Abstract
Cryptococcal meningitis (CM) contributes significantly to high early mortality in the setting of advanced HIV. In resource poor settings, the current HIV disease management approach is focused on commencing antiretroviral therapy (ART) on the same day of HIV diagnosis (‘Test and Treat’). The HIV program in Nigeria does not currently provide CrAg screening for patients with newly diagnosed and advanced HIV disease. We report a case of severe cryptococcal meningitis presenting following the commencement of ART. There is clear benefit in the early commencement of ART among HIV infected patients and to prevent patients lost to follow-up as aimed with the ‘Test & Treat’ approach. However, this approach needs to be balanced against the risk of IRIS and its associated morbidity and mortality when those patients are not being properly evaluated for opportunistic infections being present without overt symptoms.
Keywords: Cryptococcal meningitis, HIV, IRIS, Nigeria, Test and Treat
1. Introduction
Cryptococcal meningitis (CM) contributes significantly to high early mortality in the setting of advanced HIV [1,2] with 10–20% of HIV associated deaths in Africa attributable to cryptococcosis [3]. It is estimated that Cryptococcus spp. is the causative organism in up to 63% of meningitis cases among adults in Sub-Saharan Africa [3,4]. The global prevalence of asymptomatic cryptococcal antigenaemia is 6% [5], but various studies in Nigeria have shown a higher prevalence of cryptococcal antigenaemia among HIV patients ranging from 2.3 to 13.1% [6,7]. The true incidence of CM in Nigeria is not known, however, a study by Gomerep et al. conducted among HIV patients presenting with features of meningitis found that 36% of confirmed cases were due to cryptococcal infection [8].
There is clear evidence to show that early commencement of antiretroviral therapy (ART) in HIV patients improves outcomes [9], and reduces the incidence of AIDS defining illnesses (including cryptococcosis) especially in Western countries [10]. The reduction of cryptococcal infections in the setting of HIV within Sub-Saharan countries has been less remarkable due to the high burden of HIV infection coupled with late presentation of advanced disease, and low ART coverage [11]. The situation is further compounded by high rates of poverty, sub-standard living conditions and weak, over-burdened and poorly funded health systems [12]. In resource poor settings, the current approach is focused on commencing ART on the same day of HIV diagnosis [13]. This is in the bid to reduce the progression of HIV infection and overcome the high rates of loss to follow up due to long laboratory turnaround times delaying the diagnosis commonly encountered in these settings.
The point of care cryptococcal antigen (CrAg) lateral flow assay has been proven to be a cost effective tool in the detection of cryptococcal antigenaemia allowing pre-emptive therapy in HIV infected patients with severe immunosuppression [14]. In spite of this, the HIV program in Nigeria does not provide CrAg screening in patients with newly diagnosed and advanced HIV. Consequently, newly diagnosed HIV patients are being commenced on ART on the same day of diagnosis without proper evaluation for infections like cryptococcosis, putting them at risk of life-threatening immune reconstitution inflammatory syndrome (IRIS) [15].
We report a case of severe cryptococcal meningitis presenting following the commencement of ART on the day a diagnosis of HIV was made.
2. Case
A 52 year old business man, presented with 4 weeks history of fever and headache, and a 1 week history of impaired vision.
Initially been treated for malaria, but with a poor response, he presented at the peripheral hospital where he tested positive for HIV-1 antibodies and was commenced on ART consisting of tenofovir, lamivudine and efavirenz (TDF/3TC/EFV) the same day. Consequently, his headache become worse and he developed visual disturbances, for which a referral was made to our facility. At presentation (day 0), he had low grade fever with no history of drenching sweats, significant weight loss, anorexia and headaches associated with projectile vomiting. He was noticed to have become increasingly restless requiring restraint at home, and had complained of double and blurred vision with subsequent visual loss. There was no history of seizures, no differential limb weakness, and no history of preceding behavioral changes. There was no history of cough, and review of other systems was essentially normal. He was not previously known to be hypertensive or diabetic, and had a history of occasional alcohol use, however no previous tobacco use.
On examination he had a slightly elevated temperature (37.7 °C), an elevated blood pressure (170/134 mmHg) with a heart rate of 82/min. Pallor, neck stiffness and a positive Kernig's sign, and a marked restlessness was noticed. His Glasgow Coma Score was 12 (E-4, V-3, M-5). There was photophobia, sluggishly reactive unequal pupils, with a right lateral rectus palsy as well as asymmetrical right-sided facial weakness affecting the lower part of the face, corresponding to a left upper motor neuron type facial nerve palsy, but was moving all limbs. Fundoscopy showed papilloedema.
Blood cryptococcal antigen (CrAg) test (LFA) was positive. Brain MRI was reported normal, and a lumbar puncture was performed. The opening pressure was high as revealed by forceful jet of cerebrospinal fluid (CSF) (we had no access to manometers), slightly turbid, non-bloody CSF which did not form strands on standing. Qualitative CSF CrAg test was positive; however, we were unable to carry out quantitative measurement of serum and CSF CrAg due to unavailability of this test modality in our center. Gram stain revealed scanty pus cells with no organisms seen, pleocytosis with 3680 lymphocytes/μL but nil neutrophils, elevated CSF protein of 1.07 g/L, CSF glucose of 2.2 mmol/L (blood glucose 6.0 mmol/L), and CSF culture growing Cryptococcus neoformans. The CSF gene Xpert test for tuberculous meningitis was negative. HIV viral load of 1807 copies/mL (after 2 weeks on ART).
Due to initial unavailability of amphotericin B and flucytosine, he was commenced on monotherapy with intravenous fluconazole 1200mg daily in 3 divided doses (day 1). Amphotericin B was eventually procured after 8 days of monotherapy with fluconazole, and he then received a combination of amphotericin B (0.7 mg/kg/day) and fluconazole (1200mg day in 3 divided doses) for another 2 weeks (day 9 to day 25). He underwent daily therapeutic lumbar punctures for the initial four days of admission, each time draining about 15 mL of CSF with satisfactory clinical response.
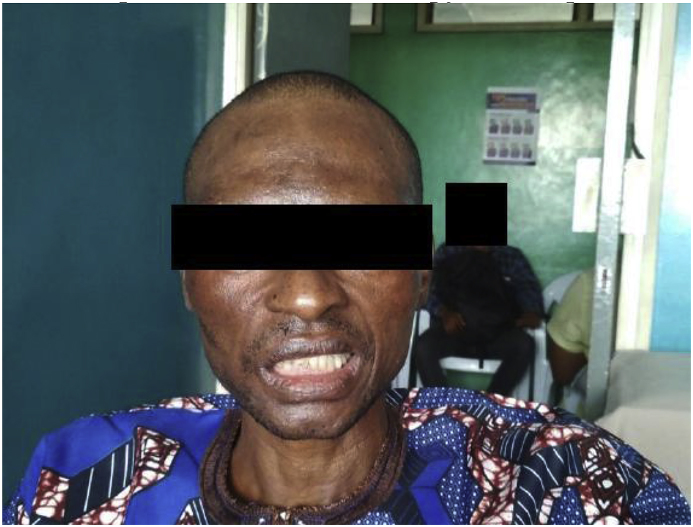
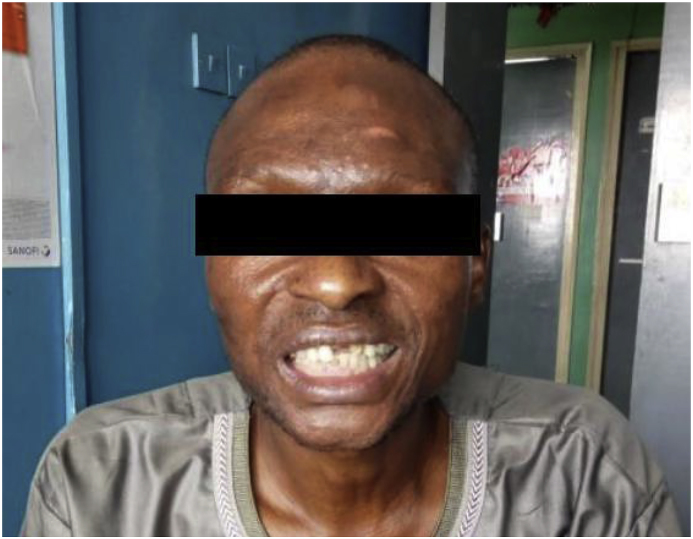
He received consolidation treatment (from day 26 onwards) consisting of 800mg fluconazole orally per day in 2 divided doses for 8 weeks, and is currently receiving maintenance phase treatment with 200mg of oral fluconazole once daily. The patient has made sustained clinical progress (day 165), with resolution of symptoms and reduction of the blood pressure. He however has residual blindness, and the facial nerve palsy is yet to completely resolve (see Figs. 1 and 2). He is still on follow up at our clinic.
Fig. 1.
Forehead wrinkling and eyelid symmetry was preserved in the patient, but he had asymmetry of the lower part of the face when he was asked to smile and bare his teeth, corresponding to a left upper motor neuron facial nerve palsy. This was still observable after completion of induction therapy with amphotericin B and fluconazole for 2 weeks.
Fig. 2.
Forehead wrinkling and eyelid symmetry was preserved in the patient, but an asymmetry of the lower part of the face was observed when he was asked to smile and bare his teeth, corresponding to a left upper motor neuron facial nerve palsy, still observed at 8 weeks since start of induction treatment (amphotericin B and fluconazole 1200 mg/d) followed by consolidation treatment with oral fluconazole (800 mg/d).
3. Discussion
This case highlights the drawback of the ‘Test & Treat’ approach; the low level of awareness/index for suspicion for cryptococcal meningitis among clinicians, the lack of a coordinated screening program for asymptomatic cryptococcal infection among newly diagnosed HIV patients and poor access to recommended antifungal medication.
The ‘Test & Treat’ approach, which is a public health algorithm, has clearly shown benefit in overcoming delay, reducing loss to follow-up commonly observed among newly diagnosed HIV-infected patients, and reversing the progressive immunosuppression and development of new opportunistic illnesses associated with HIV infection. The major drawback of the approach, especially where the implementation is not holistic, is the development of life-threatening IRIS. In Nigeria, the delay in the availability of CD4 cell counts during initial evaluation means that patients with very low CD4+ T cell counts also commence ART on the same day of treatment without the requisite evaluations/screening tests that could be lifesaving.
This case also highlights the low level of awareness and low index of suspicion among clinicians providing HIV care in Nigeria. Even though the symptoms of headache and fever were prominent when the patient initially presented to the initial hospital, those were not properly evaluated prior to commencing ART. As such, his presentation was likely a case of cryptococcal meningitis IRIS as evidenced by the observed clinical deterioration after commencement of ART, the lymphocytosis and elevated protein in the CSF [15].
Current guidelines recommend screening all HIV infected patients with CD4 cell counts <100 cells/mm3 using CrAg LFA kits [16], which have been found to be highly sensitive and specific, and cost effective in reducing the mortality of cryptococcal disease in HIV infected patients, not to mention the added benefit of preventing the occurrence of unmasking IRIS among those starting ART. The COAT trial has clearly demonstrated the benefit of starting ART 4–6 weeks after the use of antifungals in patients with cryptococcal meningitis [17].
Presently, there is no provision to routinely screen for cryptococcosis among HIV patients with advanced HIV disease in Nigeria. Where CrAg testing is available, patients pay for the tests out of pocket. In most cases however, they get prescribed low dose oral fluconazole or ART straight away. The WHO guidelines for the care of patients with advanced HIV provides for the evaluation for opportunistic infections prior to the start of ART [18]. This is geared towards reducing the mortality associated with the occurrence of IRIS in the setting of immune recovery [15]. Among patients who present with symptoms of headache and fever, the availability of CrAg tests has simplified the procedure for evaluating HIV infected patients at risk of cryptococcal infection [19]. Patients identified with asymptomatic cryptococcal antigenaemia are pre-emptively treated with oral fluconazole. Even though primary prophylaxis with low dose oral fluconazole among HIV may reduce mortality from cryptococcosis in patients without meningitis [1], there are unintended consequences, including increasing resistance of isolates to fluconazole and high costs [20].
There is therefore an urgent need to proactively institute routine screening for the detection of asymptomatic cryptococcal antigenaemia among at risk HIV infected patients who are receiving care within the Nigerian HIV treatment program. This should be implemented along with provision of point of care test kits for measurement of baseline CD4 cell counts to better identify at risk patients. Lastly, more effort is needed to increase the awareness of the Nigerian clinician about the risk and complications of cryptococcal meningitis in HIV.
In conclusion, there is clear benefit in the early commencement of ART among HIV infected patients and to prevent patients lost to follow-up as aimed with the ‘Test & Treat’ approach. Nevertheless, this approach needs to be balanced against the risk of IRIS and its associated morbidity and mortality when those patients are not being properly evaluated for opportunistic infections being present without overt symptoms. The ‘Test & Treat’ approach needs to be optimized with point-of-care screening tests being implemented to treat asymptomatic cryptococcal infections in newly diagnosed HIV infected patients.
Conflict of interest
There are none.
References
- 1.Post F.A., Szubert A.J., Prendergast A.J., Johnston V., Lyall H., Fitzgerald F. Causes and timing of mortality and morbidity among late presenters starting antiretroviral therapy in the REALITY trial. Clin. Infect. Dis. 2018;66(suppl_2):S132–S139. doi: 10.1093/cid/cix1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisson G.P., Ramchandani R., Miyahara S., Mngqibisa R., Matoga M., Ngongondo M. Risk factors for early mortality on antiretroviral therapy in advanced HIV-infected adults. AIDS. 2017;31(16):2217–2225. doi: 10.1097/QAD.0000000000001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis J.N., Meintjes G., Williams A., Brown Y., Crede T., Harrison T.S. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect. Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezeanolue E.E., Nwizu C., Greene G.S., Amusu O., Chukwuka C., Ndembi N. Brief report: geographical variation in prevalence of cryptococcal antigenemia among HIV-infected, treatment-naive patients in Nigeria: a multicenter cross-sectional study. J. Acquir. Immune Defic. Syndr. 2016;73(1):117–121. doi: 10.1097/QAI.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chukwuanukwu R., Manafa P., Iloghalu E., Onyenekwe C., Ifeanyichukwu M., Mbamalu C. 2013. Cryptococcus Neoformans Antigenemia in HIV Positive Pregnant Women Attending a PMTCT Clinic in South-East Nigeria. [Google Scholar]
- 8.Gomerep S.S., Idoko J.A., Ladep N.G., Ugoya S.O., Obaseki D., Agbaji O.A. Frequency of cryptococcal meningitis in HIV-1 infected patients in north central Nigeria. Niger. J. Med. 2010;19(4):395–399. doi: 10.4314/njm.v19i4.61963. [DOI] [PubMed] [Google Scholar]
- 9.Group I.S.S., Lundgren J.D., Babiker A.G., Gordin F., Emery S., Grund B. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lortholary O., Poizat G., Zeller V., Neuville S., Boibieux A., Alvarez M. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20(17):2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis J.N., Harrison T.S. Forgotten but not gone: HIV-associated cryptococcal meningitis. Lancet Infect. Dis. 2016;16(7):756–758. doi: 10.1016/S1473-3099(16)00128-6. [DOI] [PubMed] [Google Scholar]
- 12.Siedner M.J., Ng C.K., Bassett I.V., Katz I.T., Bangsberg D.R., Tsai A.C. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: a meta-analysis. Clin. Infect. Dis. 2015;60(7):1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig S.P., Dorvil N., Devieux J.G., Hedt-Gauthier B.L., Riviere C., Faustin M. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. 2017;14(7) doi: 10.1371/journal.pmed.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis J.N., Harrison T.S., Lawn S.D., Meintjes G., Wood R., Cleary S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco-Paredes C., Chastain D.B., Rodriguez-Morales A.J., Marcos L.A. Cryptococcal meningoencephalitis in HIV/AIDS: when to start antiretroviral therapy? Ann. Clin. Microbiol. Antimicrob. 2017;16(1):9. doi: 10.1186/s12941-017-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(WHO) WHO . 2018. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection.http://apps.who.int/iris/bitstream/handle/10665/260399/9789241550277-eng.pdf?sequence=1 Available from: [PubMed] [Google Scholar]
- 17.Boulware D.R., Meya D.B., Muzoora C., Rolfes M.A., Huppler Hullsiek K., Musubire A. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014;370(26):2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(WHO) WHO . 2017. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy.https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/ Available from: [PubMed] [Google Scholar]
- 19.Jarvis J.N., Percival A., Bauman S., Pelfrey J., Meintjes G., Williams G.N. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2011;53(10):1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongomin F., Oladele R.O., Gago S., Moore C.B., Richardson M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses. 2018;61(5):290–297. doi: 10.1111/myc.12747. [DOI] [PubMed] [Google Scholar]