Abstract
Hypoxia is an important contributor to aggressive behavior and resistance mechanisms in glioblastoma. Upregulation of hypoxia inducible transcription factors (HIFs) is the primary adaptive cellular response to a hypoxic environment. While HIF1α has been widely studied in cancer, HIF2α offers a potentially more specific and appealing target in glioblastoma given expression in glioma stem cells and not normal neural progenitors, activation in states of chronic hypoxia and expression that correlates with glioma patient survival. A first-in-class HIF2α inhibitor, PT2385, is in clinical trials for renal cell carcinoma, and provides the first opportunity to therapeutically target this important pathway in glioma biology.
Keywords: : cancer, glioblastoma, hypoxia, hypoxia-inducible factors
Oxygen is a critical substrate for homeostatic cellular processes. Failure to regulate oxygen is detrimental to normal cell survival. Cells invest themselves in redundant and competing pathways to ensure physiologic oxygen tension. In the context of cancer, a hypoxic state dominates the tumor microenvironment due to abnormal vasculature and rapid cell proliferation [1]. The hypoxic environment triggers a cascade of malignant cellular responses which lead to tumor progression, metastases, resistance to chemoradiation and characteristics of a stem-like cell phenotype [2–7].
Glioblastoma (GBM) is a valuable model to study hypoxia in cancer. Intraoperative necrosis, radiographic-decreased oxygen update and histologic vascular pathology all suggest hypoxia is pathognomonic in GBMs malignant behavior [8]. Direct intraoperative measurements of oxygen tension in patients undergoing surgery for newly diagnosed malignant brain tumors demonstrated a steep hypoxic gradient across normal and intratumoral tissue. In a seminal study of patients undergoing surgery for malignant brain tumors, the mean arterial value of oxygen was 109.2 mmHg, peritumoral values of oxygen were 59.8 ± 6.5 mmHg and intratumoral values of oxygen were 15.3 ± 2.3 mmHg, providing firm evidence that hypoxia is present in brain tumors [9]. In comparison, similar measurements within the normal brain in patients under anesthesia and inhaling an FiO2 of 100% revealed an average PO2 of 137 mmHg [10]. In neuroimaging studies such as contrast enhanced MRI, GBMs are classically defined as ring-enhancing lesions with a central necrotic core reflective of marked hypoxia central to a tumor rapidly outgrowing its blood supply. Tumor imaging with PET (positron emission tomography) tracer fluoromisonidazole (FMISO), which binds to tissue at an oxygen tension of 2–3 mmHg and below, confirms diffuse areas of hypoxia within the majority of GBM tumors [11]. In another study using the same methodology, FMISO signal was not present in low-grade gliomas confirming hypoxia is a feature unique to high-grade tumors [12]. Furthermore, in both univariate and multivariate analyses (including age and tumor volume), increasing tumor hypoxia was associated with poorer patient survival [11]. GBMs demonstrate tumor hypoxia on direct measurements and indirectly on imaging studies that correlate to patient survival.
Within GBMs, microenvironments enriched for hypoxia are defined by the presence of necrosis, which can range from focal areas of micronecrosis, larger areas of geographic necrosis and/or the classic microscopic finding of pseudopalisading necrosis, where tumor cells radially extend away from a central area of necrosis. Pseudopalisading necrosis is also negatively correlated to patient outcomes [8,13,14] and represents hypoxic tumor cells migrating away from dysfunctional vasculature [8]. Gene expression in pseudopalisading cells confirms a hypoxic response with upregulation of glycolysis, angiogenesis and cell-cycle control [15]. Specific genetic changes included vascular proliferation and permeability with overexpression of VEGF [15], OCT4 [16] and RAD51 [17], which promotes vascular proliferation and permeability, resistance to chemoradiation and altered DNA repair pathway, respectively. Just as hypoxic changes are specific to high-grade gliomas on radiographic studies, low-grade gliomas lack of central necrosis on histology also lack expression of HIF2α on immunohistochemistry analysis (small cohort from the author's unpublished work). An additional potential molecular explanation for HIF2α downregulation in low-grade gliomas is the isocitrate dehydrogenase 1 mutation in the majority of diffuse gliomas. Isocitrate dehydrogenase mutations (R132H) appear to be linked to increased hydroxylation of HIF2α, hypothesized to occur by accumulation of the R-enantiomer of hydroxygluterate activating HIF-prolyl hydroxylase (PHD), thus, initiating ubiquitin tagging and degradation, and limiting hypoxic signaling in these tumors [18].
Tumor hypoxia correlates to patient survival perhaps in part due to the biological aggressiveness but also due to the impact on treatment efficacy. Hypoxia limits the effectiveness of radiation and chemotherapeutic drugs [3], which negatively impact patient prognosis [19–21], and may be one of the mechanisms driving tumor recurrence. This review summarizes the pathophysiology of hypoxia in gliomas and highlights potential targeted therapies to this pervasive environmental stress.
Hypoxia-inducible factors
HIFs were initially described in 1992 for their role in erythropoietin gene regulation [22], yet their biologic role is rooted in primitive eukaryotic adaptations to survival in hypoxic environments through activation of glycolytic pathway to meet metabolic demands [23]. The HIFs are a family of transcription factors that mediate cellular responses to hypoxia by controlling over 40 downstream hypoxia adaptive genes. HIFs bind to hypoxia response element (HRE) sequences in gene promoters, resulting in gene expression alterations. Structurally, HIFs consist of two subunits, which combine to form a heterodimer. The α-subunits (HIF1α, HIF2α, HIF3α) are oxygen-responsive cytoplasmic proteins, while the β-subunit (HIF1β, conserved) is a constitutively expressed nuclear protein. The naming convention for active heterodimer is based on the unique α-subunit, so that active HIF1α is composed of HIF1α and HIF1β subunits while active HIF2α is a heterodimer of HIF2α and HIF1β. The majority of the literature focuses on HIF1α and HIF2α as they were discovered first [24–26]. Less is known about the function and roles of HIF3α, an isoform that antagonizes HRE gene expression [24].
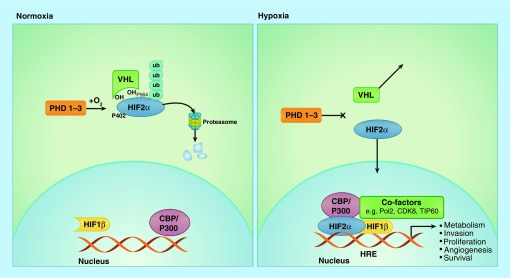
In normoxic conditions (Figure 1), cells degrade cytoplasmic HIF-α subunits via hydroxylation by PHD and polyubiquitination by Von Hippel-Lindau (VHL) and E3 ubiquitin ligase. This modified α-subunit then undergoes proteasomal degradation. In hypoxia, PHD activity is reduced leading to cytoplasmic α-HIF stabilization, accumulation and translocation to the nucleus. In the nucleus, the α-subunits dimerize with a complementary β-subunit (HIF1β, alternatively named aryl hydrocarbon receptor nuclear translocator [ARNT]). This α/β active heterodimer binds to HREs within promoter regions and drives gene expression contributing to cell proliferation, migration, metabolism, apoptosis, angiogenesis and survival [27].
Figure 1. . In normoxia, cells degrade cytoplasmic hypoxia-inducible factor 2α after hydroxylation by prolyl hydroxylases 1–3 and polyubiquitination by Von Hippel-Lindau and E3 ubiquitin ligase.
Polyubiquitinated HIF2α undergoes proteasomal degradation. In hypoxia, PHD activity is reduced leading to cytoplasmic HIF2α stabilization, accumulation and translocation to the nucleus. In the nucleus, the α subunits dimerize with a complementary β subunit (HIF1β). This α/β active heterodimer binds to HREs within DNA promoter regions and drives gene expression contributing to cell proliferation, migration, metabolism, apoptosis, angiogenesis and survival.
HRE: Hypoxic response element.
HIF1α was the first isoform identified with a function critical for cellular adaptation to low oxygen state [22,25,26,28,29]. While HIF1α was first identified in erythropoietin regulation, [22] HIF1α is widely expressed and critical in embryonic development, inflammatory/ischemic diseases and oncogenesis [26]. HIF2α was identified shortly thereafter through homology searches as 48% sequence identity is shared with HIF1α [28]. While structurally very similar, HIF1α and HIF2α demonstrate increasingly divergent biologic roles [5,30,31]. HIF1α dominates the acute response to hypoxia and is ubiquitously expressed, whereas HIF2α mediates responses to chronic hypoxia and is more selectively expressed in specific tissues [6].
The physiologic understanding of HIF signaling has expanded to include disease states with marked ischemia or inflammation [32]. More recently cancer biology has refocused on the balance of oxygen supply and consumption, hypothesizing that significant and chronic intratumoral hypoxia is the consequence of abnormal tumor vasculature, increased diffusion distances for cellular nutrient delivery and anemia of chronic disease [33]. Cells conditioned in hypoxia demonstrate a selective survival advantage during chemoradiation and clonally expand causing tumor progression and recurrence [34,35]. Given these observations, there is a growing interest in targeted inhibition of these maladaptive HIF pathways.
To date, inhibition of HIF1α has demonstrated no clear clinical evidence of antitumoral activity [19–21]. Completed studies include a Phase I study with a HIF antisense mRNA (EZN-2968) in advanced carcinoma and lymphomas and an oral HIF1α inhibitor (PX-478) in advanced solid tumors and lymphomas [36,37]. Comprehensive discussions of HIF1α in cancer biology are referenced here [38,39]. This review focuses on the role of HIF2α as an emerging target of therapeutic interest in gliomas.
Mechanisms of HIF2α upregulation
To date three distinct cellular mechanisms account for upregulation of HIF2a in an oncologic context (Table 1).
Table 1. . Mechanisms of hypoxia-inducible factor 2α upregulation in tumor cells.
| Event | Mechanism | Tumor type | Ref. |
|---|---|---|---|
| VHL gene mutation/deletion | Loss of proteasome degradation of HIF2α, HIF2α accumulates | Renal cell carcinoma | [41,44] |
| Hypoxia | Erratic blood supply and thrombosis of blood vessels contributing to hypoxic microenvironment | Glioblastoma | [7] |
| Gain of function HIF2α mutation | Missense mutations resulting in degradation failure of HIF2α | Paraganglioma and pheochromocytoma | [45] |
Loss of VHL gene results in failure to degrade HIF2α
VHL is a critical protein that targets HIF2α for proteasomal degradation. VHL is mutated in 57% or deleted (loss of heterozygosity) in 98% of renal cell carcinomas (RCCs) originally described by Gnarra et al. and confirmed in multiple other reports [40–44] and results in a condition termed pseudohypoxia – upregulation of hypoxic pathways in normoxia [45]. In pseudohypoxia, HIF2α is unable to be degraded due to an inactivating mutation of the VHL gene. HIF2α pairs with the constitutively expressed binding partner, ARNT, and drives expression of genes harboring HREs, thus mimicking a hypoxic environment [46]. The importance of VHL in regulation of the hypoxia pathways was demonstrated in gliomas through experiments modeling overexpression of VHL. Ectopic expression of VHL reduced the subcutaneous tumor growth rate in nude mice by 70% and was associated with reduced levels of HIF1α, VEGF and Bcl-2 [47].
Hypoxic upregulation of HIF2α
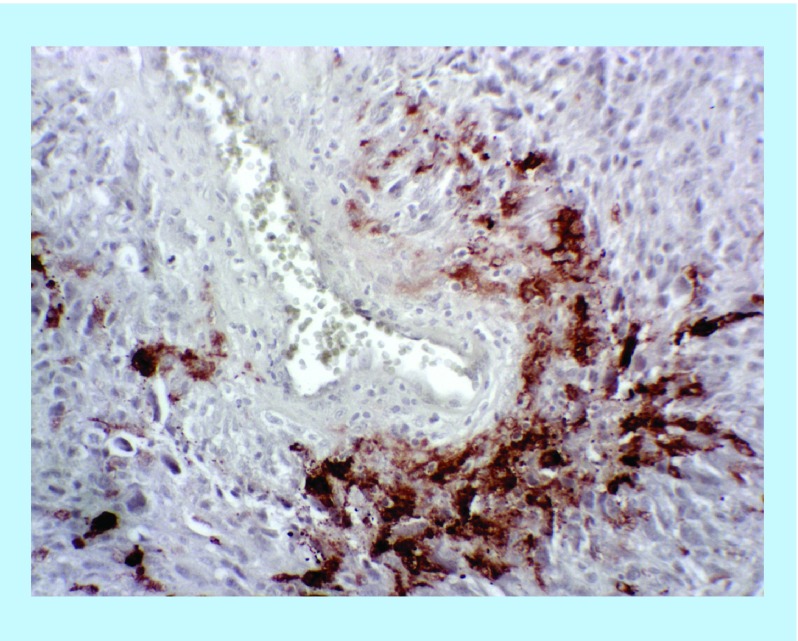
Hypoxia in cancer cells is classically attributed to accelerated clonal expansion and rapidly proliferating tumors that outgrow their blood supply. GBM is particularly predisposed to hypoxia due to irregular vasculature and high amounts of prothrombotic tissue factor. 50% of GBM specimens have observable microthrombi occluding vessels on routine histologic examination resulting in perivascular hypoxic nests [8,48]. Perivascular hypoxia is suggested by immunohistochemistry for HIF2α, which reliably and reproducibly demonstrated focal perivascular clustering of HIF2α staining (Figure 2).
Figure 2. . Immunohistochemistry for hypoxia-inducible factor 2α performed on formalin-fixed paraffin-embedded adult glioblastoma tissue demonstrates focal positive staining in the perivascular space.
This staining pattern suggests the possibility of a hypoxic gradient from dysfunctional vasculature leading to the focal upregulation of HIF2α.
At the molecular level, hypoxia shifts the balance of production and degradation resulting in upregulation of HIFs. In gliomas, the role of HIF1α and HIF2α is an area of active investigation. Immunohistochemical analysis of 50 glioma samples demonstrated a higher overall abundance of HIF2α compared with HIF1α [49]. In a TCGA (The Cancer Genome Atlas) analysis, HIF2α is associated with elevated expression mesenchymal-associated genes on chromosome 7 [50–53]. In the REMBRANT database, HIF2α but not HIF1α is inversely correlated with overall patient survival [54]. Furthermore, several studies demonstrated that HIF2α is preferentially expressed within a tumor stem cell subpopulation via CD44 and drives tumor dedifferentiation [49,54,55]. CD44 is a glycoprotein transmembrane receptor expressed on stem cells in the perivascular niche of GBMs [56]. CD44, which is transcriptionally activated by truncated glioma-associated oncogene homolog 1, can be cleaved by gamma-secretase, releasing a CD44 intracellular domain (ICD), which activates the hypoxia-response pathway [57]. Mechanistically, CD44 binds to and activates HIF2α, and not HIF1α, independent of oxygen levels and induces hypoxic signaling [58]. In a glioma mouse model, CD44 enhanced the hypoxic signature in perivascular tumor regions [58]. Inhibition of CD44 ICD both decreased HIF2α expression and reduced hypoxia-induced glioma stemness, but did not impact HIF1α expression. Thus, CD44 ICD-mediated HIF2α expression directly promotes the hypoxic signature of gliomas, independent of HIF1α [58]. Taken together, these studies suggest that HIF2α is a biologically relevant protein in glioma pathology.
In several solid malignancies, HIFs mediate signaling between macrophage and mesenchymal stem cells [59], activate malignant cell motility [60] and contribute to extracellular matrix remodeling [61,62]. In hepatocellular carcinoma, HIF2α is associated with promoting metastases via HIF2α-dependent expression in a hypoxic environment [63].
Gain-of-function mutations within HIF2α
A newly identified mechanism of HIF2α upregulation involves mutations within HIF2α that prevent its degradation. Recent studies in paragangliomas and pheochromocytomas identified 40 cases with HIF2α mutations, highlighting the first instance of HIF2α mutation in human cancers [45,64]. The majority of mutations observed were missense mutations in the oxygen-dependent degradation domain. Hotspot mutations involving proline-531 and alanine-530 account for 62% of described HIF2α mutations and result in a HIF2α gain-of-function properties [45]. In vivo mouse studies of mutant HIF2α-overexpressing HEK293T cells injected into the flank demonstrated that these gain-of-function mutations are tumorigenic [65]. This suggests that HIF2α has the capacity to be an independent oncogenic transcription factor.
HIF2α in GBM
In vitro studies have largely focused on the potential role that HIF2α plays in glioma stem cell (GSC) biology given its expression in GSCs and not normal neural progenitor cells [54]. HIF2α, not HIF1α, is selectively upregulated in GSCs, which have been implicated in driving tumor recurrence and treatment resistance [49,54]. HIF2α may also contribute to cell division and chemotherapy resistance. HIF2α regulates class III, β-tubulin expression and may play a role in microtubule dynamics [66–68]. Flow cytometry analysis of temozolomide-resistant glioma cells identified HIF2α upregulation in all chemo-resistant cell lines with increasing HIF2α levels over time [69,70]. High levels of HIF2α expression via immunohistochemistry were also noted in recurrent disease patient samples in our studies. These observations raise the question of whether standard-of-care glioma therapies induce HIF expression or whether increased HIF expression is a marker of recurrent tumor.
Functional studies tie HIF2α activity to increasing the malignant phenotype. Knockdown of HIF2α using shRNA in GSC reduced tumorsphere formation, GSC-mediated angiogenesis in vitro and increased glioma stem-like cell apoptosis [54,69]. Mao et al. confirmed HIF2α knockdown downregulated GSC oncogenes, namely zinc finger protein 217 [71]. In vivo evidence supporting the biologic significance of HIF2α targeting was demonstrated in glioma xenograft models, wherein HIF2α knockdown delayed onset of neurologic deficits and increased survival to 158 days compared with 47 days in controls [54]. Increased HIF2α expression is observed to be present in high-grade, aggressive and therapy-resistant gliomas and reducing HIF2α expression experimentally mitigates the malignant cellular phenotype and increases animal survival.
Data supporting the biologic importance of HIF2α in patients are largely retrospective. HIF2α expression, not HIF1α, was linked to patient survival using the REMBRANDT database (n = 834) [54]. In a prospective clinical trial testing bevacizumab/irinotecan in GBM patients, carbonic anhydrase 9 (CA9) and HIF2α expression were major and significant predictors of treatment effectiveness and overall survival [72]. Patients with high CA9 and HIF2α expression had the worst survival outcomes, whereas patients with negative CA9 and negative HIF2α had the best survival outcomes [72]. These genetic studies suggest that HIF2α expression may be a target of therapeutic significance.
Therapeutic implications
Targeting transcription factors in oncology has largely been overshadowed by the development of small-molecule-targeted agents against downstream targets. Within the hypoxia pathway, a VEGF-A inhibitor, bevacizumab (Avastin) gained FDA approval for colorectal cancer in 2004 [73] and accelerated approval in 2009 followed by full approval in 2017 for recurrent GBM. Since this time, numerous drugs targeting VEGF including antibodies and receptor tyrosine kinase inhibitors have been developed including sunitinib, axitinib, sorafenib and lenvatinib [74]. Unfortunately, sustained responses to these agents have not been realized in the clinical setting, refocusing interest in transcription factor inhibition. Indeed, large drug library screens have identified potential HIF inhibitors [75,76]. Clinical development of these compounds, which include echinomycin and cardiac glycosides [77], is limited by a lack of specificity of the inhibitors. Preclinical agents with nonspecific inhibition of HIF1α have been tested in GBM without significant results including bortezomib [78], echinomycin [76], amphotericin B [79] and 2-methoxyestradiol [80]. With an improved understanding of the potential biologic significance of HIF2α, drugs targeting this transcription factor were anticipated for further development.
Identifying a specific molecule to target HIF2α began in the early 2000s with magnetic resonance spectroscopy and crystallography to define the HIF2α/ARNT-binding pocket, a configuration not formed by HIF1α or HIF3α [81,82]. An extensive small-molecule screen identified 130 potential compounds of which ultimately resulted in the development of PT2385, a first-in-class HIF2α inhibitor, currently being tested in patients with clear-cell RCC by Peloton Therapeutics Inc. [83]. A Phase I dose-finding study demonstrated a favorable safety profile with anemia and fatigue being the most common treatment-emergent adverse events and determined the recommended Phase II dose (800 mg taken by mouth twice daily) [83]. The agent is orally available, has favorable blood–brain barrier penetrating properties including 90% oral bioavailability in dogs and high brain/plasma ratio (0.9) in rats [40,84].
A multi-institution Phase II study of PT2385 in patients with recurrent GBM is ongoing within the Adult Brain Tumor Consortium – ‘Single-arm, Open-label Phase II Efficacy Study of First-in-class HIF2-Alpha Inhibitor, PT2385, for Patients with Recurrent Glioblastoma.’ The optimal timing of treatment within the standard of care for patients with gliomas has yet to be defined. Synergy with radiation is suspected given hypoxic cells tend to be radioresistant and PT2385 may target this population.
Conclusion
Hypoxia is a driver of the malignant phenotype in GBM and understanding the resultant biology provides opportunities to develop novel therapeutic approaches to address this maladaptive environmental stress. Most targeted therapeutic strategies rely on enzymes or cell surface receptors; however, targeting transcription factors are gaining interest in oncology [85]. HIF2α in gliomas is an attractive candidate as expression tracks with glioma malignancy in tumor location and tumor grade. As a transcription factor, HIF2α is upstream in the signaling pathway and inhibiting its signaling should result in simultaneously downregulating multiple targets. Additionally, since HIF2α is an upstream target, being a transcription factor, resistance mechanisms including pathway redundancies or compensatory pathways may be limited. Targeting HIF2α may disrupt the tumor cells’ response to environmental signals. HIF2α is a specific and druggable target in gliomas and deserves further thorough preclinical and clinical evaluation.
Future perspective
As of this writing, specific HIF2α inhibitors are yet to be US FDA approved. Clinical trials are currently in progress and hypoxia will be a new clinically utilized therapeutic target in oncology the next 5–10 years with the FDA approval. As seen in the development of EGFR inhibitors, a library of different compounds gaining the FDA approval is anticipated. The first cancer likely to receive the FDA indication is RCC. Other solid malignancies including pancreatic and prostate cancer are likely to follow. Targeted HIF2α inhibitors will also likely be a component of rational combinational therapy in intracranial neoplasms including GBM and paragangliomas. Patients will be assessed for hypoxia before commencing treatment to increase the likelihood of a clinical response through a combination of imaging, tumor staining and serum biomarkers. Imaging (specifically PET FMISO) will play an increasingly important role in assessing tumor hypoxia prior and during treatment as this modality is under current evaluation for predicting response to therapy and outcome. Additional biomarkers to be tested for clinical response include immunohistochemistry for CA9 and the HIFs, genetic sequencing for VHL and HIF mutations, and gene copy number assays for VHL deletion. As radiation efficacy is reduced in hypoxic states, antihypoxia therapies are likely to prove synergistic with radiotherapy and to become a component of a combinational therapy approach. The delivery of antihypoxic treatments may also evolve to locoregional rather than systemic. Resistance or nonresponders to antihypoxia treatment will also be evaluated for upregulation of escape/compensatory pathways and lead to new therapy combinations or further insights on targeting the oncogenic response. Additionally, the status of tumor initiating stem-like cells will be of interest as currently these cells are hypothesized to reside in hypoxic niches. Thus, antihypoxia therapies have the potential to have broad impact as a treatment strategy in oncology.
Executive summary.
Hypoxia-inducible factors
Hypoxia is fundamental to the malignant biology of cancer including glioblastoma (GBM).
Transcription factors, hypoxia-inducible factors (HIFs), mediate the hypoxic response including HIF1α and HIF2α.
HIF2α appears to be a specific and targetable protein upregulated in human cancer.
Mechanisms of HIF2α upregulation
HIF2α is upregulated in cancer through multiple mechanisms including hypoxia, inactivation of HIF2α regulators (i.e., von Hippel-Lindau) and HIF2α genetic gain-of-function mutations.
HIF2α in GBM
HIF2α is the hypoxia response transcription factor that is specific to glioma cells and not normal neural progenitors.
HIF2α is correlated with glioma patient survival and upregulation and downregulation directly effect tumor growth in preclinical mouse models.
Therapeutic implications
A first-in-class HIF2α inhibitor, PT2385, is under investigation in renal cell carcinoma and warrants investigation in GBM.
Footnotes
Financial & competing interests disclosure
This work was supported by a Musella Foundation Grant (JJ Renfrow). Research reported in this publication was also supported by the National Cancer Institute's Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 2.Axelson H, Fredlund E, Ovenberger M, Landberg G, Pahlman S. Hypoxia-induced dedifferentiation of tumor cells – a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin. Cell Dev. Biol. 2005;16:554–563. doi: 10.1016/j.semcdb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 4.Heddleston JM, Hitomi M, Venere M, et al. Glioma stem cell maintenance: the role of the microenvironment. Curr. Pharm. Des. 2011;17(23):2386–2401. doi: 10.2174/138161211797249260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T, Tang B, Sun X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med. J. 2017;58(3):489. doi: 10.3349/ymj.2017.58.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell N, Larion M, Giles AJ, et al. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro. Oncol. 2017;19(7):887–896. doi: 10.1093/neuonc/now258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 9.Kayama T, Yoshimoto T, Fujimoto S, Sakurai Y. Intratumoral oxygen pressure in malignant brain tumor. J. Neurosurg. 1991;74(1):55–59. doi: 10.3171/jns.1991.74.1.0055. [DOI] [PubMed] [Google Scholar]
- 10.Meixensberger J, Dings J, Kuhnigk H, Roosen K. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochir. Suppl. 1993;59:58–63. doi: 10.1007/978-3-7091-9302-0_10. [DOI] [PubMed] [Google Scholar]
- 11.Gerstner ER, Zhang Z, Fink JR, et al. ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin. Cancer Res. 2016;22:5079–5086. doi: 10.1158/1078-0432.CCR-15-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Maeda Y, Kawai N, et al. Hypoxia assessed by 18F-fluoromisonidazole positron emission tomography in newly diagnosed gliomas. Nucl. Med. Commun. 2012;33(6):621–625. doi: 10.1097/MNM.0b013e3283529984. [DOI] [PubMed] [Google Scholar]
- 13.Dong S, Nutt CL, Betensky RA, et al. Histology-based expression profiling yields novel prognostic markers in human glioblastoma. J. Neuropathol. Exp. Neurol. 2005;64(11):948–955. doi: 10.1097/01.jnen.0000186940.14779.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery. 2002;51(1):2–12. doi: 10.1097/00006123-200207000-00002. discussion 12–13. [DOI] [PubMed] [Google Scholar]
- 15.Brat DJ, Mapstone TB. Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Ann. Intern. Med. 2003;138(8):659–668. doi: 10.7326/0003-4819-138-8-200304150-00014. [DOI] [PubMed] [Google Scholar]
- 16.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses hypoxia as a trigger for promoting glioblastoma stem cell formation and provides in vivo evidence for hypoxia-inducible factor 2α (HIF2α)-positive effect on flank glioma growth.
- 17.Ponnala S, Veeravalli KK, Chetty C, Dinh DH, Rao JS. Regulation of DNA repair mechanism in human glioma xenograft cells both in vitro and in vivo in nude mice. PLoS ONE. 2011;6(10):e26191. doi: 10.1371/journal.pone.0026191. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]; •• Summarizes the malignant effects of hypoxia in cancer and outlines antihypoxia therapeutic approaches including bioreductive prodrugs and molecular inhibitors of the hypoxia pathway.
- 20.Rey S, Schito L, Wouters BG, Eliasof S, Kerbel RS. Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer. 2017;3(7):529–541. doi: 10.1016/j.trecan.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Schöning JP, Monteiro M, Gu W. Drug resistance and cancer stem cells: the shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin. Exp. Pharmacol. Physiol. 2017;44(2):153–161. doi: 10.1111/1440-1681.12693. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the earliest descriptions of HIFs during their discovery.
- 23.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology. 2010;25(5):272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 24.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7(3):205–213. [PMC free article] [PubMed] [Google Scholar]
- 25.Slemc L, Kunej T. Transcription factor HIF1A: downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumor Biol. 2016;37(11):14851–14861. doi: 10.1007/s13277-016-5331-4. [DOI] [PubMed] [Google Scholar]
- 26.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol. Pharmacol. 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 27.Denko NC, Fontana LA, Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22(37):5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 28.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza GL, Agani F, Booth G, et al. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51(2):553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 30.Mole DR, Blancher C, Copley RR, et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009;284(25):16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Given emerging evidence for biologically distinct functions of HIF1α versus HIF2α genome-wide chromatin immunoprecipitation gene identified individual gene transcription targets specific to each transcription factor. Understanding the genes implicated in each HIF will aid to the biologic understanding of their unique roles in diseases including cancer.
- 31.Takeda N, O'Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24(5):491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 2014;13(11):852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl. 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 34.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 35.Littlewood TJ. The impact of hemoglobin levels on treatment outcomes in patients with cancer. Semin. Oncol. 2001;28(2 Suppl., 8):49–53. doi: 10.1016/s0093-7754(01)90213-1. [DOI] [PubMed] [Google Scholar]
- 36.Jeong W, Rapisarda A, Park SR, et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2014;73(2):343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibes R, Falchook GS, Von Hoff DD, et al. Results from a Phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1α. J. Clin. Oncol. 2010;28(15 Suppl.):3076–3076. [Google Scholar]
- 38.López-Lázaro M. Hypoxia-inducible factor 1 as a possible target for cancer chemoprevention. Cancer Epidemiol. Biomarkers Prev. 2006;15(12):2332–2335. doi: 10.1158/1055-9965.EPI-06-0369. [DOI] [PubMed] [Google Scholar]
- 39.Soni S, Padwad YS. HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. 2017;56(4):503–515. doi: 10.1080/0284186X.2017.1301680. [DOI] [PubMed] [Google Scholar]
- 40.Wallace E. 12th International VHL Medical Symposium. MA, USA: 7–9 April 2016. PT2385: HIF-2a antagonist for the treatment of VHL mutant ccRCC. Proceedings of. [Google Scholar]
- 41.Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin. Cancer Res. 2008;14(15):4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowey CL, Rathmell WK. VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr. Oncol. Rep. 2009;11(2):94–101. doi: 10.1007/s11912-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013;45(8):860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 44.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 1994;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 45.Toledo RA. New HIF2α inhibitors: potential implications as therapeutics for advanced pheochromocytomas and paragangliomas. Endocr. Relat. Cancer. 2017;24(9):C9–C19. doi: 10.1530/ERC-16-0479. [DOI] [PubMed] [Google Scholar]; •• Discusses cancers with known HIF2α mutations and the recently developed, specific HIF2α inhibitors (PT2385 and PT2399) under investigation for renal cell carcinoma and suggests these drugs may be of use in other malignancies with gain-of-function HIF2α mutations including pheochromocytoma and paragranglioma.
- 46.Schödel J, Grampp S, Maher ER, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur. Urol. 2016;69(4):646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Liu M, Wei Y, et al. Overexpression of von Hippel-Lindau tumor suppressor protein and antisense HIF-1alpha eradicates gliomas. Cancer Gene Ther. 2006;13(4):428–435. doi: 10.1038/sj.cgt.7700907. [DOI] [PubMed] [Google Scholar]
- 48.Rong Y, Durden DL, Van Meir EG, Brat DJ. “Pseudopalisading” necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006;65(6):529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 49.D'Alessio A, Proietti G, Lama G, et al. Analysis of angiogenesis related factors in glioblastoma, peritumoral tissue and their derived cancer stem cells. Oncotarget. 2016;7(48):78541–78556. doi: 10.18632/oncotarget.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper LAD, Gutman DA, Chisolm C, et al. The tumor microenvironment strongly impacts master transcriptional regulators and gene expression class of glioblastoma. Am. J. Pathol. 2012;180(5):2108–2119. doi: 10.1016/j.ajpath.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Tso C-L, Shintaku P, Chen J, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol. Cancer Res. 2006;4(9):607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y-W, Koul D, Kim SH, et al. Identification of prognostic gene signatures of glioblastoma: a study based on TCGA data analysis. Neuro. Oncol. 2013;15(7):829–839. doi: 10.1093/neuonc/not024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In vitro and in vivo evidences of HIF2α knockdown effect glioma stem cell and tumor growth along with animal survival.
- 55.Pietras A, Johnsson AS, Påhlman S. The HIF-2α-driven pseudo-hypoxic phenotype in tumor aggressiveness, differentiation, and vascularization. Curr. Top. Microbiol. Immunol. 2010;345:1–20. doi: 10.1007/82_2010_72. [DOI] [PubMed] [Google Scholar]
- 56.Pietras A, Katz AM, Ekström EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rimkus TK, Carpenter RL, Sirkisoon SR, et al. Truncated glioma-associated oncogene homolog 1 (tGLI1) mediates mesenchymal glioblastoma via transcriptional activation of CD44. Cancer Res. 2018;78(10):2589–2600. doi: 10.1158/0008-5472.CAN-17-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson E, Grassi ES, Pantazopoulou V, et al. CD44 interacts with HIF-2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Rep. 2017;20(7):1641–1653. doi: 10.1016/j.celrep.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 59.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl Acad. Sci. USA. 2014;111(20):E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilkes DM, Xiang L, Lee SJ, et al. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Natl Acad. Sci. USA. 2014;111(3):E384–E393. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Gilkes DM, Chaturvedi P, Bajpai S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73(11):3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Gilkes DM, Bajpai S, Wong CC, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 2013;11(5):456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Wang X, Dong J, Jia L, et al. HIF-2-dependent expression of stem cell factor promotes metastasis in hepatocellular carcinoma. Cancer Lett. 2017;393:113–124. doi: 10.1016/j.canlet.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang Z, Yang C, Ryska A, et al. HIF2A gain-of-function mutations detected in duodenal gangliocytic paraganglioma. Endocr. Relat. Cancer. 2016;23(5):L13–L16. doi: 10.1530/ERC-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toledo RA, Qin Y, Srikantan S, et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr. Relat. Cancer. 2013;20(3):349–359. doi: 10.1530/ERC-13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrandina G, Zannoni GF, Martinelli E, et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006;12(9):2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 67.Bordji K, Grandval A, Cuhna-Alves L, Lechapt-Zalcman E, Bernaudin M. Hypoxia-inducible factor-2α (HIF-2α) but not HIF-1α, is essential for hypoxic induction of class III β-tubulin expression in human glioblastoma cells. FEBS J. 2014;281(23):5220–5236. doi: 10.1111/febs.13062. [DOI] [PubMed] [Google Scholar]; • HIF1α dominates the early literature in hypoxia research, including hypoxia in oncology. An emerging role is being realized for HIF2α over HIF1α and this report provides evidence supporting HIF2α in glioma malignment behavior.
- 68.Katsetos CD, Draber P, Kavallaris M. Targeting βIII-tubulin in glioblastoma multiforme: from cell biology and histopathology to cancer therapeutics. Anticancer. Agents Med. Chem. 2011;11(8):719–728. doi: 10.2174/187152011797378760. [DOI] [PubMed] [Google Scholar]
- 69.Lee G, Auffinger B, Guo D, et al. Dedifferentiation of glioma cells to glioma stem-like cells by therapeutic stress-induced HIF signaling in the recurrent GBM model. Mol. Cancer Ther. 2016;15(12):3064–3076. doi: 10.1158/1535-7163.MCT-15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auffinger B, Tobias AL, Han Y, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao X, Yan M, Xue X, et al. Overexpression of ZNF217 in glioblastoma contributes to the maintenance of glioma stem cells regulated by hypoxia-inducible factors. Lab. Investig. 2011;91(7):1068–1078. doi: 10.1038/labinvest.2011.56. [DOI] [PubMed] [Google Scholar]
- 72.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J. Clin. Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin. Colorectal Cancer. 2004;4(Suppl. 2):S62–S68. doi: 10.3816/ccc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 74.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016;15(6):385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl Acad. Sci. USA. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kong D, Park EJ, Stephen AG, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65(19):9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 77.Xia Y, Choi H-K, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 78.Kaluz S, Kaluzova M, Stanbridge EJ. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1 C-terminal activation domain. Mol. Cell. Biol. 2006;26(15):5895–5907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeo E-J, Ryu J-H, Cho Y-S, et al. Amphotericin B blunts erythropoietin response to hypoxia by reinforcing FIH-mediated repression of HIF-1. Blood. 2006;107(3):916–923. doi: 10.1182/blood-2005-06-2564. [DOI] [PubMed] [Google Scholar]
- 80.Kirkpatrick J, Desjardins A, Quinn J, et al. Phase II open-label, safety, pharmacokinetic and efficacy study of 2-methoxyestradiol nanocrystal colloidal dispersion administered orally to patients with recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(18 Suppl.):2065–2065. [Google Scholar]
- 81.Erbel PJA, Card PB, Karakuzu O, Bruick RK, Gardner KH. Structural basis for PAS domain heterodimerization in the basic helix-loop-helix-PAS transcription factor hypoxia-inducible factor. Proc. Natl Acad. Sci. USA. 2003;100(26):15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheuermann TH, Tomchick DR, Machius M, Guo Y, Bruick RK, Gardner KH. Artificial ligand binding within the HIF2 PAS-B domain of the HIF2 transcription factor. Proc. Natl Acad. Sci. USA. 2009;106(2):450–455. doi: 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Courtney KD, Infante JR, Lam ET, et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J. Clin. Oncol. 2018;36(9):867–874. doi: 10.1200/JCO.2017.74.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallace EM, Rizzi JP, Han G, et al. A small-molecule antagonist of HIF2a is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76(18):5491–5500. doi: 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]; •• Renal cell carcinoma is the first malignancy for testing the newly developed HIF2α inhibitor, PT2385 and this report summarizes the preclinical evidence that led to clinical trial testing.
- 85.Bhagwat AS, Vakoc CR. Targeting transcription factors in cancer. Trends Cancer. 2015;1(1):53–65. doi: 10.1016/j.trecan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]