Abstract
Aim:
The primary objective of this study was to evaluate correlations among mortality, intensive care unit (ICU) length of stay and airway microbiotas in septic patients.
Materials & methods:
A deep-sequencing analysis of the 16S rRNA gene V4 region was performed.
Results:
The nasal microbiota in septic patients was dominated by three nasal bacterial types (Corynebacterium, Staphylococcus and Acinetobacter). The Acinetobacter type was associated with the lowest diversity and longest length of stay (median: 9 days), and the Corynebacterium type was associated with the shortest length of stay. We found that the Acinetobacter type in the >9-day group was associated with the highest mortality (33%).
Conclusion:
Septic patients have three nasal microbiota types, and the nasal microbiota is related to the length of stay and mortality.
Keywords: : 16S rRNA, bacterial type, microbiome, nose, sepsis
Sepsis is a serious public health problem with a reported mortality rate ranging from 10 to 55.7% [1–5]. The gut microbiota in patients with sepsis in intensive care units (ICUs) is currently receiving considerable attention [6,7]. However, the airway microbiome has been less extensively reported. Sepsis most often derives from the lungs [8]. Previous studies have reported that sepsis is related to lung microbial dysbiosis [9]. In addition, animal tests have shown that sepsis alters the lung microbiome, which plays a role in the development of sepsis and its exacerbation [10]. The characteristics of the airway microbiota in patients with sepsis have not been well studied using deep-sequencing analyses of the 16S rRNA gene V4 region.
Culture-dependent studies have revealed that as the hospitalization time in an ICU increases, pathogen abundance increases [11]. Moreover, these studies revealed that several pathogens colonize nasal passages after patients are transferred to an ICU, and the colonization rate was positively correlated with the length of hospital stay and infection [12]. After patients are admitted to an ICU, the risk of infection highly depends on their length of stay [13]. However, few studies have focused on the relationship between the characteristics of the nasal microbiome in patients with sepsis and patients’ length of stay in an ICU. Accordingly, we hypothesized that the nasal microbiota in patients with sepsis may be related to their length of stay in the ICU.
Recent studies have shown that nasal microbiotas can reflect deep lung infection status as the microbial community compositions in the upper and lower airways are similar [14–16]. In addition, acquiring nasal microorganisms is far less invasive than bronchoscopy. Furthermore, nasal bacteria, that is, the bacterial communities in the uppermost part of the nasal passage, can contribute to infection transmission in humans, and accumulating evidence suggests that a close relationship exists between the nasal flora and infection [17,18]. Overall, we propose that examining the nasal microbiota can benefit studies investigating airway microbiomes during sepsis. In this study, we carried out an observational study involving 89 patients with sepsis and 78 healthy subjects to describe the characteristics of the nasal flora in patients with sepsis and to explore the relationships among the nasal microbiota in septic patients, length of ICU stays and mortality. To eliminate the influence of environmental factors on the nasal microbiota, we enrolled medical staff from an ICU as controls for nasal swabs. Thus, the aim of the current study was to identify the characteristics of the nasal flora in patients with sepsis and to explore the relationships among the nasal microbiota, mortality and length of ICU stay, which may provide clinical and microbiological clues for treatment options and potential targets for preventive research.
Materials & methods
Study design & participants
We conducted an observational cross-sectional study involving participants from the hospital of Southern Medical University (SMU) in China. Approval for this study was granted by the Ethics Committee of SMU (2015-GRGLK-002). Written informed consent was obtained from all subjects or a surrogate decision maker as appropriate. The patients were enrolled between January 2015 and August 2016. In total, 167 volunteers were included. To reduce the impact of environmental microbial factors on nasal microbiomes, we enrolled 78 medical staff members from the ICU as controls for nasal swab experiments. In total, 89 patients with sepsis in the ICU were enrolled. Data regarding the patients’ demographics, antibiotic exposure, tube placements and inflammatory indicators were obtained from the study database and electronic medical records.
Sepsis is a life-threatening organ dysfunction caused by a deregulated host response to infection [19]. Organ dysfunction can be identified as an acute change ≥2 points in the total sepsis-related organ failure assessment score due to infection [20]. The baseline sepsis-related organ failure assessment scores are assumed to be zero in patients with no known pre-existing organ dysfunction.
Sample collection
The subjects either sat or laid down while fully exposing the nasal cavities. Experienced physicians used sterile swabs to collect samples. To reduce the influence of sampling at different times, we sampled from 8:00 AM to 10:00 AM. The swabs were rotated ten-times in both nostrils. For patients who were fed nasally, only a single nostril was sampled. The collected samples were temporarily stored in a biological sample transport box and then transferred to a low-temperature freezer at -80°C within 4 h until total DNA was extracted from the bacteria in the collected samples.
DNA extraction, 16S rRNA gene amplification & sequencing
Bacterial genomic DNA was extracted from nasal swab samples using a DNA Extraction Magnetic Bead Kit (Shenzhen BioEasy Biotechnologies Co., Ltd, China) according to the manufacturer's instructions and as previously described [21]. For 16S amplicon sequencing, PCR amplification spanning the bacterial V4-16S rRNA was performed [22], and the PCR products were mixed at a certain ratio by a Qubit fluorometer (Invitrogen™). The Illumina HiSeq PE250 sequencing platform was used for further sequencing. The raw 16S rRNA gene sequencing datasets are available in the European Bioinformatics Institute database (http://www.ebi.ac.uk/) under accession number PRJEB27877.
Bioinformatics & statistical analysis
We processed and analyzed the data as previously described [23]. The 16S rRNA reads were initially screened for low-quality bases and short-read lengths. Then, paired-end read pairs were assembled using SeqPrep, and the resulting consensus sequences were demultiplexed (i.e., assigned to their original sample), the artificial barcodes and primers were trimmed and chimeras were assessed using UCHIME in the closed mode implemented in Quantitative Insights Into Microbial Ecology (QIIME; release v. 1.9.1). Then, the quality trimmed sequences were clustered into Operational Taxonomic Units (OTUs) by SortMeRNA (v2.0) with GreenGene's database (v13_8) in QIIME with a minimum confidence threshold of 0.97 for the taxonomic assignments (sharing 97% similarity). SortMeRNA (v2.0) was used to classify these sequences into specific taxa using GreenGene's database (v13_8) [24].
A total of 2,356,839 sequences were obtained from 167 specimens; the lowest number obtained was 2502 sequences, and the highest was 40 810 sequences, and we normalized the sequences to 2500. α diversity was measured using a phylogenetic diversity (PD) whole-tree index, and beta diversity was estimated using the unweighted UniFrac. The linear discriminant analysis (LDA) effect size (LEfSe) was used to determine the best characterized differential genus between the groups. The LEfSe is an algorithm used for biomarker discovery, and this method measures genomic features that characterize different features in the groups analyzed. We set the threshold for the logarithmic LDA score to >3.5 and the significance to p < 0.05. We performed phenotyping according to the tutorials of ‘enterotyping’ reported by the MetaHIT consortium [25]. The detailed tutorial used to produce this work can be found on the following website: http://enterotype.embl.de/
Random forest classification models were trained by the caret R package to classify the samples from the patients with sepsis and the healthy controls [26]. The random forest algorithm generates multiple models by training multiple decision trees and then uses multiple decision trees to classify the models [27]. This method is robust with missing and unbalanced data and can predict the effects of up to several thousand explanatory variables. Training was completed through a tenfold cross-validation approach and measured as the area under the curve derived from a receiver operating characteristic analysis, which was used to overcome the problem of the two groups being unevenly distributed in this study [28].
The statistical tests were performed with R 3.0.2. As not all tested indicators passed the Shapiro–Wilk normality test or the Bartlett test of homogeneity of variances, we also applied Kruskal–Wallis tests to identify differential features between the groups. The χ2 test was used to analyze categorical variables. Because the microbiome data are multidimensional, the associations between the study groups and the microbiome composition were tested using the Adonis test implemented in QIIME (a method similar to permutational multivariate ANOVA).
Results
Patient characteristics
In this study, all 167 enrolled subjects of both sexes, including 89 patients with sepsis and 78 healthy subjects, were recruited from the ICU of the hospital at SMU. In the healthy control group, the participants’ ages ranged from 21 to 50 years, and the participants included 56 females and 22 males. The healthy control participants were all recruited from the ICU. In the group with sepsis, the ages of the participants ranged from 22 to 88 years, and the participants included 22 females and 67 males. Some septic patients suffered from bacterial, fungal or mixed infections. In 22.5% (20/89) of the patients, pathogenic bacteria were detected in blood; in 14.61% (13/89) of the patients, pathogenic bacteria were detected in sputum; in 8.99% (8/89) of the patients, pathogenic bacteria were detected in urine; and in 7.87% (7/89) of the patients, pathogenic bacteria were detected in ascites. The patient clinical characteristics are shown in Table 1.
Table 1. . Baseline characteristics of the patients with sepsis.
| Clinical variable | Baseline values (n = 89) |
|---|---|
| Age (mean ± SD; years) | 57.98 ± 16.527 |
| Sex (males/females; n) | 67/22 |
| ICU time (median; interquartile; days) | 10 (5.0–24.0) |
| Time (median; interquartile; days) | 26 (12.0–42.5) |
| ICU time before sampling (median; interquartile; days) | 4.5 (1.0–9.0) |
| Time before sampling (median; interquartile; days) | 6 (2.0–14.0) |
| ICU time after sampling (mean ± SD, days) | 7.76 ± 10.596 |
| Time after sampling (median; interquartile; days) | 13 (5.0–28.0) |
| APACHE II score (median; interquartile; n) | 16 (11.0–23.0) |
| SOFA score (median; interquartile; n) | 8 (4.0–10.0) |
| PLT (median; interquartile; n) | 164 (90–277) |
| WBC count (mean ± SD; 109/l) | 12.32 (8.76–16.35) |
| Neutrophilic granulocyte percentage (median; interquartile; %) | 90 (81–95) |
| CRP (median; interquartile;mg/l) | 68.9 (5.95–124.50) |
| PCT (median; interquartile; μg/l) | 3.02 (0.45–11.74) |
| Inhalation (yes/no) | 58/31 |
| Hormone (yes/no) | 49/40 |
| Diarrhea (yes/no) | 4/85 |
| Nasogastric tube (yes/no) | 66/23 |
| Smoking (yes/no) | 17/71 |
APACHE II: Acute physiology and chronic health evaluation II; CRP: C-reactive protein; ICU: Intensive care unit; PCT: Procalcitonin; PLT: Platelet count; SD: Standard deviation; SOFA: Sepsis-related organ failure assessment; WBC: White blood cell.
Nasal microbiome compositions in patients with sepsis
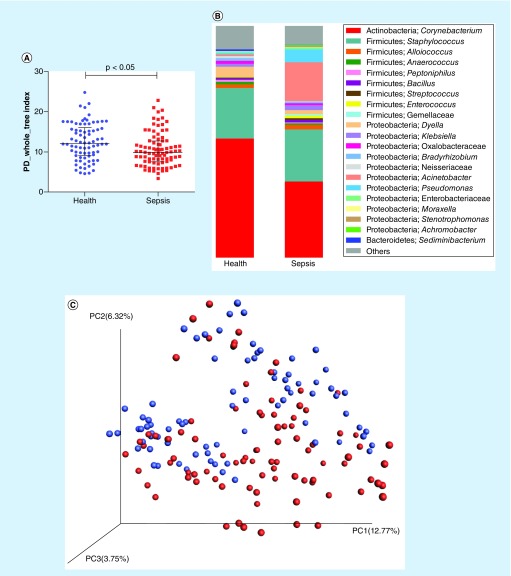
The analysis of the relative abundance levels of nasal bacterial taxa via high-throughput sequencing revealed 18 phyla and 397 nasal bacterial genera in the patients with sepsis. The top four abundant phyla detected were Actinobacteria, Proteobacteria, Firmicutes and Bacteroidetes. The relative abundance of Proteobacteria was slightly increased in the group with sepsis. The top nine genera included Corynebacterium, Staphylococcus, Dyella, Alloiococcus, Klebsiella, Bradyrhizobium, Anaerococcus, Rhodococcus and Peptoniphilus, which accounted for approximately 88% of the nasal microbiome. In addition, compared with the healthy subjects, the genera Acinetobacter, Pseudomonas and Enterococcus were enriched in the patients with sepsis (Figure 1B).
Figure 1. . Comparison of nasal microbiota between septic patients and healthy subjects.
(A) α diversity between the septic patients and healthy subjects. (B) Stacked bar chart of bacteria at the genus level between the septic patients and healthy controls. (C) Principal coordinate analysis based on unweighted UniFrac distances. The red dots represent patients with sepsis, and the blue dots represent the controls. (D) significantly different taxa between the healthy participants and septic patients were determined using the linear discriminant analysis effect size (LEfSe). The data show increasing levels of Gammaproteobacteria, Pseudomonadales, Proteobacteria, Pseudomonas, Moraxellaceae, Acinetobacter, Enterobacteriaceae and Klebsiella in the patients. (E) Machine-learning classification based on nasal microbiota using random forest algorithms.
LDA: Linear discriminant analysis.
The nasal microbiota in patients with sepsis differed from that of healthy controls
Based on the PD whole-tree index, the α diversity analysis of the nasal microbiomes indicated that the nasal microbiota diversity in the sepsis group was poorer than that in the healthy group (nasal: p < 0.05) (Figure 1A).
In addition, a principal coordinate analysis, which is a method used to determine the relationship between samples based on a distance matrix, of the unweighted UniFrac (p = 0.001) distances showed that the beta diversity in the patients with sepsis distinctly differs from that in the healthy controls (Figure 1C).
We used the microbiota data to build a machine-learning classification model, which indicated that the septic patients’ nasal microbiotas were distinct from those in the healthy group (Nasal: AUC = 89.68, 95% CI: 84.92–94.44) (Figure 1E).
Based on the LEfSe (LDA = 3.5) at the phylum level, the abundance of Proteobacteria significantly increased during sepsis. Acinetobacter, Klebsiella and unclassified Moraxellaceae, Enterobacteriaceae and Dethiosulfovibrionaceae were significantly enriched in the patients with sepsis, while Actinobacteria, Corynebacterium, Clostridia and Propionivibrio were mostly enriched in the healthy subjects (Figure 1D).
Patients with sepsis harbor different types of nasal microbiota
Group classification (stratification) is an effective method to obtain a better understanding of complex biological problems, such as human physical and mental health [29]. Different bacterial types may be related to different functions and physiological conditions. In 2011, Arumugam first proposed and defined the concept of enterotype as “microbial group characteristics gathered in a high-dimensional space, which is a limited host-microbial community structure at the time of microbial symbiosis.” Without considering age, gender, cultural background or geographic location, intestinal microbiota can be divided into several enterotypes [30]. Different enterotypes may be related to different functions and physiological and pathological conditions. Classifying gut flora by enterotype has potential clinical significance. First, this classification is helpful in diagnosing an individual's disease state, and second, this classification can be used as a risk or susceptibility index of the specific state of the human body [29]. Therefore, we also classified the nasal flora to further identify relationships between the flora and the ICU length of stay and mortality.
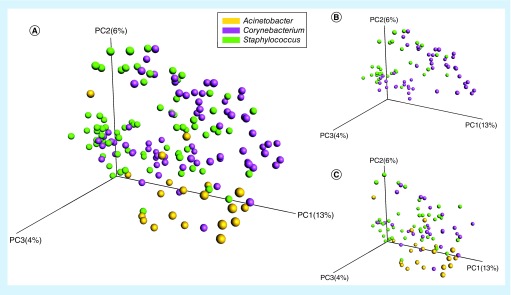
The nasal flora was clustered into three bacterial types belonging to the genera Acinetobacter, Corynebacterium and Staphylococcus (Figure 2A). The healthy subjects exhibited the Corynebacterium and Staphylococcus types of nasal flora (Figure 2B). However, the patients with sepsis exhibited the Corynebacterium, Staphylococcus and Acinetobacter nasal bacterial types (Figure 2C). All subjects with the Acinetobacter nasal bacterial type were septic patients; 72.41% of those with the Staphylococcus type were septic patients; and 24.5% of subjects with the Corynebacterium type were septic patients (p < 0.05).
Figure 2. . Differences in the results of the principal coordinate analysis of nasal microbiota between healthy subjects and patients with sepsis.
(A) Principal coordinate analysis (PCoA) of septic patients and healthy subjects based on unweighted UniFrac distances. (B) PCoA of healthy subjects based on unweighted UniFrac distances. (C) PCoA of patients with sepsis based on unweighted UniFrac distances. The orange circles represent the samples (nasal microbiota) with the Acinetobacter type, the purple circles represent the samples with the Corynebacterium type and the green circles represent the samples with the Staphylococcus type.
Different nasal microbiota types & different lengths of ICU stay among septic patients
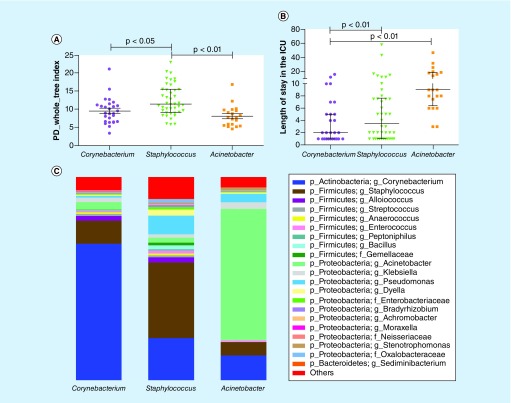
Culture-dependent studies have revealed that pathogen abundance increases with increasing hospitalization time in an ICU. We showed that patients with sepsis harbor specific types of nasal microbiota. Subsequently, we focused on determining whether specific nasal bacterial types were associated with the ICU length of stay among patients with sepsis. We found that the patients with the Acinetobacter nasal bacterial type had the longest ICU stays, with a median of 9 days, and that the patients with the Staphylococcus nasal bacterial type had the shortest ICU stays, with a median of 2 days (χ2 test, p < 0.01). Statistically significant differences were found between Acinetobacter and Corynebacterium (p < 0.01) and between Staphylococcus and Corynebacterium (p < 0.01) (Figure 3B).
Figure 3. . Relationship between the nasal bacterial type in the patients with sepsis and their length of stay in the intensive care unit.
(A) Comparison of the α diversity among the three nasal bacterial types in patients with sepsis. (B) Comparison of the length of intensive care unit stay among the three nasal bacterial types in patients with sepsis. (C) Comparison of the stacked bar charts of the genera in the three nasal bacterial types in patients with sepsis.
ICU: Intensive care unit; PD: Phylogenetic diversity.
However, the α diversity represented by the PD whole-tree index showed that the Acinetobacter type was less diverse than the Staphylococcus type (Wilcoxon test, p < 0.01), and the PD whole-tree index of the Corynebacterium type was significantly lower than that of the Staphylococcus type (Wilcoxon test, p < 0.05). No significant differences were found in the α diversity between Acinetobacter and Corynebacterium (Figure 3A).
The relative abundance of Corynebacterium was sharply increased in the group with the Corynebacterium type, and the top two genera were Corynebacterium and Staphylococcus. The genera Staphylococcus were enriched in the septic patients with the Staphylococcus type, and the top two genera were Staphylococcus and Corynebacterium. In addition, the relative abundance of Acinetobacter was dramatically increased in the group with the Acinetobacter type, and the top two genera were Acinetobacter and Corynebacterium (Figure 3C).
Antibiotics & nasal microbiota types
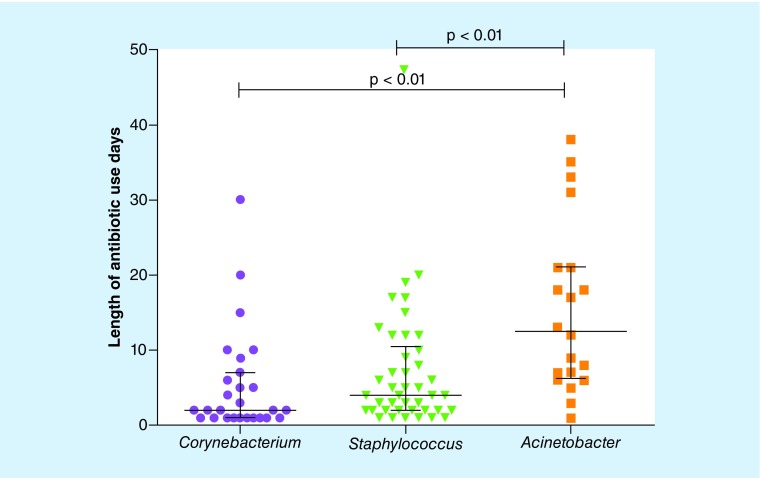
All patients were given antibiotics, and up to nine types of medications were given to individual patients during their hospitalization. Among these medications, the combined use of drugs reached 58.43% (52/89), and combinations involving three or more drugs accounted for 19.10% (17/89) of the cases. The number of days of antibiotic use varied from 1 to 47 days, with an average of 8.48 days of medication. We found significant differences in the use of antibiotics among the three bacterial types (Figure 4); the Acinetobacter type was associated with the longest duration of antibiotic use, with a median of 12.5 days, while Corynebacterium was associated with the shortest duration, with a median of 2 days (Wilcoxon test, p < 0.01). In total, 55.06% (49/89) of the patients used carbapenems.
Figure 4. . Relationship between the nasal bacterial type of patients with sepsis and the number of antibiotic use days in the intensive care unit.
In total, 64 strains were detected in blood, urine, ascitic fluid and sputum. Of these strains, Candida albicans accounted for 15.63% (10/64), Acinetobacter baumannii accounted for 9.38% (6/64), both of which were resistant to carbapenem, Staphylococcus capitis accounted for 9.38% (6/64) and was resistant to methicillin, and Pseudomonas ae ruginosa accounted for 7.81% (5/64) and was multidrug resistant.
Mortality of septic patients with different nasal microbiotas
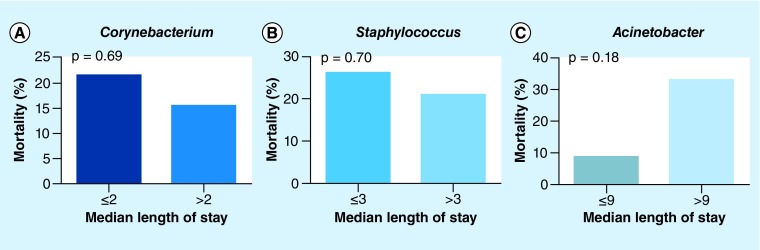
The mortality rate of the septic patients with the Acinetobacter type was 20.0%, and the mortality rates of the septic patients with the Staphylococcus type and the Corynebacterium type were 25 and 18.5%, respectively. We divided the three bacterial types into six groups by the median ICU lengths of stay associated with the three types and compared the mortality rates of the septic patients with the three bacterial types based on the different ICU lengths of stay (Table 2). In the ICU mortality group, the Acinetobacter type was found in 9.09% (1/11) of the patients in the ≤9-day group (Figure 5A) and in 33% (3/9) of the patients in the >9-day group (χ2 test, p = 0.18); the Staphylococcus type was found in 26.09% (6/23) of the patients in the ≤3-day group (Figure 5B) and in 21.05% (4/19) of the patients in the >3-day group (χ2 test, p = 0.70); finally, the Corynebacterium type was found in 21.43% (3/14) of the patients in the ≤2-day group (Figure 5C) and in 15.38% (2/13) of the patients in the >3-day group (χ2 test, p = 0.69).
Table 2. . The nasal bacterial types and its association with length of stay and mortality.
| Nose bacterial types | Length of ICU stay (days) | Death (n) | Total person (n) | Mortality (%) | p-value |
|---|---|---|---|---|---|
| Acinetobacter type | ≤9 | 1 | 11 | 9.09 | 0.18 |
| >9 | 3 | 9 | 33.00 | ||
| Staphylococcus type | ≤3 | 6 | 23 | 26.09 | 0.70 |
| >3 | 4 | 19 | 21.05 | ||
| Corynebacterium type | ≤2 | 3 | 14 | 21.43 | 0.69 |
| >2 | 2 | 13 | 15.38 | ||
ICU: Intensive care unit.
Figure 5. . Mortality rates of the three nasal bacterial types based on different lengths of intensive care unit stay.
(A) Mortality of the Corynebacterium type based on different lengths of intensive care unit (ICU) stay. (B) Mortality of the Staphylococcus type based on different lengths of ICU stay. (C) Mortality of the Acinetobacter type based on different lengths of ICU stay.
Different nasal microbiotas & clinical parameters
We analyzed the relationship between these three bacterial types (Acinetobacter, Corynebacterium and Staphylococcus) and clinical indicators (inhalation drugs, hormones, nasogastric tube, smoking, diarrhea and mechanical ventilation) and found high Acinetobacter in the diarrhea (Wilcoxon test, p = 0.02) and nasogastric tube (Wilcoxon test, p = 0.04) groups. We found low Staphylococcus in the mechanical ventilation (Wilcoxon test, p = 0.03) and nasogastric tube (Wilcoxon test, p = 0.03) groups.
Discussion
Using high-throughput approaches and sepsis-associated clinical data from the hospital of SMU, we identified the characteristics of nasal bacteria from the septic patients. This study also identified significant associations among the nasal bacterial types, mortality rates and lengths of stay in the ICU. The clustering of the nasal metagenomic samples from patients with sepsis based on the taxonomic composition using Illumina sequencing technology and 16S rRNA gene profiling data resulted in the identification of three nasal microbial community types dominated by Acinetobacter, Staphylococcus and Corynebacterium. Correlations were found between the three nasal microbial community types and the length of stay in the ICU, suggesting that a nasal community mainly composed of Acinetobacter may be an indicator of high-risk and longer hospitalization stays, and that the Corynebacterium nasal bacterial type was associated with the shortest lengths of stay in the ICU.
Staphylococcus and Corynebacterium were the most abundant species in the ICU workers’ nasal microbiome, which is consistent with a previous study [31] but inconsistent with the Wilson report suggesting that the microbiota of healthy subjects is dominated by Propionibacterium acnes and Staphylococcus epidermidis [32]. Staphylococcus, Corynebacterium and Acinetobacter were the most abundant species in the septic patients’ nasal microbiome, which is different in children. Previous literature reported that three types, Haemophilus, the Pasteurellaceae family, and Streptococcus, were relatively high in children's nasopharyngeal/oropharyngeal samples in a pediatric ICU [33]. The microbiota diversity in the patients with sepsis was significantly lower than that in the healthy subjects. The decreased microbial diversity in the patients with sepsis is consistent with reports from studies involving adult ICU patients in critical condition [34]. Sepsis caused by drug-resistant A. baumannii and other Gram-negative bacilli is a common challenge for clinicians and microbiologists [35]. Similar to the findings reported here, previous studies have found that certain species of Acinetobacter from the environment and hospitals were closely associated with lung infections [36,37]. Continuous colonization of the nasal cavity by A. baumannii may be the source of A. baumannii infection [38], especially lower respiratory tract infections, including ventilator-associated pneumonia. Furthermore, Acinetobacter is considered a hospital-associated infectious opportunistic pathogen in respiratory ICUs using mechanical ventilation [39]. Patients in ICUs are often on ventilators; the incidence of ventilator-associated pneumonia and the mortality rate associated with this type of pneumonia are 11% and 78.9%, respectively. A. baumannii and Klebsiella pneumoniae were the most common bacteria in the sputum cultures, and these bacteria were 50% resistant to frequently used antibiotics [40]. At our hospital, extensive drug resistance (XDR-AB) accounts for 49.9% of persistent infections, Carbapenem-resistant A. baumannii accounted for 70.02% of infections, The Department of Critical Care Medicine ranked first among all departments, accounting for 25.63% of all infection cases and reflecting a serious concern. Here, we provide further microbiome data complementing previous clinical findings.
Our results also show that different nasal bacterial types were associated with different ICU lengths of stay. The Acinetobacter nasal bacterial type was associated with the longest lengths of stay in the ICU, with a median of 9 days, while Corynebacterium was associated with the shortest lengths of stay. Further analysis showed that the high mortality rate associated with the Acinetobacter type was mainly due to the longer lengths of stay at the ICU. Longer ICU stays are associated with higher mortality rates. In critically ill patients, the normal nasal microbiota of the host is perturbed due to physiological stress and antibiotic treatment, causing the nasal floral structure to substantially change. The nasal flora in the patients with sepsis in this study substantially changed compared with the nasal flora in the healthy subjects, and the number of pathogenic bacteria of Acinetobacter, Pseudomonas and Enterococcus markedly increased.
The use of antibiotics is well known to affect the respiratory flora [41,42]. In our ICU, most patients, especially patients with sepsis, receive advanced antibiotics. The use of numerous antibiotics dramatically changes their flora. Our results show that the Acinetobacter type requires the most medication time. The detected pathogens of A. baumannii are resistant to carbapenem.
We found that the genus Acinetobacter was enriched in the septic patients with diarrhea and a nasogastric tube. Acinetobacter reportedly caused a case of fever, followed by diarrhea and septic shock [43]. We found that a nasogastric tube was a risk factor for the development of nosocomial pneumonia [44], and in the univariate analysis, the use of enteral feeding via a nasogastric tube was significantly associated with mortality [45]. Our results also provide new evidence that mechanical ventilation and a nasogastric tube may be important risk factors for the colonization of Acinetobacter in patients and may lead to serious consequences such as an extended ICU stay and higher mortality. Interestingly, we found septic patients with a small amount of Enterococcus, and Enterococcus is generally present in the intestine, which may be related to mechanical ventilation; Enterococcus has been detected in the respiratory tract in critically ill patients receiving mechanical ventilation [46].
The strengths of this study include the quality of the sequence-based techniques (16S rRNA), which reduce biases better than other methods, such as cultivation techniques. We found that septic patients had different nasal microbiotas and found that the Acinetobacter nasal bacterial type was associated with the lowest diversity and the longest ICU stays.
However, we recognize the limitations of our research. First, compared with some microbial studies in the intestine, our study's sample size was relatively small, and determining disease and causality was limited by the cross-sectional design. Second, as our main goal was to identify high-risk sepsis, not all bacteria from nasal or sputum specimens could be cultured. We also did not collect nasal or sputum specimens from all patients with sepsis for culturing and identification. While 16S technology can adequately detect genera, species detection is less accurate; thus, we cannot conclude that the causative agents of sepsis were Staphylococci, Corynebacteria or Acinetobacter in each of the three bacterial types described. Third, the data were obtained at an adult ICU, and some differences may exist in pediatric ICUs. This cross-sectional study suggests that a relationship exists among the bacterial type, length of hospital stay and mortality and patients who present with particular microbiota profiles, such as the Acinetobacter type, at admission may be more likely to have more serious disease or longer stays. Therefore, in the future, we may conduct longitudinal studies of nasal microorganisms in more patients with sepsis and examine the characteristics of septic patients in pediatric ICUs. In addition to 16S sequencing, we may also identify the main pathogens and drug-resistant strains in the nasal cavity or sputum by combining culturing and identification in the future.
Conclusion
This study identified the characteristics of the nasal microbiota during sepsis and revealed that the Acinetobacter type was associated with the highest mortality in the >9-day group, and that the nasal bacterial types were significantly associated with the length of stay in the ICU.
Summary points.
The nasal microbiotas in the septic patients differed from those in the healthy subjects.
The nasal microbiotas in the septic patients were dominated by three nasal bacterial types (Corynebacterium, Staphylococcus and Acinetobacter).
In the septic patients, the Acinetobacter nasal bacterial type was associated with the lowest diversity and the longest intensive care unit length of stay (median: 9 days), and the Corynebacterium type was associated with the shortest length of stay.
In the >9-day group, the Acinetobacter type was associated with the highest mortality rate of 33%.
Footnotes
Author contributions
X-L Tan designed the study; performed sample and clinical data collection, DNA extractions, PCR and bioinformatic analyses; interpreted the data; and wrote the manuscript. H-Y Liu designed and validated the 16S rRNA sample analysis pipeline and provided the associated figure. J Long, Z Jiang and X Zhao contributed to the clinical data abstraction and performed sample collection and DNA extractions. Y Luo assisted with the results interpretation and reviewed the manuscript. X Zhong, S Cai, Z Cen and J Su contributed to patient recruitment. H Zhou reviewed the findings and the manuscript.
Financial & competing interests disclosure
This work was supported by the National Natural Science Foundation of China (NSFC313220143 and NSFC31270152). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they obtained appropriate institutional review board approval and followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, for investigations involving human subjects, informed consent was obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Labelle A, Juang P, Reichley R, et al. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit. Care Med. 2012;40(7):2016–2021. doi: 10.1097/CCM.0b013e318250aa72. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Tian H, Du X, et al. Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit. Care Med. 2017;45(7):1168–1176. doi: 10.1097/CCM.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 4.Tulloch LG, Chan JD, Carlbom DJ, Kelly MJ, Dellit TH, Lynch JB. Epidemiology and microbiology of sepsis syndromes in a university-affiliated urban teaching hospital and level-1 trauma and burn center. J. Intensive Care Med. 2017;32(4):264–272. doi: 10.1177/0885066615592851. [DOI] [PubMed] [Google Scholar]
- 5.Machado FR, Cavalcanti AB, Bozza FA, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect. Dis. 2017;17(11):1180–1189. doi: 10.1016/S1473-3099(17)30322-5. [DOI] [PubMed] [Google Scholar]; •• Sepsis is a serious public health problem with a reported mortality rate of 55.7%.
- 6.Graspeuntner S, Waschina S, Kunzel S, et al. Gut dysbiosis with Bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin. Infect. Dis. 2018 doi: 10.1093/cid/ciy882. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CJ, Embleton ND, Marrs ECL, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome. 2017;5(1):75. doi: 10.1186/s40168-017-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit. Care Med. 2017;45(2):337–347. doi: 10.1097/CCM.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson RP. The microbiome and critical illness. Lancet Respir. Med. 2016;4(1):59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016;1(10):16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KA, Daneman N, Stevens VW, et al. Integrating time-varying and ecological exposures into multivariate analyses of hospital-acquired infection risk factors: a review and demonstration. Infect. Control Hosp. Epidemiol. 2016;37(4):411–419. doi: 10.1017/ice.2015.312. [DOI] [PubMed] [Google Scholar]
- 12.Ochotorena E, Hernandez Morante JJ, Canavate R, Villegas RA, Viedma I. Methicillin-resistant Staphylococcus aureus and other multidrug-resistant colonizations/infections in an intensive care unit: predictive factors. Biol. Res. Nurs. 2018 doi: 10.1177/1099800418818387. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Wolkewitz M, Cooper BS, Palomar-Martinez M, et al. Multiple time scales in modeling the incidence of infections acquired in intensive care units. BMC Med. Res. Methodol. 2016;16(1):116. doi: 10.1186/s12874-016-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• When patients are admitted to an intensive care unit, the risk of getting an infection is highly dependent on their length of stay in the intensive care unit.
- 14.Kloepfer KM, Deschamp AR, Ross SE, et al. In children, the microbiota of the nasopharynx and bronchoalveolar lavage fluid are both similar and different. Pediatr. Pulmonol. 2018;53(4):475–482. doi: 10.1002/ppul.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luna PN, Hasegawa K, Ajami NJ, et al. The association between anterior nares and nasopharyngeal microbiota in infants hospitalized for bronchiolitis. Microbiome. 2018;6(1):2. doi: 10.1186/s40168-017-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Microbiota severity associations from the nasopharynx are recapitulated in the anterior nares.
- 16.Man WH, De Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017;15(5):259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bessesen MT, Kotter CV, Wagner BD, et al. MRSA colonization and the nasal microbiome in adults at high risk of colonization and infection. J. Infect. 2015;71(6):649–657. doi: 10.1016/j.jinf.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Walsh AL, Fields AC, Dieterich JD, Chen DD, Bronson MJ, Moucha CS. Risk factors for Staphylococcus aureus nasal colonization in joint arthroplasty patients. J. Arthroplasty. 2018;33(5):1530–1533. doi: 10.1016/j.arth.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly JP, Safford MM, Shapiro NI, Baddley JW, Wang HE. Application of the third international consensus definitions for sepsis (Sepsis-3) classification: a retrospective population-based cohort study. Lancet Infect. Dis. 2017;17(6):661–670. doi: 10.1016/S1473-3099(17)30117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YE, Wang Y, He Y, et al. Homogeneity of the vaginal microbiome at the cervix, posterior fornix, and vaginal canal in pregnant Chinese women. Microb. Ecol. 2015;69(2):407–414. doi: 10.1007/s00248-014-0487-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu HY, Zhang SY, Yang WY, et al. Oropharyngeal and sputum microbiomes are similar following exacerbation of chronic obstructive pulmonary disease. Front. Microbiol. 2017;8:1163. doi: 10.3389/fmicb.2017.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Wu W, Zheng HM, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018;24(10):1532–1535. doi: 10.1038/s41591-018-0164-x. [DOI] [PubMed] [Google Scholar]
- 24.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5(2):169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The tutorials of ‘enterotyping’ reported by Arumugam.
- 26.Kuhn M. Building predictive models in R Using the caret Package. J. Stat Softw. 2008;28(5):1–26. [Google Scholar]; • Random forest classification models were trained by the caret R package to classify samples from patients with sepsis and healthy controls.
- 27.Svetnik V, Liaw A, Tong C, Culberson JC, Sheridan RP, Feuston BP. Random forest: a classification and regression tool for compound classification and QSAR modeling. J. Chem Inf. Comput. Sci. 2003;43(6):1947–1958. doi: 10.1021/ci034160g. [DOI] [PubMed] [Google Scholar]
- 28.Fawcett T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006;27(8):861–874. [Google Scholar]
- 29.Costea PI, Hildebrand F, Arumugam M, et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018;3(1):8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6(2):e00037. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports the nasal microbiota of healthy individuals.
- 32.Wilson MT, Hamilos DL. The nasal and sinus microbiome in health and disease. Curr. Allergy Asthma Rep. 2014;14(12):485. doi: 10.1007/s11882-014-0485-x. [DOI] [PubMed] [Google Scholar]; •• Reports the nasal microbiota of healthy subjects.
- 33.Pettigrew MM, Gent JF, Kong Y, et al. Association of sputum microbiota profiles with severity of community-acquired pneumonia in children. BMC Infect. Dis. 2016;16:317. doi: 10.1186/s12879-016-1670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel MD, Gail A, Ludmila K. Extreme Dysbiosis of the microbiome in critical illness. Msphere. 2016;1(4):e00199–e00116. doi: 10.1128/mSphere.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jasani B, Kannan S, Nanavati R, Gogtay NJ, Thatte U. An audit of colistin use in neonatal sepsis from a tertiary care centre of a resource-limited country. Indian J. Med. Res. 2016;144(3):433–439. doi: 10.4103/0971-5916.198682. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Sepsis caused by drug-resistant Acinetobacter baumannii and other Gram-negative bacilli is a common challenge for clinicians and microbiologists.
- 36.Raphael E, Riley LW. Infections caused by antimicrobial drug-resistant saprophytic Gram-negative bacteria in the environment. Front. Med. 2017;4:183. doi: 10.3389/fmed.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everett BR, Sitton JT, Wilson M. Efficacy and cost-benefit analysis of a global environmental cleaning algorithm on hospital-acquired infection rates. J. Patient Saf. 2017;13(4):207–210. doi: 10.1097/PTS.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 38.Liou ML, Chen KH, Yeh HL, Lai CY, Chen CH. Persistent nasal carriers of Acinetobacter baumannii in long-term-care facilities. Am. J. Infect. Control. 2017;45(7):723–727. doi: 10.1016/j.ajic.2017.02.005. [DOI] [PubMed] [Google Scholar]; •• Continuous colonization of the nasal cavity with Acinetobacter baumannii may be the source of Acinetobacter baumannii infection.
- 39.Wang HL, Sui WJ, Wang JR, et al. [Risk factors for acquired multidrug-resistant Acinetobacter baumannii colonization in respiratory intensive care unit] Zhonghua Yi Xue Za Zhi. 2012;92(14):960–963. [PubMed] [Google Scholar]
- 40.B R, B V, F A. Ventilator-associated pneumonia and its responsible germs: an epidemiological study. Emergency (Tehran) 2017;5(1):e26. [PMC free article] [PubMed] [Google Scholar]
- 41.Fodor AA, Klem ER, Gilpin DF, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS ONE. 2012;7(9):e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slater M, Rivett DW, Williams L, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2014;69(7):673–674. doi: 10.1136/thoraxjnl-2013-204517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frattari A, Parruti G, Erasmo R, et al. Recurring septic shock in a patient with blunt abdominal and pelvic trauma: how mandatory is source control surgery?: a case report. J. Med. Case Rep. 2017;11(1):49. doi: 10.1186/s13256-017-1206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajovic O, Todorovic Z, Mijalilovic Z, et al. [Incidence, risk factors and outcome of nosocomial pneumonia patients with central nervous system infections] Srp. Arh. Celok. Lek. 2011;139(7–8):476–480. doi: 10.2298/sarh1108476g. [DOI] [PubMed] [Google Scholar]
- 45.Aktar F, Tekin R, Gunes A, et al. Determining the independent risk factors and mortality rate of nosocomial infections in pediatric patients. BioMed Res. Int. 2016:7240864. doi: 10.1155/2016/7240864. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly BJ, Imai I, Bittinger K, et al. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:7. doi: 10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]