Abstract
Purpose
As a preliminary evaluation of the outcomes of implementing pharmacogenetic testing within a large rural healthcare system, patients who received pre-emptive pharmacogenetic testing and warfarin dosing were monitored until June 2017.
Summary
Over a 20-month period, 749 patients were genotyped for VKORC1 and CYP2C9 as part of the electronic Medical Records and Genomics Pharmacogenetics (eMERGE PGx) study. Of these, 27 were prescribed warfarin and received an alert for pharmacogenetic testing pertinent to warfarin; 20 patients achieved their target international normalized ratio (INR) of 2.0–3.0, and 65% of these patients achieved target dosing within the recommended pharmacogenetic alert dose (± 0.5 mg/day). Of these, 10 patients had never been on warfarin prior to the alert and were further evaluated with regard to time to first stable target INR, bleeds and thromboembolic events, hospitalizations, and mortality. There was a general trend of faster time to first stable target INR when the patient was initiated at a warfarin dose within the alert recommendation versus a dose outside of the alert recommendation with a mean (± SD) of 34 (± 28) days versus 129 (± 117) days, respectively. No trends regarding bleeds, thromboembolic events, hospitalization, or mortality were identified with respect to the pharmacogenetic alert. The pharmacogenetic alert provided pharmacogenetic dosing information to prescribing clinicians and appeared to deploy appropriately with the correct recommendation based upon patient genotype.
Conclusion
Implementing pharmacogenetic testing as a standard of care service in anticoagulation monitoring programs may improve dosage regimens for patients on anticoagulation therapy.
Keywords: clinical decision support, CYP2C9, eMERGE PGx, pharmacogenetics, VKORC1, warfarin
KEY POINTS
Pharmacogenetic alerts may aid clinicians in establishing a proper baseline dosage for warfarin regimens and can be implemented in a large rural healthcare system.
Pharmacogenetic results explain some (but not all) of a patient’s response to warfarin, thus pharmacogenetic testing should complement INR checks and dosage based upon clinical factors and does not replace these monitoring considerations.
The use of pharmacogenetic alerts may reduce the time to first stable target INR, thus potentially reducing the duration of anticoagulant bridging therapies.
Pharmacogenetics is an area of pharmacotherapy that factors individual genetic variation into dosing considerations to provide personalized or precision medicine to patients, potentially improving the effectiveness and toxicity profile of medications for a given patient, as well as saving time and costs by reducing the time needed to adjust to an optimal dose of medication for an individual. Implementation of pharmacogenetic testing has been slow, owing to several barriers—specifically, difficulty of integration into the electronic medical record (EMR), implementation of clinical decision support (CDS) tools, and interpretation and application of the results in clinical practice1; however, the practice is slowly gaining acceptance and several institutions have engaged in research to assess the full potential of pharmacogenetics in clinical practice.2,3
One such initiative is the “electronic MEdical Records and GEnomics (eMERGE) pharmacogenetics (PGx)” study which has over ten participating institutions across the country.2,3 Institutions involved in the eMERGE PGx study selected specific drug–gene interaction pairs for evaluation at their site, genotyped participating patients, and developed ways to integrate pharmacogenetic testing results into their EMR, with the goal of providing medication recommendations tailored to the patient’s genotype.2,3 At a large tertiary care facility in a rural region of the upper Midwest, 749 patients were enrolled in a pilot clinical pharmacogenetics implementation study for 3 drug–gene interaction pairs clopidogrel/CYP2C19, simvastatin/SLCO1B1, and warfarin/ CYP2C9, VKORC1. Selection criteria for study participation is discussed elsewhere but, in summary, a patient was eligible if over the age of 50 years and with no prior use of clopidogrel, simvastatin, or warfarin.3
Since there are known pharmacogenetics dosing guidelines for warfarin/CYP2C9 and VKORC1,4 warfarin was selected as one of the drug–gene interaction pairs to investigate at this institution for the eMERGE PGx study and is the focus of this case series. Warfarin is a commonly prescribed anticoagulant medication and received the most pharmacogenetics alerts among the 3 evaluated medications during the timeframe for the retrospective chart review.
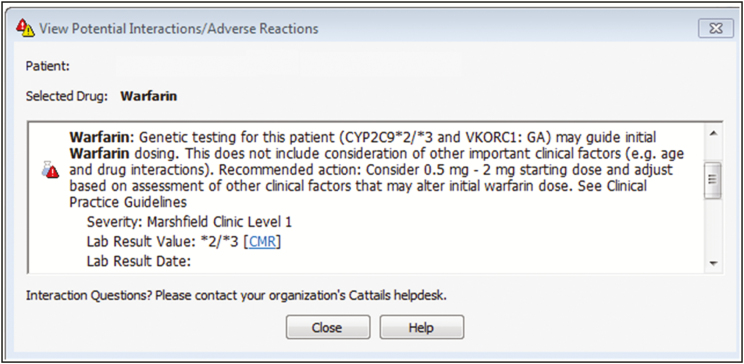
Per standard of care procedures, all patients prescribed warfarin at this institution are offered enrollment in the Anticoagulation Service (ACS), a nurse-staffed, protocol-driven program to manage patients on anticoagulation therapy. Clinicians are directed to use clinical judgement on initial warfarin dosing at the time of patient enrollment into the ACS. General guidelines include initiating treatment at a recommended starting warfarin dose of 4–5 mg/day for patients less than 75 years old or at 2–2.5 mg/day for patients 75 years and older or with comorbidities (e.g., history of liver disease, moderately to severely elevated liver function tests, elevated baseline international normalized ratio [INR] value) in addition to other patient-specific factors. When a patient with pharmacogenetic testing is prescribed warfarin, a dialog box (pharmacogenetics alert, Figure 1) is displayed on the provider’s computer screen and identifies genetic variants that alter the metabolism and clearance of warfarin from the body. The alert goes on to provide the clinician with a recommendation for initial warfarin dose based solely upon pharmacogenetic factors. Clinicians can then consider this recommendation and contextualize it with other patient-specific factors to determine a reasonable starting dose for the patient. The pharmacogenetic alerts were added to this standard of care and did not replace any component of the normal process for enrollment into the ACS, as these alerts were intended as a notification to assist the clinician with initial warfarin dosing.
Figure 1.
Pharmacogenetic alert.
While research on successful pharmacogenetics implementation is available,1,5-8 the clinical impact and outcomes of the implementation may vary from site to site due to organizational factors like pre-planning, alignment with organizational goals and objectives, leadership commitment, effective team communication, and appropriate monitoring and follow-up of the initiative.9 Regarding pharmacogenetic monitoring implementation, additional considerations may include site-specific workflows, personnel experience and expertise, and overall support pertinent to the EMR as well as interpretation and assessment of results.
Therefore, the aim of this article is to provide a preliminary description of outcomes associated with implementation of pharmacogenetic testing for warfarin administration in the eMERGE PGx patient cohort at this institution. Due to the small number of alerts (n = 27) and variability in patient-specific factors (e.g., INR goal, whether the patient was already on warfarin at the time of the alert, changes to therapy due to concurrent procedures), outcomes were quantified descriptively in a series of case studies. The primary outcomes of interest were accuracy of pharmacogenetics predicted warfarin dose, time to first stable target INR, frequency of bleeds and thromboembolic events, hospitalizations, and all-cause mortality.
Establishing an appropriate dose for anticoagulation therapy quickly and accurately is important for preventing clot formation and reducing the duration of enoxaparin bridging therapy in select patients. In this case series, 10 patients received warfarin therapy for the first time after pharmacogenetic testing. There were few bleeding events and thromboembolic (TE) events in the cohort, and 1 death associated with dysregulated coagulation, though low medication adherence and complications associated with sepsis in this patient make it difficult to draw any firm conclusions with respect to morbidity associated with warfarin or pharmacogenetics testing use. Therefore, pharmacogenetics testing prior to first-time anticoagulant administration may be a useful service for establishing baseline dosage regimens for warfarin.
Descriptive analysis
The protocol and amendments for this retrospective case series were approved by the healthcare system’s Institutional Review Board and conducted in accordance with good clinical practices. Of the 749 patients genotyped for warfarin as part of the eMERGE PGx cohort, 27 patients were prescribed warfarin, which corresponded to 27 warfarin pharmacogenetics alerts between November 2014 and June 2016; patient demographics are listed in Table 1.
Table 1.
Patient Demographics (n = 27)
| Variable | Male % (n) | Female % (n) |
|---|---|---|
| Age (years) | ||
| 51–60 | 7 (1) | 15 (2) |
| 61–70 | 14 (2) | 23 (3) |
| ≥ 71 | 79 (11) | 62 (8) |
| Ethnicity—Caucasian | 100 (14) | 100 (13) |
| Indication for warfarin | ||
| Atrial fibrillation/flutter | 50 (7) | 54 (7) |
| Thromboembolism—treatment | 21.4 (3) | 31 (4) |
| Thromboembolism—prophylaxis | 21.4 (3) | 0 (0) |
| Orthopedic thromboembolism prophylaxis | 7.2 (1) | 15 (2) |
| Total | 100 (14) | 100 (13) |
The pharmacogenetic alerts were built into the EMR to be triggered when a patient with an actionable variant was prescribed the corresponding medication; in the case of warfarin, all reported genotypes have an associated therapeutic recommendation.4,10 Pharmacogenetic recommendations for warfarin dosing were based upon the Clinical Pharmacogenetics Implementation Consortium (CPIC) recommendation on pharmacogenetic dosing at the time, which follows the FDA Warfarin Prescribing Information table on dosing by genotype, and are listed in Table 2.
Table 2.
Accuracy Among Warfarin Patients Who Achieved Stable Warfarin Dosing (n = 20)
| Variable | Dose Stable Within Pharmacogenetic Recommended Range | Dose Stable Outside Pharmacogenetic Recommended Range |
|---|---|---|
| No. (%) patients | 13 (65) | 7 (35) |
| Stratified by pharmacogenetics alert dose recommendation (no. patients) | ||
| 0.5–2 mg/day | 0 | 1 |
| 3–4 mg/day | 5 | 2 |
| 5–7 mg/day | 8 | 4 |
The 2 genes evaluated for warfarin dosing were CYP2C9 and VKORC1. Genotyping was performed for cytochrome P-450, family 2, subfamily C, poly-peptide 9 (CYP2C9) alleles *2 (p.R144C, rs1799853), *3 (p.I359L, rs1057910), *5 (p.D360E, rs28371686), and *6 (p.K273Rfs, rs9332131). The *1 allele was assigned in the absence of detection of a queried variant. Genotyping was also performed for vitamin K epoxide reductase gene, VKORC1 (c.−1639G>A, rs9923231). The CYP2C9 gene encodes the primary CYP protein involved in the metabolism of S-warfarin, and VKORC1 encodes the protein that warfarin targets; in the European population, these variants explain roughly 18% and 30% (respectively) of the variability in stable warfarin dosing.11 The pharmacogenetics recommendations for warfarin dosing were developed using an INR goal of 2.0–3.04; thus, patients with an INR value other than 2.0–3.0 (e.g., patients prescribed warfarin for orthopedic TE prophylaxis) were not included in the analysis.
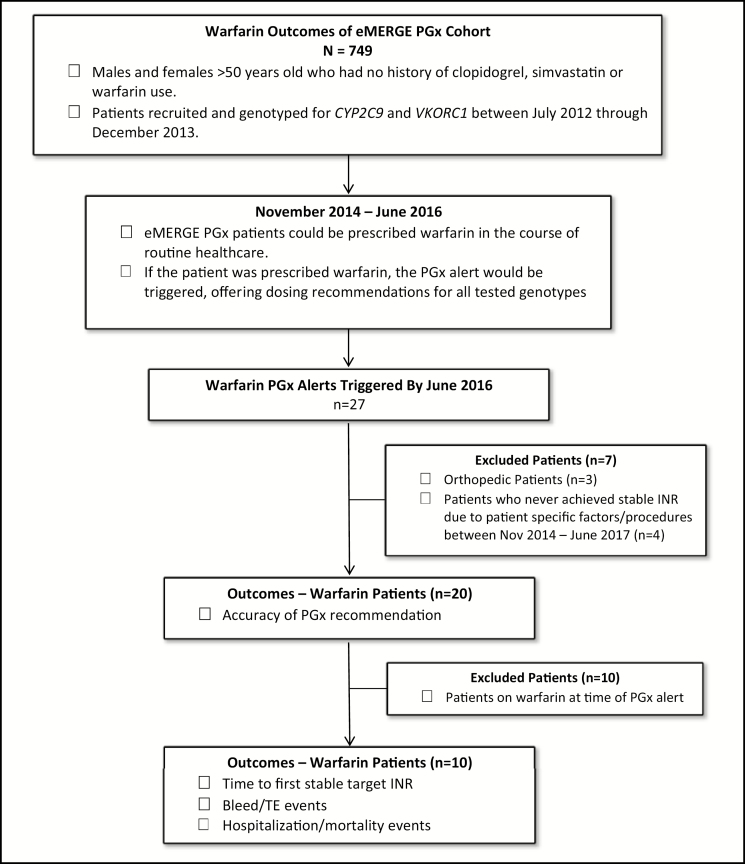
Approximately half of the patients receiving warfarin therapy were prescribed warfarin for atrial fibrillation/flutter (AF) while the remaining patients received warfarin to treat or prevent TE events. Of these 27 patients, 3 patients were prescribed warfarin for orthopedic TE prophylaxis with an INR goal value outside of 2.0–3.0 and were excluded from analysis; of the remaining 24 patients, 4 had not achieved the target INR of 2.0–3.0 at the time of analysis (Figure 2) and were also excluded. The 20 patients who achieved a target INR value were evaluated for accuracy of the pharmacogenetics predicted dose versus the empirically determined stable dose (Table 3). As the recommended dose ranges are not continuous (e.g., pharmacogenetics recommended ranges are from 0.5–2 mg/day, 3–4 mg/day and 5–7 mg/day),4,10 a margin of error for the alert recommendation of ± 0.5 mg was adopted for the analysis. Thus, a patient was considered to be “within” the alert recommendation if the stable dose was ± 0.5 mg above or below the recommended dose range. For example, if the recommendation was warfarin 3–4 mg/day, the patient would be considered “within range” if they stabilized at a dose between 2.5–4.5 mg/day. Since 10 of the 20 patients included in the initial cohort were already on warfarin at the time of the alert, these patients were excluded for additional evaluation. Therefore, the time to first stable target INR (Tables 3 and 4), rate of bleeds/TE events, hospitalization events, and all-cause mortality (Table 5) were further evaluated in the remaining 10 patients.
Figure 2.
Patient cohort outcomes.
PGx = pharmacogenetics; INR = international normalized ratio; TE event = thromboembolic event.
Table 3.
Time to First Stable Target INR Value by Initial Dosing
| Variable | Initiated at Recommended Dose in Pharmacogenetics Alert (n = 6) | Did Not Initiate at Recommended Dose in Pharmacogenetics Alert (n = 4) |
|---|---|---|
| Mean ± S.D., days | 34.3 ± 27.6 | 129.3 ± 117.0 |
| Median (range), days | 29.5 (4–73) | 107.0 (4–299) |
Table 4.
Time to First Stable Target INR (Days) Among Warfarin Patients by Recommendation and Initial Dose (n = 10)
| Variable | Dose Stable Within Recommended Range in Alert (n = 5) | Dose Stable Outside Recommended Range in Alert (n = 5) | ||
|---|---|---|---|---|
| Stratified by Initial Dose | ||||
| Was the patient initiated at a daily dose of warfarin within the dose recommended within the pharmacogenetics alert? | Yes | No | Yes | No |
| No. patients | 2 | 3 | 4 | 1 |
| Time to First Stable Target INR, days | ||||
| Mean ± S.D. | 5 ± 1.0 | 171 ± 106.2 | 49 ± 22.3 | 4 ± 0 |
| Median (range) | 5 (4–6) | 175 (39–299) | 54 (15–73) | 4 (not applicable) |
Table 5.
Warfarin Cohort Bleeding Events, Thromboembolism, Hospitalization, and Mortality Eventsa
| Case No. | Bleed(s) (Severitya) | Thromboembolism | Hospitalization Event(s) | Bleed or Thromboembolism event during stabilization period? (Y/N) | AchievedTarget Dose Within Pharmacogenetics Alert Recommendaiton? (Y/N) | Patient Initiated on Warfarin Dose Within Pharmacogenetics Alert Recommendation? (Y/N) | Initial Warfarin Dose (mg/day) | Stable Warfarin Dose(mg/day) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 (1 minor, 1 moderate) | 0 | 0 | N | N | Y | 2 | 3.3 |
| 3 | 1 (moderate) | 0 | 2 | N | Y | Y | 5 | 5.36 |
| 7b | 2 (minor) | 1c | 5d | Y | Y | N | 3e | 6 |
| 8 | 1 (minor) | 0 | 0 | N | Y | N | 5e | 2.5 |
| 9 | 1 (minor) | 0 | 1 | Y | Y | N | 2e | 5.14 |
a Severity definitions: minor bleed = bleed requiring home care; moderate bleed = bleed requiring doctor visit; major bleed = bleed requiring hospitalization or blood product transfusion.
bDeath may have been related/impacted by a possible thromboembolic event; however, death was likely multifactorial, see patient case description.
c“Possible strokes with right hemi-paresis” noted in EMR.
dOne of the 5 hospitalization events may have been related to a possible thromboembolic event (“Possible strokes with right hemi-paresis” noted in EMR).
ePatient initiated on warfarin dose outside of PGx alert recommendation.
Accuracy of the pharmacogenetics alert
To assess the accuracy of pharmacogenetics recommendations for optimal warfarin dose, the percentage of patients achieving a stable INR value within the pharmacogenetics alert recommended warfarin dose with a ± 0.5 mg/day margin of error was calculated for the total number of patients who were prescribed warfarin within the data collection period, regardless of previous warfarin exposure (Table 2). Of the 20 patients who achieved stable warfarin dosing, 13 (65%) attained a stable INR with a dose that was within the alert recommendation. This suggests that the PGx recommendations are an appropriate guide for establishing an appropriate dosage regimen for patients on warfarin.
Time to first stable target INR value
The time to first stable target INR value was defined as the number of days to the first instance of 3 consecutive in-range INRs that are at least 5 days apart without dose modification to indicate the patient has received the optimal warfarin dose for anticoagulation. The time to target INR was stratified by whether or not the patient was initiated on a warfarin dose that was within the pharmacogenetics alert recommended range (Table 3), and whether the patient’s warfarin dose stabilized within the dose recommended by the alert (Table 4).
With respect to starting dose, clinicians who followed the pharmacogenetics recommendation for warfarin dosing were able to optimize anticoagulation in their patients in a shorter period of time than clinicians who did not follow the recommendation (regardless of the dose the patient stabilized on) with a mean ± S.D. duration of 34.3 ± 27.6 days versus 129.3 ± 117.0 days (Table 3).
Among patients whose warfarin dose stabilized within the pharmacogenetics alert recommended range, the time to first stable target INR value was shorter in patients who were started at the recommended dose (n = 2) versus those who were not (n = 3) (Table 4). Of the 5 patients who stabilized outside of the alert recommendation, 4 were initiated on a dose within the alert recommendation, and these patients achieved a mean ± S.D. time to first stable target INR value of 49 ± 22.3 days. The remaining patient achieved a stable dose within 4 days.
Bleeds, thromboembolic (TE) events, hospitalizations and mortality
There were 7 bleeding events and 1 TE event among 5 of the 10 patients. Minor bleeding events occurred in 4 patients, moderate bleeding events occurred in 2 patients, and no major bleeds were reported within the patient cohort. Regarding minor bleeds, 3 of the 4 patients had a target dose within the recommended dose range, and one of the 2 patients with a moderate bleed had a target dose within the recommended range. Eight hospitalization events occurred in 3 patients, though only 1 hospitalization may have been related to a bleed or TE event. This patient (Case 7) was the only patient who died during the study period (Table 5).
Case series
Relevant clinical information for each patient is summarized in Table 6. Bleeding and TE events, hospitalization, and mortality data were collected from the time of the pharmacogenetics alert through the manual data abstraction period (through June 2017) via EMR review. Daily doses of warfarin were calculated by taking the weekly dose and dividing by 7. Drug interactions were identified with the use of a commercial drug interaction database12 and reviewed for the impact on bleeding risk/INR value.
Table 6.
Summary of eMERGE PGx Patients with a First Time Prescription of Warfarin at or After Time of Alerta
| Case No. | Patient Age (yr), sex (M/F), ethnicity | Genotype for CYP2C9, VKORC1 | Indication for Warfarin | PGx Alert Rec. Dose (mg/day) | Starting Warfarin Dose (mg/ day) | Stable Dose (mg/ day) | Patient Initiated on Warfarin Dose Within PGx Alert Rec. | Days to Stable INR | B/TE & Severity | Hosp. (Y/N) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77, M, W | *2/*3, GA | AF | 0.5–2 | 2.0 | 3.3 | Y | 15 | B—minor, moderate | N |
| 2 | 67, M, W | *1/*1, GG | AF | 5–7 | 5.0 | 5.0 | Y | 6 | None | N |
| 3 | 61, F, W | *1/*1, GA | AF | 5–7 | 5.0 | 5.4 | Y | 4 | B—moderate | Yd |
| 4 | 75, M, W | *1/*1, GG | AF | 5–7 | 5.0 | 4.3 | Y | 73 | None | N |
| 5 | 74, F, W | *1/*1, GG | TE Tx | 5–7 | 5.0 | 9.6 | Y | 64 | None | N |
| 6 | 61, F, W | *1/*1, GG | AF | 5–7 | 5.0 | 9.6 | Y | 44 | None | N |
| 7b | 77, M, W | *1/*1, GG | TE Tx | 5–7 | 3.0 | 6.0 | N | 175 | B—minor, minor; possible TE | Yc |
| 8 | 72, F, W | *1/*1, AA | AF | 3–4 | 5.0 | 2.5 | N | 299 | B—minor | N |
| 9 | 56, F, W | *1/*1, GA | AF | 5–7 | 2.0 | 5.1 | N | 39 | B—minor | Yd |
| 10 | 52, M, W | *1/*1, AA | TE PPx | 3–4 | 5.0 | 5.0 | N | 4 | None | N |
aAF = atrial fibrillation or flutter; B = bleed event; Rec. = recommended; Hosp. = hospitalization; TE = Thromboembolic event; TE Tx = thromboembolism treatment; TE PPx = thromboembolism prophylaxis; W = white/Caucasian.
bPatient died during study period.
cHospitalization was possibly TE related.
dHospitalization was not bleed or TE related.
Patients who were new to warfarin.
Case 1.
A 77-year-old Caucasian man was initiated on warfarin for AF at the time of the pharmacogenetics alert. The patient was initiated on a dose of warfarin within the alert recommendation (starting warfarin dose of 2 mg/day), and the time to first stable target INR value for this patient was 15 days. Although the alert recommended warfarin dose was 0.5–2 mg/day, the dose at which this patient achieved a stable INR value was 3.3 mg/day, which was not within the ± 0.5 mg margin of error of the dose recommendation. The only medication that could potentially interact with warfarin noted from the time of the alert to the first stable INR was fish oil, which may have increased bleed risk secondary to the inhibitory effect of fish oil on thromboxane A2 levels12; the patient’s warfarin adherence was not described in the EMR. There was no indication in the EMR of procedures during INR stabilization that required holding of warfarin, nor were other factors influencing INR values (e.g., diet, illness) reported. This patient had 1 minor and 1 moderate bleeding event and no TE events. The minor bleeding event (bleed event requiring home care) was for a pinched fingertip, while the moderate bleeding event (bleeding event requiring provider evaluation) occurred after the patient bit his tongue. The patient was not hospitalized during the data review period.
Case 2.
A 67-year-old Caucasian man was initiated on warfarin for AF, 1 day after the pharmacogenetics alert was triggered. The patient was initiated on a 5 mg/day dose of warfarin which was within the alert recommendation (5–7 mg/day), and the time to first stable target INR value for this patient was 6 days. The warfarin dose at which the patient achieved a stable INR value was 5 mg/day. Potentially interacting medications recorded at the time of the alert to the first stable INR value included fenofibrate and naproxen, both of which increase bleeding risk in patients taking warfarin12; the patient’s warfarin adherence was not noted in the EMR. No procedures were performed during INR stabilization that required holding of warfarin, nor were other factors influencing INR (e.g., diet, illness) noted. This patient had no bleeding or TE events, nor was the patient hospitalized, according to EMR review.
Case 3.
A 61-year-old Caucasian woman was initiated on warfarin for AF, 1 day after the pharmacogenetics alert. The alert recommended warfarin dose was 5–7 mg/day, and the dose at which patient achieved stable INR value was 5.36 mg/day. The patient was initiated on a dose of warfarin within the alert recommendation (starting dose of 5 mg/day), and the time to first stable target INR value for this patient was 4 days. Potentially interacting medications reported at the time of the alert to the first stable INR value included acetaminophen, aspirin, citalopram, glucosamine, and ranitidine—all of which can increase bleeding risk.12 The patient’s warfarin adherence was not described in the EMR, nor did the patient undergo procedures or other factors requiring warfarin dose adjustment. This patient had no TE events but did have 1 moderate bleeding event (bit tongue) that was treated with sutures. The patient was hospitalized twice, once for acute kidney injury and once for chronic obstructive pulmonary disease (COPD) exacerbation; however, neither of these events were related to bleeding or TE events. The patient was still alive at time of data review.
Case 4.
A 75-year-old Caucasian man was initiated on warfarin for AF at the time of the pharmacogenetics alert. The patient was initiated on a dose of warfarin within the alert recommendation (starting dose of 5 mg/day), and the time to first stable target INR value for this patient was 73 days. Although the patient initially received the alert recommended dose of 5–7 mg/day, the warfarin dose required for stabilization of the INR value was 4.29 mg/day, which was not within the ± 0.5 mg margin of error. There were no potentially interacting medications noted in the EMR; however, the patient periodically consumed alcohol during the stabilization period which may have increased or decreased the INR12 and prolonged the time to first stable target INR. The patient’s warfarin adherence was not described in the EMR. No procedures or other factors affecting INR were documented during the stabilization period. This patient had no bleeding or TE events, and the patient was not hospitalized during the data collection period.
Case 5.
A 74-year-old Caucasian woman was initiated on warfarin for deep vein thrombosis (DVT) treatment at the time of the pharmacogenetics alert. The patient was initiated on a dose of warfarin within the alert recommendation (starting dose of 5 mg/day), and the time to first stable target INR value for this patient was 64 days. However, the stable warfarin dose was 9.64 mg/day, which was not within the ± 0.5 mg margin of error (alert recommended dose: 5–7 mg/day). Potentially interacting medications noted during the stabilization period included a 10-day course of cephalexin, which can increase a patient’s risk for bleeding while on warfarin.12 The patient’s warfarin adherence may have been questionable, as the patient missed several scheduled enoxaparin injections and expressed dissatisfaction with being on warfarin. Additionally, the patient traveled several times during the stabilization period, so INR values were not always obtained at ideal intervals. No procedures or other factors influencing INR values were noted in the EMR during the stabilization period. This patient had no bleeding or TE events, and there was no record of the patient being hospitalized during the data collection period.
Case 6.
A 61-year-old Caucasian woman was initiated on warfarin for AF at the time of the pharmacogenetics alert. The patient was initiated on a dose of warfarin within the alert recommendation (starting dose was 5 mg/day), and the time to first stable target INR value for this patient was 44 days. Although the patient was initiated on warfarin therapy within the alert recommended dose of 5–7 mg/day, the stable dose was 9.64 mg/day, which was not within the ± 0.5 mg margin of error. No potentially interacting medications were noted, and no information regarding warfarin adherence or other factors influencing INR values was found during the stabilization period in the EMR. This patient had no bleeding or TE events, and the patient was not hospitalized during the data collection period.
Case 7.
A 77-year-old Caucasian man was initiated on warfarin due to chronic DVT. Although the pharmacogenetics alert recommended warfarin dose was 5–7 mg/day, the patient was not initially started on a dose within the alert recommendation (starting dose of 3 mg/day); however, the patient achieved a stable warfarin dose of 6 mg/day, which was within the alert-recommended dose range. Since the patient had stage IV lung cancer and was noted to be on many medications that could adversely impact warfarin function, the time to first stable target INR value for this patient was 175 days from the alert to stable warfarin dose. Potentially interacting medications included cisplatin, etoposide, fish oil, levofloxacin and metronidazole, all of which can increase the INR value and bleeding risk.12 This patient was also on aprepitant, which may decrease INR, and dexamethasone, which can have variable effects on the INR.12 Medication adherence was poor as reported by the patient and the patient’s caregiver. At one point in therapy, the patient’s caregiver stated they were taking the patient off of warfarin against medical advice, citing frustration with the lack of a stable dose within the target INR range. While adjusting warfarin to a stable INR value, the patient had a port placed which required holding of warfarin for 5 days. The patient experienced 2 minor bleeding events (epistaxis and hematuria) that were both treated with home cares and monitoring, and 1 possible TE event near the time of death (“possible strokes with right hemiparesis”) was noted in the EMR. During the study period, the patient was hospitalized 5 times: 4 times were for indications not related to a bleeding or TE event (small bowel obstruction, planned chest port placement, fever and diarrhea, and ileus) while the final admission was for sepsis and possible TE event. The patient died due to complications associated with sepsis and possible TE event.
Case 8.
A 72-year-old Caucasian woman was initiated on warfarin for AF, 3 days after the pharmacogenetics alert. Although the patient was not initiated on a dose of warfarin within the alert recommendation (starting dose was 5 mg/day versus 3–4 mg/day per alert recommendation), she achieved a stable dose of 2.5 mg/day, which was within the ± 0.5 mg margin of error. However, this patient had difficulty achieving a stable INR; the time to first stable target INR value for this patient was 299 days, the longest time to a stable INR value in the patient cohort. Potentially interacting medications included dronedarone (discontinued while determining stable dosing), fluconazole, and paroxetine (which was transitioned to sertraline during the stabilization period), all of which can increase INR values and risk of bleed while on warfarin.12 There was 1 documented instance of the patient missing a dose of warfarin, but no other factors affecting INR values were documented in the EMR. This patient had 1 minor bleeding event (bruising), no TE events, and was not hospitalized during the data collection period.
Case 9.
A 56-year-old Caucasian woman was initiated on warfarin for AF at the time of the pharmacogenetics alert. The patient was not initiated on a dose of warfarin within the alert recommendation (starting dose was 2 mg/day versus 5–7 mg/day per alert recommendation) but achieved a stable dose within the recommended range (5.14 mg/day); the time to first stable target INR value for this patient was 39 days. Potentially interacting medications included doxycycline, and dabigatran anticoagulation therapy (both of which can increase bleed risk in warfarin patients), and phytonadione to reverse anticoagulation post-procedure.12 The patient was transitioned from dabigatran to warfarin therapy over a 2-week period and did not receive further dabigatran after 14 days of warfarin treatment. During the titration period, the patient had a urinary stent removed at an outside facility and received phytonadione to reverse warfarin anticoagulation. The patient’s warfarin adherence was not documented in the EMR. This patient had 1 minor bleeding event (intermittent hematuria due to kidney stones) and no TE events. In addition to the urinary stent removal procedure, the patient was hospitalized at an outside facility for AF during the data collection period.
Case 10.
A 52-year-old Caucasian man was initiated on warfarin for DVT prophylaxis (due to hypercoagulable state) at the time of the pharmacogenetics alert. The alert recommended warfarin dose was 3–4 mg/day, but the patient was initiated on and achieved a stable dose of 5 mg/day, which was not within the ± 0.5 mg margin of error. The time to first stable target INR value for this patient was 4 days. No potentially interacting medications were noted; no information regarding warfarin adherence or other factors influencing INR values was reported in the EMR during the stabilization period. This patient had no bleeding or TE events, and the patient was not hospitalized during the data collection period.
Discussion
Warfarin is a coumarin-based anticoagulant that reduces clotting by inhibiting the activity of vitamin K epoxide reductase (VKORC1) to inhibit the activation of vitamin K-dependent blood coagulation proteins.13-15 Warfarin is commonly prescribed to control hematological disorders (e.g., Factor V Leiden), reduce the risk of future TE events in patients with a history of DVT, pulmonary embolism, and stroke, and is used for TE/DVT prophylaxis for patients with cardiac arrhythmias as well as patients with artificial heart valves.15,16 In 2014, over 23 million patients in the United States received a prescription for warfarin, though it is slowly being replaced by more specific oral anticoagulants.17,18
Although the anticoagulant effects of warfarin are easily reversed by phytonadione administration, its mechanism of action decreases the expression of multiple proteins and requires close monitoring by providers to prevent adverse bleeding events.15,16 Patients taking warfarin must be compliant with dosage regimens and diligently follow provider instructions regarding alterations in warfarin dosage, both pre and post surgical procedures. Since many drugs, supplements, diet, and individual factors such as age and body weight can impact warfarin metabolism and clearance, patients must exercise caution in altering their medications and supplements and monitor their dietary habits to reduce the need for frequent dose adjustments.16 Furthermore, warfarin effectiveness is also dependent in part on an individual’s genotype for drug metabolizing enzymes such as CYP2C9.4,19 Therefore, establishing a stable warfarin dose in the early phase of anticoagulant therapy requires a significant amount of patient and provider effort that could be reduced by pharmacogenetic testing.
In the patient cohort analyzed for the primary outcomes, pharmacogenetic recommendations were usually accurate and timely for establishing a stable dosage regimen for warfarin. The alert recommendation accurately predicted stable dosing for 65% of our cohort with a target INR value of 2.0–3.0 (n = 20). Regarding patients who were new to warfarin, the time to first stable target INR value was also shorter in patients who were initiated at the alert recommended dose versus those who were not. For patients who stabilized within the pharmacogenetic recommended dosing, those who received an initial warfarin dose within the alert recommended range had a shorter time to target INR value than the patients who were not initiated at a dose within the recommended range. The pharmacogenetics alert recommendation may remain valuable even if the patient does not stabilize in the genotype predicted dosing range: starting the patient at the alert recommended dosing resulted in a mean time to first stable target INR of 49 days in these patients (n = 4), which is still shorter than for the patients who stabilized within the range and were not initiated at the recommended dose (171 days, n = 3). It is postulated that patients who initially received warfarin at a dose outside of the pharmacogenetics alert recommendation would have stabilized faster if they had been initiated on a dose within the recommended dose range (e.g., Cases 7, 8, and 9), though it is important to note that these patients were on multiple medications that can adversely impact warfarin dosage. Medication adherence was of particular concern for Cases 5 and 7, as reflected in comments in the EMR; however, Case 5 did eventually achieve a stable warfarin dose, despite the need for a much higher daily warfarin dose. Aside from noted nonadherence to warfarin treatment and potential drug and supplement interactions, patients may achieve a stable warfarin dose outside of the alert recommendation due to genotypic variations in unanalyzed proteins, and/or global alterations in protein expression and/or activity due to disease or medication use.15,19 For example, patients were not genotyped for variations in CYP4F2, a cytochrome P450 enzyme that hydroxylates vitamin K to reduce the amount of available vitamin K.4 Caldwell and colleagues determined that a C>T variant in CYP4F2 (rs2108622, p.V433M) is linked to reduced protein function, and necessitated an increase in warfarin dose by about 1 mg/day in patients homozygous for the T allele within their cohort.20 Patients with reduced CYP4F2 activity require higher warfarin doses to achieve therapeutic INR to surmount the increased level of vitamin K in circulation.19 This genotypic variant may have played a role in Cases 5 and 6, as these patients required higher daily doses of warfarin.
Of the relevant drug-genotype interactions assessed, 1 patient (Case 1) had a genotype (CYP2C9*2/*3, VKORC1 G/A) and thus alert recommendation that diverged significantly from the standard dosage regimen (alert recommendation of 0.5–2 mg/day versus 5–7 mg/day, respectively). The remaining patients appeared to receive dosage per standard recommendation (no pharmacogenetic considerations) but still required an adjustment period to stabilize dosage. Since most patients did not require alterations in warfarin dosing for genotype, dosing changes were likely made in response to external factors such as interacting medications, surgical procedures, and overall health status of the individual. Although there were no apparent associations between bleeding events, TE episodes, or hospitalization/mortality events and warfarin dose, these adverse events appeared to occur more frequently in patients with multiple comorbidities; however, the patient cohort was too small for rigorous statistical analysis.
Two commonly used measures for characterizing warfarin dosage regimens are “time in therapeutic range” and “time to first therapeutic INR.” Although “time in therapeutic range” (usually defined as a percentage of time patient is in their therapeutic INR range) is the more widely used measure for the purposes of assessing warfarin dosing, this unit of measure would not accurately assess the current patient cohort, as additional factors like supplements and lifestyle factors influence warfarin dosing independently of pharmacogenetics. In contrast, reporting “time to first therapeutic INR” without consideration of pharmacogenetics factors would provide information about the duration of time required to get a patient to his or her target INR value; however, this measurement did not account for the stability of dosing and was not an appropriate comparison for this data.
Therefore, “time to first stable target INR” was assessed to better represent the use of pharmacogenetics to establish a starting dose and account for adjustments in warfarin dosing during the stabilization period. “Time to first therapeutic INR” has been used to assess the need for duration of bridging therapy among different anticoagulants,21,22 but since only 1 patient in our cohort underwent bridging therapy, no conclusions can be made with respect to this clinical factor.
A recent large clinical trial that evaluated pharmacogenetics-guided warfarin dosing is the Genetic Informatics Trial (GIFT), a multicenter study of 1597 patients randomized to either genotype-guided warfarin dosing or clinically guided warfarin dosing; the authors concluded that genotype-guided warfarin dosing reduced major bleeds, INR value greater than 4, and venous thromboembolism (VTE).23 Our case series complements the GIFT study results, as our data supports the accuracy of the published dosing recommendations, as well as a shorter time to first stable target INR when patients were initiated within the pharmacogenetics recommended dosing range.
Analyzing patient outcomes in the context of pharmacogenetics testing for warfarin dosing was challenging and resulted in a few important study limitations including ethnic homogeneity, small sample size, outliers, retrospective data evaluation, and limited clinician education prior to implementation. As is often the case in many rural Midwest settings, most of the analyzed population identified themselves as non-Hispanic Caucasian, which reduces the generalizability of these findings to other ethnic groups.19 However, even in the context of a Caucasian population, the analyzed population is very homogeneous due to the regional makeup of the area. The majority of the analyzed population (70%, or 7 patients) did not have a genotype warranting deviation from standard dosing (5 mg/day for warfarin) and received the recommendation to initiate warfarin at a dose between 5–7 mg daily. This genetic homogeneity was not surprising given the average allele frequencies of CYP2C9 *2 (rs1799853) and *3 alleles (rs1057910) as well as VKORC1 G>A (rs9923231) among Caucasian individuals (13%, 7%, and 39% respectively) in the analyzed population.11
In addition to a small, homogeneous patient population, outcome assessment was also challenging due to the presence of a few significant outlier values in time to first stable target INR and lack of warfarin naïve patients (10 out of 20 patients had received warfarin therapy in the past). It was also somewhat surprising that within a cohort of 749 patients, only 27 patients received warfarin pharmacogenetics alerts. However, there was a delay in genotyping which postponed pharmacogenetics alert deployment. Several study patients received their first warfarin therapy prior to the alert deployment, thus reducing our numbers. The study was designed to target patients likely to need clopidogrel, simvastatin, or warfarin within a 3-year period (as predicted by age greater than 50 years); most patients were enrolled due to potentially needing simvastatin (as opposed to needing clopidogrel or warfarin). A larger, more diverse sample population is needed to assess adverse outcomes associated with warfarin use in patients with various genotypes and in the context of first-time warfarin use.
Due to the retrospective nature of the case series, it is difficult to verify and assess warfarin adherence solely from EMR data. Patient-specific situations and clinical factors such as surgical procedures and hospitalizations may have impacted diet, medication use, and INR testing during the data collection period. Furthermore, medical care received outside of the institution may not be captured in the ACS portion of the EMR unless the patient (or their provider) alerted the ACS to these events. Analyzing data from additional sources such as physician notes or patient questionnaires may be necessary for a comprehensive study of patient outcomes associated with pharmacogenetics testing for warfarin dosage.
Furthermore, integration and deployment of pharmacogenetics testing involves a coordinated effort of clinicians, information technology experts, research/genetics/pharmacist support staff, and many other stakeholders. Unfortunately, clinician involvement was limited for this project. General education was made available via Grand Rounds presentations and email communications. While clinician focus groups were conducted prior to the implementation of testing for select patient groups, attendance was sparse (fewer than 10 clinicians attended); thus most, if not all, of the clinicians who received a pharmacogenetics alert had no foreknowledge or experience with the EMR alert. The standard care practice warrants a warfarin dose of 5–7 mg/day if the genotype is unknown. The clinician’s lack of knowledge may have impacted their decision-making and comprehension in response to the alert, though with such a low number of alerts and no way to track if the alert had an impact on the clinician’s prescribing, it is unclear as to what impact the alert had on clinician prescribing habits. Despite this, the alerts appeared to be triggered appropriately in the EMR and were responded to by clinicians. Research is ongoing regarding clinician use of pharmacogenetic alerts in clinical practice at this institution, and future educational opportunities such as computer-based training modules, Grand Rounds, and presentations are currently under development, with the aim of increasing awareness and comprehension of the alerts.
Conclusion
Pharmacogenetic alerts usually established an appropriate baseline warfarin dosage regimen and decreased time to first stable target INR value among patients who were initiated on warfarin therapy at this institution.
Acknowledgments
We would like to acknowledge the assistance of the following individuals without which this work would not be possible: Laurel Verhaugen and Steffani Roush, programmer analysts; Deb Kempf, resident research facilitator; Connie Folz, Pharm.D., resident research advisor; Emily Andreae, Ph.D. and Marie Fleisner, manuscript editing and submission.
Disclosures
This project was partially supported by the following grants: NIH NH6RI U01HG8701, NCATS 1UL1TR002373, Marshfield Clinic Health System Funds, Marshfield Clinic Research Institute, and Marshfield Clinic Division of Education Resident Research Program. The authors have declared no potential conflicts of interest.
References
- 1. Dunnenberger HM, Crews KR, Hoffman JM et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015; 55:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herr TM, Bielinski SJ, Bottinger E et al. Practical considerations in genomic decision support: the emerge experience. J Pathol Inform. 2015; 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasmussen-Torvik LJ, Stallings SC, Gordon AS et al. Design and anticipated outcomes of the EMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014; 96:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson JA, Caudle KE, Gong L et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017; 102:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crews KR, Cross SJ, McCormick JN et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health-Syst Pharm. 2011; 68:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunnenberger HM, Biszewski M, Bell GC et al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am J Health-Syst Pharm. 2016; 73:1956–66. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman JM, Haidar CE, Wilkinson MR et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014; 166C:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson JA, Elsey AR, Clare-Salzler MJ et al. Institutional profile: University of Florida and Shands Hospital personalized medicine program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013; 14:723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGreevy M. Why change works sometimes. Industrial and Commercial Training. 2009; 41:305–13. [Google Scholar]
- 10. Bristol-Myers Squibb Company. Coumadin (warfarin) prescribing information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009218s107lbl.pdf (accessed 2017 Sep 09).
- 11. Johnson JA, Gong L, Whirl-Carrillo M et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011; 90:625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. IBM Watson Health. IBM Micromedex® Drug Interaction Checking. Colorado, USA: Greenwood Village; 2018. https://www.micromedexsolutions.com/ (accessed 2018 Feb 1). [Google Scholar]
- 13. Bell RG, Sadowski JA, Matschiner JT. Mechanism of action of warfarin. Warfarin and metabolism of vitamin K 1. Biochemistry. 1972; 11:1959–61. [DOI] [PubMed] [Google Scholar]
- 14. Vainieri H, Wingard LB Jr. Effect of warfarin on the kinetics of the vitamin K-dependent clotting factors in rats. J Pharmacol Exp Ther. 1977; 201:507–17. [PubMed] [Google Scholar]
- 15. Wadelius M, Chen LY, Eriksson N et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007; 121:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Minno A, Frigerio B, Spadarella G et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 2017; 31:193–203. [DOI] [PubMed] [Google Scholar]
- 17. ClinCalc DrugStats Database. Warfarin. Drug usage statistics, United States, 2006–2016.http://clincalc.com/DrugStats/Drugs/Warfarin (accessed 2018 Jan 30).
- 18. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015; 128:1300–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaye JB, Schultz LE, Steiner HE et al. Warfarin pharmacogenomics in diverse populations. Pharmacotherapy. 2017; 37:1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caldwell MD, Awad T, Johnson JA et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008; 111:4106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison L, Johnston M, Massicotte MP et al. Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy. Ann Intern Med. 1997; 126:133–6. [DOI] [PubMed] [Google Scholar]
- 22. Kahlon P, Nabi S, Arshad A et al. Warfarin dosing and time required to reach therapeutic international normalized ratio in patients with hypercoagulable conditions. Turk J Haematol. 2016; 33:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gage BF, Bass AR, Lin H et al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT randomized clinical trial. JAMA. 2017; 318:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]