Abstract
CCL28 is a mucosal chemokine that has been involved in various responses, including IgA production. We have analyzed its production in human tissues using a comprehensive microarray database. Its highest expression is in the salivary gland, indicating that it is an important component of saliva. It is also expressed in the trachea, bronchus, and in the mammary gland upon onset of lactation. We have also characterized a Ccl28−/− mouse that exhibits very low IgA levels in milk, and the IgA levels in feces are also reduced. These observations confirm a role for the CCL28/CCR10 chemokine axis in the recruitment of IgA plasmablasts to the lactating mammary gland. CCL28 is also expressed in the vomeronasal organ. We also detected olfactory defects (anosmia) in a Ccl28−/− mouse suggesting that CCL28 is involved in the function/development of olfaction. Importantly, Ccl28−/− mice are highly susceptible to Salmonella enterica serovar Typhimurium in an acute model of infection, indicating that CCL28 plays a major role in innate immunity against Salmonella in the gut. Finally, microbiome studies revealed modest differences in the gut microbiota between Ccl28−/− mice and their cohoused wild-type littermates. The latter observation suggests that under homeostatic conditions, CCL28 plays a limited role in shaping the gut microbiome.
Keywords: chemokines, vomeronasal organ, Salmonella, innate immunity, gut
Introduction
Chemokines are small secreted cytokines that represent an important family of chemotactic mediators. They arose through evolution from an ancestral chemokine gene that gave rise to a superfamily that has been divided into inflammatory and homeostatic chemokines depending on their expression patterns (Zlotnik and Yoshie 2012). While chemokines are best known for their chemotactic activities, they are now known to be involved in many other important processes, including development (Raz 2003), cancer and metastasis (Zlotnik and others 2011), and mobilization of hematopoietic stem cells (Stover and others 2017), among others. There are 48 human chemokines known, of which 4 represent chemokines with predominant expression in mucosal tissues (Hernandez-Ruiz and Zlotnik 2017).
These include CCL25, CCL28, CXCL14, and CXCL17. Probably the best characterized of these is CCL25, which is expressed in the small intestine and recruits CCR9+ T cells (Svensson and others 2002). The function of the rest has only recently started to be characterized. Based on the phenotype of a Cxcl14−/− mouse, Hara and Tanegashima (2012) concluded that CXCL14 is involved in metabolic responses and feeding behavior, whereas CXCL17 has emerged as an important macrophage chemotactic factor that recruits macrophages to mucosal tissues (Burkhardt and others 2014).
CCL28 is a mucosal chemokine produced by epithelial cells that binds a chemokine receptor called CCR10 (Wang and others 2000). It is expressed by epithelial cells in many mucosal tissues, including the respiratory tract, the digestive system, and the female reproductive system. One of its properties is wide-spectrum antimicrobial activity, including candidacidal activity mediated by a Histidine motif found at the carboxy terminus of its sequence (Hieshima and others 2003). CCL28 has been identified as responsible for the recruitment of IgA producing plasmablasts (Wilson and Butcher 2004) to the mammary gland upon the onset of lactation, allowing them to produce the IgA that will be transferred to the newborn in the milk. Supporting this conclusion is the fact that the Ccl28 gene only exists in mammalian genomes (unpublished observation), suggesting mammalian-specific functions.
There have been 2 studies describing the phenotype of Ccr10−/− mice, and both reported that Ccr10−/− mice exhibit defects in IgA production (Morteau and others 2008; Hu and others 2011). Matsuo and others (2017) have recently reported the production and phenotyping of a Ccl28−/− mouse, which exhibits reduced IgA and an altered microbiota in the colon.
In the present study, we have also characterized a Ccl28−/− mouse. These mice develop normally but exhibit abnormalities in their reproductive behavior, which we have mapped to defects in olfaction. We also show that Ccl28 is expressed in the vomeronasal organ (VNO), suggesting that Ccl28−/− mice have abnormal development or function of this organ. This is consistent with reports that have documented that CCL28 is expressed in the nasal mucosa (Bourges and others 2007). In addition, Ccl28−/− mice also exhibit defects in IgA production in the milk, a finding that, as we mentioned above, mirrors the phenotype of Ccr10−/− mice. Finally, we observed that Ccl28−/− mice are highly susceptible to Salmonella enterica serovar Typhimurium in an acute infection model, indicating that CCL28 plays a pivotal protective role in enteric infections.
Taken together, these observations highlight the important roles that CCL28 plays in both mucosal homeostasis and in innate/adaptive immune responses.
Materials and Methods
Mice
Mice were obtained at the heterozygote stage (Ccl28+/−) from Deltagen (www.deltagen.com/target/deltabase.html). Briefly, the Ccl28 gene was replaced with a Neo cassette by homologous recombination resulting in a whole-body knockout mouse in the C57Bl/6 background. Unless otherwise specified, controls for most experiments were wild-type (WT) littermates obtained in the process of maintaining the Ccl28−/− mouse colony.
Mice were bred to obtain homozygote Ccl28-deficient mice (Ccl28−/−) or WT mice with 2 functional copies of Ccl28. All mice used were housed in the same specific pathogen-free facility with a 12-h dark/12-h light cycle with autoclaved bedding and irradiated food. All breeding and handling of the mice were performed under an approved IACUC protocol.
Genotyping polymerase chain reaction
Ear punches were collected from 3-week-old mice upon weaning. DNA was extracted from the ear punches using a kit (Bioland Scientific, Paramount, CA). Primers specific to the Ccl28-targeting construct were used in a 35-cycle conventional polymerase chain reaction (PCR) to determine the genotype of each mouse. The amplified DNA products were analyzed on a 1% agarose gel. Primers: Ccl28 forward 5′-AAGATGAGGACAGGCTGGTACTCTG-3′, Ccl28 reverse 5′-CTACAGTTGCAGACATGTTGG-3′, Neo forward 5′-GGGTGGGATTAGATAAATGCCTGCTCT-3′, Common reverse 5′-ATTTCGCATGTCCTTGCTGAGGGAC-3′.
Collection of mouse samples for studies
Several biological samples were collected from WT, Ccl28+/−, or Ccl28−/− mice for secreted molecule studies by enzyme-linked immunosorbent assay (ELISA). Serum was collected following centrifugation of blood collected through cardiac punch. Fecal samples were collected from the colons of mice during postmortem dissection. Milk was collected from the stomachs of 9-day-old mice during postmortem dissection. All samples were stored at −20°C until their analysis by ELISA.
Isolation of the mouse VNO
VNO isolation was performed according to instructions from the JOVE video “An Effective Manual Deboning Method To Prepare Intact Mouse Nasal Tissue With Preserved Anatomical Organization” (DOI 10.3791/50538). Briefly, mice were euthanized with lethal doses of CO2 followed by cervical dislocation. To gain access to the soft palate on the roof of the mouth, scissors were used to separate the upper and lower jaws from each other. The upper portion of the skull was removed from the rest of the animal and placed, soft palate side down, in a Petri dish of cold 1 × PBS until ready to perform complete dissection. Using a dissection microscope, the soft palate was cut open and pulled back to reveal the underlying bony structure containing the VNO. Using scissors, the vomer bone was then cut at the caudal and rostral side and gently removed using fine forceps; the VNO was attached. Gentle dissection separated the VNO from the vomer bone. Any remaining cartilage or bone fragments still attached were carefully removed. The VNO was then placed in RNALater for RNA extraction.
IgA ELISAs
IgA ELISAs were performed using the Ready-SET-Go! Mouse IgA ELISA Kit (eBioscience, San Diego, CA). Assays were performed according to the manufacturer's instructions and the absorbance at 450 nm was read using a BioTek ELx800 machine (BioTek, Winooski, VT). Data were processed in Excel and analyzed using GraphPad Prism (www.graphpad.com).
CCL28 ELISA
CCL28 ELISA was performed using the Mouse CCL28 ELISA MAX Deluxe Kit from BioLegend (BioLegend, San Diego, CA). Assays were performed according to the manufacturer's instructions and the absorbance at 450 nm was read using a BioTek ELx800 machine (BioTek). Data were processed in Excel and analyzed using GraphPad Prism (www.graphpad.com).
Quantitative real-time PCR analysis
Total RNA was extracted from mouse tissues using TRIzol (Invitrogen, Carlsbad, CA) and subsequently purified using Qiagen's RNEasy columns and DNase digest (Qiagen, Valencia, CA). Equal concentrations of RNA were used for each tissue sample in a reverse transcription reaction to synthesize cDNA (Qiagen). Each reaction used cDNA synthesized from 1 μg of total RNA, gene-specific primers, and gene-specific UPL probes to quantitatively detect Ccl28 and control gene transcripts in each tissue sample. The results were processed in Excel and analyzed using GraphPad Prism (www.graphpad.com).
Olfaction test
These assays were performed as described (Del Punta and others 2002; Kurtenbach and others 2013). Briefly, an Oreo cookie was hidden under the corncob bedding in the back left corner of a standard mouse cage. The mice to be tested were placed in the cage at the opposite corner. Time was measured, in seconds, starting from the time the mice were placed in the cage until they took a bite of the cookie. If after 400 s a mouse had not found the hidden cookie, time was stopped, the mouse was removed from the cage and the animal was assumed to have defects in olfaction compared with WT controls. Fresh cages and fresh cookies were used for each experiment to prevent crosscontamination. Control mice were either WT or heterozygous Ccl28+/− littermates of Ccl28−/− mice.
Analysis of the microbiota
Fecal samples were collected from Ccl28−/− mice and wild-type, cohoused littermate mice. The samples were snap frozen in liquid nitrogen, and then DNA was later extracted using the QIAamp DNA Stool Kit (Qiagen) according to the manufacturer's instructions with modifications as previously described (Behnsen and others 2014). DNA extracted from fecal samples was amplified by PCR of 16S rDNA (V4 region) with primers 515F and 806R modified by the addition of barcodes for multiplexing, then sequenced on an Illumina MiSeq system (UC Davis HMSB Facility). Sequences were processed and analyzed by employing the QIIME pipeline v1.9.1 with default settings, except as noted. In brief, paired-end sequences were joined, quality filtered, and chimera filtered (usearch61 option; RDP gold database); operational taxonomic units were picked de novo (“pick_otus” options: enable_rev_strand_match True, otu_picking_method usearch61) at 97% similarity, employing the SILVA rRNA gene database v123 (align_seqs:template_fp core_alignment_SILVA123.fasta; “filter_alignment” options: allowed_gap_frac 0.80, entropy_threshold 0.10, suppress_lane_mask_filter True); taxonomy was assigned with the RDP classifier (“assign_taxonomy” options: assignment_method rdp, confidence 0.8, rdp_max_memory 24000, reference_seqs_fp 97_otus_16S.fasta, id_to_taxonomy_fp consensus_taxonomy_7_levels.txt). Samples were rarefied to 10,000 reads and then alpha (Shannon index) and beta (unweighted and weighted UniFrac) diversity were assessed through QIIME. Prism 7 software (GraphPad) was used for statistical analyses (Mann–Whitney U test).
Salmonella infection
Mice were infected as previously described using an acute infection model (Barthel and others 2003; Raffatellu and others 2008, 2009). Briefly, mice were pretreated with streptomycin (0.1 mL of a 200 mg/mL solution in sterile water) intragastrically before oral inoculation with fully virulent Salmonella enterica serovar Typhimurium strain IR715 (1 × 109 colony-forming units (CFUs)/mouse) and mouse survival was monitored at 24, 48, and 72 h postinfection.
In vitro growth curves
Salmonella enterica serovar Typhimurium strain IR715 cultures were grown overnight at 37°C in LB supplemented with 50 μg/mL of nalidixic acid (Nal). The following day, cultures were diluted 1:100 in LB and grown at 37°C for 3 h, subsequently diluted to ∼0.5 × 106 CFU/mL in 1 mM potassium phosphate buffer (pH 7.2) as previously described (Hieshima and others 2003), and incubated at 37°C in the presence or absence of recombinant human or murine CCL28 (BioLegend). After 2 h, samples were plated onto LB Nal agar to enumerate viable bacteria.
Results
CCL28 is a mucosal chemokine highly expressed in salivary glands
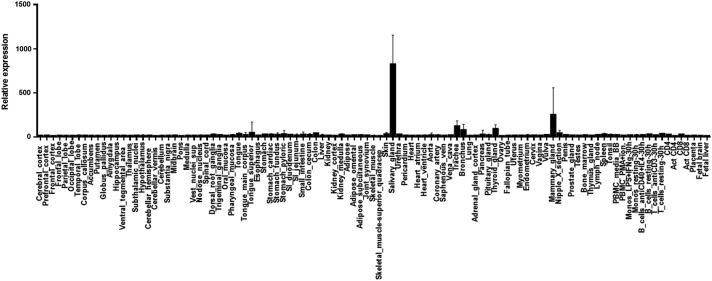
CCL28 has been recognized to be a mucosal chemokine. However, a comprehensive study of its expression in the human body has not been reported. We analyzed the expression of CCL28 in the BIGE (Body Index of Gene Expression) database that represents 130 tissues from 4 human males and 4 females (Lee and others 2005; Roth and others 2006). As shown in Fig. 1, the highest expression of CCL28 in the human body is in the salivary glands. In support of this conclusion, CCL28 is readily detectable in saliva from normal humans (Hieshima and others 2003; Hernandez-Molina and others 2015). The second site of strong expression is the mammary gland, a finding that supports a role for CCL28 in lactation [it has been reported to mediate the recruitment of CCR10+ IgA-producing plasmablasts to the maturing mammary gland (Wilson and Butcher 2004)].
FIG. 1.
Expression of CCL28 in the human body (BIGE database). Hybridization Units in the Y axis to tissue-specific cDNAs (X axis) correspond to probeset 224027_at from the U133 2.0 Affymetrix human genearray. Significant expression was observed in (highest to lowest) salivary gland; mammary gland; trachea; bronchus; and thyroid.
We have confirmed that Ccl28 is induced in the mouse mammary gland upon the onset of lactation (not shown). Rounding up the top expression sites are trachea and bronchus, and the thyroid gland. Hybridization values for the top 14 human tissues that exhibit significant expression of CCL28 are shown in Table 1. Other noteworthy sites of expression include tongue superior [CCL28 is expressed by the lingual epithelium (Hevezi and others 2009)], colon, urethra, small intestine, and skin. CCL28 has been recognized to be a mucosal chemokine that plays a role in both lung and digestive tract physiology (Hernandez-Ruiz and Zlotnik 2017). In the digestive tract, Matsuo and others (2017) have shown that it plays a role in IgA production and likely shaping of the microbiome through its antimicrobial activity and in the lung it may play a role in eosinophil recruitment during allergic inflammation (John and others 2005).
Table 1.
Significant Expression of CCL28 in Human Tissues (from the BIGE Database)
| Human tissue | CCL28 expressiona |
|---|---|
| Salivary gland | 823.06 ± 158.35 |
| Mammary gland | 251.50 ± 177.23 |
| Trachea | 122.46 ± 32.63 |
| Bronchus | 79.02 ± 24.89 |
| Thyroid | 92.85 ± 14.29 |
| Tongue superior | 47.58 ± 43.64 |
| Nipple | 43.653 ± 29.18 |
| Colon | 42.64 ± 7.36 |
| Tongue | 35.56 ± 10.41 |
| Skin | 32.64 ± 16.39 |
| Stomach pyloric | 32.35 ± 8.85 |
| Urethra | 29.06 ± 6.46 |
| Small intestine | 27.92 ± 2.48 |
| Kidney medulla | 27.17 ± 6.00 |
Hybridization Units of probeset 224027_at (Affymetrix U133 2.0 genearray) corresponding to human CCL28 to cDNAs from each tissue in the BIGE database (20); mean ± standard deviation. Background hybridization units averaged a mean of 20.
BIGE, Body Index of Gene Expression (Database of human microarray data).
However, this expression pattern suggests that its main role in the human digestive tract may be as an important component of saliva, of which an estimated 1 L is produced every day, and it continuously covers the oral cavity and esophagus. It is also expressed in the small intestine and colon; however, its expression in these tissues likely involves only specific subsets of epithelial cells.
CCL28 is required for the recruitment of IgA plasmablasts to the mammary gland
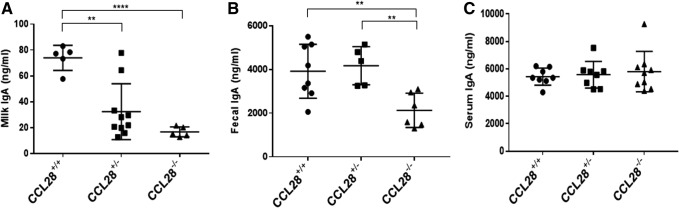
We obtained a Ccl28−/− mouse, which was produced by replacing the Ccl28 mouse gene with a Neo cassette by homologous recombination, resulting in a whole-body Ccl28 knockout mouse. We confirmed that Ccl28 expression was not detected in some organs where Ccl28 is normally expressed (salivary gland, small intestine) (using quantitative PCR, data not shown). Initial phenotyping of this mouse indicated that it develops normally, and is fertile. Given the reported role of CCL28 in the recruitment of IgA+ B cell plasmablasts, we measured the levels of immunoglobulins in serum, milk, and feces of Ccl28−/− mice or WT littermates. As shown in Fig. 2, there was no significant difference in IgA levels in the serum of Ccl28−/− mice, an ∼50% drop in IgA levels in the feces of Ccl28−/− mice, but the biggest decrease in IgA levels was observed in milk from Ccl28−/− mice. All other immunoglobulin isotypes were normal (data not shown). These observations indicate that Ccl28−/− mice has a similar phenotype to Ccr10−/− mice, which also exhibit a paucity of IgA in the milk (Morteau and others 2008; Hu and others 2011) and confirm that the CCL28/CCR10 axis is responsible for the recruitment of IgA plasmablasts to the mammary gland upon the onset of lactation.
FIG. 2.
(A) IgA levels in milk from Ccl28+/+ WT, Ccl28+/− heterozygote, or Ccl28−/− mice. (B) IgA levels in feces from Ccl28+/+ WT, Ccl28+/− heterozygote, or Ccl28−/− mice. (C) IgA levels in serum from Ccl28+/+ WT, Ccl28+/− heterozygote, or Ccl28−/− mice. Shown is a representative experiment (out of 3). **P < 0.01; ****P < 0.001.
We have also studied peripheral B cell subsets in the Ccl28−/− mouse and have observed no abnormalities in splenic or peritoneal B cell subsets (data not shown).
CCL28 expression is required for efficient olfaction
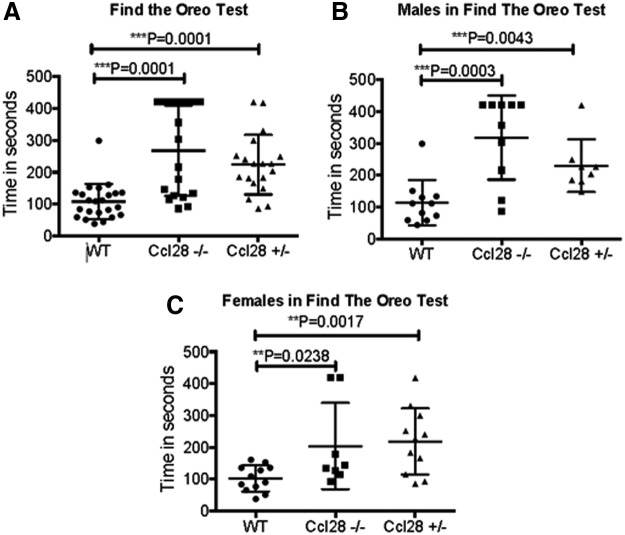
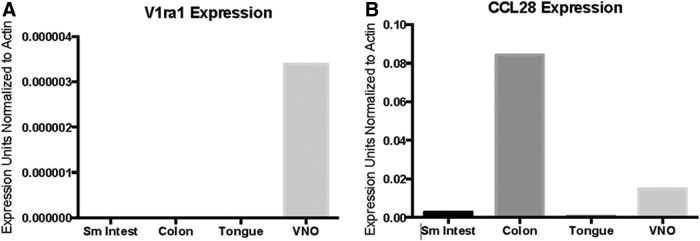
Interestingly, however, Ccl28−/− mice displayed abnormal reproductive behavior. Both males and females were observed mounting their same-sex cagemates during the daytime. A review of the literature indicated that similar behavior has been observed in mice where 16 V1R genes were deleted (Del Punta and others 2002). V1r and V2r genes encode 7 transmembrane receptors that represent a superfamily of genes involved in the detection of pheromones that are part of the olfactory system in mice and are expressed in the VNO (Del Punta and others 2002). These observations suggested that Ccl28−/− mice may exhibit abnormal olfaction. To test this hypothesis, we used a behavioral test that depends on olfaction (cookie test) (Dawson and others 2005; Kurtenbach and others 2013). In this test, an Oreo cookie is hidden under the bedding of a cage and a mouse is placed in the cage. Time measurements are taken from the time the mouse is placed in the cage until it finds the cookie. As shown in Fig. 3, Ccl28−/− mice show a marked deficiency in this test, indicating that they have deficient olfaction. We then hypothesized that CCL28 may be necessary for efficient development of the mouse olfactory system (VNO). As shown in Fig. 4, CCL28 is expressed in the VNO. Taken together, these observations suggest that CCL28 may play a role in its function/development.
FIG. 3.
Find the Oreo Test Results for WT, Ccl28+/−, and Ccl28−/− Mice. (A) Combined data from male and female mice that were tested for the time (in seconds) needed to find the Oreo cookie buried in the cage bedding. (B) Time (in seconds) it took WT, Ccl28+/−, and Ccl28−/− male mice to find the Oreo. (C) Time (in seconds) it took Ccl28+/− and Ccl28−/− female mice to find the Oreo. For both genders, Ccl28+/− and Ccl28−/− mice took significantly longer to locate the Oreo compared with WT littermates, indicating anosmia. These experiments were repeated twice, with at least 6 mice per group each experiment, using fresh mice for each experiment to control for learned behavior. WT, wild type.
FIG. 4.
Expression of the VNO-specific gene V1ra1 and Ccl28 in WT mouse mucosal tissues by qPCR. (A) Expression of the VNO-specific gene Vomeronasal Receptor A1 (V1ra1) was used as positive control to confirm the successful isolation of mouse VNO tissue. (B) Expression of Ccl28 in the VNO in addition to confirming its previously reported expression in mucosal tissues, including small intestine (Sm Intest) and colon. Representative experiment (out of 2) is shown. qPCR, quantitative polymerase chain reaction; VNO, vomeronasal organ; WT, wild type.
CCL28 is involved in the host response against enteric infections
CCL28 is expressed in the normal small intestine and colon (Table 1) and an increase in CCL28 expression has been associated with epithelial inflammation (Wang and others 2000; Ogawa and others 2004); in line with these findings, CCL28 expression is increased in the colon of patients with inflammatory bowel disease and returns to normal levels when patients are treated with infliximab (anti-TNFα) (Arijs and others 2011). We, therefore, hypothesized that CCL28 may play a role in protection against the enteric pathogen Salmonella enterica serovar Typhimurium. Human infections with nontyphoidal Salmonella serovars are characterized by rapidly induced acute inflammation in the terminal ileum and colon (Zhang and others 2003). This acute infection can be modeled in mice by pretreatment with streptomycin 24 h before infection (colitis model) (Raffatellu and others 2008, 2009). We sought to investigate a possible role for CCL28 in resistance against Salmonella using this model. To this end, we infected WT and Ccl28−/− littermate mice with Salmonella enterica serovar Typhimurium orally and followed the course of the infection to evaluate the survival of the mice. As shown in Fig. 5, Ccl28−/− mice are extremely susceptible to Salmonella in this acute infection model. Ccl28−/− mice started to succumb to the infection within 24 h following the bacterial inoculation, and only 40% of the Ccl28−/− mice survived by 72 h postinfection, whereas 100% of the WT mice were alive at this time point postinfection (Fig. 5). There was also a trend toward higher CFU in the spleen of Ccl28−/− mice (Table 2 shows CFUs in the spleen from a representative experiment). These data indicate that CCL28 is a pivotal chemokine mediating innate immunity against Salmonella infection in the gut.
FIG. 5.
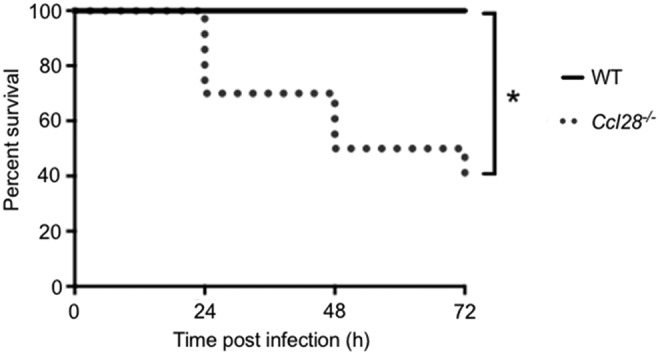
Survival curve of Ccl28−/− (n = 10) and WT (n = 7) littermate control mice infected orogastrically with 1 × 109 fully virulent Salmonella (IR715). Data shown are combined results from 2 independent experiments. *P < 0.05. WT, wild type.
Table 2.
Colony-Forming Units at 72 H Postinfection
| Mouse No. | CFUs per mg of spleen | |
|---|---|---|
| WT | Ccl28−/− | |
| 1 | 1481 | |
| 2 | 2735 | |
| 3 | 1804 | |
| 4 | 272487 | |
| 5 | 6835 | |
| 6 | a | |
| 7 | 21546390 | |
| 8 | a | |
| 9 | 392857 | |
| 10 | 492958 | |
| 11 | 8019 | |
| 12 | 1222 | |
| 13 | a | |
| 14 | a | |
| 15 | 56250000 | |
| 16 | a | |
| 17 | a | |
| Mean | 10478 | 1225710 |
Mouse was moribund and had to be sacrificed before 72 h postinfection (pi)
P = 0.1091 (Mann–Whitney test).
CFU, colony-forming units; WT, wild type.
Microbiome changes in Ccl28−/− mice
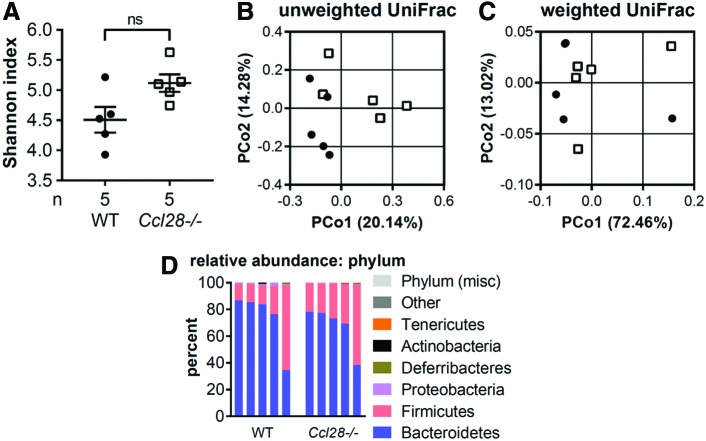
CCL28 has been reported to have antimicrobial activity (Hieshima and others 2003). We, therefore, sought to analyze the gut microbiome of Ccl28−/− mice and compare them to their cohoused wild-type littermates. Although the intrasample Eubacterial diversity was increased in Ccl28−/− mice, the difference was not significant (P < 0.095; Fig. 6A). Similarly, although the intersample community composition is trending toward being different between the 2 groups of mice (Fig. 6B), they are not distinct groupings, and the overall community structures fully overlap (Fig. 6C). When analyzed at the phylum level, the gut microbiota reflects what is usually observed under homeostatic conditions: a predominance of the phyla Bacteroidetes and Firmicutes (Fig. 6D). Although there was a slight average increase in the abundance of Firmicutes with a concomitant decrease of Bacteroidetes in the absence of Ccl28, the differences were not significant (Fig. 6D). Elsewhere, we did see a few significant differences among taxa that each represented on average ≤1% of the microbiota. In Ccl28−/− mice, there were decreases of ∼2-fold in the phylum Proteobacteria and ∼10-fold in the family Peptostreptococcaceae; conversely, we saw an increase of ∼6-fold in the Lachnospiraceae genus UCG-006. Taken together, these data suggest that although there are small changes in the microbial composition in the gut of Ccl28−/− mice, the absence of Ccl28 did not have a big impact on the gut microbiota of cohoused littermates.
FIG. 6.
16S sequence analysis of fecal DNA from WT and Ccl28−/− mice. (A) Alpha diversity (Shannon index) in the gut microbiota of WT (black circles) or Ccl28−/− (white squares) mice. (B, C) Beta diversity represented by a PCoA (principal coordinate analysis) plot based on the (B) unweighted or (C) weighted UniFrac metric. (D) Bar chart of phylum relative abundance for each sample. ns = no significant change (Mann–Whitney-U) relative to WT control. WT, wild type. Color images are available online.
Discussion
Chemokines represent a superfamily of chemotactic cytokines that have been studied for the last 30 years. While initially their chemotactic activities were the main focus of studies on chemokines, they are now known to be involved in multiple biological processes. Of the 48 human chemokines (Zlotnik and Yoshie 2012), 4 exhibit a predominantly mucosal expression pattern (Hernandez-Ruiz and Zlotnik 2017). These include CXCL14, CCL25, CCL28, and CXCL17. The best characterized of these are CCL25 and CCL28. However, even for these better-characterized mucosal chemokines, many questions remain unanswered. In this study, we have focused on the function of CCL28 through the characterization of its expression pattern in humans as well as the functional characterization of a Ccl28−/− mouse.
The first important observation we report in this study is that the main expression site of CCL28 in humans is in the salivary glands (Fig. 1). This important observation is confirmed by the fact that it is normally abundant in human saliva (Hernandez-Molina and others 2015). Matsuo et al. first reported the production of CCL28 by human salivary glands and further mapped it to epithelial cells of the parotid glands (Hieshima and others 2003). Our survey of the BIGE database confirms these data and further indicate that the salivary glands are the main site of expression of CCL28 in the human body. Other studies have documented that it is expressed in the small intestine (Bourges and others 2007), and female reproductive tissues (Cha and others 2011). However, data from the BIGE database indicate that the expression of CCL28 in the salivary glands is much higher than in other mucosal tissues (Fig. 1).
Humans produce around 1 L of saliva a day, and it “bathes” the digestive tract. This observation suggests that it may have important effects in the oropharyngeal cavity, for example, where its antimicrobial activity may be important against pathogens such as Candida albicans (Hieshima and others 2003). In support of this, we have reported lower levels of CCL28 in saliva from patients with Sjögren's syndrome (Hernandez-Molina and others 2015), suggesting that its absence could account for the greater incidence of Candidiasis in patients with this disease (Yan and others 2011). If this is correct, then supplementing artificial saliva with CCL28 may bring therapeutic benefit to these patients. Surprisingly, the BIGE database also indicates that CCL28 is expressed in the thyroid gland (Fig. 1 and Table 1). In support of this, CCL28 has been detected in a study of genes associated with thyroid carcinoma (Huang and others 2017). However, there is currently no information on a possible function of CCL28 in the thyroid.
Ccl28 starts to be expressed in the mammary gland upon the onset of lactation where it chemoattracts CCR10+ IgA plasmablasts, which will produce the IgA that will be present in the milk that will be transferred to infants (Lazarus and others 2003; Wilson and Butcher 2004). Two previous studies have documented that Ccr10−/− mice have very low or no IgA in the milk (Morteau and others 2008; Hu and others 2011). This observation led to the conclusion that Ccl28 normally recruits IgA plasmablasts to the mammary gland upon the onset of lactation (Lazarus and others 2003; Wilson and Butcher 2004). In support of this conclusion, we observed that the Ccl28−/− mouse also exhibits minimal IgA levels in the milk and lower levels in the gut (Fig. 2).
One of these previous studies (Hu and others 2011) reported similar results for gut IgA in a Ccr10−/− mouse, but found that the repertoire of the fecal IgA antibodies in the CCR10−/− mice was limited compared with WT mice. The similar IgA phenotype (to CCR10−/− mice) exhibited by Ccl28−/− mice confirms that the CCL28/CCR10 axis mediates the recruitment of IgA plasmablasts to the lactating mammary gland. The latter point is important because another chemokine (CCL27), is also able to bind CCR10 (Homey and others 2000). Similarly, CCL28 has also been reported to be able to bind CCR3 (Pan and others 2000). Taken together, our data indicate that there is no other CCR10 ligand that can substitute for CCL28 in this function, and that the CCL28/CCR10 chemokine axis recruits IgA plasmablasts to the lactating mammary gland.
A Ccl28−/− mouse is viable and develops normally. However, we noticed abnormal reproductive behavior. Both males and females were observed mounting their same-sex cagemates during the daytime. When we reviewed the literature, we noticed that similar behavior has been observed in mice engineered to have defects in the VNO, which mediates olfaction in mice (Del Punta and others 2002). We, therefore, tested the olfactory ability of Ccl28−/− mice and observed abnormal olfactory ability suggesting anosmia (Fig. 3). Since Ccl28 is expressed in both olfactory epithelial mucosa (Bourges and others 2007; Nagakubo and others 2016) and in the VNO (Fig. 4), we hypothesize that it may be required for normal development and/or function of the olfactory system. Its absence during development in the Ccl28−/− mouse is likely responsible for this abnormal behavior and anosmic phenotype.
We also tested the ability of the Ccl28−/− mouse to handle infection with Salmonella in an acute infection model. In this model, infection develops in a few days and therefore resistance is dependent on innate immunity mechanisms operational in the gut and does not depend on the development of IgA responses (Behnsen and others 2014) because of its short duration (just a few days). As shown in Fig. 5, Ccl28−/− mice are very susceptible to Salmonella in this acute infection model and succumb to the infection rapidly (starting at 24 h). To explain this surprising phenotype, we hypothesized that CCL28 may be involved in an important mechanism of resistance to Salmonella in 2 possible ways: first, CCL28 may be active against Salmonella through its wide-spectrum antimicrobial activity (Hieshima and others 2003). Second, it may be necessary for the recruitment of certain cell(s) of the immune system to the infected intestine that mediate innate resistance to Salmonella. If the latter is the case, we predict that these cells should express either CCR10 or alternatively CCR3 [which has also been reported to bind CCL28 (Pan and others 2000)].
We favor the second hypothesis, because we have observed that recombinant CCL28 does not show significant antimicrobial activity against the Salmonella strain we used for this model (data not shown), and a knockout mouse of the also mucosal chemokine Cxcl17 does not exhibit enhanced susceptibility to Salmonella in the same acute infection model (data not shown) even though Cxcl17 (like Ccl28) also exhibits broad antimicrobial activity (Burkhardt and others 2012).
Furthermore, we have recently reported the identification of a novel B cell-associated cytokine called interleukin 40 (IL-40). An Il-40−/− mouse exhibits significant defects in gut IgA production, especially in the gut (Catalan-Dibene and others 2017). We have tested Il-40−/− mice in the same Salmonella infection model, and observed that Il-40−/− mice did not show increased susceptibility. By 72 h after infection, 100% of Il-40−/− mice were alive (data not shown), whereas, as shown in Fig. 5, only 40% of Ccl28−/− mice were alive at the same time point (72 h) postinfection. These observations confirm that IgA is not involved in the mechanism of susceptibility to Salmonella observed in the Ccl28−/− mouse. Taken together, these observations strongly suggest that CCL28 is part of an important innate immunity mechanism operating in the gut that renders mice resistant to Salmonella in this acute infection model.
Finally, we analyzed the fecal microbiome of Ccl28−/− mice. Although we did detect some differences in the microbiome of the Ccl28−/− mice relative to cohoused wild-type littermates (Fig. 6), they were relatively modest and the only significant changes were among a few taxa that individually represent ≤1% of the microbiota. A recent report also showed only minor changes in the microbiota of another Ccl28−/− mouse and cohoused wild-type mice (not littermates) (Matsuo and others 2018). This study identified a modest but significant expansion of class Bacilli in Ccl28−/− mice (Matsuo and others 2018), which we did not observe in our colony. Importantly, microbiota analyses are known to be influenced by several factors, including housing and diet, even when mice have the same genetic background (Rausch and others 2016). While Matsuo and others (2018) compared Ccl28−/− mice with WT mice from a different colony that were cohoused at weaning, our control mice were WT littermates that were housed together for an extended period of time before obtaining the samples for analyses.
From another perspective, it is not uncommon for animals in different facilities to be colonized with different strains of the same microbial taxa, so the susceptibility profiles of the microbes could also be different. Another factor that could influence microbiota studies is the fact that in the gut, CCL28 acts like an inflammatory chemokine; that is, its expression in the normal gut is detectable but not high. Instead, it is strongly induced in gut epithelial cells during inflammation (Arijs and others 2011). Furthermore, as shown in Fig. 1 and Table 1, CCL28 is normally more abundant in the oral cavity than in the intestinal gut. Therefore, its antimicrobial role may be more important in shaping the microbiome of the oral cavity than in the intestinal mucosa. Its particular ability to kill C. albicans (Hieshima and others 2003) also lends support to this hypothesis.
We conclude that CCL28 is a very versatile multifunctional chemokine. Our data indicate that it is involved in several processes, including recruitment of IgA plasmablasts to the mammary gland, an unexpected function in the olfactory process, and finally, it represents a critical factor in gut innate immunity against Salmonella. Our data confirm that the recruitment of IgA plasmablasts to the mammary gland is mediated by the CCL28/CCR10 chemokine axis. Further studies are needed to fully understand the role of CCL28 in the olfactory process and its role in host defense against enteric infections.
Acknowledgments
This work was supported by Public Health Service Grant AI121928 to M.R. and A.Z. Work in the AZ laboratory was also supported by Public Health Service Grant AI093548. Work in M.R. laboratory is also supported by Public Health Service Grant AI126277, AI126465, and AI114625. M.R. also holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Welcome Fund. The authors want to thank Monica Vazquez for help with the Ccl28−/− mouse colony.
Author Disclosure Statement
No competing financial interests exist.
References
- Arijs I, De Hertogh G, Machiels K, Van Steen K, Lemaire K, Schraenen A, Van Lommel L, Quintens R, Van Assche G, Vermeire S, Schuit F, Rutgeerts P. 2011. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am J Gastroenterol 106(4):748–761 [DOI] [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71(5):2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M. 2014. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40(2):262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourges D, Chevaleyre C, Wang C, Berri M, Zhang X, Nicaise L, Meurens F, Salmon H. 2007. Differential expression of adhesion molecules and chemokines between nasal and small intestinal mucosae: implications for T- and sIgA+ B-lymphocyte recruitment. Immunology 122(4):551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt AM, Maravillas-Montero JL, Carnevale CD, Vilches-Cisneros N, Flores JP, Hevezi PA, Zlotnik A. 2014. CXCL17 is a major chemotactic factor for lung macrophages. J Immunol 193(3):1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, Zlotnik A. 2012. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol 188(12):6399–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan-Dibene J, Vazquez MI, Luu VP, Nuccio SP, Karimzadeh A, Kastenschmidt JM, Villalta SA, Ushach I, Pone EJ, Casali P, Raffatellu M, Burkhardt AM, Hernandez-Ruiz M, Heller G, Hevezi PA, Zlotnik A. 2017. Identification of IL-40, a novel B cell-associated cytokine. J Immunol 199(9):3326–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU, Cuburu N, Ryu S, Kim S, Kweon MN. 2011. Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. J Immunol 187(6):3044–3052 [DOI] [PubMed] [Google Scholar]
- Dawson PA, Steane SE, Markovich D. 2005. Impaired memory and olfactory performance in NaSi-1 sulphate transporter deficient mice. Behav Brain Res 159(1):15–20 [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. 2002. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419(6902):70–74 [DOI] [PubMed] [Google Scholar]
- Hara T, Tanegashima K. 2012. Pleiotropic functions of the CXC-type chemokine CXCL14 in mammals. J Biochem 151(5):469–476 [DOI] [PubMed] [Google Scholar]
- Hernandez-Molina G, Burkhardt AM, Lima G, Zlotnik A, Betanzos JL, Bahena S, Llorente L. 2015. Absence of salivary CCL28 in primary Sjogren's syndrome. Rheumatol Int 35(8):1431–1434 [DOI] [PubMed] [Google Scholar]
- Hernandez-Ruiz M, Zlotnik A. 2017. Mucosal chemokines. J Interferon Cytokine Res 37(2):62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, Yeh SA, Zoller M, Zlotnik A. 2009. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One 4(7):e6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O. 2003. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol 170(3):1452–1461 [DOI] [PubMed] [Google Scholar]
- Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. 2000. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol 164(7):3465–3470 [DOI] [PubMed] [Google Scholar]
- Hu S, Yang K, Yang J, Li M, Xiong N. 2011. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc Natl Acad Sci U S A 108(45):E1035–E1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tao Y, Li X, Chang S, Jiang B, Li F, Wang ZM. 2017. Bioinformatics analysis of key genes and latent pathway interactions based on the anaplastic thyroid carcinoma gene expression profile. Oncol Lett 13(1):167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John AE, Thomas MS, Berlin AA, Lukacs NW. 2005. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am J Pathol 166(2):345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach S, Wewering S, Hatt H, Neuhaus EM, Lubbert H. 2013. Olfaction in three genetic and two MPTP-induced Parkinson's disease mouse models. PLoS One 8(10):e77509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol 170(7):3799–3805 [DOI] [PubMed] [Google Scholar]
- Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. 2005. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J 19(10):1356–1358 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nagakubo D, Yamamoto S, Shigeta A, Tomida S, Fujita M, Hirata T, Tsunoda I, Nakayama T, Yoshie O. 2018. CCL28-Deficient Mice Have Reduced IgA Antibody-Secreting Cells and an Altered Microbiota in the Colon. J Immunol 200(2):800–809 [DOI] [PubMed] [Google Scholar]
- Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, Law Y, Distelhorst K, Nielsen EM, Hill ED, Kwan R, Lazarus NH, Butcher EC, Wilson E. 2008. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol 181(9):6309–6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakubo D, Yoshie O, Hirata T. 2016. Upregulated CCL28 expression in the nasal mucosa in experimental allergic rhinitis: implication for CD4(+) memory T cell recruitment. Cell Immunol 302:58–62 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Iimura M, Eckmann L, Kagnoff MF. 2004. Regulated production of the chemokine CCL28 in human colon epithelium. Am J Physiol Gastrointest Liver Physiol 287(5):G1062–G1069 [DOI] [PubMed] [Google Scholar]
- Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. 2000. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol 165(6):2943–2949 [DOI] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5(5):476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14(4):421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P, Basic M, Batra A, Bischoff SC, Blaut M, Clavel T, Glasner J, Gopalakrishnan S, Grassl GA, Gunther C, Haller D, Hirose M, Ibrahim S, Loh G, Mattner J, Nagel S, Pabst O, Schmidt F, Siegmund B, Strowig T, Volynets V, Wirtz S, Zeissig S, Zeissig Y, Bleich A, Baines JF. 2016. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int J Med Microbiol 306(5):343–355 [DOI] [PubMed] [Google Scholar]
- Raz E. 2003. Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet 4(9):690–700 [DOI] [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. 2006. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7(2):67–80 [DOI] [PubMed] [Google Scholar]
- Stover JT, Shaw JR, Kuchibhatla M, Horwitz ME, Engemann AM. 2017. Evaluation of hematopoietic stem cell mobilization rates with early plerixafor administration for adult stem cell transplantation. Biol Blood Marrow Transplant 23(8):1290–1294 [DOI] [PubMed] [Google Scholar]
- Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, Agace WW. 2002. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest 110(8):1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, Abrams J, Kershenovich D, Smith K, McClanahan T, Vicari AP, Zlotnik A. 2000. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem 275(29):22313–22323 [DOI] [PubMed] [Google Scholar]
- Wilson E, Butcher EC. 2004. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med 200(6):805–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Young AL, Hua H, Xu Y. 2011. Multiple oral Candida infections in patients with Sjogren's syndrome—prevalence and clinical and drug susceptibility profiles. J Rheumatol 38(11):2428–2431 [DOI] [PubMed] [Google Scholar]
- Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J, Nunes J, Tsolis RM, Adams LG, Baumler AJ. 2003. Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea. Infect Immun 71(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. 2011. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11(9):597–606 [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. 2012. The chemokine superfamily revisited. Immunity 36(5):705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]