Abstract
Aim:
Serum uric acid (UA) is associated with many health conditions, including kidney, cardiovascular, and metabolic disorders. We examined the validity and stability of salivary UA as a noninvasive measure of serum UA.
Materials & methods:
Using serum and salivary UA data from healthy adults (n = 99), we examined the UA serum–saliva correlation, and UA associations with adiponectin and C-reactive protein. Using longitudinal data from young adults (n = 182), we examined salivary UA stability.
Results:
We found robust positive serum–saliva correlations for UA. UA and adiponectin were inversely related in serum and saliva. Salivary UA was relatively stable; 62–66% of variance could be attributed to a latent trait-like component.
Conclusion:
Salivary UA may be an important biomarker indexing health and disease risk.
Keywords: : adiponectin, biomarker, body mass index, C-reactive protein, latent state-trait modeling, saliva, serum, uric acid
Technical advances enable the measurement of a broad spectrum of biologic markers and analytes in oral fluids. These advances create opportunities to noninvasively assess individual differences in the psychobiology of stress, inflammation, reproductive hormones, and infectious disease exposure (see [1] for review). Among the more recent advances is a series of studies revealing the capacity to measure uric acid (UA) in saliva [2–5]. UA is produced during the breakdown of purine nucleotides. UA has both anti- and pro-oxidant effects on a wide range of peripheral tissues and the central nervous system [6–8], making relations between systemic UA levels and disease complex. However, many studies reveal that high levels of UA (hyperuricemia) are associated with hypertension, stroke, and chronic kidney disease, as well as increased risk for metabolic syndrome, insulin-resistance, obesity, and Type-2 diabetes [6,7,9–11]. Elevated levels of UA may be caused by high dietary intake of high-fructose corn syrup and table sugar, and/or reduced excretion from the kidneys [7,9,12,13]. Emerging literature, however, also links elevated levels of serum UA to beneficial outcomes in neurodegenerative disorders, including age-related mild cognitive impairment, and Alzheimer's, Parkinson's and Huntington's disease [14,15]. UA has also been associated with emotion-related psychopathology, such as anxiety and mood disorders [16–20], disinhibition, impulsivity, and hyperactivity [21,22]. There is also some evidence from functional magnetic resonance imaging studies demonstrating UA is involved in regulating key components of the psychobiology of the stress response [23].
Given UA's association with a wide spectrum of conditions, disorders, and health behaviors, a noninvasive measure of UA would be particularly useful in biobehavioral health and clinical research. A noninvasive method for assessing UA-related risk could increase participation in screening, monitoring, and treatment programs. A handful of studies confirm a modest-to-strong positive association between circulating and salivary levels of UA [5,24]. Moreover, studies reveal that salivary UA levels correlate in the same direction as circulating UA with established measures of cardiovascular risk, such as blood pressure, triglyceride levels, high-density lipoprotein, fasting blood glucose levels, and body mass index (BMI), and may be useful for monitoring UA levels during dialysis [2,25,26]. Taken together, these findings highlight the potential for oral fluid to be a noninvasive specimen for examining differences and intra-individual change in UA.
To our knowledge, neither the validity nor stability of salivary UA has been rigorously examined by previous investigations [3–5]. Using a sample of healthy young adults, we address these issues by examining the association between salivary and systemic UA, and interrogating the strength and nature of this correlation when adjusting for potential sources of confounding, such as blood contamination in saliva, salivary flow rate, and indices of oral health and inflammation. We thoroughly examine associations of salivary UA with oral health and inflammation because positive associations between circulating UA and inflammatory markers such as IL-6, TNFα, IL-1β, and C-reactive protein (CRP) have been reported in the literature [6,8,27]. We further examine the relations between subject characteristics, such as sex, age, race, and BMI, and salivary UA levels. We also examine the short- and long-term stability of salivary UA and decompose variation in salivary UA into a stable person-specific component, a changing situation-specific component, and measurement error in a sample of healthy young adults.
Given the strong associations between UA and cardiovascular and metabolic disorders reported in the literature [28–33], we also examine associations between salivary UA and other biomarkers associated with these conditions. Specifically, we examine relations between salivary UA and adiponectin, a hormone that modulates glucose and lipid metabolism in insulin-sensitive tissues [34], and CRP, an indicator of general inflammation and marker of cardiovascular disease risk [35]. Based on their relations in serum, we expected inverse relations between adiponectin and UA and positive relations between CRP and UA in saliva [6,7,36–38].
Materials & methods
Participants & procedures
To examine the validity of salivary UA as a marker of systemic UA and associations between salivary UA and cardiovascular and metabolic biomarkers, we used data from a sample of 99 healthy young adults (53 males; aged 18–36 years [mean (M): 23.76 years, standard deviation (SD): 4.57]). These participants were mostly white/Caucasian (n = 45; 45%); 18 were black/African–American, five were South East Indian, five were native Hawaiian and 27 were of other or multiple races. Eligible participants were young adults not currently under a physician's care for any acute or chronic medical condition, not taking prescription or over the counter medication (excluding oral contraceptives), without open wounds and sores in the mouth, and without recent history of dental surgery.
Participants provided written informed consent and the Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved all study procedures. Data were collected in 2012 and 2013. During a single 45-min assessment, participants completed a demographic survey and provided blood and saliva samples. Participants reported on their oral hygiene practices; they indicated their frequency of flossing and brushing their teeth, and if their saliva had a red/pinkish color when they brushed their teeth (yes/no). Participants were compensated US$50 for their time.
To examine the short- and long-term stability of salivary UA, we used data from a sample of 205 undergraduate student members of the Arizona State University marching band. Written informed consent was obtained from all participants. Study procedures were approved by the Arizona State University Institutional Review Board. Data were collected on two days approximately two months apart (day 1 in September and day 2 in November, 2014; see [39] for details). On each day of data collection, participants provided two whole unstimulated saliva samples; one sample was collected at 3:00 PM (afternoon sample) and one at 6:00 PM (evening sample). On each data collection day, participants also completed a 30-minute survey that included questions about participant sex (male/female), age (years), racial/ethnic background (African–American/black, Asian–American, white, Hispanic or Latino, native American and other), and height and weight. Twenty-three participants were missing salivary UA data at all time points and were excluded from the analyses. Overall, these participants were not different than the 182 participants in the study sample on measures of age, sex, race/ethnicity, and BMI. The study sample was 63% white, 20% Hispanic/Latino, 4% African–American/Black, 5% Asian–American, 4% Native American, and 3% other race/ethnicities or missing race. Race/ethnicity was not evenly distributed, so this variable was dichotomized (white/nonwhite). Participant BMI was computed as weight (lb)/height (in) × 703.
Biospecimen collection
Blood was drawn by venipuncture into 2 ml lavender/EDTA tubes. EDTA/whole blood was mixed well by inversion and spun at 900 × g for 15 min. The top plasma layer was transferred into 4 × 1 ml aliquots and snap frozen and stored at -80°C. Additionally, a SST Tiger serum separator tube (BD, Becton-Dickinson) of blood was drawn for serum isolation. Serum was mixed by inversion five-times and allowed to clot at room temperature for 30 min (no longer than 1 h). After clot activation, serum was centrifuged at 2000 r.p.m. for 10 min. Serum was removed and aliquoted into 2 ml cryovial tubes and frozen at -80°C until assay.
Following Granger and colleagues [40], whole unstimulated saliva was collected by passive drool and aliquoted into 2 ml cryogenic vials. Saliva samples were stored at -80°C until assayed.
Biomarker determination
Saliva was assayed in duplicate for UA using a commercially available colorimetric enzymatic reaction kit designed for the use with saliva (Catalog #1–3802, Salimetrics, CA, USA). The amount of UA in the sample was assessed by measuring the production of red chromogen during the assay process. The average inter- and intra-assay coefficients of variation were 2.0 and 2.6%, respectively. Serum UA levels were determined using Pointe Scientific UA enzymatic assay kit (Catalog #U7581, MI, USA). The average inter- and intra-assay coefficients of variation were 4.1 and 5.5%, respectively.
Salivary and serum cytokines were measured using 96-well format multiplex electrochemiluminescence immunoassays manufactured by Meso Scale Discovery (MSD, MD, USA). The assay was run following the manufacturer's protocol and using standard diluent (MSD #R51BB).
Salivary MMP-8 was measured as a marker of oral health [41] using a commercially available immunoassay development kit (Catalog #DY908, DuoSet ELISA, R&D Systems, MN, USA) following the manufacturer's guidelines.
Saliva samples were assayed for blood contamination using a commercially available enzyme immunoassay kit for transferrin (Catalog #1-1302, Salimetrics, PA, USA) [42].
Salivary total protein was determined using a commercial protein assay kit (Catalog #23225, Pierce™ BCA ThermoScientific) without modification to the manufacturer's guidelines for the microplate procedure.
Salivary adiponectin was measured using multiplex electrochemiluminescence assay (MSD, MD, USA). Serum adiponectin was measured using a commercial kit (Catalog #EZHADP-61K, Linco) without modification to the manufacturer's protocol.
Saliva was assayed for CRP using a commercially available immunoassay without modification to the manufacturer's protocol (Salimetrics, CA, USA) [43]. Serum CRP was determined using the guidelines outlined in a commercial enzyme immunoassay kit by ALPCO Diagnostics, Inc. (Catalog #30–9710-S, MN, USA).
Additional details on biomarker determination are provided in the Supplementary Methods.
Preanalytic issues
For serum and saliva biomarker data from the sample of 99 young adults, undetectable samples were changed to zero to examine descriptive statistics and missing for statistical analyses. In addition, concentrations greater than four standard deviations from the analyte mean were Winsorized, and analytes with skewness greater than two were log transformed to improve normality. Data from the saliva samples of the 182 participants were Winsorized at three standard deviations above the mean, and all data met assumptions for normality [44].
Analytic plan
Validity of salivary UA as a marker of systemic UA activity: paired samples t-tests were used to compare UA concentrations in serum and saliva, and the unadjusted correlation between serum and salivary UA was examined using Pearson correlation. To further interrogate the serum–saliva correlation for UA, partial correlations adjusting for biologic indicators of blood leakage into saliva (transferrin), total protein, oral inflammatory activity (cytokines) and oral health biomarkers (MMP-8) were performed.
Associations between salivary UA and measures of oral and systemic inflammatory activity were examined with Pearson correlations between salivary UA and each cytokine in saliva and serum separately. We conducted a principal components analysis (PCA) to determine if the four salivary cytokines (IL-1β, IL-6, IL-8, and TNFα) could be combined into a single composite measure representing oral inflammatory activity. A single factor solution for the four salivary cytokines resulted in high component loadings (0.83–0.92) and accounted for 76% of the variance in analyte determinations. Therefore, a composite score representing oral inflammatory activity was derived for each participant using the regression method. A Pearson correlation was then used to assess the relation between salivary UA and oral inflammatory activity using the composite score. Furthermore, partial correlations were used to examine if transferrin and total protein confounded the association between salivary UA and oral inflammatory activity.
The relation between oral health and salivary UA was examined using both self-reported and biologic measures of oral health. Pearson correlations examined relations between salivary UA and self-reported frequency of flossing per week. A Kruskal–Wallis test examined differences in salivary UA by the frequency of brushing per day, and a t-test examined whether salivary UA levels were different between participants who reported red/pink tinted saliva when brushing and those that did not. A Pearson correlation also examined the association between salivary UA and MMP-8, a biologic marker of oral health.
We examined the associations between salivary UA and sex, age, race/ethnicity and BMI using data from the sample of 182 individuals because these data allowed for both simple and advanced statistical testing of these associations. First, Wald tests assessed mean differences in each salivary UA sample concentration by sex and race/ethnicity, and Pearson correlations assessed relations between salivary UA and age and BMI. To further examine these associations, advanced statistical modeling (latent state-trait [LST] and structural equation modeling discussed below) was used to examine associations between BMI and sex with stable, person-specific salivary UA components. The full information maximum likelihood estimator was used to account for missing data.
Short- and long-term stability of salivary UA: Preliminary analyses examined the stability of UA determinations using bivariate correlations. We assessed the short-term stability of salivary UA using Pearson correlations examining the association between salivary UA determinations from the same day (samples three hours apart), and long-term stability using Pearson correlations examining the association between afternoon and evening determinations from separate days of data collection (samples 56 days apart).
To further examine the stability of salivary UA, we used latent state-trait (LST) modeling, an advanced statistical approach, to determine the proportion of variance in salivary UA attributable to a stable, person-specific (‘trait’) component, a changing, occasion-specific (‘state’) component, and an error component. We examined factor invariance (FI) to construct an LST model and used multiple fit indices to determine the appropriate model for salivary UA. We accepted the model fit as adequate when at least three of the following criteria were met: a p-value for the χ2 test greater than 0.05; an SRMR close to or less than 0.08; an RMSEA close to or less than 0.06; and a CFI close to or greater than 0.95 [45,46]. The full information maximum likelihood estimator was used to account for missing data (additional details on LST analyses and procedures can be found in the Supplementary Methods).
Salivary UA and cardiovascular and metabolic biomarkers: Relations between UA and adiponectin and CRP were examined using Pearson correlations. These correlations were conducted with both serum and salivary determinations of UA, adiponectin and CRP. Partial correlations controlling for blood leakage, total protein, oral inflammatory activity, and oral health were also examined.
The criterion for significance for nondirectional tests was two-tailed, and directional tests (UA with adiponectin, and UA with CRP) were one-tailed; p < 0.05. Analyses were conducted in Mplus 7.11 (Muthén and Muthén, 2012, CA, USA) and SPSS 23 (IBM Corp., 2014, IBM SPSS Statistics for Windows, Version 23.0, NY, USA).
Results
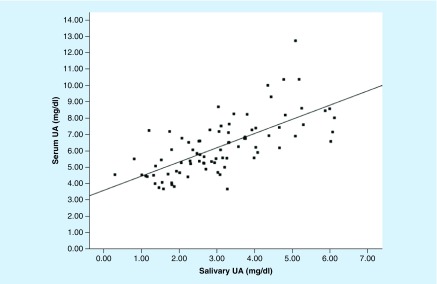
Validity of salivary UA as a marker of systemic UA activity: Levels of UA were higher in serum than in saliva (Table 1; t[81] = 22.84; p < 0.01), and UA determinations in serum and saliva were significantly, positively correlated (r[81] = 0.69; p < 0.01; Cohen's d = 1.91; Figure 1). The UA serum–saliva correlation remained significant in partial correlations controlling for transferrin (r[77] = 0.68; p < 0.01; d = 1.85), total protein (r[80] = 0.70; p < 0.01; d = 1.96), the oral inflammatory activity composite score (r[80] = 0.69; p < 0.01; d = 1.91) and MMP-8 (r[68] = 0.73; p < 0.01; d = 2.14).
Table 1. . Descriptive statistics of all analytes included in validity analyses in healthy young adults.
| Analyte | Mean | SD | Minimum | Maximum | n |
|---|---|---|---|---|---|
| Saliva | |||||
| Uric acid (mg/dl) | 3.01 | 1.41 | 0.00 | 6.12 | 97 |
| Adiponectin (ng/ml) | 9.87 | 10.75 | 0.33 | 59.22 | 96 |
| MMP-8 (pg/ml) | 62,815.17 | 40,410.80 | 5380.34 | 169,479.62 | 84 |
| IL-1β (pg/ml) | 264.04 | 255.85 | 9.10 | 1591.18 | 99 |
| IL-6 (pg/ml) | 10.09 | 16.72 | 0.35 | 120.93 | 99 |
| IL-8 (pg/ml) | 935.77 | 714.76 | 109.11 | 3374.14 | 99 |
| TNF-α (pg/ml) | 5.13 | 11.95 | 0.16 | 110.67 | 99 |
| Total protein (mg/ml) | 700.76 | 364.09 | 148.50 | 1911.20 | 99 |
| Transferrin (mg/dl) | 0.72 | 0.85 | 0.00 | 4.69 | 96 |
| CRP (pg/ml) | 3878.29 | 4235.14 | 808.84 | 23,286.58 | 76 |
| Serum | |||||
| Uric acid (mg/dl) | 6.24 | 1.73 | 3.67 | 12.74 | 85 |
| Adiponectin (μg/ml) | 11.06 | 5.07 | 2.82 | 32.61 | 99 |
| IL-1β (pg/ml) | 1.20 | 0.77 | 0.28 | 6.23 | 99 |
| IL-6 (pg/ml) | 1.25 | 2.45 | 0.17 | 18.90 | 99 |
| IL-8 (pg/ml) | 15.43 | 33.29 | 2.26 | 246.20 | 99 |
| TNF-α (pg/ml) | 4.77 | 12.55 | 0.93 | 111.72 | 99 |
| CRP (μg/ml) | 2.67 | 6.04 | 0.02 | 50.56 | 99 |
Two salivary uric acid and three transferrin determinations were not detectable (i.e., below the lower limit of detection) and set to zero for the purpose of these descriptive statistics only. Three salivary adiponectin determinations are missing due to insufficient saliva samples. Fourteen serum uric acid samples are missing due to sample quality or type (i.e., whole blood vs. serum). Fifteen MMP-8 and 23 salivary CRP determinations had CVs >15% and were set as missing in all analyses.
CRP: C-reactive protein; CV: Coefficient of variation; IL-1β: interleukin-1β; IL-6: interleukin-6; IL-8: interleukin-8; MMP-8: Matrix metallopeptidase 8; SD: Standard deviation; TNF-α: tumor necrosis factor alpha.
Figure 1. . Scatterplot of salivary and serum concentrations of uric acid in healthy young adults (n = 83).
Concentrations of salivary and serum uric acid were positively correlated (R2 = 0.48; p < 0.01).
UA: Uric acid.
Salivary UA was not associated with any of the four cytokine determinations in either serum or saliva. There was also no association between salivary UA and the oral inflammatory activity composite score, and this relation remained nonsignificant when controlling for transferrin and total protein in separate analyses. Salivary UA was also not significantly related to any oral health measure, including salivary MMP-8, self-reported brushing and flossing frequencies, and reports of pink/red saliva when brushing.
The average age of the participants used to examine salivary UA associations with age, sex, race/ethnicity and BMI was 20.05 years (SD: 1.57, range: 18–31) and the sample was 46% male and 63% white. BMI ranged from 15.55 to 46.59 (day 1: M: 24.26, SD: 5.26; day 2: M: 24.53, SD: 5.23), and did not differ significantly between males (Mday1: 24.53, SDday1: 5.35; Mday2: 24.89, SDday2: 5.28) and females (Mday1: 24.02, SDday1: 5.19; Mday2: 24.19, SDday2: 5.19).
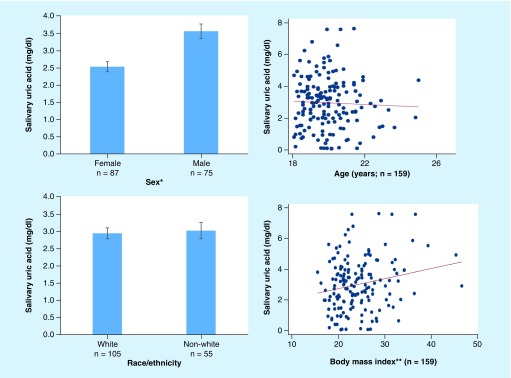
Pearson correlations and Wald tests showed salivary UA was not associated with age and did not vary by race/ethnicity (Figure 2). Males had significantly higher mean levels of salivary UA than females (day 1 afternoon sample: MMales: 3.45 mg/dl, MFemales: 2.52 mg/dl, Wald[1] = 13.66; p < 0.001; evening sample: MMales: 3.18 mg/dl, MFemales: 2.19 mg/dl, Wald[1] = 13.66; p < 0.001; day 2 afternoon sample: MMales: 4.29 mg/dl, MFemales: 2.88 mg/dl, Wald[1] = 20.17; p < 0.001; evening sample: MMales: 3.51 mg/dl, MFemales: 2.44 mg/dl, Wald[1] = 16.14; p < 0.001; Figure 2). In general, BMI was positively correlated with salivary UA levels (day 1 afternoon sample: r[180] = 0.21; p < 0.01; evening sample: r[180] = 0.07; p = 0.35; day 2 afternoon sample: r[180] = 0.24; p < 0.01; evening sample (r[180] = 0.15; p < 0.05; Figure 2).
Figure 2. . Salivary uric acid and participant characteristics in healthy young adults.
Salivary uric acid was higher in males than females and positively correlated with BMI. For illustrative purposes, all measurements are from the first day of data collection and the afternoon saliva sample. The relation between BMI and salivary UA was not consistent across all saliva samples. See text for full results.
*Wald(1) = 13.66; p < 0.001; **Pearson's r(180) = 0.21; p < 0.01.
These findings were confirmed using advanced modeling methods. The SEM model used had adequate fit (χ2; [41] = 61.64; p < 0.05; RMSEA = 0.05 [90% CI: 0.02–0.08]; CFI: 0.99; SRMR = 0.04). Results showed that BMI was significantly positively associated with stable, person-specific, trait salivary UA (b = 0.05; p < 0.05; β = 0.19). Sex was also significantly related to stable, person-specific, trait salivary UA (b = -0.89; p < 0.01; β = -0.33) with males exhibiting higher levels of salivary UA than females. Overall, sex and BMI accounted for approximately 15% of the variance in stable, person-specific, trait salivary UA (R2 = 0.15; p < 0.01).
Short- and long-term stability of salivary UA: Salivary UA levels were positively correlated across the day (day 1: r[180] = 0.68; p < 0.001; day 2: r[180] = 0.69; p < 0.001; descriptive statistics shown in Table 2). Salivary UA levels were also positively correlated across days approximately 2 months apart (afternoon samples: r[180] = 0.73; p < 0.001; evening samples: r[180] = 0.49; p < 0.001).
Table 2. . Descriptive statistics for salivary uric acid collected at four time points from healthy young adults for stability analyses (mg/dl).
| Saliva sample | Mean | SD | Minimum | Maximum | n |
|---|---|---|---|---|---|
| Day 1 Afternoon Evening |
2.98 2.69 |
1.68 1.76 |
0.08 0.03 |
7.63 7.56 |
163 164 |
| Day 2 Afternoon Evening |
3.55 2.93 |
2.13 1.77 |
0.03 0.01 |
9.94 8.03 |
167 174 |
Afternoon and evening saliva samples were 3 hours apart. Days 1 and 2 were 56 days apart. n = 182; missing data were addressed using a full information maximum likelihood estimator in the final statistical models.
SD: Standard deviation.
Advanced statistical modeling provided an LST model with adequate fit that allowed us to examine the proportion of salivary UA variance attributable to a stable, person-specific trait component, a changing state component and measurement error (additional details provided in Supplementary Results, Supplementary Table 1 and Supplementary Figure 1). Results indicated the majority (62–68%) of the variance in salivary UA levels was attributed to stable, person-specific trait salivary UA and 31–37% of the variance was attributed to changing state salivary UA. Trait and state components of salivary UA accounted for over 99% of the variance in salivary UA with less than 1% of error variance.
Salivary UA and cardiovascular and metabolic biomarkers: There were significant inverse correlations between salivary UA and serum adiponectin (r[95] = -0.25; p < 0.01; d = 0.52), and between serum UA and serum and salivary adiponectin (r[85] = -0.34; p < 0.01; d = 0.72; r(83) = -0.22; p < 0.05; d = 0.45, respectively). The bivariate association between salivary UA and salivary adiponectin was not significant. Partial correlations controlling for transferrin, total protein and MMP-8 (separately) revealed similar relations as the unadjusted correlations (salivary UA and serum adiponectin: r's[80–94] = -0.26 to -0.24; p-values < 0.05, d's = 0.49–0.54; serum UA and serum adiponectin: r's[66–78] = -0.39 to -0.30; p-values <0.01, d's = 0.68–0.87; serum UA and salivary adiponectin: controlling for transferrin – nonsignificant, controlling for total protein r[78] = -0.21; p < 0.05, d = 0.43, controlling for MMP-8 r[66] = -0.34; p < 0.01; d = 0.72; salivary UA and salivary adiponectin: controlling for transferrin – nonsignificant, controlling for total protein – nonsignificant, controlling for MMP-8 r[64] = -0.22; p < 0.05, d = 0.45). The partial correlation between salivary UA and salivary adiponectin was also significant after controlling for the oral inflammatory activity composite score (r[76] = -0.20; p < 0.05; d = 0.41; Figure 3). Relations between salivary UA and serum adiponectin and between serum UA and serum and salivary adiponectin remained significant after controlling for the oral inflammatory activity composite score (Figure 3). Serum and salivary adiponectin were also significantly positively related (r[94] = 0.39; p < 0.01; d = 0.84) and remained significantly correlated after controlling for the oral inflammatory activity composite score (Figure 3).
Figure 3. . Relations between uric acid and adiponectin in both saliva and serum controlling for oral inflammatory activity.
Values along the arrows show Pearson's partial correlation coefficients for the measures connected by the arrows controlling for oral inflammation. Among participants with complete uric acid, adiponectin and oral inflammation data (n = 81), uric acid and adiponectin were inversely correlated regardless of whether these analytes were measured in serum or saliva. All partial correlations are one-tailed (df = 78).
**p ≤ 0.01; *p < 0.05.
Salivary UA was not associated with salivary or serum CRP. Similarly, serum UA was not associated with salivary or serum CRP. These relations remained not significant when controlling for transferrin, total protein, the oral inflammatory activity composite score and MMP-8 in separate models.
Discussion
Findings from these studies provide new insight and support for salivary UA as a valid, stable and useful index of systemic UA activity. In our sample of healthy young adults, we found a robust, positive serum–saliva correlation for UA. Although the salivary UA levels and associations with serum UA found in our study are consistent with previous reports of UA in serum and saliva [2–5,23], our findings provide novel evidence regarding the strength of the serum–saliva correlation for UA. Importantly, we observed that neither the direction nor the magnitude of this association was significantly influenced by salivary total protein, blood leakage into saliva, proinflammatory cytokine activity, or biomarkers related to oral health. We also found strong positive correlations between salivary UA levels within and across days in healthy young adults, suggesting that salivary UA levels are relatively stable within individuals over time. Advanced statistical modeling further confirmed these results revealing that the majority of the variance in salivary UA levels could be estimated using a latent trait component that is stable and person-specific.
Consistent with previous findings, we found differences in salivary UA between males and females and by BMI [2,16,47]. BMI was positively associated with salivary UA levels and males displayed higher salivary UA levels than females. Furthermore, BMI and sex were significantly associated with stable, person-specific and trait components of salivary UA. To the best of our knowledge, this is the first demonstration that BMI and sex are associated with a stable trait-like component of salivary UA. Taken together, the findings suggest that the serum–saliva correlation for UA is robust and salivary UA may have utility as a noninvasive means of monitoring circulating UA.
To our knowledge, this is the first study to examine the influence of the oral immune environment and oral health on salivary UA. Salivary UA was not associated with inflammatory cytokines, MMP-8, a biomarker of oral health [41], nor self-reported measures of oral hygiene practices. The finding that salivary UA levels are not confounded by inflammatory activity in the oral cavity is important for salivary bioscience research focusing on salivary UA as a marker of cardiometabolic risk. Unlike many commonly investigated salivary analytes (e.g., cortisol), the serum–saliva association for most cytokines is negligible, and the vast majority of variation in salivary cytokine levels is produced locally in the oral cavity [48–50]. Given the unique immune environment of the oral cavity, it was important to test whether salivary UA levels are influenced by, or index, differences in oral inflammatory activity. The finding that salivary UA concentrations were not significantly associated with salivary cytokine levels nor oral health measures is of more than passing interest because the physiological correlates of several diseases associated with UA, such as metabolic syndrome and Type-2 diabetes, include periodontal disease [51,52]. The finding that salivary UA is not related to oral health and inflammatory indices suggests that the co-variation of inflammation, oral health, and risk for cardiometabolic disease does not significantly compromise the interpretation of salivary UA levels as a marker of circulating UA.
Given the strong association between UA and cardiovascular and metabolic disorders, we also tested whether salivary UA was associated with other biomarkers related to these conditions. We found that UA and adiponectin are inversely correlated regardless of whether these analytes were measured in serum or saliva. The pattern of associations between UA and adiponectin extended across biospecimens. That is, saliva levels of one were not only related to saliva levels of the other, but also serum levels of the other, and vice versa. However, for this overall pattern of co-variation to be revealed, tests of association had to account for measures of oral health or inflammatory activity. This is likely due to an association between adiponectin and oral inflammation [49], as there were no significant associations between UA and inflammatory markers and indices of oral health in these data. In a previous study, we reported on the validity and utility of salivary adiponectin [53]. Using data from the same sample as used in the current study, we found salivary adiponectin was strongly correlated with measures of the oral immune environment (salivary cytokines) and oral health (MMP-8), and not significantly related to sex or BMI. By controlling for oral health and immune markers in the current analyses, we examine the UA–adiponectin relations, while adjusting for potential immune-related changes in salivary adiponectin that may be specific to the oral immune environment and unrelated to systemic adiponectin levels. Importantly, others have shown adiponectin levels are lower in patients with essential hypertension and diabetes, and levels are associated with insulin resistance, triglycerides, and HDL cholesterol levels [34,54–56]. Furthermore, the combination of hyperuricemia and hypoadiponectinemia may be an indicator of risk for cardiometabolic disorders [57]. Measuring both salivary UA and salivary adiponectin in biobehavioral research may expand research opportunities focused on cardiometabolic risk.
In contrast, we found no significant associations between UA and CRP in our sample regardless of the biospecimen examined. Similarly, as noted above, salivary UA was not related to inflammatory activity indexed by salivary and serum cytokines. Given UA's role as both a pro- and anti-oxidant agent and the complex relations between UA and the immune system, it is not surprising that among young healthy adults with good oral health we failed to see significant relations between UA and markers of inflammation. Future studies should continue to examine UA-immune relations among healthy and at risk populations to fully understand the role UA plays in health and disease.
Although we found the majority of the variance in salivary UA was attributed to an individual-specific latent trait, approximately a third of the salivary UA variance was associated with a changing, state component. Previous studies suggest several factors have the potential to contribute to the observed state variance in salivary UA. For example, physical exercise [58,59], and food intake may influence UA levels [11]. Participants in this study attended marching band practice between the saliva samples and eating was not restricted. Additional research on state-specific influences of salivary UA is needed to fully understand the meaning of changes in salivary UA. It would be particularly interesting to implement the sampling and LST approach used here in studies of clinical populations (e.g., patients with metabolic syndrome) where the range of cardiovascular risk factors is more extreme. Additional measures of cardiovascular risk factors (e.g., body fat percentage and waist to hip ratio) should also be examined as predictors of stable trait-like variance in salivary UA to establish salivary UA as a biomarker of metabolic risk. Similarly, elucidating the associations between trait components of salivary UA and pathological factors (e.g., neuroimaging for tau or amyloid plaque pathology) in other diseases associated with oxidative stress would be a worthwhile next step.
Limitations
The analyses presented herein complement each other with each helping to address the limitations of the other. The findings from analyses examining the robustness of the serum–saliva correlation are strengthened by the use of several biomarkers in both serum and saliva which allowed us to rigorously interrogate the UA serum–saliva correlation. However, the cross-sectional nature of these analyses is a limitation to interpreting the findings. This limitation is addressed by the findings from our stability analyses supporting the relatively stable nature of salivary UA levels within individuals. Similarly, our stability analyses could not adjust for several of the factors typically thought to influence salivary analyte levels, however, we saw in the validity analyses that many of these factors (i.e., blood leakage, total protein, oral inflammation, and oral health) did not impact the validity of the salivary UA determinations.
Due to the composition of our study samples, the generalizability of our findings is limited to healthy young adults with good oral hygiene practices. To be included in the study sample used for the validity analyses, participants could not be under a physician's care for acute or chronic medical conditions. The sample used for the stability analyses was also comprised of generally healthy young adults; approximately 11% of participants reported an acute or chronic medical condition. The most common health condition was asthma (n = 5). The nature of our samples prohibits us from examining relations between UA and relevant cardiometabolic conditions and medication use. The associations found in the current study, including relations between UA and other biomarkers and participant characteristics (e.g., sex and BMI), may not be observed when examined in clinical or at-risk populations. Although a limitation, this also represents a contribution to the field as previous studies of salivary UA have predominantly examined small samples or focused on individuals from select populations or with specific disorders (e.g., [2,58–65]). Our findings contribute to the growing knowledge of salivary UA by expanding our understanding to the healthy population. Advancing the study of UA with longitudinal research focusing on clinical or at-risk populations is an important next step to develop our understanding of UA as a biomarker for heath and disease risk.
In addition, the use of self-reported participant characteristics (e.g., age, sex, race/ethnicity, height, and weight) introduce the possibility of reporting biases. BMI has been shown to be particularly vulnerable to under-reporting, especially by female and overweight/obese participants [66–68]. Although an important limitation, we found no sex differences in BMI in our sample. Also, the under-reporting of weight would have reduced BMI variability, making it harder to detect the significant relations between salivary UA and BMI found in our study. Future studies using objective measures of body composition are needed to fully understand relations between salivary UA and BMI.
The analysis of adiponectin also introduces a unique limitation. Adiponectin circulates in three molecular weight complexes [69]. In saliva, however, the primary species is possibly a more complex super high molecular weight oligomer [70]. The adiponectin assays employed may not have recognized each species with the same affinity and may differ in avidity effects with the various complexes of adiponectin. This potential artifact may be reflected in the modest serum–saliva association for adiponectin observed. In examining relations between adiponectin and UA, we controlled for several factors known to influence salivary biomarker concentrations, including indices of oral health and inflammatory activity. We did not however adjust for sex or BMI, both of which were related to UA concentrations. To test the influence of sex and BMI on the observed associations, we explored relations between UA and adiponectin in serum and saliva controlling for the oral inflammatory activity composite score as well as sex and BMI. These analyses revealed the same pattern of associations between UA and adiponectin as described above, although, possibly due to reductions in power and sample size, the associations were weaker and no longer statistically significant. Future studies with larger, more diverse samples are needed to fully understand the relations between UA and adiponectin. In addition, researchers examining salivary adiponectin should consider the limitations regarding assay recognition and analyte immunosensitivity when designing their studies and interpreting their findings.
Conclusion
Technical advances that enable the measurement of a broad spectrum of analytes in oral fluids create opportunity. These advances should be received with excitement and enthusiasm. Nonetheless, just because an analyte can be measured in oral fluid does not necessarily mean that it should be measured, or that the interpretation of variation in that analyte's levels in saliva will be straightforward or meaningful. Our experience reveals the importance of approaching these advances with cautious enthusiasm [71,72]. Taken together, the present findings move us more toward enthusiasm than caution by suggesting moderate-to-strong measurement validity and stability when UA is measured in saliva, and evidence suggesting salivary UA may be a robust indicator of systemic UA activity.
Summary points.
Uric acid (UA) is associated with cardiovascular and metabolic disorders, as well as a wide range of other health conditions and behaviors.
A noninvasive measure of UA would be particularly useful in biobehavioral health and clinical research.
Using salivary and serum UA data from healthy young adults, we examined the validity and stability of salivary UA and its utility as a noninvasive measure of circulating UA.
We found a strong positive association between salivary and serum UA, and, importantly, neither the direction nor the magnitude of this association was related to total protein in saliva, blood leakage into oral fluid, proinflammatory cytokines, or biobehavioral indices of poor oral health.
Results also revealed robust inverse associations between UA and adiponectin in both serum and saliva.
Salivary UA levels were highly correlated within and between assessment points, and advanced statistical modeling showed the majority (62–66%) of the variance in salivary UA could be attributed to a latent trait component suggesting relative stability in salivary UA levels.
BMI and sex were associated with the stable trait-like component of salivary UA.
The findings demonstrate strong measurement validity and stability when UA is measured in saliva, and provide evidence supporting salivary UA as a robust indicator of systemic UA activity.
The finding suggests that salivary UA could serve as a biomarker for a wide range of potential conditions and disease states.
Supplementary Material
Acknowledgements
The authors thank M Twomley and W Worley for coordination of biospecimen collection; J Peterson and SW Granger at Salimetrics for technical advice; and Salimetrics (Carlsbad, CA, USA) for contributing some reagents. We greatly appreciate the participation by members of the 2013–2014 Arizona State University marching band and the efforts of S Weren, G Hill and JG Hudson in coordinating and facilitating data collection. We also acknowledge A Reinhard, J Bayer, and T Hand for biotechnical support with salivary assays. We thank J Goenaga, C Yee, M Pelaez, K Granger and R Field for assistance with data collection, and E Thomas for comments on earlier drafts of this manuscript.
Footnotes
Financial & competing interests disclosure
This work was supported by a formative research project grant from the National Children's Study (NICHD research project LOI2-BIO-19, PI Douglas Granger); a seed grant from the Herberger School of Music at ASU; and reagents were graciously donated by Salimetrics LLC (CA, USA). O Kornienko was supported in part by National Institutes of Health Loan Repayment Award (L30 DA042448). In the interest of full disclosure, DA Granger is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Both studies were conducted with the approval of institutional review boards and written informed consent was obtained from all participants.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Granger DA, Johnson SB, Szanton SL, Out D, Schumann LL. Incorporating salivary biomarkers into nursing research: an overview and review of best practices. Biol. Res. Nurs. 2012;14:347–356. doi: 10.1177/1099800412443892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soukup M, Biesiada I, Henderson A, et al. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol. Metab. Syndr. 2012;4:14. doi: 10.1186/1758-5996-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Original research study finding salivary uric acid is associated with metabolic syndrome and cardiometabolic risk factors.
- 3.Shibasaki K, Kimura M, Ikarashi R, Yamaguchi A, Watanabe T. Uric acid concentration in saliva and its changes with the patients receiving treatment for hyperuricemia. Metabolomics. 2012;8:484–491. [Google Scholar]
- 4.Xia Y, Peng C, Zhou Z, et al. Clinical significance of saliva urea, creatinine, and uric acid levels in patients with chronic kidney disease. J. Cent. South Univ. Med. Sci. 2012;37:1171–1176. doi: 10.3969/j.issn.1672-7347.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Nunes LAS, Brenzikofer R, Macedo DV. Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin. Biochem. 2011;44:1440–1444. doi: 10.1016/j.clinbiochem.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Biscaglia S, Ceconi C, Malagù M, Pavasini R, Ferrari R. Uric acid and coronary artery disease: an elusive link deserving further attention. Int. J. Cardiol. 2015;213:28–32. doi: 10.1016/j.ijcard.2015.08.086. [DOI] [PubMed] [Google Scholar]; • Review article summarizing the evidence regarding uric acid and cardiovascular disease.
- 7.Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur. J. Intern. Med. 2016;29:3–8. doi: 10.1016/j.ejim.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu SH, Shu XO, Milne G, et al. Uric acid correlates to oxidation and inflammation in opposite directions in women. Biomarkers. 2015;20:225–231. doi: 10.3109/1354750X.2015.1068852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushiyama A, Tanaka K, Hara S, Kawazu S. Linking uric acid metabolism to diabetic complications. World J. Diabetes. 2014;5:787–795. doi: 10.4239/wjd.v5.i6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin. Chim. Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira EEP, Burini RCR. High plasma uric acid concentration: causes and consequences. Diabetol. Metab. Syndr. 2012;4:12. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dornas WC, De Lima WG, Pedrosa ML, Silva ME. Health implications of high-fructose intake and current research. Advances in Nutrition. 2015;6(6):729–737. doi: 10.3945/an.114.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad Sah OS, Qing YX. Associations between hyperuricemia and chronic kidney disease: a review. Nephrourol. Mon. 2015;7(3) doi: 10.5812/numonthly.7(3)2015.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang P, Li X, Luo JJ, Wang H, Yang X-F. A double-edged sword: uric acid and neurological disorders. Brain Disord. Ther. 2013;2(2):109. doi: 10.4172/2168-975X.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinaldi P, Polidori MC, Metastasio A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol. Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 16.Lyngdoh T, Bochud M, Glaus J, et al. Associations of serum uric acid and SLC2A9 variant with depressive and anxiety disorders: a population-based study. PLoS ONE. 2013;8:e76336. doi: 10.1371/journal.pone.0076336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado-Vieira R, Soares JC, Lara DR, et al. A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J. Clin. Psychiatry. 2008;69:1237–1245. doi: 10.4088/jcp.v69n0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert U, De Cori D, Aguglia A, Barbaro F, Bogetto F, Maina G. Increased uric acid levels in bipolar disorder subjects during different phases of illness. J. Affect. Disord. 2015;173:170–175. doi: 10.1016/j.jad.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Jahangard L, Soroush S, Haghighi M, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant allopurinol improved symptoms of mania in in-patients suffering from bipolar disorder. Eur. Neuropsychopharmacol. 2014;24:1210–1221. doi: 10.1016/j.euroneuro.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Kesebir S, Tatlıdil Yaylacı E, Süner O, Gültekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J. Affect. Disord. 2014;165:131–134. doi: 10.1016/j.jad.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 21.Sutin AR, Cutler RG, Camandola S, et al. Impulsivity is associated with uric acid: evidence from humans and mice. Biol. Psychiatry. 2014;75:31–37. doi: 10.1016/j.biopsych.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJR, Lanaspa MAM, Gaucher EA. Uric acid: a danger signal from the RNA world that may hava a role in the epidemic of obesity, metabolic syndrome and cardiorenal disease: evolutionary considerations. Semin. Nephrol. 2011;31:394–399. doi: 10.1016/j.semnephrol.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman AM, Wheelock MD, Harnett NG, Mrug S, Granger DA, Knight DC. The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience. 2016;339:396–401. doi: 10.1016/j.neuroscience.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Original research study finding salivary uric acid is associated with hippocampal function in response to stress.
- 24.Cheng P, Xia Y, Peng C, Zhou Z. Evaluation of dialysis in patients with end-stage renal disease by salivary urea, creatinine and uric acid. J. South Cent. Univ. Med. Coll. 2013;38:1260–1263. doi: 10.3969/j.issn.1672-7347.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Blicharz TM, Rissin DM, Bowden M, et al. Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: a proof of principle. Clin. Chem. 2008;54:1473–1480. doi: 10.1373/clinchem.2008.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez AD, Ruelas L, Granger DA. Association between BMI and salivary uric acid among Mexican-origin infants, youth and adults: gender and developmental differences. Dev. Psychobiol. 2017;59:225–234. doi: 10.1002/dev.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie H, Sun J, Chen Y, Zong M, Li S, Wang Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/214836. https://www.hindawi.com/journals/omcl/2015/214836/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niskanen L, Laaksonen D, Nyysonen K. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men. ACC Curr. J. Rev. 2004;13:18–19. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality. JAMA. 2000;283(18):2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]; • Original research study using large-scale epidemiologic data and finding a significant association between serum uric acid and cardiovascular mortality.
- 30.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.2215/01.CJN.0000926948.20608.e5. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int. J. Cardiol. 2016;213:4–7. doi: 10.1016/j.ijcard.2015.08.110. [DOI] [PubMed] [Google Scholar]
- 32.Babio N, Martinez-Gonzalez MA, Estruch R, et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2015;25:173–180. doi: 10.1016/j.numecd.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Nejatinamini S, Ataie-Jafari A, Qorbani M, et al. Association between serum uric acid level and metabolic syndrome components. J. Diabetes Metab. Disord. 2015;14:70. doi: 10.1186/s40200-015-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 35.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 36.Tamba S, Nishizawa H, Funahashi T, et al. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern. Med. 2008;47:1175–1180. doi: 10.2169/internalmedicine.47.0603. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Kang S, Ahn CW, Cha BS, Kim KR, Lee HC. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas. 2012;73:344–348. doi: 10.1016/j.maturitas.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 39.Kornienko O, Schaefer DR, Weren S, Hill GW, Granger DA. Cortisol and testosterone associations with social network dynamics. Horm. Behav. 2016;80:92–102. doi: 10.1016/j.yhbeh.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Granger DA, Kivlighan KT, Fortunato C, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol. Behav. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 42.Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Horm. Behav. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Out D, Hall R, Granger G, Page G, Woods S. Assessing salivary C-reactive protein: longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain. Behav. Immun. 2012;26:543–551. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabachnick B, Fidell L. Using Multivariate Statistics: International Edition. Pearson; NJ, USA: 2012. [Google Scholar]
- 45.Brown T. Confirmatory Factor Analysis For Applied Research. Guilford Publications; NY, USA: 2014. [Google Scholar]
- 46.Hu L, Bentler P. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol. Methods. 1988;3:424. [Google Scholar]
- 47.Woo J, Swaminathan R, Cockram C, Lau E, Chan A. Association between serum uric acid and some cardiovascular risk factors in a Chinese population. Postgr. Med. J. 1994;70:486–491. doi: 10.1136/pgmj.70.825.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riis JL, Granger DA, DiPietro JA, Bandeen-Roche K, Johnson SB. Salivary cytokines as a minimally-invasive measure of immune functioning in young children: correlates of individual differences and sensitivity to laboratory stress. Dev. Psychobiol. 2015;57:153–67. doi: 10.1002/dev.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riis JL, Out D, Dorn LD, et al. Salivary cytokines in healthy adolescent girls: intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol. 2014;56:797–811. doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granger DA, Granger GA, Granger SW. Immunology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Volume Two: Developmental Neuroscience. John Wiley and Sons; NY, USA: 2006. pp. 677–709. [Google Scholar]
- 51.Hatipoglu H, Yaylak F, Gungor Y. A brief review on the periodontal health in metabolic syndrome patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2015;9:124–126. doi: 10.1016/j.dsx.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe V, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch. Oral Biol. 2014;59:855–870. doi: 10.1016/j.archoralbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riis JL, Bryce CI, Ha T, et al. Adiponectin: serum–saliva associations and relations with oral and systemic markers of inflammation. Peptides. 2017;91:58–64. doi: 10.1016/j.peptides.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Stojanović S, Ilić MD, Ilić S, Petrović D, Djukić S. The significance of adiponectin as a biomarker in metabolic syndrome and/or coronary artery disease. Vojnosanit. Pregl. 2015;72:779–784. doi: 10.2298/vsp140531067s. [DOI] [PubMed] [Google Scholar]
- 55.Pilz S, Horejsi R, Moller R, et al. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J. Clin. Endocrinol. Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- 56.Ebrahimi-Mamaeghani M, Mohammadi S, Arefhosseini SR, Fallah P, Bazi Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 2015;11:55–70. doi: 10.2147/VHRM.S48753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mascarenhas-Melo F, Palavra F, Marado D, et al. Emergent biomarkers of residual cardiovascular risk in patients with low HDL-c and/or high triglycerides and average LDL-c concentrations: focus on HDL subpopulations, oxidized LDL, adiponectin, and uric acid. Sci. World J. 2013 doi: 10.1155/2013/387849. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green HJ, Fraser IG. Differential effects of exercise intensity on serum uric acid concentration. Med. Sci. Sports Exerc. 1988;20:55–59. doi: 10.1249/00005768-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Wiecek M, Maciejczyk M, Szymura J, Szygula Z, Kantorowicz M. Changes in non-enzymatic antioxidants in the blood following anaerobic exercise in men and women. PLoS ONE. 2015;10:1–16. doi: 10.1371/journal.pone.0143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sedigheh Bakhtiari MB, Toosi P, Samadi S. Assessment of uric acid level in the saliva of patients with oral lichen planus. Med. Princ. Pract. 2017;26:56–60. doi: 10.1159/000452133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zloczower M, Reznick AZ, Zouby RO, Nagler RM. Relationship of flow rate, uric acid, peroxidase, and superoxide dismutase activity levels with complications in diabetic patients: can saliva be used to diagnose diabetes? Antioxid. Redox Signal. 2007;9:765–773. doi: 10.1089/ars.2007.1515. [DOI] [PubMed] [Google Scholar]
- 62.Miricescu D, Totan A, Calenic B, et al. Salivary biomarkers: relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol. Scand. 2014;72:42–47. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J, Huang Y. Salivary uric acid as a noninvasive biomarker for monitoring the efficacy of urate-lowering therapy in a patient with chronic gouty arthropathy. Clin. Chim. Acta. 2015;450:115–120. doi: 10.1016/j.cca.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Ahmadi-Motamayel F, Vaziri-Amjad S, Goodarzi M, Poorolajal J. Evaluation of salivary uric acid and pH in human immunodeficiency virus infected patients: a historical cohort study. Infect. Disord. Drug Targets. 2017;17 doi: 10.2174/1871526517666170428122405. [DOI] [PubMed] [Google Scholar]
- 65.Fatima G, Uppin RB, Kasagani S, Tapshetty R, Rao A. Comparison of salivary uric acid level among healthy individuals without periodontitis with that of smokers and non-smokers with periodontitis. JoAOR. 2016;7 [Google Scholar]
- 66.Wang Z, Patterson CM, Hills AP. A comparison of self-reported and measured height, weight and BMI in Australian adolescents. Aust. NZ J. Public Health. 2002;26:473–478. doi: 10.1111/j.1467-842x.2002.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 67.Boring AL, Peeters A, Freak-Poli R, Lim MSC, Halliard M. Measuring the accuracy of self-reported height and weight in a community-based sample of young people. BMC Med. Res. Methodol. 2012;12:175–182. doi: 10.1186/1471-2288-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danubio ME, Miranda G, Vinciguerra MG, Vecchi E, Rufo F. Comparison of self-reported and measured height and weight: implications for obesity research among young adults. Econ. Hum. Biol. 2008;6:181–190. doi: 10.1016/j.ehb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:25–31. doi: 10.1016/j.beem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nigro E, Piombino P, Scudiero O, et al. Evaluation of salivary adiponectin profile in obese patients. Peptides. 2015;63:150–155. doi: 10.1016/j.peptides.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Horvat-Gordon M, Granger DA, Schwartz EB, Nelson VJ, Kivlighan KT. Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiol. Behav. 2005;84:445–448. doi: 10.1016/j.physbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Matin MJ, Li D, Peterson J, et al. Measuring nerve growth factor in saliva by immunoassay: a cautionary note. Psychoneuroendocrinology. 2016;63:235–237. doi: 10.1016/j.psyneuen.2015.09.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.