Abstract
Purpose: This study evaluated oral medication adherence among adolescents and young adults (AYAs) with cancer during a trial of a smartphone-based medication reminder application (app).
Methods: Twenty-three AYAs receiving at least one prescribed, scheduled oral medication related to their outpatient cancer treatment participated in this 12-week single-group interrupted time series longitudinal design study. Baseline oral medication adherence was monitored using electronic monitoring caps for 4 weeks. Participants then used a medication reminder app and continued to have their oral medication adherence monitored for 8 weeks. Participants completed an electronically administered weekly survey addressing perceived adherence and reasons for nonadherence.
Results: Four adherence phenotypes were identified using visual graphical analysis of individual participants' weekly adherence: (1) high adherence during the preintervention and intervention periods (n = 13), (2) low preintervention adherence and improved adherence during the intervention period (n = 3), (3) low adherence during both periods (n = 6), and (4) high preintervention adherence and low adherence during the intervention period (n = 1). Growth curve models did not show significant changes in adherence by preintervention versus intervention trajectories (p > 0.05); however, the variance in adherence during the intervention narrowed for more highly adherent AYAs. “Forgetfulness” was the most frequently reported reason for nonadherence.
Conclusion: Although overall adherence did not improve following use of the app, the variance decreased for more highly adherent participants. Additional or alternative interventions are needed for AYAs with persistently poor adherence. Assessment of adherence patterns may support individualized recommendation of tailored interventions.
Keywords: adherence, intervention, technology, oral medications, mobile technology
Introduction/Background
The increasing use of oral medications for cancer treatment and supportive care allow adolescents and young adults (AYAs) to receive more ambulatory-based treatment. While oral medication use has reduced the need for some inpatient services, a greater burden of responsibility to implement the medical regimen rests with patients, creating more opportunities for nonadherence.1,2 Medication nonadherence is ∼50% for AYAs with cancer and other chronic health conditions.3–8 Less than 95% adherence to 6-mercaptopurine is associated with a 2.7-fold increased risk of relapse among children and adolescents with acute lymphoblastic leukemia.9 Poorer adherence to sulfamethoxazole/trimethoprim has been associated with decreased long-term survival.10
Numerous factors contribute to suboptimal adherence among AYAs. Although many larger studies addressing adherence among AYAs have emphasized noncancer conditions, developmental similarities across these groups provide a foundation for understanding reasons for nonadherence and guiding interventions. AYAs frequently cite “forgetfulness” as a reason for nonadherence, and many are tackling complex life changes that pose challenges to adherence.11–13 Changing insurance status, new pharmacies or care providers, costly medications, and complicated dosing regimens may contribute to nonadherence.3,14 AYAs' experience of medication-related side effects, and medication regimens that disrupt AYAs' usual activities, particularly interactions with friends, further influence adherence-related decision-making.11 AYAs with cancer endorse the desire for skills to facilitate adherence, including resources that prompt them to take medications.11
Phenotypic patterns of self-reported compared with electronically monitored adherence have been described among children and adolescents receiving 6-mercaptopurine therapy during maintenance therapy for acute lymphoblastic leukemia.15 Participants with “over-reporter” phenotypes, those whose self-reported adherence was greater than that identified through electronic cap monitoring for ≥15 days/month for ≥50% of monitored months, were more likely to have suboptimal adherence. Further identification and description of adherence phenotypes may define subgroups that may benefit from a given intervention.
Over 90% of 18- to 29-year-olds in the United States own a smartphone.16 Mobile health (mHealth) interventions have demonstrated feasibility among AYAs with cancer17–21; however, evidence regarding their efficacy and clinical utility is limited.22,23 mHealth interventions have the advantage of directly supporting AYAs' growing independence in treatment-related decision-making.
Purpose
This article describes oral medication adherence among AYAs with cancer during a 4-week baseline period and an 8-week trial of using a smartphone-based medication reminder application (app). This study also explored adherence phenotypes and AYAs' reasons for nonadherence.
Methods
Design
This mixed methods study used a single-group intervention longitudinal design over a 12-week period, during which oral medication adherence was monitored electronically.24 Baseline adherence was monitored for 4 weeks. Participants then used a smartphone-based medication reminder app for the 8-week intervention period. Other studies evaluating adherence-related interventions in AYAs with other chronic conditions have likewise used shorter baseline monitoring periods relative to the intervention.25,26 Participants also completed weekly questionnaires addressing reasons for nonadherence.
Study sample and setting
Participants were AYAs, 15–29 years of age, receiving treatment for cancer. A narrower age range relative to the National Cancer Institute (NCI) definition of AYAs (15–39 years of age)27 was selected to recruit a more developmentally homogenous sample. Participants were recruited from two clinical sites in the Intermountain West of the United States: Primary Children's Hospital (PCH), a Children's Oncology Group-affiliated hospital and Huntsman Cancer Institute (HCI), an NCI-designated Comprehensive Cancer Center.
Participants were receiving at least one prescribed, scheduled oral medication related to their cancer in the ambulatory setting. Eligible medications included both cancer-directed (e.g., 6-mercaptopurine and imatinib) and supportive care medications (e.g., sulfamethoxazole–trimethoprim and gabapentin). Participants had completed at least 1 month of therapy and were anticipated to continue therapy an additional 3 months. They were required to speak and read English and have an iOS- or Android-based smartphone. Individuals who had previously used a smartphone-based medication reminder app were ineligible, as were individuals with cognitive or physical disabilities preventing completion of study-related activities.
Study intervention
The study intervention was the Dosecast Pro mobile app,28 accessible via Apple's App Store and Google Play from Montuno Software. Participants received an individual passcode that provided 1 year of access. Dosecast Pro allowed participants to establish a detailed, personalized schedule to receive reminders to take medications and track adherence. Reminders within the app prompt users to: “take dose now,” “postpone,” or “skip.”
Study procedures and measures
Institutional Review Board approval was granted. Patients scheduled for ambulatory clinic visits or inpatient admissions for chemotherapy were screened for initial eligibility. Sixty-four AYAs were approached, 54 met eligibility criteria, and 32 were enrolled. AYAs <18 years of age provided written assent, and parents provided written permission for participation. AYAs aged 18 years and older provided written consent. Recruitment was 67% at PCH and 50% at HCI. The most frequent reasons for nonparticipation included “not interested” (n = 9), and “too busy” (n = 7). Retention was 65% for PCH and 83% for HCI. The most frequent reasons for attrition included a preference for another medication reminder system (n = 2), and a sense of “having too much going on” (n = 2). All attrition occurred during the preintervention period.
Baseline questionnaire
Participants completed baseline demographic questionnaires via the University of Utah's Research Electronic Data Capture (REDCap) tool.29 Clinical data, scheduled medications, and doses were verified via the electronic medical record.
Adherence
Weekly adherence was measured using eCap™ electronic monitoring caps.24 eCaps contain a computer chip embedded in a cap that fits a standard medication vial. The chip records each date and time the vial is opened. Electronic monitoring caps have been used as an objective measure of medication adherence and to evaluate the efficacy of interventions.15,30–32
Participants received eCaps for their scheduled oral medications. They were also asked to keep a log of eCap openings not associated with taking a medication (e.g., transferring medication from another vial). During the 4-week preintervention period, participants used the eCaps with their scheduled oral medications and completed weekly questionnaires via REDCap using an electronic link.
Reasons for nonadherence
Participants completed a weekly questionnaire that included an open-ended item: “The reason(s) I did not take all my scheduled oral medications exactly as prescribed this week were __________.”
Study visits
Participants brought their medication bottles and met with a study team member during a scheduled clinic visit or inpatient admission following the 4-week preintervention period. eCaps were scanned to retrieve preintervention adherence data. Study staff assisted participants in downloading the Dosecast app and entering their medication-related information to receive reminders.
Participants were instructed to use the Dosecast app during the 8-week intervention period. They also completed weekly questionnaires and continued to use the eCaps for scheduled oral medications. At the study conclusion, participants returned the eCaps, which were again scanned to determine how many times they were opened.
Data management
Questionnaire responses were retrieved from REDCap and compiled into Excel for analysis. eCap data were downloaded via a CertiScan® RFID desktop reader and exported as a .csv file.
Data analysis procedures
Descriptive statistics characterized participants' demographic characteristics. Weekly adherence for each medication was calculated as a percentage based on number of doses taken (eCap openings) relative to the number of anticipated doses. Weekly adherence was summarized across all monitored medications. Medications were categorized as being (1) cancer-directed, (2) antibiotics, and (3) other supportive care medications.
Multilevel interrupted time series compared electronically monitored adherence during the preintervention and intervention periods for repeated individual measurements. Multilevel unconditional growth curve modeling estimated patient-to-patient variation during the preintervention and intervention phases. Results were considered statistically significant if p < 0.05 and are only reported explicitly where significant, to avoid overinterpretation of nonsignificant findings. Statistical analyses and graphics were generated using R, version 3.5.0.33
Individual profile plots were constructed to illustrate weekly adherence rates across the study period. Visual graphic analysis (VGA) techniques were applied to individual profile plots to evaluate trends in adherence across the study period and distinguish adherence phenotypes, that is, subgroups with similar adherence characteristics.34 VGA techniques are useful to explore similarities and differences among participants when the capacity to measure statistically significant differences is limited. A threshold of 80%, which has been used to define adequate adherence among youth and adults,35–37 distinguished “high” from “low” adherence for each week of this exploratory study.
Reasons for nonadherence were analyzed using qualitative content analysis procedures.38 Each response was treated as an individual unit of analysis. Two authors independently assigned one or more codes for each response. A constant comparative approach was used to organize codes into categories and subcategories, which were confirmed by the author team. Chi-square tests assessed differences in categorical variables and reasons for nonadherence.
Results
Participant characteristics
Participants who completed the study were a median of 19 years of age (range 15–29 years), and nearly two-thirds were 18 years or older. Participants were a median of 10 months from their initial cancer diagnosis (range 1–83) (Table 1).
Table 1.
Participant Demographic Characteristics (N = 23)
| N | % | ||
|---|---|---|---|
| Gender | |||
| Male | 14 | 60.9 | |
| Female | 9 | 39.1 | |
| Age group | |||
| Adolescent (15–18 years) | 10 | 34.8 | |
| Young adult (19–29 years) | 13 | 65.2 | |
| Ethnicity | |||
| Hispanic/Latino | 2 | 8.7 | |
| Not Hispanic/Latino | 21 | 91.3 | |
| Race | |||
| White | 21 | 91.3 | |
| Asian | 1 | 4.3 | |
| Native Hawaiian/Pacific Islander | 1 | 4.3 | |
| Diagnosis | |||
| Leukemia | 8 | 34.8 | |
| Lymphoma | 4 | 17.4 | |
| Sarcoma | 4 | 17.4 | |
| Brain tumor | 4 | 17.4 | |
| Other solid tumor | 3 | 13 | |
| Disease stage | |||
| Primary disease | 18 | 78.3 | |
| Recurrent disease | 5 | 21.7 | |
| Currently living witha | |||
| Parents | 18 | 78.3 | |
| Significant other/spouse | 3 | 13 | |
| Roommate/friends | 2 | 8.7 | |
| Alone | 1 | 4.3 | |
| Median | Mean (SD) | Range | |
|---|---|---|---|
| Age | 19.05 | 20.2 (4.3) | 15–29 |
| Household income | $60,000–79,000 | ≤$20,000 to ≥$100,000 |
One participant indicated living with both parents and significant other.
SD, standard deviation.
Medications
Adherence was monitored for a median of two medications (range 1–3) per participant. Twenty-two participants (96%) were receiving at least one scheduled medication for supportive care needs related to their cancer diagnosis. Sulfamethoxazole–trimethoprim was the most frequently monitored medication (n = 13). Nine participants (39%) were receiving at least one oral cancer-directed medication. The most common cancer-directed medications were temozolamide (n = 3), tyrosine kinase inhibitors (n = 3), 6-mercaptopurine (n = 2), and methotrexate (n = 2).
Adherence
Electronically monitored weekly adherence for participants ranged from 0% to 121% across prescribed medications. Weekly adherence for individual medications ranged from 0% to 200%. Twenty-two participants (96%) missed at least one scheduled medication dose. Weekly adherence was measured at ≥100% for 10 participants (43%) at least once. Overall adherence did not differ during the 8-week intervention period compared with the preintervention period (p > 0.05).
Adherence phenotypes
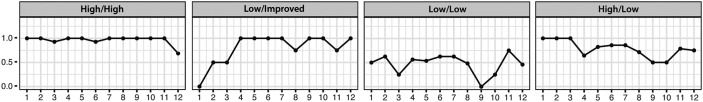
Four adherence phenotypes were identified using VGA (Fig. 1). These included (1) high adherence during both the preintervention and intervention periods (n = 13), (2) low adherence during the preintervention period and improved adherence during the intervention period (n = 3), (3) low adherence during both the preintervention and intervention periods (n = 6), and (4) high adherence during the preintervention period and low adherence during the intervention period (n = 1). Table 2 summarizes demographic and clinical characteristics of participants based on adherence phenotype. Individual profiles for medication types are presented in Supplementary Figure S1. VGA techniques did not identify differences in adherence based on medication type among participants with more than one monitored medication.
FIG. 1.
Example profile plots of adherence phenotypes.
Table 2.
Demographic and Clinical Characteristics Based on Adherence Phenotype
| High/high (n = 13) | Low/improved (n = 3) | Low/low (n = 6) | High/low (n = 1) | |
|---|---|---|---|---|
| Gender | ||||
| Male (n = 14) | 10 | 1 | 3 | 0 |
| Female (n = 9) | 3 | 2 | 3 | 1 |
| Age group | ||||
| 15–18 Years (n = 10) | 4 | 2 | 3 | 1 |
| ≥19 Years (n = 13) | 9 | 1 | 3 | 0 |
| Diagnostic group | ||||
| Leukemia (n = 9) | 4 | 1 | 4 | 0 |
| Lymphoma (n = 5) | 2 | 2 | 1 | 0 |
| Brain tumor (n = 4) | 3 | 0 | 1 | 0 |
| Other (n = 5) | 4 | 0 | 0 | 1 |
| Medication class | ||||
| Cancer-directed (n = 9) | 4 | 0 | 4 | 1 |
| Antibiotic (n = 14) | 6 | 3 | 5 | 0 |
| Other (n = 9) | 7 | 0 | 1 | 1 |
| No. of monitored medications | ||||
| One (n = 15) | 10 | 3 | 2 | 0 |
| More than one (n = 8) | 3 | 0 | 4 | 1 |
Definitions: High adherence during the 4-week preintervention period was defined as at least 3 weeks with 80% or greater adherence. High adherence during the 8-week intervention period was defined as at least 7 weeks with 80% or greater adherence. Improved adherence during the 8-week intervention period was defined as 6 weeks with 80% or greater adherence. Low adherence during the 8-week intervention period was defined as 5 or fewer weeks with at least 80% adherence.
Adherence based on adherence phenotype
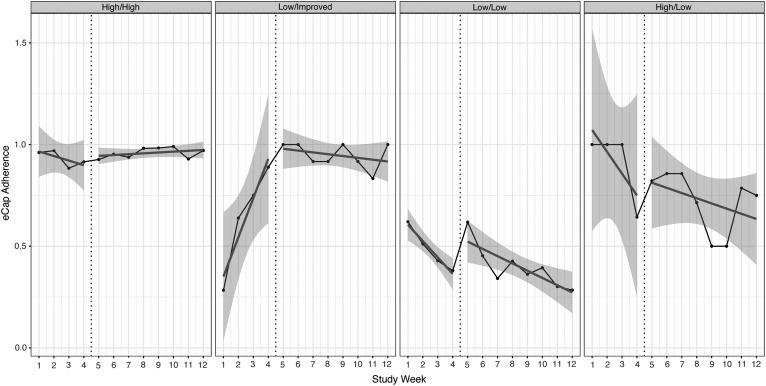
Table 3 summarizes percent adherence during preintervention and intervention periods based on adherence phenotype. Mean adherence did not exceed 80% for any participants in the low/low phenotype during either the preintervention or intervention period. Figure 2 illustrates variation in oral medication adherence based on adherence phenotype. Adherence was unchanged for participants with persistently high adherence during the intervention period (p > 0.05), however, the variance in adherence decreased. The estimated patient-to-patient variation in the slope corresponded to a standard deviation of about 0.042/week before intervention (range: −0.0453 to 0.0387) and 0.007/week (range: −0.0063 to 0.0077) with intervention. The slope during the intervention period was positive compared with a negative slope during the preintervention period.
Table 3.
Percent Adherence During the Preintervention and Intervention Periods Based on Phenotype
| High/high (n = 13) | Low/improved (n = 3) | Low/low (n = 6) | High/low (n = 1) | |
|---|---|---|---|---|
| Preintervention | ||||
| Mean (SD) | 94.43 (6.99) | 64.03 (12.78) | 44.19 (27.16) | 91.07 (N/A) |
| Range | 80.95–103.97 | 50–67.09 | 7.86–77.38 | N/A |
| Intervention | ||||
| Mean (SD) | 94.52 (6.95) | 94.79 (1.81) | 43.07 (18.57) | 74.64 (N/A) |
| Range | 82.81–103.57 | 93.75–96.88 | 19.49–73.33 | N/A |
FIG. 2.
Comparison of variance in adherence during the preintervention and intervention periods based on adherence phenotype.
Adherence was unchanged for participants with low preintervention adherence and improved adherence during the intervention period (p > 0.05). The patient-to-patient variation also narrowed during the intervention period (0.01/week; range: 0–0.02). For participants with persistently low adherence, adherence was unchanged following implementation of the intervention (p > 0.05). In contrast to other phenotypes, patient-to-patient variation increased during the intervention period (0.031/week; range: −0.048 to 0.014) compared with the preintervention period (0.018/week; range: −0.05 to 0.014).
Reasons for nonadherence
Nineteen participants provided 76 free-text responses representing 101 individual codes identifying reasons for medication nonadherence. Reasons for missing data included not having responded to weekly questionnaires (n = 27 occasions involving 10 participants, 6 of whom had a high/high phenotype) and leaving the item blank (n = 41) occasions involving 9 participants, 6 of whom had a high/high phenotype). For other occurrences, patients denied missed medication doses by responding “0” or “none.”
Codes were organized into 5 categories and 15 subcategories (Table 4). The most frequent category was “Forgetfulness,” (n = 47). Some AYAs provided insight into reasons for their forgetfulness, “Morning dose is tough to remember,” and “I forgot that I have to take two pills.” Responses also provided perspectives regarding the complexity of AYAs' daily lives and the added challenges associated with oral medication regimens, for example, “I forgot to take them with me when I didn't have dinner at home,” and “… during treatments I stay with family and friends and am thus in an unfamiliar environment.” Cancer- and treatment-related symptoms (n = 13) affected AYAs' perceived ability to tolerate oral medications, “I was nauseous before taking my medication and I was not in a good place for possible vomiting so I chose to skip my medication.” AYAs also reported running out of medication, “Ran out and had to call in refill,” and financial barriers, “… my insurance premiums were late so I couldn't afford to refill at the time,” as contributing to nonadherence.
Table 4.
Categories and Subcategories of Reasons for Nonadherence
| Frequency | |
|---|---|
| Forgetfulness | |
| Patient forgot | 43 |
| Caregiver forgot | 2 |
| Late to take a dose | 2 |
| Lifestyle/schedule | |
| Environment | 14 |
| Busy | 6 |
| Schedule | 5 |
| Physical symptoms/managing illness | |
| Feeling sick | 9 |
| Fell asleep/tired | 3 |
| Provider's request | 1 |
| Access to medication | |
| Ran out | 8 |
| Financial difficulty | 3 |
| Could not find medication | 1 |
| Other reasons | |
| Did not receive application alert | 2 |
| Lazy | 1 |
| Not sure | 1 |
Chi-square analyses did not identify differences based on gender, preintervention versus intervention period, or diagnostic group. The frequency of reported reasons for nonadherence differed by age group (χ2 = 15.32; p = 0.004). Adolescents more frequently reported lifestyle/schedule issues (adjusted standardized residual [ASR] = 3.5). Young adults more frequently reported access to medication as a reason for nonadherence (ASR = 2.3). Frequencies in reasons for nonadherence also differed based on adherence phenotype (χ2 = 32.43; p = 0.001) Participants whose adherence improved during the intervention period more frequently reported forgetfulness (ASR = 2.1), those with consistently high adherence more frequently cited lifestyle/schedule issues, and those with persistently low adherence more frequently reported lack of access to medication.
Discussion
Adherence to oral medications remains challenging for AYAs with cancer. Oral medication regimens can be complex and include both cancer-directed and supportive care medications. Participants endorsed the acceptability21 of the medication reminder app, but overall, oral medication adherence remained unchanged with use of the app.
Adherence phenotypes across the 12-week study period were identified with the aid of visual graphical analysis. One small subset of participants (Low/High) with improved adherence was identified. These three participants more frequently named “forgetfulness” as a reason for nonadherence, suggesting that a medication reminder may be an effective intervention for patients endorsing this self-reported reason for nonadherence.
Of potential clinical relevance, the variance in adherence narrowed among patients with High/High and Low/High adherence phenotypes. Adherence, even among more highly adherent participants, reflected ongoing challenges related to oral medications. Although mean adherence for those with High/High and improved (Low/High) adherence during the intervention period approached 95%, mean adherence for some individuals in these groups was <85%. The latter is suboptimal for cancer-directed therapies, indicating that even some more highly adherent participants were at risk for inferior outcomes.9,15
This study identified potential occasions of overadherence, a clinically relevant aspect of oral medication nonadherence39 that has been less explored among AYAs. Overadherence occurs when patients take more than the scheduled number of medication doses. Review of the raw data, however, did not identify cases of systematic overadherence. Participants were asked to record instances when the medication bottle was opened without taking a dose; however, this task may not have occurred. Some instances of overadherence could have been artifact related to opening the medication bottle without taking a dose.
Qualitative analyses support future exploration of reasons for nonadherence. Similar to previous studies, forgetfulness was the most frequently cited reason for nonadherence.3,7,13,40 Responses also provided insights into AYA lifestyle factors that could guide future intervention, including reports of being away from home when medication doses were missed. Interventions could help AYAs develop proactive plans for medication-taking for times that they are away from home or out of their usual routine. Optimizing symptom management,41 including nausea, may support adherence. AYAs reported skipping scheduled medications because of not feeling well or fearing potential side effects of the medication, particularly if they would be away from home.
Access to medication emerged as an important issue among young adults. Whether or not they live with parents, young adults may be assuming greater physical and/or financial accountability for acquiring medications. Three individuals (50%) in the Low/Low group reported access to medication as a reason for nonadherence at least once. Two of these individuals reported living apart from parents. These AYAs may have encountered systemic barriers to adherence, such as financial toxicity, that could not be addressed with app use.
Limitations
Study limitations included a single-group design using a small sample heterogeneous with regard to cancer diagnosis, stage of treatment, and prescribed medications. The study sample had limited racial and ethnic diversity and did not include participants receiving treatment at community-based sites. This small sample did not permit additional comparisons based on duration of therapy at the time of study enrollment. Adherence behaviors among patients who had been receiving treatment for longer periods of time may have been less amenable to change. Although electronic medication caps were used to provide a more objective measure of adherence than self-report, actual medication consumption was not directly observed.
An open-ended question explored AYAs' reasons for nonadherence. Given the small sample size, all domains of reasons for nonadherence may not have been elicited.
Future research
Directions for future studies include a sample size sufficiently powered to distinguish adherence phenotypes and to predict adherence phenotype early in the course of treatment. Such risk stratification could facilitate implementation of tailored interventions before nonadherence behaviors become established.
Research is needed to better understand which patients benefit most from mobile technology-based interventions and how app software can be tailored to maximize desired health-promoting effects. Incorporating reminder app data into electronic health record systems could be part of a broader strategy to increase providers' engagement in supporting adherence,42 identifying patient-specific adherence barriers in real-time. Other future studies should evaluate the role of mobile technology interventions on adherence-related outcomes, including survival, relapse risk, maintenance of therapeutic levels, symptoms, hospitalizations for supportive care needs, and quality of life.
Conclusion
Promoting excellent oral medication adherence is essential to optimize cancer survival for AYAs. Study results support an understanding of adherence phenotypes and provide a framework for future adherence-related research. Mobile technology-based reminder apps may increase adherence among some AYAs receiving oral medications. More research is needed to address adherence barriers identified by AYAs, discover which AYAs are most likely to benefit from a given intervention, and find additional strategies to support patients with very poor adherence.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Aisner J. Overview of the changing paradigm in cancer treatment: oral chemotherapy. Am J Health Syst Pharm. 2007;64(Suppl 5):S4–7 [DOI] [PubMed] [Google Scholar]
- 2. Rudnitzki T, McMahon D. Oral agents for cancer: safety challenges and recommendations. Clin J Oncol Nurs. 2015;19:41–6 [DOI] [PubMed] [Google Scholar]
- 3. Butow P, Palmer S, Pai A, et al. Review of adherence related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–9 [DOI] [PubMed] [Google Scholar]
- 4. Johnson MA, Javalkar K, van Tilburg M, et al. The relationship of transition readiness, self-efficacy, and adherence to preferred health learning methods by youths with chronic conditions. J Pediatr Nurs. 2015;30(5):e83–90 [DOI] [PubMed] [Google Scholar]
- 5. Joyce N, Eaton CB, Wellenius GA, et al. Patterns and predictors of medication adherence to lipid lowering therapy in children ages 8 to 20. J Clin Lipidol. 2016;10(4):824–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narayanan S, Mainz JG, Gala S, et al. Adherence to therapies in cystic fibrosis: a targeted literature review. Expert Rev Respir Med. 2017;11(2):129–45 [DOI] [PubMed] [Google Scholar]
- 7. Simons LE, McCormick ML, Mee LL, et al. Parent and patient perspectives on barriers to medication adherence in adolescent transplant recipients. Pediatr Transplant. 2009;13:338–47 [DOI] [PubMed] [Google Scholar]
- 8. Tae CH, Jung SA, Moon HS, et al. Importance of patients' knowledge of their prescribed medication in improving treatment adherence in inflammatory bowel disease. J Clin Gastroenterol. 2016;50(2):157–62 [DOI] [PubMed] [Google Scholar]
- 9. Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a Children's Oncology Group study. JAMA Oncol. 2015;1:287–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennard BD, Stewart SM, Olvera R, et al. Nonadherence in adolescent oncology patients: preliminary data on psychological risk factors and relationships to outcome. J Clin Psychol Med Settings. 2004;11(1):31–9 [Google Scholar]
- 11. McGrady ME, Brown GA, Pai ALH. Medication adherence decision-making among adolescents and young adults with cancer. Euro J Oncol Nurs. 2016;20:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blaakman SW, Cohen A, Fagnano M, et al. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators, and experiences with school-based care. J Asthma. 2014;51(5):522–9 [DOI] [PubMed] [Google Scholar]
- 13. Koster ES, Philbert D, de Vries TW, et al. “I just forget to take it”: asthma self-management needs and preferences in adolescents. J Asthma. 2015;52(8):831–7 [DOI] [PubMed] [Google Scholar]
- 14. Wood L. A review on adherence management in patients on oral cancer therapies. Eur J Oncol Nurs, 2012;16:432–8 [DOI] [PubMed] [Google Scholar]
- 15. Landier W, Chen Y, Hageman L, et al. Comparison of self-report and electronic monitoring of 6-MP intake in childhood ALL: a Children's Oncology Group study. Blood. 2017;129(14):1919–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pew Research Center. Who owns cellphones and smartphones. Washington, DC; 2017. Accessed September27, 2018 from: http://www.pewinternet.org/chart/who-owns-cellphones-and-smartphones [Google Scholar]
- 17. Baggott C, Gibson F, Coll B, et al. Initial evaluation of an electronic symptom diary for adolescents with cancer. J Med Internet Res Protocols. 2012;1(2):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kock AK, Kaya R, Muller C, et al. A mobile application to manage and minimise the risk of late effects caused by childhood cancer. Stud Health Technol Inform. 2015;210:798–802 [PubMed] [Google Scholar]
- 19. Macpherson CF, Linder LA, Ameringer S, et al. Feasibility and acceptability of an iPad application to explore symptom clusters in adolescents and young adults with cancer. Pediatr Blood Cancer. 2014;61(11):1996–2003 [DOI] [PubMed] [Google Scholar]
- 20. Wesley KM, Fizur PJ. A review of mobile applications to help adolescent and young adult cancer patients. Adolesc Health Med Ther. 2015;6:141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu YP, Linder LA, Kanokvimankul P, et al. Use of a smartphone application for prompting oral medication adherence among adolescents and young adults with cancer. Oncol Nurs Forum. 2018;45(1):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017;177(5):624–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21(3):354–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Information Mediary Corp. eCAP™ Medication adherence innovations. 2017. Accessed September27, 2018 from: https://ecm.cachefly.net/pdf/eCAPBrochure2015sm.pdf
- 25. Foster BJ, Pai ALH, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT). Am J Kidney Dis. 2018;72(1):30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mimiaga MJ, Kuhns LM. Biello KB, et al. , Positive STEPS—a randomized controlled efficacy trial of an adaptive intervention for strengthening adherence to antiretroviral HIV treatment among youth: study protocol. BMC Public Health. 2018;18(1):867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Cancer Institute. Adolescents and young adults with cancer. 2018. Accessed September27, 2018 from: www.cancer.gov/types/aya
- 28. Montuno software about Dosecast. 2017. Accessed September27, 2018 from: http://www.montunosoftware.com/about
- 29. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eakin MN, Brady T, Kandasamy V, et al. Disparities in antihypertensive medications in adolescents. Pediatr Nephrol. 2013;28:1267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greenley RN, Kunz JH, Blank V, et al. Identifying youth nonadherence in clinical settings: data-based recommendations for children and adolescents with inflammatory bowel disease. Inflam Bowel Dis. 2012;18:1254–9 [DOI] [PubMed] [Google Scholar]
- 32. Safren SA, Bedoya CA, O'Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3:e529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The R Foundation for Statistical Computing. 2018. Accessed September27, 2018 from: www.r-project.org
- 34. Brown CG, McGuire DB, Beck SL, et al. Visual graphical analysis: a technique to investigate symptom trajectories over time. Nurs Res. 2007;56(3):195–201 [DOI] [PubMed] [Google Scholar]
- 35. Osterberg L, Blashke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97 [DOI] [PubMed] [Google Scholar]
- 36. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katz LL, Anderson BJ, McKay SV, et al. Correlates of medication adherence in the TODAY cohort of youth with type 2 diabetes. Diabetes Care. 2016;39:1956–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–15 [DOI] [PubMed] [Google Scholar]
- 39. Spoelstra SL, Given BA, Given CW, et al. An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nurs. 2013;36:18–28 [DOI] [PubMed] [Google Scholar]
- 40. Muluneh B, Deal A, Alexander MD, et al. Patient perspectives on the barriers associated with medication adherence to oral chemotherapy. J Oncol Pharm Pract. 2018;24(2):98–109 [DOI] [PubMed] [Google Scholar]
- 41. Linder L, Erickson JM, Stegenga K, et al. Symptom self-management strategies reported by adolescents and young adults with cancer receiving chemotherapy. Support Care Cancer. 2017;25(12):3793–806 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez G, Utate MA, Joseph G, et al. Oral chemotherapy adherence: a novel nursing intervention using an electronic health record workflow. Clin J Oncol Nurs. 2017;21(2):165–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.