) for each “CL” and “IL Difference” time course. Note the similarity of the “CL” and “IL Difference” time courses in terms of periodicity in each patient, as well as the relatively low amplitude of IL BOLD signal compared with CL signal, reflecting the effects of NVU on the voxels that were selected for correction. ALFF, amplitude of low frequency fluctuations; a.u., arbitrary units; ROI, region of interest. Color images are available online.
Of the 12 cases included in this study, we have electrophysiological confirmation of the precentral gyrus localization in 75% of the cases (9/12), which confirms the specificity of newly detected activation in the PMC and therefore provides an effective validation of our correction technique.
Discussion
Our preliminary study with a cohort of de novo brain tumor patients demonstrates the feasibility of minimizing spuriously decreased motor fMRI activation, through use of a novel resting-state ALFF-based NVU correction method. In addition, functional specificity of the cortical correction was confirmed with intraoperative mapping and use of parcellation that specifically identified PMC.
NVU is characterized by the presence of abnormally decreased fMRI activation in the IL hemisphere when the patient neither exhibits substantial neurological deficit that would be indicative of tumor-related destruction of eloquent cortex nor displays inability to perform the required tasks adequately (Zaca et al., 2014). BOLD fMRI only indirectly measures neuronal activity since it only detects alterations in deoxyhemoglobin concentration influenced by hemodynamic factors that occur in response to neuronal activity. Since the change in cerebral blood flow in response to a vasodilatory or vasoconstrictive stimulus is measured by CVR mapping, evaluating CVR can be useful for identifying cortical regions of NVU (Pillai and Mikulis, 2015).
CVR mapping is typically performed using hypercapnic gas inhalation (Lu et al., 2014; Wise et al., 2007; Yezhuvath et al., 2009) as a vasoactive challenge while collecting BOLD images; however, the need for a gas inhalation apparatus setup is a practical obstacle to its in routine clinical application (Spano et al., 2013). Breath-holding (BH) has been used to induce hypercapnia as the BH technique is easier to implement and produces similarly useful BOLD CVR maps as those achieved by using gas inhalation techniques (Kastrup et al., 2001; Magon et al., 2009). In this study, all patients underwent BH CVR mapping as part of their clinical presurgical mapping fMRI examinations, and the BH CVR maps displayed concordant IL decreases in CVR that correspond to tumor-induced NVU (Fig. 1).
Studies have also been conducted using resting-state fMRI for CVR assessment; resting-state fluctuation of amplitude (RSFA) has been described as an alternative approach to evaluate CVR (Jahanian et al., 2017; Kannurpatti and Biswal, 2008; Kannurpatti et al., 2011; Wang et al., 2016; Wise et al., 2004). These studies examined the correlation between end-tidal CO2 fluctuations and low-frequency BOLD fluctuations available from rsfMRI and established RSFA as a strong CVR correlate. A recent study (Liu et al., 2017) proposed a resting-state CVR map that exploits the natural variation in respiration to map CVR using resting-state BOLD data.
However, to our knowledge, there have been no effective methods published to date that specifically address the correction for impaired BOLD activation due to brain tumor-induced NVU. Since previous studies (Agarwal et al., 2017b; Agarwal et al., 2016a,b; Mallela et al., 2016) have demonstrated that brain tumor-induced NVU can impair the ability to depict resting-state networks similar to decreased fMRI task activation (Zaca et al., 2014), we wished to develop a technique based on ALFF obtained from rsfMRI data to address this important unresolved problem of need for BOLD signal correction in the setting of NVU. This approach seemed to be a viable strategy for NVU correction since we have recently shown that brain tumor-induced NVU can be detected on ALFF and fALFF maps (Agarwal et al., 2017a).
In this novel approach proposed in this study, we considered using the ratio of CL mean ALFF (reflecting normal eloquent cortex) to IL ALFF (reflecting NVU-affected tissue) as a potential correction factor for the effects of NVU in IL primary sensorimotor cortex. The proposed NVU correction technique was applied only to those voxels in the IL ROI that demonstrated subthreshold activation, since there was no need to apply a correction to activated suprathreshold voxels in a baseline activation map (which by definition are not affected by NVU).
To keep the threshold uniform across all subjects, we used the AMPLE normalization method (Voyvodic et al., 2009) of operator-independent thresholding with 50% of maximum Z-score in analyzed ROIs as the cutoff for generating thresholded activation maps in each individual case. In this manner, internal normalization was achieved across all subjects with the CL maximal Z-score serving as an internal reference standard in each subject. This method has been demonstrated to provide highly reproducible BOLD fMRI activation maps (Voyvodic, 2012).
To provide quantitative and objective measurements of activation, we adopted operator-independent, identical atlas-based parcellation-derived ROIs (including pre- and postcentral gyri only) instead of arbitrary (operator-dependent) manual ROI tracings. This ROI approach permitted us to directly evaluate the group performance of our proposed NVU correction method and allowed us to maintain motor network functional specificity with respect to the application of this correction algorithm (as confirmed via intraoperative electrophysiologic mapping).
In the proposed NVU correction technique, we used 25% of a local cluster Z-score maximum as the lower limit for selecting the eloquent cortical voxels most likely affected by NVU, since voxels with subthreshold Z-scores by this criterion are likely to represent a mixture of noise voxels/spurious activation (in which case overcorrection would lead to potential false positives) and voxels affected by such severe NVU that effective correction may not be possible.
We chose the 50% AMPLE threshold for display of activation maps in light of previously published work by Voyvodic and colleagues (2009), suggesting that it may represent an optimal threshold for task activation. Any voxels in the PMC with Z-scores above this 50% AMPLE threshold are considered suprathreshold and thereby unaffected by NVU. Thus, we felt that the 25–50% zone for AMPLE thresholds represented a reasonable estimate for cortical voxels affected by NVU that may benefit from application of this correction algorithm. Since there is no previously published reference for such voxel selection criteria in brain tumor patients, our newly proposed methodology needs to be validated with larger cohorts of patients in future investigations.
This particular cohort of de novo brain tumor patients represents a rare data set that was difficult to acquire due to the relatively low prevalence of perirolandic gliomas exhibiting motor system NVU. In all 12 cases reported in this study, in which we applied these selection criteria, we obtained effective separation of NVU-affected eloquent cortical voxels from the remainder of the voxels in our analyzed IL ROIs, as demonstrated by both visual inspection of results and quantitative assessment at single-subject level. Moreover, as these were de novo, treatment-naive patients, we are confident that the neurovasculature was unaffected by the effects of conventional chemo- or radiotherapy.
Figures 1 and 2, which display representative cases of severe, moderate, and mild NVU, demonstrate the effectiveness of the ALFF correction algorithm across the entire spectrum of severity of NVU. While Figure 1 displays newly detectable activation in the IL sensorimotor cortex following application of the correction algorithm, as shown in the “IL Difference” maps, Figure 2 displays the effects of NVU on the BOLD signal time courses in each case.
The similarity of BOLD signal time courses in terms of periodicity and the relatively low amplitude of BOLD signal in the voxels for which the ALFF correction algorithm had been applied (as shown on the “IL Difference” maps), compared with CL-activated voxels, suggest that these selected voxels actually represent NVU-affected voxels rather than just spurious noise voxels. If the selected voxels had represented noise voxels, we would have expected to see random BOLD signal fluctuations rather than time courses of similar periodicity and comparable overall fluctuations with that of the CL-activated voxels. In the case of severe NVU (Patient 1), the averaged CL voxel time course demonstrates similar periodicity to that of the averaged voxel IL voxel time course, although amplitude remains relatively decreased and less prominent signal increases are seen during active epochs relative to baseline epochs.
For Patients 2 and 3, however, the IL ROI mean voxel time course appears more strikingly similar to that of the CL ROI mean voxel time course, suggesting that NVU may have a less profound effect on the raw BOLD signal time courses in such cases of milder NVU. Interestingly, as shown in Figure 2, the IL BOLD signal time course demonstrated a linear trend (upward BOLD signal drift over time) in the case of severe NVU, absent in the cases of mild and moderate NVU. The exact significance of this finding is unclear, but future work may help to elucidate this phenomenon.
Figure 3 displays the average voxel BOLD signal time courses within the CL, IL pre-ALFF correction and IL post-ALFF correction Heschl's gyrus (main regions in the auditory network) in each case. Notice the randomness of the averaged CL and IL time courses in Heschl's gyri. Notice the same random BOLD signal fluctuations following correction that we have seen precorrection in the IL ROI. This strongly contrasts with the highly task-correlated periodicity of the BOLD signal time course in the NVU-affected IL voxels in the sensorimotor network, as shown in Figure 2.
FIG. 3.
The average fMRI time courses of Heschl's gyrus (a key component of the auditory network) in CL and IL hemispheres in three cases displaying severe NVU, moderate NVU, and mild NVU, respectively, are plotted in blue (CL) and red (IL), respectively. Note the randomness of both the “CL” and “IL” BOLD signal time courses, that is, absence of task-correlated periodicity, in each patient. Also, in the last column, the BOLD signal time course post-ALFF correction in IL is also plotted (black-colored line) along with pre-ALFF-corrected IL time course (in red). Note the same random BOLD signal fluctuations following correction that we have seen pre-correction in the IL ROI. Color images are available online.
There are a few limitations to this study. First of all, in all cases, our neurosurgeons used evoked potential recordings rather than an Ojemann stimulator for intraoperative mapping, so it is not possible to have a voxel by voxel correlation between the fMRI activation maps and the identified cortical areas as assessed in the operating room for exact computation of the sensitivity and specificity of our method with respect to an intraoperative electrophysiological criterion standard. Nevertheless, the use of anatomic parcellation of exclusively perirolandic cortex, coupled with intraoperative confirmation of motor cortex localization, provides strong evidence of the high functional specificity of the activation maps that we have generated, both pre- and post-ALFF correction.
In addition, unlike the language system, there is much lower intersubject variability in cortical representation in the sensorimotor network, and thus, in the absence of cortical plasticity, the anatomic parcellation is reliable. Although we did not specifically explore plasticity in these cases, even in the setting of such plasticity, our intraoperative mapping confirmed fMRI-concordant localization of the PMC in all cases. Another limitation is that the use of a 25% local cluster Z-score maxima as the lower cutoff for voxel selection for application of this correction method is somewhat arbitrary. However, since this approach is novel and no previous studies can be cited for this purpose, we relied on empirical data for establishing this standard with the considerations described above in mind.
Another limitation of our study is the relatively small sample size of our patient cohort. It should be borne in mind that such data are challenging to acquire because of the relatively low prevalence of de novo perirolandic gliomas exhibiting NVU, even in a large tertiary care referral center such as ours. We selected these 12 reported cases from among hundreds of tumor patients who had been referred to our academic center over the last 5 years for presurgical fMRI. Nevertheless, this preliminary study may serve as a catalyst for future development of similar and possibly more robust methods for the correction of NVU.
In future studies, we will apply this correction technique to more lateralized networks and brain networks with greater intersubject variability. Since ALFF maps derived from rsfMRI provide whole-brain coverage, theoretically they should be effective for correction of BOLD signal within any chosen brain functional network.
Conclusion
In conclusion, we have demonstrated the feasibility of a novel ALFF-based NVU correction technique to enhance the detectability of sensorimotor activation in the setting of perirolandic brain tumors.
Acknowledgment
This work is partially supported by NIH grant R42 CA173976-02 (NCI).
Author Disclosure Statement
No competing financial interests exist.
References
- Agarwal S, Lu H, Pillai JJ. 2017a. Value of frequency domain resting-state functional magnetic resonance imaging metrics amplitude of low-frequency fluctuation and functional amplitude of low-frequency fluctuation in the assessment of brain tumor-induced neurovascular uncoupling. Brain Connect 7:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sair HI, Airan R, Hua J, Jones CK, Heo HY, et al. 2016a. Demonstration of brain tumor-induced neurovascular uncoupling in resting-state fMRI at ultrahigh field. Brain Connect 6:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sair HI, Pillai JJ. 2017b. The resting-state functional magnetic resonance imaging regional homogeneity metrics-kendall's coefficient of concordance-regional homogeneity and coherence-regional homogeneity-are valid indicators of tumor-related neurovascular uncoupling. Brain Connect 7:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sair HI, Yahyavi-Firouz-Abadi N, Airan R, Pillai JJ. 2016b. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J Magn Reson Imaging 43:620–626 [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. 2010. Glial and neuronal control of brain blood flow. Nature 468:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- DeYoe E, Ulmer JL. 2008. Method for measuring neurovascular uncoupling in fMRI. Google Patents [Google Scholar]
- Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. 2000. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 21:1415–1422 [PMC free article] [PubMed] [Google Scholar]
- Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. 2006. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 32:489–497 [DOI] [PubMed] [Google Scholar]
- Jahanian H, Christen T, Moseley ME, Pajewski NM, Wright CB, Tamura MK, et al. 2017. Measuring vascular reactivity with resting-state blood oxygenation level-dependent (BOLD) signal fluctuations: a potential alternative to the breath-holding challenge? J Cereb Blood Flow Metab 37:2526–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Krainik A, David O, Salon C, Tropres I, Hoffmann D, et al. 2010. Impaired fMRI activation in patients with primary brain tumors. Neuroimage 52:538–548 [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. 2008. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage 40:1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti SS, Motes MA, Rypma B, Biswal BB. 2011. Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum Brain Mapp 32:1125–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Neumann-Haefelin T, Moseley ME. 2001. Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: comparison of CO(2) and breath holding. Magn Reson Imaging 19:13–20 [DOI] [PubMed] [Google Scholar]
- Lee J, Lund-Smith C, Borboa A, Gonzalez AM, Baird A, Eliceiri BP. 2009. Glioma-induced remodeling of the neurovascular unit. Brain Res 1288:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li Y, Pinho M, Park DC, Welch BG, Lu H. 2017. Cerebrovascular reactivity mapping without gas challenges. Neuroimage 146:320–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. 2014. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp DOI: 10.3791/52306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magon S, Basso G, Farace P, Ricciardi GK, Beltramello A, Sbarbati A. 2009. Reproducibility of BOLD signal change induced by breath holding. Neuroimage 45:702–712 [DOI] [PubMed] [Google Scholar]
- Mallela AN, Peck KK, Petrovich-Brennan NM, Zhang Z, Lou W, Holodny AI. 2016. Altered resting-state functional connectivity in the hand motor network in glioma patients. Brain Connect [Epub ahead of print]; DOI: 10.1089/brain.2016.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak RW, Hadjiabadi DH, Senarathna J, Agarwal S, Thakor NV, Pillai JJ, Pathak AP. 2017. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J Cereb Blood Flow Metab 37:3475–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Mikulis DJ. 2015. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am J Neuroradiol 36:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Zaca D. 2011. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J Clin Oncol 2:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Zaca D. 2012. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol Cancer Res Treat 11:361–374 [DOI] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp 17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo X N, Zhu CZ, et al. 2011. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano VR, Mandell DM, Poublanc J, Sam K, Battisti-Charbonney A, Pucci O, et al. 2013. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology 266:592–598 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. 2003. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol 24:213–217 [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Dirnagl U. 1995. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev 7:240–276 [PubMed] [Google Scholar]
- Voyvodic JT. 2012. Reproducibility of single-subject fMRI language mapping with AMPLE normalization. J Magn Reson Imaging 36:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT, Petrella JR, Friedman AH. 2009. fMRI activation mapping as a percentage of local excitation: consistent presurgical motor maps without threshold adjustment. J Magn Reson Imaging 29:751–759 [DOI] [PubMed] [Google Scholar]
- Wang P, Hou P, Kesler S, Colen R, Kumar A, Prabhu S, Liu H. 2016. SU-G-IeP1-11: resting-state fluctuation of BOLD signal amplitude for mapping cerebrovascular reactivity in presurgical functional MRI. Med Phys 43:3646–3647 [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. 2004. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21:1652–1664 [DOI] [PubMed] [Google Scholar]
- Wise RG, Pattinson KT, Bulte DP, Chiarelli PA, Mayhew SD, Balanos GM, et al. 2007. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab 27:1521–1532 [DOI] [PubMed] [Google Scholar]
- Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. 2009. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed 22:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaca D, Hua J, Pillai JJ. 2011. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol 2:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaca D, Jovicich J, Nadar SR, Voyvodic JT, Pillai JJ. 2014. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J Magn Reson Imaging 40:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91 [DOI] [PubMed] [Google Scholar]





