Abstract
Aims
Our objectives were to compare effectiveness and long-term prognosis after epicardial thoracoscopic atrial fibrillation (AF) ablation vs. endocardial catheter ablation, in patients with prior failed catheter ablation or high risk of failure.
Methods and results
Patients were randomized to thoracoscopic or catheter ablation, consisting of pulmonary vein isolation with optional additional lines (2007–2010). Patients were reassessed in 2016/2017, and those without documented AF recurrence underwent 7-day ambulatory electrocardiography. The primary rhythm outcome was recurrence of any atrial arrhythmia lasting >30 s. The primary clinical endpoint was a composite of death, myocardial infarction, or cerebrovascular event, analysed with adjusted Cox proportional hazard ratios (HRs). One hundred and 24 patients were randomized with 34% persistent AF and mean age 56 years. Arrhythmia recurrence was common at mean follow-up of 7.0 years, but substantially lower with thoracoscopic ablation: 34/61 (56%) compared with 55/63 (87%) with catheter ablation [adjusted HR 0.40, 95% confidence interval (CI) 0.25–0.64; P < 0.001]. Additional ablation procedures were performed in 8 patients (13%) compared with 31 (49%), respectively (P < 0.001). Eleven patients (19%) were on anti-arrhythmic drugs at end of follow-up with thoracoscopy vs. 24 (39%) with catheter ablation (P = 0.012). There was no difference in the composite clinical outcome: 9 patients (15%) in the thoracoscopy arm vs. 10 patients (16%) with catheter ablation (HR 1.11, 95% CI 0.40–3.10; P = 0.84). Pacemaker implantation was required in 6 patients (10%) undergoing thoracoscopy and 3 (5%) in the catheter group (P = 0.27).
Conclusion
Thoracoscopic AF ablation demonstrated more consistent maintenance of sinus rhythm than catheter ablation, with similar long-term clinical event rates.
Keywords: Atrial fibrillation, Ablation, Surgery, Thoracoscopy, Catheter, Mortality, Rhythm
What’s new?
The first long-term results after randomization of patients to thoracoscopic vs. catheter ablation for atrial fibrillation (AF).
Excellent and similar long-term freedom from death, myocardial infarction, and cerebrovascular events with both thoracoscopic and catheter ablation.
Accounting for older technologies used, there was still a high rate of atrial arrhythmia recurrence in both arms.
Thoracoscopic AF ablation was associated with more durable maintenance of sinus rhythm over a 7-year period in this patient group who had initial, or high-risk of future failure from catheter ablation.
Introduction
Atrial fibrillation (AF) is a common health problem with rising incidence and prevalence, and high rates of stroke and mortality.1 Rhythm control with AF ablation is commonly used to treat symptomatic and drug-resistant patients and to improve quality of life.2 Percutaneous catheter ablation is most often the first-line approach, but long-term durability is modest.3 Other alternatives include surgical AF ablation using open heart surgery (Cox Maze), or a totally thoracoscopic procedure, which retains high success rates but with a minimally invasive approach.4
The FAST trial was the first randomized comparison of thoracoscopic and catheter ablation for AF in drug-refractory patients more likely to fail catheter ablation, either with a history of previously failed catheter ablation or factors suggesting an increased risk of failure of endocardial ablation, including hypertension with left atrial dilatation. One-year results confirmed a greater freedom from AF with thoracoscopic ablation, but a higher peri-procedural rate of adverse events than with catheter ablation.5 However, data on longer-term outcomes directly comparing thoracoscopic and catheter ablation are not currently available. Recent joint guidelines from the European Society of Cardiology and the European Association of Cardiothoracic Surgeons recommend that minimally invasive surgery should be considered in patients with symptomatic AF when catheter ablation has failed (IIaB). In symptomatic patients with persistent or long-standing persistent AF that is drug refractory, catheter and surgical ablation have the same level of recommendation, but only based on consensus opinion (IIaC).2
The present study extends the follow-up of patients randomized in the FAST trial to examine long-term outcomes relating to the success of rhythm control and major cardiovascular events. Our aim was to determine the comparative efficacy of thoracoscopic vs. catheter ablation in this patient group that is at higher risk of failure of catheter ablation. Although we are not adequately powered for clinical endpoints, our analysis provides the only long-term data, and alongside other ongoing clinical studies will provide additional information to clinicians and patients about treatment options for symptomatic AF.
Methods
A prospective randomized clinical trial was designed to compare catheter and thoracoscopic ablation in patients with drug-refractory AF referred for further rhythm control. The study was performed at St. Antonius Hospital in Nieuwegein, the Netherlands, and the Hospital Clínic in Barcelona, Spain. The trial was registered (https://www.clinicaltrials.gov/ct2/show/NCT00662701) and conducted in accordance with the Helsinki Declaration. Enrolment started in July 2007 and was completed in June 2010. Ethical approval for the original trial was obtained by both hospitals and the Dutch Central Trial Registration Organization (VCMO/CCMO). Ethical approval was again obtained for this extended follow-up study by the Research Ethical Committees of both sites: Comité Ético de Investigación Clínica del Hospital Clínic de Barcelona, Spain (HCB/2017/0085) and Medical Research Ethics Committees United, Nieuwegein, the Netherlands (NL55162.100.15).
Trial participants
Full details of the trial have previously been published.5 In brief, inclusion criteria were symptomatic paroxysmal AF for more than 12 months, or persistent AF confirmed by 7-day ambulatory electrocardiogram (ECG). Patients were refractory to, or intolerant of, at least one anti-arrhythmic drug, aged between 30 years and 70 years, and able to give informed consent, with either failure of a prior catheter ablation, left atrial (LA) diameter ≥45 mm, or hypertension with LA 40–44 mm. Major exclusion criteria were AF >1 year in duration, catheter or surgical procedures within the last 3 months, previous stroke or transient ischaemic attack, LA thrombus, LA diameter >65 mm, left ventricular ejection fraction <45%, significant valve disease, and other major cardiovascular or non-cardiovascular conditions.
Randomization and procedures
Block randomization was performed with concealed allocation using sealed envelopes. Patients randomized to thoracoscopy underwent general anaesthesia and pulmonary vein isolation (PVI) using radiofrequency ablation under direct view through a video-assisted thoracoscopic approach as previously described,6 with optional additional ablation lines and ganglionic plexus ablation. The LA appendage (LAA) was stapled and excised in thoracoscopic patients under direct vision and echocardiographic control. In the catheter ablation group, patients underwent PVI with conscious sedation. Procedural details differed at each centre,5 but all patients received radiofrequency ablation with optional lines at the discretion of the operator. All the patients with previous catheter ablation were found to have at least one pulmonary vein with intact conduction to left atrium before re-isolation. In both groups, patients were initially treated with vitamin K antagonist oral anticoagulation for three months, which was then continued at the discretion of the treating cardiologist based on the CHADS2 and later CHA2DS2-VASc score. A blanking period of 3 months was established in which pharmacological or electrical cardioversion was allowed. After this period, all antiarrhythmic drugs were suspended and any cardioversion after this period was considered a procedural failure.
Patient follow-up
A 7-day ambulatory ECG was performed as part of the original study protocol at 6 and 12 months after randomization. Clinical follow-up was similar in both thoracoscopic and catheter-treated patients. Long-term follow-up for this study in both centres consisted of a phone call or personal visit at the outpatient clinic in 2016, 12-lead ECG and documentation of current therapy (two patients in each arm were missing data on medications). Patients with no history of AF recurrence at that time point underwent a further 7-day ambulatory ECG. Major adverse clinical outcomes were confirmed by medical records locally and through central government records (Història Clínica Compartida, Departament de Salut, Generalitat de Catalunya, Spain, and Municipal Personal Records Database, The Netherlands). The European Society of Cardiology definitions for myocardial infarction (MI), transient cerebral ischaemic attack, and stroke were used to document cardiovascular events. Data were registered by independent researchers at both sites and Principal Investigators had no access to data until the database was locked for analysis.
Study outcomes
The primary rhythm endpoint was the first recurrence of any documented atrial arrhythmia lasting >30 s during follow-up. We also analysed atrial arrhythmia recurrence in relevant subgroups (patients on and off antiarrhythmic drugs at final follow-up, and according to prior catheter ablation treatment, age, gender, and type of AF at baseline). Where long-term follow-up was unavailable due to death, loss to follow-up or lack of ambulatory ECG, the rhythm endpoint was censored to the last available 7-day ambulatory ECG to avoid bias (typically at 12 months).
The primary clinical endpoints were the composite cumulative frequency of death, MI, or cerebrovascular event (transient ischaemic attack or ischaemic/haemorrhagic stroke), and the time to the first of these events. Secondary clinical endpoints were all-cause mortality and the need for permanent pacemaker implantation. We also assessed for bleeding requiring transfusion or surgery (not including peri-procedural events).
Endpoints were defined prior to statistical analysis, based on knowledge of available data and the prior publication of 1-year outcomes.5
Statistical analysis
Summary results are presented as percentages, or mean and standard deviation (SD). All analyses followed the principle of intention-to-treat. Categorical outcomes were compared using the χ2 test. Outcomes during follow-up were analysed using a Cox proportional hazards regression model, adjusted for baseline age, sex, AF type, and history of prior failed catheter ablation. Analyses were stratified by enrolment site and censored at 2400 days. Hazard ratios (HRs) and 95% confidence intervals (CIs) are presented, along with corresponding P-values. Kaplan–Meier plots were used to graph results according to treatment arm, with log-rank tests for comparison stratified by site. A post hoc analysis was performed to account for the competing risk of death in relation to arrhythmia recurrence using the method of Fine and Gray.7 There was no evidence of violation of the proportional hazards assumption in any multivariable model as determined by Schoenfeld residuals.
Aggregate data meta-analysis of the FAST trial data with two other prospective randomized controlled trials8,9 was performed using the method of Bagos and Nikolopoulos10 to account for differing follow-up periods. In brief, incidence rates were calculated for each trial arm by multiplying the rate of arrhythmia recurrence with the person-months of follow-up, and then incidence rate ratios calculated by comparing thoracoscopic with catheter ablation. Values were then pooled using a fixed effects model. Risk of bias was assessed using the Cochrane tool11 by two investigators working independently and a third for adjudication.
A two-tailed P-value of 0.05 was considered statistically significant. Analyses were performed on Stata Version 14.2 (StataCorp LP, TX, USA).
Results
One hundred and 24 patients were included in the study, with 63 randomized and receiving catheter ablation and 61 to thoracoscopy (Figure 1). Mean age was 56 ± 8 years at the time of inclusion, 81% were male, and AF type was persistent in 34% (Table 1). The majority of patients were included due to previous failed catheter ablation (67%).
Figure 1.
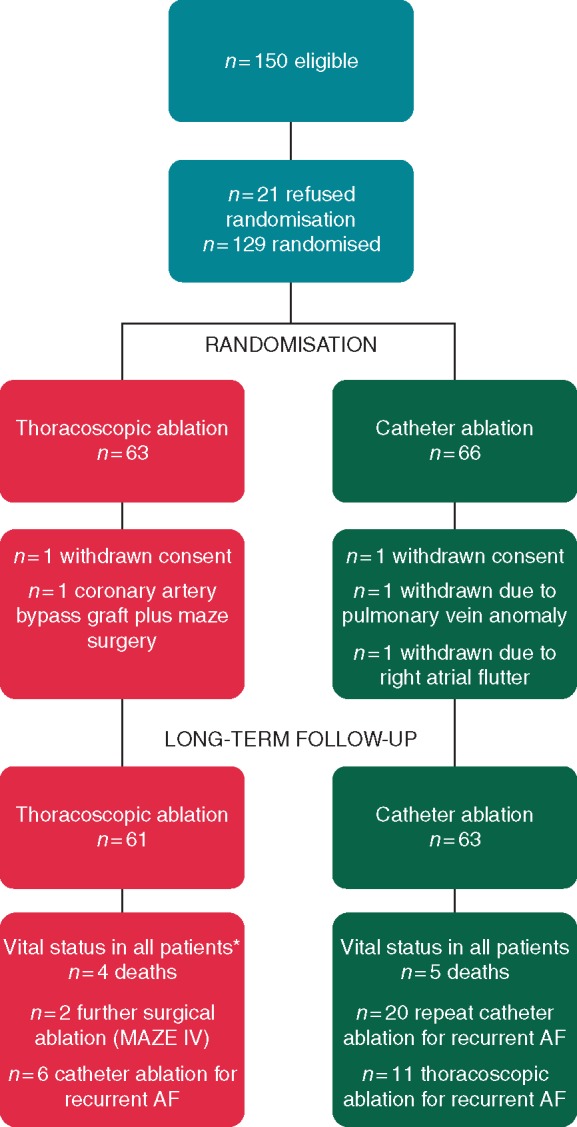
Study flowchart. *For rhythm outcome in thoracoscopic arm, n = 3 censored at 1 year (n = 2 unwilling to have 7-day ECG on follow-up due to lack of AF or symptoms and n = 1 moved abroad). AF, atrial fibrillation; ECG, electrocardiogram.
Table 1.
Baseline characteristics
| Characteristics | Thoracoscopic ablation (N = 61) | Catheter ablation (N = 63) |
|---|---|---|
| Male | 45 (74%) | 55 (87%) |
| Age (years), mean ± SD | 56.1 ± 8.0 | 56.0 ± 7.2 |
| Body mass index (kg/m2), mean ± SD | 27.8 ± 4.6 | 28.6 ± 3.5 |
| Prior myocardial infarction | 0 | 2 (3.2%) |
| Left ventricular ejection fraction (%), mean ± SD | 57.7 ± 6.8% | 55.5 ± 8.2% |
| LA diameter (mm), mean ± SD | 42.5 ± 6.5 | 43.2 ± 4.8 |
| Reason for randomization | ||
| Prior failed catheter ablation | 45 (74%) | 38 (60%) |
| LA diameter 40–45 mm and hypertension | 8 (13%) | 15 (24%) |
| LA diameter ≥45 mm | 8 (13%) | 10 (16%) |
| AF type | ||
| Paroxysmal AF | 45 (74%) | 37 (59%) |
| Persistent AF | 16 (26%) | 26 (41%) |
AF, atrial fibrillation; LA, left atrial; SD, standard deviation.
Median duration of hospitalization was 5.5 days for thoracoscopy vs. 2.0 days for catheter ablation (P < 0.001). Procedural complications and peri-procedural adverse events have previously been described and were higher with thoracoscopy.5 To summarize, in those randomized to thoracoscopy, there were conversions to median sternotomy for bleeding (n = 1), abandoned procedure with prolonged hospitalization (n = 1), haemothorax (n = 1), conservatively-treated pneumothorax (n = 6), stroke (n = 1), pericardial tamponade (n = 1), and rib fracture (n = 1). In the catheter ablation arm, one patient developed a pericardial effusion after transeptal puncture, one patient had a transient ischaemic attack, and four patients developed an uncomplicated (minor) groin haematoma.
Long-term follow-up for vital status was successfully obtained in all patients (Figure 1). The mean follow-up period was 7.0 years from randomization (SD 1.5 years). At final follow-up, 11 patients (19%) initially randomized to thoracoscopy were on anti-arrhythmic drugs, vs. 24 (39%) allocated to catheter ablation (P = 0.012). Corresponding numbers for oral anticoagulation were 19 patients (32%) in the thoracoscopy arm, vs. 32 patients (52%) with catheter ablation (P = 0.024).
Rhythm outcomes
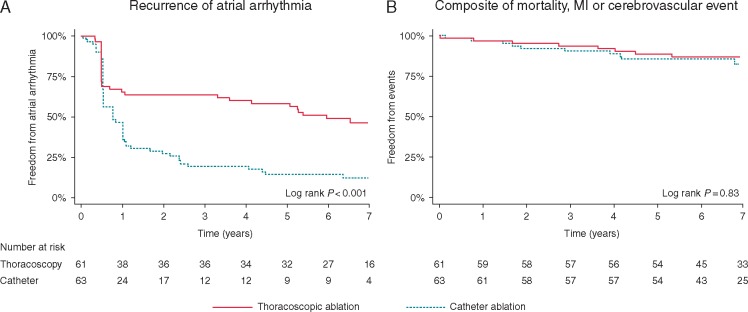
Across all recruited participants, recurrence of atrial arrhythmias was common, occurring in 89/124 patients (72%). Patients randomized to thoracoscopic ablation had lower recurrence rates during extended follow-up; 34/61 (56%) compared with 55/63 (87%) with catheter ablation. The adjusted HR was 0.40, 95% CI 0.25–0.64; P < 0.001 (Table 2). Kaplan–Meier curves are presented in Figure 2A, showing high early failure in both groups. This was followed by a high rate of arrhythmia recurrence in the catheter ablation arm up to 3 years of follow-up, much greater than in those randomized to thoracoscopy. All arrhythmia recurrences were AF in the catheter ablation group. With thoracoscopy, recurrence was AF in 32 patients (94%) and left atrial flutter in 2 patients (6%).
Table 2.
Multivariate analysis
| Outcomes | Thoracoscopic ablation, events (n) | Catheter ablation, events (n) | Thoracoscopic vs. catheter ablation; hazard ratio (95% CI)a | P-value |
|---|---|---|---|---|
| Atrial arrhythmia recurrence | 34/61 (56%) | 55/63 (87%) | 0.40 (0.25–0.64) | <0.001 |
| Atrial arrhythmia recurrence accounting for the competing risk of death | – | – | 0.43 (0.27–0.70) | 0.001 |
| Death, myocardial infarction or cerebrovascular event | 9/61 (15%) | 10/63 (16%) | 1.11 (0.40–3.10) | 0.84 |
| All-cause mortality | 4/61 (7%) | 5/63 (8%) | 0.94 (0.18–4.94) | 0.95 |
Adjusted for baseline age, sex, AF type and history of prior failed catheter ablation, stratified by recruitment site.
Figure 2.
Kaplan–Meier curves for the primary rhythm and clinical endpoints. (A) Time to atrial arrhythmia recurrence after the blanking period. (B) Time to first event—death, MI, or cerebrovascular event (transient ischaemic attack, ischaemic, or haemorrhagic stroke). MI, myocardial infarction.
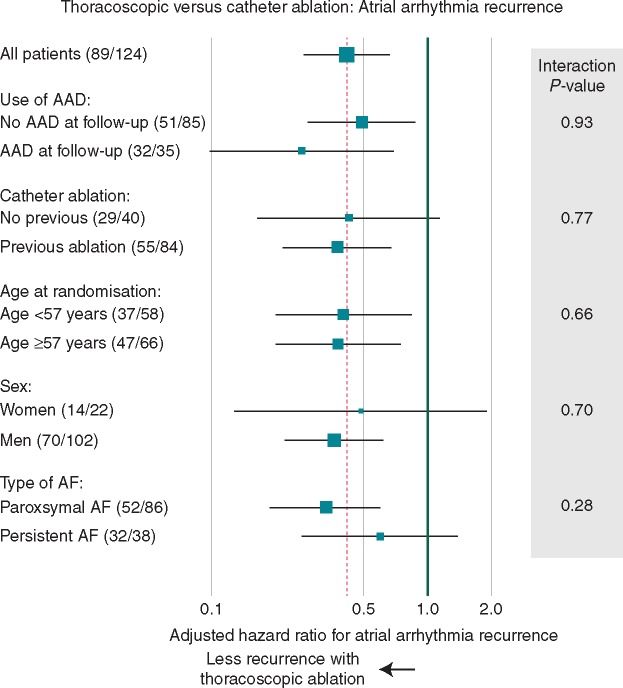
Additional ablation procedures (catheter or surgical) were performed in 8 patients (13%) randomized to thoracoscopy and 31 (49%) for to catheter ablation (P < 0.001) (Figure 1). In patients free of anti-arrhythmic drug use on final follow-up, the difference between thoracoscopy and catheter ablation for arrhythmia recurrence was consistent with the overall results (adjusted HR 0.49, 95% CI 0.27–0.89; P = 0.019). Atrial arrhythmia recurrence was lower in thoracoscopy patients compared with catheter ablation for all patient subgroups, including those with an without prior catheter ablation, and by age, sex and type of AF (Figure 3; all interaction P-values non-significant).
Figure 3.
Thoracoscopic vs. catheter ablation: arrhythmia recurrence by subgroup. Numbers in brackets are the number of patients with recurrence/total number in that subgroup. AAD, antiarrhythmic drugs; AF, atrial fibrillation.
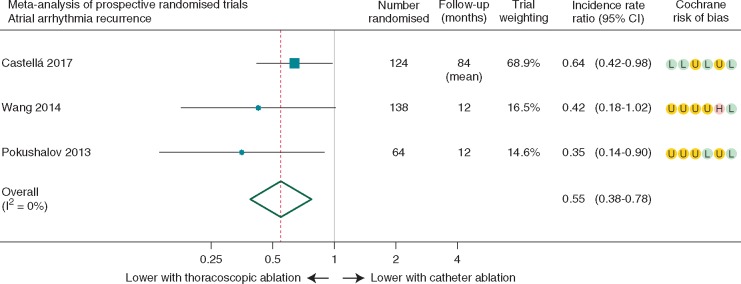
Meta-analysis combining our long-term study with 12-month data from two other prospective randomized trials confirmed a lower rate of incident atrial arrhythmia recurrence after thoracoscopic vs. catheter ablation. The pooled incidence rate ratio was 0.55, 95% CI 0.38–0.78, with P-value of 0.001, no heterogeneity between trials (I2 = 0%; P = 0.43), but variable risk of bias (Figure 4).
Figure 4.
Meta-analysis of incident rate ratios for arrhythmia recurrence in prospective randomized trials. Summary data for the three randomized controlled trials of thoracoscopic vs. catheter ablation for AF. Note that each study had different inclusion criteria, ablation strategies, and use of antiarrhythmic drug therapy during follow-up. The incidence rate ratio is the rate of arrhythmia recurrence weighted by person-months of follow-up comparing thoracoscopic with catheter ablation. Cochrane risk of bias domains are (from left to right): sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other threats to validity; scored as low risk (L), unclear risk (U), or high risk (H) of bias. AF, atrial fibrillation; CI, confidence interval; I2, heterogeneity.
Clinical outcomes
Beyond the initial procedural complications, there were no differences in adverse clinical events between groups. The composite of death, MI, or cerebrovascular event (transient ischaemic attack, ischaemic or haemorrhagic stroke) occurred in 9/61 patients (15%) assigned to thoracoscopy and 10/63 (16%) to catheter ablation. The adjusted HR for time to first event was 1.11, 95% CI 0.40–3.10; P = 0.84 (Figure 2B).
Although we were not powered to detect a different in all-cause mortality, this was similar in both groups (adjusted HR 0.94, 95% CI 0.18–4.94; P = 0.95). Four patients (7%) died in the thoracoscopic arm, with one due to cardiovascular causes. Five patients (8%) died in the catheter group, with four due to cardiovascular causes. Major bleeding requiring transfusion or surgery (not including peri-procedural events) occurred in 0 patients (0%) receiving thoracoscopic ablation and 1 patient (2%) with catheter ablation. Pacemaker implantation was required in 6 patients (10%) in the thoracoscopy arm and 3 (5%) randomized to catheter ablation (P = 0.27). A list of all outcomes by treatment arm is presented in Table 3.
Table 3.
Endpoints for thoracoscopic and catheter ablation
| Outcomes | Thoracoscopic ablation (N = 61) | Catheter ablation (N = 63) |
|---|---|---|
| All-cause mortality | 4 (7%) | 5 (8%) |
| Cardiovascular death | 1 (2%) | 4 (6%) |
| Non-cardiovascular death | 2 (3%) | 0 (0%) |
| Unknown cause of death | 1 (2%) | 1 (2%) |
| Myocardial infarction | 1 (2%) | 0 (0%) |
| Cerebrovascular eventa,b | 5 (8%) | 6 (10%) |
| Stroke | 4 (7%) | 2 (3%) |
| Transient ischaemic attack | 2 (3%) | 4 (6%) |
| Intracranial haemorrhage | 0 (0%) | 1 (2%) |
| Bleeding requiring transfusion or surgery | 0 (0%) | 1 (2%) |
| Permanent pacemaker implantation | 6 (10%) | 3 (5%) |
Outcomes are from the day of the procedure to last follow-up.
One patient in each group had both a transient ischaemic attack and stroke.
One patient in each group had a fatal cerebrovascular event and so are also included in the cardiovascular death outcome.
Discussion
The FAST trial is the only randomized evaluation of thoracoscopic vs. catheter ablation for AF with long-term follow-up (mean of 7 years). Thoracoscopic ablation after prior catheter ablation failure or in patients with dilated left atrium and hypertension was associated with higher rates of sinus rhythm maintenance, and significantly less patients required additional ablation or antiarrhythmic medication compared with those in the catheter ablation group. Whereas thoracoscopic ablation was associated with higher rates of peri-procedural adverse events, there were no differences in the long-term clinical composite endpoint of mortality, MI, or cerebrovascular events compared to catheter ablation. Our results provide reassurance for the safety of both procedures in symptomatic patients refractory or intolerant to anti-arrhythmic drugs.
Recurrence of atrial arrhythmias was common in this trial. We have to emphasize the high standard for failure in this trial, a single 30 s episode of atrial tachyarrhythmia in 7 years searched with 7-day ambulatory ECG. Typically, ‘success’ of AF ablation strategies (including in the FAST trial) has been the maintenance of sinus rhythm, despite the knowledge of only modest rates of sinus rhythm in long-term studies.3 Our trial confirms that arrhythmia recurrence is frequent in the long-term after ablation, even if AF burden and symptoms may improve. The risk of AF recurrence, despite multiple ablation procedures, has important implications on stroke prevention, in particular the continuation of anticoagulation even after apparently successful ablation, as reflected in the ESC/EACTS 2016 Guidelines for AF management.2 The similar rate of thromboembolic events in our study in the two groups, despite LAA exclusion in the thoracoscopic arm and lower rates of arrhythmia recurrence, should remind us that not all thromboembolic events arise from the LAA, and that AF is an inflammatory and pro-thrombotic condition.
Contemporary rates for maintenance of sinus rhythm after ablation are higher than we document due to recent advances, including new technologies such as better mapping or catheters. In the FIRE and ICE trial of paroxysmal AF patients, freedom from atrial arrhythmia or need for further treatment was 65% at 12 months.12 In the STAR-AF trial of persistent AF patients, there was 49% freedom from AF recurrence at 18 months.13 Second generation cryoballoon have shown 64% freedom from AF at 12 months in persistent AF patients.14 Our catheter ablation results using monopolar radiofrequency catheters are broadly similar to other published data in the same period.15 Surgical techniques have also improved during our follow-up period, including bipolar clamps able to perform wider ablation lines, and standardized conductance-based protocols that allow for more applications and better transmurality than during the FAST trial. Hence, success rates from ablation are likely higher now for both catheter and thoracoscopic ablation.
Our findings were consistent in meta-analysis pooling the two other prospective randomized controlled trials.8,9 Albeit with shorter 12-month follow-up than our data, the absence of heterogeneity in treatment effect comparing thoracoscopy and catheter ablation with different procedures and patient populations is reassuring. Although our sample size limits full assessment, we found no evidence of any difference in patient subgroups, with all patients having lower arrhythmia recurrence with thoracoscopic compared to catheter ablation. This included patients with persistent forms of AF, where the interaction P-value was non-significant but we clearly lacked power. The optimal strategy of ablation in these patients remains to be identified. Studies have demonstrated the superiority of the full Cox Maze lesion set as the most effective pattern in maintaining sinus rhythm in persistent AF when lesions are performed surgically with bipolar radiofrequency or cryothermy.16 However, recent studies in the surgical and catheter field suggest that additional lines, when added to PVI, do not necessarily improve results in patients with persistent AF.13 New approaches to AF management, including enhanced clinical phenotyping and identification of atrial function and atrial damage (fibrosis, dilatation, complex or aberrant focal ECGs) may result in better classification and stratification of therapy in the future.17
Availability of thoracoscopic ablation remains limited globally and due to the invasive approach, it is unlikely this could be recommended for initial treatment of AF in most patients at present. Instead, guidelines have suggested that thoracoscopic ablation is a useful treatment option in those patients refractory to other therapy.2 The FAST trial included patients at higher risk of catheter ablation failure, which in addition to the epicardial ablation and LAA excision afforded by the thoracoscopic approach, may have led to better rhythm outcomes. Like other interventional procedures, there is evidence of a learning curve with thoracoscopic ablation, and also that complications can be reduced in expert hands to a similar level as seen with percutaneous approaches.18 If this trend continues, along with better technologies and patient selection, there may be a wider role for thoracoscopic procedures in the future.19 Although recently presented data did not identify a clinical advantage from catheter ablation over drug therapy,20 further study data are awaited to establish if early control of rhythm can lead to improved prognosis for patients with AF. Our findings show better rhythm outcomes for thoracoscopic ablation, but future studies also need to demonstrate whether these approaches can robustly improve quality of life.
Limitations
Not all patients enrolled in the FAST trial had prior catheter ablation, reflecting clinical practice whereby it may not be ethical to proceed with an endocardial intervention in patients at increased risk of treatment failure. Those that underwent prior ablation were often treated in other referring centres, and so information on the number of previous procedures was not always available. This study describes the longest follow-up of patients randomized to thoracoscopic vs. catheter ablation. However, the original study protocol was one year, and subsequent follow-up was not performed in a regular, protocol-driven manner, but rather at a fixed time point. For this reason, we were unable to calculate time periods on and off antiarrhythmic drugs. We used a strict definition of recurrence of any single episode of atrial arrhythmia >30 s in duration, consistent with consensus documents,21 although does not differentiate between AF and other rhythms such as atrial macro re-entrant tachycardia. Further, we performed 7-day ambulatory ECG in all patients without prior clinical recurrence of AF. However, we were not able to assess AF burden, and some atrial arrhythmia episodes may not necessarily reflect AF, which may have contributed to the low overall rates for maintenance of sinus rhythm. We do not present data on the type of arrhythmia recurrence, which in clinical practice could differ depending on endocardial or epicardial approaches. By chance, there was a lower number of persistent AF patients in the thoracoscopic ablation group, and despite no evidence of interaction in treatment effect, further randomized data in this population is clearly warranted. The meta-analysis is limited by the small number of randomized trials in this field, the variable risk of bias, and the relatively short duration of the other trials that required an incidence rate assessment for atrial arrhythmia recurrence. As already noted, the technologies used are now outdated have now been superseded both for thoracoscopic and catheter ablation. Finally, the sample size is clearly insufficient to power for clinical outcomes even over this long time period, although we demonstrate reassuringly low rates of death, MI and cerebrovascular events after both thoracoscopic and catheter ablation.
Conclusion
For symptomatic AF patients with previous failed catheter ablation or structural changes, thoracoscopic ablation is associated with lower rates of recurrence of atrial arrhythmias compared to catheter ablation. Thoracoscopic PVI and LAA excision was associated with higher rates of peri-procedural events and a longer initial hospital stay. Both thoracoscopic and catheter ablation patients had low rates of major adverse clinical outcomes during 7-year follow-up.
Acknowledgements
We are indebted to the patients, clinicians, and research teams involved in the FAST trial.
Funding
The participating institutions funded the study. AtriCure provided a partial grant to facilitate the original trial. AtriCure had no part in any aspect of follow-up, including design, and did not have access to any part of the data, including collection or interpretation. D.K. is funded by a National Institute for Health Research (NIHR) Career Development Fellowship (CDF-2015-08-074). The opinions expressed are those of the authors and do not represent the NIHR or the UK Department of Health.
Conflict of interest: All authors have completed the ICMJE conflict of interest statement and report the following: M.C. reports personal fees and consultancy from Atricure and Medtronic, outside the submitted work. W.J.v.B. reports grants from Atricure during the conduct of the study; and personal fees from Atricure outside the submitted work. L.M. reports grants from Johnson & Johnson and St Jude Medical/Abbott, during the conduct of the study; and personal fees from St Jude Medical/Abbott, Medtronic and Boston Scientific, outside the submitted work. B.P.v.P. reports an unrestricted research grant from Atricure during the conduct of the study; and personal fees from Atricure for consulting and proctoring activities, outside the submitted work. All other authors declared no conflict of interest.
References
- 1. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Wynn GJ, El-Kadri M, Haq I, Das M, Modi S, Snowdon R. et al. Long-term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Heart 2016;3:e000394.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Laar C, Kelder J, van Putte BP.. The totally thoracoscopic maze procedure for the treatment of atrial fibrillation. Interact Cardiovasc Thorac Surg 2017;24:102–11. [DOI] [PubMed] [Google Scholar]
- 5. Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M. et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23–30. [DOI] [PubMed] [Google Scholar]
- 6. Edgerton JR, Jackman WM, Mack MJ.. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88:1655–7. [DOI] [PubMed] [Google Scholar]
- 7. Fine J, Gray R.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 8. Pokushalov E, Romanov A, Elesin D, Bogachev-Prokophiev A, Losik D, Bairamova S. et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol 2013;24:1338–43. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Liu L, Zou C.. Comparative study of video-assisted thoracoscopic surgery ablation and radiofrequency catheter ablation on treating paroxysmal atrial fibrillation: a randomized, controlled short-term trial. Chin Med J 2014;127:2567–70. [PubMed] [Google Scholar]
- 10. Bagos PG, Nikolopoulos GK.. Mixed-effects poisson regression models for meta-analysis of follow-up studies with constant or varying durations. Int J Biostat 2009;5:1; Article 21. [Google Scholar]
- 11. Higgins JPT, Altman DG, Sterne JAC (eds). Chapter 8: assessing risk of bias in included studies In Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org. [Google Scholar]
- 12. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR. et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 13. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 14. Mortsell D, Jansson V, Malmborg H, Lonnerholm S, Blomstrom-Lundqvist C.. Clinical outcome of the 2nd generation cryoballoon for pulmonary vein isolation in patients with persistent atrial fibrillation—a sub-study of the randomized trial evaluating single versus dual cryoballoon applications. Int J Cardiol 2018;doi:10.1016/j.ijcard.2018.10.097. [DOI] [PubMed] [Google Scholar]
- 15. Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F. et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57:160–6. [DOI] [PubMed] [Google Scholar]
- 16. Bogachev-Prokophiev A, Zheleznev S, Pivkin A, Pokushalov E, Romanov A, Nazarov V. et al. Assessment of concomitant paroxysmal atrial fibrillation ablation in mitral valve surgery patients based on continuous monitoring: does a different lesion set matter? Interact Cardiovasc Thorac Surg 2014;18:177–81; discussion 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A. et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 18. Vos LM, Kotecha D, Geuzebroek GSC, Hofman FN, van Boven WJP, Kelder J. et al. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace 2018;20:1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Laar C, Verberkmoes NJ, van Es HW, Lewalter T, Dunnington G, Stark S. et al. Thoracoscopic left atrial appendage clipping: a multicenter cohort analysis. JACC Clin Electrophysiol 2018;4:893–901. [DOI] [PubMed] [Google Scholar]
- 20. Packer DL. Catheter ABlation vs ANtiarrhythmic Drug Therapy in Atrial Fibrillation—CABANA. In Heart Rhythm Society Scientific Session, May 10. Boston, MA, USA: Heart Rhythm Society; 2018. https://www.acc.org/latest-in-cardiology/clinical-trials/2018/05/10/15/57/cabana. [Google Scholar]
- 21. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]