Abstract
The platypus (Ornithorhynchus anatinus) is one of the world’s most evolutionarily distinct mammals, one of five extant species of egg-laying mammals, and the only living species within the family Ornithorhynchidae. Modern platypuses are endemic to eastern mainland Australia, Tasmania, and adjacent King Island, with a small introduced population on Kangaroo Island, South Australia, and are widely distributed in permanent river systems from tropical to alpine environments. Accumulating knowledge and technological advancements have provided insights into many aspects of its evolutionary history and biology but have also raised concern about significant knowledge gaps surrounding distribution, population sizes, and trends. The platypus’ distribution coincides with many of Australia’s major threatening processes, including highly regulated and disrupted rivers, intensive habitat destruction, and fragmentation, and they were extensively hunted for their fur until the early 20th century. Emerging evidence of local population declines and extinctions identifies that ecological thresholds have been crossed in some populations and, if threats are not addressed, the species will continue to decline. In 2016, the IUCN Red Listing for the platypus was elevated to “Near Threatened,” but the platypus remains unlisted on threatened species schedules of any Australian state, apart from South Australia, or nationally. In this synthesis, we review the evolutionary history, genetics, biology, and ecology of this extraordinary mammal and highlight prevailing threats. We also outline future research directions and challenges that need to be met to help conserve the species.
Keywords: Australia, conservation management, freshwater biology, Monotremata, Ornithorhynchus anatinus
The platypus (Ornithorhynchus anatinus) is one of five extant species of egg-laying mammals in the subclass Monotremata and the only living species within the family Ornithorhynchidae (Fig. 1). As one of the world’s most evolutionarily distinct mammals, the platypus has long been regarded to be of exceptional scientific importance as well as a globally unique component of Australia’s biodiversity. The modern platypus is endemic to eastern mainland Australia, Tasmania, and adjacent King Island, with a small introduced population on Kangaroo Island, South Australia (Fig. 2). Platypuses are widely distributed in permanent river systems from tropical to alpine environments. However, relatively little is known about the species’ past and present distribution and numbers, limiting accurate evaluation of its conservation status and future population trajectories. Recent documented local declines and extinctions identify that the species is facing considerable threats in some areas (Lintermans 1998; Lunney et al. 1998; Serena et al. 1998; Rohweder and Baverstock 1999; Otley 2001; Milione and Harding 2009). However, estimates of population sizes are particularly difficult to obtain, given low recapture rates and the substantial effort required (Grant 2004a; Serena and Williams 2012a). In South Australia, the species is nearly extinct and is “Endangered” (National Parks and Wildlife Act 1972). The current drivers of declining platypus distribution and population are many, widespread, and synergistic, including predominantly regulation of river flows (Kingsford 2000; Grant and Fanning 2007) and extensive riparian and lotic habitat degradation by land clearing for agriculture and urbanization (Grant and Temple-Smith 2003). In light of documented local declines, the conservation status of the platypus was elevated by the IUCN to “Near Threatened” in 2016 (Woinarski and Burbidge 2016). Given the extent and severity of the threatening processes, coupled with lack of knowledge of past and present trends, there is an urgent need to re-assess the conservation status of the species and establish a national monitoring program. Apart from South Australia, platypuses are not currently listed on the threatened species schedules of any Australian state or nationally (i.e., Environment Protection and Biodiversity Conservation Act 1999). In this synthesis, which was initiated during a workshop attended by many of Australia’s platypus researchers, we review the current knowledge of the platypus’ life history and identify threats to its existence, priorities for conservation, and research challenges required to conserve this unique species. Prior to assessing these topics, we provide a summary of Aboriginal knowledge and use of the species.
Fig. 1.
A platypus, Ornithorhynchus anatinus returning back to the Upper Tarago River in Victoria, Australia after having been measured and tagged. Photo by Doug Gimesy.
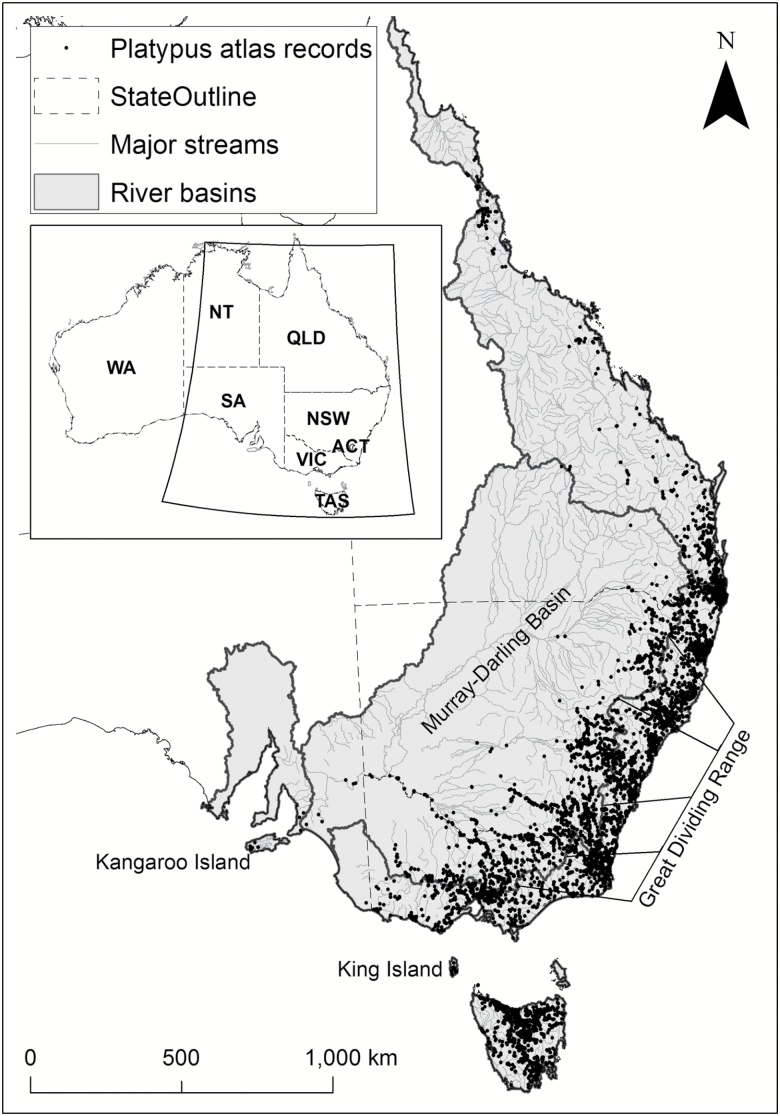
Fig. 2.
Distribution of the platypus (Ornithorhynchus anatinus) based on 11,830 records from Australian state government fauna atlases and the Atlas of Living Australia (www.ala.org.au) between 1760 and 2017.
Aboriginal Knowledge and Use
Platypuses have many Aboriginal names including Mallangong, Tambreet, Gaya-dari, Boonaburra, and Lare-re-lar (Pike 1997; McKay et al. 2001). They were hunted for food (Marshall 1992; Cosgrove and Allen 2001) by digging them from their burrows or spearing them while swimming (Robinson and Plomley 2008), providing a food resource rich in polyunsaturated fats (Naughton et al. 1986), particularly important in cold conditions (Marshall 1992; Cosgrove and Allen 2001). Aboriginal people had also developed a deep biocultural or ecological knowledge of platypuses, which was largely overlooked by early naturalists. A dreamtime story of the platypus from the upper reaches of the Darling River (McKay et al. 2001) begins with a young duck who disregarded her tribe’s warning of Mulloka (or Waaway), the water devil (Pike 1997). The duck, venturing down the creek far from her tribe, was abducted by Biggoon, a large water-rat who took the duck as his wife. The duck eventually escaped and returned to her tribe, where she laid two eggs which hatched as platypuses. They had soft fur instead of feathers, four webbed feet instead of two, and spurs on their hind legs, like Biggoon’s spear. The duck and her two different children were banished by her tribe, choosing to live far away in the mountains where she could hide from her tribe and Biggoon. A second dreaming from the Central Coast in New South Wales (McKay et al. 2001) begins with the Ancestor Spirits deciding on totems. The birds, marsupials, and fish each implore the platypus to join their particular family. After consulting with the echidna, the platypus graciously declines, explaining that it shares traits with all groups and wishes to remain friends with all of them, rather than belong to one single group. The platypus commemorates the Great Spirit for making all the animals different and respecting its wisdom.
Researching the Platypus
After Europeans first encountered platypuses in 1797, several specimens arrived in Britain and Europe, prompting taxonomic description (Shaw 1799) and anatomical studies (Griffiths 1978; Hobbins 2015), including the confirmation of functional mammary glands (Meckel 1823). Despite strong evidence for oviparity, including Aboriginal knowledge, the idea was strongly resisted by the conservative establishment as it supported the theory of transformism in nature along with all its social implications (Nicol 2018). Whereas Étienne Geoffroy Saint-Hilaire argued that platypuses were egg-laying but could not possibly lactate (Geoffroy Sàint-Hilaire 1829), Richard Owen the pre-eminent comparative anatomist for much of the 19th century and the leading authority on the anatomy of monotremes and marsupials, argued that they lactated but could not possibly lay eggs. Early ideas of evolution, or transformism, were attractive to radical thinkers, whereas social conservatives were anxious to show that the boundaries between types of animals, just like the boundaries between social classes, were erected by God and could not be crossed (Nicol 2018). Owen’s continued denial of oviparity was likely the most important of many impediments to conclusively settling the nature of monotreme reproduction and scientific acceptance of oviparity (Caldwell 1884; Nicol 2018).
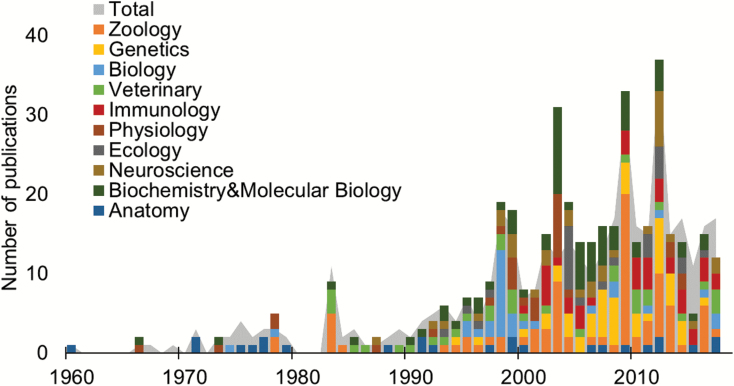
In the late 19th and early 20th century, platypuses were widely hunted for fur and sport. They were legally protected in all states by 1912 (Victoria—1892; New South Wales—1901; Queensland—1906; Tasmania—1907; South Australia—1912), stimulating considerable study of their natural history (Burrell 1927; Barrett 1944; Fleay 1944). Studies of reproduction, physiology, ecology, and behavior began in the 1970s (Temple-Smith 1973; Grant 1976; Griffiths 1978) and gained pace in the 1990s and early 2000s (Augee 1992; Grant 1995; Manger and Pettigrew 1998; Temple-Smith and Grant 2001), while health-related studies emerged in the 1980s (Munday and Peel 1983; Whittington and Grant 1983; Fig. 2). Dedicated symposia and special issues in peer-reviewed journals from 1978 to 2009 provided publication outlets for platypus-focused research, coinciding with peaks in peer-reviewed papers (Fig. 3). The surge in research in the 21st century was driven by more researchers and new technological developments, including smaller telemetry and data logging devices, use of passive integrated transponder tags (“microchipping”) for permanent marking (Grant and Whittington 1991), as well as DNA technologies and sequencing (Warren et al. 2008; Martin et al. 2018; Fig. 3). A study of familial relationships marked the beginning of research using the species’ DNA (Gemmell et al. 1992, 1995). Investigations of many aspects of biology using molecular genetic technologies quickly followed (Warren and Grützner 2009), expanding with progressive development and reduction in the cost of new technologies. In the late 1980s, studies of local, state, and national populations were sparked by interest in possible changes in the distribution or numbers of the platypus since the arrival of Europeans (Grant 1991, 1992; Grant and Denny 1991; Grant and Fanning 2007). Emergence of mucormycosis, an ulcerative skin condition in Tasmanian platypuses, also raised concern (Connolly 2009). By the mid-2000s, increasing concern about the status of local platypus populations (Woinarski et al. 2014) culminated in the IUCN raising its conservation status to “Near Threatened” in 2016 (Woinarski and Burbidge 2016).
Fig. 3.
The number of peer-reviewed publications (gray fill, n = 404) on the platypus (Ornithorhynchus anatinus) grouped by year (1960–2017) and stratified by the top ten research areas (color bars) in the Web of Science database with “Ornithorhynchus anatinus” in either title, abstract, keywords, or keywords plus (https://www.isiknowledge.com).
Despite this increasing research effort, key knowledge gaps remain, particularly with regards to the species’ past and present distribution and numbers, and the impacts of threatening processes on population viability. These gaps limit our ability to assess the current status and to develop conservation strategies for safeguarding the future of platypus populations. Platypuses are cryptic, and predominantly nocturnal and crepuscular, thereby impeding investigations of distribution, numbers, health, reproduction, recruitment, and movements. Trapping platypuses is time- and labor-intensive and is highly dependent on the depth and flow of water. In deep pools (> 2 m), unweighted mesh nets, often set an hour before dark, require continuous monitoring to ensure the welfare of platypuses and non-target species (e.g., fish). Fyke nets (checked every 2–4 h and allowing unimpeded access to air) are effective in small streams, although capture rates may vary substantially by age, sex, and season, and may also be affected by learned avoidance (Serena and Williams 2012b; Griffiths et al. 2013). Mark–recapture methods that take into account detection probabilities can produce robust estimates of population size (Bino et al. 2015). Environmental DNA (Ficetola et al. 2008; Lugg et al. 2018) is now used to detect the presence of platypuses and visual survey techniques provide useful information for assessing and monitoring population activity and relative abundance (Easton et al. 2008).
Platypus movements have been investigated using capture-recapture studies (Serena and Williams 2012a), radiotracking (Grant et al. 1992; McLeod 1993; Serena 1994; Gardner and Serena 1995; Gust and Handasyde 1995; Serena et al. 1998, 2001; Otley et al. 2000), microchip implantation (Macgregor et al. 2015), and externally attached (Griffiths et al. 2013) or implanted (Grant et al. 1992; Bino et al. 2018) acoustic tags. Unlike other freshwater mammals, the use of collars or harnesses for fine-scale telemetry (GPS, radio, or acoustic) is impractical, given the high risk of strangulation or drowning as platypuses forage between submerged roots and branches and dig their burrows between tree roots (Grant and Fanning 2007). Gluing radio and acoustic trackers to the body surface provides limited temporal data before they detach as fur regrows (Griffiths et al. 2014; Griffiths and Weeks 2015). By comparison, implanted telemetry devices (subcutaneous, intraperitoneal) can generate results for up to a year (G. Bino, pers. obs.). Subcutaneously implanted passive integrated transponders (Grant and Whittington 1991) extend tracking duration but have short detection distances (< 1 m), limiting their application to narrow streams (Macgregor et al. 2015).
Research into platypus diets is mostly based on sampling the contents of cheek pouches (Grant and Carrick 1978; Faragher et al. 1979; McLachlan-Troup et al. 2010; Marchant and Grant 2015) or analysis of captive nutrition (Thomas et al. 2018b), with more recent application of stable isotope analysis of platypus fur indicating that a combination of cheek pouch and stable isotope analyses is the most thorough approach (Klamt et al. 2016). Feeding behavior of captive platypuses indicates that preferences are shaped by prey mobility and increased energy consumption associated with preparing for and recovering from breeding (Thomas et al. 2018b). Body condition can be measured using tail volume and fur condition (Grant and Carrick 1978), but portable ultrasound devices offer new and potentially more accurate indices of body condition (Macgregor et al. 2017a). Assessing the reproductive status of females remains reliant on inducing milk let-down using injected synthetic oxytocin (Grant et al. 2004). Health assessments include external physical examination, collection of parasites, sampling excreta for reproductive hormones, corticosteroid analysis or pathogens, and sampling blood for hematology, serum biochemistry, and serology (e.g., Mucor enzyme-linked immunosorbent assay [ELISA], Leptospira serovar antibody titers—Connolly et al. 1998; Macgregor 2015; Macgregor et al. 2017b).
Fossil Record and Phylogeography
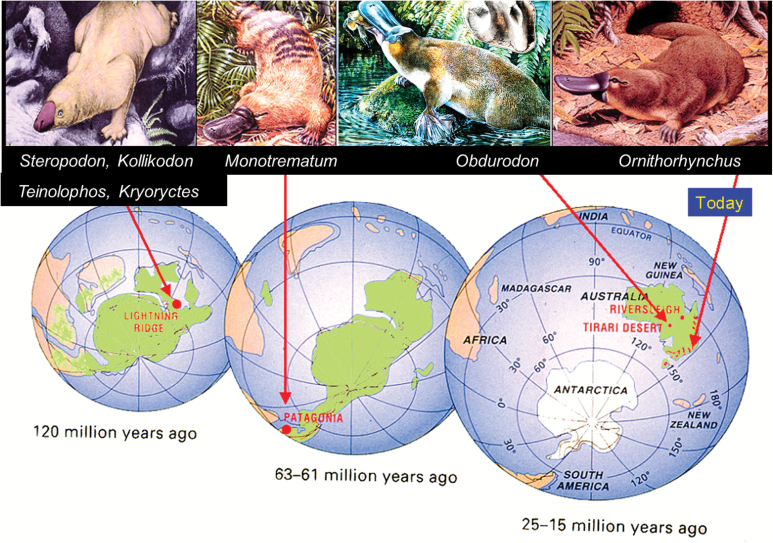
Prior to 1971, no extinct ornithorhynchids were known. A fragment of a Pliocene platypus, Ornithorhynchus agilis (De Vis 1885), may be the oldest known record (~3.8 million years ago [Mya]) of the living O. anatinus (Archer et al. 1978). The first breakthrough came with discovery of two well-formed teeth in Oligocene (~26 Mya) clay deposits in central South Australia (Fig. 4B) named Obdurodon insignis (Woodburne and Tedford 1975). Subsequent discovery of a dentary fragment and an ilium confirmed the ornithorhynchid nature of this taxon (Archer et al. 1978). In 1985, teeth and a nearly complete skull (Fig. 4A and 4B) of a species named Obdurodon dicksoni were discovered in Middle Miocene freshwater limestones (~15 Mya) in the Riversleigh World Heritage Area, Queensland (Lester and Archer 1986; Archer et al. 1991, 1992, 1993, 2000; Musser and Archer 1998; Macrini et al. 2006; Asahara et al. 2016). A second, much larger, Riversleigh species, Obdurodon tharalkooschild, was later discovered (Pian et al. 2013). The Riversleigh species appears to have lived in pools within cool, temperate, lowland rainforest (Archer et al. 1995, 2000).
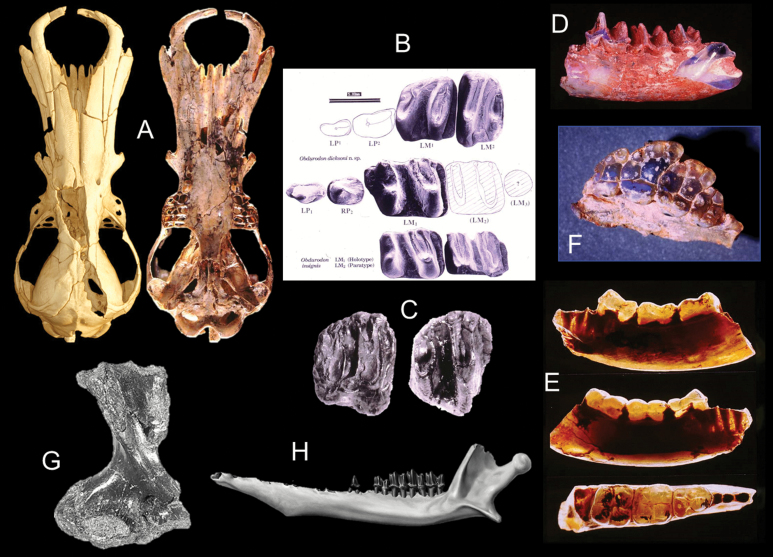
Fig. 4.
A) Dorsal and ventral views of the skull of Obdurodon dicksoni from Middle Miocene sediments in the Riversleigh World Heritage area (left image, dorsal view, micro-CT image courtesy T. Rowe, the University of Texas; right image, ventral view, photo Ross Arnett). B) Dentition of O. dicksoni (upper two rows) and Obdurodon insignis (bottom row—Archer et al. 1993). C) A right upper molar (RM2) of Monotrematum sudamericanum (left) compared with a slightly damaged RM2 (right) of O. dicksoni (Pascual et al. 1992b). D) Left dentary fragment with LM1-3, of Steropodon galmani (photo by John Field—Archer et al. 1985). E) Three views of a lower right dentary fragment with RM1-3 of Kollikodon ritchiei. F) Upper left maxillary fragment with LP4 to M4 of K. Ritchie (photo by John Field). G) Right humerus of Kryoryctes cadburyi (photo by Steven Morton—Pridmore et al. 2005). H) Left dentary of Teinolophos trusleri retaining one premolar (of four) and four (of five) molars (composition reconstruction by Peter Trusler—Rich et al. 2016).
With the discovery of Monotrematum sudamericanum (Pascual et al. 1992a, 1992b, 2002; Archer et al. 1995; Forasiepi and Martinelli 2013; Fig. 4C) from Paleocene deposits (63–61 Mya) in Argentina, it became clear that platypuses formerly existed on a continent that is now far beyond Australia (Fig. 5). Based on close overall similarity, this South American platypus could well be regarded as a species of Obdurodon (Musser 2013). The presence of fossils of the Queensland lungfish (Neoceratodus forsteri) and myobatrachid frogs in the same Patagonian deposit further demonstrated the strong faunal links that united Gondwana until at least the Eocene (about 50 Mya).
Fig. 5.
Long-term decline in geographic distribution and species’ diversity in monotremes and their early descendants. Cretaceous monotremes probably occurred throughout much of eastern Gondwana. By the early Paleocene, ornithorhynchids were geographically as widespread across Gondwana as Patagonia in southern South America. By the late Oligocene/Miocene (25–15 Mya), at least three ornithorhynchids occurred across the continent of Australia but none survived on other continents. Today, the platypus (Ornithorhynchus anatinus) maintains an even more restricted area, the river systems of eastern Australia (modified after Archer 1995; Steropodon image by Peter Schouten; Monotrematum image by James McKinnon—Archer 1995; Obdurodon image by Peter Schouten—Pian et al. 2013; Ornithorhynchus artwork by Rod Scott, Australian Geographic Magazine).
None of the older monotreme fossils now known from the Early Cretaceous (146–100 Mya) such as Steropodon galmani, Kollikodon ritchiei, Kryoryctes cadburyi, and Teinolophos trusleri (Archer et al. 1985; Flannery et al. 1995; Rich et al. 1999, 2001, 2016; Pridmore et al. 2005; Pian et al. 2016; Fig. 4D–H) have been demonstrated to be ornithorhynchids or tachyglossids. In particular, T. trusleri, which appears to be the oldest known monotreme, apparently did not have a bill nor other features characteristic of ornithorhynchids. There have been arguments based on fossils and molecular data that ornithorhynchids and tachyglossids may have diverged from one another prior to the Cenozoic (Pridmore et al. 2005; Rowe et al. 2008; Springer and Krajewski 2009). However, there are counter arguments that Ornithorhynchidae may well be paraphyletic, with tachyglossids having evolved from ancestral ornithorhynchids sometime during the Cenozoic (Pascual et al. 1992b; Phillips et al. 2009). Unfortunately, because of a lack of fossil tachyglossids more plesiomorphic than the extant long-beaked (Zaglossus spp.) and short-beaked (Tachyglossus aculeatus) echidnas, uncertainty remains about the origins of echidnas and their relationships to platypuses, other than that both groups are monotremes (Camens 2010; Phillips et al. 2010; Musser 2013).
Taken together (Fig. 5), the fossil record of ornithorhynchids provides a disquieting deep-time perspective on the conservation status of the living platypus that suggests that the species may be less environmentally resilient than commonly presumed. Overall, the record appears to be one of continuous geographic and taxonomic decline with representatives disappearing from Patagonia within the last 60 million years and from most of mainland Australia, apart from the relatively well-watered eastern coast, within approximately the last 15 million years (Fig. 5). Of the five ornithorhynchid species identified over the last 63 million years, only one survives today. At the same time, ornithorhynchids have evolved morphologically, transforming from cranially robust, toothed forms in the Miocene to cranially fused skulls lacking adult teeth in extant O. anatinus. Although juveniles have rudimentary, poorly formed, rootless molar teeth, these are shed about a month after the young leave the nesting burrow (Griffiths 1978). From a paleontological perspective, lineages that undergo declines over time of this magnitude in geographic distribution, species diversity, and functional morphology are more likely to suffer extinction than lineages that exhibit increasing geographic distribution, taxonomic diversity, and non-degenerating morphology (Archer et al. 1992).
Population Genetic Structure and Diversity
Molecular clock estimates suggest that the echidna and platypus families diverged from their common ancestor ~17–90 Mya based on different genes and traits, and fossil calibrations (Rowe et al. 2008; Phillips et al. 2009). The oldest estimate suggests this split may have occurred during the mid-Cretaceous (~80–100 Mya—Musser 2003). However, there is no evidence based on fossil biochronology that echidnas even existed as a distinct family prior to about 15 Mya. Mitochondrial DNA (mtDNA—Gongora et al. 2012) and whole-genome data (Martin et al. 2018) suggest that the emergence of modern platypus populations can be traced back to at least ~0.7–0.8 Mya. Given that O. agilis DeVis, which is a junior synonym of O. anatinus, is known to have existed in the middle Pliocene at ~3.8 Mya, the possibility cannot be excluded that other events of divergence may have occurred at some point that genetic studies of modern specimens are unable to shed light on.
The phylogeography and population structure of extant platypuses have been investigated using retrotransposon, mtDNA, and microsatellite loci (Akiyama 1998; Warren et al. 2008; Furlan et al. 2010; Gongora et al. 2012; Martin et al. 2018). These studies found that river basins act as discrete population units on the Australian mainland, with greater differences between than within river systems (Gemmell and Westerman 1994; Akiyama 1998; Kolomyjec et al. 2009; Gongora et al. 2012; Furlan et al. 2013). However, there is little genetic differentiation between platypuses on either side of the Great Dividing Range in Victoria (Furlan et al. 2013). Genetic studies also indicate limited gene flow between proximal rivers on the mainland, in contrast to Tasmania where there is less genetic structuring (Kolomyjec et al. 2009, 2013; Furlan et al. 2013).
At the regional level, two divergent evolutionary groups have been identified, one from mainland Australia and the other from Tasmania–King Island (Akiyama 1998; Warren et al. 2008; Furlan et al. 2010; Gongora et al. 2012; Martin et al. 2018). This split may have occurred on mainland Australia before platypuses colonized Tasmania. However, given that platypuses had to be in Tasmania for millions of years as part of the “Gondwanan link” (Lagabrielle et al. 2009), the location of that split is difficult to pinpoint. Furthermore, across the modern range of the platypus, a considerable level of differentiation has been found, with three to four phylogenetic clades based on mtDNA: New South Wales–Victoria, central Queensland, north Queensland, and Tasmania (Gongora et al. 2012). This conclusion is consistent with the number of significant units that have been defined based on microsatellites and whole-genome sequencing (Kolomyjec et al. 2013; Martin et al. 2018).
The recent whole-genome sequencing of 57 platypuses from populations sampled throughout the range of the species confirms a very strong genetic structure in the platypus (O. anatinus) over 0.8 Mya, but found no evidence of gene flow between river systems (Martin et al. 2018). In addition, these analyses suggest a historical genetic bottleneck in both north and central Queensland populations. In contrast, individuals from New South Wales appear to have had higher and relatively stable genetic diversity through their history. Furthermore, it was possible to establish the relatedness among 28 of the individuals from the same river system and estimate a de novo mutation rate of 4.1 × 10−9–1.2 × 10−8/bp/generation, considered intermediate for a mammal, lower than humans and chimpanzees but higher than laboratory mice (Martin et al. 2018).
Life History
Morphology.
Platypuses are sexually dimorphic, with males approximately 40% heavier and 15% longer than females (Burrell 1927; Temple-Smith 1973; Furlan et al. 2012; Bino et al. 2015), suggesting that males probably compete for territory, females, and other resources (Brown et al. 2013). There is a clinal increase in size from north Queensland (~700–1,100 g) to Tasmania (1,200–3,000 g—Connolly et al. 1998; Kolomyjec 2010; Gust and Griffiths 2011; Furlan et al. 2012; Bino et al. 2015). However, at finer geographical scales, size variation is confounded by inconsistencies, suggesting involvement of other environmental factors (Kolomyjec 2010; Furlan et al. 2012). The maximum recorded longevity in the wild is 21 and 25 years in captivity (J. Thomas, pers. obs.), although most animals survive approximately 6–15 years (Grant et al. 2004; Serena et al. 2014; Bino et al. 2015).
Temperature regulation.
The pelage consists of an undercoat of dense, short, and finely kinked hairs and an outer layer of spatulate-shaped guard hairs. The underfur retains air during dives, providing efficient insulation against heat loss, which is aided by a counter-current heat exchange in the cardiovascular system supplying the bare extremities (Grant and Dawson 1978). The body temperature of the platypus is maintained close to 32°C in air and water, with an ambient temperature tolerance of 0–30°C (Grant and Dawson 1978; Grant 1983; Grant et al. 1992). Despite sweat glands in the skin, platypuses are not able to withstand environmental temperatures exceeding 30°C (Robinson 1954); its crepuscular and nocturnal activities and burrow use during the day are likely strategies to avoid extreme heat (Grant and Dawson 1978; Bethge et al. 2004).
Senses.
Skin furrows on each side of the head house both the eye and the external ear opening. When submerged these furrows close, as do the nostrils, so that the senses of sight, hearing, and olfaction are absent or reduced (Burrell 1927). The auditory and visual areas of the cerebral cortex are relatively small compared to those receiving neural input from the bill (Bohringer and Rowe 1977). The eyes are small, ~6 mm diameter, with round pupils and flattened corneas characteristic of aquatic vertebrates, perhaps indicating aquatic ancestry. The retina is rod-dominated with some red and blue cones, rhodopsin is the dominant pigment, and there are double cones not found in marsupials or eutherians (Griffiths 1978; Zeiss et al. 2011). Observations of the platypus in the wild suggest acute eyesight, especially sensitive to movement (Burrell 1927). The ear is encased in cartilage rather than bone (Griffiths 1978), there is no external pinna, and no obvious mechanism to conduct water-borne sound to the inner ear (Pettigrew et al. 1998). The cochlea is less sensitive than in other mammals, being most sensitive to frequencies around 4 kHz but responding to frequencies up to 15 kHz (Gates et al. 1974; Krubitzer 1998; Pettigrew et al. 1998).
Platypuses have a pair of cervical scent glands that produce a musky odor and increase in size during the breeding season, suggesting an olfactory role in reproduction (Temple-Smith 1973). Olfaction is unlikely to be important in foraging, as the nares are closed when the platypus is submerged. However, considerable genetic representation of the vomeronasal system has been identified in the platypus genome (Grus et al. 2007; Keller and Vosshall 2008), possibility indicating this system may be used underwater to detect chemicals produced by prey or other platypuses. A vomeronasal (Jacobson’s) organ inside the front of the upper bill opens into the oral cavity (Griffiths 1978).
Specialized sensory structures housed in pores on the skin over the bill and frontal shield are supplied by the trigeminal nerve. Longitudinal rows of these electroreceptors, and uniformly distributed mechanoreceptors, provide electric and tactile senses, presumed to allow platypuses to navigate and locate weak electric fields produced by macroinvertebrate prey species (Scheich et al. 1986; Gregory et al. 1987; Iggo et al. 1992; Manger et al. 1996; Proske et al. 1998; Pettigrew 1999). Although the electric field strengths of common prey species are not within the range detectable by the electroreceptors (Taylor et al. 1992), the signal may be amplified by large numbers of receptors in the bill being stimulated at the same time. Discerning prey direction and location may be achieved by comparing signal strength during side-to-side movements of the bill, along with the input from mechanoreceptors (Pettigrew et al. 1998; Proske and Gregory 2003, 2004).
Diving and diving physiology.
Compared to other mammals, the platypus has a high hematocrit, erythrocyte count, and hemoglobin level, a low mean corpuscular volume, and a high mean corpuscular hemoglobin concentration, which suggests an adaptation to avoid hypoxia during diving (Whittington and Grant 1983; Evans et al. 1994). Platypuses show bradycardia on submersion, from a normal heart rate of 140–230 beats per minute (BPM) to 10–120 BPM. Blood oxygen levels fall rapidly during diving, with rapid restoration of arterial O2 saturation following dives (Johansen et al. 1966; Evans et al. 1994). Dives have an aerobic limit of 40–59 s (Bethge et al. 2001) and foraging dives in the wild last 30–140 s with around 10–15 s spent on the surface between dives. The frequency of foraging dives is around 75 dives/h (Bethge et al. 2003).
Foraging and energetics.
A pair of cheek pouches lateral to the maxillary and mandibular keratinous grinding pads, which replace the juvenile teeth, store prey items collected underwater for mastication on return to the surface (Griffiths 1978). The digestive tract is relatively short, and its structure is simple (Harrop and Hume 1980). Both the oesophagus and presumptive stomach are small, thin-walled, and lined with non-keratinizing stratified squamous epithelium. Gastric glands and the genes involved in gastric function are absent, and there is therefore no acid secreted and peptic digestion, but Brunner’s glands are present at the end of the stomach (Krause 1971). The short small intestine has no villi, but groups of intestinal glands drain into lumena between these numerous surface folds (Krause 1975), and the general structure of the pancreas is similar to other mammals (He et al. 2013). A small caecum (Hill and Rewell 1954) joins the short large intestine, which connects to the rectum, which is of greater diameter than the rest of the tract. Little is known about digestive physiology in platypuses although the diet suggests high proteolytic activity in the secretions of both the pancreas and the intestinal wall (Harrop and Hume 1980).
Unlike most aquatic mammals, the platypus swims using alternate strokes of its large webbed front limbs, swimming at 0.7–3.6 km/h (Grant and Fanning 2007). Collecting predominantly small organisms on each short dive, foraging normally lasts for 8–16 h per day (Serena 1994; Gust and Handasyde 1995; Otley et al. 2000; Bethge et al. 2003, 2009), although, particularly in winter, animals may forage continuously for more than 30 h per bout (Bethge 2002). When walking, the limbs are splayed away from the body, which is not continuously held above the ground surface, and the energy required for walking is 19–27% higher than for most terrestrial mammals of similar size (Bethge et al. 2001; Fish et al. 2001). The tail is relatively sparsely furred and acts mainly as a fat storage organ (Temple-Smith 1973), containing approximately 40% of the total body fat (Hulbert and Grant 1983), with seasonal changes of body fat occurring during periods of high metabolic demand, especially in winter and during breeding (Temple-Smith 1973; Grant and Carrick 1978; Connolly et al. 2016).
Venom.
Male platypuses are one of few extant venomous mammals (Ligabue-Braun et al. 2012). They have paired venom glands on the dorsocaudal surface of the pelvis, connected via ducts to hollow, keratinous extratarsal spurs on each hind leg; juvenile females have vestigial spur sheaths, lost within the first year of life (Temple-Smith 1973; Grant and Fanning 2007; Williams et al. 2013). The male venom gland may be a derived sweat gland, which enlarges during the breeding season along with increased venom production and male aggressiveness (Temple-Smith 1973). Although the spurs and venom may have had a defensive function in evolutionary history, as hypothesized for Mesozoic mammals (Ligabue-Braun et al. 2012), platypuses currently have few known native predators (Burrell 1927; Grant and Fanning 2007). The venom has a chemically complex composition (de Plater et al. 1995; de Plater 1998; Torres et al. 1999, 2000, 2002b; Torres and Kuchel 2004; Koh et al. 2009) produced by 88 toxin genes (Whittington et al. 2010; Wong et al. 2012). The venom disrupts hemostasis (Martin and Tidswell 1895; Kellaway and Le Messurier 1935), cell membranes (Kourie 1999; Torres et al. 2002a), and nociception (Kourie 1999; de Plater et al. 2001). Venom may have a primarily reproductive function, when males fight each other over access to breeding females, as indicated by cyclic venom production (Temple-Smith 1973; Whittington and Belov 2014) and fresh spur wounds and possible temporary partial paralysis in envenomated males during the breeding season (Fleay 1950; Temple-Smith 1973). Spur wounds heal, indicating that intraspecific envenomation hampers or temporarily disables competitors; death has been recorded only in captive conditions due to multiple spurring (Temple-Smith 1973; Grant and Fanning 2007). The venom causes excruciating local pain in humans that can effectively be reduced using a nerve blocker (Temple-Smith 1973; Fenner et al. 1992). Platypus venom may provide clinically useful substances and improve understanding and treatment of novel pain pathways (Fenner et al. 1992; Whittington and Belov 2014, 2016).
Habitat.
Platypuses are amphibious, inhabiting creeks, rivers, shallow lakes, wetlands, and their riparian margins, in agricultural land, urban areas, and natural environments (Connolly et al. 1998; Goldney 1998; Grant and Temple-Smith 1998a; Otley et al. 2000; Rakick et al. 2001; Munks et al. 2004; Bethge et al. 2009). Mid and lower river reaches in Australia’s eastern flowing rivers are generally more favored than upper reaches of rivers (Serena et al. 1998, 2001; Turnbull 1998; Rohweder and Baverstock 1999; Koch et al. 2006; Olsson Herrin 2009; Macgregor et al. 2015). Home ranges vary spatially and temporally with breeding season, age, and sex (Grant et al. 1992; McLeod 1993; Serena 1994; Gust and Handasyde 1995; Serena et al. 1998; Otley et al. 2000; Serena and Williams 2012a; Bino et al. 2018). Preferred habitat tends to include consolidated earth banks with large trees in the riparian zone, vegetation overhanging the stream channel, wide streams with in-stream organic matter, shallow pools, coarse woody debris, and coarse channel substrates, but platypuses still occur in habitats without some of these features, often in quite degraded agricultural settings (Rohweder 1992; Bryant 1993; Ellem et al. 1998; Serena et al. 2001; Milione and Harding 2009). In a Tasmanian study (Lunn 2015), catchment-scale factors (e.g., nearest large stream, catchment area) were found to be more useful indicators of platypus presence in high-order streams, whereas “finer-scale” local habitat variables (e.g., substrate, in-stream barriers) were particularly important indicators of stream use by platypuses in small headwater streams. This result highlights the importance of considering multiple spatial scales in any research into the effect of ecological disturbance on platypuses.
Movement.
Platypuses feed exclusively in the water and rest in burrows, typically in the banks of waterbodies (Grant et al. 1992; McLeod 1993; Serena 1994; Gardner and Serena 1995; Gust and Handasyde 1995; Serena et al. 1998; Otley et al. 2000; Serena et al. 2001); in Tasmania, platypuses are more terrestrial and have been found up to 250 m from water (Otley et al. 2000; Munks et al. 2004). Their activity patterns follow a circadian rhythm, cued by light (Otley 1996; Francis et al. 1999; Bethge et al. 2009). Platypuses are predominantly nocturnal (Grant et al. 1992; McLeod 1993; Gardner and Serena 1995; Francis et al. 1999), although some foraging occurs during daylight, particularly in winter or during lactation (Grant et al. 1992; Grigg et al. 1992; Serena 1994; Gardner and Serena 1995; Gust and Handasyde 1995; Serena et al. 1998; Bino et al. 2018). Individuals may also sometimes align activity patterns with the lunar cycle, synchronizing with moonrise and moonset (Bethge 2002; Bethge et al. 2009). Activity levels also vary throughout the year. In a lake in northern Tasmania, platypuses were most active between late winter and early spring, and least active in mid-summer. Peak activity coincided with the breeding season, mate searching, and increased foraging by lactating females in late spring–summer (Bethge 2002). Interactions between platypuses may also affect temporal partitioning of movements (Hawkins 1998; Bethge et al. 2009). In particular, it has been posited that subordinate individuals may tend to adopt a more variable or fragmented activity pattern or spend relatively more time active during daylight hours (Gust and Handasyde 1995; Hawkins 1998; Bethge et al. 2009).
Most adults appear to maintain reasonably stable home ranges over periods up to several years but these can vary in size between 0.5 and 15 km in linear habitats, with males typically occupying larger home ranges, particularly prior to breeding and as juveniles (Grant and Carrick 1978; Grant et al. 1992; Serena 1994; Gardner and Serena 1995; Gust and Handasyde 1995; Otley 1996; Serena et al. 1998; Bethge 2002; Serena and Williams 2012a; Bino et al. 2018). However, during lactation, females have sometimes been found to forage over larger areas than those used by males (Griffiths et al. 2014). In a subalpine Tasmanian lake, radiotagged individuals occupied areas of 2–58 ha over periods of 22–90 days (Otley et al. 2000). Juvenile males have been found to travel greater distances (Bino et al. 2018) and disperse over 40 km (Serena and Williams 2012a). Although animals typically use only a fraction (e.g., a mean 24–70%) of their home range in a given 24-h period (Serena 1994), adult males and females have, respectively, been documented to travel up to 10.4 km (including backtracking) and 4.0 km overnight (Serena et al. 1998). Platypuses occasionally move overland between water bodies (Taylor et al. 1991; Scott and Grant 1997; Munks and Nicol 2000; Otley et al. 2000) and between river catchments, based on genetic evidence (Kolomyjec et al. 2009; Furlan et al. 2013; Kolomyjec et al. 2013).
Feeding.
The platypus has a distinctive foraging behavior (Bethge 2002) and almost complete reliance on aquatic invertebrates as a food source (Faragher et al. 1979; Grant 1982; McLachlan-Troup et al. 2010). Platypuses forage in both slow-moving pools and faster-moving riffles within streams, and prefer depths of less than 5 m and coarse bottom substrates (Serena et al. 2001; Grant 2004b) that may improve foraging efficiency compared to fine sediment substrates or greater diving depths. Analysis of stomach contents provides little insight into diet and feeding habits of platypuses. Ejection of chitinous parts of macroinvertebrates and crustacean exoskeletons during mastication produces a fine particulate matter that lacks identifiable structures. Most of the useful information on diet has been obtained from analysis of cheek pouch contents. Both sexes feed opportunistically on a similarly wide range of benthic macroinvertebrates of varying sizes (McLachlan-Troup et al. 2010), consuming most invertebrates of a reasonable size, according to availability (Faragher et al. 1979; Grant 1982; McLachlan-Troup et al. 2010; Marchant and Grant 2015). Platypus diets are often dominated by relatively large aquatic macroinvertebrates from the orders Trichoptera, Ephemeroptera, and Odonata (Faragher et al. 1979; Grant 1982; McLachlan-Troup et al. 2010), though small chironomid species may also be important in the diet (McLachlan-Troup et al. 2010; Marchant and Grant 2015; Klamt et al. 2016). Diets including small prey may reflect reduced abundance of preferred larger prey items or increased abundance of smaller items (Marchant and Grant 2015). Platypuses also feed on freshwater crayfish (normally genus Cherax) in captivity and on members of this and other genera in the wild, although this is less common (Krueger et al. 1992; Bethge 2002). Daily food consumption is 13–28% of body weight (non-breeding individuals—Krueger et al. 1992; Munks et al. 2000; Bethge et al. 2001; Thomas et al. 2018b), but is much higher (90–100%) in lactating females (Holland and Jackson 2002), suggesting increased energy expenditure. The platypus can exert top-down influence in aquatic environments, but to a variable extent (McLachlan-Troup 2007). For example, platypuses were found to feed at a low trophic level during drought and on few items in urban environments, indicated by a narrower isotopic niche width as compared to agricultural and forested areas (Klamt 2016). Understanding how these relationships vary temporally and spatially in relation to different environmental conditions, invertebrate biomass, and productivity is still lacking (Marchant and Grant 2015).
Breeding.
Platypuses are seasonal breeders, breeding earlier in lower latitudes (Munks et al. 2000; Temple-Smith and Grant 2001; Grant et al. 2004). In New South Wales, breeding, with the onset of courtship followed by nesting behavior by females, begins around August and continues until young emerge from nesting burrows the following late January to early March. By comparison, in Tasmania, breeding starts 2 months later (Connolly and Obendorf 1998; Munks et al. 2000; Temple-Smith and Grant 2001). Though prey preferences suggest opportunism (see “Feeding” section), the timing of breeding may align with peak food availability during summer months, similar to terrestrial marsupials (Fisher et al. 2013; Lancaster and Downes 2018), but this remains speculative at present. During courtship, female and male platypuses engage in a dance, during which the male holds the tail of the female with his bill, and the female leads them both through a series of slow circles, twists, and turns on the surface of the water, followed by mating (Holland and Jackson 2002; Hawkins and Battaglia 2009; Thomas et al. 2018c). In captivity, reproductive behavior is controlled by the female (Thomas et al. 2018c). Afterwards, female platypuses construct a nesting burrow where they lay one to three eggs ~12–15 mm diameter and ~15–17 mm long (Burrell 1927), which they incubate ~10 days (Griffiths 1978) before the young hatch. Hatchlings are ~15 mm, unfurred, altricial (Manger et al. 1998), and are suckled for 120–140 days based on observations in captivity (Hawkins and Battaglia 2009; Thomas et al. 2018a). Platypus milk contains an antimicrobial protein unique to monotremes, monotreme lactation protein (MLP), which likely evolved to mitigate microbial infection in response to the lack of nipples (Enjapoori et al. 2014) and may perform the functions of other mammalian milk antimicrobials not present in platypus milk (Whittington and Belov 2009).
Nesting burrows are often complex structures with multiple openings, long tunnels (~5 m), a nesting chamber, and “pugs” (sections of burrow backfilled with soil—Burrell 1927). In a large (~0.06 m3), ovoid nesting chamber, the female creates a nest of wet vegetation, mostly grasses, leaves, and bark, dragged into the burrow with her tail (Holland and Jackson 2002; Thomas et al. 2018a). Two nest-like mounds had a dry recess, along a stream cave in Tasmania, which was made of fibrous roots and small amounts of leaf material and branchlets of moss (Munks et al. 2004). The burrow and its collected vegetation provide security and suitable microclimate conditions for incubation and hatching of the eggs and development of the young. Newly emerged juvenile platypuses are 65–70% of their adult mass and 83–87% of their adult length (Grant and Temple-Smith 1998b). In captivity, juveniles are weaned within days of emergence and feed on available aquatic invertebrates similar to adults (Thomas et al. 2018c). Dispersal of juveniles remains poorly known, except for two studied populations where 78% of females and 94% of males (Bino et al. 2015), and 75% of females and 88% of males (Serena et al. 2014) were not recaptured after their first year, suggesting high dispersal or mortality (Bino et al. 2015).
Threats
Historical accounts of past numbers of the platypus resemble those of many other previously common species that have subsequently declined (Gaston 2011). Globally, there is growing concern that extinction risk to common and widespread species is rapidly increasing, with little analysis or implementation of conservation assessment and actions. Conserving only the formally designated threatened species, while neglecting all other native fauna, hinders and even undermines biodiversity conservation, as is the case for the platypus (Lunney 2017a, 2017b). Platypus populations are at risk of declines and local extinctions because of the many and synergistic threats to their survival, compounded by our current lack of information, particularly of population dynamics and the impacts of anthropogenic activities (Lunney et al. 2008; Woinarski et al. 2014). Impacts of threatening processes and evidence of declines across the species’ entire range rely almost entirely on two long-term studies of densities, reproduction, age structure, and survival (Grant 2004a; Serena et al. 2014). Quantifying the effects of threatening processes and their impacts on population viability is very difficult for a species like the platypus, remaining a key knowledge gap essential for developing rigorous risk assessments that can guide effective conservation actions (Mace et al. 2008).
The drivers of declining distribution and population size for the platypus are many, widespread, and synergistic. Distribution of the platypus coincides with major threatening processes (Kingsford et al. 2009), including highly regulated and disrupted rivers (Kingsford 2000; Grant and Fanning 2007), extensive riparian and lotic habitat degradation by agriculture and urbanization (Grant and Temple-Smith 2003), and fragmentation by dams and other in-stream structures (Kolomyjec 2010; Furlan et al. 2013). By-catch mortality in fishing gear (Grant and Fanning 2007; Serena and Williams 2010a), diseases, and predation by invasive foxes and feral dogs (Serena 1994; Connolly and Obendorf 1998; Grant and Fanning 2007) also impact platypus populations.
Projected climate change will likely affect platypus distribution and numbers, even though platypuses occupy a broad environmental gradient. Projected decreased precipitation and increased evapotranspiration have significant implications for habitat availability by reducing thermally suitable habitat (Klamt et al. 2011). Current projections predict both drought frequencies and severity are likely to increase (CSIRO and Bureau of Meteorology 2015), further threatening small and isolated populations. Extended droughts can dry up creeks, likely reducing the extent of critical refugia, forcing platypuses to move overland where risk of predation is high, and exacerbating competition within decreasing numbers of pools.
Past fur trade and hunting.
Platypuses were hunted for the fur trade in the late 19th and early 20th century, driven by ongoing demand and high commercial prices for platypus skins, until nation-wide protection by 1912. A single rug or garment needed more than 50 platypus skins (75 platypus skins were used for a rug on display at the Australian Museum), making their skins more valuable than any other Australian animal (Goulburn Herald 1905). Skins were common in the Sydney market, with 754–2,356 sold annually between 1891 and 1899 (Sydney Wool and Produce Journal and Sydney Wool and Stock Journal). Some were also exported to London, although many more were undoubtedly smuggled, disguised as other small mammal skins (Burrell 1927). One furrier reported selling single-handedly over 29,000 skins before World War I (The Nowra Leader 1938). Sportsmen also shot hundreds of platypuses (The Don Dorrigo Gazette and Guy Fawkes Advocate 1919), some making a living from this activity (Grant and Denny 1991). One person shot many thousands over 32 years work (Grant and Fanning 2007). Given records of skin sales account for 10–100% of current population estimates (30,000–300,000—Woinarski and Burbidge 2016) and the slow reproductive rate of platypuses (1.5 young per year, with only half of females breeding in a given year—Bino et al. 2015), the impacts of the fur trade were probably never reversed, leaving many populations vulnerable to the many increasing threatening processes.
Habitat destruction.
Widespread land clearing and degradation of ecological function (disruption of water, nutrient, mineral, and carbon cycles) are major present-day drivers of declines and local extinctions of platypuses. Platypuses require stable banks of rivers and creeks to build burrows for resting and breeding purposes (Serena et al. 2001). Clearing, grazing, and watering access by livestock have severely degraded river banks and riparian vegetation (Lunney et al. 2004), increasing sedimentation, which in turn smothers stream beds and further degrades foraging habitat (Klamt 2016). Catchment-scale modification is also a significant threat, impacting food availability (Magierowski et al. 2012) and restricting overland movements. Degraded landscapes can overheat and dry out rapidly due to the loss of soil carbon, reducing habitat size and destroying drought refugia (Bauer and Goldney 1999; Kerle et al. 2014). In turn, runoff has increased dramatically, adding to soil loss and in-channel sedimentation (Walker et al. 1993; Minella et al. 2018).
Water resource development, including the building of dams, extraction of water, and development of floodplains, has caused widespread degradation of freshwater habitats within the platypus’ range (Kingsford 2000; Grant and Fanning 2007). River regulation alters the natural flow regime including both magnitude and frequency of flow events, degrading the ecological health of impacted river sections (Gilligan and Williams 2008). This process includes channel habitat destruction, accumulation of fine sediment (Coleman and Williams 2017), and encroachment of terrestrial vegetation into river channels. Altering the natural flow regime can impact resources required by platypuses, as well as reproduction (Serena and Grant 2017).
Hydrologic connectivity critically maintains the ecological integrity of river systems, mediating transfer of organic and non-organic matter, energy, as well as organisms (Pringle 2003). Platypuses make extensive movements that are almost certainly affected by the many weirs and large dams (Bino et al. 2016) that impede connectivity between populations. Dams are significant physical barriers (e.g., Dartmouth Dam on the Mitta Mitta River in the state of Victoria is 120 m in height) but also potentially ecological barriers, given the significant sizes of dam reservoirs (e.g., Dartmouth Dam reservoir, ~33 km long) and their limited food resources for platypuses, given their depth. Loss of connectivity between populations restricts source-sink dynamics (Hanski 1999) in cases of localized extinction as well as reducing genetic viability, adversely impacting conservation outcomes (Frankham et al. 2017).
Although platypuses still occur in urban and peri-urban environments, declines and localized extinctions in the most heavily urbanized areas indicate platypuses are sensitive to urbanization (Grant 1992; Grant 1998; Lintermans 1998; Serena et al. 2014). Throughout Melbourne, platypus distribution has been found to be limited by catchment imperviousness (Serena and Pettigrove 2005; Martin et al. 2014), indicating platypuses are impacted by the altered flow regimes of urban streams. Urban streams typically suffer from high flow variability, with increased magnitude and frequency of high flows, and reduced and extended baseflows (Walsh et al. 2005, 2012). High flow events may increase foraging energetics for platypuses (Gust and Handasyde 1995); summer flood events can reduce recruitment (Serena et al. 2014; Bino et al. 2015; Serena and Grant 2017), and decreased baseflow during drought lowers overall habitat availability for platypuses and their prey (Marchant and Grant 2015), also increasing predation risk and reducing longitudinal connectivity and fragmenting populations. Urbanization is also associated with increased water pollution, increased risks of predation, litter entanglement, road kill, and greater disturbance from human activities (Connolly et al. 1998; Serena and Williams 1998, 2010a).
Fishing by-catch.
From the late 1880s, a commercial fishery extended over much of the Murray-Darling Basin, especially in the middle and lower reaches of the Murray and Murrumbidgee Rivers (Grant 1993). Use of small mesh net sizes almost certainly impacted platypus numbers in these rivers, until larger mesh was introduced from the mid-1900s to reduce the capture of larger breeding fish. Following the introduction of trout species, the use of nets or traps in headwater streams was banned in New South Wales in 1902, providing some protection to platypuses in these regions (Grant 1993). In Victoria, where mortality was tracked and could be assigned, 56% of 186 platypus mortalities (1980–2009) were caused by drowning in illegal nets or enclosed traps (also referred to as opera house traps) set to capture fish or crustaceans (Serena and Williams 2010a). Such enclosed traps, which are left unattended in the water for extended periods, have relatively small openings (7.5–10 cm diameter) at the ends of internal funnels to prevent animals from escaping. Platypuses enter these traps either by accident or because they are attracted to trapped prey. Consequently, Victorian Fisheries Authority announced a state-wide ban on use of enclosed traps from 2019 (VFA 2018).
Invasive and feral species.
During overland movements, such as juvenile dispersal or searching for refugia during dry periods, platypuses are particularly vulnerable to predation by invasive terrestrial carnivores: red foxes (Vulpes vulpes), feral dogs (Canis familiaris), and feral house cats (Felis catus—Grant and Fanning 2007), as well as by native Tasmanian devils (Sarcophilus harrisii) in Tasmania. In the mid-1990s in Tasmania, 40% of platypus deaths were due to attacks by domestic dogs (Connolly et al. 1998), based on necropsies of 25 carcasses. In Victoria, predation by raptors, dogs, or foxes accounted for 13% (n = 24) of documented platypus deaths (Serena and Williams 2010a). Interactions with European carp (Cyprinus carpio) remain unknown, although there are likely indirect effects such as increased sedimentation and reduced benthic food availability (Serena and Williams 2010b).
Pollution.
Platypuses can get stuck in in-stream structures, such as pipes or hydroelectric turbines (Serena and Williams 2010a). Platypuses are incapable of using their highly specialized front feet to remove litter wedged around their body, which ultimately causes deep lesions. They frequently get entangled in discarded hooks and loops of fishing line (Serena and Williams 2010a). Plastic or rubber loops (e.g., canning jar rings, engine gaskets, cable-ties, tamper-proof seals from plastic-lidded food jars, child’s plastic bracelets, hair bands) have been recovered from the neck or torso of up to nearly 40% of animals captured in some suburban streams near Melbourne (Serena and Williams 1998, 2010a).
Disease.
Many infectious agents have been isolated from platypuses but relatively few cause serious disease (Whittington et al. 1992; Booth and Connolly 2008; Supplementary Data SD1). The exception is mucormycosis caused by Mucor amphibiorum, an environmental fungus first detected in platypuses in 1982, which causes a lethal infection in Tasmanian platypuses and is a considerable threat to mainland platypus populations if introduced (Munday and Peel 1983; Gust et al. 2009; Gust and Griffiths 2011). Mucormycosis causes a severe granulomatous and often ulcerative dermatitis, sometimes progressing to underlying tissues or disseminating to the lungs (Connolly 2009). Death may also result from secondary bacterial infections or impaired thermoregulation and mobility. Diagnosis is based on culturing the dimorphic fungus from platypus lesions, supported by morphological, molecular, or serological tests, such as an ELISA (Whittington et al. 2002; Connolly et al. 2010). Mucormycosis may also be detectable clinically or via signs or presence of spherules in cytology or histology of lesions, but this is less accurate than culture. Corynebacterium ulcerans or non-Mucor fungal skin disease can cause similar infections and cutaneous foreign body reactions (Connolly et al. 1998; Macgregor et al. 2017b). Mucormycosis was accidentally introduced to toads and frogs on the Australian mainland (Northern Territory, Queensland, New South Wales) by captive frogs from Melbourne and Perth, but the infection has not been recorded in mainland platypuses. Isolates of M. amphibiorum are susceptible to Amphotericin B and some disinfectants, with some hope of future vaccine development (Connolly et al. 1998; Connolly 2009; Webb et al. 2012).
An adenovirus-like virus causes a cytomegalic inclusion renal disease (Whittington et al. 1990) and a putative papilloma virus causes webbing papules (Booth and Connolly 2008). Between 47% and 66% of platypuses in New South Wales and 10% in Tasmania had leptospirosis based on serology, while in Victoria, 25% of platypus necropsied showed suggestive nephritis histologically (McColl and Whittington 1985; Loewenstein et al. 2008; Macgregor et al. 2017b). The Ixodes ornithorhynchi tick is common and may cause a mild dermatitis but importantly, it can be a vector of the hemoparasites Theileria ornithorhynchi and Trypanosoma binneyi (Booth and Connolly 2008), with the former sometimes causing hemolytic anemia in immunocompromised platypuses (Kessell et al. 2014).
Conservation and Research Priorities
Effective conservation of platypus populations hinges on controlling threatening processes, supported by investment in systematic long-term monitoring of trends in population sizes, demographics, distribution, genetics, and diseases. These priorities are particularly important in small streams, where populations are small and permanent drought refugia may not persist as they do in larger streams. Our understanding of how threatening processes impact individual health, population dynamics (e.g., survival, dispersal), and habitat quality remains largely qualitative in nature, demanding we develop quantitative models that allow predicting population viabilities, critical for prioritizing conservation management strategies. Whether dams and roads impede connectivity between platypus populations remains unevaluated, potentially affecting genetic diversity and severely degrading adaptive potential (Holderegger and Wagner 2006; Frankham et al. 2017). Synergistic impacts of habitat destruction and barriers, along with forecasted increasing frequency and intensity of droughts due to climate change that will reduce thermally suitable habitat (Klamt et al. 2011), will further jeopardize genetic and population viability in fragmented and isolated populations (Martin et al. 2018). Delineating the thermal tolerance of the species is needed to better predict the impacts of increasing temperatures (Kearney and Porter 2009). Use of novel genetic technologies (e.g., genomics, transcriptomics, proteomics, metabolomics, metagenomics, and epigenomics) can offer significant insights into many aspects of life history as well as the capacity of platypuses to adapt in response to changing climates and diseases (Amato et al. 2009).
Reducing the extent and intensity of identified threats is required to increase the likelihood of long-term survival of platypuses across eastern Australia. Restoration of riparian habitat through rehabilitation of river banks by replanting trees and restricting livestock access should become a priority. Improving water quality and restoration of natural flow regimes could improve functioning and food-web structures, while maintaining longitudinal connectivity and drought refugia. Environmental flows (i.e., dedicated flows) could also be used as a management action to ensure that these refugia do not dry out. Connectivity between populations must also be assessed and maintained by limiting and removing in-stream barriers (e.g., weirs, dams, roads) wherever possible and potentially developing “platypus-ways” across barriers, which require dedicated design planning and research. Considerable uncertainty remains regarding the dispersal behavior of juveniles both in terms of timing and distances, critical knowledge gaps for understanding metapopulation dynamics. Platypuses need to be protected from invasive predators when they move overland during dry periods or when dispersing. There should be a nation-wide ban of closed traps targeting crustaceans or fish in freshwater habitats, along with reduction in pollution to reduce mortality. Zoos play an important role in platypus conservation by conducting research, contributing to better public awareness of threatening processes, and establishing insurance populations to secure genetic diversity, particularly when considering potential impacts of climate change and the increased likelihood of severe droughts. Success of captive breeding remains sporadic with only four females breeding in zoos to date (J. Thomas, pers. obs.; J. O’Brien, Taronga Zoo, pers. comm., 2018), suggesting more directed efforts are needed to understand breeding requirements, including habitat and mate selection.
Given increasing support for research on charismatic species (Lunney 2012), the iconic platypus can be a focus for citizen science wildlife surveys that can improve knowledge of distribution as well as establish baselines for long-term monitoring (Grant and Llewellyn 1992; Lunney et al. 1998, 2004; Turnbull 1998; Grant et al. 2000; Otley 2001). Relying on sightings in the form of citizen science or community-based surveys requires scrutiny, such as concurrent systematic surveys (Lunney et al. 2004) to establish a reliable estimate of detection biases and how these can inform on population densities. We conclude that conserving the platypus, an Australian icon and an evolutionarily unique animal (Isaac et al. 2007), must become a priority at all levels of government and for the public through increased community awareness of threats. An appropriate level of listing for the platypus on State and Federal threatened species schedules (e.g., Environment Protection and Biodiversity Conservation Act 1999) is also needed based on improved understanding of distributional patterns and demographic processes, focused research, and management of the many threats raised in this review.
Supplementary Material
Acknowledgments
This review began with a conference and workshop at Taronga Zoo that brought together many of Australia’s platypus researchers to discuss the current status and challenges for this unique species. It was supported by Taronga Zoo and New South Wales Department of Primary Industries Conference Sponsorship Program 2017. This study was funded by an Australian Research Council Linkage grant LP150100093 and the Marcia Evelyn Williams Bequest, School of Veterinary Science, University of Sydney.
Supplementary Data
Supplementary Data SD1.—Infectious disease agents of the platypus.
Literature Cited
- Akiyama S. 1998. Molecular ecology of the platypus (Ornithorhynchus anatinus). Ph.D. thesis, La Trobe University, Melbourne, Victoria, Australia. [Google Scholar]
- Amato G., Ryder O., Rosenbaum H., and DeSalle R. (eds.). 2009. Conservation genetics in the age of genomics. Columbia University Press, New York. [Google Scholar]
- Archer M. 1995. Prehistoric platypus fits the bill. Australian geographic 38:86–103. [Google Scholar]
- Archer M., Flannery T. F., Ritchie A., and Molnar R.. 1985. First Mesozoic mammal from Australia—an early Cretaceous monotreme. Nature 318:363. [Google Scholar]
- Archer M., Hand S. J., and Godthelp H.. 1991. Back to the future: the contribution of palaeontology to the conservation of Australian forest faunas. Pp. 67–80 in Conservation of Australia’s forest fauna Royal Zoological Society of New South Wales, Sydney (Lunney D., ed.). Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Archer M., Hand S. J., and Godthelp H.. 2000. Australia’s lost world: prehistoric animals of Riversleigh. Indiana University Press, Bloomington. [Google Scholar]
- Archer M., Hand S. J., and Godthelp H.. 1995. Tertiary environmental and biotic change in Australia. Pp. 77–90 in Paleoclimate and evolution, with emphasis on human origins (Vrba E., Denton G. H., Partridge T. C., and Burckle L. H., eds.). Yale University Press, New Haven, Connecticut. [Google Scholar]
- Archer M., Jenkins F. A. Jr., Hand S. J., Murray P., and Godthelp H.. 1992. Description of the skull and non-vestigial dentition of a Miocene platypus (Obdurodon dicksoni n. sp.) from Riversleigh, Australia, and the problem of monotreme origins. Pp. 15–27 in Platypus and echidnas (Augee M. L., ed.). Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Archer M., Murray P., Hand S. J., and Godthelp H.. 1993. Reconsideration of monotreme relationships based on the skull and dentition of the Miocene Obdurodon dicksoni. Pp. 75–94 in Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials (Szalay F. S., Novacek M. J., and McKenna M. C., eds.). Springer, New York. [Google Scholar]
- Archer M., Plane M., and Pledge N.. 1978. Additional evidence for interpreting the Miocene Obdurodon insignis Woodburne and Tedford, 1975, to be a fossil platypus (Ornithorhynchidae: Monotremata) and a reconsideration of the status of Ornithorhynchus agilis De Vis, 1885. Australian Zoologist 20:9–19. [Google Scholar]
- Asahara M., Koizumi M., Macrini T. E., Hand S. J., and Archer M.. 2016. Comparative cranial morphology in living and extinct platypuses: feeding behavior, electroreception, and loss of teeth. Science Advances 2:e1601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augee M. L. (ed.). 1992. Platypus and echidnas. The Royal Zoological Society of New South Wales, Sydney: https://www.worldcat.org/title/platypus-and-echidnas/oclc/26247374. [Google Scholar]
- Barrett C. 1944. The platypus. Robertson & Mullens, Melbourne, Victoria, Australia. [Google Scholar]
- Bauer J., and Goldney D.. 1999. Extinction processes in a transitional agricultural landscape system. In Temperate eucalypt woodlands in Australia: biology, conservation, management and restoration (Hobbs R. and Yates C. J., eds.). Surrey Beatty, Chipping Norton, New South Wales, Australia. [Google Scholar]
- Bethge P. 2002. Energetics and foraging behaviour of the platypus. Ph.D. thesis, University of Tasmania, Hobart, Tasmania, Australia. [Google Scholar]
- Bethge P., Munks S., and Nicol S.. 2001. Energetics of foraging and locomotion in the platypus Ornithorhynchus anatinus. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 171:497–506. [DOI] [PubMed] [Google Scholar]
- Bethge P., Munks S., Otley H., and Nicol S.. 2003. Diving behaviour, dive cycles and aerobic dive limit in the platypus Ornithorhynchus anatinus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 136:799–809. [DOI] [PubMed] [Google Scholar]
- Bethge P., Munks S., Otley H., and Nicol S.. 2004. Platypus burrow temperatures at a subalpine Tasmanian lake. Pp. 273 in Proceedings of the Linnean Society of New South Wales 125:273–276. [Google Scholar]
- Bethge P., Munks S., Otley H., and Nicol S.. 2009. Activity patterns and sharing of time and space of platypuses, Ornithorhynchus anatinus, in a subalpine Tasmanian lake. Journal of Mammalogy 90:1350–1356. [Google Scholar]
- Bino G., Grant T. R., and Kingsford R. T.. 2015. Life history and dynamics of a platypus (Ornithorhynchus anatinus) population: four decades of mark-recapture surveys. Scientific Reports 5:16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bino G., Kingsford R., and Brandis K.. 2016. Australia’s wetlands–learning from the past to manage for the future. Pacific Conservation Biology 22:116–129. [Google Scholar]
- Bino G., Kingsford R. T., Grant T., Taylor M. D., and Vogelnest L.. 2018. Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Scientific Reports 8:5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohringer R. C., and Rowe M. J.. 1977. The organisation of the sensory and motor areas of the cerebral cortex in the platypus (Ornithorhynchus anatinus). Journal of Comparative Neurology 174:1–14. [DOI] [PubMed] [Google Scholar]
- Booth R., and Connolly J. H.. 2008. Platypuses. Pp. 103–132 in Medicine of Australian mammals (Vogelnest L. and Woods R., eds.). CSIRO Publishing, Collingwood, Victoria, Australia. [Google Scholar]
- Brown J. H., Kodric-Brown A., and Sibly R. M.. 2013. On body size and life history of mammals. Pp. 206–234 in Animal body size: linking pattern and process across space, time, and taxonomic group (Smith F. A. and Lyons S. K., eds.). Univ. of Chicago Press, Chicago, Illinois. [Google Scholar]
- Bryant A. G. 1993. An evaluation of the habitat characteristics of pools used by platypus (Ornithorhynchus anatinus) in the upper Macquarie River system, New South Wales. B.Appl.Sc. (Honours) thesis, Charles Sturt University, Bathurst, New South Wales, Australia. [Google Scholar]
- Burrell H. 1927. The platypus. Angus and Robertson, Sydney, New South Wales, Australia. [Google Scholar]
- Caldwell W. 1884. Telegram: monotremes oviparous, ovum meroblastic. Read in Montreal on September 2.
- Camens A. B. 2010. Were early Tertiary monotremes really all aquatic? Inferring paleobiology and phylogeny from a depauperate fossil record. Proceedings of the National Academy of Sciences of the USA 107:E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D., and Williams S.. 2017. Mobilising fine sediment in a highly regulated upland snowmelt river using hydrological scaled experimental floods. Marine and Freshwater Research 68:146–158. [Google Scholar]
- Connolly J. H. 2009. A review of mucormycosis in the platypus (Ornithorhynchus anatinus). Australian Journal of Zoology 57:235–244. [Google Scholar]
- Connolly J. H., Claridge T., Cordell S. M., Nielsen S., and Dutton G. J.. 2016. Distribution and characteristics of the platypus (Ornithorhynchus anatinus) in the Murrumbidgee catchment. Australian Mammalogy 38:58–67. [Google Scholar]
- Connolly J., and Obendorf D.. 1998. Distribution, captures and physical characteristics of the platypus (Ornithorhynchus anatinus) in Tasmania. Australian Mammalogy 20:231–237. [Google Scholar]
- Connolly J., Obendorf D., Whittington R., and Muir D.. 1998. Causes of morbidity and mortality in platypus (Ornithorhynchus anatinus) from Tasmania, with particular reference to Mucor amphibiorum infection. Australian Mammalogy 20:177–187. [Google Scholar]
- Connolly J., Stodart B., and Ash G.. 2010. Genotypic analysis of Mucor from the platypus in Australia. Journal of Wildlife Diseases 46:55–69. [DOI] [PubMed] [Google Scholar]
- Cosgrove R., and Allen J.. 2001. Prey choice and hunting strategies in the Late Pleistocene: evidence from Southwest Tasmania. Pp. 397–430 in Histories of old ages: essays in honour of Rhys Jones (Anderson A., Lilley I., and O’Connor S., eds.). Research School of Pacific and Asian Studies, Australian National University, Canberra, Australian Capital Territory, Australia. [Google Scholar]
- CSIRO and Bureau of Meteorology 2015. Climate change in Australia information for Australia’s natural resource management regions: technical report. CSIRO and Bureau of Meteorology, Australia. https://www.climatechangeinaustralia.gov.au/media/ccia/2.1.6/cms_page_media/168/CCIA_2015_NRM_TechnicalReport_WEB.pdf. [Google Scholar]
- de Plater G. 1998. Fractionation, primary structural characterisation and biological activities of polypeptides from the venom of the platypus (Ornithorhynchus anatinus). Australian National University, Canberra, Australia. [Google Scholar]
- de Plater G., Martin R. L., and Milburn P. J.. 1995. A pharmacological and biochemical investigation of the venom from the platypus (Ornithorhynchus anatinus). Toxicon 33:157–169. [DOI] [PubMed] [Google Scholar]
- de Plater G. M., Milburn P. J., and Martin R. L.. 2001. Venom from the platypus, Ornithorhynchus anatinus, induces a calcium-dependent current in cultured dorsal root ganglion cells. Journal of Neurophysiology 85:1340–1345. [DOI] [PubMed] [Google Scholar]
- Easton L., Williams G., and Serena M.. 2008. Monthly variation in observed activity of the platypus ‘Ornithorhynchus Anatinus’. Victorian Naturalist 125:104. [Google Scholar]
- Ellem B., Bryant A., and O’Connor A.. 1998. Statistical modelling of platypus (Ornithorhynchus anatinus) habitat preferences using generalised linear models. Australian Mammalogy 20:281–285. [Google Scholar]
- Enjapoori A. K., Grant T. R., Nicol S. C., Lefevre C. M., Nicholas K. R., and Sharp J. A.. 2014. Monotreme lactation protein is highly expressed in monotreme milk and provides antimicrobial protection. Genome Biology and Evolution 6:2754–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B., Jones D., Baldwin J., and Gabbott G.. 1994. Diving ability of the platypus. Australian Journal of Zoology 42:17–27. [Google Scholar]
- Faragher R., Grant T., and Carrick F.. 1979. Food of the platypus (Ornithorhynchus anatinus) with notes on the food of brown trout (Salmo trutta) in the Shoalhaven River, NSW. Australian Journal of Ecology 4:171–179. [Google Scholar]
- Fenner P. J., Williamson J. A., and Myers D.. 1992. Platypus envenomation—a painful learning experience. Medical Journal of Australia 157:829–832. [DOI] [PubMed] [Google Scholar]
- Ficetola G. F., Miaud C., Pompanon F., and Taberlet P.. 2008. Species detection using environmental DNA from water samples. Biology Letters 4:423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish F. E., Frappell P. B., Baudinette R. V., and MacFarlane P.. 2001. Energetics of terrestrial locomotion of the platypus Ornithorhynchus anatinus. Journal of Experimental Biology 204:797–803. [DOI] [PubMed] [Google Scholar]
- Fisher D. O., Dickman C. R., Jones M. E., and Blomberg S. P.. 2013. Sperm competition drives the evolution of suicidal reproduction in mammals. Proceedings of the National Academy of Sciences of the USA 110:17910–17914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery T. F., Archer M., Rich T. H., and Jones R.. 1995. A new family of monotremes from the Cretaceous of Australia. Nature 377:418–420. [Google Scholar]
- Fleay D. H. 1944. We breed the platypus. Robertson & Mullens, Melbourne, Victoria, Australia. [Google Scholar]
- Fleay D. 1950. Further notes on the Badger Creek platypuses. Victorian Naturalist 67:81–87. [Google Scholar]
- Forasiepi A. M., and Martinelli A. G.. 2013. Femur of a monotreme (Mammalia, Monotremata) from the Early Paleocene Salamanca Formation of Patagonia, Argentina. Ameghiniana 40:625–630. [Google Scholar]
- Francis A. J., de Alwis C., Peach L., and Redman J. R.. 1999. Circadian activity rhythms in the Australian platypus, Ornithorhynchus anatinus (Monotremata). Biological Rhythm Research 30:91–103. [Google Scholar]
- Frankham R., et al. 2017. Genetic management of fragmented animal and plant populations. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Furlan E., et al. 2012. Is body size variation in the platypus (Ornithorhynchus anatinus) associated with environmental variables? Australian Journal of Zoology 59:201–215. [Google Scholar]
- Furlan E., Umina P. A., Mitrovski P. J., Gust N., Griffiths J., and Weeks A. R.. 2010. High levels of genetic divergence between Tasmanian and Victorian platypuses, Ornithorhynchus anatinus, as revealed by microsatellite loci. Conservation Genetics 11:319–323. [Google Scholar]
- Furlan E. M., et al. 2013. Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conservation Genetics 14:837–853. [Google Scholar]
- Gardner J., and Serena M.. 1995. Spatial-organization and movement patterns of adult male platypus, Ornithorhynchus anatinus (Monotremata, Ornithorhynchidae). Australian Journal of Zoology 43:91–103. [Google Scholar]
- Gaston K. J. 2011. Common ecology. Bioscience 61:354–362. [Google Scholar]
- Gates G. R., Saunders J. C., and Boek G. R.. 1974. Peripheral auditory function in the platypus, Ornithorhynchus anatinus. Journal of the Acoustical Society of America 56:152–156. [DOI] [PubMed] [Google Scholar]
- Gemmell N. J., Grant T. R., Western P. S., Watson J. M., Murray N. D., and Graves J. A. M.. 1992. Preliminary molecular studies of platypus family and population structure. Pp. 232–254 in Platypus and echidnas (Augee M. L., ed.). The Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Gemmell N. J., et al. 1995. Determining platypus relationships. Australian Journal of Zoology 43:283–291. [Google Scholar]
- Gemmell N. J., and Westerman M.. 1994. Phylogenetic relationships within the class Mammalia: a study using mitochondrial 12S RNA sequences. Journal of Mammalian Evolution 2:3–23. [Google Scholar]
- Geoffroy Sàint-Hilaire É. 1829. Considérations sur des oeufs d’Ornithorinque, formant de nouveaux documens pour la question de la classification des Monotrêmes. Annales des Sciences Naturelles 18:157–164. [Google Scholar]
- Gilligan D., and Williams S.. 2008. Changes in fish assemblages after the first flow releases to the Snowy River downstream of Jindabyne Dam. In: Snowy River flow response monitoring. Department of Water and Energy, Sydney, New South Wales, Australia. [Google Scholar]
- Goldney D. 1998. The distribution and abundance of platypuses in the Thredbo River–Lake Jindabyne system. Australian Mammalogy 20:302–303. [Google Scholar]
- Gongora J., et al. 2012. Genetic structure and phylogeography of platypuses revealed by mitochondrial DNA. Journal of Zoology 286:110–119. [Google Scholar]
- Goulburn Herald 1905. Destruction of Australian fauna. Pp. 2 in Goulburn Herald. W.R. Riley & Co., Goulburn, New South Wales, Australia. [Google Scholar]
- Grant T. R. 1976. Thermoregulation of the platypus, Ornithorhynchus anatinus. Ph.D. thesis, University of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Grant T. 1982. Food of the platypus, Ornithorhynchus anatinus (Ornithorhynchidae: Monotremata) from various water bodies in New South Wales. Australian Mammalogy 5:135–136. [Google Scholar]
- Grant T. R. 1983. Body temperatures of free-ranging platypuses, Ornithorhynchus anatinus (Monotremata), with observations on their use of burrows. Australian Journal of Zoology 31:117–122. [Google Scholar]
- Grant T. R. 1991. The biology and management of the platypus (Ornithorhynchus anatinus) in NSW. Species Management Report No. 5. NSW National Parks and Wildlife Service, Hurstville, New South Wales, Australia. [Google Scholar]
- Grant T. R. 1992. Historical and current distribution of the platypus, Ornithorhynchus anatinus, in Australia. Pp. 232–254 in Platypus and echidnas (Augee M, L., ed.). Royal Zoological Society of NSW, Sydney, New South Wales, Australia. [Google Scholar]
- Grant T. 1993. The past and present freshwater fishery in New South Wales and the distribution and status of the platypus Omithorhynchus anatinus. Australian Zoologist 29:105–113. [Google Scholar]
- Grant T. R. 1995. The platypus: a unique mammal. 2nd ed. University of New South Wales Press Ltd, Sydney, New South Wales, Australia. [Google Scholar]
- Grant T. R. 1998. Current and historical occurrence of platypuses, Ornithorhynchus anatinus, around Sydney. Australian Mammalogy 20:257–266. [Google Scholar]
- Grant T. R. 2004a. Captures, capture mortality, age and sex ratios of platypuses, Ornithorhynchus anatinus, during studies over 30 years in the Upper Shoalhaven River in New South Wales. Proceedings of the Linnean Society of New South Wales 125:217–226. [Google Scholar]
- Grant T. R. 2004b. Depth and substrate selection by platypuses, Ornithorhynchus anatinus, in the lower Hastings River, New South Wales. Proceedings of the Linnean Society of New South Wales 125:235. [Google Scholar]
- Grant T. R., and Carrick F.. 1978. Some aspects of the ecology of the platypus, Ornithorhynchus anatinus, in the upper Shoalhaven River. Australian Zoologist 20:181–199. [Google Scholar]
- Grant T. R., and Dawson T. J.. 1978. Temperature regulation in the platypus, Ornithorhynchus anatinus, production and loss of metabolic heat in air and water. Physiological Zoology 51:315–332. [Google Scholar]
- Grant T. R., and Denny M. J. S.. 1991. Historical and current distribution of the platypus in Australia, with guidelines for the management and conservation of the species. Australian National Parks and Wildlife Service by Mt. King Ecological Surveys, Oberon, New South Wales, Australia.. [Google Scholar]
- Grant T. R., and Fanning D.. 2007. Platypus. 4th ed. CSIRO Publishing, Collingwood, Victoria, Australia. [Google Scholar]
- Grant T., Gehrke P., Harris J., and Hartley S.. 2000. Distribution of the platypus (Ornithorhynchus anatinus) in NSW: results of the 1994–96 NSW Rivers Survey. Australian Mammalogy 21:177–184. [Google Scholar]
- Grant T. R., Griffiths M., and Temple-Smith P. D.. 2004. Breeding in a free-ranging population of platypuses, Ornithorhynchus anatinus, in the Upper Shoalhaven River, New South Wales—a 27 year study. Proceedings of the Linnean Society of New South Wales 125:227. [Google Scholar]
- Grant T. R., Grigg G. C., Beard L. A., and Augee M. L.. 1992. Movements and burrow use by platypuses, Ornithorhynchus anatinus, in the Thredbo River, New South Wales. Pp. 263–267 in Platypus and echidnas (Augee M. L., ed.). Royal Zoological Society of NSW, Sydney, New South Wales, Australia. [Google Scholar]
- Grant T. R., and Llewellyn L. C.. 1992. Draft plan of management for the platypus, Ornithorhynchus anatinus, in New South Wales. NSW National Parks and Wildlife Service, Sydney, New South Wales, Australia. [Google Scholar]
- Grant T., and Temple-Smith P.. 1998a. Field biology of the platypus (Ornithorhynchus anatinus): historical and current perspectives. Philosophical Transactions of the Royal Society of London B: Biological Sciences 353:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant T., and Temple-Smith P.. 1998b. Growth of nestling and juvenile platypuses (Ornithorhynchus anatinus). Australian Mammalogy 20:221–230. [Google Scholar]
- Grant T. R., and Temple-Smith P.. 2003. Conservation of the platypus, Ornithorhynchus anatinus: threats and challenges. Aquatic Ecosystem Health & Management 6:5–18. [Google Scholar]
- Grant T. R., and Whittington R. J.. 1991. The use of freeze-branding and implanted transponder tags as a permanent marking method for platypuses, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae). Australian Mammalogy 14:147–150. [Google Scholar]
- Gregory J., Iggo A., McIntyre A., and Proske U.. 1987. Electroreceptors in the platypus. Nature 326:386. [DOI] [PubMed] [Google Scholar]
- Griffiths M. 1978. The biology of monotremes. Academic Press Inc., New York. [Google Scholar]
- Griffiths J., Kelly T., and Weeks A.. 2013. Net-avoidance behaviour in platypuses. Australian Mammalogy 35:245–247. [Google Scholar]
- Griffiths J., Kelly T., and Weeks A.. 2014. Impacts of high flows on platypus movements and habitat use in an urban stream. Report to Melbourne Water. Cesar, Parkville, Victoria, Australia. [Google Scholar]
- Griffiths J., and Weeks A.. 2015. Impact of environmental flows on platypuses in a regulated river. Cesar, Parkville, Victoria, Australia. [Google Scholar]
- Grigg G., Beard L., Grant T., and Augee M.. 1992. Body-temperature and diurnal activity patterns in the platypus (Ornithorhynchus anatinus) during winter. Australian Journal of Zoology 40:135–142. [Google Scholar]
- Grus W. E., Shi P., and Zhang J.. 2007. Largest vertebrate vomeronasal type 1 receptor gene repertoire in the semiaquatic platypus. Molecular Biology and Evolution 24:2153–2157. [DOI] [PubMed] [Google Scholar]
- Gust N., and Griffiths J.. 2011. Platypus (Ornithorhynchus anatinus) body size, condition and population structure in Tasmanian river catchments: variability and potential mucormycosis impacts. Wildlife Research 38:271–289. [Google Scholar]
- Gust N., Griffiths J., Driessen M., Philips A., Stewart N., and Geraghty D.. 2009. Distribution, prevalence and persistence of mucormycosis in Tasmanian platypuses (Ornithorhynchus anatinus). Australian Journal of Zoology 57:245–254. [Google Scholar]
- Gust N., and Handasyde K.. 1995. Seasonal-variation in the ranging behavior of the platypus (Ornithorhynchus-anatinus) on the Goulburn River, Victoria. Australian Journal of Zoology 43:193–208. [Google Scholar]
- Hanski I. 1999. Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 87:209–219. [Google Scholar]
- Harrop C. J. F., and Hume I. D.. 1980. Digestive tract and digestive function in monotremes and nonmacropod marsupials. Pp. 63–77 in Comparative physiology: primitive mammals (Schmidt-Nielsen K., Bolis L., and Taylor C. R., eds.). Cambridge University Press, New York. [Google Scholar]
- Hawkins M. 1998. Time and space sharing between platypuses (Ornithorhynchus anatinus) in captivity. Australian Mammalogy 20:195–205. [Google Scholar]
- Hawkins M., and Battaglia A.. 2009. Breeding behaviour of the platypus (Ornithorhynchus anatinus) in captivity. Australian Journal of Zoology 57:283–293. [Google Scholar]
- He C., Tsend-Ayush E., Myers M. A., Forbes B. E., and Grützner F.. 2013. Changes in the ghrelin hormone pathway maybe part of an unusual gastric system in monotremes. General and Comparative Endocrinology 191:74–82. [DOI] [PubMed] [Google Scholar]
- Hill W. O., and Rewell R. E.. 1954. The caecum of monotremes and marsupials. The Transactions of the Zoological Society of London 28:185–240. [Google Scholar]
- Hobbins P. 2015. A spur to atavism: placing platypus poison. Journal of the History of Biology 48:499–537. [DOI] [PubMed] [Google Scholar]
- Holderegger R., and Wagner H. H.. 2006. A brief guide to landscape genetics. Landscape Ecology 21:793–796. [Google Scholar]
- Holland N., and Jackson S. M.. 2002. Reproductive behaviour and food consumption associated with the captive breeding of platypus (Ornithorhynchus anatinus). Journal of Zoology 256:279–288. [Google Scholar]
- Hulbert A., and Grant T.. 1983. A seasonal study of body condition and water turnover in a free-living population of platypuses, Ornithorhynchus anatinus (Monotremata). Australian Journal of Zoology 31:109–116. [Google Scholar]
- Iggo A., Gregory J., and Proske U.. 1992. The central projection of electrosensory information in the platypus. The Journal of Physiology 447:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac N. J., Turvey S. T., Collen B., Waterman C., and Baillie J. E.. 2007. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 2:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K., Lenfant C., and Grigg G.. 1966. Respiratory properties of blood and responses to diving of the platypus, Ornithorhynchus anatinus (Shaw). Comparative Biochemistry and Physiology 18:597–608. [DOI] [PubMed] [Google Scholar]
- Kearney M., and Porter W.. 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecology Letters 12:334–350. [DOI] [PubMed] [Google Scholar]
- Kellaway C. H., and Le Messurier D. H.. 1935. The venom of the platypus (Ornithorhynchus anatinus). Australian Journal of Experimental Biology and Medical Science 13:205–221. [Google Scholar]
- Keller A., and Vosshall L. B.. 2008. Better smelling through genetics: mammalian odor perception. Current Opinion in Neurobiology 18:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerle A., Goldney D., and Fleming M.. 2014. Habitat loss and landscape degradation: the disastrous outlook for vertebrate fauna in central western NSW. Australian Zoologist 37:40–74. [Google Scholar]
- Kessell A., et al. 2014. Haemolytic anaemia associated with Theileria sp. in an orphaned platypus. Australian Veterinary Journal 92:443–449. [DOI] [PubMed] [Google Scholar]
- Kingsford R. T. 2000. Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology 25:109–127. [Google Scholar]
- Kingsford R., et al. 2009. Major conservation policy issues for biodiversity in Oceania. Conservation Biology 23:834–840. [DOI] [PubMed] [Google Scholar]
- Klamt M. 2016. Platypus in a changing world: impacts of land use and climate. Ph.D. thesis, Monash University, Melbourne, Victoria, Australia. [Google Scholar]
- Klamt M., Davis J., Thompson R., Marchant R., and Grant T.. 2016. Trophic relationships of the platypus: insights from stable isotope and cheek pouch dietary analyses. Marine and Freshwater Research 67:1196–1204. [Google Scholar]
- Klamt M., Thompson R., and Davis J.. 2011. Early response of the platypus to climate warming. Global Change Biology 17:3011–3018. [Google Scholar]
- Koch N., Munks S., Utesch M., Davies P., and McIntosh P.. 2006. The platypus Ornithorhynchus anatinus in headwater streams, and effects of pre-Code forest clearfelling, in the South Esk River catchment, Tasmania, Australia. Australian Zoologist 33:458–473. [Google Scholar]
- Koh J. M. S., Bansal P. S., Torres A. M., and Kuchel P. W.. 2009. Platypus venom: source of novel compounds. Australian Journal of Zoology 57:203–210. [Google Scholar]
- Kolomyjec S. H., et al. 2009. Population genetics of the platypus (Ornithorhynchus anatinus): a fine-scale look at adjacent river systems. Australian Journal of Zoology 57:225–234. [Google Scholar]
- Kolomyjec S. 2010. The history and relationships of northern platypus (Ornithorhynchus anatinus) populations: a molecular approach. Ph.D. thesis, James Cook University, Townsville, Australia. [Google Scholar]
- Kolomyjec S., Grant T. R., Johnson C. N., and Blair D.. 2013. Regional population structuring and conservation units in the platypus (Ornithorhynchus anatinus). Australian Journal of Zoology 61:378–385. [Google Scholar]
- Kourie J. I. 1999. A component of platypus (Ornithorhynchus anatinus) venom forms slow-kinetic cation channels. Journal of Membrane Biology 172:37–45. [DOI] [PubMed] [Google Scholar]
- Krause W. J. 1971. Brunner’s glands of the duckbilled platypus (Ornithorhynchus anatinus). American Journal of Anatomy 132:147–165. [DOI] [PubMed] [Google Scholar]
- Krause W. J. 1975. Intestinal mucosa of the platypus, Ornithorhynchus anatinus. The Anatomical Record 181:251–265. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. 1998. What can monotremes tell us about brain evolution? Philosophical Transactions of the Royal Society B 353:1127–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B., Hunter S., and Serena M.. 1992. Husbandry, diet and behaviour of platypus Ornithorhynchus anatinus at Healesville Sanctuary. International Zoo Yearbook 31:64–71. [Google Scholar]
- Lagabrielle Y., Goddéris Y., Donnadieu Y., Malavieille J., and Suarez M.. 2009. The tectonic history of Drake Passage and its possible impacts on global climate. Earth and Planetary Science Letters 279:197–211. [Google Scholar]
- Lancaster J., and Downes B.. 2018. Aquatic versus terrestrial insects: real or presumed differences in population dynamics? Insects 9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester K. S., and Archer M.. 1986. A description of the molar enamel of a middle Miocene monotreme (Obdurodon, Ornithorhynchidae). Anatomy and Embryology 174:145–151. [DOI] [PubMed] [Google Scholar]
- Ligabue-Braun R., Verli H., and Carlini C. R.. 2012. Venomous mammals: a review. Toxicon 59:680–695. [DOI] [PubMed] [Google Scholar]
- Lintermans M. 1998. The status and distribution of the platypus (Ornithorhynchus anatinus) in the Australian Capital Territory with notes on some localised declines. Australian Mammalogy 20:306. [Google Scholar]
- Loewenstein L., McLachlan‐Troup T., Hartley M., and English A.. 2008. Serological survey for evidence of Leptospira interrogans in free‐living platypuses (Ornithorhynchus anatinus). Australian Veterinary Journal 86:242–245. [DOI] [PubMed] [Google Scholar]
- Lugg W. H., Griffiths J., van Rooyen A. R., Weeks A. R., and Tingley R.. 2018. Optimal survey designs for environmental DNA sampling. Methods in Ecology and Evolution 9:1049–1059. [Google Scholar]
- Lunn T. 2015. Causal processes of a complex system: modelling stream use and disturbance influence on the platypus (Ornithorhynchus anatinus). Honours thesis, University of Tasmania, Hobart, Tasmania, Australia. [Google Scholar]
- Lunney D. 2012. Charismatic megafauna. Pp. 63–66 in The encyclopedia of sustainability: Vol. 5. Ecosystem management and sustainability (Craig R. K., Pardy B., Nagle J. C., Schmitz O., and Smith W., eds.). Berkshire Publishing, Great Barrington, Massachusetts. [Google Scholar]
- Lunney D. 2017a. A dangerous idea in action: the hegemony of endangered species legislation and how it hinders biodiversity conservation. Australian Zoologist 38:289–307. [Google Scholar]
- Lunney D. 2017b. Dangerous? Necessary: we must conserve all our native fauna. Australian Zoologist 38:281–288. [Google Scholar]
- Lunney D., et al. 2008. Ornithorhynchus anatinus. IUCN Red List of Threatened Species. [Google Scholar]
- Lunney D., Grant T., and Matthews A.. 2004. Distribution of the platypus in the Bellinger catchment from community knowledge and field survey and its relationship to river disturbance. Proceedings of the Linnean Society of New South Wales 125:243–258. [Google Scholar]
- Lunney D., Grant T. R., Matthews A., Esson C., Moon C., and Ellis M.. 1998. Determining the distribution of the platypus (Ornithorhynchus anatinus) in the Eden region of south-eastern New South Wales through community-based surveys. Australian Mammalogy 20:239–250. [Google Scholar]
- Mace G. M., et al. 2008. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology 22:1424–1442. [DOI] [PubMed] [Google Scholar]
- Macgregor J. W. 2015. Conservation of the platypus (Ornithorhynchus anatinus): development of a framework to assess the health of wild platypus populations. Ph.D. thesis, Murdoch University, Murdoch, Western Australia. [Google Scholar]
- Macgregor J., et al. 2015. Corrigendum to: novel use of in-stream microchip readers to monitor wild platypuses. Pacific Conservation Biology 21:101. [Google Scholar]
- Macgregor J., et al. 2017a. Assessing body condition in the platypus (Ornithorhynchus anatinus): a comparison of new and old methods. Australian Journal of Zoology 64:421–429. [Google Scholar]
- Macgregor J. W., et al. 2017b. Investigation into individual health and exposure to infectious agents of platypuses (Ornithorhynchus anatinus) in two river catchments in northwest Tasmania. Journal of Wildlife Diseases 53:258–271. [DOI] [PubMed] [Google Scholar]
- Macrini T. E., Rowe T., and Archer M.. 2006. Description of a cranial endocast from a fossil platypus, Obdurodon dicksoni (Monotremata, Ornithorhynchidae), and the relevance of endocranial characters to monotreme monophyly. Journal of Morphology 267:1000–1015. [DOI] [PubMed] [Google Scholar]
- Magierowski R. H., Davies P. E., Read S. M., and Horrigan N.. 2012. Impacts of land use on the structure of river macroinvertebrate communities across Tasmania, Australia: spatial scales and thresholds. Marine and Freshwater Research 63:762–776. [Google Scholar]
- Manger P. R., Calford M. B., and Pettigrew J. D.. 1996. Properties of electrosensory neurons in the cortex of the platypus (Ornithorhynchus anatinus). Implications for processing electrosensory stimuli. Proceedings of the Royal Society London B: Biological Sciences 263:611–617. [Google Scholar]
- Manger P. R., Hall L. S., and Pettigrew J. D.. 1998. The development of the external features of the platypus (Ornithorhynchus anatinus). Philosophical Transactions of the Royal Society B: Biological Sciences 353:1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger P. R., and Pettigrew J. D.. 1998. Platypus biology: recent advances and reviews. Philosophical Transactions: Biological Sciences B 353:1057–1237. [Google Scholar]
- Marchant R., and Grant T. R.. 2015. The productivity of the macroinvertebrate prey of the platypus in the upper Shoalhaven River, New South Wales. Marine and Freshwater Research 66:1128–1137. [Google Scholar]
- Marshall B. 1992. Late Pleistocene human exploitation of the platypus in southern Tasmania. Pp. 268–276 in Platypus and echidnas (Augee M. L., ed.). Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Martin C. J., and Tidswell F.. 1895. Observations on the femoral gland of Ornithorhynchus and its secretion; together with an experimental enquiry concerning its supposed toxic action. Proceedings of the Linnean Society of New South Wales 9:471–500. [Google Scholar]
- Martin E. H., Walsh C. J., Serena M., and Webb J. A.. 2014. Urban stormwater runoff limits distribution of platypus. Austral Ecology 39:337–345. [Google Scholar]
- Martin H. C., et al. 2018. Insights into platypus population structure and history from whole-genome sequencing. Molecular Biology and Evolution 35:1238–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl K. A., and Whittington R. J.. 1985. Leptospiral titres in wild platypuses (Ornithorhynchus anatinus) in New South Wales. Australian Veterinary Journal 62:66–67. [DOI] [PubMed] [Google Scholar]
- McKay H. F., McLeod P. E., Jones F. F., and Barber J. E.. 2001. Gadi Mirrabooka: Australian Aboriginal tales from the dreaming. Libraries Unlimited, Santa Barbara, California. [Google Scholar]
- McLachlan-Troup T. 2007. The ecology and functional importance of the platypus (Ornithorhynchus anatinus) in Australian freshwater habitats. Ph.D. thesis, University of Sydney, Sydney, New South Wales, Australia. [Google Scholar]
- McLachlan-Troup T. A., Dickman C. R., and Grant T. R.. 2010. Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. Journal of Zoology 280:237–246. [Google Scholar]
- McLeod A. L. 1993. Movement, home range and burrow usage, diel activity and juvenile dispersal of platypuses, Ornithorhynchus anatinus, on the Duckmaloi Weir, NSW. Bachelor of Applied Science (Honors) thesis, Charles Sturt University, Bathurst, New South Wales, Australia. [Google Scholar]
- Meckel V. J. 1823. Ueber den stachel und das giftorgan des Ornithorhynchus. Deutsches Archives fur die Physiologie 8:592–595. [Google Scholar]
- Milione M., and Harding E.. 2009. Habitat use by platypus (Ornithorhynchus anatinus) in a modified Australian Wet Tropics catchment, north-eastern Queensland. Australian Mammalogy 31:35–46. [Google Scholar]
- Minella J. P., et al. 2018. Long‐term sediment yield from a small catchment in southern Brazil affected by land use and soil management changes. Hydrological Processes 32:200–211. [Google Scholar]
- Munday B., and Peel B.. 1983. Severe ulcerative dermatitis in platypus (Ornithorhynchus anatinus). Journal of Wildlife Diseases 19:363–365. [DOI] [PubMed] [Google Scholar]
- Munks S., Eberhard R., and Duhig N.. 2004. Nests of the platypus Ornithorhynchus anatinus in a Tasmanian cave. The Tasmanian Naturalist 126:55–58. [Google Scholar]
- Munks S., and Nicol S.. 2000. Current research on the platypus, Ornithorhynchus anatinus in Tasmania: abstracts from the 1999 ‘Tasmanian Platypus Workshop’. Australian Mammalogy 21:259–266. [Google Scholar]
- Munks S., Otley H., Bethge P., and Jackson J.. 2000. Reproduction, diet and daily energy expenditure of the platypus in a sub-alpine Tasmanian lake. Australian Mammalogy 21:260–261. [Google Scholar]
- Musser A. M. 2003. Review of the monotreme fossil record and comparison of palaeontological and molecular data. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 136:927–942. [DOI] [PubMed] [Google Scholar]
- Musser A. 2013. Classification and evolution of the monotremes. Pp. 1–16 in Neurobiology of monotremes: brain evolution in our distant mammalian cousins (Ashwell K., ed.). CSIRO Publishing, Collingwood, Victoria, Australia. [Google Scholar]
- Musser A. M., and Archer M.. 1998. New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philosophical Transactions of the Royal Society B: Biological Sciences 353:1063–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton J. M., O’Dea K., and Sinclair A. J.. 1986. Animal foods in traditional Australian Aboriginal diets: polyunsaturated and low in fat. Lipids 21:684–690. [DOI] [PubMed] [Google Scholar]
- Nicol S. 2018. Étienne Geoffroy Saint-Hilaire, Richard Owen and monotreme oviparity. Australian Zoologist, in press. doi:10.7882/AZ.2018.033. [Google Scholar]
- Olsson Herrin R. 2009. Distribution and individual characteristics of the platypus (Ornithorhynchus anatinus) in the Plenty River, southeast Tasmania. M.Sc. thesis, Lund University, Lund, Sweden. [Google Scholar]
- Otley H. 1996. Aspects of platypus foraging ecology in a subalpine Tasmanian lake system. Honours thesis, University of Tasmania, Hobart, Tasmania, Australia. [Google Scholar]
- Otley H. M. 2001. The use of a community-based survey to determine the distribution of the platypus Ornithorhynchus anatinus in the Huon River catchment, southern Tasmania. Australian Zoologist 31:632–641. [Google Scholar]
- Otley H. M., Munks S. A., and Hindell M. A.. 2000. Activity patterns, movements and burrows of platypuses (Ornithorhynchus anatinus) in a sub-alpine Tasmanian lake. Australian Journal of Zoology 48:701–713. [Google Scholar]
- Pascual R., Archer M., Jaureguizar E. O., Prado J. L., Godthelp H., and Hand S. J.. 1992a. First discovery of monotremes in South America. Nature 356:704. [Google Scholar]
- Pascual R., Archer M., Ortiz-Jaureguizar E., Prado J., Godthelp H., and Hand S. J.. 1992b. The first non-Australian monotreme: an early Paleocene South American platypus (Monotremata, Ornitorhynchidae). Pp. 1–14 in Platypus and echidnas (Augee M., ed.). Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Pascual R., Goin F. J., Balarino L., and Udrizar Sauthier D.. 2002. New data on the Paleocene monotreme Monotrematum sudamericanum, and the convergent evolution of triangulate molars. Acta Palaeontologica Polonica 47:487–492. [Google Scholar]
- Pettigrew J. D. 1999. Electroreception in monotremes. Journal of Experimental Biology 202:1447–1154. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Manger P. R., and Fine S.. 1998. The sensory world of the platypus. Philosophical Transactions of the Royal Society of London B: Biological Sciences 353:1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Bennett T. H., and Lee M. S.. 2009. Molecules, morphology, and ecology indicate a recent, amphibious ancestry for echidnas. Proceedings of the National Academy of Sciences of the USA 106:17089–17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Bennett T., and Lee M. S.. 2010. Reply to Camens: how recently did modern monotremes diversify? Proceedings of the National Academy of Sciences of the USA 107:E13–E13. [Google Scholar]
- Pian R., Archer M., and Hand S. J.. 2013. A new, giant platypus, Obdurodon tharalkooschild, sp. nov. (Monotremata, Ornithorhynchidae), from the Riversleigh World Heritage Area, Australia. Journal of Vertebrate Paleontology 33:1255–1259. [Google Scholar]
- Pian R., Archer M., Hand S. J., Beck R. M. D., and Cody A.. 2016. The upper dentition and relationships of the enigmatic Australian Cretaceous mammal Kollikodon ritchiei. Memoirs of Museum Victoria 74:97–105. [Google Scholar]
- Pike E. 1997. Parable of the platypus dreaming. Nelen Yubu 66:33–37. [Google Scholar]
- Pridmore P. A., Rich T. H., Vickers-Rich P., and Gambaryan P. P.. 2005. A tachyglossid-like humerus from the Early Cretaceous of south-eastern Australia. Journal of Mammalian Evolution 12:359–378. [Google Scholar]
- Pringle C. M. 2003. What is hydrologic connectivity and why is it ecologically important? Hydrological Processes 17:2685–2689. [Google Scholar]
- Proske U., and Gregory E.. 2003. Electrolocation in the platypus—some speculations. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 136:821–825. [DOI] [PubMed] [Google Scholar]
- Proske U., and Gregory J.. 2004. The role of push rods in platypus and echidna - some speculations. Proceedings of the Linnean Society of New South Wales 125:319–326.
- Proske U., Gregory J. E., and Iggo A.. 1998. Sensory receptors in monotremes. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 353:1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakick R., Rakick B., Cook L., and Munks S.. 2001. Observations of a platypus foraging in the sea and hunting by a wedge-tailed eagle. Tasmanian Naturalist 123:3–4. [Google Scholar]
- Rich T., Vickers-Rich P., Constantine A., Flannery T., Kool L., and Van Klaveren N.. 1999. Early Cretaceous mammals from Flat Rocks, Victoria, Australia. Records of the Queen Victoria Museum 106:1–35. [Google Scholar]
- Rich T. H., et al. 2016. The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa: An Australasian Journal of Palaeontology 40:475–501. [Google Scholar]
- Rich T. H., et al. 2001. Monotreme nature of the Australian Early Cretaceous mammal Teinolophos. Acta Palaeontologica Polonica 46:113–118. [Google Scholar]
- Robinson K. W. 1954. Heat tolerances of Australian monotremes and marsupials. Australian Journal of Biological Sciences 7:348–360. [DOI] [PubMed] [Google Scholar]
- Robinson G. A., and Plomley N. J. B.. 2008. Friendly mission: the Tasmanian journals and papers of George Augustus Robinson, 1829–1834. 2nd ed. Queen Victoria Museum and Art Gallery, Hobart, Quintus, Launceston, Tasmania. [Google Scholar]
- Rohweder D. 1992. Management of platypus in the Richmond River catchment, northern New South Wales. B.AS. (Hons) thesis, University of New England, Northern Rivers, Lismore, New South Wales, Australia. [Google Scholar]
- Rohweder D. A., and Baverstock P. R.. 1999. Distribution of platypus Ornithorhynchus anatinus in the Richmond River catchment, northern New South Wales. Australian Zoologist 31:30–37. [Google Scholar]
- Rowe T., Rich T. H., Vickers-Rich P., Springer M., and Woodburne M. O.. 2008. The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proceedings of the National Academy of Sciences of the USA 105:1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich H., Langner G., Tidemann C., Coles R. B., and Guppy A.. 1986. Electroreception and electrolocation in platypus. Nature 319:401. [DOI] [PubMed] [Google Scholar]
- Scott A. C., and Grant T.. 1997. Impacts of water management in the Murray-Darling Basin on the platypus (Ornithorhynchus anatinus) and the water rat (Hydromys chrysogaster). CSIRO Land and Water, Murray-Darling Basin Commission, National River Health Program, Canberra, Australia. [Google Scholar]
- Serena M. 1994. Use of time and space by platypus (Ornithorhynchus anatinus: Monotremata) along a Victorian stream. Journal of Zoology 232:117–131. [Google Scholar]
- Serena M., and Grant T.. 2017. Effect of flow on platypus (Ornithorhynchus anatinus) reproduction and related population processes in the upper Shoalhaven River. Australian Journal of Zoology 65:130–139. [Google Scholar]
- Serena M., and Pettigrove V.. 2005. Relationship of sediment toxicants and water quality to the distribution of platypus populations in urban streams. Journal of the North American Benthological Society 24:679–689. [Google Scholar]
- Serena M., Thomas J. L., Williams G. A., and Officer R. C. E.. 1998. Use of stream and river habitats by the platypus, Ornithorhynchus anatinus, in an urban fringe environment. Australian Journal of Zoology 46:267–282. [Google Scholar]
- Serena M., and Williams G.. 1998. Rubber and plastic rubbish: a summary of the hazard posed to platypus Ornithorhynchus anatinus in suburban habitats. Victorian Naturalist 115:47–49. [Google Scholar]
- Serena M., and Williams G.. 2010a. Factors contributing to platypus mortality in Victoria. The Victorian Naturalist 127:178. [Google Scholar]
- Serena M., and Williams G.. 2010b. Platypus population assessment and recommended management actions along Broken Creek. Report to Goulburn-Broken Catchment Management Authority. Australian Platypus Conservancy, Wiseleigh, Victoria, Australia. [Google Scholar]
- Serena M., and Williams G.. 2012a. Movements and cumulative range size of the platypus (Ornithorhynchus anatinus) inferred from mark–recapture studies. Australian Journal of Zoology 60:352–359. [Google Scholar]
- Serena M., and Williams G. A.. 2012b. Effect of sex and age on temporal variation in the frequency and direction of platypus (Ornithorhynchus anatinus) captures in fyke nets. Australian Mammalogy 34:75–82. [Google Scholar]
- Serena M., Williams G., Weeks A., and Griffiths J.. 2014. Variation in platypus (Ornithorhynchus anatinus) life history attributes and population trajectories in urban streams. Australian Journal of Zoology 62:223–234. [Google Scholar]
- Serena M., Worley M., Swinnerton M., and Williams G.. 2001. Effect of food availability and habitat on the distribution of platypus (Ornithorhynchus anatinus) foraging activity. Australian Journal of Zoology 49:263–277. [Google Scholar]
- Shaw G. 1799. The duck-billed platypus. The Naturalists’ Miscellany. Nodder & Co., London, United Kingdom. [Google Scholar]
- Springer M. S., and Krajewski C.. 2009. Monotremes (Prototheria). Pp. 462–465 in The timetree of life (Hedges S. B. and Kumar S., eds.). Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Taylor N., Manger P., Pettigrew J., and Hall L.. 1992. Electromyogenic potentials of a variety of platypus prey items: an amplitude and frequency analysis. Pp. 216–224 in Platypus and echidnas (Augee M. L., ed.). Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia. [Google Scholar]
- Taylor R., Mooney N., and Lange K.. 1991. Observations on platypus. The Tasmanian Naturalist 105:1–3. [Google Scholar]
- Temple-Smith P. D. 1973. Seasonal breeding biology of the platypus, Ornithorhynchus anatinus (Shaw, 1799), with special reference to the male. Ph.D. thesis, Australian National University, Canberra, Australia. [Google Scholar]
- Temple-Smith P., and Grant T.. 2001. Uncertain breeding: a short history of reproduction in monotremes. Reproduction, Fertility and Development 13:487–497. [DOI] [PubMed] [Google Scholar]
- The Don Dorrigo Gazette and Guy Fawkes Advocate 1919. The platypus. Pp. 6 in The Don Dorrigo Gazette and Guy Fawkes Advocate, Roy and Reginald Vincent, Dorrigo, New South Wales, Australia. [Google Scholar]
- The Nowra Leader 1938. Bank the Butcher’s shop. Pp. 2 in The Nowra Leader, Connolly, H.J., Nowra, New South Wales, Australia. [Google Scholar]
- Thomas J., Handasyde K., Parrott M. L., and Temple-Smith P.. 2018a. The platypus nest: burrow structure and nesting behaviour in captivity. Australian Journal of Zoology 65:347–356. [Google Scholar]
- Thomas J. L., Handasyde K. A., Temple-Smith P., and Parrott M. L.. 2018b. Seasonal changes in food selection and nutrition of captive platypuses (Ornithorhynchus anatinus). Australian Journal of Zoology 65:319–327. [Google Scholar]
- Thomas J. L., Parrott M. L., Handasyde K. A., and Temple-Smith P.. 2018c. Female control of reproductive behaviour in the platypus (Ornithorhynchus anatinus), with notes on female competition for mating. Behaviour 155:27–53. [Google Scholar]
- Torres A. M., Alewood D., Alewood P. F., Gallagher C. H., and Kuchel P. W.. 2002a. Conformations of platypus venom C-type natriuretic peptide in aqueous solution and sodium dodecyl sulfate micelles. Toxicon 40:711–719. [DOI] [PubMed] [Google Scholar]
- Torres A. M., et al. 2000. Defensin-like peptide-2 from platypus venom: member of a class of peptides with a distinct structural fold. Biochemical Journal 348:649–656. [PMC free article] [PubMed] [Google Scholar]
- Torres A. M., and Kuchel P. W.. 2004. The beta-defensin-fold family of polypeptides. Toxicon 44:581–588. [DOI] [PubMed] [Google Scholar]
- Torres A. M., et al. 2002b. D-amino acid residue in the C-type natriuretic peptide from the venom of the mammal, Ornithorhynchus anatinus, the Australian platypus. FEBS Letters 524:172–176. [DOI] [PubMed] [Google Scholar]
- Torres A. M., et al. 1999. Solution structure of a defensin-like peptide from platypus venom. Biochemical Journal 341:785–794. [PMC free article] [PubMed] [Google Scholar]
- Turnbull R. 1998. Distribution of the platypus (Ornithorhynchus anatinus) in the Bombala River catchment, south-eastern New South Wales. Australian Mammalogy 20:251–256. [Google Scholar]
- VFA 2018. Changes ahead for yabby fishing gear.https://vfa.vic.gov.au/recreational-fishing/changes-ahead-for-yabby-fishing-gear. Accessed July 2018.
- Walker J., Bullen F., and Williams B.. 1993. Ecohydrological changes in the Murray-Darling Basin. I. The number of trees cleared over two centuries. Journal of Applied Ecology 30:265–273. [Google Scholar]
- Walsh C. J., Fletcher T. D., and Burns M. J.. 2012. Urban stormwater runoff: a new class of environmental flow problem. PLoS ONE 7:e45814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. J., Roy A. H., Feminella J. W., Cottingham P. D., Groffman P. M., and Morgan R. P.. 2005. The urban stream syndrome: current knowledge and search for a cure. Journal of the North American Benthological Society 24:706–723. [Google Scholar]
- Warren W. C., and Grützner F.. 2009. The enigma of the platypus genome. Australian Journal of Zoology 57:157–165. [Google Scholar]
- Warren W. C., et al. 2008. Genome analysis of the platypus reveals unique signatures of evolution. Nature 453:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R., Philips A., Speare R., Connolly J., and Berger L.. 2012. Controlling wildlife fungal disease spread: in vitro efficacy of disinfectants against Batrachochytrium dendrobatidis and Mucor amphibiorum. Diseases of Aquatic Organisms 99:119–125. [DOI] [PubMed] [Google Scholar]
- Whittington C. M., and Belov K.. 2009. Platypus venom genes expressed in non-venom tissues. Australian Journal of Zoology 57:199–202. [Google Scholar]
- Whittington C., and Belov K.. 2014. Tracing monotreme venom evolution in the genomics era. Toxins 6:1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington C. M., and Belov K.. 2016. The platypus: a venomous mammal. Pp. 169–183 in Venom genomics and proteomics: toxinology (Gopalakrishnakone P. and Calvete J. J., eds.). Springer, New York. [Google Scholar]
- Whittington R., and Grant T.. 1983. Haematology and blood chemistry of the free-living platypus, Ornithorhynchus anatinus (Shaw) (Monotremata: Ornithorhynchidae). Australian Journal of Zoology 31:475–482. [Google Scholar]
- Whittington C. M., et al. 2010. Novel venom gene discovery in the platypus. Genome Biology 11:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington R., Bell I., and Searson J.. 1990. A viral infection causing cytomegalic inclusion disease in the renal epithelium of the platypus (Ornithorhynchus anatinus). Journal of Wildlife Diseases 26:55–61. [DOI] [PubMed] [Google Scholar]
- Whittington R., et al. 1992. Sparganosis in the monotremes Tachyglossus aculeatus and Ornithorhynchus anatinus in Australia. Journal of Wildlife Diseases 28:636–640. [DOI] [PubMed] [Google Scholar]
- Whittington R. J., Connolly J. H., Obendorf D. L., Emmins J., Grant T. R., and Handasyde K. A.. 2002. Serological responses against the pathogenic dimorphic fungus Mucor amphibiorum in populations of platypus (Ornithorhynchus anatinus) with and without ulcerative mycotic dermatitis. Veterinary Microbiology 87:59–71. [DOI] [PubMed] [Google Scholar]
- Williams G. A., Serena M., and Grant T. R.. 2013. Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus). Australian Mammalogy 35:107–114. [Google Scholar]
- Woinarski J. and Burbidge A.. 2016. Ornithorhynchus anatinus. The IUCN Red List of Threatened Species 2016: e.T40488A21964009 https://www.iucnredlist.org/species/40488/21964009. Accessed September 2018.
- Woinarski J., Burbidge A., and Harrison P.. 2014. Action plan for Australian mammals 2012. CSIRO. [Google Scholar]
- Wong E. S. W., et al. 2012. Proteomics and deep sequencing comparison of seasonally active venom glands in the platypus reveals novel venom peptides and distinct expression profiles. Molecular & Cellular Proteomics 11:1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne M. O., and Tedford R. H.. 1975. The first Tertiary monotreme from Australia. American Museum Novitates 2588:1–11. [Google Scholar]
- Zeiss C. J., Schwab I. R., Murphy C. J., and Dubielzig R. W.. 2011. Comparative retinal morphology of the platypus. Journal of Morphology 272:949–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.