Abstract
Mild traumatic brain injury (mild TBI) is a growing public concern, as evidence mounts that even brain injuries classified as “mild” can result in persistent neurological dysfunction. Multiple brain injuries heighten the likelihood of worsened or more prolonged symptomatology and may trigger long-term neurodegeneration. Animal models provide a logical platform to identify key parameters, such as loading forces, duration between injuries, and number of injuries, which contribute to additive or synergistic damage after repeated mild TBI. Despite the tremendous increase in research productivity in the field of repeated mild TBI, relatively few studies have been designed in such a way as to provide experimental-based insights into the dependence of cellular and functional outcomes on the prescribed parameters of mild TBI. In this review, we summarize how standard models of TBI have been adapted to produce mild TBI and highlight commonly observed aspects of neuropathology replicated in rodent models of mild TBI. The complexity of designing studies of repeated TBI is discussed, including challenges of incorporating appropriate control groups, informative experimental design, and relevant outcome measures. We then feature studies that provide a well-controlled, within-study design varying either the number of injuries or the interinjury interval. Harnessing the power of experimental models of TBI to elucidate which injury parameters are critical contributors to acute and chronic damage after repeated injury can further efforts at prevention and provide improved models for testing mechanisms and therapeutic interventions.
Keywords: axonal injury, cortex, hippocampus, memory loss, neuroinflammation, rodent behavior
Classification and Incidence of Traumatic Brain Injury
Traumatic brain injury (TBI) occurs after a direct or indirect insult to the head resulting in damage to the brain that interrupts normal brain function.1 Among the leading causes of TBI are falls, being struck by or against an object, and motor vehicle accidents.1 The variable manner in which forces are imparted to the brain during TBI, in terms of the type of loading (e.g., impact, acceleration/deceleration), the magnitude, and the location, yields a spectrum of pathophysiological outcomes. Clinically, signs of altered brain function after a head injury may include loss of consciousness, amnesia for events either immediately before or immediately after the injury, difficulties with motor control such as muscle weakness or balance, changes in vision or speech, confusion, disorientation, and/or problems with concentration.2
The Glasgow Coma Scale (GCS) is a widely used neurological assessment that enables the classification of TBI along the spectrum of mild to severe. The GCS comprises a set of prescribed outcome measures including a patient's eye, verbal, and motor responses to auditory and painful stimuli, yielding a score from 3 to 15, where 3 is deep unconsciousness and 15 is normal consciousness. In general, a patient with a GCS score of 3–8 falls under the categorization of severe TBI, while a GCS score of 9–12 is considered a moderate TBI and a GCS score of 13–15 is classified as a mild TBI. Within any of these severity categories, however, the pathophysiological features of TBIs are heterogeneous, including contusion, hemorrhage, diffuse axonal injury (DAI), diffuse swelling, and/or hematoma.3
Between 1.9 and 3.8 million TBIs are estimated to occur every year in the United States,4 resulting in upward of 300,000 hospitalizations1 and $60 billion in direct healthcare costs.5 Approximately 80% of TBIs fall within the mild category6 and do not require hospitalization. A classification of “mild” belies the potential for long-term disabilities, however, such as deficits in working memory.7,8 Symptoms of TBI can resolve quickly or last indefinitely, depending on the severity of the injury. For the majority of individuals with mild TBI, symptoms resolve within a week. For some, however, symptoms of mild TBI such as cognitive deficits can last for months.9–14
A TBI, including mild TBI, also increases an individual's risk of additional health concerns such as binge drinking,15,16 depression,17 neurodegenerative diseases,18 and post-traumatic epilepsy.19 Because the persistence of cognitive impairment after mild TBI is highly variable, it is critical to understand what aspects of the injury are the major contributing factors of long-term dysfunction and diminished quality of life.
Neuroimaging of Mild TBI
In the clinical setting, magnetic resonance imaging (MRI) and computed tomography (CT) scans are used routinely to assess damage associated with moderate and severe TBI such as contusion, hemorrhage, edema, and skull fracture. In an estimated 95% of mild TBI cases, however, minimal, if any, pathological findings are observed with these standard neuroimaging techniques.20 Indeed, the current clinical consensus is that patients presenting with GCS of 13–15 should not undergo neuroimaging.21–23 However, the CT scans are warranted for those with GCS <15 and clinical evidence of skull fractures, focal neurological signs, seizures, persistent vomiting, retrograde amnesia, age >60 years, presence of coagulopathy, high likelihood mechanism (fall from height, pedestrian motor vehicle accident), chronic alcohol use, or previous neurosurgical procedure.24
Cases of mild TBI (GCS score 13–15) with intracranial abnormalities on day-of-injury CT or MRI scans that can require neurosurgical intervention have been coined “complicated” mild TBI.25 Patients with complicated mild TBI typically display worse neuropsychological outcomes in weeks to months after injury compared with those with uncomplicated mild TBI.26–28
Other neuroimaging techniques can detect subtle changes that may occur with uncomplicated mild TBI. Diffusion tensor imaging (DTI) measures the directionality of water diffusion. In white matter regions, uninjured axon fibers restrict lateral flow of water creating directionality, or anisotropy, of flow parallel to the fiber tracts. After injury, the flow of water along axon tracts is hindered by axon retraction bulbs, edema, and/or disrupted integrity of myelin surrounding the axon, thereby reducing anisotropy. Changes in fractional anisotropy (FA) or other DTI parameters, then, may reflect axonal damage after mild TBI. Because axonal injury is an important component of mild TBI,29–33 DTI can provide crucial information about the localization and extent of axonal injury over time both in clinical studies and in animal models, as reviewed below.
Additional cutting-edge imaging techniques evaluate parameters such as regional blood flow (dynamic susceptibility contrast MRI or arterial spin labeling), indirect measures of neuronal activation (functional MRI) and brain metabolism (MR spectroscopy) that are relevant to the study of mild TBI.34 Increased implementation of sensitive neuroimaging tools in pre-clinical studies provides improved model validation and allows investigation of the cellular and molecular underpinnings of functional alterations detected after mild TBI.
Repeated TBI
The risk of sustaining a brain injury is two to five times higher for individuals with a history of TBI.35,36 Athletes and military personnel are particularly at risk for multiple brain injuries.37,38 The incidence of sports-related TBIs is difficult to estimate, both because a large proportion of mild brain injuries go unreported39 and because current data systems place TBIs occurring during sports or recreational activities under “falls” or “struck by/against” categories, making it difficult to estimate what percentage of these categories are sports-related.1
Although reported symptoms often subside within a week after mild TBI,37,40,41 increasing evidence suggests that subsequent brain injuries are associated with more persistent symptomatology and even emotional, cognitive, and motor dysfunction that worsen over time.35,42–45 Return-to-play guidelines in contact sports and return-to-duty guidelines for military service members have been developed to prevent additional injury during recovery.46–48 The standardized assessment of concussion (SAC) is used in athletics to measure changes in immediate and delayed memory recall and ability to concentrate and orient with time compared with a baseline (pre-game/pre-season) assessment. This approach presumes that cellular dysfunction is transient and, once a person is symptom free, the brain is no longer vulnerable to the additive effects of a second or repeated TBI.
Symptom resolution, however, is not necessarily synonymous with full recovery of function at a cellular level, according to clinical reports.49 The lack of diagnostic imaging or biomarkers for cellular responses to mild TBI currently hampers the ability to assess sufficient brain recovery after mild TBI.
Although the increased utilization of concussion databases can facilitate understanding of the causes and consequences of multiple TBIs in humans, it remains difficult to draw conclusions across different types and severities of brain injury, numbers of injuries, interinjury intervals, ages, and pre-existing health conditions. Many questions remain. Does brain damage increase linearly or exponentially with each TBI? Is there a threshold or plateau for increased damage with repeated TBI? How does this relationship change with injury severity? How long is the injured brain at heightened risk for a subsequent TBI? How are the risks of repeated injury dependent on sex, age, and other genetic or health-related factors? Animal model studies of mild TBI provide a suitable platform in which to control many of these variables while systematically varying a single parameter to identify which variables are most critical in affecting outcomes after repeated mild TBI.
Experimental Models of TBI
Animal models developed over the past several decades reproduce many aspects of the pathobiological features of human TBI. Well-established small animal models of TBI include fluid percussion injury (FPI),50,51 controlled cortical impact (CCI),52 weight drop (WD),53,54 and impact acceleration (IA).55,56 Each model was designed to simulate a specific type of brain injury to characterize the physiological, behavioral, and histopathological consequences of TBI.
Midline FPI and IA are often used as models of human DAI whereas CCI, WD, and lateral FPI mimic characteristics of contusion brain injury. Behavioral consequences of TBI in animals may include loss of consciousness, deficits in learning and memory, difficulties with motor coordination and sensory function, anxiety, and depression. Neuronal death, axonal injury, cerebral edema, and blood–brain barrier (BBB) disruption can be replicated, as well as secondary mechanisms of injury such as excitotoxicity, oxidative damage, and inflammation. Similar to the heterogeneity of symptomology and pathology in human TBI, however, every model of TBI does not encompass all of these outcomes. Further, behavioral, physiological, and histological features observed in animals may depend on both the severity and location of injury.
For many years, rodent TBI studies of secondary injury mechanisms and evaluation of potential therapeutics were conducted typically using injury levels designed to induce moderate or severe trauma. The emphasis on moderate/severe TBI was largely an effort to model the population of patients with TBI (often GCS 3–8) typically enrolled in pharmacological intervention clinical trials. In the current review, however, we focus on models of closed head trauma developed to mimic the majority of mild TBI observed clinically. We refer the reader to other reviews of blast injury and penetrating TBI.57–59
Experimental Models of Mild TBI
A growing appreciation for the potential for persistent neurological impairment after mild TBI and the identification of repeated TBI as a risk factor for the neurodegenerative disease chronic traumatic encephalopathy (CTE) has fueled interest in the development, characterization, and implementation of mild TBI and repeated mild TBI models. One common strategy has been to modify existing models to reduce the severity of injury. Injury severity can be reduced by scaling mechanical input parameters such as the depth or speed of impact60 or the mass or drop distance of a weight.61,62
Alternatively, the manner in which force is delivered to the head or brain can be altered to minimize impact severity. For example, instead of inducing a focal injury by directly impacting the brain surface exposed after a craniectomy, impact onto the closed skull or head creates a more diffuse, and typically milder, injury.63,64 Moreover, as compared with impact to the skull after scalp reflection,65,66 impact to the intact scalp or a helmet/disk affixed to the head further attenuates and distributes the impact force.62,66 Although impact to the scalp or an affixed helmet reduces the incidence of skull fracture, the inability to visualize anatomical landmarks on the skull (i.e., lambda, bregma, midline) increases variability in positioning the impact. The risk of skull fracture with direct impact to the skull can be mitigated by modifying the impactor tip through the addition of silicone or rubber to the tip.67,68 In addition, increasing the surface area of the impacting object can reduce fracture risk and create a more diffuse, and potentially milder, injury.
The location of impact influences the location, distribution, and severity of histopathological damage as well as the animal's response to injury. Impacts directed midline as opposed to lateral, or over the frontal cortex as opposed to parietal cortex, can produce different patterns of regional damage and modify the physiological response of the animal. For example, axonal impairment occurs predominantly in one hemisphere with a lateral impact69,70 while axonal injury is observed diffusely after impact at midline.63,68,71 The type of head support given and extent of head movement allowed during impact can also influence the mechanical input to the brain. To create a more reproducible injury during impact, original WD models and some CCI models prevent head motion during impact by use of a stereotaxic frame or by placing the head on a hard surface.63,64,67 The IA model introduced limited head movement during impact by placing the rodent's head on a deformable foam pad.62,72
More recent variants of the IA model allow free movement of the head by placing the animal on a temporary surface (Kimwipe or foil) that gives way during head impact to create acceleration of the brain accompanying movement of the entire body.73–76 A new model based on the IA concept and termed the closed-head impact model of engineered rotational acceleration, or CHIMERA, allows free movement of the head after impact to the intact scalp by a piston barrel while constraining the torso of the mouse to accentuate rotational movement of the head.77
Over the years, the goal of mild TBI models has shifted from (a) producing a scaled-down, less severe injury, typically documented through cell death and overt behavioral deficits, than standard moderate to severe TBI to (b) inducing transient behavioral or physiological impairment accompanied by cellular dysfunction with little or no cell death or hemorrhage. This shift has been driven in part by clinical neuroimaging studies that reveal minimal, if any, focal lesions and by the transient nature of symptoms in the vast majority of mild TBI cases. Because defining criteria for mild TBI have not been established in rodent models, the reader is advised to interpret published studies with caution.
Because of the large number of injury models used to study mild TBI and variance in the implementation of these models across laboratories, mild TBI cannot be defined solely based on mechanical injury parameters. DeWitt and colleagues78 suggested that mild TBI be categorized based on righting reflex (RR) response after injury. Although RR is lengthened after mild TBI, other physiological parameters, such as blood pressure, do not appear to change in models of mild TBI,61,67 nor is there a decrease in the cerebrovasculor response to the neurotransmitter acetylcholine.62 The RR in rodents is an established correlate for loss of consciousness in humans and, as such, RR values under 15 min in animals are proposed to reflect mild TBI severity.63,78 Because RR seems to be influenced by anesthesia protocol and impact location, inclusion of other behavioral or histological parameters to verify injury severity is advisable.
This review presents an overview of selected single and repeated mild TBI studies, highlighting commonly reported outcome measures after injury including axonal damage, neuroinflammation, cell death, and behavioral deficits. Although neuroinflammation is a constellation of events, here we focus on the most commonly reported features related to astrocyte and microglia reactivity, such as morphological alterations/hypertrophy, increased protein expression or numbers, and migration. Because there are numerous published studies on single-impact mild TBI models, we direct the reader to a more extensive review of these studies prepared by DeWitt and colleagues.79
We then focus on experimental work comparing effects of repeated mild TBI with single mild TBI within the same study (Table 1), and, importantly, compare different numbers of impacts (Table 2) and different interinjury intervals (Table 3) within the same study. Each table contains a column labeled “Injury model,” with information regarding the species of animals, the injury model (e.g., closed head injury [CHI]), the location of injury (e.g., midline), whether the head was fixed or unfixed, and information regarding the object used to impact the head/brain.
Table 1.
Studies Comparing Outcomes after Repeated Mild Traumatic Brain Injury with Those after Single Mild Traumatic Brain Injury
| Ref | Injury model | # of injuries | Interinjury Interval | Axonal injury | Cell death | Inflammation | Behavioral deficits |
|---|---|---|---|---|---|---|---|
| (Laurer, et al. 2001) 67 | Mice CHI Lateral Fixed 6 mm rubber |
1 | – | APP: none at 7 d; mild in external capsule at 28, 56 d | Nissl: no overt damage at 7 or 56 d | – | Neuroscore: deficit at 3 d; none at 7 or 14 d Rotarod: no deficit across 3, 7, 14, 28, and 56 d Rotating pole test: no deficits at 3, 7, 14, 28, or 56 d MWM: no deficit in learning or memory at 1 or 8 wks |
| 2 | 24 h | APP: none at 7 d; pronounced at 28 d; subsiding at 56 d | Nissl: no overt cell loss at 7 or 56 d | – | Neuroscore: worse than single at 3 and 7 d Rotarod: worse than single at 3, 7, 14, 28, and 56 d Rotating pole test: worse than single only at 3 and 56 d MWM: no deficit in learning or memory at 1 or 8 wks |
||
| (Shitaka, et al. 2011) 64 | Mice CHI Lateral Fixed 9 mm rubber |
1 | – | APP: none at 1, 2 or 7 d; SS and EM: minimal WM at 7 d | Nissl: no overt damage at 7 d | Iba-1: none at 7 d | MWM: no deficit in learning or memory at 2–5 d |
| 2 | 24 h | APP, SS, EM: cortex and WM at 7 d SS: HP and thalamus at 7 d |
Nissl: no overt damage at 7 d | Iba-1: ↑ at 7 d in cortex, HP, and thalamus | MWM: learning deficits at 2–5 d, memory deficit at 5 d | ||
| (Cheng, et al. 2014) 105 | Mice CHI Frontal Fixed 56 g metal ball at 71 cm height |
1 | – | – | – | – | Barnes Maze: no learning or memory deficit at 1 mo |
| 2 | 48 h | – | – | GFAP and Iba-1: ∼sham at 12 mo in CC | Barnes Maze: learning deficits at 1 mo compared with sham; memory deficit compared with single Y-Maze, EPM, beam balance, FS, OF: no deficits compared to sham |
||
| (Uryu, et al. 2002) 65 | 9 mo Mice CHI Lateral Fixed 6 mm rubber |
1 | – | – | – | GFAP: very mild in WM at 16 wks | Neuroscore: no deficit at 16 wks MWM: no deficit in learning at 16 wks |
| 3 | 24 h | – | – | GFAP; mild ↑ over single below impact site at 2 d, 9 and 16 wk | Neuroscore: no deficit at 16 wks MWM: no deficit in learning at 16 wks |
||
| (Nichols, et al. 2016) 72 | Mice IA Unfixed-foam Steel helmet |
1 | – | – | – | – | MWM: memory deficit at 3 wks; Marble-burying: ∼sham at 10 d; Nestlet shredding: ∼sham at 11 d EPM: no deficit at 9 d; FS: no change at 10 d |
| 3 | 24 h | – | – | – | MWM: learning deficit at 1, 2, and 3 wks; memory deficit ∼single at 3 wks Marble-burying: ↓ compared with sham at 10 d Nestlet shredding: ↓ compared with sham at 11 d EPM: no deficit at 9d; FS: no change at 10 d |
||
| (Tyburski, et al. 2017) 110 | Rat CHI Lateral Unfixed - foam 10 mm rubber |
1 | – | – | – | GFAP: ∼sham in ipsi somatosensory cortex at 7 d; Iba-1: ∼sham in TNC at 7 d | – |
| 3 | 24 h | – | – | GFAP: > single in the ipsi somatosensory cortex at 7 d; Iba-1: > sham but not single in the TNC at 7 d | – | ||
| (Jamnia, et al. 2017) 127 | Rat CHI Lateral Unfixed - foam 5 mm metal |
1 | – | APP: modest at 8 d in CC; 16% ↓ in CC thickness | NeuN: 8% ↓ volume at 8 d in cortex | Iba-1: modest ↑ at 8 d compared with single | OF: activity deficit at 5 d, not at 1 d; no anxiety deficit Foot Fault: deficit at 5 d but not at 1 d, 7 d NOR: memory deficit at 3 d but not 7 d |
| 3 | 48 h | APP: ∼single in CC; CC thickness: ∼single | NeuN: cortex volume ∼single | Iba-1: ∼single | OF: ∼single in activity; anxiety deficit at 1 d compared with 1 d after single Foot Fault: ∼sham at 1, 3 d after last injury NOR: ∼single time matched to last injury |
||
| (McAteer, et al. 2016) 103 | Rats IA Midline Unfixed-foam Steel disc on head 450 g weight |
1 | – | APP: ∼sham at 1 d, mild ↑ in cortex at 12 wk compared with sham | – | Iba-1: ↑ in cortex at 12 wk but not 24 h | Rotarod: no deficit at 6 or 12 wks Barnes Maze: mild memory deficit at 6 wk but not 12 wks Y-maze: no deficit at 6 or 12 wks OF: less exploration at 6 wk but not 12 wks |
| 3 | 5 d | APP: ∼sham at 1 d, >sham in cortex, HP, and thalamus at 12 wk | – | Iba-1: ↑ in cortex at 24 h and 12 wk | Rotarod: no deficit at 6 or 12 wks Barnes Maze: memory deficit at 6 wk, mild deficit at 12 wks Y-maze: mild deficit at 12 wks but not 6 wks OF: ↓ exploration at 6 and 12 wk |
||
| (Mountney, et al. 2017) 108 | Rats CHI Lateral Unfixed Carbon-fiber helmet 3.52 g steel projectile |
1 | – | H&E: no change in CC thickness at 3 mo | FJ: positive in subset of animals in ipsi cortex at 24 h | GFAP: ↑ in ipsi cortex and CSF but not HP at 24 h. No change at 3 mo. | NSS: deficit at 1 h and 3 mo, none at 24 h and 72 h Rotarod: mild deficit at 15 min and 1 h, similar to sham at 24 h, 7 d, 14 d, and 3 mo CatWalk: stance duration ↑ at 2 h, 24 h, and 3 mo, none at 3, 9 and 28 d; gait balance/ataxia at 2 and 24 h MWM: no learning deficit at 3 mo |
| 4 | 1 h | H&E: CC thinning at 3 mo compared with single and sham | FJ: present in the ipsi cortex of every animal at 24 h | GFAP: ↑ in ipsi cortex, HP and CSF at 24 h, > single at 3 mo. | NSS: deficit at 1 h, 4 h, 3 mo compared with sham; none at 24 h or 72 h Rotarod: deficit at 15 min and 1 h compared with single and 3 mo compared with sham; none at 1, 7, 14 d CatWalk: stance duration ↑ at 2 h, 24 h, 3 and 9 d compared with single, at 3 mo compared with sham; gait balance/ataxia at 2 h and 24 h compared with single, at 3, 9, and 28 d compared with sham MWM: no learning deficit at 3 mo |
||
| (Mouzon, et al. 2012) 98 | Mice CHI Midline Fixed 5 mm metal |
1 | – | APP: ↑ in white matter at 24 h; none at 10 d | Nissl: cerebellar damage at 10 d, not 1 d | GFAP: ↑ in CC from 1–10 d; Iba-1: > sham in CC at 1 d, 10 d | Rotarod: deficit out to 7 d Barnes Maze: learning and memory deficit at 7 d |
| 5 | 48 h | APP: < single at 24 h in CC, > single in brainstem | Nissl: ∼single at 10 d in cerebellum | GFAP and Iba-1: ↑ in CC and HP compared with single | Rotarod: not different from single Barnes Maze: trend toward worsened learning and memory, but not different from single EPM: ↓ inhibition compared with sham at 12 mo |
||
| (Mouzon, et al. 2014) 100 | Mice CHI Midline Fixed 5 mm metal |
1 | – | APP: mild at 12 mo in CC; LFB/CV: 10% ↓ in CC thickness | – | GFAP: ∼sham in HP and CC at 12 mo Iba-1: > sham in CC |
Rotarod: no deficit at 6 mo Barnes Maze: learning deficit at 12 mo and 18 mo; no memory deficit EPM: ↓ inhibition compared with sham at 12 mo |
| 5 | 48 h | APP: > single at 12 mo in CC; LFB/CV: 15% ↓ in CC thickness compared with single | – | GFAP: > single in HP at 12 wks and in CC at 6 and 12 wks Iba-1: > sham at 12 wks |
Rotarod: no deficit at 6 mo Barnes Maze: learning deficit at 6 mo and 18 mo compared with single; memory deficit at 6 mo and 12 mo compared with sham |
||
| (Winston, et al. 2016) 109 | Rat CHI Midline Unfixed - gel mold 10 mm Teflon |
1 | – | APP, SS, FJ: no axonal injury at 1 d in the optic tract | – | GFAP, Iba-1, CD68: ∼sham at 1 d in the optic tract | – |
| 30 | 24 h (5 d/wk) | FJ, SS: positive in the optic tract at 1 d; APP: none at 1 d | – | GFAP, Iba-1, CD68: qualitatively > single at 1 d in optic tract | – | ||
| (Petraglia, et al. 2014) 66,128 | Mice CHI Lateral Unfixed 6 mm rubber helmet |
1 | – | – | – | GFAP: ↑ at 7d in cortex and amygdala; ↓ at 30 d in HP; ↑ at 6 mo in HP; CD68: ∼sham at 7 d, 30 d, 6 mo |
NSS: deficit at 1 h, 4 h, 24 h, 72 h, not at 7 d and 30 d MWM: learning deficit at 1–5 d and 1 mo, but not 6 mo EPM: deficit at 2 wks, none at 1 or 6 mo FS: no change at 1 mo |
| 42 | 2 h (6/d for 7 d) | – | – | GFAP: ∼single, except ↑ at 6 mo in cortex and amygdala; CD68: ↑ at 7 d, 30 d, and 6 mo in cortex, amygdala, and HP |
NSS: deficit compared with single up to 1 mo MWM: learning deficit 1–5 d compared with single; ∼single at 1 mo; no deficit at 6 mo EPM: deficit at 2 wks, 1 mo, 6 mo FS: change at 1 mo compared with single |
– indicates the pathology or behavioral dysfunction was not evaluated. APP, amyloid precursor protein; CC, corpus callosum; CHI, closed head injury; CSF, cerebrospinal fluid; EM, electron microscopy; EPM, elevated plus maze; FJ, Fluoro-Jade; FS, forced swim; GFAP, glial fibrillary acidic protein; H&E, hematoxylin and eosin; HP, hippocampus; IA, impact acceleration; Iba-1, ionized calcium-binding adaptor molecule-1; LFB/CV, luxol fast blue/cresyl violet; MWM, Morris Water Maze; NOR, novel object recognition; NSS, neurological severity score; OF, open field; SS, silver stain; TNC, trigeminal nucleus caudalis; WM, white matter.
Table 2.
Studies Comparing the Effects of Varying Numbers of Repeated Mild Traumatic Brain Injuries
| Ref | Injury model | # of injuries | Interinjury interval | Axonal injury | Cell death | Inflammation | Behavioral deficits |
|---|---|---|---|---|---|---|---|
| (Yates, et al. 2017) 76 | Female Rats IA Midline Unfixed-Kimwipe 250 g weight |
1 | – | LFB: no change in CC thickness at 4d (∼ sham) | – | GFAP and Iba-1: ∼ sham at 4 d in CC | NSS: no deficit days 1–4 (∼ sham) MWM: no learning deficit days 1–3; no memory deficit at 4 d |
| 2 | 24 h | LFB: ∼ sham | – | GFAP and Iba-1: ∼ sham | NSS: ∼ sham MWM: learning ∼ sham; memory deficit at 4d compared with sham |
||
| 3 | LFB: ∼ sham | – | GFAP and Iba-1: ∼ sham | NSS: ∼ sham MWM: no deficits (∼ sham, single) |
|||
| (Shultz, et al. 2012) 112 | Rats FPI Lateral |
1 | – | – | NeuN: ∼ sham with very little cortical damage | ED1: mild, ∼ sham at 1 d, 8 wks | MWM: learning deficit at 24 h but not 8 wks EPM: no change at 24 h or 8 wks FS: immobility duration similar to sham at 24 h Beam task: no motor deficit at 24 h or 8 wks OF: no locomotor deficit at 24 h or 8 wks |
| 3 | 5 d | – | NeuN: Most had moderate cortical damage | ED1: ↑ at 1 d, not 8 wks | MWM: learning deficit at 24 h compared with sham and at 8 wks compared with sham and single EPM: ∼ single; FS: ∼ single Beam task: ∼ single; OF: ∼ single |
||
| 5 | – | NeuN: Larger percentage with cortical damage | ED1: ↑ at 1 d, 8 wks | MWM: learning deficit compared with sham and single 24 h and similar to 3 TBI at 8 wks EPM: change at 24 h compared with sham and 8 wks compared with all other groups FS: ↑ immobility compared with sham at 8 wks; Beam task: ∼ single; OF: ∼ single |
|||
| (Meehan, et al. 2012) 74 | Mice IA Midline Unfixed-Kimwipe 53g weight |
1 | – | APP: none | H&E: no cell loss FJ, TUNEL: none |
– | MWM: no learning deficit at 24 h compared with sham |
| 3 | 24 h | APP: none | none | – | MWM: learning deficit at 24 h compared with sham; groups were not compared with single or other repeated TBI groups | ||
| 5 | APP: none | none | – | ||||
| 10 | APP: none | none | – |
– indicates the pathology or behavioral dysfunction was not evaluated. APP, amyloid precursor protein; CC, corpus callosum; EPM, elevated plus maze; FJ, Fluoro-Jade; FPI, fluid percussion injury; FS, forced swim; GFAP, glial fibrillary acidic protein; H&E, hematoxylin and eosin; IA, impact acceleration; Iba-1, ionized calcium-binding adaptor molecule-1; LFB, luxol fast blue; MWM, Morris Water Maze; NSS, neurological severity score; OF, open field; TBI, traumatic brain injury; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Table 3.
Studies Comparing the Effects of Varying Interinjury Intervals in Repeated Mild Traumatic Brain Injury Designs
| Ref | Injury model | # of injuries | Interinjury interval | Axonal injury | Cell death | Inflammation | Behavioral deficits |
|---|---|---|---|---|---|---|---|
| (Fujita, et al. 2012) 62 | Rats IA Midline Unfixed-foam Metal weight helmet |
1 | – | APP: minimal AI at 4 h in corticospinal tract | – | – | – |
| 2 | 3 h | APP: > single at 4 h in corticospinal tract | – | – | – | ||
| 5 h | APP: > single but <3 h interval cohort | – | – | – | |||
| 10 h | APP: > single, but <3 h and 5 h interval cohorts | – | – | – | |||
| (Acabchuk, et al. 2016) 129 | Mice IA Midline Unfixed-trap door 95 g weight |
5 |
6 h/18 h | MBP: no change at 2 wks SMI-32: no axonal damage at 2 wks |
– | Iba-1: ↑ in LS and HP at 1 h but not 2 or 6 wks GFAP: ↑ in LS and CC at 1 h, 2 wks, 6 wks, and in HP at 1 h, 2 wks |
– |
| 3 d | MBP: no change at 2 wks SMI-32: no axonal damage at 2 wks |
– | Iba-1: ∼sham at 1 h, 2 wks, 6 wks GFAP: ↑ in LS at 1 h but not 2 or 6 wks; no change in HP at 1 h, 2 wks, 6 wks |
– | |||
| (Meehan, et al. 2012) 74 | Mice IA Midline Unfixed-Kimwipe 53 g weight |
1 | – | see Table 2 for details on single injury | |||
| 5 | 24 h | APP: none | H&E: no cell loss at 1 d, 7 wks FJ, TUNEL: none at 1 h, 1 d |
– | MWM: learning deficit at 1 d, 1 mo, and 1 y | ||
| 1 wk | APP: none | H&E, FJ, TUNEL: none | – | MWM: learning deficit at 1 d, 1 mo, resolved by 1 y | |||
| 1 mo | APP: none | H&E, FJ, TUNEL: none | – | MWM: learning deficit at 1 d, resolved by 1 mo | |||
| (Mannix, et al. 2013) 114 | Mice IA Midline Unfixed-Kimwipe 53 g weight |
1 | – | – | – | – | MWM: learning ∼sham at 6 mo |
| 5 | 24 h | – | Hoescht: no overt cell loss in HP compared with sham | Iba-1: ∼sham at 6 mo GFAP: ↑ # cells at 6 mo in cortex, CC and HP |
MWM: learning deficit at 3 d, 2 mo, 6 mo, and 1 y compared with sham | ||
| 1 wk | – | – | – | MWM: learning deficit at 6 mo compared with sham | |||
| 2 wks | – | – | – | MWM: learning ∼sham at 6 mo | |||
| 1 mo | – | – | – | MWM: learning ∼sham at 6 mo | |||
| (Bolton and Saatman 2014) 63 | Mice CHI Midline Fixed 5 mm silicone |
1 | – | APP: transient (24 h to 9 d) in external capsule | FJ: minimal in entorhinal cortex at 1 d Nissl: no overt cell loss |
GFAP: > sham at 9 d, but not 1, 5 d in entorhinal cortex Iba-1: ∼sham at 1, 5, and 9 d |
– |
| 5 | 24 h | APP: ∼single at 1 d after last injury | FJ: > single Nissl: hemorrhage in entorhinal cortex |
GFAP: > sham and single at 24 h after last injury Iba-1: > single at 24 h after last injury |
– | ||
| 48 h | APP: ∼single at 1 d after last injury | FJ: ∼single Nissl: no overt cell loss |
GFAP and Iba-1: ∼single | – | |||
| (Bolton Hall, et al. 2016) 104 | Mice CHI Midline Fixed 5 mm-silicone |
5 | 24 h | SS: > sham at 10 wks in optic tract NF200: < sham at 10 wks in optic nerve |
– | GFAP and Iba-1: > sham in cerebellar WM, optic tract, and optic nerve | Beam Balance: deficit over 10 wks compared with sham NOR: memory deficit over 10 wks compared with sham. |
| 48 h | SS: ∼24 h interval NF200: ∼24 h interval |
– | GFAP and Iba-1: ∼24 h interval group | Beam Balance, NOR: ∼24h interval group | |||
| (Longhi, et al. 2005) 68 | Mice CHI Lateral Fixed 9 mm silicone |
1 | – | APP: in WM at 3, 7 d | FJ: in ipsi cortex at 3 d | – | Rotarod: deficit at 4 d compared with sham; MWM: no learning deficit over days 1–3 |
| 2 | 3 d | APP: > single at 3 d | FJ: ∼single at 3 d | – | Rotarod: deficit at 4 d compared with sham and to all other CHI groups MWM: learning deficit over days 1–3 compared with sham or single |
||
| 5 d | – | – | – | Rotarod: ∼single at 4 d MWM: learning deficit over days 1–3 compared with single, but not sham |
|||
| 7 d | – | – | – | Rotarod: ∼single at 4 d MWM: no learning deficit over days 1–3 |
|||
| (Weil, et al. 2014) 95 | Rats IA Lateral Unfixed 36 g weight onto plastic plunger |
1 | SS: WM degeneration at 40 d in CC, HP, and caudate putamen | FJ: no positive cells | Iba-1: > sham at 40 d in ipsi cortex, HP, and thalamus GFAP: ∼sham |
Barnes Maze: learning deficit at ∼1 mo | |
|
2 |
3 d | SS: > sham and single | FJ: no positive cells | Iba-1 and GFAP: > sham in ipsi hemisphere at 40 d | Barnes Maze: memory and learning deficits at ∼1 mo from first injury | ||
| 20 d | SS: ∼single | FJ: no positive cells | Iba-1: > sham bilaterally at 40d after 1st injury GFAP: > sham at 40 d after 1st inj. |
Barnes Maze: learning deficit at ∼1 mo from 1st injury | |||
– indicates the pathology or behavioral dysfunction was not evaluated. AI, axonal injury; APP, amyloid precursor protein; CC, corpus callosum; CHI, closed head injury; FJ, Fluoro-Jade; GFAP, glial fibrillary acidic protein; H&E, hematoxylin and eosin; HP, hippocampus; IA, impact acceleration; Iba-1, ionized calcium-binding adaptor molecule-1; LS, lateral septum; MBP, myelin basic protein; MWM, Morris Water Maze; NF200, neurofilament 200; NOR, novel object recognition; SS, silver stain; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; WM, white matter.
All tables detail repeated mild TBI models and include the number and interinjury interval of the experimental design. Time points for pathological and behavioral outcomes are indicated within their respective sections. Only adult rodent models of mild TBI are reviewed, although larger animal models,79,80 particularly swine,81–83 are also utilized to study mild TBI. Although sex is an important biological factor that may contribute to a differential response to TBI,84–88 the vast majority of published studies to date have incorporated males only. Thus, unless otherwise noted, the studies referred to within this review include males only in their experimental design. Data related to pediatric rodent studies are not within the scope of this review; readers are referred to previous publications.89,90 Finally, aspects of studies concerning therapeutic intervention are not included.
Histopathology of Experimental Single Mild TBI
Despite the numerous ways in which to induce a mild TBI in animal models, common histopathological and behavioral features have emerged. Because the vast majority of human mild TBIs do not result in skull fracture or contusion, mild TBI models are typically optimized to avoid these outcomes. Intraparenchymal hemorrhage and BBB breakdown accompanying more severe injury91–93 are uncommon in models of mild TBI.67,75 Indeed, current mild TBI models present with limited visible cell death using traditional histological stains such as Nissl or hematoxylin and eosin.63,64,67,74 In some models, sparsely distributed degenerating neurons can be detected by Fluoro-Jade (FJ) or silver stain (SS).63,64,68,71,94 Other mild TBI studies report no cell death identified by FJ labeling,74,95 supporting the premise that mild TBI results in neurological impairment without overt cell death. Given that neuroimaging techniques currently lack the resolution to detect scattered or diffuse neuron loss in human mild TBI, neither the presence nor absence of neuron death is a requisite for modeling mild TBI. Rather, models of mild TBI without neuronal loss and those with limited neuron death together may help identify injury mechanisms and therapeutic targets.
DAI presents clinically after mild TBI96,97 because of mechanical loading along axons. In rodent models of mild TBI, axonal injury is observed commonly in white matter tracts, particularly the subcortical white matter and brainstem,63,71,98 but is also observed in gray matter regions such as the cortex or thalamus.63,64,73 Axonal injury and/or axonal degeneration are evaluated after TBI using several markers including amyloid precursor protein (APP), SS, and FJ labeling. After mechanical trauma to the axon, APP, normally synthesized in the cell body and transported to the axon terminal, accumulates at the sites of microtubule disruption where the axon swells. The amino cupric SS developed by de Olmos and colleagues99 labels parts of damaged neurons that become argyrophilic (axons, lysosomes, etc.), allowing for easy visualization of degenerating cell bodies and processes. FJ also labels both cell bodies and axons of neurons undergoing degeneration through a poorly understood mechanism.
Longitudinal studies have shown APP positive labeling in white matter tracts as early as one day after injury that progressively decreases over one to two weeks.63,71 In contrast, FJ positive axons have been observed in white matter tracts of the corpus callosum and cerebellum as late as 14 days.71 In addition, low level APP accumulations have been reported as late as 12 months after CHI,100 suggesting that APP accumulation is useful as a marker of early axonal injury in rodent models as well as chronic axonal injury. A number of studies also report negative findings with APP after a single mild TBI at different time points (24 h and 7 days) using different models (CHI and IA).64,67,74 In a study of rotational acceleration, DTI detected a reduction in FA at the internal capsule at four days post-injury with increased acceleration magnitude, suggesting axonal disruption.102
Neuroinflammation accompanying mild TBI may be triggered by membrane damage, increases in reactive oxygen species, excitotoxicity, and the release of other toxic byproducts from damaged cells. Astrocytosis is documented routinely by immunolabeling for glial fibrillary acidic protein (GFAP), an intermediate filament protein upregulated in reactive astrocytes. Expression of GFAP is upregulated as early as 24 h after injury,63,65,69,73 and progressively increases over the first two weeks63,98 before resolving by 12 months.100 Astrogliosis occurs even in the absence of notable axonal injury or cell death,63,73,98 suggesting it may be a useful marker for identifying regions of sublethal neuronal damage or dysfunction. Astrocytic activation has been shown to inversely correlate with mean diffusivity detected by DTI in the cortex, suggesting that elevated numbers or hypertrophy of astrocytes restricts diffusion in the gray matter, as early as three days after injury.102
Resident microglia within the brain respond quickly to injury, contributing to the neuroinflammatory response. On activation, microglial cell bodies swell and the processes thicken. Such morphological changes can be appreciated by immunolabeling for microglial-specific proteins, such as ionized calcium-binding adaptor molecule-1 (Iba-1). Alternatively, markers that selectively detect activated microglia, such as ED1 for rat tissue and cluster of differentiation-68 (CD68) for mouse tissue, provide greater sensitivity through clearer differentiation of reactive microglia from resting microglia. Microgliosis is reported in many73,95,98,103,104 but not all64,105 experimental models of single-impact mild TBI.
In our original characterization of the CHI model, we utilized Iba-1 immunohistochemistry and observed no changes in microglial morphology between 24 h and nine days.63 Yet in our later studies, we incorporated CD68 as a marker for microgliosis and observed a delayed (5 days post-injury) increase in optic tract microgliosis that was not appreciated with Iba-1.104 This delayed onset of microgliosis has been viewed by others after a single mild TBI.100,103 Microglial reactivity without astrocytosis has been reported to be sustained in white matter for as long as 12 months after a single injury,100 providing evidence for chronic microglial reactivity. Single mild TBI studies do not concur on the extent of overlap between astrogliosis and microgliosis, although some find evidence of these pathologies in the same region and at the same time point after a single mild TBI.73,98
Behavioral Phenotypes Associated with Single Mild TBI
Neurological deficits commonly reported after mild TBI, including problems with memory or learning, attention deficits, and difficulties with motor coordination, have been replicated in animal models, while others such as headache or nausea are not easily mimicked in rodents. Tests of post-traumatic deficits such as depression and anxiety are increasingly used in studies of mild TBI and repeated mild TBI because of interest in post-traumatic stress disorder; these assays will be discussed later in the review. Rotarod, beam walking, and gait analysis are among the tests utilized to establish motor dysfunction as an acute feature of single mild TBI models66,69,106 that appears to resolve within a week.66,73,98 Evaluation of motor dysfunction at two months after injury, or later, does not reveal deficits,66,101 consistent with transient motor dysfunction after mild TBI in humans.
Cognitive dysfunction in rodent models of TBI is assessed most commonly using the Morris Water Maze (MWM) and the novel object recognition task (NOR). The MWM is used to evaluate visuospatial learning and memory abilities whereas the NOR is a test of non-spatial memory. Other behavioral assays of cognition include the radial arm water maze (RAWM) (working and reference memory), the Y-maze (cued and contextual memory), and the Barnes maze (spatial reference memory). Different models of single-impact mild TBI are associated with varying degrees of cognitive dysfunction, with reports of no significant impairment compared with controls,64,65,67,69 transient deficits,71 or long-lasting impairment.100,105,107 Disparate cognitive outcomes may be because of differences in injury location, injury severity, or cognitive test procedures.
Task sensitivity is critical for detection of mild TBI-induced deficits. Many tests developed to detect more stark deficits in models of moderate and severe TBI, or even repeated mild TBI as discussed later, may fail to detect subtler cognitive deficits that might result from a single mild TBI. Continued development of sensitive behavioral measures is therefore essential. Nevertheless, models of mild TBI that do not produce a behavioral phenotype or yield only transient dysfunction may provide opportunities to examine physiological and molecular changes important in determining vulnerability to subsequent TBIs. Such models may represent the majority of clinical mild TBI in which individuals do not have long-lasting behavioral changes.
Experimental Models of Repeated Mild TBI
As clinical evidence mounts that repeated brain injuries pose an increased risk for greater morbidity and for long-term neurodegenerative pathology, the number of animal studies of repeated mild TBI has expanded rapidly. A large proportion of pre-clinical studies, however, report outcomes after repeated mild TBI in the absence of a single TBI comparator group, making it difficult to ascribe the consequences to repetitive brain injury, per se. To specifically implicate repeated injury as a causative factor, it is critical that animal model studies of repeated mild TBI directly compare single and repeated mild TBI within the same study. In this section, we review a subset of studies that compare effects of repeated mild TBI with those of a single mild TBI (Table 1).
In most studies presented in this section, even repeated injuries result in minimal cell death,64,67,95 in comparison with single contusion injury models. In some studies, however, a small amount of regional neuron death was identified by activated caspase-3 or FJ after repeated mild TBIs.63,68,108 In models where no axonal injury was observed after a single mild TBI, mild TBIs repeated at 24 h intervals induced axonal injury,64,67,109 and in models where axonal injury is present after a single mild TBI, repeated mild TBIs have exacerbated acute (4–24 h)62,63 or chronic100 axonal injury. Further, two studies have reported a delayed (1–3 months) onset for axonal injury in the thalamus after repeated but not after single closed head impact.67,103 Repeated mild TBI also increased atrophy of the corpus callosum in the chronic post-injury period,100,108 which could reflect loss of myelin and/or axon degeneration. Together, these data suggest ongoing secondary injury processes contributing to axonal pathology.
With respect to neuroinflammation, repeated mild TBI increases and/or prolongs astrocytosis and microgliosis throughout gray and white matter regions of the brain (cortex, hippocampus, amygdala, corpus callosum) compared with that in animals with a single injury.63,65,98,100,108–110 In some studies, repeated mild TBI also appears to induce an earlier onset of neuroinflammation65,103,108; however, we advise caution for this interpretation. In our comparison of repeated mild TBI with single TBI,63 a critical component included single injury controls for both the first and last injury of the repeated mild TBI cohort. While the number of GFAP-positive astrocytes was significantly increased after repeated mild TBI compared with the single mild TBI cohort matched to the last injury, repeated mild TBI displayed similar numbers compared with the single mild TBI cohort matched to the first injury. Because pathological differences observed between repeated mild TBI and single mild TBI, especially acute differences, can be influenced by time elapsed after injury, appropriate single mild TBI control groups should be factored into experimental design.
A number of studies detected motor or memory dysfunction after multiple, but not single, mild TBIs.64,105 In other studies, motor or cognitive dysfunction was worsened or prolonged after repeated injury compared with a single injury,66,67 suggesting that cellular dysfunction mediates behavioral impairments observed in these models and should be the therapeutic target.
In summary, repeated mild TBIs can amplify the neuropathological damage and neurobehavioral deficits created by a single mild TBI or induce aspects of pathology and behavioral dysfunction not observed after a single injury. Because mild TBI and repeated mild TBI result in a considerable range of clinical symptoms and brain pathology, different phenotypes across animal models may represent opportunities to isolate and study specific features of mild TBI. Because of differences in injury models, numbers of injuries, and interinjury intervals used to create repeated mild TBI, however, it is not possible to compare across studies to isolate the contribution of these experimental factors. Therefore, studies that vary a single parameter provide unique insights into the contribution of the number of insults or the interval between insults to the ultimate outcome of repeated mild TBI.
Increasing Number of Injuries
Although it is presumed widely that morbidity after repeated TBI will be proportional to the number of brain injuries sustained, the nature of how neuropathological damage or behavioral dysfunction scales with the number of injuries is not clear. Under certain circumstances, adaptive changes triggered by mild TBI could potentially decrease risk for additive damage, akin to the protection provided by ischemic pre-conditioning.111 It is possible that long-lasting cellular pathology and chronic behavioral deficits require a critical threshold in terms of numbers of head injuries. To date, only a very small number of studies have examined how pathological and/or behavioral outcomes vary as a function of the number of insults to the brain (Table 2).
In a large study by Meehan and associates,74 mice receiving one, three, five, or 10 mild TBI using an IA model did not exhibit axonal injury or cell death 24 h after the final injury. Acute memory function was impaired after all repeated injury paradigms but not after one injury, supporting increased cognitive dysfunction with repeated compared with single mild TBI. Although memory deficits appeared to increase with the number of injuries, this relationship was not tested explicitly. Further, because the functional assessment was very early (24 h) after the last injury and no single injury controls with survival times matched to the repeated TBI groups' first injuries (i.e., 4, 6, or 11 days) were included, impairment noted in the repeated TBI groups could reflect a delayed manifestation of memory deficits rather than memory deficits caused by repeated injuries.
In a female rat model of IA injury, one, two, or three mild TBIs failed to result in corpus callosum thinning or gliosis or in acute motor deficits.76 Mild deficits in memory (compared with the sham group) were observed after two but not after three mild TBIs. No learning deficits were observed among groups; however, mice that received multiple brain injuries were evaluated for learning deficits after each injury, which may have confounded the learning assessment. Further, in utilizing this study design, mice that received two mild TBI were evaluated in the probe trial approximately 48 h after their final injury, whereas mice that received three mild TBI were evaluated at 24 h after injury, highlighting the importance of time-matched controls after injury because ongoing pathology can influence cognitive decline. In addition, in these two studies of IA injury, repeated mild TBI induced minimal, if any, axonal damage, cell death, and neuroinflammation, limiting insights into how the number of TBIs per se affects neuropathological outcomes.
Shultz and coworkers112 conducted the only study, to our knowledge, to highlight a scaled response with increasing numbers of injuries. While one mild FPI resulted in little cortical damage, the incidence of cortical damage increased in cohorts with three FPI and more so in those with five FPI.112 Five TBIs were associated with persistent microglial activation, whereas three TBIs only activated microglia transiently, and one TBI had no significant effect on microglial ED-1 staining. Memory deficits were greater and more prolonged after three or five repeated FPI compared with a single FPI, while only the rats with five FPI exhibited changes in anxiety-like and depressive behaviors.
These data begin to shed light on how injury outcomes depend on numbers of mild TBIs sustained and suggest that this relationship may differ for cellular and functional measures. A great deal more work is needed to better understand the relative risks assumed by individuals exposed to higher numbers of concussions or mild TBIs.
Interinjury Interval
The transient nature of many symptoms of mild TBI lends credence to the idea that the risk of worsened damage or dysfunction because of another TBI can be obviated by ensuring sufficient recovery time after the initial injury. Thus, the length of time between injuries may be a more important variable than the actual number of injuries experienced. Currently, recovery periods after concussion are determined individually, based on symptom severity, persistence of those symptoms, and medical history.113 Evidence-based guidelines would help to inform post-concussion management and return-to-play decisions. Pre-clinical models of mild TBI allow investigation of the cellular underpinnings of neurological dysfunction and the consequences of sustaining another brain injury before neural function returns to pre-injury levels. Comparison of data across studies employing different interinjury intervals can be instructive, but variables such as the spectrum of mild TBI severity, species/strain employed, different models of mild TBI, number of injuries, and time points post-injury complicate interpretation. This complexity advocates for examination of studies that independently vary the interinjury interval while holding other variables constant (Table 3).
Studies have shown that mild TBI repeated at interinjury intervals of 24 h or less increases axonal injury64,67 and worsens motor dysfunction66,67 compared with a single TBI. Even TBI repeated at longer intervals such as 48 h and five days, however, induces greater and/or longer lasting cognitive dysfunction compared with a single CHI.105 An interinjury interval study comparing two IA injuries separated by three, five, or 10 h evaluated changes in axonal injury and vascular reactivity to acetylcholine.62 The amount of axonal injury decreased progressively with longer interinjury intervals but, even at a 10 h interval, repeated IA exacerbated axonal injury compared with a single IA. Two IA at 3 h intervals caused a decrease in vascular reactivity to acetylcholine, but vascular reactivity was closer to normal with a 5 h interinjury interval and back to sham levels with a 10 h interinjury interval.
A subsequent study demonstrated that increased axonal injury and GFAP staining observed 40 days after the first of two IA injuries delivered three days apart was reduced by lengthening the interval between injuries to 20 days.95 This longer interval did not mitigate microgliosis, because levels of Iba-1 were increased compared with sham at 40 days similar to repeated injuries at three day intervals.95 Further analysis of the neuroinflammatory markers interleukin (IL)-1β and tumor necrosis factor (TNF)α, however, showed significant increases with repeated mild TBI at a three day interval compared with single injury controls, and that lengthening the interinjury interval to 20 days reduced gene expression of these inflammatory markers to that of a single injury. It is possible that some responses (axonal damage, gene expression) can be lessened by a certain increase in the time between impacts (i.e., 20 days) while other measures (glial reactivity) require a longer time interval between impacts (i.e., >20 days) to be mitigated.
In our evaluation of axonal injury after repeated mild TBIs at 24 h or 48 h interinjury intervals, five mild TBI every 24 h increased the number of FJ positive cells and enhanced acute microglial activation and astrocytosis in the entorhinal cortex compared with a single CHI matched to either the first or last injury. Lengthening the interinjury interval to 48 h prevented the additive effects of repeated CHI, yielding minimal neuron death and inflammatory responses similar to that resulting from a single CHI.63 These results suggest that a short interval (24 h) between repeated impacts resulted in enhanced neuropathology while a longer recovery period of 48 h between impacts mitigated the additive damage observed 24 h after the final injury.
A follow-up study, however, found similar levels of inflammation and neurodegeneration in white matter tracts 10 weeks after five CHIs repeated at either 24 h or 48 h intervals104 placing a 48 h interinjury interval within the window of vulnerability for long-lasting pathology in the brain. Together, our studies highlight the importance of evaluating neuropathological outcomes at multiple time points to assess the effects of interinjury interval for repeated mild TBI.
Two studies have evaluated motor dysfunction in relation to interinjury interval. Longhi and colleagues68 reported that two mild TBI at a three day interval worsened motor function at 24 h compared with single mild TBI, but function returned to sham levels by one week after the final injury. In comparison, two mild TBI at intervals of five days or seven days yielded motor function equivalent to that after a single injury. In our study, deficits were observed in a beam walking task across 10 weeks after five mild TBIs at intervals of 24 h.104 Lengthening the interval to 48 h between brain injuries did not significantly reduce the motor deficits, although there was no comparison with single mild TBI. Results from this follow-up behavioral study argue further for the use of all controls even when evaluating a specific variable of repeated mild TBI, because the lack of pathology at an acute time point does not prohibit the development of chronic neuropathology or functional/behavioral deficits. Regardless, these data suggest that in mice, interinjury intervals of three days or less may lead to greater motor impairment.
In relation to cognitive dysfunction, shorter interinjury intervals result in more persistent memory deficits. For example, mild TBIs at a 24 h interinjury interval were reported to induce memory deficits persisting one year after injury, whereas with a one week interinjury interval, memory dysfunction resolved between one month and one year after injury.74 A subsequent study by the same group extended these findings to show that lengthening the interinjury interval to two weeks led to normalization of cognitive function by six months,114 while a four week interinjury interval resulted in no additional deficit compared with a single mild TBI by one month after the final injury.74 This suggests that injuries more than two weeks apart minimize the persistence of cognitive deficits.
In support, Weil and coworkers95 found memory deficits at one month after two repeated injuries separated by a three day but not a 20 day interval or after a single mild TBI. Using a model of CHI, Longhi and associates68 reported that an interinjury interval of three days or five days, but not seven days, induced memory deficits acutely after injury. The finding by Longhi and associates68 that injuries should be more than five days to minimize additive cognitive dysfunction differs from the findings by Meehan and colleagues74 and Mannix and coworkers114 that injuries should be separated by more than two weeks. Two primary factors may contribute to these differences: (1) the variation in injury models (CHI vs. IA) and (2) the number of impacts (two vs. five, respectively), highlighting the difficulty of comparing between different studies.
Collectively, rodent studies suggest that the interinjury interval required to prevent worsened or more prolonged damage/impairment with repeated mild TBI is longer for cognitive measures than for outcomes such as axonal injury and motor function. Given the relatively small number of published studies independently varying injury interval within their design and the likelihood that vulnerable periods will vary depending on the biological variable assessed, development of evidenced-based guidelines will require additional studies.
Tau in Models of Repeated TBI
Repetitive mild TBI has been implicated in the latent development of neurological disorders including CTE. Clinically, symptoms of CTE are progressive in nature and present after sustaining multiple traumatic brain insults.33,115,116 The core clinical features of CTE include difficulties in cognition, emotional distress, physical violence, and changes in mood such as depression.119 Impulsivity, anxiety, apathy, paranoia, suicidality, headache, and motor deficits, however, are also reported in those suspected to have CTE.117
Because of overlap in symptomatology with other neurodegenerative disorders, CTE can only be diagnosed definitively post-mortem, but work continues to further distinguish CTE through the use of imaging and biomarkers.118 The histopathological features of CTE—namely, phosphorylated-tau (p-tau) aggregation—are progressive. Tau is a microtubule-associated protein present within neurons that stabilizes microtubules. In disease states, hyperphosphorylation of tau reduces binding affinity for microtubules and promotes aggregation within the neuron.
At the onset of CTE, p-tau neurofibrillary tangles are observed around small vessels at the depths of the sulci in the human brain.116,119 In the second stage of CTE, tau pathology spreads from the focal sulci areas to the superficial cortical layers. By stage three, the p-tau pathology is widespread through the cortices and includes the hippocampus, entorhinal cortex, and amygdala. In the final stage, stage four, p-tau pathology is severe and widespread. While many confirmed cases of CTE involve a history of repeated brain injuries (i.e., diagnosed concussions), cases have also been reported in individuals sustaining repeated subconcussive blows.120 The specific parameters of head injury or repeated head injuries responsible for triggering CTE are difficult to elucidate from clinical studies.
Interest in the cellular mechanisms underlying CTE and the development of therapeutics has stimulated investigation of tau pathology in animal models of repetitive mild TBI. While numerous studies in small animal models of repetitive mild TBI have failed to find evidence of pathological tau,63,100,114,121 others report increased p-tau levels by Western blot or immunohistochemistry.75,77,122 To increase the likelihood of tau-related neurodegeneration, mice expressing human, mutant, or supraphysiological levels of tau have been employed. For example, transgenic mice expressing a human tau isoform exhibit increased cortical and hippocampal tau pathology after repeated mild TBI.123 For more detail regarding animal models of CTE, see recent reviews by Ojo and associates,124 McAteer and colleagues,125 and Wojnarowicz and coworkers.126
Challenges and Recommendations for Modeling Repeated Mild TBI
The field of experimental mild TBI is growing rapidly. In the absence of a standardized injury model, laboratories often design and characterize their own mild TBI models. Use of different models, species, and strains hinders comparisons across studies; therefore, it is essential to design experiments that allow meaningful comparisons within a given study. While the majority of published rodent studies contrast the effects of repeated mild TBI with single mild TBI, numerous studies documenting pathological and behavioral responses after repeated mild TBI lack single injury groups, limiting the ability to identify repeated injury, per se, as a causative factor in reported outcomes.
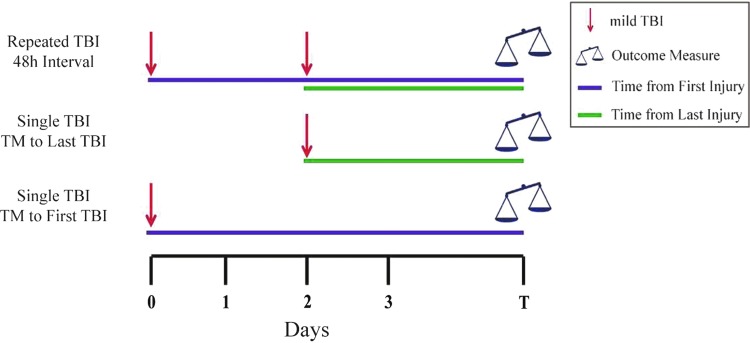
While inclusion of a single mild TBI comparator group is optimal, selection of evaluation time points to match the repeated mild TBI group(s) can be challenging. Typically, studies assess outcomes at a certain time point after the last injury received (e.g.,74,112; see also Fig. 1). In some instances, however, specifically studies with acute outcomes, time matching only to the last injury received could fail to consider adequately the contribution of delayed or progressive pathology initiated by the first injury sustained. Inclusion of another single injury group with an assessment time matched to the first injury in the repeated injury paradigm is recommended, as depicted in Figure 1.
FIG. 1.
Representative experimental design comparing outcomes after repeated mild traumatic brain injury (TBI) with those after single mild TBI. The repeated mild TBI group could be any number of injuries at any given interinjury interval, typically determined by laboratory-specific characterization. Single mild TBI groups included are time-matched (TM) with the first and last injuries of the repeated mild TBI group. Color image is available online.
Several published studies have included single mild TBI controls matched to both the first and last TBI of the repeated group.63,98,127 If the evaluation time point is chronic (e.g., weeks or months after the injury/injuries), then the need for separate single mild TBI groups is diminished, and one single mild TBI group may suffice for comparison with the repeated mild TBI group. It may also be useful to include both single sham128 and repeated sham groups129 to account for effects of handling, surgical procedures, and anesthesia.
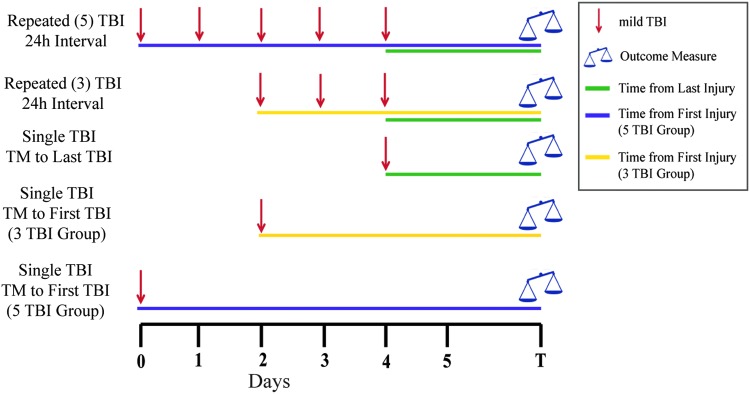
To determine whether the number of impacts is critical in producing a worsened outcome after mild TBI, a design such as that depicted in Figure 2 could be utilized. In this example, groups receiving one, three, or five mild TBIs are compared, with additional single injury groups included to time match to the first and last injury of each repeated mild TBI group. Again, the importance of multiple single mild TBI comparator groups declines when outcomes are measured at time points much longer than the interval between injuries. Given that anesthesia administered before or with TBI may be neurotherapeutic,130–133 anesthesia should be administered at the same frequency in the sham control groups as in the repeated TBI group(s). It is arguable whether the single TBI group should receive subsequent anesthesia bouts to match the anesthetic exposure of the repeated TBI group or whether that additional anesthesia exposure interferes with interpretation of the natural course of single TBI responses. This issue is not addressed easily and is likely dependent on the outcomes of interest.
FIG. 2.
Representative experimental design isolating the effect of the number of injuries in a repeated mild traumatic brain injury (TBI) paradigm with single mild TBI as a reference. This type of experiment would compare outcomes from different numbers of mild TBIs (e.g., 1 vs. 3 vs. 5) at a certain interinjury interval. Single mild TBI groups included are time-matched (TM) with the first and last injuries of both repeated mild TBI groups. Color image is available online.
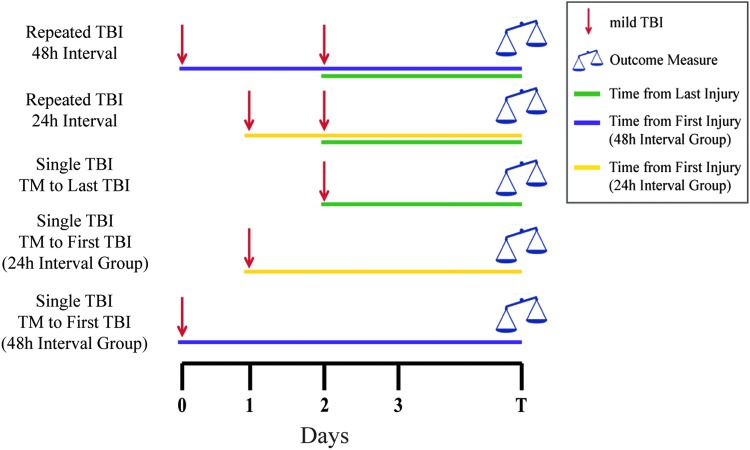
An example of a design to test the role of interinjury interval in exacerbating the effects of mild TBI is illustrated in Figure 3, showing two intervals (here, 24 h and 48 h) and the necessary single mild TBI comparator groups. Investigators of repeated TBI face a difficult balance between designing experiments that adequately test their hypothesis and not overburdening the financial, technical, and physical constraints of the project. Therefore, the number of injury/sham groups needed to test the hypothesis of the respective study should be considered carefully. In addition, because by design many outcome measures will show small or no change after mild TBI, preliminary data demonstrating greater changes after repeated mild TBI compared with single mild TBI may be needed to perform meaningful power analysis to design a larger or more complex study. Further, additional power and larger group sizes likely will be required to differentiate responses between repeated mild TBI conditions (e.g., different numbers of injuries or different intervals) or therapeutic interventions, because these variations likely will show subtle alterations in pathological or behavioral outcomes.
FIG. 3.
Representative experimental design isolating the effect of interinjury interval in a repeated mild traumatic brain injury (TBI) paradigm with single mild TBI as a reference. This experimental design compares outcomes from a certain number of repeated mild TBIs produced at different interinjury intervals (e.g., 24 h vs. 48 h). Single mild TBI groups included are time-matched (TM) with the first and last injuries of both repeated mild TBI groups. Color image is available online.
Implementation of well-controlled experiments in which the number of variables is limited helps to better link cause and effect. Nonetheless, it is important to note that induction of repeated TBI through a series of injuries with identical parameters (severity) and location and a pre-determined regular interval limits somewhat the translational relevance to the human condition, in which repeated mild TBI may involve different magnitude and location insults occurring at irregular intervals. Further, findings from small animal models related to temporal aspects of damage or recovery, interinjury intervals, or age should be interpreted with caution when translating to humans, because the rate of pathology development and progression may differ substantially in rodents and humans. Rather, the identification of time windows of vulnerability is useful in guiding investigations of underlying cellular mechanisms to be verified in larger animal models and in man.
While it is important to understand whether acute pathology/behavior is altered by repeated head injuries to identify early therapeutic targets, a pressing need in the mild TBI field is to identify which injury scenarios cause long-term dysfunction or progressively deteriorating outcomes. Therefore, studies of repeated mild TBI that do not include chronic evaluation time points lack the full picture regarding risk mitigation.
An additional challenge inherent in studies of repeated mild TBI is the interpretation and expectation of what mild TBI “looks like” in animal models. While repeated mild TBI can induce long-lasting neurological consequences clinically, the majority of individuals who have mild TBI recover within days or may exhibit no notable physiological or behavioral changes. Therefore, one could debate whether an ideal model of mild TBI is one that produces consistent, albeit mild, deficits in all subjects or one that yields no damage or symptoms in most subjects with only a subset of animals showing pathology/dysfunction, more akin to the clinical situation. If mild TBI models yield only small, transient changes in a few pathological or behavioral parameters, studies would likely produce large quantities of negative data, much of which might go unpublished. This increases the chance of positive published findings reflecting type I error and, alternatively, may bias researchers toward models that produce consistent or easily measured changes.
While there are many challenges to overcome, such as complex experimental design and variability across injury models, the field of mild TBI can make great strides with logically formulated injury paradigms and inclusion of both acute and long-term outcome measures. Proper interpretation of the effects of repeated mild TBI requires careful comparisons with appropriate controls. In this way, discovery of fundamental aspects of the molecular, cellular, and behavioral responses to mild TBI and repeated TBI can be accelerated, guiding prevention and treatment efforts.
Acknowledgments
The authors would like to thank and acknowledge the funding sources for this review: NIH NINDS F31 NS087878 (ABH), Kentucky Spinal Cord & Head Injury Research Trust 14-13A (KES), and National Science Foundation Grant No. 1539068 - EPSCoR Seed Grant 4978/111315 (WBH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention; Atlanta, GA [Google Scholar]
- 2. Menon D.K., Schwab K., Wright D.W., and Maas A.I.; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. (2010). Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1637–164021044706 [Google Scholar]
- 3. Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T.; Workshop Scientific Team and Advisory Panel Members. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 5. Finkelstein E., Corso P., and Miller T. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford University Press: New York [Google Scholar]
- 6. Kraus J.F. and Nourjah P. (1988). The epidemiology of mild, uncomplicated brain injury. J. Trauma 28, 1637–1643 [DOI] [PubMed] [Google Scholar]
- 7. McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., and Saykin A.J. (2001). Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 8. Alves W., Macciocchi S., and Barth J. (1993). Postconcussive symptoms after uncomplicated mild head injury. J. Head Trauma Rehabil. 8, 48–59 [Google Scholar]
- 9. Rimel R.W., Giordani B., Barth J.T., Boll T.J., and Jane J.A. (1981). Disability caused by minor head injury. Neurosurgery 9, 221–228 [PubMed] [Google Scholar]
- 10. McMahon P., Hricik A., Yue J.K., Puccio A.M., Inoue T., Lingsma H.F., Beers S.R., Gordon W.A., Valadka A.B., Manley G.T., Okonkwo D.O., and TRACK-TBI Investigators T.T. (2014). Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J. Neurotrauma 31, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dean P.J. and Sterr A. (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansson B., Berglund P., and Ronnback L. (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 23, 1027–1040 [DOI] [PubMed] [Google Scholar]
- 13. Ponsford J., Cameron P., Fitzgerald M., Grant M., and Mikocka-Walus A. (2011). Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J. Neurotrauma 28, 937–946 [DOI] [PubMed] [Google Scholar]
- 14. Catale C., Marique P., Closset A., and Meulemans T. (2009). Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J. Clin Exp. Neuropsychol. 31, 331–338 [DOI] [PubMed] [Google Scholar]
- 15. Horner M.D., Ferguson P.L., Selassie A.W., Labbate L.A., Kniele K., and Corrigan J.D. (2005). Patterns of alcohol use 1 year after traumatic brain injury: a population-based, epidemiological study. J. Int. Neuropsychol. Soc. 11, 322–330 [DOI] [PubMed] [Google Scholar]
- 16. Grossbard J., Malte C.A., Lapham G., Pagulayan K., Turner A.P., Rubinsky A.D., Bradley K.A., Saxon A.J., and Hawkins E.J. (2017). Prevalence of alcohol misuse and follow-up care in a national sample of OEF/OIF VA patients with and without TBI. Psychiatr. Serv. 68, 48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holsinger T., Steffens D.C., Phillips C., Helms M.J., Havlik R.J., Breitner J.C., Guralnik J.M., and Plassman B.L. (2002). Head injury in early adulthood and the lifetime risk of depression. Arch. Gen. Psychiatry 59, 17–22 [DOI] [PubMed] [Google Scholar]
- 18. Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 19. Agrawal A., Timothy J., Pandit L., and Manju M. (2006). Post-traumatic epilepsy: an overview. Clin. Neurol. Neurosurg. 108, 433–439 [DOI] [PubMed] [Google Scholar]
- 20. Borg J., Holm L., Cassidy J.D., Peloso P.M., Carroll L.J., von Holst H., and Ericson K. (2004). Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, Suppl, 61–75 [DOI] [PubMed] [Google Scholar]
- 21. Harmon K.G., Drezner J.A., Gammons M., Guskiewicz K.M., Halstead M., Herring S.A., Kutcher J.S., Pana A., Putukian M., and Roberts W.O. (2013). American Medical Society for Sports Medicine position statement: concussion in sport. Br. J. Sports Med. 47, 15–26 [DOI] [PubMed] [Google Scholar]
- 22. Giza C.C., Kutcher J.S., Ashwal S., Barth J., Getchius T.S., Gioia G.A., Gronseth G.S., Guskiewicz K., Mandel S., Manley G., McKeag D.B., Thurman D.J., and Zafonte R. (2013). Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 80, 2250–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorak J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 24. Pandor A., Harnan S., Goodacre S., Pickering A., Fitzgerald P., and Rees A. (2012). Diagnostic accuracy of clinical characteristics for identifying CT abnormality after minor brain injury: a systematic review and meta-analysis. J. Neurotrauma 29, 707–718 [DOI] [PubMed] [Google Scholar]
- 25. Williams D.H., Levin H.S,. and Eisenberg H.M. (1990). Mild head injury classification. Neurosurgery 27, 422–428 [DOI] [PubMed] [Google Scholar]
- 26. Iverson G.L. (2006). Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj. 20, 1335–1344 [DOI] [PubMed] [Google Scholar]
- 27. Borgaro S.R., Prigatano G.P., Kwasnica C., and Rexer J.L. (2003). Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Inj. 17, 189–198 [DOI] [PubMed] [Google Scholar]
- 28. Kurca E., Sivak S., and Kucera P. (2006). Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic magnetic resonance imaging. Neuroradiology 48, 661–669 [DOI] [PubMed] [Google Scholar]
- 29. Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., and Peterson D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 [DOI] [PubMed] [Google Scholar]
- 30. Huang M.X., Theilmann R.J., Robb A., Angeles A., Nichols S., Drake A., D'Andrea J., Levy M., Holland M., Song T., Ge S., Hwang E., Yoo K., Cui L., Baker D.G., Trauner D., Coimbra R., and Lee R.R. (2009). Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 31. Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O., and Grossman R.I. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg 103, 298–303 [DOI] [PubMed] [Google Scholar]
- 32. Messe A., Caplain S., Paradot G., Garrigue D., Mineo J.F., Soto Ares G., Ducreux D., Vignaud F., Rozec G., Desal H., Pelegrini-Issac M., Montreuil M., Benali H., and Lehericy S. (2011). Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 32, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKee A.C., Daneshvar D.H., Alvarez V.E., and Stein T.D. (2014). The neuropathology of sport. Acta Neuropathol. 127, 29–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koerte I.K., Hufschmidt J., Muehlmann M., Lin A.P., and Shenton M.E. (2016). Advanced neuroimaging of mild traumatic brain injury, in: Translational Research in Traumatic Brain Injury. Laskowitz D., and Grant G. (eds). CRC Press/Taylor and Francis Group: Boca Raton, FL: [PubMed] [Google Scholar]
- 35. Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Harding H.P., Jr, Matthews A., Mihalik J.R., and Cantu R.C. (2007). Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 39, 903–909 [DOI] [PubMed] [Google Scholar]
- 36. Emery C., Kang J., Shrier I., Goulet C., Hagel B., Benson B., Nettel-Aguirre A., McAllister J., and Meeuwisse W. (2011). Risk of injury associated with bodychecking experience among youth hockey players. CMAJ 183, 1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marar M., McIlvain N.M., Fields S.K., and Comstock R.D. (2012). Epidemiology of concussions among United States high school athletes in 20 sports. Am. J. Sports Med. 40, 747–755 [DOI] [PubMed] [Google Scholar]
- 38. Guskiewicz K.M., Weaver N.L., Padua D.A., and Garrett W.E., Jr (2000). Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 28, 643–650 [DOI] [PubMed] [Google Scholar]
- 39. McCrea M., Hammeke T., Olsen G., Leo P., and Guskiewicz K. (2004). Unreported concussion in high school football players: implications for prevention. Clin. J. Sport Med. 14, 13–17 [DOI] [PubMed] [Google Scholar]
- 40. McCrea M., Barr W.B., Guskiewicz K., Randolph C., Marshall S.W., Cantu R., Onate J.A., and Kelly J.P. (2005). Standard regression-based methods for measuring recovery after sport-related concussion. J. Int. Neuropsychol. Soc. 11, 58–69 [DOI] [PubMed] [Google Scholar]
- 41. McCrory P., Johnston K., Meeuwisse W., Aubry M., Cantu R., Dvorak J., Graf-Baumann T., Kelly J., Lovell M., and Schamasch P. (2005). Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br. J. Sports Med. 39, 196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gronwall D. and Wrightson P. (1975). Cumulative effect of concussion. Lancet 2, 995–997 [DOI] [PubMed] [Google Scholar]
- 43. Slobounov S., Slobounov E., Sebastianelli W., Cao C., and Newell K. (2007). Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery 61, 338–344 [DOI] [PubMed] [Google Scholar]
- 44. McAllister T., and McCrea M. (2017). Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J Alth Train 52, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Covassin T., Elbin R., Kontos A., and Larson E. (2010). Investigating baseline neurocognitive performance between male and female athletes with a history of multiple concussion. J. Neurol. Neurosurg. Psychiatry 81, 597–601 [DOI] [PubMed] [Google Scholar]
- 46. Kerr Z.Y., Zuckerman S.L., Wasserman E.B., Covassin T., Djoko A., and Dompier T.P. (2016). Concussion symptoms and return to play time in youth, high school, and college American football athletes. JAMA Pediatr. 170, 647–653 [DOI] [PubMed] [Google Scholar]
- 47. Scherer M.R., Weightman M.M., Radomski M.V., Davidson L.F., and McCulloch K.L. (2013). Returning service members to duty following mild traumatic brain injury: exploring the use of dual-task and multitask assessment methods. Phys. Ther. 93, 1254–1267 [DOI] [PubMed] [Google Scholar]
- 48. Mayers L. (2012). Return-to-play interval after sport-related concussion: background review and current issues. Curr. Sports Med. Rep. 11, 277–279 [DOI] [PubMed] [Google Scholar]
- 49. Vagnozzi R., Signoretti S., Tavazzi B., Floris R., Ludovici A., Marziali S., Tarascio G., Amorini A.M., Di Pietro V., Delfini R., and Lazzarino G. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery 62, 1286–1295 [DOI] [PubMed] [Google Scholar]
- 50. McIntosh T.K., Noble L., Andrews B., and Faden A.I. (1987). Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134 [DOI] [PubMed] [Google Scholar]
- 51. Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 52. Lighthall J.W. (1988). Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma 5, 1–15 [DOI] [PubMed] [Google Scholar]
- 53. Shapira Y., Shohami E., Sidi A., Soffer D., Freeman S., and Cotev S. (1988). Experimental closed head injury in rats: Mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 16, 258–265 [DOI] [PubMed] [Google Scholar]
- 54. Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 55. Foda M.A. and Marmarou A. (1994). A new model of diffuse brain injury in rats. Part II: Morphological characterization. J. Neurosurg. 80, 301–313 [DOI] [PubMed] [Google Scholar]
- 56. Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., and Demetriadou K. (1994). A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 80, 291–300 [DOI] [PubMed] [Google Scholar]
- 57. Cernak I., Savic J., Malicevic Z., Zunic G., Radosevic P., Ivanovic I., and Davidovic L. (1996). Involvement of the central nervous system in the general response to pulmonary blast injury. J. Trauma 40, Suppl 3, S100–S104 [DOI] [PubMed] [Google Scholar]
- 58. Leung L.Y., VandeVord P.J., Dal Cengio A.L., Bir C., Yang K.H., and King A.I. (2008). Blast related neurotrauma: a review of cellular injury. Mol. Cell Biomech. 5, 155–168 [PubMed] [Google Scholar]
- 59. Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen Y., Mao H., Yang K.H., Abel T., and Meaney D.F. (2014). A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front. Neurol. 5, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DeFord S.M., Wilson M.S., Rice A.C., Clausen T., Rice L.K., Barabnova A., Bullock R., and Hamm R.J. (2002). Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma 19, 427–438 [DOI] [PubMed] [Google Scholar]
- 62. Fujita M., Wei E.P., and Povlishock J.T. (2012). Intensity- and interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. J. Neurotrauma 29, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bolton A.N. and Saatman K.E. (2014). Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J. Neuropathol. Exp. Neurol. 73, 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shitaka Y., Tran H.T., Bennett R.E., Sanchez L., Levy M.A., Dikranian K., and Brody D.L. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 70, 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Uryu K., Laurer H., McIntosh T., Pratico D., Martinez D., Leight S., Lee V.M., and Trojanowski J.Q. (2002). Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Petraglia A.L., Plog B.A., Dayawansa S., Chen M., Dashnaw M.L., Czerniecka K., Walker C.T., Viterise T., Hyrien O., Iliff J.J., Deane R., Nedergaard M.A., and Huang J.H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laurer H.L., Bareyre F.M., Lee V.M., Trojanowski J.Q., Longhi L., Hoover R., Saatman K.E., Raghupathi R., Hoshino S., Grady M.S., and McIntosh T.K. (2001). Mild head injury increasing the brain's vulnerability to a second concussive impact. J. Neurosurg 95, 859–870 [DOI] [PubMed] [Google Scholar]
- 68. Longhi L., Saatman K.E., Fujimoto S., Raghupathi R., Meaney D.F., Davis J., McMillan B.S., Conte V., Laurer H.L., Stein S., Stocchetti N., and McIntosh T.K. (2005). Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery 56, 364–374 [DOI] [PubMed] [Google Scholar]
- 69. Chen Z., Leung L.Y., Mountney A., Liao Z., Yang W., Lu X.C., Dave J., Deng-Bryant Y., Wei G., Schmid K., Shear D.A., and Tortella F.C. (2012). A novel animal model of closed-head concussive-induced mild traumatic brain injury: development, implementation, and characterization. J. Neurotrauma 29, 268–280 [DOI] [PubMed] [Google Scholar]
- 70. Meaney D.F., Morrison B., and Dale Bass C. (2014). The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J. Biomech. Eng. 136, 021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Creed J.A., DiLeonardi A.M., Fox D.P., Tessler A.R., and Raghupathi R. (2011). Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J. Neurotrauma 28, 547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nichols J.N., Deshane A.S., Niedzielko T.L., Smith C.D., and Floyd C.L. (2016). Greater neurobehavioral deficits occur in adult mice after repeated, as compared to single, mild traumatic brain injury (mTBI). Behav. Brain Res. 298, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khuman J., Meehan W.P., 3rd Zhu, X., Qiu J., Hoffmann U., Zhang J., Giovannone E., Lo E.H. and Whalen M.J. (2011). Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 31, 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meehan W.P., 3rd, Zhang J., Mannix R., and Whalen M.J. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kane M.J., Angoa-Perez M., Briggs D.I., Viano D.C., Kreipke C.W., and Kuhn D.M. (2012). A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 203, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yates N.J., Lydiard S., Fehily B., Weir G., Chin A., Bartlett C.A., Alderson J., and Fitzgerald M. (2017). Repeated mild traumatic brain injury in female rats increases lipid peroxidation in neurons. Exp. Brain Res. 235, 2133–2149 [DOI] [PubMed] [Google Scholar]
- 77. Namjoshi D.R., Cheng W.H., McInnes K.A., Martens K.M., Carr M., Wilkinson A., Fan J., Robert J., Hayat A., Cripton P.A., and Wellington C.L. (2014). Merging pathology with biomechanics using chimera (closed-head impact model of engineered rotational acceleration): a novel, surgery-free model of traumatic brain injury. Mol. Neurodegener 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dewitt D.S., Perez-Polo R., Hulsebosch C.E., Dash P.K., and Robertson C.S. (2013). Challenges in the development of rodent models of mild traumatic brain injury. J. Neurotrauma 30, 688–701 [DOI] [PubMed] [Google Scholar]
- 79. Friess S.H., Ichord R.N., Ralston J., Ryall K., Helfaer M.A., Smith C., and Margulies S.S. (2009). Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dai J.X., Ma Y.B., Le N.Y., Cao J., and Wang Y. (2018). Large animal models of traumatic brain injury. Int. J. Neurosci. 128, 243–254 [DOI] [PubMed] [Google Scholar]
- 81. Wolf J.A., Johnson B.N., Johnson V.E., Putt M.E., Browne K.D., Mietus C.J., Brown D.P., Wofford K.L., Smith D.H., Grady M.S., Cohen A.S., and Cullen D.K. (2017). Concussion induces hippocampal circuitry disruption in swine. J. Neurotrauma 34, 2303–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lafrenaye A.D., Todani M., Walker S.A., and Povlishock J.T. (2015). Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation 12, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glass T.F., Fabian M.J., Schweitzer J.B., Weinberg J.A., and Proctor K.G. (1999). Secondary neurologic injury resulting from nonhypotensive hemorrhage combined with mild traumatic brain injury. J. Neurotrauma 16, 771–782 [DOI] [PubMed] [Google Scholar]
- 84. Bazarian J.J., Blyth B., Mookerjee S., He H., and McDermott M.P. (2010). Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 27, 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Broshek D.K., Kaushik T., Freeman J.R., Erlanger D., Webbe F., and Barth J.T. (2005). Sex differences in outcome following sports-related concussion. J. Neurosurg/source>. 102, 856–863 [DOI] [PubMed] [Google Scholar]
- 86. Doran S.J., Ritzel R., Glaser E., Henry R., Faden A., and Loane D.J. (2018). Sex differences in acute neuroinflammation are mediated by infiltrating myeloid cells. J. Neurotrauma [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ferguson S.A., Mouzon B.C., Lynch C., Lungmus C., Morin A., Crynen G., Carper B., Bieler G., Mufson E.J., Stewart W., Mullan M., and Crawford F. (2017). Negative impact of female sex on outcomes from repetitive mild traumatic brain injury in hTau mice is age dependent: A chronic effects of neurotrauma consortium study. Front. Aging Neurosci. 9, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tucker L.B., Fu A.H., and McCabe J.T. (2016). Performance of male and female c57bl/6j mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma 33, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Prins M.L. and Giza C.C. (2012). Repeat traumatic brain injury in the developing brain. Int. J. Dev. Neurosci. 30, 185–190 [DOI] [PubMed] [Google Scholar]
- 90. Kochanek P.M., Wallisch J.S., Bayir H., and Clark R.S. (2017). Pre-clinical models in pediatric traumatic brain injury—challenges and lessons learned. Childs Nerv. Syst. 33, 1693–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saatman K.E., Feeko K.J., Pape R.L., and Raghupathi R. (2006). Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 92. Dietrich W.D., Alonso O., and Halley M. (1994). Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma 11, 289–301 [DOI] [PubMed] [Google Scholar]
- 93. Fukuda K., Tanno H., Okimura Y., Nakamura M., and Yamaura A. (1995). The blood-brain barrier disruption to circulating proteins in the early period after fluid percussion brain injury in rats. J Neurotrauma 12, 315–324 [DOI] [PubMed] [Google Scholar]
- 94. Huh J.W., Widing A.G., and Raghupathi R. (2008). Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp. Neurol. 213, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weil Z.M., Gaier K.R., and Karelina K. (2014). Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 70, 108–116 [DOI] [PubMed] [Google Scholar]
- 96. Benson R.R., Meda S.A., Vasudevan S., Kou Z., Govindarajan K.A., Hanks R.A., Millis S.R., Makki M., Latif Z., Coplin W., Meythaler J., and Haacke E.M. (2007). Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J. Neurotrauma 24, 446–459 [DOI] [PubMed] [Google Scholar]
- 97. Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., and Crawford F. (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 29, 2761–2773 [DOI] [PubMed] [Google Scholar]
- 99. de Olmos J.S., Beltramino C.A., and de Olmos de Lorenzo S. (1994). Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol. Teratol. 16, 545–561 [DOI] [PubMed] [Google Scholar]
- 100. Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 101. Stemper B.D., Shah A.S., Pintar F.A., McCrea M., Kurpad S.N., Glavaski-Joksimovic A., Olsen C., and Budde M.D. (2015). Head rotational acceleration characteristics influence behavioral and diffusion tensor imaging outcomes following concussion. Ann. Biomed. Eng. 43, 1071–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Singh K., Trivedi R., Devi M.M., Tripathi R.P., and Khushu S. (2016). Longitudinal changes in the DTI measures, anti-GFAP expression and levels of serum inflammatory cytokines following mild traumatic brain injury. Exp. Neurol. 275, Pt 3, 427–435 [DOI] [PubMed] [Google Scholar]
- 103. McAteer K.M., Corrigan F., Thornton E., Turner R.J., and Vink R. (2016). Short and long term behavioral and pathological changes in a novel rodent model of repetitive mild traumatic brain injury. PLoS One 11, e0160220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bolton Hall A.N., Joseph B., Brelsfoard J.M., and Saatman K.E. (2016). Repeated closed head injury in mice results in sustained motor and memory deficits and chronic cellular changes. PLoS One 11, e0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cheng J.S., Craft R., Yu G.Q., Ho K., Wang X., Mohan G., Mangnitsky S., Ponnusamy R., and Mucke L. (2014). Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS One 9, e115765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mychasiuk R., Hehar H., Candy S., Ma I., and Esser M.J. (2016). The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci. Methods 257, 168–178 [DOI] [PubMed] [Google Scholar]
- 107. Zohar O., Rubovitch V., Milman A., Schreiber S., and Pick C.G. (2011). Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol. Exp. (Wars) 71, 36–45 [DOI] [PubMed] [Google Scholar]
- 108. Mountney A., Boutte A.M., Cartagena C.M., Flerlage W.F., Johnson W.D., Rho C., Lu X.C., Yarnell A., Marcsisin S., Sousa J., Vuong C., Zottig V., Leung L.Y., Deng-Bryant Y., Gilsdorf J., Tortella F.C., and Shear D.A. (2017). Functional and molecular correlates after single and repeated rat closed-head concussion: indices of vulnerability after brain injury. J. Neurotrauma 34, 2768–2789 [DOI] [PubMed] [Google Scholar]
- 109. Winston C.N., Noël A., Neustadtl A., Parsadanian M., Barton D.J., Chellappa D., Wilkins T.E., Alikhani A.D., Zapple D.N., Villapol S., Planel E. and Burns M.P. (2016). Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am. J. Pathol. 186, 552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tyburski A.L., Cheng L., Assari S., Darvish K., and Elliott M.B. (2017). Frequent mild head injury promotes trigeminal sensitivity concomitant with microglial proliferation, astrocytosis, and increased neuropeptide levels in the trigeminal pain system. J. Headache Pain 18, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kitagawa K., Matsumoto M., Tagaya M., Hata R., Ueda H., Niinobe M., Handa N., Fukunaga R., Kimura K., Mikoshiba K., et al. (1990). 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 528, 21–24 [DOI] [PubMed] [Google Scholar]
- 112. Shultz S.R., Bao F., Omana V., Chiu C., Brown A., and Cain D.P. (2012). Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma 29, 281–294 [DOI] [PubMed] [Google Scholar]
- 113. Leddy J.J., Sandhu H., Sodhi V., Baker J.G., and Willer B. (2012). Rehabilitation of concussion and post-concussion syndrome. Sports Health 4, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mannix R., Meehan W.P., Mandeville J., Grant P.E., Gray T., Berglass J., Zhang J., Bryant J., Rezaie S., Chung J.Y., Peters N.V., Lee C., Tien L.W., Kaplan D.L., Feany M., and Whalen M. (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 74, 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gavett B.E., Stern R.A., and McKee A.C. (2011). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 30, 179–188, xi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O., Fritts N.G., Stamm J.M., Robbins C.A., McHale L., Simkin I., Stein T.D., Alvarez V.E., Goldstein L.E., Budson A.E., Kowall N.W., Nowinski C.J., Cantu R.C., and McKee A.C. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Montenigro P.H., Baugh C.M., Daneshvar D.H., Mez J., Budson A.E., Au R., Katz D.I., Cantu R.C., and Stern R.A. (2014). Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res. Ther. 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang J., Puvenna V., and Janigro D. (2016). Biomarkers of traumatic brain injury and their relationship to pathology, in: Translational Research in Traumatic Brain Injury. Laskowitz D., and Grant G. (eds). CRC Press/Taylor and Francis Group: Boca Raton, FL: [PubMed] [Google Scholar]
- 119. McKee A.C., Stein T.D., Kiernan P.T., and Alvarez V.E. (2015). The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stein T.D., Alvarez V.E., and McKee A.C. (2015). Concussion in chronic traumatic encephalopathy. Curr. Pain Headache Rep. 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu L., Nguyen J.V., Lehar M., Menon A., Rha E., Arena J., Ryu J., Marsh-Armstrong N., Marmarou C.R., and Koliatsos V.E. (2016). Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp. Neurol. 275, Pt 3, 436–449 [DOI] [PubMed] [Google Scholar]
- 122. Iliff J.J., Chen M.J., Plog B.A., Zeppenfeld D.M., Soltero M., Yang L., Singh I., Deane R., and Nedergaard M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ojo J.O., Mouzon B., Greenberg M.B., Bachmeier C., Mullan M., and Crawford F. (2013). Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 72, 137–151 [DOI] [PubMed] [Google Scholar]
- 124. Ojo J.O., Mouzon B.C., and Crawford F. (2016). Repetitive head trauma, chronic traumatic encephalopathy and tau: challenges in translating from mice to men. Exp. Neurol. 275, Pt 3, 389–404 [DOI] [PubMed] [Google Scholar]
- 125. McAteer K.M., Turner R.J., and Corrigan F. (2017). Animal models of chronic traumatic encephalopathy. Concussion 2, CNC32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wojnarowicz M.W., Fisher A.M., Minaeva O., and Goldstein L.E. (2017). Considerations for experimental animal models of concussion, traumatic brain injury, and chronic traumatic encephalopathy—these matters matter. Front. Neurol. 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jamnia N., Urban J.H., Stutzmann G.E., Chiren S.G., Reisenbigler E., Marr R., Peterson D.A., and Kozlowski D.A. (2017). A clinically relevant closed-head model of single and repeat concussive injury in the adult rat using a controlled cortical impact device. J. Neurotrauma 34, 1351–1363 [DOI] [PubMed] [Google Scholar]
- 128. Petraglia A.L., Plog B.A., Dayawansa S., Dashnaw M.L., Czerniecka K., Walker C.T., Chen M., Hyrien O., Iliff J.J., Deane R., Huang J.H., and Nedergaard M. (2014). The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg. Neurol. Int. 5, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Acabchuk R., Briggs D.I., Angoa-Perez M., Powers M., Wolferz R., Jr., Soloway M., Stern M., Talbot L.R., Kuhn D.M., and Conover J.C. (2016). Repeated mild traumatic brain injury causes focal response in lateral septum and hippocampus. Concussion 1, CNC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kabadi S.V. and Faden A.I. (2014). Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int. J. Mol. Sci. 15, 1216–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Statler K.D., Alexander H., Vagni V., Holubkov R., Dixon C.E., Clark R.S, Jenkins L., and Kochanek P.M. (2006). Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res, 1076, 216–224 [DOI] [PubMed] [Google Scholar]
- 132. Statler K.D., Alexander H., Vagni V., Dixon C.E., Clark R.S., Jenkins L., and Kochanek P.M. (2006). Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J. Neurotrauma 23, 97–108 [DOI] [PubMed] [Google Scholar]
- 133. Stocchetti N., Taccone F.S., Citerio G., Pepe P.E., Le Roux P.D., Oddo M., Polderman K.H., Stevens R.D., Barsan W., Maas A.I., Meyfroidt G., Bell M.J., Silbergleit R., Vespa P.M., Faden A.I., Helbok R., Tisherman S., Zanier E.R., Valenzuela T., Wendon J., Menon D.K., and Vincent J.L. (2015). Neuroprotection in acute brain injury: an up-to-date review. Crit. Care 19, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]