Abstract
Mechanical ventilation is usually required for saving lives in critically ill patients; however, it can cause ventilator-induced lung injury (VILI). As VEGF-secreting Ly6Chigh monocytes are involved in VILI pathogenesis, we investigated whether cyclooxygenase-2 (COX-2) activity regulates the recruitment of VEGF-secreting Ly6Chigh monocytes during VILI. The clinically relevant two-hit mouse model of VILI, which involves the intravenous injection of lipopolysaccharide prior to high tidal volume (HTV)-mechanical ventilation, was used in this study. To investigate the role of COX-2 in the recruitment of VEGF-secreting Ly6Chigh monocytes during VILI, celecoxib, which is a clinical COX-2 inhibitor, was administered 1 h prior to HTV-mechanical ventilation. Pulmonary vascular permeability and leakage, inflammatory leukocyte infiltration, and lung oxygenation levels were measured to assess the severity of VILI. HTV-mechanical ventilation significantly increased the recruitment of COX-2-expressing Ly6Chigh, but not Ly6Clow, monocytes. Celecoxib significantly diminished the recruitment of Ly6Chigh monocytes, attenuated the levels of VEGF and total protein in bronchoalveolar lavage fluid, and restored pulmonary oxygenation during VILI. Our findings demonstrate that COX-2 activity is important in the recruitment of VEGF-secreting Ly6Chigh monocytes, which are involved in VILI pathogenesis, and indicate that the suppression of COX-2 activity might be a useful strategy in mitigating VILI.
Keywords: cyclooxygenase-2, Ly6Chigh monocytes, ventilator-induced lung injury
1. Introduction
Mechanical ventilation is required for patients that suffer from acute respiratory failure or acute respiratory distress syndrome (ARDS), which can be triggered by direct pulmonary insult and indirect extra-pulmonary insult [1]. However, mechanical ventilation can potentially exacerbate the condition by overly distending the regional pulmonary alveoli, particularly with large tidal volumes, which causes ventilator-induced lung injury (VILI) [2,3]. VILI may occur in healthy lungs or worsen pre-existing pulmonary disease, such as ARDS [4,5], which is characterized by up-regulating the recruitment of numerous subsets of leukocytes that overproduce inflammatory cytokines for increasing pulmonary-vasculature permeability, causing protein-rich pulmonary edema, and ultimately worsening gas exchange [6,7]. The recruitment of inflammatory leukocytes, particularly alveolar macrophages [8] and neutrophils [9,10], which migrate to the pulmonary microenvironment and release inflammatory and injurious mediators, plays a critical role in the pathogenesis of VILI [4]. Moreover, recent studies showed that various subsets of monocytes are also involved in the development of VILI [11,12,13].
The inflammatory subsets of monocytes, which are heterogeneous and pluripotent cells, consists of at least two phenotypically distinct cells that can be distinguished by Ly6C expression that serves as a specific marker for the monocytic lineage in mice [14,15,16]. Ly6ChighCCR2highCX3CR1low monocytes, which express high levels of Ly6C (Ly6Chigh) and C-C chemokine receptor 2 (CCR2high) and low levels of CX3C chemokine receptor 1 (CX3CR1low), are designated as the inflammatory subset, and they are derived from bone marrow macrophage-dendritic precursor cells that migrate to inflammatory sites during injury [14,15]. In contrast, the Ly6ClowCCR2lowCX3CR1high monocytes are designated as the resident subset and they can enter tissues, irrespective of inflammation [15]. Studies have reported that the rapid recruitment of monocytes, particularly the Ly6Chigh subset, to the lung microvasculature, contributes to pulmonary-vascular injury in a murine model of systemic endotoxemia [17,18].
Cyclooxygenases (COXs), including the constitutive COX-1, are expressed in most tissues, and the inducible COX-2 expressed in lung resident inflammatory, endothelial, and epithelial cells are responsible for catalyzing the conversion of arachidonic acid to prostanoids, which are involved in inflammation, vascular homeostasis, and vascular permeability [19]. Recent evidence has shown that COX-2 is a potent mediator of VILI progression [20,21]. The expression and activity of COX-2, but not of COX-1, are induced in lungs that were subjected to high tidal volume (HTV)-mechanical ventilation, and the expression of COX-2 is limited to alveolar monocytes and macrophages [21]. Furthermore, CAY10404, a COX-2-specific inhibitor, potently diminishes tpulmonary permeability, neutrophil infiltration, and cytokine production during VILI [21]. However, the more precise role of COX-2 expression and activity in the pulmonary monocytes, a complex and heterogenous monocytic subset, which modulate VILI, is unknown. Furthermore, studies have shown that activated Ly6Chigh monocytes are recruited to the lung in VILI, which participate in a decrease of lung compliance [11,12]. Our recent study also demonstrates that Ly6Chigh monocytes through enhancing vascular endothelial growth factor (VEGF) secretion for increasing pulmonary vascular permeability on causing VILI [13], indicating the critical role of the recruitment of VEGF-secreting Ly6Chigh monocytes during VILI. Thus, we sought to investigate the functional role and therapeutic potential of COX-2 in VEGF-secreting Ly6Chigh monocytes during VILI.
Presently, we explore the kinetic changes of COX-2-expressing Ly6Chigh monocytes in a clinically relevant two-hit VILI mouse model. Moreover, using celecoxib, which is a COX-2-specific inhibitor, we demonstrated that COX-2 activity is important in mediating the recruitment of VEGF-secreting Ly6Chigh monocytes that contribute to the increase of total protein in bronchoalveolar lavage fluid (BALF) during the development of VILI. Thus, the COX-2 blockade may have a therapeutic value in the treatment of VILI.
2. Results
2.1. Celecoxib, a Clinical COX-2 Inhibitor, Attenuates VILI
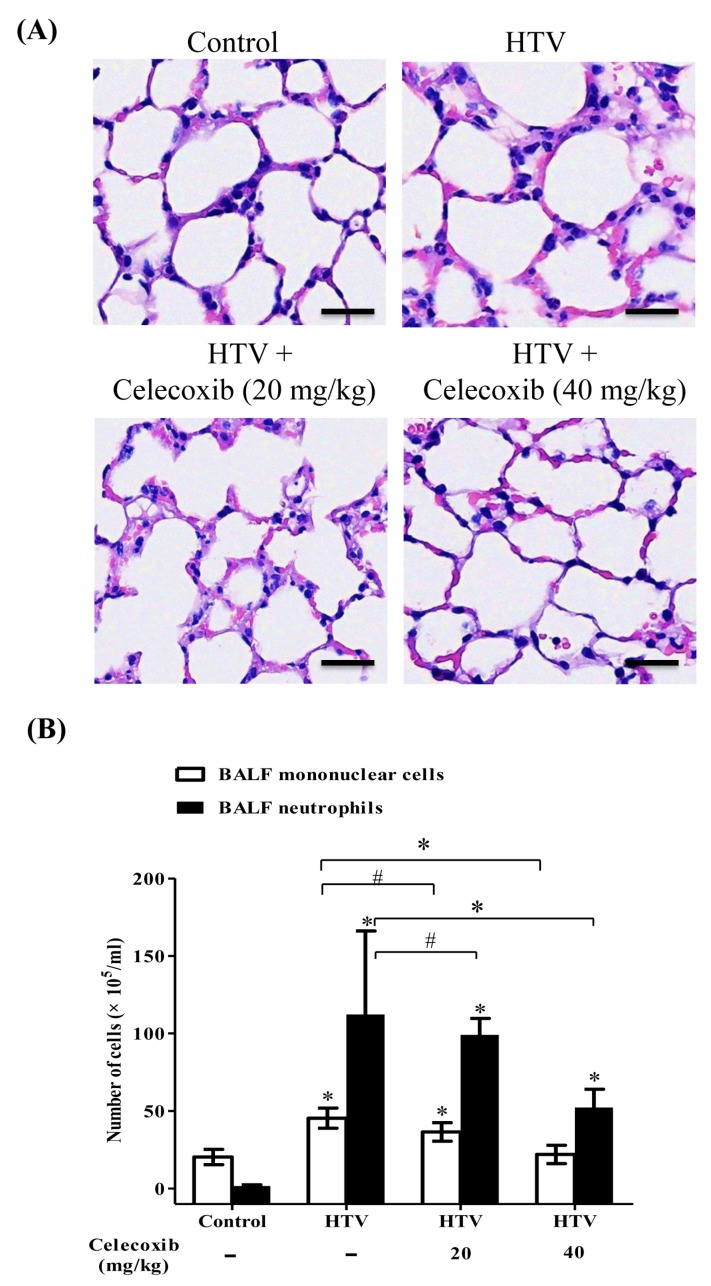
While a previous study has demonstrated that the expression and activity of COX-2 are critically involved in the pathogenesis of VILI [21], the detailed functional mechanism of COX-2 in Ly6C monocytes during VILI is unclear, and the therapeutic significance of celecoxib, which is a clinical COX-2 specific inhibitor, in VILI remains to be studied. To investigate the effect of the COX-2 pathway in VILI, our previously established murine model of VILI [13], which consisted of intraperitoneal administration of celecoxib (20 or 40 mg/kg) 1 h prior to HTV-mechanical ventilation, was used. In our preliminary experiments, the small-scale doses of celecoxib had been screened in mouse. We found that the doses of celecoxib that were lower than 10 mg/kg had no significant effect on VILI, and celecoxib (20 and 40 mg/kg) had better action on VILI in our system; thus, celecoxib (20 and 40 mg/kg) was applied to this study. The results (Figure 1A) showed that HTV caused significant lung injury with the infiltration of inflammatory leukocytes into the alveolus. The administration of celecoxib significantly diminished the severity of lung injury in HTV-ventilated mice (Figure 1A). Moreover, Figure 1B demonstrates that celecoxib notably reduced the infiltration of neutrophils and mononuclear cells into BALF during VILI. These results indicate the importance of COX-2 and the clinical benefit of celecoxib in treating VILI.
Figure 1.
Celecoxib attenuated ventilator-induced lung injury (VILI) and decreased the infiltration of leukocytes in BALF during VILI. (A) Representative micrographs of hematoxylin-and-eosin-stained sections of the lung. Scale bar = 100 μm. (B) Cytological analyses of mononuclear cells and polymorphonuclear neutrophils (PMN) in bronchoalveolar lavage fluid (BALF). Values represent the mean ± SD (n = 6). # p < 0.05 and * p < 0.01, when compared with the control or between groups.
2.2. The Recruitment of COX-2-Expressing Neutrophils and Ly6Chigh, but Not Ly6Clow, Monocytes Is Enhanced during VILI
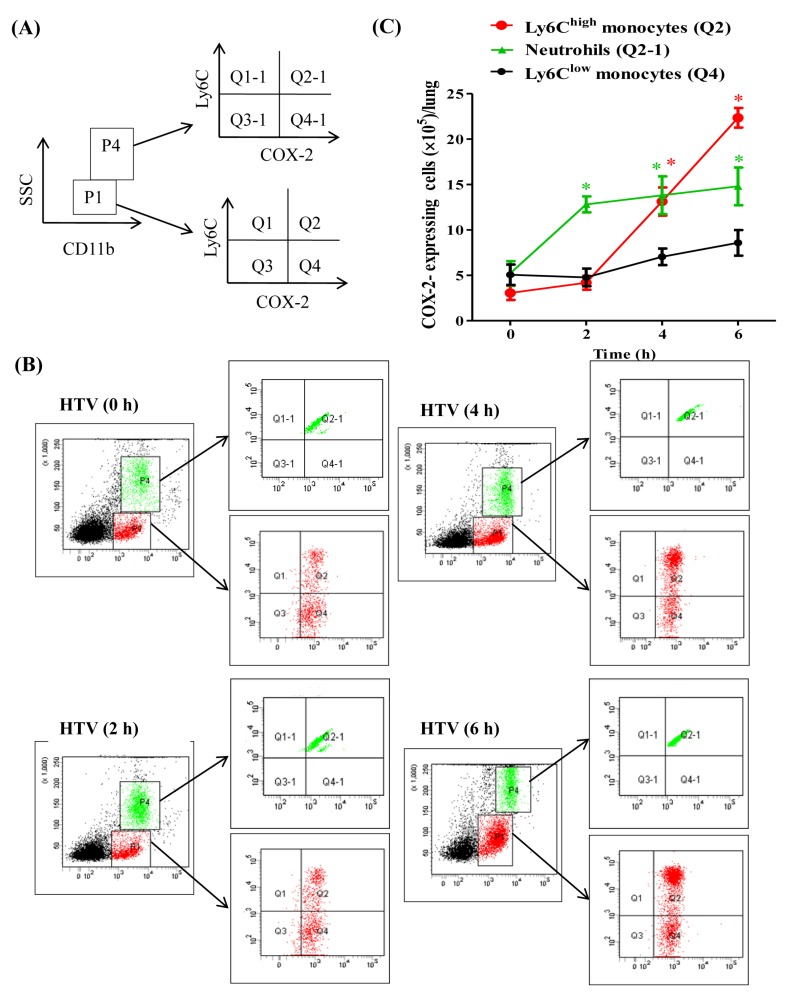
A previous study demonstrated that HTV-mechanical ventilation induced the recruitment of COX-2-expressing mononuclear cells to the injured lung alveolus [21]. As monocytes are a subset of complex and heterogeneous cells, it is important to investigate the role of COX-2 in accurate monocytes. Accordingly, our recent study indicated that VEGF-expressing Ly6Chigh monocytes are recruited during VILI [13]. Therefore, we aimed to determine the expression and activity of COX-2 in the recruitment of Ly6C monocytes during VILI. Thus, the time course recruitment of COX-2-expressing cells in response to the development of VILI was investigated. The staining and gating strategies of flow cytometry for identifying cell types is shown (Figure 2A,B). The low side scatter (SSC) and CD11b-positive events (P1) were gated in the analysis of monocytes with Ly6C and COX-2 expressions. COX-2-expressing Ly6Chigh monocytes were COX-2-positive and they showed high Ly6C expression (Q2). COX-2-expressing Ly6Clow monocytes were COX-2-positive and revealed low Ly6C expression (Q4). A high degree of SSC and high levels of CD11b expression (P4) were used for the gating and analysis of neutrophils with Ly6C and COX-2 expressions. COX-2-expressing neutrophils were COX-2-positive and showed high Ly6C expression (Q2-1). The quantification of time course recruitment of COX-2-expressing Ly6C monocytes and neutrophils during VILI is shown (Figure 2C). The recruitment of COX-2-expressing neutrophils significantly increased by 2 h and plateaued from 4 to 6 h, but the changes in COX-2-expressing neutrophil recruitment from 2 to 4, or 6 h were not significant, indicating that COX-2-expressing neutrophil recruitment occurs early in VILI. Moreover, the recruitment of COX-2-expressing Ly6Chigh monocytes in response to HTV-mechanical ventilation occurred later than COX-2-expressing neutrophil recruitment, increasing significantly by 4 h and peaking at 6 h. However, there was no significant change in the number of COX-2-expressing Ly6Clow monocytes during these time points. These data revealed the critical involvement of COX-2-expressing Ly6Chigh monocytes in the progression of VILI (Figure 2). Furthermore, when compared to our previous publication [19], it is worth noting that the tendency of the time course recruitment of COX-2-expressing Ly6Chigh monocytes is similar to the VEGF-expressing Ly6Chigh monocytes during VILI development on VEGF production and pulmonary-vasculature leakage, suggesting that COX-2 activity might associate with VEGF secretion in the Ly6Chigh monocytes for causing VILI.
Figure 2.
Cyclooxygenase-2 (COX-2)-expressing Ly6Chigh monocytes recruited into the lung during VILI. (A,B) Gating strategy of flow cytometry analysis. (C) Time course recruitment of COX-2-expressing Ly6Chigh monocytes, Ly6Clow monocytes, and neutrophils during VILI. Values represent the mean ± SD (n = 6). * p < 0.01 as compared with the control at that time point.
2.3. Celecoxib Aignificantly Mitigates the Recruitment of Ly6Chigh, but Not Ly6Clow, Monocytes in VILI
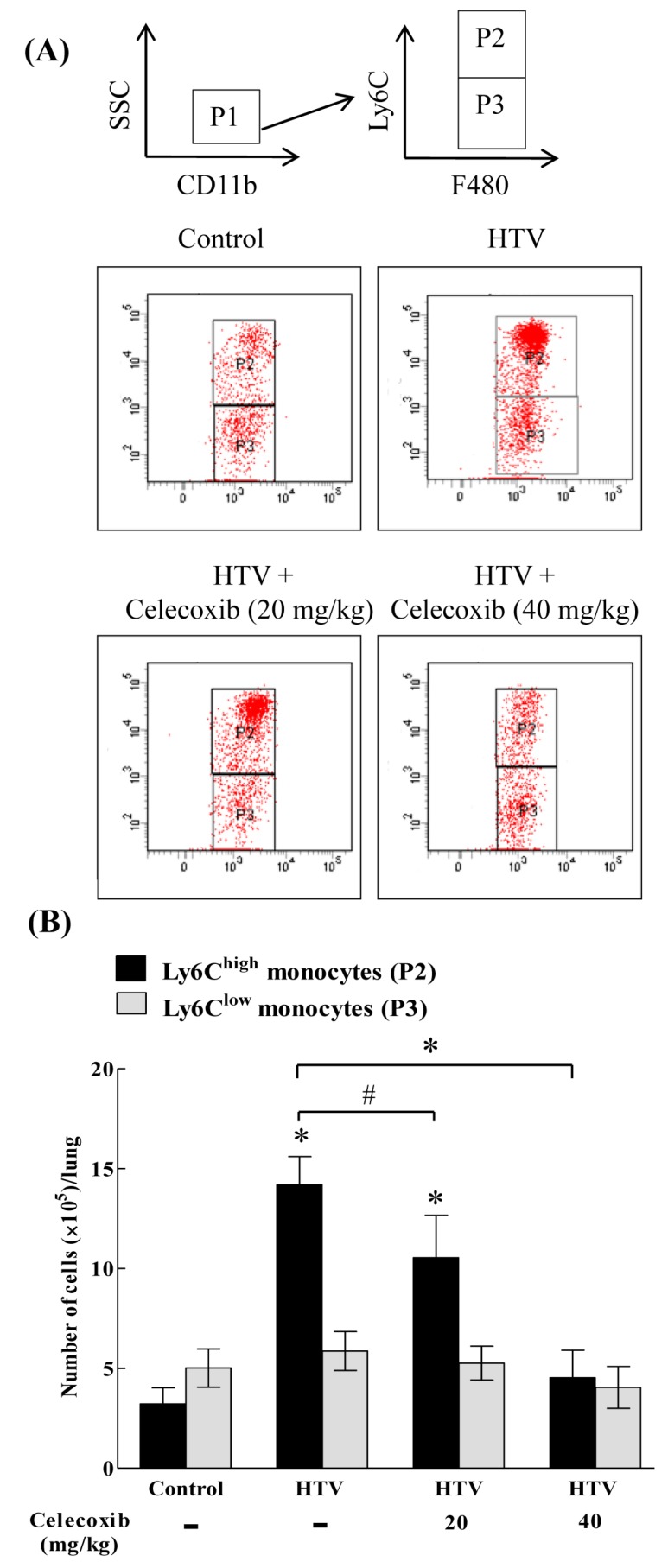
Although COX-2 inhibition potently restrains VILI [21], the functional effect of a COX-2 signaling blockade on the recruitment of Ly6Chigh monocytes in VILI is unknown. Figure 3A shows the flow cytometric technique for quantifying the recruitment of specific Ly6C monocytes in response to COX-2 inhibition. Low SSC and CD11b-positive events (P1) were gated to analyze Ly6C and F4/80 expression. Ly6Chigh monocytes were F4/80-positive and they showed high Ly6C expression (P2). Ly6Clow monocytes were F4/80-positive and showed low Ly6C expression (P3). Figure 3B shows the quantification of Ly6Chigh and Ly6Clow monocytes. The number of Ly6Chigh, but not Ly6Clow, monocytes was higher in the HTV-injured lung than in the lungs of control, non-ventilated mice. Celecoxib had no significant effect on the recruitment of Ly6Clow monocytes (Figure 3B). However, celecoxib treatment diminished the recruitment of Ly6Chigh monocytes in a dose-dependent manner (Figure 3B), indicating the importance of COX-2 in mediating the recruitment of Ly6Chigh monocytes during VILI.
Figure 3.
Celecoxib significantly diminished the recruitment of Ly6Chigh, but not Ly6Clow, monocytes in VILI. (A) Gating strategy of flow cytometry analysis. (B) The numbers of Ly6C monocytes in lung homogenates. Values represent the mean ± SD (n = 6). # p < 0.05 and * p < 0.01, when compared with the control or between groups.
2.4. Celecoxib Reduces Alveolar Protein Outflow and VEGF Secretion into Bronchoalveolar Lavage Fluid (BALF) and Improves Pulmonary Oxygenation in VILI
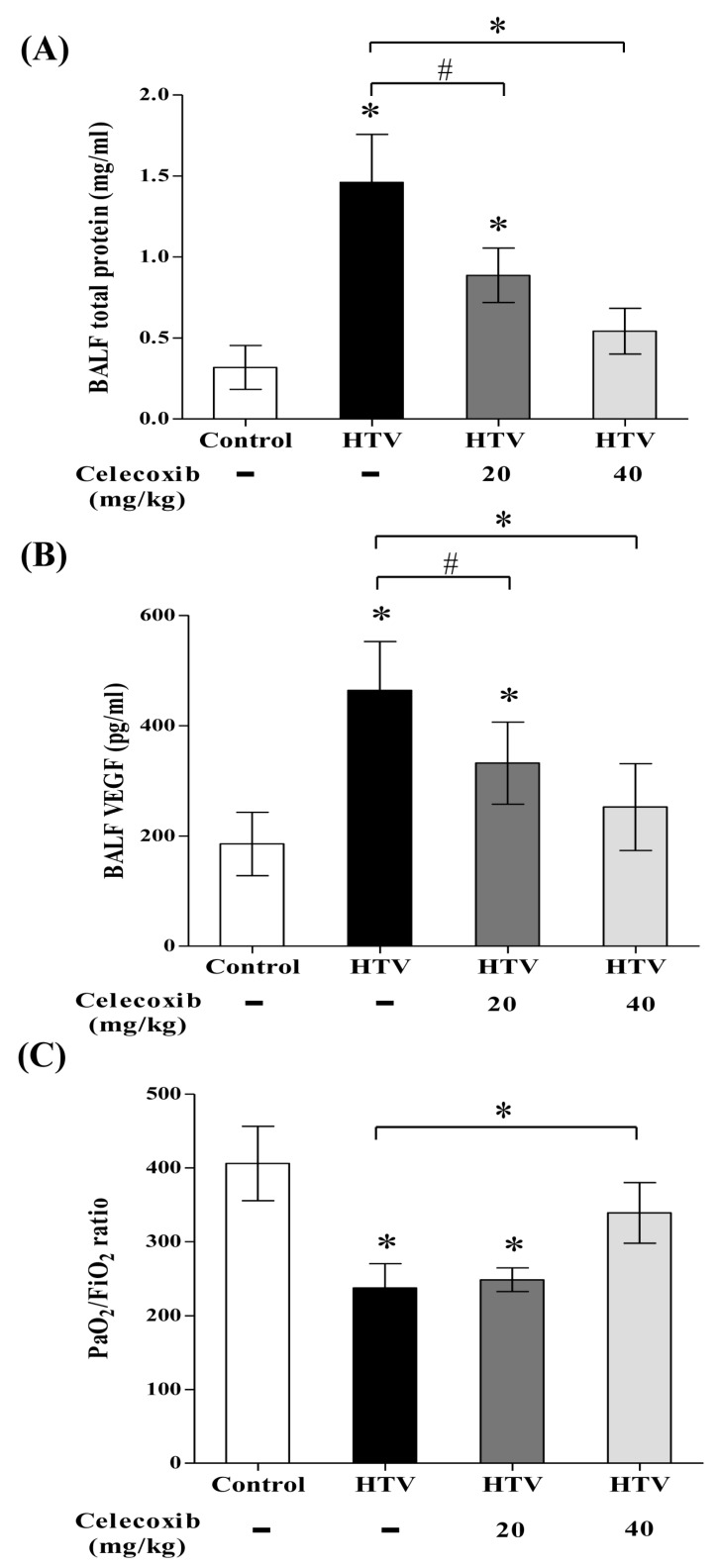
The total quantity of proteins and VEGF in BALF are an indicator of pulmonary-vasculature permeability [13,22]. Assaying total proteins and VEGF secretion on reflecting pulmonary-vasculature leakage during VILI evaluated the therapeutic effect of celecoxib. Figure 4A,B show that VEGF and total protein levels in BALF were significantly increased with HTV-mechanical ventilation. Celecoxib treatment meaningfully blunted the increase of total protein outflow and VEGF secretion in BALF after VILI in a dose-dependent manner (Figure 4A,B). Moreover, an analysis of cardiac blood samples to investigate the status of pulmonary oxygenation showed that PaO2 (Table 1) and the PaO2/FIO2 ratio (Figure 4C) were low in HTV-mechanical ventilation, indicating acute lung injury and hypoxemia. However, the level of pulmonary oxygenation was preserved by celecoxib treatment after HTV-mechanical ventilation. No significant differences in pH, PaCO2, or HCO3 were found among the four experimental groups (Table 1), indicating that there was no severe metabolic acidosis during the experiments. This data reveals that celecoxib exerts a therapeutic benefit in VILI by preventing pulmonary-vasculature damage and preserving pulmonary oxygenation.
Figure 4.
Celecoxib significantly reduced pulmonary-vasculature permeability and improved pulmonary oxygenation in VILI. (A) BALF total protein. (B) BALF vascular endothelial growth factor (VEGF). (C) PaO2/FiO2. Values represent the mean ± SD (n = 6). # p < 0.05 and * p < 0.01, as compared with the control or between groups.
Table 1.
Analysis of cardiac blood gas.
| Control | HTV | HTV | HTV | |
|---|---|---|---|---|
| Celecoxib (mg/kg) | — | — | +20 | +40 |
| pH | 7.371 ± 0.031 | 7.322 ± 0.026 | 7.307 ± 0.02 | 7.37 ± 0.025 |
| PaCO2 (mm Hg) | 35.83 ± 5.56 | 30.5 ± 3.72 | 34.9 ± 4.23 | 36.23 ± 2.15 |
| HCO3 (mmol/l) | 21.48 ± 1.75 | 17.51 ± 1.30 | 18.8 ± 0.89 | 21.3 ± 1.45 |
| PaO2 (mm Hg) | 82.83 ± 11.28 | 51.0 ± 6.89 * | 52.3 ± 3.31 | 71.21 ± 8.6 # |
Values represent the mean ± SD (n = 6). * p < 0.05 compared with control group. # p < 0.05 compared with HTV group.
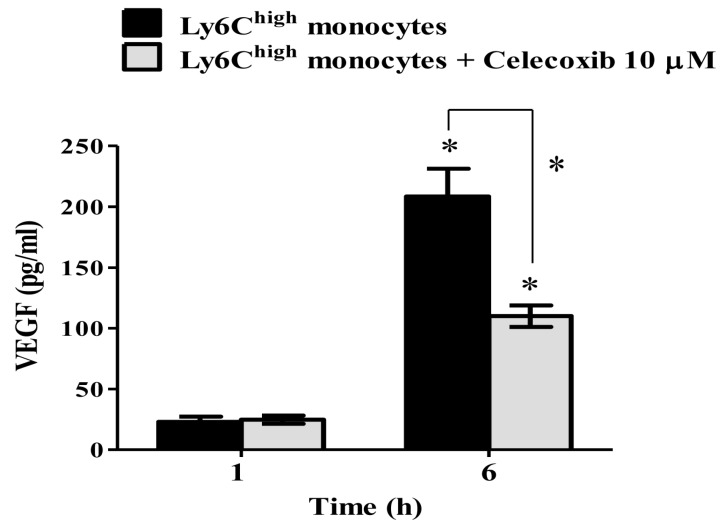
2.5. VEGF Secretion in Ly6Chigh Monocytes Is COX-2-Dependent
The recruitment of VEGF-secreting Ly6Chigh monocytes plays an important role in the pathogenesis of VILI [13]. However, it is unclear whether COX-2 activity directly regulates VEGF secretion in Ly6Chigh monocytes on VILI. The Ly6Chigh monocytes were sorted from HTV-injured lungs for testing the involvement of COX-2 on VEGF secretion ex vivo. Figure 5 shows that VEGF production by sorted Ly6Chigh monocytes was significantly increased 6 h after culture (Figure 5). However, this increment of VEGF secretion by the sorted Ly6Chigh monocytes was considerably suppressed in the presence of celecoxib (10 µM). This result denotes the importance of COX-2 and VEGF functional axis in Ly6Chigh monocytes during the development of VILI, and it provides the clinical insights of celecoxib on targeting COX-2-expressing and VEGF-secreting Ly6Chigh monocytes for treating VILI in patients.
Figure 5.
The secretion of VEGF by sorted Ly6Chigh monocytes was COX-2-dependent. Values represent the mean ± SD (n = 4). * p < 0.01 compared to individual group of 1 h or compared with group untreated with celecoxib.
3. Discussion
Mechanical ventilation is used as supportive therapy for patients with acute respiratory failure or ARDS. However, due to the morphological variation of patients’ lungs, even when protective ventilation is used, and the uneven overdistention of regional alveoli, which leads to the exacerbation of VILI [23], suggests that more detailed mechanistic and pharmacologic investigations are required in identifying the therapeutic window for improving the clinical outcomes in ventilated patients. By using our previous established VILI murine model [13], here we demonstrate that COX-2 activity is critically important in the recruitment of functional, VEGF-secreting Ly6Chigh, but not Ly6Clow, monocytes; this contributes to the pathogenesis of VILI via enhancing pulmonary-capillary permeability and -vasculature leakage in reducing pulmonary oxygenation. Celecoxib, which is a clinically used COX-2 inhibitor that affects functional COX-2 and VEGF expressions in Ly6Chigh monocytes exhibits therapeutic value in treating and/or preventing VILI in patients.
Neutrophils and monocytes are the important cell types in inflammation and VILI [9,11]. In our VILI model, the COX-2-expressing neutrophils were significantly recruited into HTV-injured lungs after 2 h and recruitment had plateaued by 4 to 6 h. Moreover, COX-2-expressing Ly6Chigh monocytes were not significantly elevated after 2 h, increased by 4 h, and then significantly increased at 6 h At 0 and 2 h, there were fewer COX-2-expressing Ly6Chigh monocytes than neutrophils; at 4 h, there were equivalent numbers of COX-2-expressing monocytes and neutrophils; and at 6 h, there were more COX-2-expressing monocytes than neutrophils. This result indicated that the COX-2-expressing neutrophils are the first cells to arrive in the ventilator-injured lung and they may employ paracrine signaling to recruit the wave of COX-2-expressing Ly6Chigh monocytes that further amplify the acute phase of lung-injured cascade. Since the role of neutrophils in the process of lung injury has been well-investigated [9], the importance of COX-2 expression in mononuclear cells is established [21]. As the COX-2-specific inhibitor CAY10404 and parecoxib can potently diminish VILI [21,24], the precise role of COX-2 activity and expression in Ly6Chigh monocytes was therefore focused on the pathogenesis of VILI in this study.
Ly6ChighCCR2highCX3CR1low monocytes have been demonstrated to be recruited into inflamed tissues through the activation of CCR2, which is a receptor of chemokine C-C-chemokine ligand 2 (CCL2) [25]. However, the underlying mechanism of the recruitment and activity of Ly6Chigh monocytes in VILI is unknown. Moreover, how COX-2-activated prostaglandins or other mediators, including cytokines, chemokines, and growth factors, mobilize and activate Ly6Chigh monocytes in the pathogenesis of VILI remains unclear. CCL2 is required in guiding the migration of CCR2-expressing monocytes into inflamed tissues [26], and CCL2 has been shown to accumulate in BALF in a model of LPS-induced VILI [27]. Further, the induction of COX-2 is required for CCL2 expression by monocytes during the progression of atherosclerosis [2]. In CCR2 knockout mice, the accumulation of Ly6Chigh monocytes in injured alveoli is suppressed, protecting the mouse from fibrosis [28]. Moreover, the plasma and BALF levels of interleukin-1β (IL-1β), a specific inducer of COX-2 expression [29], are associated with clinical VILI, and the blockade of IL-1β ameliorates VILI [30]. Here, we show that COX-2 inhibition inhibited the recruitment of Ly6Chigh monocytes that have been reported to express CCR2 [31], resulting in the mitigation of VILI. These findings indicate the activation of COX-2 from a wide variety of HTV-activated resident cells in the lung for regulating the recruitment and activation of Ly6Chigh monocytes. During VILI, COX-2 activation by proinflammatory mediators may stimulate the secretion of CCL2 to govern the lung margination of inflammatory cells, such as Ly6Chigh monocytes. Moreover, these findings contribute therapeutic insights into the inhibitory effect of celecoxib on the IL-1β-COX-2-CCL2 signaling axis, which suppresses the mobilization of Ly6Chigh monocytes, thereby attenuating VILI.
Further, we show that the inhibition of COX-2 activity by celecoxib can significantly diminish the VEGF and BALF total protein levels. It is known that there is a high correlation between the expression of COX-2 and VEGF, and that NS-398, a COX-2 inhibitor, inhibits VEGF expression in cancer cells [32]. Additionally, celecoxib reduces the transcriptional activity of Sp1, which down-regulates VEGF expression by cancer cells [33]. Our recent study also determined that the Ly6Chigh monocytes enhance VEGF expression for increasing pulmonary vascular permeability, which leads to VILI [13]. Presently, our data further showed that Ly6Chigh monocytes time-dependently secreted VEGF, and this VEGF secretion was inhibited by celecoxib, indicating that VEGF secretion by Ly6Chigh monocytes during VILI is COX-2-dependent. Furthermore, these results reveal the importance of COX-2 inhibition by clinical COX-2 inhibitors that are used for treating and/or preventing VILI, via the interruption of the COX-2-VEGF functional axis in Ly6Chigh monocytes.
Here, we focused on the COX-2-expressing Ly6Chigh monocytes, but the role of COX-2-expressing neutrophils in VILI cannot be ignored, because COX-2-expressing neutrophils and Ly6Chigh monocytes may sequentially interplay with each other during VILI. A previous study showed that a COX-2 inhibitor potently inhibits the recruitment of neutrophils during VILI [21]. In our study, mononuclear cells and neutrophils were accumulated in HTV-injured lungs, and this effect was also significantly blocked by celecoxib treatment. Therefore, the inhibition of COX-2 activity may provide a wide range of therapeutic effects, such as the inhibition of early recruitment of COX-2-expressing neutrophils, thereby reducing the recruitment of VEGF-secreting Ly6Chigh monocytes in late to prevent the VEGF-stimulated pulmonary permeability increment. A previous study showed that the expression of induced form of COX-2 is relatively low in mouse lung tissue, but it is strongly upregulated after ventilation, especially HTV for 4 h, indicating an acute phase injury of ventilation on upregulating COX-2 expression. Furthermore, the COX-2 expressing cells in ventilation-injured lung were verified using immunohistochemically staining, which show a significant positivity staining of pulmonary alveolar and interstitial mononuclear cells (CD45 and CD68 positive monocyte/macrophage lineage), rather than bronchiolar epithelium on COX-2 expression [21]. Accordingly, our result was consistent with this study and it provided more detailed information that the subset of mononuclear cells, Ly6Chigh-COX-2-expressing monocytes, are critically involved in VEGF secretion during VILI. Due to pulmonary epithelial and endothelial cells being evidenced to express COX-2 after various stimulations on resulting lung injury [19,34,35,36], therefore, we cannot exclude the involvement of COX-2 on pulmonary epithelial and endothelial cells for contributing to exacerbate VILI in the late phase other than the early acute phase. However, the temporal, reciprocal, and sequential expression of COX-2 between inflammatory leukocyte subsets, alveolar epithelium, and pulmonary endothelium during VILI development are unknown and are worthy of further investigation.
COX-2 acts as a key role in arachidonic acid metabolism in both physiological and pathological conditions [37]. It is constitutively expressed in human kidney and brain tissues and is inducible in various cells, including monocytes/macrophages, endothelial cells, epithelial cells, and cancer cells during stimulations of inflammatory cytokines, laminar shear stress, growth factors, and VILI [21,38]. Furthermore, the activity of COX-2 on generating prostaglandin E2 (PGE2) and prostacyclin are involved in various biological processes, including renal hemodynamics, the control of blood pressure, endothelial thromboresistance, pain and inflammation, and VILI [21,38]. Hence, it is predictable that COX-2 inhibition by COX-2 inhibitors, such as celecoxib, may exhibit multifaceted clinical outcomes other than diminishing VILI, ranging from increasing cardiovascular hazard [39], blood pressure [40], and atherothrombotic risk [41]. However, due to patients’ variations and disease status, the depiction of the practical consequences of COX-2 inhibition in humans has been problematic and has generated many controversial findings [42]. Besides, stroke volume and cardiac output reductions may alter the ventilation/perfusion ratio in the lungs and compromise the gas exchange [43]. In our experimental system, the cardiovascular hazards, such as hypoxemia and metabolic acidosis, were not found in different doses of celecoxib (20 and 40 mg/kg) administration during a short period. Even though, it is unclear whether a long period or higher doses of celecoxib administration will affect cardiovascular-related functions. Therefore, the long-term application of the COX-2 inhibitor should be very carefully evaluated, especially on the cardiovascular hazard issues.
4. Materials and Methods
4.1. Animals
Six to eight-week-old, C57BL/6 male mice (20–25 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan). All of the animal experiments were conducted according to the National Institutes of Health guidelines (Guides for the Care and Use of Experimental Animals) that Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital, Chiayi, Taiwan approved (Approval number: 2013070301 (23 October 2013) and 2017030202 (15 May 2017)).
4.2. Experimental Model of Ventilation-Induced Lung Injury
We used our previously established murine model of VILI [13] for studying the molecular mechanism of COX-2 on Ly6Chigh monocytes in VILI. Celecoxib (20 or 40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally administered 1 h prior to mechanical ventilation. Following this, lipopolysaccharide (LPS; O111B4; Sigma-Aldrich) (20 ng per mouse) was intravenously administered, immediately prior to mechanical ventilation. After tracheostomy, a 20-gauge angiocatheter was introduced into the tracheostomy orifice under general anesthesia by intraperitoneal injection with Zoletil 50 (80 mg/kg; Tiletamine-Zolazepam, Virbac, Carros, France). Anesthesia was maintained with an intraperitoneal injection of Zoletil 50 (10 mg/kg/h) during mechanical ventilation. The mice were placed in a supine position on a heated blanket and then attached to a specialized rodent ventilator (SAR-830/AP; CWE Inc., Ardmore, PA, USA) that was ventilated with HTV (VT 20 mL/kg, 60 breaths/min) for 6 h while the mice were breathing room air with zero end-expiratory pressure. At the end of experiment, the mice were sacrificed via an overdose of anesthetic. The non-ventilated mice served as control. Terminal blood samples of mice were obtained by cardiac puncture and were used for blood gas analysis to assess pulmonary oxygenation and the severity of acute lung injury [44].
4.3. Analysis of BALF
At the end of the experiment, the catheter that had been inserted into the trachea was lavaged three times with 1 mL of phosphate buffered saline to collect BALF. BALF was centrifuged at 300 × g for 10 min and the supernatant was collected for a measurement of total protein and VEGF levels. Furthermore, the total number of leukocytes in the sediment was calculated while using a hemocytometer (Paul Marienfeld GmbH, Lauda-Koenigshofen, Germany). A 100 μL aliquot of BALF sediment was further smeared on a glass slide for staining with Liu’s stain, which can differentiate and quantify the leukocyte subsets [13]. The VEGF level in BALF was measured by enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA). BALF total protein was determined while using a Pierce protein assay kit (Pierce, Rockford, IL, USA). Both of the analyses were conducted according to the manufacturer’s instructions.
4.4. Classification Strategy of Flow Cytometry
As described previously [13], the mouse lung homogenate was processed to produce a single cell suspension for flow cytometry. Briefly, lungs that were excised from each group of mice were mechanically disrupted and prepared as single cell suspensions by passing through a 40-μm strainer (BD Biosciences, San Jose, CA, USA). The pulmonary single cell suspension was stained using fluorophore-conjugated anti-mouse antibodies (eBioscence, San Diego, CA, USA) against CD11b (Clone M1/70, anti-mouse CD11b FITC; 1:200), Ly6C (Clone HK1.4, anti-mouse Ly6C APC; 1:200), F4/80 (Clone BM8, anti-mouse F4/80 PE; 1:300), or an appropriate isotype-matched control. To analyze the intracellular proteins, the pulmonary single cell preparation was permeabilized while using Cytofix/Cytoperm solution and Perm/Wash Buffer (BD Biosciences). The pulmonary single cell preparation was further stained with fluorophore-conjugated anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) against COX-2. Fluorescence was determined using FACSCanto II and analyzed with FACSDiva software version 7.0 (BD Biosciences). The viable Ly6Chigh monocytes were sorted using the FACSAria Fusion cell sorter and analyzed with FACSDiva software version 8.0 (BD Biosciences).
4.5. Ex vivo VEGF Secretion Assay
Ly6Chigh monocytes that were sorted from the HTV-injured lungs were seeded (1 × 105 cells per well) in the wells of a 96-well plate and cultured in an RPMI medium containing 10% fetal bovine serum in the presence or absence of celecoxib (10 μM). After 1 and 6 h of culture, Ly6Chigh monocyte-conditioned medium was separately collected for measuring the secretion of VEGF by Ly6Chigh monocytes while using an enzyme-linked immunosorbent assay (R&D Systems).
4.6. Statistical Analysis
All the data are expressed as the mean plus or minus the standard deviation (SD). Statistical significance was examined using unpaired Student’s t-test. Comparisons among multiple groups were made by one-way analysis of variance with Bonferroni-corrected pairwise post hoc comparisons. All of the statistical analyses were performed with GraphPad Prism software version 5.0 (GraphPad Software, La Jolla, CA, USA). A p-value of <0.05 was considered to be statistically significant.
5. Conclusions
The present study defines the time course and mechanistic significance of a COX-2-dependent mechanism for the recruitment of activated, VEGF-secreting Ly6Chigh monocytes, which increase the pulmonary-vasculature permeability and contribute to the pathogenesis of a clinically relevant murine model of VILI with HTV-mechanical ventilation. Therefore, the adjuvant pharmacologic application of COX-2 inhibitors to target the recruitment of COX-2- and VEGF-expressing Ly6Chigh monocytes might provide novel therapeutic insights that will facilitate the treatment and/or prevention of clinical VILI.
Acknowledgments
We are grateful for the use of the Common Laboratory and the Laboratory Animal Center, Chang Gung Memorial Hospital, Chiayi, Taiwan, and acknowledge the BD FACSCanto II flow cytometer service and BD FACSAria Fusion cell sorter provided by the Precious Instrumentation Core Laboratory, Chang Gung Memorial Hospital, Chiayi.
Abbreviations
| VILI | ventilator-induced lung injury |
| HTV | high tidal volume |
| BALF | bronchoalveolar lavage fluid |
| VEGF | vascular endothelial growth factor |
Author Contributions
T.-H.H. designed the study, performed experiments, and drafted the manuscript. P.-H.F. interpretation of data and edited the manuscript. J.-M.L. preformed experiments and analyzed data. H.-Y.L. performed animal experiments. C.-M.L. and C.-S.S. conceived and supervised the study and edited the manuscript. All authors have read and approved the final manuscript.
Funding
This research was funded by grant number (CMRPG6D0021 and CMRPG6G0361) from the Chang Gung Memorial Hospital Research Foundation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.Y., Kim S.D., Shim J.W., Lee S.Y., Lee H., Cho K.H., Yun J., Bae Y.S. Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J. Immunol. 2008;181:4332–4339. doi: 10.4049/jimmunol.181.6.4332. [DOI] [PubMed] [Google Scholar]
- 3.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.International consensus conferences in intensive care medicine: Ventilator-associated Lung Injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societe de Reanimation de Langue Francaise, and was approved by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 1999;160:2118–2124. doi: 10.1164/ajrccm.160.6.ats16060. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss D., Basset G., Soler P., Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am. Rev. Respir. Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 6.Maniatis N.A., Kotanidou A., Catravas J.D., Orfanos S.E. Endothelial pathomechanisms in acute lung injury. Vasc. Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker J.C., Hernandez L.A., Peevy K.J. Mechanisms of ventilator-induced lung injury. Crit. Care Med. 1993;21:131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Frank J.A., Wray C.M., McAuley D.F., Schwendener R., Matthay M.A. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1191–L1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury S., Wilson M.R., Goddard M.E., O’Dea K.P., Takata M. Mechanisms of early pulmonary neutrophil sequestration in ventilator-induced lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L902–L910. doi: 10.1152/ajplung.00187.2004. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 11.Wilson M.R., O’Dea K.P., Zhang D., Shearman A.D., van Rooijen N., Takata M. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2009;179:914–922. doi: 10.1164/rccm.200806-877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller H.C., Hellwig K., Rosseau S., Tschernig T., Schmiedl A., Gutbier B., Schmeck B., Hippenstiel S., Peters H., Morawietz L., et al. Simvastatin attenuates ventilator-induced lung injury in mice. Crit. Care. 2010;14:R143. doi: 10.1186/cc9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C.S., Huang T.H., Lin C.K., Li J.M., Chen M.H., Tsai M.L., Chang C.C. VEGF Production by Ly6C+high Monocytes Contributes to Ventilator-Induced Lung Injury. PLoS ONE. 2016;11:e0165317. doi: 10.1371/journal.pone.0165317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose S., Misharin A., Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytom. Part A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Dea K.P., Young A.J., Yamamoto H., Robotham J.L., Brennan F.M., Takata M. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am. J. Respir. Crit. Care Med. 2005;172:1119–1127. doi: 10.1164/rccm.200504-605OC. [DOI] [PubMed] [Google Scholar]
- 18.O’Dea K.P., Wilson M.R., Dokpesi J.O., Wakabayashi K., Tatton L., van Rooijen N., Takata M. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J. Immunol. 2009;182:1155–1166. doi: 10.4049/jimmunol.182.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ermert L., Ermert M., Merkle M., Goppelt-Struebe M., Duncker H.R., Grimminger F., Seeger W. Rat pulmonary cyclooxygenase-2 expression in response to endotoxin challenge: Differential regulation in the various types of cells in the lung. Am. J. Pathol. 2000;156:1275–1287. doi: 10.1016/S0002-9440(10)64998-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonas S.A., Moreno-Vinasco L., Ma S.F., Jacobson J.R., Desai A.A., Dudek S.M., Flores C., Hassoun P.M., Sam L., Ye S.Q., et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L292–L302. doi: 10.1152/ajplung.00481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson J.A., Sauer D., Gold J.A., Nonas S.A. The role of cyclooxygenase-2 in mechanical ventilation-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2012;47:387–394. doi: 10.1165/rcmb.2011-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L.-F., Huang C.-C., Liu Y.-Y., Lin H.-C., Kao K.-C., Yang C.-T., Liao S.-K. Hydroxyethyl starch reduces high stretch ventilation-augmented lung injury via vascular endothelial growth factor. Transl. Res. 2011;157:293–305. doi: 10.1016/j.trsl.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Puybasset L., Gusman P., Muller J.C., Cluzel P., Coriat P., Rouby J.J. Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med. 2000;26:1215–1227. doi: 10.1007/s001340051340. [DOI] [PubMed] [Google Scholar]
- 24.Meng F.-Y., Gao W., Ju Y.-N. Parecoxib reduced ventilation induced lung injury in acute respiratory distress syndrome. BMC Pharm. Toxicol. 2017;18:25. doi: 10.1186/s40360-017-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pflucke D., Hackel D., Mousa S.A., Partheil A., Neumann A., Brack A., Rittner H.L. The molecular link between C-C-chemokine ligand 2-induced leukocyte recruitment and hyperalgesia. J. Pain. 2013;14:897–910. doi: 10.1016/j.jpain.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Altemeier W.A., Matute-Bello G., Frevert C.W., Kawata Y., Kajikawa O., Martin T.R., Glenny R.W. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L533–L542. doi: 10.1152/ajplung.00004.2004. [DOI] [PubMed] [Google Scholar]
- 28.Osterholzer J.J., Olszewski M.A., Murdock B.J., Chen G.H., Erb-Downward J.R., Subbotina N., Browning K., Lin Y., Morey R.E., Dayrit J.K., et al. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J. Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G.S., Liu D.S., Dai C.W., Li R.J. Antitumor effects of celecoxib on K562 leukemia cells are mediated by cell-cycle arrest, caspase-3 activation, and downregulation of Cox-2 expression and are synergistic with hydroxyurea or imatinib. Am. J. Hematol. 2006;81:242–255. doi: 10.1002/ajh.20542. [DOI] [PubMed] [Google Scholar]
- 30.Frank J.A., Pittet J.F., Wray C., Matthay M.A. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63:147–153. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z., Zhou Q., Gu C., Li D., Zhu L. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017;312:L231–L242. doi: 10.1152/ajplung.00389.2016. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y.M., Azahri N.S., Yu D.C., Woll P.J. Effects of COX-2 inhibition on expression of vascular endothelial growth factor and interleukin-8 in lung cancer cells. BMC Cancer. 2008;8:218. doi: 10.1186/1471-2407-8-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei D., Wang L., He Y., Xiong H.Q., Abbruzzese J.L., Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.CAN-03-1945. [DOI] [PubMed] [Google Scholar]
- 34.Gust R., Kozlowski J.K., Stephenson A.H., Schuster D.P. Role of cyclooxygenase-2 in oleic acid-induced acute lung injury. Am. J. Respir. Crit. Care Med. 1999;160:1165–1170. doi: 10.1164/ajrccm.160.4.9811073. [DOI] [PubMed] [Google Scholar]
- 35.Hodges R.J., Jenkins R.G., Wheeler-Jones C.P.D., Copeman D.M., Bottoms S.E., Bellingan G.J., Nanthakumar C.B., Laurent G.J., Hart S.L., Foster M.L., et al. Severity of Lung Injury in Cyclooxygenase-2-Deficient Mice Is Dependent on Reduced Prostaglandin E2 Production. Am. J. Pathol. 2004;165:1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien G., Shields C.J., Winter D.C., Dillon J.P., Kirwan W.O., Redmond H.P. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat. Dis. Int. 2005;4:126–129. [PubMed] [Google Scholar]
- 37.Smith W.L., Garavito R.M., DeWitt D.L. Prostaglandin Endoperoxide H Synthases (Cyclooxygenases)-1 and −2. J. Boil. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 38.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Boil. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong D., Wang M., Cheng Y., FitzGerald G.A. Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr. Opin. Pharm. 2005;5:204–210. doi: 10.1016/j.coph.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Patrono C., Baigent C. Nonsteroidal Anti-Inflammatory Drugs and the Heart. Circulation. 2014;129:907–916. doi: 10.1161/CIRCULATIONAHA.113.004480. [DOI] [PubMed] [Google Scholar]
- 41.Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharm. 2016;82:957–964. doi: 10.1111/bcp.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal Melo M.F. Effect of cardiac output on pulmonary gas exchange: Role of diffusion limitation with Va/Q mismatch. Respir. Physiol. 1998;113:23–32. doi: 10.1016/S0034-5687(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 44.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]