Abstract
Platycodon grandiflorus is a plant widely used in traditional Chinese medicine, of which polysaccharides are reported to be the main components responsible for its bio-functions. In this work, the inulin-type fructan (PGF) was obtained by DEAE anion exchange chromatography from the water extracted from P. grandifloras. Characterization was performed with methanolysis, methylation, and NMR and the results showed that PGF is a β-(2-1) linked fructan, with terminal glucose and with a degree of polymerization of 2–10. In order to study its biofunctions, the prebiotic and immunomodulation properties were assayed. We found that PGF exhibited good prebiotic activity, as shown by a promotion on six strains of lactobacillus proliferation. Additionally, the PGF also displayed direct immunomodulation on intestinal epithelial cells and stimulated the expressions of anti-inflammatory factors. These results indicated that the inulin from P. grandiflorus is a potential natural source of prebiotics as well as a potential intestinal immunomodulator, which will be valuable for further studies and new applications.
Keywords: Platycodon grandiflorus, inulin-type fructan, prebiotic activity, immunomodulation
1. Introduction
The immune system is responsible for the initiation of immune response and is of critical importance for the recognition or elimination of foreign substances to keep an immune balance [1]. The gastrointestinal tract is the interface between host and environment and it acts as the largest immune organ of human beings [2]. Numerous commensal bacteria are housed in the gastrointestinal tract and are thus implicated in the modification of the immune response in the gastrointestinal tract [3,4,5]. Among these commensal bacteria, probiotics, which are defined as ‘live microorganisms that confer a health benefit on the host’, are, with large evidence, emerging as important participants in promoting a non-immunologic gut defense barrier and improving the intestine’s immunological barrier [6,7]. Therefore, probiotics are widely used in gastrointestinal disorders, especially in intestinal inflammation resulting from imbalance of the intestinal microflora. Oral probiotics are the most used method, but defects and limitations of these have elicited interest in exploring biomolecules with prebiotic activity to modulate the intestinal immune response.
Several polysaccharides, especially the neutral ones, were reported to function as prebiotics. The inulin-type fructan, which is widely distributed in medical plants and is most abundant in Compositae and in Platycodon grandifloras [8,9], is one of the neutral types of polysaccharides. As a natural storage polysaccharide with a variety of prospects in food and pharmaceutical applications, inulin is a linear plant oligo- and poly-saccharide, which consists of a minimum two fructose-units and at least one β-(2-1) fructosyl-fructose glycosydic bond [10,11]. A variety of bio-functions have been demonstrated for inulin, including as an anti-oxidant [12,13], immune and metabolism regulation [9,11,14,15], and for intestinal microbial balance improvement [16]. A beneficial effect of inulin was reported to selectively stimulate the growth and/or activity of one or a limited number of bacteria in the colon [17]. Inulin from different plants, such as Burdock [18], Stevia rebaudiana [19], and Codonopsis pilosula [20], showed prebiotic activity on lactobacilli in vitro. In addition to functioning as a prebiotic to indirectly modulate intestinal immune response, inulin could be detected by gut dendritic cells or intestinal epithelial cells and directly stimulate the anti-inflammatory cytokines release implicated in immune response [21,22,23]. Therefore, a great prospective application of inulin could be predicted for the treatment of diseases related to intestinal microbial imbalance or immune dysfunction.
Platycodonis Radix, the root of Platycodon grandiflorus (Jacq.) A. DC., is a traditional herb widely used for the treatment for sore throats, excessive phlegm, and coughing in Eastern Asia. Modern pharmacology studies have further expanded its applications for anti-asthmatic, hepatoprotective, anti-oxidant, immunoregulation, and anti-tumor effects [24,25]. As both an edible and a pharmaceutical material, P. grandiflorus not only contains nutrient substances, such as amino acids, saponins, flavonoids, anthocyanins, and phenolics, but is also rich in polysaccharides [25,26]. Previous studies have demonstrated that the polysaccharide is one of the major active ingredients of P. grandiflorus and the function studies of them have displayed bio-activities, including as an anti-oxidative [27], for anti-angiogenesis [28], and especially for immunoregulation [29]. For example, P. grandiflorus polysaccharides were reported to induce the maturation of dendritic cells or macrophage, accompanied with increased expressions of MHC-I/II, co-stimulatory molecules, and cytokines [29,30]. However, the composition and structure of these polysaccharides, including the inulin-type fructan, have not been clearly identified and characterized. The neutral polysaccharides, but not inulin, from P. grandiflorus were isolated by DEAE-cellulose chromatography before, but its immunomodulating bioactivity and prebiotic activity are not well defined for now [28,31].
In this study, we aim to isolate a fructan from P. grandifloras using water extraction and ethanol precipitation, followed by ion exchange chromatography, to further characterize its structure and investigate its potential prebiotic and immunomodulating activity in vitro.
2. Results and Discussions
2.1. Purification of Inulin from P. grandiflorus
The crude polysaccharide from P. grandiflorus (PG) was obtained by using water extraction and ethanol precipitation, which is different from other studies, as they used a 70% ethanol extraction [32]. The crude polysaccharide PG (1.2 g) was dissolved in distilled water and subjected to DEAE ion exchange chromatography six times (200 mg for each time). The distilled water elutes (PGF) were collected and combined, concentrated, and lyophilized, with a yield of 59.4% (0.7127 g PGF from 1.2 g PG). Oka et al. purified several inulin-type fructans from P. grandifloras, with different degrees of polymerization, using ion exchange chromatography, but they used a different elution buffer and more steps for purification [32].
2.2. Chemical Characterization of PGF
The results of methanolysis and the Urea-HCl colorimetric method indicated that PGF only contains glucose (Glc) and fructose (Fru). The results of a Folin–Ciocalteu assay and a Bio-Rad protein assay indicated that there were no phenolic compounds and trace amount of protein was detected in PGF. The inter-glycosidic linkages were identified by GC-MS, after methylation analysis, and the results showed that PGF was composed of mainly terminal-Fruf, terminal-Glcp, and 2, 1-linked Fruf, with a molar ratio of 1, 2, and 7, respectively.
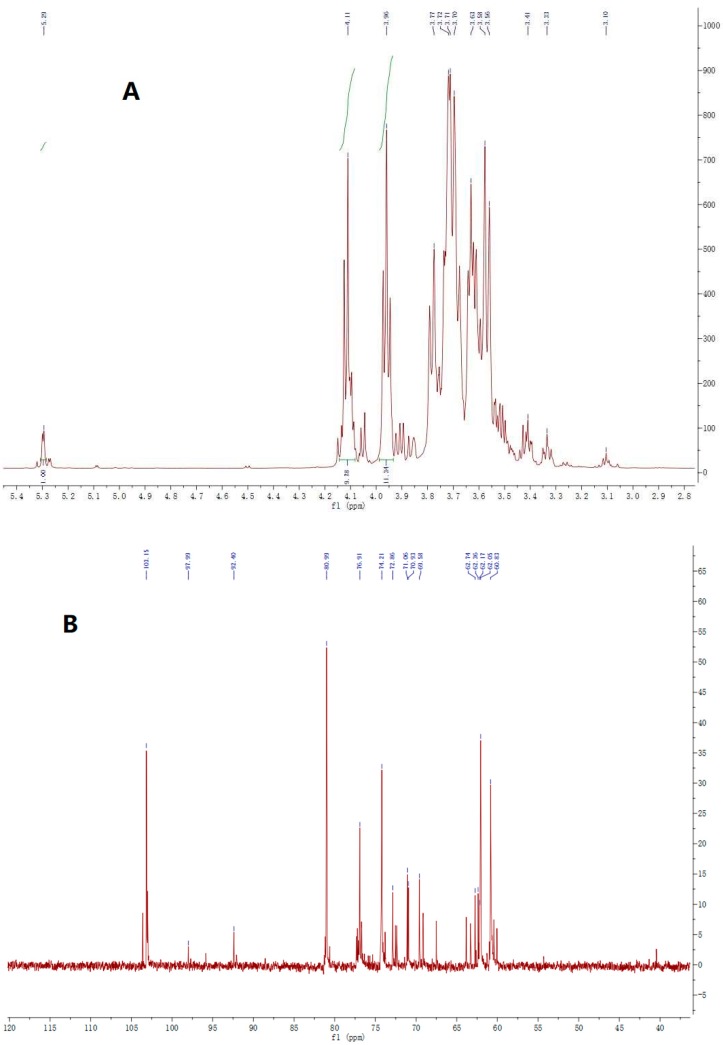
The NMR signals were characterized and compared with chemical shift values from literature [20,32,33,34]. The 1H NMR spectrum of PGF contained a main anomeric proton at 5.29 ppm, belonging to the H1 of terminal linked Glcp (Figure 1A). The other signals at δ 4.11 and 3.96 corresponded to the H3-Fruf and H4-Fruf, respectively. The degree of polymerization (DP) was calculated by the mean ratio between the integral proton signal (H3-Fruf and H4-Fruf) and the integral of the Glcp signal (H1-Glcp) [35], with the result suggesting the DP of PGF was about 10. The DP of PGF, obtained in the present study, is similar to those extracted from P. grandiflorus using a 70% ethanol extraction method, in which the DP ranged from 2 to 9 [32]. The signals between δ 3.56–3.77 corresponded to H1-Fruf, H5-Fruf, and H6-Fruf (Figure 1A). The 13C NMR spectrum contained six major signals, one major signal at 103.15 ppm, assigned to the C2 carbon of Fruf, and five signals at 60.83, 76.91, 74.21, 80.99, and 62.05 ppm, as the signals of C1-Fruf, C3-Fruf, C4-Fruf, C5-Fruf, and C6-Fruf, respectively (Figure 1B). The carbon atom signal at 92.39 ppm was assigned to Glcp because signals beyond 100 ppm pointed to ketose residues. The other signals between 60.42–71.06 ppm belonged to the C2–C6 of the Glcp (Figure 1B). All these results indicated that the PGF was linked on C2 of Fruf and C1 of the terminal-Glcp, with a backbone of (2→1)-Fruf, identified as a typical inulin-type fructan, as follows: A β-(2→1)-linked configuration at the anomeric carbon of fructosyl residues, connected with terminal Glcp residue by an α-d-(1→2) bond, combing the results of monosaccharide compositions and glycosidic linkage. Those results indicated the structure of PGF as α-d-Glcp-(1→2)–(β-d-Fruf-(2→1)-β-d-Fruf)n-(2→1)-β-d-Fruf, an inulin-type fructan.
Figure 1.
The1H-NMR (A) and 13C-NMR (B) spectrum of PGF.
2.3. Prebiotic Activity of PGF
The inulin-type fructan cannot be hydrolyzed or absorbed in the upper part of the gastrointestinal tract (GIT), but is degraded by the gut microbiota in the large intestine through processes of fermentation [9,11]. Inulin fermentation will improve the composition of the gut microbiota and is beneficial for the probiotic’s enrichment. Additionally, short-chain fatty acids (SCFAs) will be produced during fermentation and implicated in multiple cellular and physiological processes, such as energy metabolism, hormone release, and immune response [36,37]. In our study, PGF (DPn 2–10) was separately fermented with six strains of lactobacilli and significantly higher bacterial optical densities were detected in the medium with PGF than that in the basal medium (without sugar) (Table 1). In this study, two available commercial prebiotics, P95s (DPn 2–9) and Orafti®® HP (DPav ≥ 23), were selected to compare with PGF. The bacterial optical densities in the medium containing Orafti®® HP were almost the same as in the basal medium, suggesting that Orafti®® HP displayed no prebiotic effect on all the lactobacilli. Differently, the bacterial optical density in the medium containing P95s was dramatically increased. Among them, except for the L. gasseri KQ11-1, the optical density of the other bacteria was higher than that in the PGF group. These results demonstrated that the activity of probiotics on lactobacilli was related to its DP, which was consistent with previous reports [19,35]. Similarly, besides L. rhamnosus LGG, the pH values of the PGF medium, after incubation with other strains of lactobacilli, were lower than the basal medium and were the lowest in P95s group. Additionally, of course, the pH was changed with the species of lactobacilli (Table 2). The reductions of pH in the PGF or P95s groups were probably due to the metabolites, like lactic acid and acetic acid, produced by lactobacilli, or the SCFAs, the fermentation products from inulin [11,38]. All these results indicated that the PGF was a potential prebiotic, stimulating the growth of lactobacilli and with a lower pH.
Table 1.
Capacity to ferment PGF and commercial prebiotics by lactobacilli.
| Groups | The Bacterial Density (△A600, n = 4) | ||||
|---|---|---|---|---|---|
| PGF | Orafti®® HP | P95s | Basal Medium | Glucose | |
| L. Johnsonii BS15 | 0.037 ± 0.001 a,b,A | 0.015 ± 0.001 a,A | 0.051 ± 0.033 b,A | 0.016 ± 0.002 a,A | 0.060 ± 0.003 b,A |
| L. plantarum BS10 | 0.056 ± 0.001 a,A | 0.030 ± 0.001 b,B | 0.198 ± 0.006 c,B | 0.031 ± 0.003 b,B | 0.177 ± 0.00 d,B |
| plantarum BSGP201683 | 0.12 ± 0.025 a,B | 0.04 ± 0.001 b,C | 0.24 ± 0.002 c,C | 0.04 ± 0.003 b,C | 0.39 ± 0.005 d,C |
| L. rhamnosus LGG | 0.045 ± 0.002 a,A | 0.027 ± 0.005 b,B | 0.031 ± 0.002 b,A | 0.027 ± 0.004 b,B | 0.196 ± 0.007 c,B |
| L. gasseri KQ11-1 | 0.104 ± 0.004 a,B,C | 0.088 ± 0.001 b,D | 0.091 ± 0.002 b,D | 0.084 ± 0.002 b,D | 0.324 ± 0.014 c,D |
| Weissella confusa X3 | 0.085 ± 0.005 a,C | 0.040 ± 0.002 b,E | 0.224 ± 0.004 c,C | 0.037 ± 0.002 b,E | 0.395 ± 0.026 d,C |
Notes: Data are expressed as the increase in A600 of the bacterial suspension with 24 h incubations; values are means from triplicate determination ± standard deviation. a–d Data in lines with different superscripts are significantly (p < 0.05). A–E Data in columns with different superscripts are significantly (p < 0.05).
Table 2.
The final pH of mediums containing PGF and commercial prebiotics by lactobacilli.
| Groups | pH (n = 4) | ||||
|---|---|---|---|---|---|
| PGF | Orafti®® HP | P95s | Basal Medium | Glucose | |
| L. Johnsonii BS15 | 5.87 ± 0.15 a,A,B | 6.03 + 0.06 b,A | 5.67 + 0.05 c,A | 6.10 + 0.00 b,A | 5.03 + 0.06 d,A |
| L. plantarum BS10 | 5.93 ± 0.02 a,A | 6.08 ± 0.01 b,A | 5.49 ± 0.01 c,B | 6.08 ± 0.02 b,A | 5.03 ± 0.01 d,A |
| L. plantarum BSGP201683 | 5.54 ± 0.03 a,C | 5.68 ± 0.02 b,B | 5.37 ± 0.02 c,C | 5.78 ± 0.02 d,B | 5.06 ± 0.01,e,A |
| L. rhamnosus LGG | 5.76 ± 0.06 a,B,D | 5.82 ± 0.12 a,C | 5.82 ± 0.06 a,D | 5.89 ± 0.05 a,C | 4.64 ± 0.05 b,B |
| L. gasseri KQ11-1 | 5.40 ± 0.05 a,E | 5.51 ± 0.01 a,D | 5.50 ± 0.09 a,B | 5.48 ± 0.09 a,D | 4.45 ± 0.07 b,C |
| Weissella confusa X3 | 5.70 ± 0.00 a,D | 5.80 ± 0.00 b,C | 5.40 ± 0.00 c,C | 5.80 ± 0.00 b,B | 5.07 ± 0.06 d,A |
Notes: Data are expressed as the final pH of the medium after 24 h incubations; values are means from triplicate determination ± standard deviation. a–d Data in lines with different superscripts are significantly (p < 0.05). A–E Data in columns with different superscripts are significantly (p < 0.05).
As shown above, the PGF displayed prebiotic activity on all the strains of lactobacilli, but with unequal effects. The bacterial optical density of BSGP201683 in the medium with PGF increased 3-fold compared to the basal medium, but that of KQ11-1 was just 1.2-fold. The difference in inulin utilization between bacteria may be due to their enzymatic equipment, especially the hydrolases and transportases, which are responsible for the hydrolysis of fructan in position β-(2→1) [11,38].
2.4. PGF Promote the Anti-Inflammatory Factors Release of IPEC-J2 Cells
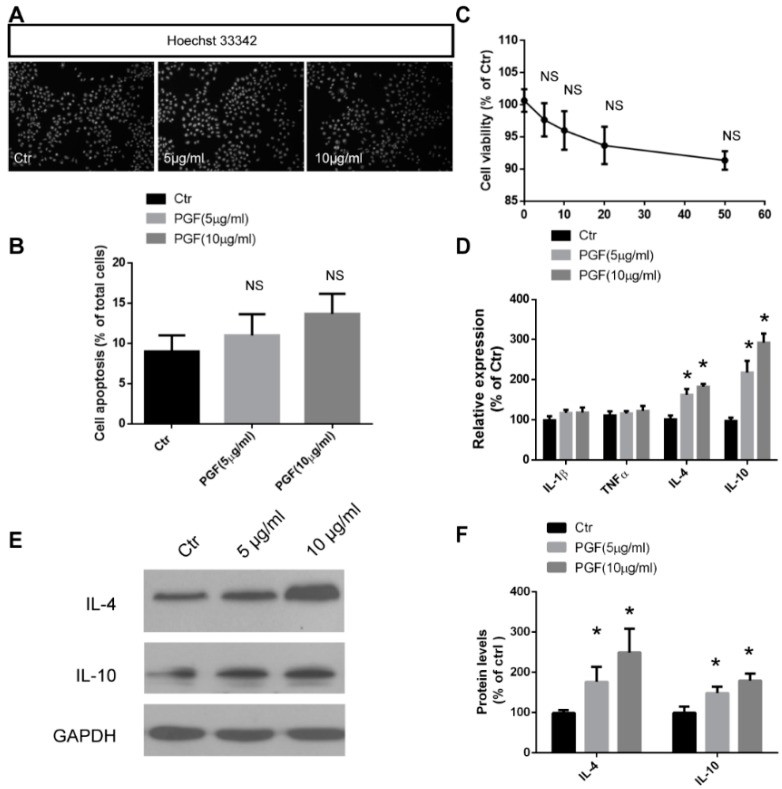
Recent studies have noticed that prebiotic carbohydrates, such as inulin, may elicit additional direct effects on immunomodulation [1,21]. This may occur via direct contact between inulin and intestinal epithelial cells, to modify epithelial tight junction integrity, or the release of pro- or anti-inflammatory factors [39]. To evaluate the immunomodulating effects of PGF on intestinal epithelial cells, in vitro assays were performed with a porcine jejunum epithelial cell line (IPEC-J2). Firstly, the cytotoxicity of the PGF was evaluated and we found that the additional PGF exhibited no cellular toxicity up to 50 μg/mL, which was indicated by CCK-8 assays and Hoechst 33342 staining (Figure 2A–C). Then, the expressions of pro- or anti-inflammatory factors were detected in IPEC-J2 cells after PGF treatment. It was found that anti-inflammatory factor (IL-4 and IL-10) mRNA levels were significantly increased in a dose dependent manner, but little pro-inflammatory change (IL-1β and TNF-α) was present (Figure 2D). Additionally, protein levels of IL-4 and IL-10 were further evaluated by western blots, which found the same results as with mRNA levels (Figure 2E,F). These data demonstrated that, except functioning as probiotics, PGF may be implicated in the intestinal immune response directly through an activation of the anti-inflammatory factor’s expression or even release. However, the exact mechanism behind this effect is not well understood and needs to be further studied. It is suggested that pathogen recognition receptors (PRRs), such as toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs), possess carbohydrate binding properties and are involved in the perception of carbohydrates in the intestine [40,41,42]. When carbohydrates ligate with PRRs, cellular signals are elicited and further modulate intestinal barrier function and immune response. It is also reported that inulin-type fructans may even be inserted into the lipid membrane and subsequent signal transduction may be induced [43,44,45]. Therefore, more studies are needed to clarify whether PGF could bind to some PRRs, or insert into the lipid membrane to directly stimulate the intestinal immune response.
Figure 2.
PGF promote the anti-inflammatory factors’ release of IPEC-J2 cells. (A) Representative images display the nuclear morphology of IPEC-J2 cells under different doses of PGF treatment for 24 h, labeled with Hoechst 33342. (B) Quantification shows that PGF doesn’t induce cell apoptosis in IPEC-J2 cells, according to Hoechst staining. (C) Quantification shows the cell viability of IPEC-J2 cells treated with different concentrations of PGF for 24 h. (D) Quantification shows increased expression levels of anti-inflammatory factors, but not pro-inflammatory factors, after the treatment of PGF (5 μg/mL and 10 μg/mL). (E and F) Western blots and quantification show increased protein levels of anti-inflammatory factors after the treatment of PGF (5 μg/mL and 10 μg/mL). Error bars indicate SEM. * p < 0.05, NS, not significant.
3. Materials and Methods
3.1. Materials and Reagents
The roots of P. grandiflorus were purchased from the Lotus Pond Chinese herbal medicine market, and identified as Platycodon grandiflorus (Jacq.) A. DC. by Dr. Yuan-Feng Zou of Sichuan Agricultural University. The roots were dried in a drying oven (DHG-9420A, Yi-heng Technology Co., Ltd., Shanghai, China) at 40 °C overnight and pulverized to a fine powder by a mechanical grinder, then passed through 0.25 mm mesh.
The MRS medium (HB0384-1), peptone (HB8276), and tryptone (HB8270) were purchased from Hopebio Biotechnology Co., Ltd. (Qingdao, China); the yeast extract powder (JM-500) was purchased from Biotopped Science and Technology Co., Ltd. (Beijing, China); the McIntosh Turbidimetric tube (G60346) was obtained from Wenzhou Kangtai Biotechnology Co., Ltd. (Wenzhou, Zhejiang, China); and the standards fructo-oligosaccharide (QHT-FOS-P95S) and inulin (Orafti®® HP) were purchased from Quantum Hi-Tech Biological Co., Ltd. (Jiangmen, China) and Beneo-Orafti (Oreye, Belgium), respectively.
The standards of fructose (Fru) and glucose (Glc) were purchased from Solarbio (Beijing, China). All other chemicals, such as phenol, sulfuric acid, acetone, boric acid, glycerin, etc., were of analytical grade, obtained from the Chengdu Kelong chemical factory (Chengdu, China).
3.2. Isolation of Inulin from P. grandiflorus
Seventy grams of P. grandiflorus powder were extracted by refluxing petroleum ether to remove low molecular weight and lipid soluble compounds. The residue was dried and extracted three times, with distilled water (water/material, 30 mL/1 g) for 2 h each time. These extracts were filtered and concentrated, then precipitated with 3-fold volumes of ethanol at 4 °C for 24 h. After centrifugation at 1000 rpm for 15 min, the precipitate was freeze-dried, named PG (the crude polysaccharides from P. grandiflorus).
The crude polysaccharide PG (1.2 g) was dissolved in distilled water and then centrifuged (1000 rpm, 10 min) to remove insoluble impurities. The supernatant was filtered through a 0.45 μm filter for purification. These PG solutions were applied to a DEAE-Sepharose (Fast Flow, FF) column (50 mm × 40 cm, Beijing Rui Da Heng Hui Science Technology Development Co., Ltd., Beijing, China), and eluted with distilled water. The flow rate was 2.0 mL/min. It was collected until no sugar was detected by the PABR method (Boratyński, 1984). Then, the eluate was concentrated and lyophilized and named PGF.
3.3. Characterization of Inulin from P. grandiflorus
The monosaccharide composition of PGF was determined using methanolysis. Approximately 1 mg of PGF was dissolved in 3 M hydrochloric acids in anhydrous methanol for 24 h at 80 °C and then derivatized by trimethylchlorsilane (TMS). The derivatives were analyzed using gas chromatography [46,47]. Mannitol was added to the samples as the internal standard. The presence of fructose was tested with the Urea-HCl colorimetric method [48].
The total amount of phenolic compounds in the purified polysaccharide fractions were quantitatively determined using the Folin–Ciocalteu assay [49]. The protein content of the polysaccharide fractions was determined by the Bio-Rad protein assay, based on the method of Bradford [50].
The glycisidic linkages were determined by methylation. The derivatives were analyzed by a GCMS-QP2010 (Shimadzu, Kyoto, Japan). The relative amount of each type of linkage was determined based on the area of each compound and related to the monosaccharide composition of each compound [47].
Approximately 20 mg of PGF was first deuterium exchanged three times by freeze-drying in D2O (10 mg/mL). The 1H NMR and 13C NMR spectra of PGF were recorded on a Bruker AV600 instrument (Bruker, Rheinstetten, Germany) at 25 °C.
3.4. Prebiotic Activity
3.4.1. Bacterial Strains
Six strains of lactobacilli were used to determine the prebiotic activity in vitro. The L. johnsonii (BS15, CCTCC: M2013663), L. plantarum (BS10, CCTCC: M2012487), L. plantarum (BSGP201683, CCTCC: M2016425), L. rhamnosus GG (LGG, ATCC53103) and Weissella confusa X3 were gifts from professor Xue-Qin Ni of Animal Microecology Institute, College of Veterinary Medicine, Sichuan Agricultural University, China. L. gasseri (KQ11-1, ATCC 33323) was a gift from Dr. Bing-Zhao Zhang of Shenzhen Institutes of Advanced Technology, Chinese Academy of Science, China. They were stored at −80 °C in the MRS medium, with 20% glycerin.
3.4.2. Bacterial Growth
The basal medium (10 g tryptone, 10 g peptone, 5 g yeast extract, 1 mL of Tween 80, 0.5 g/L-cysteine hydrochloride, 1 g/L carbohydrate source, and 1 L of distilled water, pH 6.5) and the MRS medium were autoclaved at 121 °C for 20 min. The PGF (10 mg/mL in saline, the same below), P95s, Orafti®® HP, and glucose were used as carbon sources after being filtered through a sterile filter (0.22 μm), in which the P95s (96.1% fructo-oligosaccharides, DPn 2–9, with 2.7% glucose, fructose, and sucrose, product of the partial enzymatic hydrolysis of chicory inulin) and Orafti®® HP (99.8% inulin, DPav ≥ 23, with 0.2% glucose, fructose, and sucrose), commercially available prebiotics, were used for comparison with PGF. Meanwhile, the basal medium, without carbohydrates, was used as the negative control [35,51].
These six strains of lactobacilli were incubated in 50 mL of the MRS medium at 37 °C overnight in an anaerobic chamber (Thermo Scientific 1029, in 85% N2, 10% H2, 5% CO2), then centrifuged (2129 g, 10 min) and resuspended in the saline and basal mediums to remove the carbon source. Finally, they were resuspended with the basal medium containing these four different carbon sources above (PGF, P95s, Orafti®® HP and glucose) at a concentration of 107–108 CFU/mL bacteria [38], adjusted by a McIntosh Turbidimetric tube. The 5 milliliter bacterial suspensions were divided into test tubes and then incubated at 37 °C for 0 h and 24 h. All the test tubes were set in triplicate. After 0 h and 24 h of incubation, 200 μL of the basal medium was added to the 96-well plates and the densities of the bacteria were measured at the wave-length of 600 nm (A600) by a Multiscan Spectrum (Thermo Scientific, Varioskan Flash, Waltham, MA, USA). The bacterial growth was presented as the increment in A600 (ΔA600) during 24 h of incubation in anaerobic chamber. The pH was measured by a pH meter (A115200, Lichen Instrument Technology Co. Ltd., Changsha, Hunan, China) after removing bacteria by centrifuge, at 2231g for 20 min after 24 h of incubation. Each tube was tested three times to ensure high accuracy and precision.
3.5. Cell Culture
The IPEC-J2 was obtained from the Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences (Shanghai, China). IPEC-J2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, GE Healthcare Life Sciences, Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin–streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were maintained at 37 °C in a humidified atmosphere (5% CO2).
3.6. Cell Viability Assay
Cell viability was determined by CCK-8 assay. The cells were seeded with a density of 5 × 103 cells/well in 96-well plates and incubated, at 37 °C, in a complete medium (DMEM with 10% FBS), with serial dilutions of PGF, for 24 h. CCK-8 was added to each well of the 96-well plate (CCK-8, plus fresh medium at a fixed ratio of 1:10, 100 μL/well), followed by further incubation for 1 h. The absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
3.7. Hoechst 33342 Staining
Briefly, IPEC-J2 were plated in 12-well plates with a density of 5 × 104 cells/well and treated with PGF at 37 °C for 24 h. The cells were washed in PBS three times and incubated in the Hoechst 33342 solution (10 μg/mL) for 30min at 4 °C. Finally, fluorescence microscopy was performed to observe the nuclear changes of IPEC-J2 cells. For each treatment group, ≥1000 cells were analyzed in triplicate.
3.8. Quantitative Realtime PCR
RNA extraction from IPEC-J2 cells and real time PCR for anti- or pro-inflammatory factor detection was performed as previously reported [52]. Briefly, IPEC-J2 cells were lysed with Trizol Regent (Invitrogen, Waltham, MA, USA) and the total RNA was extracted from the IPEC-J2 cells, according to the manufacturer’s instructions. The quality of RNA was assessed by agarose gel and the concentration was measured with a spectrophotometer (NanoDrop 2000, Thermo Scientific, Shanghai, China). The total RNA was subjected to reverse transcription with a reverse transcriptase, according to the manufacturer’s instructions (Fermentas, Waltham, MA, USA). Quantitative real-time PCR was performed using the Bio-Rad CFX96 system and the relative gene expression was normalized to the internal control actin. Primer sequences for SYBR Green probes of the target genes are described in Table 3.
Table 3.
Primer sequences for qRT-PCR.
| Gene | Primers |
|---|---|
| IL-1β | Fr 5′-GGCCGCCAAGATATAACTGA-3′ |
| Rv 5′-GGACCTCTGGGTATGGCTTTC-3′ | |
| TNF-α | Fr 5′-CGCCCACGTTGTAGCCAATGT-3′ |
| Rv 5′-CAGATAGTCGGGCAGGTTGATCTC-3′ | |
| IL-4 | Fr 5′-TACCAGCAACTTCGTCCAC-3′ |
| Rv 5′-ATCGTCTTTAGCCTTTCCAA-3′ | |
| IL-10 | Fr 5′-AACAAGAGCAAGGCCGTG-3′ |
| Rv 5′-AATAGTTCACAGAGAGGCTCGG-3′ | |
| β-Actin | Fr 5′-GTACCACTGGCATTGTGATG-3′ |
| Rv 5′-ATCTTCATGGTGCTAGGAGC-3′ |
3.9. Statistical Analysis
All data obtained above were analyzed by SPSS statistics software (Version 20.0, USA), including the one-way analysis of variance with Duncan’s test, presented as the mean ± standard deviation (SD). The differences between the groups were evaluated at the statistically significant level of p < 0.05.
4. Conclusions
In summary, a purified inulin-type fructan from P. grandiflorus was obtained by DEAE ion exchange chromatography. Structural analysis by methanolysis, methylation, and NMR identified PGF as a typical inulin-type fructan having (2-1) linkages. Moreover, this PGF was shown to be an effective prebiotic on lactobacilli and an immunomodulator on intestinal epithelial cells. However, more in vivo studies are needed prior to the further advancement of PGF applications.
Acknowledgments
We acknowledge the support by Key Laboratory of Animal Disease and Human Health of Sichuan Province, and the GC-MS determination support by the Department of Pharmaceutical Chemistry, University of Oslo. This work was partly supported by the Research Council of Norway through the Norwegian NMR Platform, NNP (226244/F50).
Author Contributions
Conceptualization, B.F., C.H., and Y.-F.Z.; Data collection, D.-J.P., M.-L.C., Y.-P.F., and F.R.; Formal analysis, Y.-P.F. and X.S.; Funding acquisition, Z.-Q.Y., Y.-L.C., and Y.-F.Z.; Methodology, M.-L.C., B.F., X.-Q.N., and Y.-P.F.; Project administration, Z.-Q.Y., R.-Y.J.; Resources, L.-X.L. and Y.-L.C.; Supervision, C.H., R.-Y.J., and Z.-L.C.; Writing—original draft, D.-J.P., C.H., M.-L.C., and Y.-F.Z.; Writing—review & editing, B.F., B.S.P., R.-Y.J., and Y.-F.Z.
Funding
This work was supported in part by the International Cooperation Projects of Science & Technology Department of Sichuan Province (2017HH0093), a General Financial Grant from the China Postdoctoral Science Foundation (2016M602704), The Foundation from Sichuan province (2019CZZX29), and Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds PGF is available from the authors.
References
- 1.Kober M.M., Bowe W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Womens Dermatol. 2015;1:85–89. doi: 10.1016/j.ijwd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mowat A.M., Viney J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 2010;156:145–166. doi: 10.1111/j.1600-065X.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff S.C., KräMer S. Human mast cells, bacteria, and intestinal immunity. Immunol. Rev. 2010;217:329–337. doi: 10.1111/j.1600-065X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 4.Hill D.A., Artis D. Intestinal Bacteria and the Regulation of Immune Cell Homeostasis. Annu. Rev. Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 6.Bron P.A., Kleerebezem M., Brummer R.-J., Cani P.D., Mercenier A., MacDonald T.T., Garcia-Ródenas C.L., Wells J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017;117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borchers A.T., Selmi C., Meyers F.J., Keen C.L., Gershwin M.E. Probiotics and immunity. J. Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 8.Apolinario A.C., de Lima Damasceno B.P.G., de Macedo Beltrao N.E., Pessoa A., Converti A., da Silva J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014;101:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 9.Shoaib M., Shehzad A., Omar M., Rakha A., Raza H., Sharif H.R., Shakeel A., Ansari A., Niazi S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016;147:444–454. doi: 10.1016/j.carbpol.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Kelly G. Inulin-type prebiotics—A review: Part I. Altern. Med. Rev. 2008;13:315–330. [PubMed] [Google Scholar]
- 11.Wilson B., Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.-X., Zhou J.-D., Cao F., Yu Z.-L., Zhang F.-R., Huang R.-X. Extraction, Purification and Polymerization Degree Distribution of Inulin from Jerusalem artichoke Grown in Saline-alkaline Soil. Food Sci. 2011;12:164–168. [Google Scholar]
- 13.Liu D., Ping W.U. Studies on the Antioxidant Activity of Inulin and Its Mechanism. J. Food Sci. Biotechnol. 2015;9:1002–1007. [Google Scholar]
- 14.Salazar N., Dewulf E.M., Neyrinck A.M., Bindels L.B., Cani P.D., Mahillon J., de Vos W.M., Thissen J.-P., Gueimonde M., Clara G. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015;34:501–507. doi: 10.1016/j.clnu.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Z.W., Dong Q., Wang Q. Effect of inulin bread on blood glucose and lipid of mice with diabetes induced by STZ. Sci. Technol. Food Ind. 2015;20:368–370. [Google Scholar]
- 16.Xu X., Xu P., Ma C., Tang J., Zhang X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013;31:318–337. doi: 10.1016/j.biotechadv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Morris C., Morris G.A. The effect of inulin and fructo-oligosaccharide supplementation on the textural, rheological and sensory properties of bread and their role in weight management: A review. Food Chem. 2012;133:237–248. doi: 10.1016/j.foodchem.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Du Y.-J., Xie C.-P. The Inulin Extraction from Burdock and Bacteriostasis Research. Food Res. Dev. 2011;12:32–36. [Google Scholar]
- 19.Lopes S.M.S., Francisco M.G., Higashi B., de Almeida R.T.R., Krausová G., Pilau E.J., Gonçalves J.E., Gonçalves R.A.C., de Oliveira A.J.B. Chemical characterization and prebiotic activity of fructo-oligosaccharides from Stevia rebaudiana (Bertoni) roots and in vitro adventitious root cultures. Carbohydr. Polym. 2016;152:718–725. doi: 10.1016/j.carbpol.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y.-P., Li L.-X., Zhang B.-Z., Paulsen B.S., Yin Z.-Q., Huang C., Feng B., Chen X.-F., Jia R.-R., Song X. Characterization and prebiotic activity in vitro of inulin-type fructan from Codonopsis pilosula roots. Carbohydr. Polym. 2018;193:212–220. doi: 10.1016/j.carbpol.2018.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Vogt L., Meyer D., Pullens G., Faas M., Smelt M., Venema K., Ramasamy U., Schols H.A., De Vos P. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 2015;55:414–436. doi: 10.1080/10408398.2012.656772. [DOI] [PubMed] [Google Scholar]
- 22.Hapfelmeier S., Müller A.J., Stecher B., Kaiser P., Barthel M., Endt K., Eberhard M., Robbiani R., Jacobi C.A., Heikenwalder M. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent stepin ΔinvG S. Typhimurium colitis. J. Exp. Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eiwegger T., Stahl B., Haidl P., Schmitt J., Boehm G., Dehlink E., Urbanek R., Szépfalusi Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 2010;21:1179–1188. doi: 10.1111/j.1399-3038.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.State Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Chemical Industry Press; Beijing, China: 2015. [Google Scholar]
- 25.Zhang L., Wang Y., Yang D., Zhang C., Zhang N., Li M., Liu Y. Platycodon grandiflorus–An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015;164:147–161. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Zhang W., Tang P., Jing W., Liu Y., Tang Z. Detection of Main Components and Antioxidant Activity of Platycodon grandiflorum Extracts. Chin. Agric. Sci. Bull. 2017;33:139–144. [Google Scholar]
- 27.Sheng Y., Liu G., Wang M., Lv Z., Du P. A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress. Int. J. Boil. Macromol. 2017;104:393–399. doi: 10.1016/j.ijbiomac.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Dong Q., Qiu H., Cong R., Ding K. Structural characterization of an arabinogalactan from Platycodon grandiflorum roots and antiangiogenic activity of its sulfated derivative. Biomacromolecules. 2010;11:2558–2566. doi: 10.1021/bm100402n. [DOI] [PubMed] [Google Scholar]
- 29.Zheng P., Fan W., Wang S., Hao P., Wang Y., Wan H., Hao Z., Liu J., Zhao X. Characterization of polysaccharides extracted from Platycodon grandiflorus (Jacq.) A. DC. affecting activation of chicken peritoneal macrophages. Int. J. Boil. Macromol. 2017;96:775–785. doi: 10.1016/j.ijbiomac.2016.12.077. [DOI] [PubMed] [Google Scholar]
- 30.Park M.J., Ryu H.S., Kim J.S., Lee H.K., Kang J.S., Yun J., Kim S.Y., Lee M.K., Hong J.T., Kim Y. Platycodon grandiflorum polysaccharide induces dendritic cell maturation via TLR4 signaling. Food Chem. Toxicol. 2014;72:212–220. doi: 10.1016/j.fct.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Liu W., Liu H., Han M. Polysaccharides from Platycodon grandiflorum. Chem. Nat. Compd. 2013;48:927–929. doi: 10.1007/s10600-013-0430-6. [DOI] [Google Scholar]
- 32.Oka M., Ota N., Mino Y., Iwashita T., Komura H. Studies on the conformational aspects of inulin oligomers. Chem. Pharm. Bull. 1992;40:1203–1207. doi: 10.1248/cpb.40.1203. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Zhang X., Cao L., Ji J., Gao J. Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules. 2018;23:3123. doi: 10.3390/molecules23123123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontes A.G.O., Silva K.L., da Cruz Fonseca S.G., Soares A.A., de Andrade Feitosa J.P., Braz-Filho R., Romero N.R., Bandeira M.A.M. Identification and determination of the inulin content in the roots of the Northeast Brazilian species Pombalia calceolaria L. Carbohydr. Polym. 2016;149:391–398. doi: 10.1016/j.carbpol.2016.04.108. [DOI] [PubMed] [Google Scholar]
- 35.Caleffi E.R., Krausová G., Hyršlová I., Paredes L.L.R., dos Santos M.M., Sassaki G.L., Gonçalves R.A.C., de Oliveira A.J.B. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Boil. Macromol. 2015;80:392–399. doi: 10.1016/j.ijbiomac.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 36.Weitkunat K., Schumann S., Petzke K.J., Blaut M., Loh G., Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015;26:929–937. doi: 10.1016/j.jnutbio.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Carlson J., Erickson J., Hess J., Gould T., Slavin J. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, Inulin and Xylooligosaccharide. Nutrients. 2017;9:1361. doi: 10.3390/nu9121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes S.M.S., Krausová G., Carneiro J.W.P., Gonçalves J.E., Gonçalves R.A.C., de Oliveira A.J.B. A new natural source for obtainment of inulin and fructo-oligosaccharides from industrial waste of Stevia rebaudiana Bertoni. Food Chem. 2017;225:154–161. doi: 10.1016/j.foodchem.2016.12.100. [DOI] [PubMed] [Google Scholar]
- 39.De Kivit S., Kraneveld A.D., Garssen J., Willemsen L.E. Glycan recognition at the interface of the intestinal immune system: Target for immune modulation via dietary components. Eur. J. Pharmacol. 2011;668:S124–S132. doi: 10.1016/j.ejphar.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 40.Osorio F., e Sousa C.R. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Elinav E., Strowig T., Henao-Mejia J., Flavell R.A. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Loo Y.-M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figdor C.G., van Spriel A.B. Fungal pattern-recognition receptors and tetraspanins: Partners on antigen-presenting cells. Trends Immunol. 2010;31:91–96. doi: 10.1016/j.it.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Vereyken I.J., Chupin V., Hoekstra F.A., Smeekens S.C., de Kruijff B. The effect of fructan on membrane lipid organization and dynamics in the dry state. Biophys. J. 2003;84:3759–3766. doi: 10.1016/S0006-3495(03)75104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vereyken I.J., Van Kuik J.A., Evers T.H., Rijken P.J., de Kruijff B. Structural requirements of the fructan-lipid interaction. Biophys. J. 2003;84:3147–3154. doi: 10.1016/S0006-3495(03)70039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austarheim I., Christensen B.E., Hegna I.K., Petersen B.O., Duus J.O., Bye R., Michaelsen T.E., Diallo D., Inngjerdingen M., Paulsen B.S. Chemical and biological characterization of pectin-like polysaccharides from the bark of the Malian medicinal tree Cola cordifolia. Carbohydr. Polym. 2012;89:259–268. doi: 10.1016/j.carbpol.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Zou Y.-F., Chen X.-F., Malterud K.E., Rise F., Barsett H., Inngjerdingen K.T., Michaelsen T.E., Paulsen B.S. Structural features and complement fixing activity of polysaccharides from Codonopsis pilosula Nannf. var. modesta LT Shen roots. Carbohydr. Polym. 2014;113:420–429. doi: 10.1016/j.carbpol.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 48.Dedonder R. Carbohydrates of the Jerusalem artichoke. I. Demonstration of a series of glucofructosans in the tubers. Isolation, analysis and structure of the less polymerised members of the series. Bull. Soc. Chim Biol. 1952;34:144–156. [PubMed] [Google Scholar]
- 49.Singleton V., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 50.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Li W., Zhang J., Yu C., Li Q., Dong F., Wang G., Gu G., Guo Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015;121:315–319. doi: 10.1016/j.carbpol.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 52.Hu J., Cao X., Pang D., Luo Q., Zou Y., Feng B., Li L., Chen Z., Huang C. Tumor grade related expression of neuroglobin is negatively regulated by PPARγ and confers antioxidant activity in glioma progression. Redox Boil. 2017;12:682–689. doi: 10.1016/j.redox.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]