Abstract
Carbonates and bicarbonates are two groups of accelerators which can be used in sprayed concrete. In this study, the effects of the two accelerators sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3) (0%, 1%, 2%, 3%, and 4% by weight of ordinary Portland cement OPC) on the properties of OPC paste were compared. The results show that both of them could accelerate the initial and final setting time of OPC paste, but the effect of the two accelerators on the compressive strength were different. After 1 day, sodium bicarbonate at 3% had the highest strength while sodium carbonate at 1% had the highest strength. After 7 days, both of the two accelerators at 1% had the highest compressive strength. After 28 days, the compressive strength decreased with the increase of the two. The improved strength at 1 and 7 days was caused by the accelerated formation of ettringite and the formation of CaCO3 through the reactions between the two with portlandite. The decrease of strength was caused by the Na+ could reduce the adhesion between C-S-H gel by replacing the Ca2+. NaHCO3 was found be a better accelerator than Na2CO3.
Keywords: NaHCO3, Na2CO3, portland cement, compressive strength, setting time
1. Introduction
Rapid hardening ability and high early strength are essential properties for shotcrete or sprayed concrete. Different accelerators were usually used in order to meet these requirements [1,2,3,4,5,6,7,8,9]. The mostly used accelerators including alkali carbonates, alkali hydroxide, alkali silicate, and alkali aluminate. For example, the sodium silicate was found to be able to modify the ITZ between the cement paste and aggregates and decrease the porosity of mortar [10]. Sodium aluminate and potassium aluminate can accelerate the formation of ettringite in the cement paste, and thus cause a rapid hardening effect [11]. Sodium aluminate was reported to be able to modify the pore structure of cement paste at an early age, improve the resistance to chloride ingress, and increase early-age compressive strength [12]. Carbonates [7,13,14,15,16,17] and bicarbonates with alkali were also among the most-used accelerators, such as Na2CO3 and NaHCO3 [18,19,20,21,22]. Mathur and Sharma [23] reported that the NaHCO3 can improve the strength and porosity of cement paste. Chandrawat and Yadav [24] found that the Na2CO3 could enhance the compressive strength and durability of cement paste. The work of Kunther et al. [25] showed that the bicarbonate ions could reduce the expansion of mortar and improve the sulfate resistance of mortar when subjected to sulfate attack. Yang et al. [26] reported that the addition of NaHCO3 and calcium lignosulphonate together could accelerate the formation of ettringite in the fly ash cement paste by changing the liquid-phase composition and the status of ettringite crystallization. Jang et al. [20] showed that the addition of 1% and 2% NaHCO3 could accelerate the hydration of cement paste and improve both flexural and compressive strength of the mortar specimen, but the addition of above 5% NaHCO3 caused the adverse effect on the strength development because of the formation of strong alkali NaOH in the specimen. Reddy et al. [27], however, reported that the both of Na2CO3 and NaHCO3 could decrease the compressive and tensile strength of concrete regardless of the content added or test age, and they also reported a significant reduction of strength after 28 days. However, Reddy and Krishna [28] reported that either Na2CO3 or NaHCO3 could increase the early age strength at 3 and 7 days but decrease significantly the strength after 28 days, besides, they reported that Na2CO3 accelerated the setting time whereas the NaHCO3 retarded the setting time. In addition, the structure and shape of the interface transition zone between the slurry and the aggregate in the cement composite material is a complicated problem. It has been well accepted that [29,30] the cement-based interface transition zone of the coarse aggregate is the weakest unit in the concrete, and the fly ash as mineral additive has a positive impact on the performance improvement of the interface transition zone.
It can be seen that there exist conflicted findings on the influence of Na2CO3 and NaHCO3 on the setting time and physical properties of cementitious materials. It is necessary to carry out a comprehensive study on the effects of the two accelerators on the properties of cement paste and make a comparison between the two. In order to investigate and compare the effects of the two accelerators on the properties of OPC paste, the same amount of Na2CO3 and NaHCO3 with 0%, 1%, 2%, 3%, and 4% weight of OPC were added into different mixes and the setting time and compressive strength at ages of 1, 7, and 28 days were studied, besides, the related hydration mechanism and hydration products were investigated through hydration heat, Thermogravimetry-Differential Thermal Analysis (TG-DTA), X-ray Diffraction (XRD), and Scanning Electron Microscopy (SEM) tests.
2. Materials and Methods
2.1. Materials
P.O. 42.5 ordinary Portland cement (OPC) in accordance with a Chinese standard GB175-2007 [31] was used. The physical properties and chemical composition of OPC are shown Table 1 and Table 2. The mineral composition of OPC is shown in Table 3. The NaHCO3 and Na2CO3 used were in powder form and the purity was >99.5% and >99.8%, respectively. A superplasticizer used was polycarboxylate. The mixing water was deionized water.
Table 1.
Physical properties of ordinary Portland cement (OPC).
| Fineness/% | Stability | Setting Time/min | Flexural Strength/MPa | Compressive Strength/MPa | |||
|---|---|---|---|---|---|---|---|
| Initial | Final | 3 days | 28 days | 3 days | 28 days | ||
| 1.5 | Qualified | 181 | 378 | 5.1 | 9.3 | 25.3 | 51.6 |
Table 2.
Chemical composition of OPC/%.
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|
| 22.96 | 63.87 | 5.73 | 3.31 | 2.64 | 0.32 | 0.23 | 0.18 |
Table 3.
Mineral composition of OPC/%.
| C3S | C2S | C3A | C4AF |
|---|---|---|---|
| 54.5 | 19.23 | 8.36 | 10.14 |
2.2. Methods
The mix design is shown in Table 4. Water-cement ratio (w/c) was kept same as 0.35. The superplasticizer was kept same as 0.5% by weight of OPC. OPC was firstly mixed with NaHCO3 or Na2CO3 in dry state. The water and superplasticizer were then added and mixing speed was at 60 rpm for 2 min followed by 120 rpm for another 2 min. The weight of NaHCO3 and Na2CO3 were added as 1%, 2%, 3%, and 4% by weight of cement in different mixes. Specimens were cured under s standard curing condition (20 °C, 97% R.H.).
Table 4.
Mix design.
| Mix ID | OPC/% | Superplasticizer/% | NaHCO3/% | Na2CO3/% | W/C |
|---|---|---|---|---|---|
| 1 | 100 | 0.5 | 0 | 0 | 0.35 |
| 2 | 1 | - | |||
| 3 | 2 | - | |||
| 4 | 3 | - | |||
| 5 | 4 | - | |||
| 6 | - | 1 | |||
| 7 | - | 2 | |||
| 8 | - | 3 | |||
| 9 | - | 4 |
The setting time of cement paste were tested according to a Chinese standard JC477-2005 [32]. A multichannel microcalorimeter was used for hydration heat test and it lasted for 24 h. Cubic samples with a dimension of 40 mm × 40 mm × 40 mm were used for compressive strength test. Compressive strength, TG-DTA, and SEM tests were conducted at ages of 1, 7, and 28 days. Powder samples were collected and the hydration was terminated by immersing into absolute ethyl alcohol for 24 h. The powder samples were then dried at 40 °C in a vacuum oven for 4 h. The samples were furtherly grounded by a pestle and mortar to pass a sieve with an aperture of 75 µm. The final powder samples were used for TG-DTA and XRD tests. The TG-DTA tests were conducted in a N2 environment with a simultaneous thermal analyzer system (HENVEN, Beijing, China) and the temperature was increased from 20 °C to 800 °C at a rate of 10 °C/min. Each time, one gram of the powder sample was used for XRD test. The XRD tests were conducted using a Rigaku SmartLab X-ray diffractometer (Rigaku, Tokyo, Japan) under a voltage of 40 kV and a current of 150 mA. The scanning rate was 10°/min from 5° to 70°. A MERLIN Compactfield Emission Scanning Electron Microscope (ZEISS, Jena, Germany) was used for SEM observations. The selected samples for SEM observations were at a size of around 5 mm in length and width with a fresh broken surface after the compression tests at ages of 1, 7, and 28 days. The samples were gold coated under vacuum condition before observation. The detailed procedures of hydration heat, compressive strength, TG-DTA, XRD, and SEM can also be found in our previous paper [33].
3. Results
3.1. Influence of NaHCO3/Na2CO3 on the Setting Time of OPC Paste
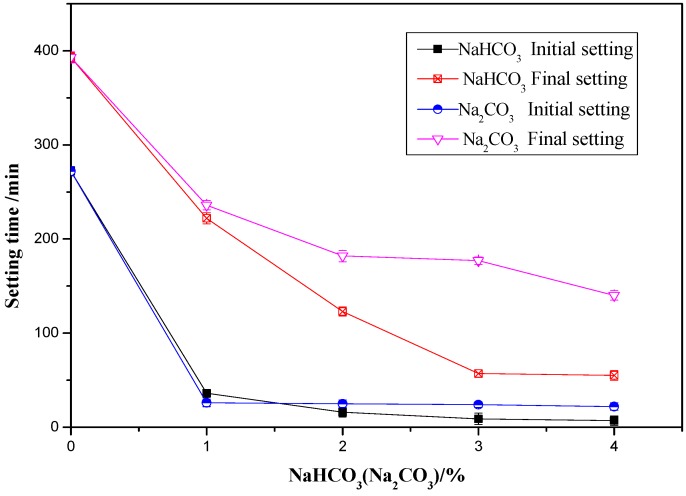
The influence of 0%, 1%, 2%, 3%, and 4% of NaHCO3/Na2CO3 on the initial and final setting time of OPC paste is shown in Figure 1. The results show that both of the initial and final setting time of the OPC paste decreased with the increase of NaHCO3 or Na2CO3 content. The initial setting time of OPC paste with 1%, 2%, 3%, and 4% NaHCO3 decreased by 86.76%, 94.12%, 96.69%, and 97.43% respectively compared to that of pure OPC paste. The final setting time of OPC paste with 1%, 2%, 3%, and 4% NaHCO3 decreased by 43.51%, 68.70%, 85.50%, and 86.01% respectively compared to that of pure OPC paste. It can be seen that the addition of 1–2% NaHCO3 significantly deceased the initial and final setting time of OPC paste. Further increase of NaHCO3 beyond 1% up to 4% showed little influence on the initial setting time, and further increase of NaHCO3 beyond 2% up to 4% showed little influence on the final setting time.
Figure 1.
Effects of NaHCO3 and Na2CO3 on the setting time of OPC paste.
The initial setting time of OPC paste with 1%, 2%, 3%, and 4% Na2CO3 decreased by 90.44%, 90.80%, 91.18%, and 91.91% respectively compared to that of pure OPC paste. The final setting time of OPC paste with 1%, 2%, 3%, and 4% Na2CO3 decreased by 39.95%, 53.69%, 54.96%, and 64.38% respectively compared to that of pure OPC paste. It can be seen that the influence of Na2CO3 on the initial setting time was more significant than the final setting time. The Na2CO3 showed similar effect as NaHCO3 on the initial setting time but its influence on the final setting time was less than the NaHCO3. The related mechanisms will be discussed later.
3.2. Influence of NaHCO3/Na2CO3 on the Compressive Strength of OPC Paste
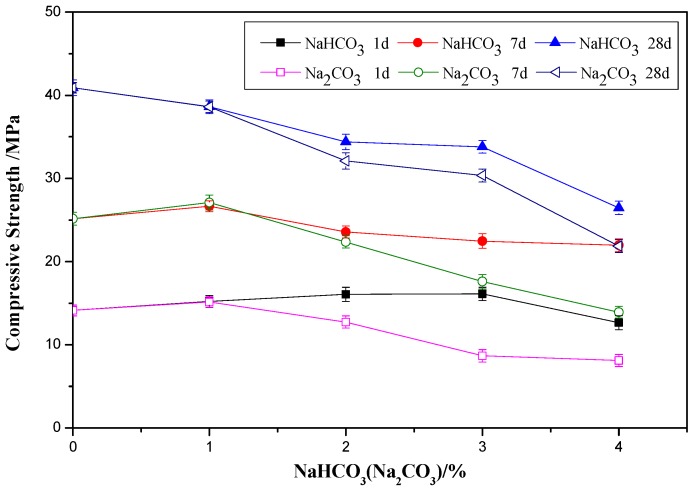
The effect of 0%, 1%, 2%, 3%, and 4% of NaHCO3 and Na2CO3 on the compressive strength of OPC paste specimen at the ages of 1, 7, and 28 days is shown in Figure 2. At the age of 1 day, with the increasing content of NaHCO3, the compressive strength of OPC paste increased initially and then decreased. The highest compressive strength happened in the mix with 3% NaHCO3. The strength of the mix with 3% NaHCO3 at the age of 1 day was 14% higher than that of the paste with no NaHCO3. At the age of 7 days, with the increase of NaHCO3, the compressive strength of OPC paste increased initially and then decreased with the highest strength happened in the mix with 1% NaHCO3. The strength of the mix with 1% NaHCO3 at the age of 7 days was 6% higher than that of OPC. At 28 days, the compressive strength of cement paste deceased continuously with the increase of NaHCO3. It can be seen that below 1% NaHCO3 can increase the early age strength but higher content of could decrease the later age strength significantly. This can be caused by the formation of NaOH [20], which is a strong alkali and could react with the silica sand in the paste specimen.
Figure 2.
Effects of NaHCO3 and Na2CO3 on the compressive strength of OPC paste.
For the pastes with Na2CO3, the compressive strength at ages of 1 and 7 days firstly increased and then decreased with the increase of Na2CO3 content, and the paste with 1% Na2CO3 had the highest compressive strength. The compressive strength of paste with 1% Na2CO3 was 7.2% higher at age of 1 day and 7.7% higher at age of 7 days compared to that of OPC paste. Similarly to NaHCO3, the compressive strength of pastes with Na2CO3 at age of 28 days decreased continuously with the increase of Na2CO3. The reason could be that the formation of NaOH caused the decrease of compressive strength. From Figure 2 it can be seen that the NaHCO3 had the similar beneficial effect as Na2CO3 on the early age strength when the addition was below 1%, but much worse effect than Na2CO3 on the strength development when the addition was above 1%.
3.3. Hydration Heat
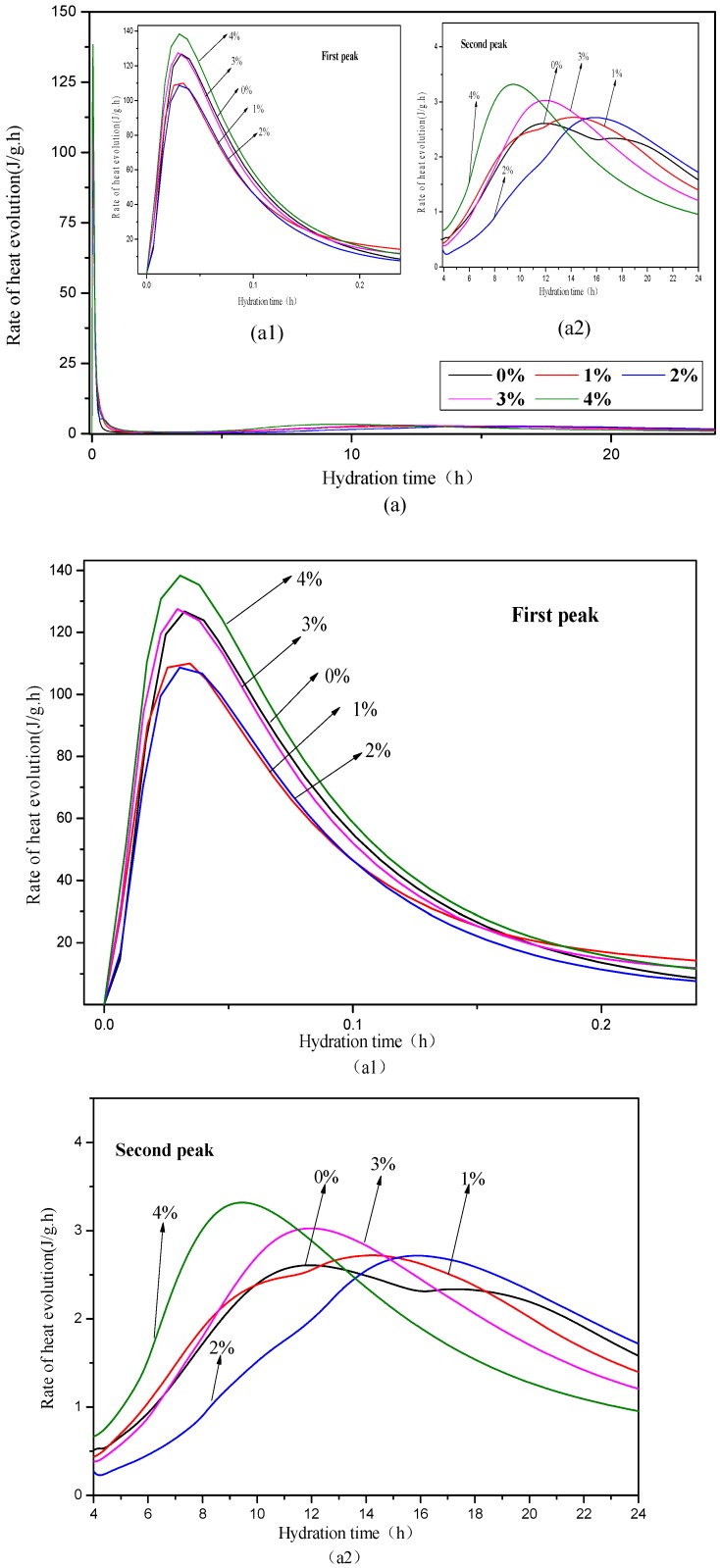
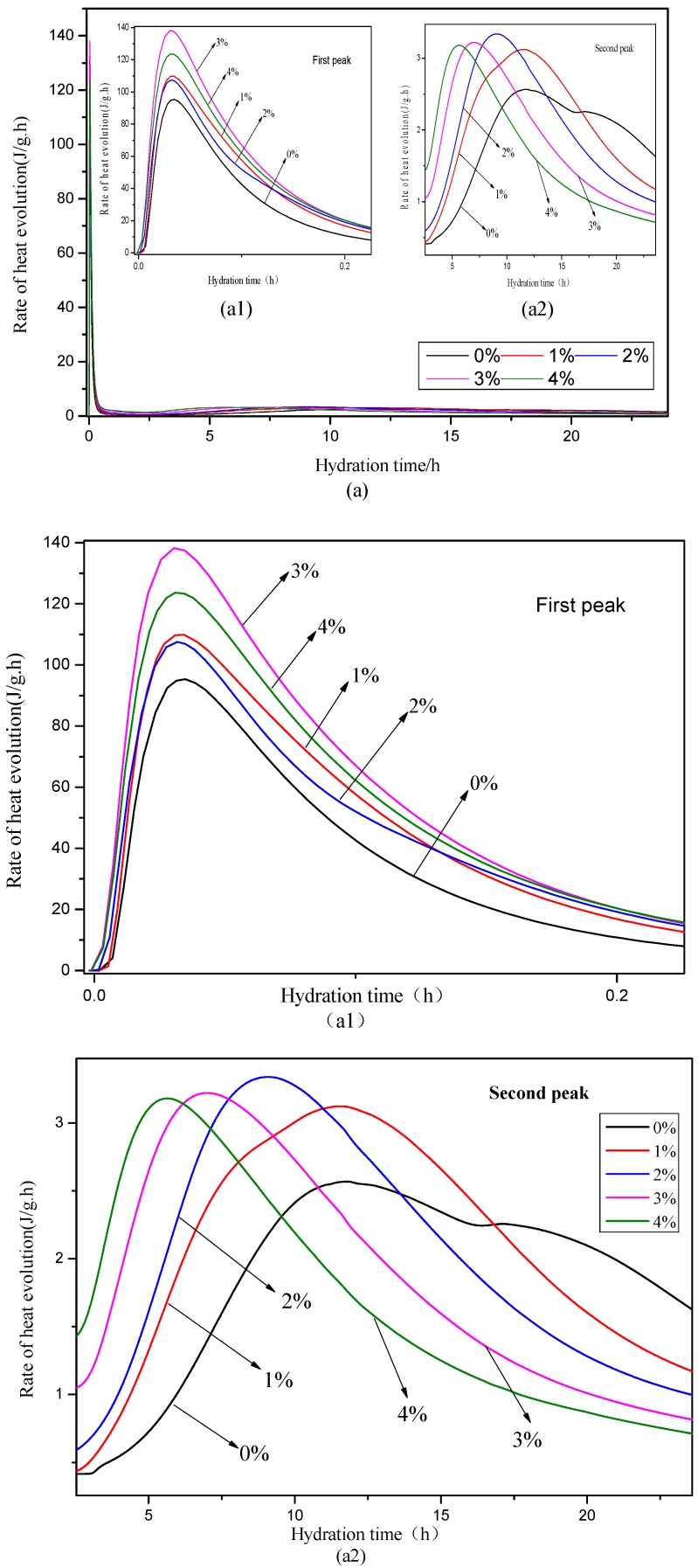
Figure 3 shows the hydration heat rate and accumulated hydration heat of the OPC pastes with 0%, 1%, 2%, 3%, and 4% NaHCO3. The first peak of hydration heat rate at around 0.05 h in Figure 3(a1) firstly decreased with the increase of NaHCO3 up to 2% and then increased with the further increase of NaHCO3 up to 4%. The first peak associated with the formation ettringite (AFt) [34,35], and it is suggested that the addition of 1–2% NaHCO3 refrained the formation of AFt in OPC paste but the further addition of NaHCO3 up to 4% accelerated the formation of AFt. The initial decrease of the AFt could be caused by the possible reaction or adhesion between the NaHCO3 and the aluminum phases, but the later increase of the AFt in the mix with 4% NaHCO3 could be caused by the increased CO32− content [17].
Figure 3.
Hydration heat rate (a) and accumulated heat (b) of the pastes with different contents of NaHCO3.
Different from the trend of the first peak of hydration heat rate with content of NaHCO3, as shown in Figure 3(a2), the peak height of the second peak of the hydration heat rate at 8–15 h increased continuously with the increase of NaHCO3. The second peak associated with the hydration of C3S and C2S and the formation of C-S-H and portlandite. It can be seen that the addition of increased the peak height of the second peak and it was indicated that there could be more hydration of C3S and C2S at 8–15 h in the mix with more NaHCO3. However, the peak time of the second peak was delayed in the mixes with 1% and 2% NaHCO3 but it was earlier in the mixes with 3% and 4% NaHCO3 compared to the control group with no NaHCO3. It suggested that the addition of NaHCO3 up to 2% delayed the hydration of C3S and C2S but further increase of NaHCO3 up to 4% accelerated the hydration of C3S and C2S in the initial 24 h.
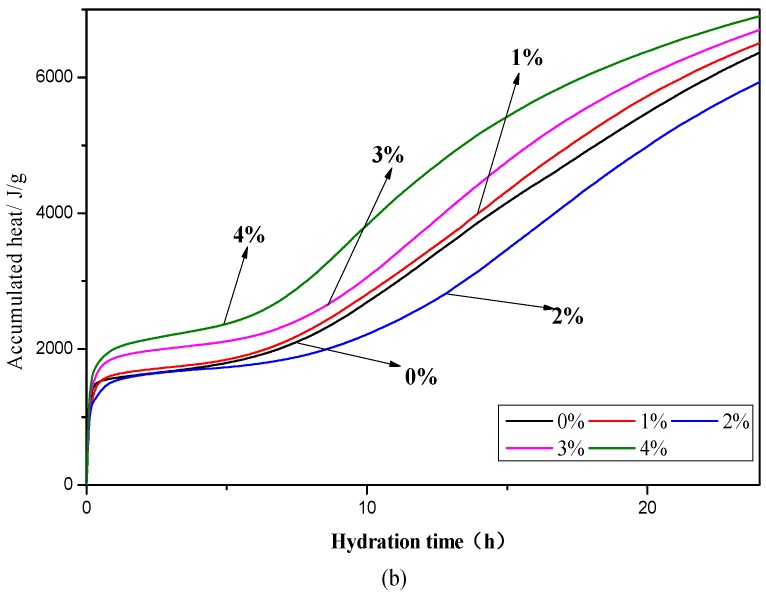
The total accumulated hydration heat in the initial 24 h is shown in Figure 3b, and it shows that the difference between the total hydration heat of OPC paste and that of the mix with 1% NaHCO3 was not significant, the mix with 2% had a much lower hydration heat than the OPC paste, but the mixes with 3% and 4% had a significantly higher total hydration heat than the OPC paste. This was mainly caused by the previously described delayed effect on the hydration heat in the mix with 2% NaHCO3 and the accelerated effect on the hydration heat in the mixes with 3% and 4% NaHCO3.
The hydration heat rate and accumulated hydration heat of the mixes with 0%, 1%, 2%, 3%, and 4% Na2CO3 are shown in Figure 4. The results show that the mixes with Na2CO3 had a higher first peak height of the hydration heat rate, as in Figure 4(a1), than the OPC paste with no Na2CO3. The highest first peak height happened in the mix with 3% Na2CO3 and there was a slightly decrease of the peak height in the mix with 4% Na2CO3. It is indicated that the addition of Na2CO3 accelerated the hydration of C3A and the formation of AFt.
Figure 4.
Hydration heat rate (a) and accumulated heat (b) of the pastes with different contents of Na2CO3.
As for the second peak, in Figure 4(a2), the addition of Na2CO3 increased the peak height and accelerated the peak time compared to the OPC paste with no Na2CO3. It suggested that the Na2CO3 accelerated and increased the hydration of C3S and C2S. This agrees with the findings in literature [33]. There was a shoulder peak at around 18 h after the second peak in the control group, which was cause by the secondary formation of AFt [36], but this shoulder peak did not appear in any mix with Na2CO3. This suggested that, in the mixes with Na2CO3, the initial accelerated formation of AFt in the first peak might consumed most of the C3A and formation of most AFt was finished at that time.
The accumulated hydration heat of the mixes with different contents of Na2CO3 is shown in Figure 4b. It can be seen that the mix with Na2CO3 had a much higher accumulated hydration heat compared to the control group. At 5–10 h, the mix with 4% Na2CO3 had the highest accumulated hydration heat and the higher content of Na2CO3 caused a higher accumulated hydration heat. At the end of 24 h, the mix with 1% Na2CO3 had the highest total hydration heat, followed by the mixes with 3%, 2%, 4%, and 0% Na2CO3. After 15 h, the increase rate of the accumulated hydration heat in the mixes with 3% and 4% Na2CO3 decreased obviously compared to the mixes with 1% and 2% Na2CO3. There was a tendency that the total hydration heat of the mixes with 3% and 4% Na2CO3 could be lower than the control group in the long term.
3.4. TG-DTA Results
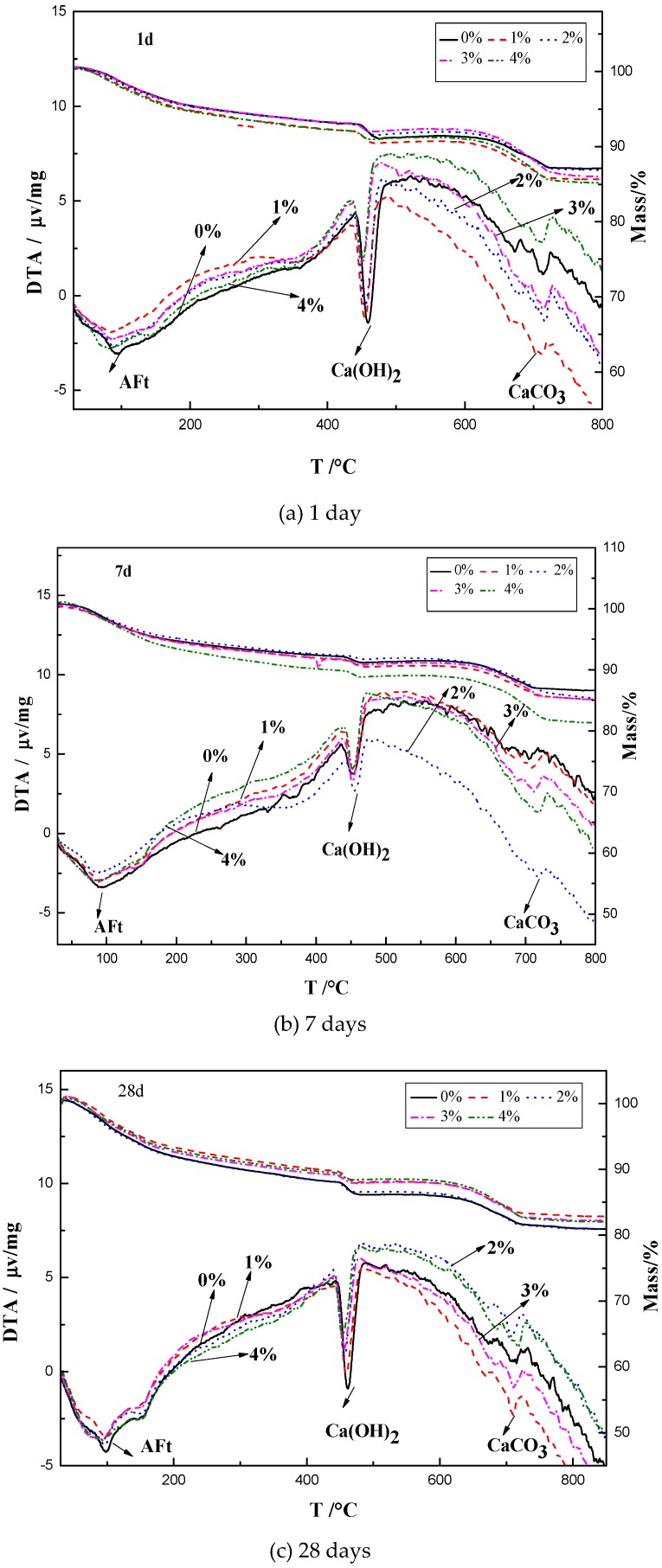
The TG-DTA results of the mixes with 0%, 1%, 2%, 3%, and 4% NaHCO3 are shown in Figure 5. There were three main DTA peaks at around 100 °C, 460 °C, and 700°C, which indicated the composition of AFt, portlandite and CaCO3 respectively. At the age of 1 day, the weight loss at the peak of AFt was 2.6%, 2.9%, 2.7%, 2.6%, and 3.1% in the mix with 0%, 1%, 2%, 3%, and 4% NaHCO3 respectively. The weight loss at the peak of portlandite was 2.0%, 1.6%, 1.5%, 1.2%, and 1.0% in the mix with 0%, 1%, 2%, 3%, and 4% NaHCO3 respectively. The weight loss at the peak of CaCO3 was 3.5%, 3.9%, 4.0%, 4.8%, and 4.7% in the mix with 0%, 1%, 2%, 3%, and 4% NaHCO3 respectively. It can be seen that the addition of NaHCO3 increased the formation of AFt and CaCO3 at the age of 1 day but decreased the portlandite. At the age of 7 and 28 days, the weight losses at the peaks of AFt, portlandite and CaCO3 showed similar trend as that at age of 1 day. It can be seen that the addition of NaHCO3 increased the formation of AFt and CaCO3 and decreased the portlandite at all the ages.
Figure 5.
TG-DTA results of the pastes with different contents of NaHCO3 at ages of (a) 1, (b) 7, and (c) 28 days.
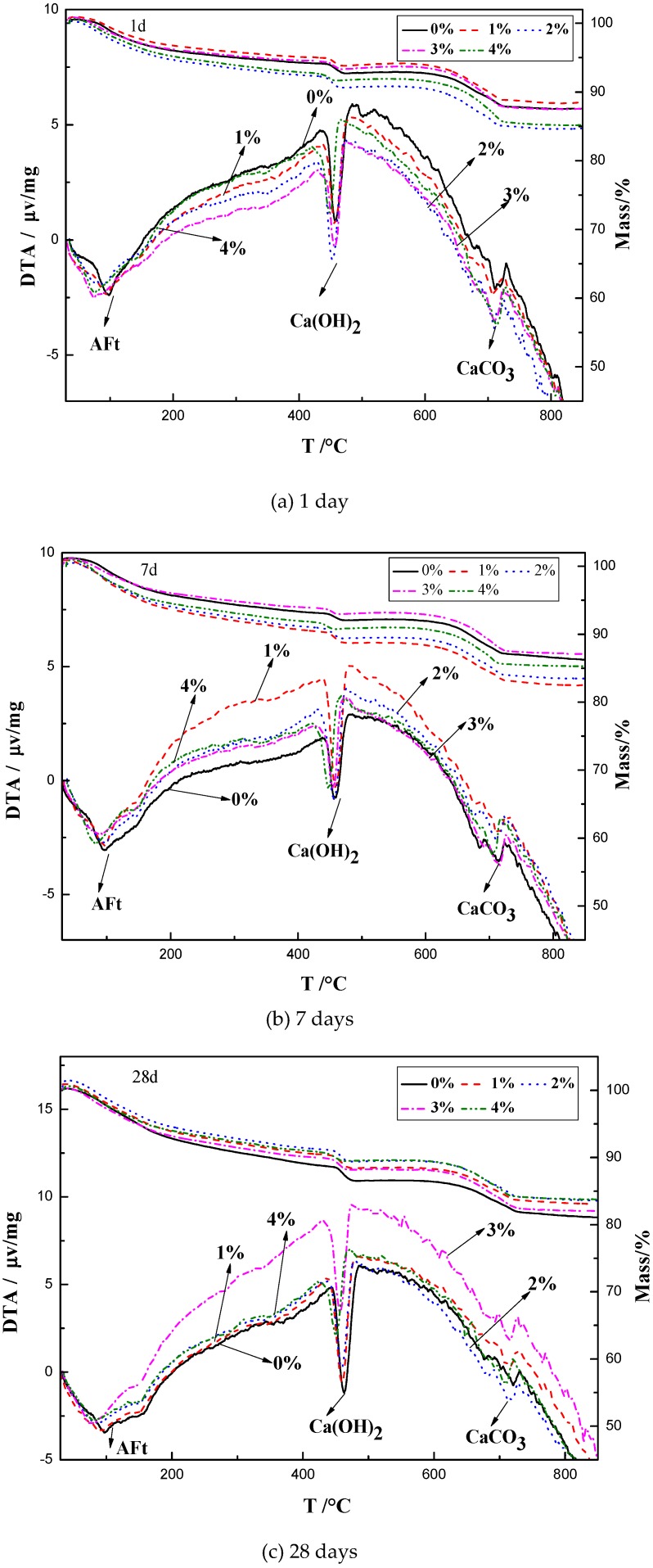
Figure 6 shows the TG-DTA results of the mixes with 0%, 1%, 2%, 3%, and 4% Na2CO3 at the ages of 1, 7, and 28 days. At the age of 1 day, the weight loss at the peak of AFt was 2.6%, 2.6%, 3.0%, 2.8%, and 3.0% in the mix with 0%, 1%, 2%, 3%, and 4% Na2CO3 respectively. The weight loss at the peak of portlandite was 2.0%, 1.6%, 1.4%, 1.1%, and 0.9% in the mix with 0%, 1%, 2%, 3%, and 4% Na2CO3 respectively. The weight loss at the peak of CaCO3 was 3.5%, 3.9%, 4.0%, 4.8%, and 4.7% in the mix with 0%, 1%, 2%, 3%, and 4% Na2CO3 respectively. It can be seen that, similar as the NaHCO3, the addition of Na2CO3 increased the formation of AFt and CaCO3 and decreased the portlandite at the age of 1 day. This trend was similar at the ages of 7 and 28 days.
Figure 6.
TG-DTA results of the pastes with different contents of Na2CO3 at ages of (a) 1, (b) 7, and (c) 28 days.
These results showed that the influence of NaHCO3 on the formation of AFt, portlandite and CaCO3 was similar as Na2CO3. The mix with the highest amount of NaHCO3 or Na2CO3 had the highest amount of AFt and CaCO3 but the lowest amount of portlandite. As can be seen from Figure 5 and Figure 6, the addition of NaHCO3 or Na2CO3 made the overall weight loss of the blended paste higher than the control group at the age of 1 and 7 days but the lower than the control group at the age of 28 days.
3.5. XRD Results
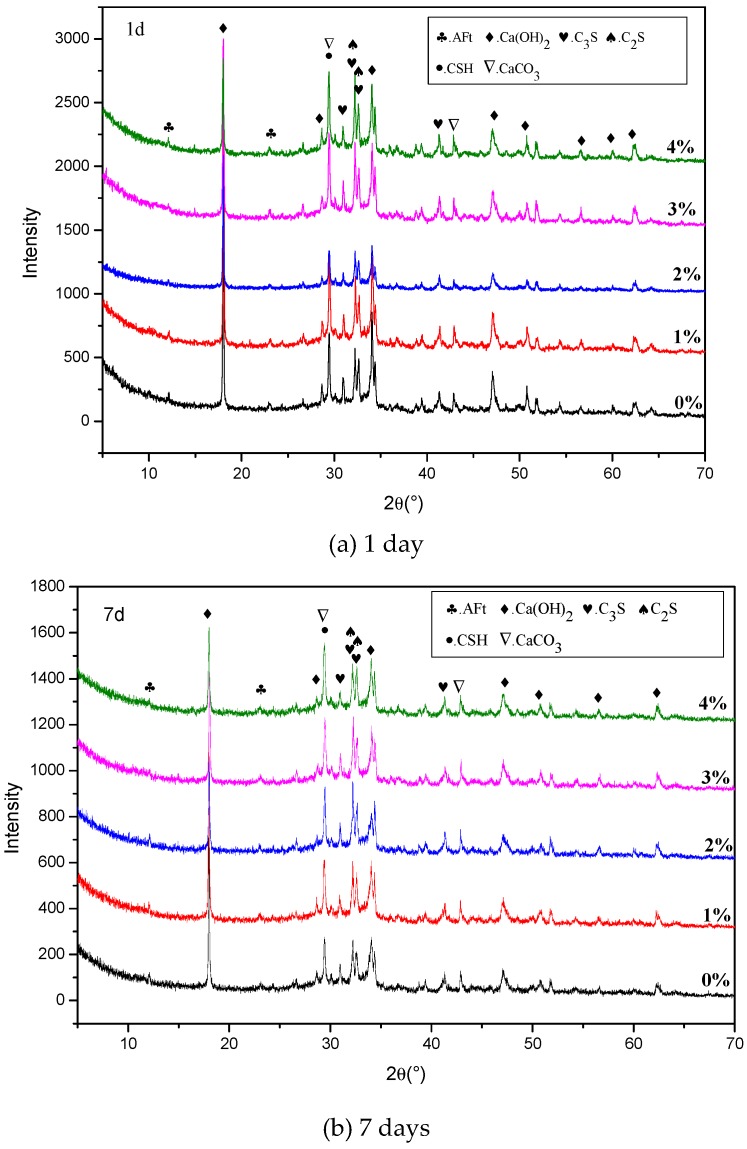
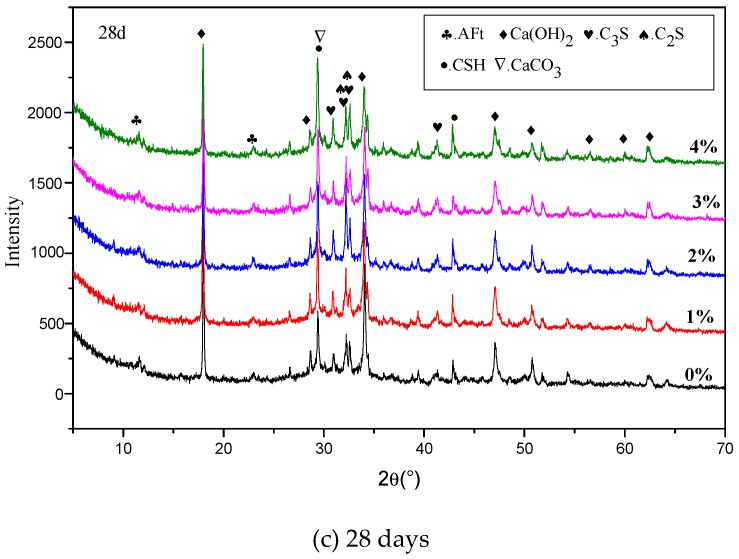
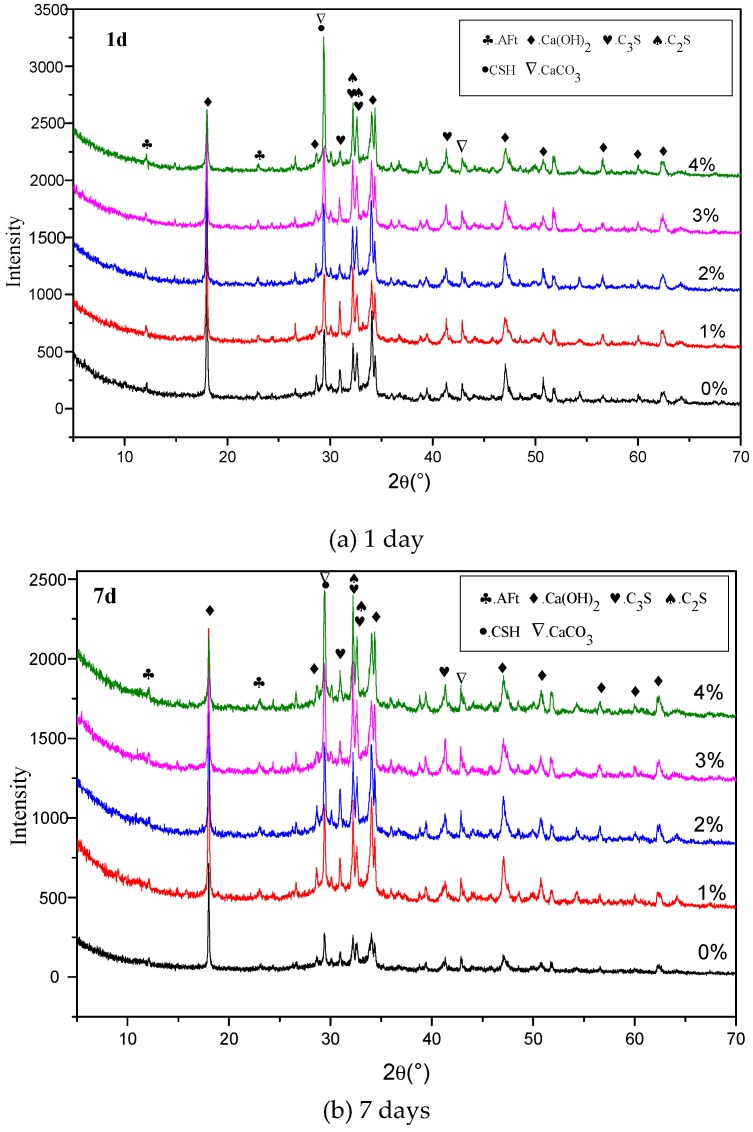
The XRD results of the pastes with 0%, 1%, 2%, 3%, and 4% NaHCO3 at the ages of 1, 7, and 28 days are shown in Figure 7. It can be seen that, at the age of 1 day, the peaks of Ca(OH)2 at 2θ = 34° and 47° decreased with the increase of NaHCO3. At the ages of 7 and 28 days, the peaks of portlandite changed in the same way as that in 1 day, besides, the peak of C-S-H and CaCO3 at 2θ = 29° increased with the increase of NaHCO3. These results all agree with the previously reported findings in the TG-DTA results. The change of AFt in the XRD spectrum was not obvious for the mixes with different contents of NaHCO3.
Figure 7.
XRD spectrum results of the mixes with different contents of NaHCO3 at ages of (a) 1, (b) 7, and (c) 28 days.
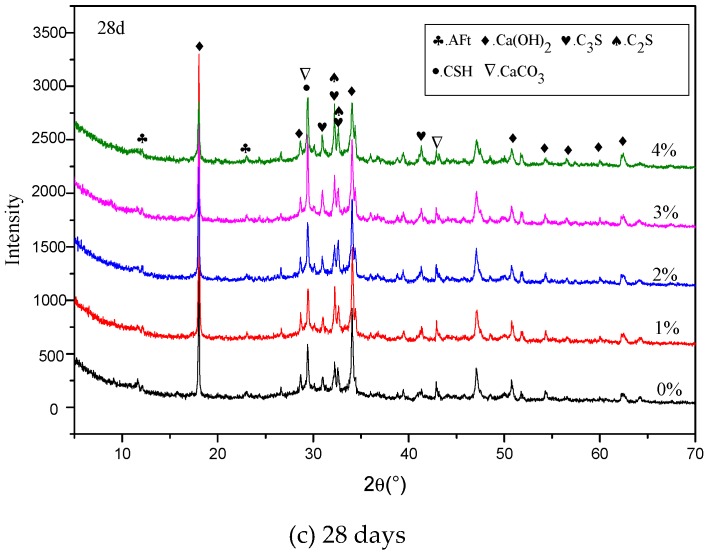
Figure 8 shows the XRD results of the pastes with 0%, 1%, 2%, 3%, and 4% Na2CO3 at the ages of 1, 7, and 28 days. The results show that the peaks of C-S-H and CaCO3 increased with the increase of Na2CO3 content at all ages, at the same time, the portlandite decreased gradually with the increase of Na2CO3 content. This again agrees with the findings in TG-DTA results.
Figure 8.
XRD spectrum results of the mixes with different contents of Na2CO3 at ages of (a) 1, (b) 7, and (c) 28 days.
3.6. SEM Results
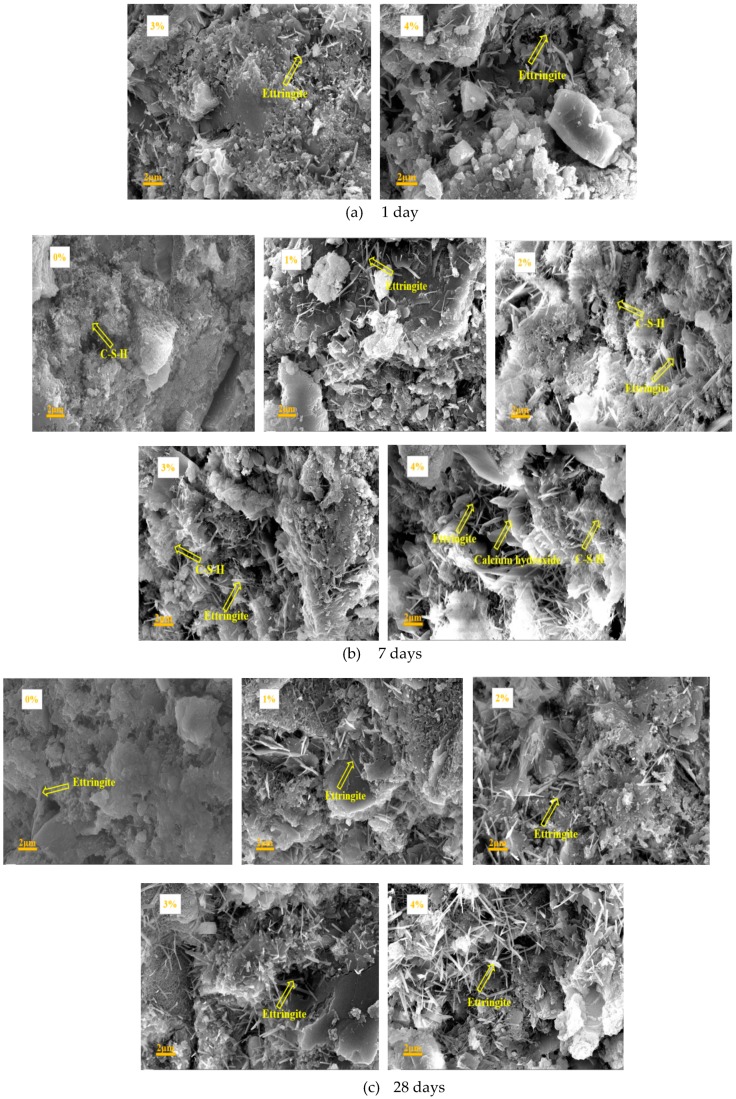
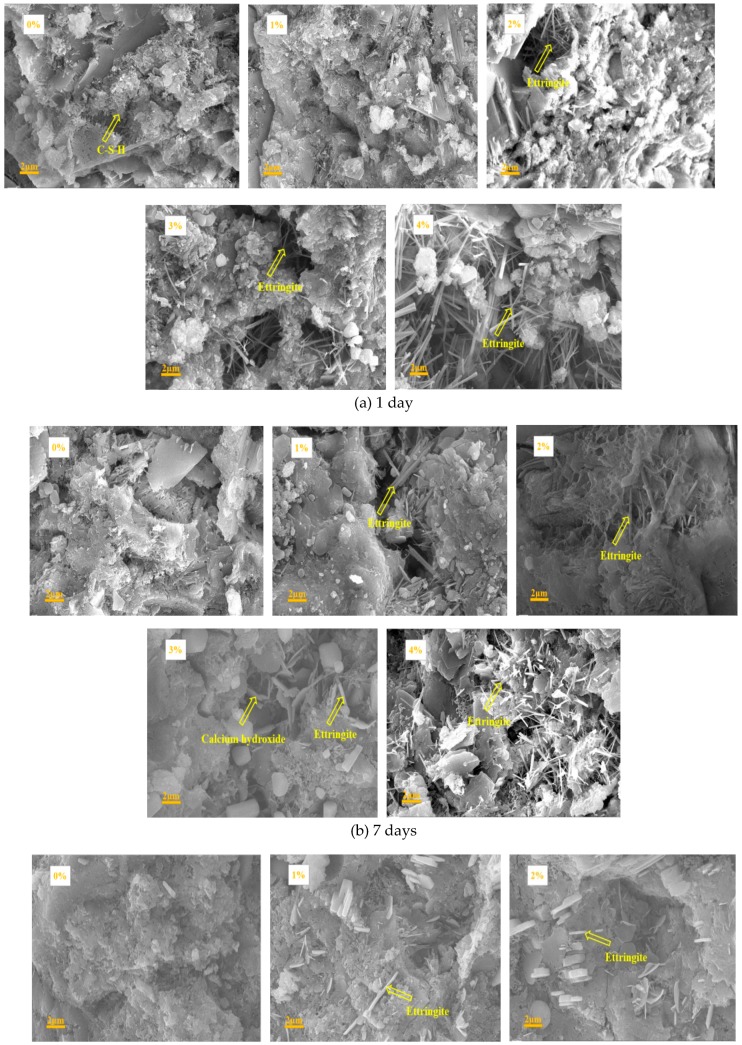
The SEM results of the pastes with 0%, 1%, 2%, 3%, and 4% NaHCO3 at the ages of 1, 7, and 28 days are shown in Figure 9. At the age of 1 day, it can be seen that the amount of needle-shaped ettringite in the mixes with NaHCO3 was higher than that in the pure OPC paste. The microstructure of the C-S-H gel in the mixes with 1%, 2%, and 3% was denser than that in the pure OPC paste, but the C-S-H gel in the mix with 4% was a bit loose compared to the other groups. These agrees with the changing trend of the compressive strength with NaHCO3 at 1 day in Figure 2. At the age of 7 days, the mix with 1% NaHCO3 had more ettringite and denser C-S-H gel than the pure OPC paste, but further increase of NaHCO3 made the C-S-H gel become loose although the amount of ettringite was increased. At the age of 28 days, the ettringite in the mixes with NaHCO3 was still higher than that in pure OPC paste but the C-S-H gel in the mixes with NaHCO3 was looser than that in pure OPC paste. These agree with the results of XRD and compressive strength.
Figure 9.
SEM results of the mixes with different contents of NaHCO3 at ages of (a) 1, (b) 7, and (c) 28 days.
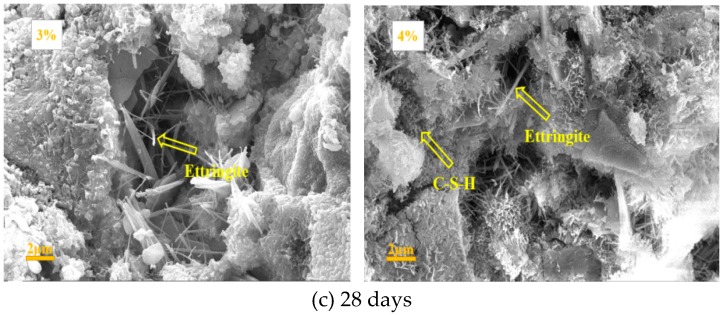
Figure 10 shows the SEM images of the mixes with 0%, 1%, 2%, 3%, and 4% Na2CO3 at the ages of 1, 7, and 28 days. At the age of 1 and 7 days, the mix with 1% Na2CO3 had more ettringite and a denser C-S-H structure than the pure OPC paste. The mixes with 2–4% had more ettringite but a worse C-S-H gel structure than the pure OPC paste. At the age of 28 days, the C-S-H gel became worse with the increase of Na2CO3 content compared to the OPC paste with no Na2CO3. These results agree with the compressive strength results as shown in Figure 2. It could be indicated that the early age strength at 1 day was mainly influenced by both ettringite and C-S-H gel, and the later age strength, such as 28 days, was mainly influenced by C-S-H gel structure.
Figure 10.
SEM results of the mixes with different contents of Na2CO3 at ages of (a) 1, (b) 7, and (c) 28 days.
4. Discussion
4.1. Influence of Na2CO3/NaHCO3 on the PH of OPC Paste
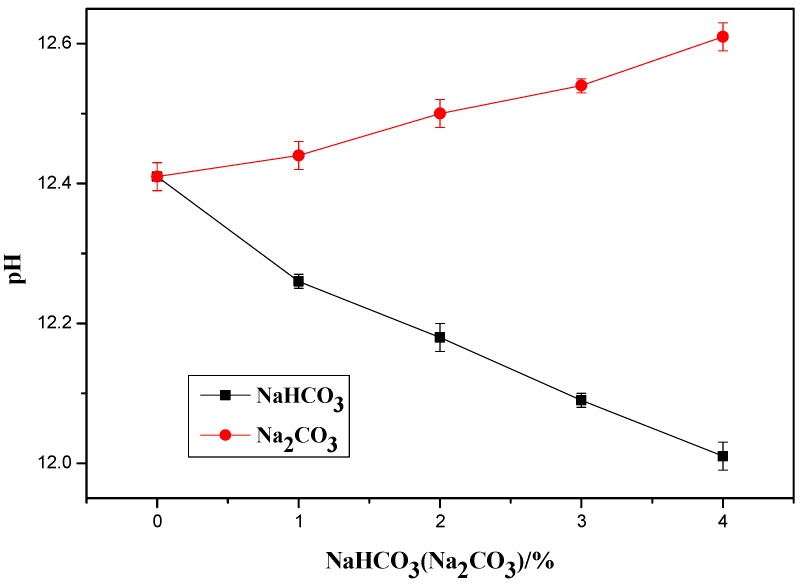
It is known that the both Na2CO3 and NaHCO3 are soluble and their main difference is that the Na2CO3 dissolves into Na+ and CO32− and the NaHCO3 dissolves into Na+ and HCO3− in water as shown in Equations (1) and (2). Solutions of Na2CO3 or NaHCO3 have a PH > 7, and the PH of Na2CO3 solution is higher than that of bicarbonate solution when the same content of the two are added. For example, under the same concentration 1 mmol/L (25 °C and 1 atm), the pH values of Na2CO3 and NaHCO3 solutions are 10.52 and 8.27 respectively. When they are added in cement paste, both of them can react with the portlandite, which is a hydration product of cement, and form CaCO3, as Equations (3) and (4). Cement slurry was prepared for pH measurements with a water-cement ratio of 0.5, a water reducing agent of 0.5%, and Na2CO3 and NaHCO3 of 0%, 1%, 2%, 3%, and 4%. The pH meter was initially calibrated with a neutral solution (pH = 7) and then with an alkaline solution with a known pH. After the calibration is completed, the electrode of the pH meter was immersed into the cement slurry and the slurry was gently vibrated to reach a uniform state during the measurements. The pH value was recorded after the reading was stable. The measured pH results are shown in Figure 11. It can be seen the pH of the OPC paste increased with the increase of Na2CO3 but it decreased with the increase of NaHCO3. This was caused by the different pH of the solutions with the same amount of Na2CO3 and NaHCO3. There could be a risk of alkali silica reaction in the concrete with a high amount of Na2CO3 because of the increased pH. There could be a decay of the C-S-H gel in the concrete with a high amount of NaHCO3 because of the decreased pH.
Figure 11.
pH of fresh cement paste with Na2CO3 or NaHCO3.
| Na2CO3 → 2Na+ + CO32− | (1) |
| NaHCO3 → Na+ + HCO3− | (2) |
| Na2CO3 + Ca(OH)2 = CaCO3 ↓ + 2NaOH | (3) |
| NaHCO3 + Ca(OH)2 = NaOH + CaCO3 ↓ + H2O | (4) |
4.2. Influence of Na2CO3/NaHCO3 on the Introduced CO2
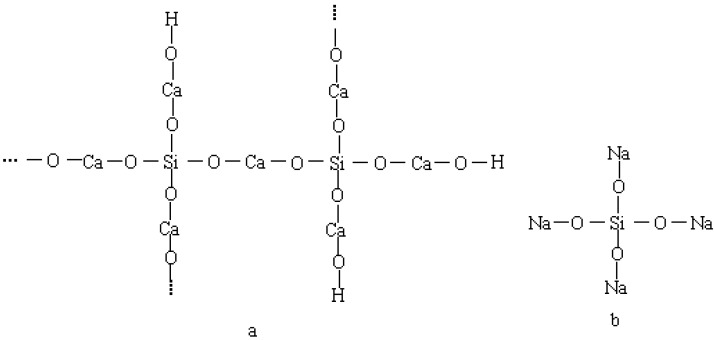
In Na2CO3 the weight of Na+ is 43.4% and the weight of CO2 is 41.5%. In NaHCO3 the weight of Na+ is 27.4% and the weight of CO2 is 52.4%. It can be seen that, when the same weight of the two are used, NaHCO3 brings 16% less Na+ and 10.9% more CO2 into the cement paste. Although the Na+ is believed to accelerate the initial hydration and early age strength [37], it is thought that Na+ is responsible for the adverse effect on the later age strength development of the cement paste with salts containing Na+ [20]. The adverse effect of Na+ on the strength development can be explained that the Na+ could affect the adhesion between C-S-H gel structure (Figure 12a) by reaction with the silica phase in the cement paste and form sodium orthosilicate (Figure 12b) [38,39]. The difference of Na+ introduced by the two was thought to be the main reason that the paste with NaHCO3 had a better later stage strength development than that with Na2CO3.
Figure 12.
Sketch of the structure of C-S-H gel (a) and sodium orthosilicate (b).
4.3. Influence of Na2CO3/NaHCO3 on the Formation of Ettringite and CaCO3
The initial difference between the effects of the Na2CO3 and NaHCO3 on the setting time and early age strength can be explained by the following reasons. In the paste with Na2CO3 the initial reactions were as Equations (3) and (5)–(7), and there was formation of both CaCO3 and ettringite. While in the paste with NaHCO3 the initial reactions were as Equations (4), (5), and (7), and there was no initial formation of CaCO3 and there was only formation of ettringite. There could be less ettringite formed in the paste with Na2CO3 compared to that with NaHCO3 because of the initial consumption of Ca2+ with CO32−. This could contribute to the better initial performance and shorter setting time of the paste with NaHCO3. In the paste with NaHCO3 the formation of CaCO3 happened at a later stage as Equations (8) and (9), and the later stage formed CaCO3 particles could contribute to fill the micro- and nano-pores of the C-S-H gel.
| CaSO4•2H2O → Ca2+ + SO42− + 2H2O | (5) |
| CO32− + Ca2+ → CaCO3 ↓ | (6) |
| 3CaO•Al2O3 + 3(CaSO4•2H2O) + 26H2O → 3CaO•Al2O3•3CaSO4•32H2O | (7) |
| C3S + H2O → C-S-H + Ca2+ + OH− | (8) |
| HCO3− + Ca2+ + OH− → CaCO3 ↓ + H2O | (9) |
4.4. Influence of Na2CO3/NaHCO3 on the Enthalpies of the Reactions with C3S
In order to further investigate the effect of Na2CO3 and NaHCO3 on the hydration of C3S, the enthalpies of the reactions are calculated. The enthalpies of all the reactants and products were calculated by the first-principles and the module of total energy pseudopotential calculations in the Vienna Ab initio Simulation Package (VASP) [40] was used for the calculations.
| 2(3CaO·SiO2) + 6H2O = 3CaO·2SiO2·3H2O + 3Ca(OH)2 | (10) |
| Ca(OH)2 + Na2CO3 = CaCO3 + 2NaOH | (11) |
| Ca(OH)2 + NaHCO3 = CaCO3 + NaOH + H2O | (12) |
| 3CaO·2SiO2·3H2O + 2NaOH = Na2O·2CaO·2SiO2·H2O + Ca(OH)2 + H2O | (13) |
By combing Equations (10), (11) and (13), resulting Equation (14)
| 2(3CaO·SiO2) + Na2CO3 + 4H2O = Na2O·2CaO·2SiO2·H2O + CaCO3 + 3Ca(OH)2 | (14) |
By combing Equations (10), (12) and (13), resulting Equation (15)
| 2(3CaO·SiO2) + 2NaHCO3 + 2H2O = Na2O·2CaO·2SiO2·H2O + 2CaCO3 + 2Ca(OH)2 | (15) |
The enthalpy of the reactions in Equation (14) (Ereaction14) can be calculated by Equation (16) and the value was −0.02903 eV/atom. The enthalpy of the reactions in Equation (15) (Ereaction15) can be calculated by Equation (17) and the value was −0.04306 eV/atom. These negative values suggest that the reactions in Equations (14) and (15) are both exothermic and can proceed spontaneously in thermodynamics. The reaction in Equation (15) had a more negative value than that in Equation (14) and it means that the reaction in Equation (15) is much easier to happen than that in Equation (14), which suggests that the reaction between C3S and NaHCO3 is much easier that the reaction between C3S and Na2CO3.
| Ereaction14 = (ENa2O·2CaO·2SiO2·H2O × 16 + ECaCO3 × 5 + ECa(OH)2 × 15-E3CaO·SiO2 × 18-ENa2CO3 × 6-EH2O × 12)/36 | (16) |
| Ereaction15 = (ENa2O·2CaO·2SiO2·H2O × 16 + ECaCO3 × 10 + ECa(OH)2 × 10-E3CaO·SiO2 × 18-ENaHCO3 × 18-EH2O × 6)/36 | (17) |
where E3CaO·SiO2, ENa2CO3, ENaHCO3, EH2O, ENa2O·2CaO·2SiO2·H2O, ECaCO3, ECa(OH)2 are the enthalpies of 3CaO·SiO2, Na2CO3, NaHCO3, H2O, Na2O·2CaO·2SiO2·H2O, CaCO3, and Ca(OH)2 molecules in unit of eV/atom, respectively.
5. Conclusions
The influence of NaHCO3 and Na2CO3 as additional additives on the setting time and compressive strength of OPC paste was investigated and the related effect on the hydration mechanism was studied through TG-DTA, XRD, and SEM tests. The following conclusions can be drawn.
-
(1)
The initial and final setting time of OPC paste decreased with the increase of either NaHCO3 or Na2CO3.
-
(2)
The addition of either NaHCO3 or Na2CO3 could increase the early age compressive strength (1 and 7 days) depending on the content added but they could decrease the compressive strength at later ages, such as 28 days, with the increase of content added.
-
(3)
As an accelerator, the optimum content of NaHCO3 and Na2CO3 were found to be in the same level as 1% of the weight of OPC. The addition 1% of either of the two accelerators could significantly shorten the setting time, increase the early age strength and did not have an obvious detrimental effect on the later age strength.
-
(4)
Further increase of NaHCO3 and Na2CO3 above 1% could decrease the compressive strength of OPC paste although the ettringite formation was accelerated and increased. This decay was mainly caused by the Na+ ions introduced and the Na+ could partly replace the Ca2+ in the C-S-H gel and cause the discontinuity of the C-S-H gel.
-
(5)
NaHCO3 was seen to be a better option as an accelerator compared to Na2CO3. The reaction between NaHCO3 and C3S was found to be much easier than the reaction between Na2CO3 and C3S. The same amount addition of NaHCO3 resulted a higher compressive strength at all ages compared to NaHCO3. Besides, NaHCO3 the introduced less Na+ and more CO2 in the cementitious system than the Na2CO3 when the same amount of the two were used.
Author Contributions
Conceptualization and Supervision: J.W., Q.H.; Methodology: J.W., Y.W., F.H.; Analysis: J.W., F.H., and Y.W., Writing and Editing: Y.W., F.H., and J.W.
Funding
The supports from the National Key Research and Development Plan of China (2017YFC0603004), the National Natural Science Foundation of China (51678220), the Program for Innovation Scientists and Technicians Troop Construction Projects of Henan Province in China (CXTD2017088) and the Program for Innovative Research Team (in Science and Technology) (19IRTSTHN027) in Henan Polytechnic University are appreciated. The authors appreciate the support from the Engineering division in New York University Abu Dhabi, UAE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ma B., Ma M., Shen X., Li X., Wu X. Compatibility between a polycarboxylate superplasticizer and the belite-rich sulfoaluminate cement: Setting time and the hydration properties. Constr. Build. Mater. 2014;51:47–54. doi: 10.1016/j.conbuildmat.2013.10.028. [DOI] [Google Scholar]
- 2.Bamonte P., Gambarova P.G., Nafarieh A. High-temperature behavior of structural and non-structural shotcretes. Cem. Concr. Compos. 2016;73:42–53. doi: 10.1016/j.cemconcomp.2016.06.009. [DOI] [Google Scholar]
- 3.De Belie N., Grosse C.U., Kurz J., Reinhardt H. Ultrasound monitoring of the influence of different accelerating admixtures and cement types for shotcrete on setting and hardening behaviour. Cem. Concr. Res. 2005;35:2087–2094. doi: 10.1016/j.cemconres.2005.03.011. [DOI] [Google Scholar]
- 4.Won J., Choi B., Lee J. Experimental and statistical analysis of the alkali–silica reaction of accelerating admixtures in shotcrete. Constr. Build. Mater. 2012;30:330–339. doi: 10.1016/j.conbuildmat.2011.11.017. [DOI] [Google Scholar]
- 5.Xie J., Wang J., Rao R., Wang C., Fang C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. B Eng. 2019;164:179–190. doi: 10.1016/j.compositesb.2018.11.067. [DOI] [Google Scholar]
- 6.Hou P., Kawashima S., Kong D., Corr D.J., Qian J., Shah S.P. Modification effects of colloidal nano SiO2 on cement hydration and its gel property. Compos. B Eng. 2013;45:440–448. doi: 10.1016/j.compositesb.2012.05.056. [DOI] [Google Scholar]
- 7.Meng T., Yu Y., Wang Z. Effect of nano-CaCO3 slurry on the mechanical properties and micro-structure of concrete with and without fly ash. Compos. B Eng. 2017;117:124–129. doi: 10.1016/j.compositesb.2017.02.030. [DOI] [Google Scholar]
- 8.Nazari A., Riahi S. The effects of zinc dioxide nanoparticles on flexural strength of self-compacting concrete. Compos. B Eng. 2011;42:167–175. doi: 10.1016/j.compositesb.2010.09.001. [DOI] [Google Scholar]
- 9.Jalal M., Ramezanianpour A.A., Pool M.K. Split tensile strength of binary blended self compacting concrete containing low volume fly ash and TiO2 nanoparticles. Compos B Eng. 2013;55:324–337. doi: 10.1016/j.compositesb.2013.05.050. [DOI] [Google Scholar]
- 10.Brough A.R., Atkinson A. Sodium silicate-based. alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002;32:865–879. doi: 10.1016/S0008-8846(02)00717-2. [DOI] [Google Scholar]
- 11.Li G., Li C., Zhou W., Wu Y. Factors affecting the liquid sodium aluminate accelerated agent. Concrete. 2005;7:54–58. [Google Scholar]
- 12.Han J., Wang K., Shi J., Wang Y. Influence of sodium aluminate on cement hydration and concrete properties. Constr. Build. Mater. 2014;64:342–349. doi: 10.1016/j.conbuildmat.2014.04.089. [DOI] [Google Scholar]
- 13.Tu Z., Shi C., Farzadnia N. Effect of Limestone Powder Content on the Early-Age Properties of CO2-Cured Concrete. J. Mater. Civ. Eng. 2018;30:04018164. doi: 10.1061/(ASCE)MT.1943-5533.0002232. [DOI] [Google Scholar]
- 14.Chong L., Shi C., Yang J., Jia H. Effect of limestone powder on the water stability of magnesium phosphate cement-based materials. Constr. Build. Mater. 2017;148:590–598. doi: 10.1016/j.conbuildmat.2017.04.207. [DOI] [Google Scholar]
- 15.Wu Z., Shi C., Khayat K.H. Multi-scale investigation of microstructure, fiber pullout behavior, and mechanical properties of ultra-high performance concrete with nano-CaCO3 particles. Cem. Concr. Compos. 2018;86:255–265. doi: 10.1016/j.cemconcomp.2017.11.014. [DOI] [Google Scholar]
- 16.Li W., Huang Z., Zu T., Shi C., Duan W.H., Shah S.P. Influence of Nanolimestone on the Hydration, Mechanical Strength, and Autogenous Shrinkage of Ultrahigh-Performance Concrete. J. Mater. Civ. Eng. 2016;28:04015068. doi: 10.1061/(ASCE)MT.1943-5533.0001327. [DOI] [Google Scholar]
- 17.Tu Z., Guo M., Poon C.S., Shi C. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 2016;72:9–16. doi: 10.1016/j.cemconcomp.2016.05.019. [DOI] [Google Scholar]
- 18.Monosi S., Moriconi G., Collepardi M. Combined effect of lignosulfonate and carbonate on pure portland clinker compounds hydration. III. Hydration of tricalcium silicate alone and in the presence of tricalcium aluminate. Cem. Concr. Res. 1982;12:425–435. doi: 10.1016/0008-8846(82)90057-6. [DOI] [Google Scholar]
- 19.Krismahariyanto M., Saing B., Widodo H. The Effects of Sodium Carbonate on the Properties and the Hydration of the Cement Mixed with the Hardening Accelerator Based on Calcium-Aluminate. AIP Conf. Proc. 2017;1877:090002. [Google Scholar]
- 20.Jang J.G., Kim H.J., Park S.M., Lee H.K. The influence of sodium hydrogen carbonate on the hydration of cement. Constr. Build. Mater. 2015;94:746–749. doi: 10.1016/j.conbuildmat.2015.07.121. [DOI] [Google Scholar]
- 21.Yang H., Che Y. Effects of Nano-CaCO3/Limestone Composite Particles on the Hydration Products and Pore Structure of Cementitious Materials. Adv. Mater. Sci. Eng. 2018;2018:8. doi: 10.1155/2018/5732352. [DOI] [Google Scholar]
- 22.Bernardi A., Bortoluzzi E.A., Felippe W.T., Felippe M.C.S., Wan W.S., Teixeira C.S. Effects of the addition of nanoparticulate calcium carbonate on setting time, dimensional change, compressive strength, solubility and pH of MTA. Int. Endod. J. 2017;50:97–105. doi: 10.1111/iej.12594. [DOI] [PubMed] [Google Scholar]
- 23.Mathur R., Sharma S.K. Magnesium oxysulphate cement: Change in properties on admixing sodium bicarbonate as an additive. Rasayan J.Chem. 2008;1:620–630. [Google Scholar]
- 24.Chandrawat M.P.S., Yadav R.N. Effect of sodium carbonate on some properties of magnesia cement. J. Indian Chem. Soc. 2001;78:389–391. [Google Scholar]
- 25.Kunther W., Lothenbach B., Scrivener K. Influence of bicarbonate ions on the deterioration of mortar bars in sulfate solutions. Cem. Concr. Res. 2013;44:77–86. doi: 10.1016/j.cemconres.2012.10.016. [DOI] [Google Scholar]
- 26.Kerui Y., Caiwen Z., Zhigang L. The influence of calcium lignosulphonate–sodium bicarbonate on the status of ettringite crystallization in fly ash cement paste. Cem. Concr. Res. 2002;32:51–56. doi: 10.1016/S0008-8846(01)00628-7. [DOI] [Google Scholar]
- 27.Reddy V.V., Rao H.S., Jayaveera K.N. Influence of strong alkaline substances (sodium carbonate and sodium bicarbonate) in mixing water on strength and setting properties of concrete. Indian J. Eng. Mater. Soc. 2006;13:123–128. [Google Scholar]
- 28.Reddy L.V.G., Krishna B. Influence of Na2CO3, NaHCO3 and Organic Substances (Algae) in Water on Physical Properties of Blended Pozzolonic Portland Cement. Int. J. Eng. Res. Technol. (IJERT). 2014;3:35–41. [Google Scholar]
- 29.Golewski G.L. Evaluation of morphology and size of cracks of the Interfacial Transition Zone (ITZ) in concrete containing fly ash (FA) J. Hazard. Mater. 2018;357:298–304. doi: 10.1016/j.jhazmat.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Golewski G.L. An assessment of microcracks in the Interfacial Transition Zone of durable concrete composites with fly ash additives. Compos. Struct. 2018;200:515–520. doi: 10.1016/j.compstruct.2018.05.144. [DOI] [Google Scholar]
- 31.Standardization Administration of the People’s Republic of China . GB175-2007: Common Portland Cement. China Architecture and Building Press; Beijing, China: 2007. [Google Scholar]
- 32.Ministry of Housing and Urban-Rural Development . JC 477-2005 Flash Setting Admixtures for Shotcrete. Ministry of Housing and Urban-Rural Development; Beijing, China: 2005. [Google Scholar]
- 33.Zhang Y., Wang Y., Li T., Xiong Z., Sun Y. Effects of lithium carbonate on performances of sulphoaluminate cement-based dual liquid high water material and its mechanisms. Constr. Build. Mater. 2018;161:374–380. doi: 10.1016/j.conbuildmat.2017.11.130. [DOI] [Google Scholar]
- 34.Xiong Z., Wang P., Wang Y. Hydration Behaviors of Portland Cement with Different Lithologic Stone Powders. Int. J. Concr. Struct. Mater. 2015;9:55–60. doi: 10.1007/s40069-014-0086-z. [DOI] [Google Scholar]
- 35.Bensted J., Barnes P. Structure & Performance of Cements. Taylor & Francis e-Library; London, UK: New York, NY, USA: 2008. pp. 90–91. [Google Scholar]
- 36.Bullard J.W., Jennings H.M., Livingston R.A., Nonat A., Scherer G.W., Schweitzer J.S., Scrivener K.L., Thomas J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011;41:1208–1223. doi: 10.1016/j.cemconres.2010.09.011. [DOI] [Google Scholar]
- 37.Edwards G.C., Angstadt R.L. The effect of some soluble inorganic admixtures on the early hydration of portland cement. J. Appl. Chem. 2007;16:166–168. doi: 10.1002/jctb.5010160508. [DOI] [Google Scholar]
- 38.Abdalqader A.F., Jin F., Al-Tabbaa A. Development of greener alkali-activated cement: Utilisation of sodium carbonate for activating slag and fly ash mixtures. J. Clean. Prod. 2016;113:66–75. doi: 10.1016/j.jclepro.2015.12.010. [DOI] [Google Scholar]
- 39.Yuan B., Straub C., Segers S., Yu Q.L., Brouwers H.J.H. Sodium carbonate activated slag as cement replacement in autoclaved aerated concrete. Ceram. Int. 2017;43:6039–6047. doi: 10.1016/j.ceramint.2017.01.144. [DOI] [Google Scholar]
- 40.Kresse G., Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]