Abstract
Patients with inflammatory bowel disease (IBD) are susceptible to varieties of opportunistic infections due to immunological changes in the setting of their disease and drug-induced immunosuppression. Even though numerous infections can be prevented by vaccine, vaccination in IBD patients is inadequate. Data showed only 9% were vaccinated against pneumococcal infection and 28% described commonly receiving influenza vaccine. This review article discusses the recent immunizations against influenza virus; pneumococcal infection; human papilloma virus; tetanus, diphtheria and pertussis; measles, mumps and rubella; varicella zoster; and herpes zoster for individuals diagnosed with IBD and those patients with drug-related immunosuppression. In addition, this review discusses concerns about IBD patients planning to travel abroad. Immunization status and screening for opportunistic infection need to be addressed in IBD patients at the time of diagnosis and they should be vaccinated accordingly. Generally, standard vaccination strategies should be pursued in IBD patients, although live vaccines should be avoided while they are not immunocompetent.
Keywords: Inflammatory bowel disease, opportunistic infections, immunization
Introduction
Inflammatory bowel diseases (IBD) are a group of chronic inflammatory conditions of the colon and small intestinal tract [1]. Immunomodulators and biologic agents are approved for treating this group of patients and current data support their introduction early in the disease course [2]. Biologic medications and immunomodulators, or a combination of both, are used as the maintenance therapy in IBD [3]. Immunosuppression increases the risk of infections, some of which are preventable with routine immunization [4]. IBD patients on immunosuppressive treatment have a considerably weaker reaction to routine vaccinations. The greatest effect is seen in patients on a combination of anti-tumor necrosis factor (TNF) and immunosuppressive therapy [5]. Given the risk of vaccine-related infection, live vaccines are contraindicated in immunodeficient IBD patients (Table 1) [6].
Table 1.
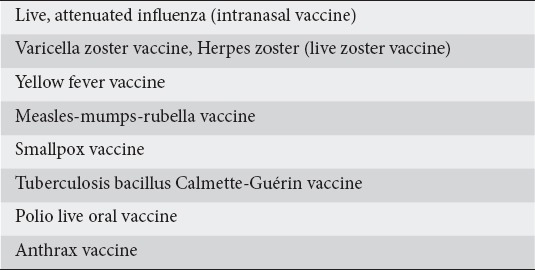
Data have shown poor counselling about vaccinations by gastroenterologists or primary care physicians [7]. Prior studies have proven that physician counseling is a strong predictor of being vaccinated and other preventive care interventions [8]. Therefore, these findings draw attention to the need for a thorough and organized assessment of immunization status at the time of diagnosis of IBD or prior to starting any biologic agents. The aim of this review is to improve gastroenterologists’ knowledge of the importance of preventive healthcare within the IBD patient population.
Materials and methods
MEDLINE records were explored through PubMed with search strategies using search keywords “IBD”, “immunization”, “vaccination recommendation”, “influenza”, “Europe”, “HPV”, “pneumococcal”, “herpes zoster”, “varicella”, “Tdap” and “MMR” to identify studies published between the years 1987 and 2018. Articles were selected from case-control studies, randomized trails, cohort research, and case reports. In addition, abstracts of conferences from important congresses in the gastroenterology field, United European Gastroenterology Week, and the European Crohn’s and Colitis Organisation were searched. Adults with IBD receiving any vaccine type and at any dose were included. Studies related to non-humans or not in the English language were excluded from our review. Abstracts of the articles found by the preliminary search were reviewed by the authors for pertinence to IBD, and all potentially related data were selected and evaluated in detail.
Definition of immunocompromised in IBD
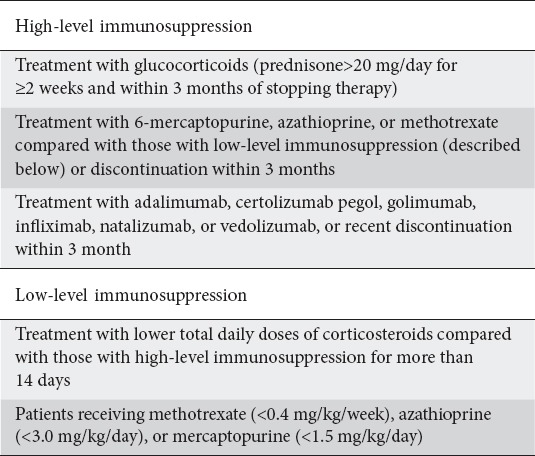
The criteria for impaired immune systems in IBD patients are: 1) patients on glucocorticoid therapy ≥20 mg of prednisone for longer than 2 weeks; 2) patients taking immunomodulators, including azathioprine, mercaptopurine and/or methotrexate, calcineurin inhibitors or anti-TNF (infliximab, adalimumab or others); 3) undernourished patients and patients with any condition leading to impaired immune systems, such as asplenia or human immunodeficiency virus infection [4,9]. Another classification specifies high or low levels of immunosuppression according to the strength of the immunosuppressive agents (Table 2) [10].
Table 2.
Level of immunosuppression based upon strength of immunosuppressive medication [10]
Screening test for infectious disease
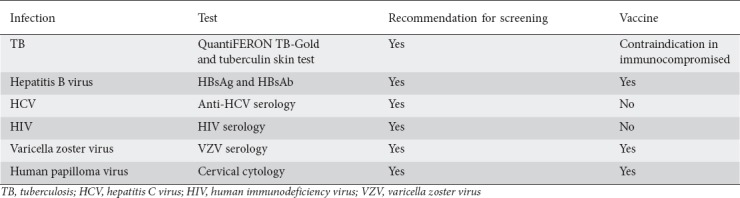
The use of biological medications and immunomodulators for IBD is connected to an increased risk of opportunistic infections. Therefore, screening for immunity to serious infection is recommended, but compliance with these recommendations is unknown. It is essential that gastroenterologists involved in IBD care execute a vigilant investigation for infectious disease before starting immunomodulation. Vigilant screening allows the physician to avoid having to stop a biological medication because of the presence of infections with the risk of recurrence of the underlying disease [11] (Table 3).
Table 3.
Rate of infection in IBD patients
The risk of opportunist infections is very high in IBD patients. Kirchgesner et al in 2018 showed that, among 190,694 patients with IBD, serious infections occurred in 8561 of them, while 674 patients were dealing with opportunistic infections. The investigators reported that combination therapy was accompanied by higher risks of serious infection (hazard ratio [HR] 1.23, 95% confidence interval [CI] 1.05-1.45) and opportunistic infection (HR 1.96, 95%CI 1.32-2.91), compared with anti-TNF monotherapy [12]. Reactivation of the hepatitis B virus (HBV) has been documented at rates of 16-36% in IBD patients with HBsAg-positive. Longstanding use (defined as more than 3 months) of immunosuppressive therapy and combination therapy without being immunized with antiviral vaccine prophylactically are associated with the risk of HBV reactivation [13]. Huang et al showed that the rate of hepatitis C virus (HCV) infection in patients with IBD was not statistically different from that in the general population. Among 714 patients with IBD, the rate of HCV infection was 0.42% compared with 0.36% (P=0.80) in non-IBD individuals. This outcome was in line with another study conducted in Italy [14,15]. The latest data indicate that IBD patients have a 1.65% chance of developing a tuberculosis infection, even after latent tuberculosis infection screening, before the initiation of anti TNF-α therapy [16].
Vaccination rate in IBD patients
The vaccination rate among IBD patients is still suboptimal. A survey by Melmed et al showed that, among 146 IBD patients, only 41 (28%) had received an influenza vaccine and 13 (9%) reported being vaccinated against pneumococcal infection with a history of application of immunosuppressive agents. A lack of awareness (49%) and fear of side effects (18%) are the most common reasons for non-immunization with the influenza vaccine [17]. Malhi et al found that in Canada the rate of self-reported vaccinations among IBD patients is significantly low. The vaccination rates were reported as influenza 61.3%, pneumococcus 10.3%, HBV 61.0%, hepatitis A virus 52.0%, varicella 26.0%, meningococcus 20.7%, herpes zoster (HZ) 5.3%, and human papillomavirus (HPV) 11.0%. Among IBD patients, insufficient counseling by providers, ambiguity about indications and fears concerning vaccine safety are the most common reasons for non-uptake (22.0%, 20.7% and 5.3%, respectively) [7]. Additionally, physician uncertainties over whether vaccination is indicated in IBD patients and a lack of knowledge about immunizations on the part of providers have been reported [18,19]. Regrettably, data showed only 30% of family medicine specialists felt comfortable managing routine maintenance issues including immunization in the IBD patients, especially when they were immunocompromised [8,20].
Vaccination recommendations
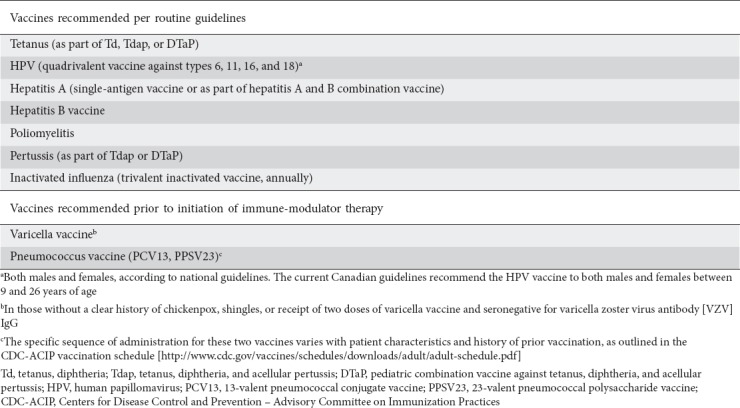
Current practice recommendations proposed by the second European evidence-based consensus for routine vaccinations in IBD patients are presented in (Table 4) [21].
Table 4.
Summary of current practice recommendations for routine vaccinations in patients with inflammatory bowel disease [21]
Influenza
All patients diagnosed with IBD should be immunized with the influenza vaccine yearly [22]. There are two forms of influenza vaccines: an inactivated form injected intramuscularly and intradermally, and a live form administered intranasally [3]. The inactivated influenza vaccine is safe to be given to patients on immunomodulators or biologic therapy. However, the live intranasal vaccination should be avoided in patients who are immunosuppressed [23].
DeBruyn et al showed in a randomized study that, in 137 patients with IBD, serologic protection against the influenza vaccine was reached by around 45-80% on maintenance infliximab therapy, varying by antigen. Essentially, vaccine timing relative to infliximab infusion did not affect the attainment of serologic protection and the influenza vaccine was well tolerated. Consequently, vaccination against influenza is recommended at any point throughout infliximab scheduling [24]. Cullen et al reported that, among 108 IBD patients taking the 2009 H1N1 influenza vaccine, the proportion with seroprotection was considerably lower among individuals on combination immunosuppression therapy compared to those not treated with immunosuppressive medications (36% vs. 64%, P=0.02) [25]. Additionally, Hiroko et al, in a prospective randomized controlled trial, found that booster doses of the trivalent influenza vaccine were not able to induce a significant immune response in adult IBD patients [26]. Importantly, data showed that the higher dose of influenza vaccine in persons 65 years of age or older triggered significantly higher antibody responses and offered better protection against laboratory-confirmed influenza disease [25].
Pneumococcal infection
Streptococcus pneumoniae is a pathological microorganism that can cause severe infections, such as pneumonia or meningitis [27]. A study in Denmark reported that, even before the diagnosis of IBD, this group of patients was prone to be infected with pneumococcal pneumonia, signifying that the existence of IBD increases the chance of infection [28,29]. In a retrospective cohort study performed among IBD patients who matched non-IBD individuals, the IBD group had a higher risk of developing pneumonia than did patients without IBD (incidence rate ratio [IRR] 1.82, 95%CI 1.75-1.88). It was shown that the use of biologic medications (OR 1.28, 95% CI1.08-1.52), steroids (OR 3.62, 95%CI 3.30-3.98) or proton pump inhibitors (OR 1.14, 95%CI 1.03-1.25) within 120 days was strongly related to pneumonia [30]. Therefore, the immunization status should be updated even before the initiation of immunosuppressive therapy [28,31].
Presently, there are two forms of pneumococcal vaccines available: 23-valent polysaccharide vaccine (PPSV23) and 13-valent conjugate vaccine (PCV13). According to a recommendation of the Advisory Committee on Immunization Practices (ACIP), all IBD patients should be vaccinated with both PCV13 and PPSV23. A single dose of PCV13 should be given to all IBD patients, followed by a dose of PPSV23 at least 8 weeks later in immunodeficient patients, or after 1 year in immunocompetent patients. A second dose of PPSV23 should be given 5 years after the first dose and needs to be repeated in adults aged 65 years or older. If IBD patients previously received the PPSV23 vaccine, a single dose of the PVC13 vaccine should be administered at least 1 year later, regardless of the patient’s immune status [32,33]. The recommendations of the European consensus on opportunistic infection in IBD patients are parallel to those in the USA, where all patients with IBD treated with immunosuppressive medications, and who have not previously been vaccinated with PCV13 or PPV23, should be given a single dose of PCV13, followed by a dose of PPV23 at least 8 weeks later. A second dose of PPV23 vaccine is suggested 5 years later. Patients who have previously received PPV23 should be vaccinated with a PCV13 dose 12 months after their last PPV23 [21].
HZ
Varicella zoster virus (VZV) is a worldwide pathogen that only infects humans. Primary infection by VZV leads to varicella (chickenpox) disorder and subsequently the virus remains dormant in the nervous system. Treatment with immunosuppressive agents can debilitate cell-medicated immunity to VZV, leading to its reactivation [34]. VZV infection risk is high for IBD patients [35,36]. A retrospective cohort study by Long et al showed that the IBD cohort had a higher risk of HZ infection compared with the general population (IRR 1.68, 95%CI 1.60-1.76) [37]. Moreover, in another study by Gupta et al, patients with ulcerative colitis and Crohn’s disease had a higher incidence of zoster infection compared with their matched controls. The investigators reported that the use of azathioprine/6-mercaptopurine medications (adjusted OR 3.1, 95%CI 1.7-5.6) in corticosteroid recipients (adjusted OR 1.5, 95%CI 1.1-2.2) was associated with a higher chance of developing shingles [38]. In line with previous studies, Cullen et al showed that VZV can be associated with a significant risk of morbidity and mortality in immunosuppressed patients, especially those under treatment with corticosteroids and combination immunosuppression, including methotrexate and azathioprine [39].
Immunosuppression increases the risk, but not all immunosuppression might carry the same risk, as vedolizumab has been reported to have a low incidence of serious infections [40]. Moreover, another study by Papp et al demonstrated that the rates of serious infection for infliximab and other biologics were significantly greater than that for ustekinumab [41].
The HZ vaccine is recommended in IBD patients aged 60 years and older, regardless of whether they have had a previous zoster episode. The vaccination is effective for reducing the incidence of HZ by 51% and post-herpetic neuralgia by 67% [42]. However, the zoster vaccine is contraindicated while IBD patients are on biological agents, given the fact that the zoster vaccine is a live, attenuated vaccine. Given the risk of developing HZ infections, this vaccine should be considered even in those managed with low-dose immunosuppression, such as low-dose prednisone (<20 mg/daily), 6-mercaptopurine (<1.5 mg/kg/day) or azathioprine (<2.5 mg/kg/day) [43]. Therefore, the vaccine needs to be given ≥3 weeks prior to the initiation of any immunosuppressant medication [44]. However, Khan et al showed that, in 59 patients treated with anti-TNF medication, of whom 12 (20%) were also using thiopurine, once they received the vaccine no case of HZ infection was seen within 0-42 days after its administration [45].
Varicella
It was suggested by the Centers for Disease Control and Prevention (CDC) that all individuals who lack evidence of VZV immunity by serology testing should receive two doses of varicella vaccine, given at least 4-8 weeks apart [46]. All IBD patients need to be screened for immunity to VZV at the time of diagnosis. Unimmunized and immunocompetent IBD patients should be offered vaccination with a 2-dose series of live varicella vaccine at least 3 weeks prior to the start of immunosuppressive therapy. Because varicella vaccine is a live virus vaccine, it should be administered in immunocompromised patients at least 3 months after the immunosuppressive treatment is discontinued [4,47]. In line with this, the European Crohn’s and Colitis Organisation suggests administering the live vaccine either 3 weeks prior to starting treatment or 3-6 months after discontinuation of immunosuppressive agents [21,48]. Lindsey et al showed that the HZ vaccination (Zostavax) was safe in patients with rheumatoid, psoriatic arthritis and spondyloarthropathies who were on infused biologics [49].
Because the inactivated zoster vaccine (ZVIN) is not a live vaccine, no issues should be expected when it is administered to immunocompromised patients. Parrino et al showed that, in patients with hematologic malignancies receiving anti-CD20 monoclonal antibodies, ZVIN was tolerated well and caused statistically significant VZV-specific T-cell immune responses [50]. Additionally, another study reported that, in patients with autoimmune diseases, ZVIN produced statistically significant immune responses [51].
The presence of VZV antibodies shows a prior infection with varicella and protection against this virus. VZV-specific antibody testing to measure immunity in those previously immunized with the varicella vaccine is not indorsed by the Advisory Committee on Immunization Practices [52]. As data showed, the reason for this is that the varicella vaccine produces lower VZV antigen-antibody concentration compared to natural immunity after varicella infection.
The recent commercial antibody, enzyme-linked immunosorbent assay (ELISA), is not sensitive enough to assess vaccine-induced VZV antibody levels in all patients, particularly those with a distant history of vaccination. Investigators from the CDC discovered that their ELISA, comparable with or more sensitive than commercial assays, had a 34% false-negative rate when compared with the glycoprotein ELISA developed by Merck [52,53].
Gastroenterologists need to be aware of the limitations of VZV serology using the commercially available immunoassays when they measure varicella immunity in those immunized. We need to rely more upon the patient’s history of immunization, rather than current commercial ELISA, to evaluate the immunity to varicella [54].
Tetanus, diphtheria, acellular pertussis
Diphtheria and tetanus have become uncommon infections in developed countries but outbreaks have occurred in former Soviet Republics [55]. As per ACIP recommendations, adults who did not receive primary vaccination, or who did not complete the primary series, should begin or complete the primary vaccination series with three doses of tetanus- and diphtheria-containing vaccines, one of which should be a Tdap dose. All IBD patients should be given the combined tetanus and diphtheria toxoids (Td) booster every 10 years, regardless of their immunosuppression status [56]. In 2014, 40,727 cases of pertussis were reported to The European Surveillance System (TESSy) by 29 countries of the European Union (EU) or European Economic Area (EEA) [57]. Additionally, in 2015, state health departments reported 20,762 cases of pertussis to the CDC [58,56]. Regardless of previous Tdap vaccination records, Tdap was recommended by ACIP for all pregnant women in the third trimester of their pregnancies [59]. The Tdap vaccine was recommended for all pregnant women in the United Kingdom and Ireland (Table 5) [60,61]. Dezfoli et al, in a controlled trial, evaluated the immunogenicity of the Tdap vaccine and found that, regardless of immunosuppressive regimen, patients have a normal booster response. They suggested patients with IBD should be vaccinated with Tdap prior to starting immunomodulators, especially once combination therapy with anti-TNF is started [62]. Additionally, in a cross-sectional study, Caldera et al showed that IBD patients on combination therapy or biological monotherapy had lower sustained pertussis antibody concentration [63]. Brogan et al suggested that patients with IBD had significantly impaired in vitro production of anti-tetanus toxoid antibody during an 8-day pokeweed mitogen-stimulated culture period. They reported that their results indicated many IBD patients have an impaired humoral immune response to tetanus toxoid booster immunization. This impaired immune response may be due to an inability to generate B cell precursors of anti-tetanus toxoid IgG-producing B cells, rather than to abnormal circulating helper or suppressor T-cell activity or natural killer cell regulatory activity [64].
Table 5.
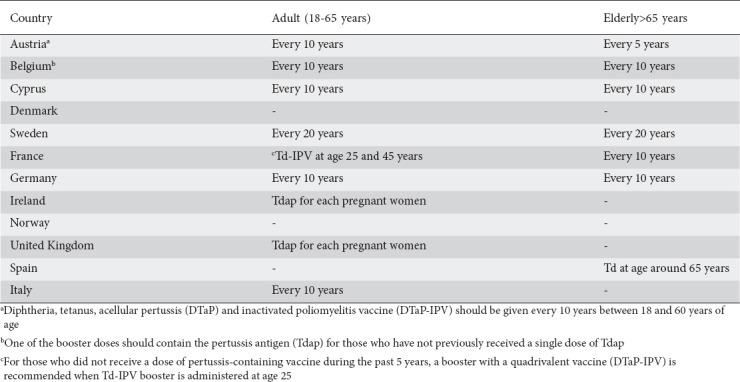
Measles, mumps, rubella (MMR)
According to The Regional Verification Commission for Measles and Rubella Elimination at the WHO Regional Office for Europe, measles elimination was not reached in 14 of the 53 member states (26%) of the WHO European Region at the end of 2015 [65]. In January-February 2017, 10 EU/EEA countries reported more than double the number of cases compared to the same period in 2016 [66].
The effectiveness of the measles-component of the MMR vaccine was reported as 95-98% after a single dose and more than 99% following the second dose of the vaccine [67]. Similarly to measles protection, the MMR vaccine achieves more than 95% seroprotection against rubella after 1 dose and more than 99% following 2 doses [67,68]. However, the potency of the MMR vaccine against mumps is not as effective as against measles and rubella: seroconversion for mumps is 64-95% with 1 dose of vaccine and 88-95% following 2 doses [67,68].
Cleveland et al, in a prospective study, showed that a significant number of IBD patients lack immunity to measles [69]. The MMR vaccine is only available as a live, attenuated vaccine. Therefore, immunocompromised IBD patients should not be vaccinated with MMR [70]. Consequently, this vaccine should be administered at least 1 month before the start of immunosuppressive agents [71]. The MMR vaccine can be given safely to the household or close contacts of an immunocompromised IBD patient [67].
HPV
The International Agency for Research on Cancer (IARC) has shown proof of a strong association between HPV and cancer sites such as anus and cervix [72]. HPV might be accountable for other malignancies, including cancer of the esophagus, oral cavity and lip. However, a causal role for HPV has not been recognized [73]. The incidence of HPV-related cancers is typically higher in immunosuppressed patients [74]. Shah et al demonstrated that there was a trend toward abnormal anal Papanicolaou in IBD subjects compared with a healthy control. There was no difference based on immunosuppression [75].
The quadrivalent HPV (qHPV) vaccine has been proven to prevent vaccine-related persistent anal HPV infections in addition to anal intraepithelial neoplasia [76]. A handful of studies have examined the prevalence of cervical dysplasia in IBD patients. Jacobson et al reported that IBD patients aged 9-26 on immunosuppressive therapy showed a proper immune response with 100% seroconversion to the HPV4 quadrivalent vaccine (against HPV types 16, 18, 6, and 11). They did not find any serious side-effects or worsening of disease activity due to the HPV4 vaccine [77]. Allegretti at al showed that IBD patients on immunosuppressive medication have a higher risk of high-grade cervical dysplasia and cervical cancer (OR 1.34, 95%CI 1.23-1.46) compared with the general population [78]. Likewise, Singh et al documented that combination therapy with steroids and immunosuppressive agents can increase the risk of cervical abnormalities (OR 1.46, 95%CI 1.09-1.81) compared with IBD patients who are not on these medications [79]. The recommendation for the HPV vaccination schedules was updated by the CDC in October 2016. It was advised 2 doses of HPV vaccine for individuals younger than 15 years and 3 doses of HPV vaccine series for those aged 15 or older and have certain immunodeficiency conditions. The CDC continues to recommend routine HPV vaccination series for girls and boys aged 11 or 12 years. For immunocompromised patients aged 9 to 26 years, 3 doses of HPV vaccine (0, 1-2, 6 months) are recommended. The HPV vaccination series can be started at age 9 years and is also suggested for women through age 26 years and men through age 21 years. Individuals whose immune responses might be insufficient or lower (because of HIV infection, malignancy, autoimmune disorder, or use of immunosuppressant medications) should receive 3 doses to ensure they receive the most benefit [80]. In Europe there are two vaccines (Cervarix© and Gardasil©) against HPV authorized centrally by the European Medicines Agency. Cervarix is itemized to be administered with the reduced schedule in girls aged 9-14 years and Gardasil received positive feedback for use in 9-13-year-old adolescent girls and boys [81].
IBD vaccination and travel
All IBD patients who plan to travel overseas need to check what specific vaccinations they need according to where they are planning to travel [82]. It is important that they discuss their travel plans with a traveler’s clinic beforehand and to go over the required vaccinations. They should familiarize themselves with the endemic infections of the specific areas they plan to visit by reviewing the traveler’s health information from the World Health Organization [83]. Yellow fever is one of the biggest challenges for IBD patients who plan to visit South America and sub-Saharan Africa. Unfortunately, the yellow fever vaccine is a live vaccine and should be avoided in drug-induced immunocompromised IBD patients [82]. The yellow fever vaccine can be given to this group of patients if immunosuppressive medications are discontinued for at least 4 months before vaccination [23,84]. Otherwise, if their immunosuppressive medications cannot be terminated because of medical necessity, they need to be instructed against visiting areas where yellow fever is endemic [23]. Enteric fever is another serious disease that IBD patients should be concerned about if they plan to travel to the Indian subcontinent. Accordingly, all patients with IBD need to be vaccinated with the parenteral inactive typhoid vaccine (Vi vaccine) before they travel [85]. Rabies is a fatal disease that is broadly distributed throughout the world and cell-culture–derived vaccines are available for use by IBD patients traveling to high risk areas such as Africa, Asia and Latin America [86,87]. Regarding HBV, the immune status needs to be screened if there is intention to visit areas where HBV is endemic, such as Africa, China and Southeast Asia. HBV booster should be offered to those who are immunocompromised and whose immune titers are less than 10 mIU/mL [88].
Viral meningoencephalitis is mostly caused by Japanese encephalitis (JE) in large parts of Asia [89]. Patients on anti-TNF therapy or dealing with a chronic medical condition may qualify for the JE vaccine [18]. An inactivated Japanese encephalitis vaccine (IXIARO®) has been approved in Europe and the United States and can be safely offered to IBD patients [82,90,91].
Concluding remarks
Proper immunizations are an essential part of medical management in IBD patients [20]. Because immunomodulators are used to treat IBD patients, these patients are susceptible to infection, with a high rate of morbidity and mortality [92,93]. Therefore, this group of patients should be immunized prophylactically against these infections [94], preferably upon initial presentation and once the start of immunosuppressive agents is planned [95]. Given the increased risk of vaccine-related infections, live vaccines should be avoided in immunocompromised patients. Importantly, the majority of immunocompromised patients exhibit a proper and sufficient seroconversion once they are vaccinated [3].
However, vaccination rates for these preventable diseases continue to be suboptimal in the face of a decade of research proving that IBD patients are at an increased risk of vaccine-preventable infections [96]. Prior data have shown that recommendations from physicians are the most important factor for receiving preventative health services such as vaccination and screening for cancer [97]. As per previous data, in the majority of IBD patients the screening test for HBV serology was missed by their gastroenterologists. This suggests that providers may not be effectively instructed and do not regularly recommend screening for HBV and vaccination for their IBD patients, whether they are on or off immunosuppressive medications [98]. Vaccination assessments yearly, and prior to initiation of treatment with immunosuppressive agents, were important predictors of vaccination completion [7].
In conclusion, taking care of patients with IBD often includes making complex medical decisions. Gastroenterologists are usually the primary providers for patients with IBD; consequently, it is critical to have a broad knowledge of the issues surrounding the administration of vaccines to patients with IBD. The vaccination recommendation should be tailored to each patient, taking into account his/her age, comorbidities, nutritional status, IBD disease severity, immunosuppressive therapy, risk of exposure to pathogens and geographic clustering. Moreover, vaccination should not delay urgent medical therapy and gastroenterologists should involve infectious specialists when they face a challenging situation not addressed by guidelines.
Biography
Banner University Medical Center, University of Arizona, Tucson; MedStar Georgetown University Hospital, Washington, DC, USA
Footnotes
Conflict of Interest: None
References
- 1.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation:inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013;62:1523–1528. doi: 10.1136/gutjnl-2013-305300. [DOI] [PubMed] [Google Scholar]
- 3.Chaudrey K, Salvaggio M, Ahmed A, Mahmood S, Ali T. Updates in vaccination:recommendations for adult inflammatory bowel disease patients. World J Gastroenterol. 2015;21:3184–3196. doi: 10.3748/wjg.v21.i11.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677–692. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations:a meta-analysis. Dig Dis Sci. 2015;60:2446–2453. doi: 10.1007/s10620-015-3631-y. [DOI] [PubMed] [Google Scholar]
- 6.Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis. 2009;15:1410–1416. doi: 10.1002/ibd.20943. [DOI] [PubMed] [Google Scholar]
- 7.Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients:attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439–444. doi: 10.1093/ecco-jcc/jjv064. [DOI] [PubMed] [Google Scholar]
- 8.Selby L, Hoellein A, Wilson JF. Are primary care providers uncomfortable providing routine preventive care for inflammatory bowel disease patients? Dig Dis Sci. 2011;56:819–824. doi: 10.1007/s10620-010-1329-8. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Tembleque MD, Corella C, Pérez-Calle JL. Vaccines and recommendations for their use in inflammatory bowel disease. World J Gastroenterol. 2013;19:1354–1358. doi: 10.3748/wjg.v19.i9.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 11.Andrisani G, Armuzzi A, Marzo M, et al. What is the best way to manage screening for infections and vaccination of inflammatory bowel disease patients? World J Gastrointest Pharmacol Ther. 2016;7:387–396. doi: 10.4292/wjgpt.v7.i3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Liu XQ, Qin L. Treatment strategy of inflammatory bowel disease associated with viral hepatitis. Chin J Gastroenterol Hepatol. 2016;25:1091–1093. [Google Scholar]
- 14.Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China:a retrospective study. J Crohns Colitis. 2014;8:282–287. doi: 10.1016/j.crohns.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Papa A, Felice C, Marzo M, et al. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-αagents. J Crohns Colitis. 2013;7:113–119. doi: 10.1016/j.crohns.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Macrae FA, Gibson PR. Vaccination and screening for infections in patients with inflammatory bowel disease:a survey of Australian gastroenterologists. Intern Med J. 2011;41:462–467. doi: 10.1111/j.1445-5994.2009.02114.x. [DOI] [PubMed] [Google Scholar]
- 19.Jung YS, Park JH, Kim HJ, et al. Insufficient knowledge of Korean gastroenterologists regarding the vaccination of patients with inflammatory bowel disease. Gut Liver. 2014;8:242–247. doi: 10.5009/gnl.2014.8.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasan SK, Calderwood AH, Long MD, Kappelman MD, Sandler RS, Farraye FA. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease:an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246–250. doi: 10.1097/01.MIB.0000437737.68841.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahier JF, Magro F, Abreu C, et al. European Crohn's and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Harmandar F, Çekin AH. Preventive care in inflammatory bowel disease. Turk J Gastroenterol. 2017;28:307–310. doi: 10.5152/tjg.2017.190617. [DOI] [PubMed] [Google Scholar]
- 23.Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2016;12:540–546. [PMC free article] [PubMed] [Google Scholar]
- 24.deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance Infliximab therapy:a randomized trial. Inflamm Bowel Dis. 2016;22:638–647. doi: 10.1097/MIB.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 25.Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. doi: 10.1136/gutjnl-2011-300256. [DOI] [PubMed] [Google Scholar]
- 26.Hiroko M, Watanabe K, Morimoto K, et al. Booster doses of the trivalent influenza vaccine do not elicit a significant immune response in patients with inflammatory bowel disease:a prospective randomized controlled trial. Gastroenterology. 2014;146(Suppl 1):S–80. [Google Scholar]
- 27.Marín AC, Gisbert JP, Chaparro M. Immunogenicity and mechanisms impairing the response to vaccines in inflammatory bowel disease. World J Gastroenterol. 2015;21:11273–11281. doi: 10.3748/wjg.v21.i40.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farraye FA. Vaccination of patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2017;13:431–434. [PMC free article] [PubMed] [Google Scholar]
- 29.Kantsø B, Simonsen J, Hoffmann S, Valentiner-Branth P, Petersen AM, Jess T. Inflammatory bowel disease patients are at increased risk of invasive pneumococcal disease:a Nationwide Danish Cohort Study 1977-2013. Am J Gastroenterol. 2015;110:1582–1587. doi: 10.1038/ajg.2015.284. [DOI] [PubMed] [Google Scholar]
- 30.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240–248. doi: 10.1038/ajg.2012.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Bousvaros A. Immunizations in children with inflammatory bowel disease treated with immunosuppressive therapy. Gastroenterol Hepatol (N Y) 2014;10:355–363. [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions:recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–819. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions:recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2015;62:521–524. [PMC free article] [PubMed] [Google Scholar]
- 34.Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol. 2013;15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease:a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 36.Ma C, Walters B, Fedorak RN. Varicella zoster meningitis complicating combined anti-tumor necrosis factor and corticosteroid therapy in Crohn's disease. World J Gastroenterol. 2013;19:3347–3351. doi: 10.3748/wjg.v19.i21.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420–429. doi: 10.1111/apt.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–1490. doi: 10.1016/j.cgh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2392–2403. doi: 10.1002/ibd.22950. [DOI] [PubMed] [Google Scholar]
- 40.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839–851. doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR) J Drugs Dermatol. 2015;14:706–714. [PubMed] [Google Scholar]
- 42.Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 43.Harpaz R, Ortega-Sanchez IR, Seward JF Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster:recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 44.Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99. doi: 10.2147/DHPS.S28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan N, Shah Y, Trivedi C, Lewis JD. Safety of herpes zoster vaccination among inflammatory bowel disease patients being treated with anti-TNF medications. Aliment Pharmacol Ther. 2017;46:668–672. doi: 10.1111/apt.14257. [DOI] [PubMed] [Google Scholar]
- 46.Kim DK, Riley LE, Hunter P. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older –United States. MMWR Morb Mortal Wkly Rep. 2018;67:158–160. doi: 10.15585/mmwr.mm6705e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahier JF, Moutschen M, Van Gompel A, et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford) 2010;49:1815–1827. doi: 10.1093/rheumatology/keq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Côté-Daigneault J, Peerani F, MacMahon E, Delaporte E, Rahier JF, Colombel JF. Management and prevention of Herpes Zoster in the immunocompromised inflammatory bowel disease patient:a clinical quandary. Inflamm Bowel Dis. 2016;22:2538–2547. doi: 10.1097/MIB.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 49.Lindsey S, Oufnac B, Walker H. Safety of Zoster vaccination administration in rheumatic patients on current biologic therapy. ACR/ARHP Annual Meeting. 2014;1836 [Google Scholar]
- 50.Parrino J, McNeil SA, Lawrence SJ, et al. Safety and immunogenicity of inactivated varicella-zoster virus vaccine in adults with hematologic malignancies receiving treatment with anti-CD20 monoclonal antibodies. Vaccine. 2017;35:1764–1769. doi: 10.1016/j.vaccine.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 51.Eberhardson M, Hall S, Papp KA, et al. Safety and immunogenicity of inactivated Varicella-Zoster virus vaccine in adults with autoimmune disease:a phase 2, randomized, double-blind, placebo-controlled clinical trial. Clin Infect Dis. 2017;65:1174–1182. doi: 10.1093/cid/cix484. [DOI] [PubMed] [Google Scholar]
- 52.Marin M, Güris D, Chaves SS, Schmid S, Seward JF Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC) Prevention of varicella. Recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 53.Caldera F, Wasan SK, Farraye FA, Hayney MS. Caution when assessing immunity to varicella through antibody testing in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:E50–E51. doi: 10.1097/MIB.0000000000001239. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Ye X, Jia J, et al. Serological evaluation of immunity to the varicella-zoster virus based on a novel competitive enzyme-linked immunosorbent assay. Sci Rep. 2016;6:20577. doi: 10.1038/srep20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golaz A, Hardy IR, Strebel P, et al. Epidemic diphtheria in the newly independent states of the former Soviet Union:implications for diphtheria control in the United States. J Infect Dis. 2000;181(Suppl 1):S237–S243. doi: 10.1086/315569. [DOI] [PubMed] [Google Scholar]
- 56.Kim DK, Bridges CB, Harriman KH. Advisory Committee on Immunization Practices (ACIP):Advisory Committee on Immunization Practices Recommended Immunization schedule for adults aged 19 years or older – United States 2016. MMWR Recomm Rep. 2016;65:88–90. doi: 10.15585/mmwr.mm6504a5. [DOI] [PubMed] [Google Scholar]
- 57.European Centre for Disease Prevention and Control. Annual Epidemiological Report 2016 –Pertussis. 2016. [[Accessed January 1, 2019]]. https://ecdc.europa.eu/sites/portal/files/documents/AER-for-2016-pertussis.pdf .
- 58.Centers for Disease Control and Prevention (CDC) Pertussis (whooping cough):surveillance and reporting. [[Accessed January 1, 2019]]. https://www.cdc.gov/pertussis/outbreaks/trends.html .
- 59.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–135. [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. WHO vaccine-preventable diseases:monitoring system:2016 global summary. Geneva: World Health Organization; 2016. [[Accessed January 1, 2019]]. http://apps.who.int/immunization_monitoring/globalsummary . [Google Scholar]
- 61.European Centre for Disease Prevention and Control Vaccine; European Centre for Disease Prevention and Control. 2016. [[Accessed January 1, 2019]]. http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx .
- 62.Dezfoli S, Horton HA, Thepyasuwan N, et al. Combined immunosuppression impairs immunogenicity to tetanus and pertussis vaccination among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1754–1760. doi: 10.1097/MIB.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 63.Caldera F, Saha S, Wald A, et al. Lower sustained diphtheria and pertussis antibody concentrations in inflammatory bowel disease patients. Dig Dis Sci. 2018;63:1532–1540. doi: 10.1007/s10620-018-5043-2. [DOI] [PubMed] [Google Scholar]
- 64.Brogan MD, Shanahan F, Oliver M, Stevens RH, Targan SR. Defective memory B cell formation in patients with inflammatory bowel disease following tetanus toxoid booster immunization. J Clin Lab Immunol. 1987;24:69–74. [PubMed] [Google Scholar]
- 65.World Health Organization (WHO) Regional Office for Europe. 5thMeeting of the European Regional Verification Commission for Measles and Rubella Elimination (RVC) Copenhagen: WHO/Europe; 2016. [[Accessed January 1, 2019]]. http://www.euro.who.int/__data/assets/pdf_file/0005/330917/5th-RVC-meeting-report.pdf?ua=1 . [Google Scholar]
- 66.European Centre for Disease Prevention and Control (ECDC) Monthly measles epidemiological updates. Stockholm: ECDC; [[Accessed January 1, 2019]]. http://ecdc.europa.eu/en/healthtopics/measles/epidemiological_data/Pages/measles_surveillance_reports.aspx . [Google Scholar]
- 67.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps:recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47:1–57. [PubMed] [Google Scholar]
- 68.White SJ, Boldt KL, Holditch SJ, Poland GA, Jacobson RM. Measles, mumps, and rubella. Clin Obstet Gynecol. 2012;55:550–559. doi: 10.1097/GRF.0b013e31824df256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cleveland NK, Rodriquez D, Wichman A, Pan I, Melmed GY, Rubin DT. Many inflammatory bowel disease patients are not immune to measles or pertussis. Dig Dis Sci. 2016;61:2972–2976. doi: 10.1007/s10620-016-4275-2. [DOI] [PubMed] [Google Scholar]
- 70.Kotton CN. Vaccines and inflammatory bowel disease. Dig Dis. 2010;28:525–535. doi: 10.1159/000320412. [DOI] [PubMed] [Google Scholar]
- 71.Wasan SK, Baker SE, Skolnik PR, Farraye FA. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol. 2010;105:1231–1238. doi: 10.1038/ajg.2009.733. [DOI] [PubMed] [Google Scholar]
- 72.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 73.Chaturvedi AK. Beyond cervical cancer:burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46:S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Bucchi D, Stracci F, Buonora N, Masanotti G. Human papillomavirus and gastrointestinal cancer:A review. World J Gastroenterol. 2016;22:7415–7430. doi: 10.3748/wjg.v22.i33.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah SB, Pickham D, Araya H, et al. Prevalence of anal dysplasia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:1955–1961. e1. doi: 10.1016/j.cgh.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 76.Stier EA, Chigurupati NL, Fung L. Prophylactic HPV vaccination and anal cancer. Hum Vaccin Immunother. 2016;12:1348–1351. doi: 10.1080/21645515.2016.1149274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobson DL, Bousvaros A, Ashworth L, et al. Immunogenicity and tolerability to human papillomavirus-like particle vaccine in girls and young women with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1441–1449. doi: 10.1097/MIB.0b013e318281341b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer?A meta-analysis. Inflamm Bowel Dis. 2015;21:1089–1097. doi: 10.1097/MIB.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh H, Demers AA, Nugent Z, Mahmud SM, Kliewer EV, Bernstein CN. Risk of cervical abnormalities in women with inflammatory bowel disease:a population-based nested case-control study. Gastroenterology. 2009;136:451–458. doi: 10.1053/j.gastro.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years –United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;66:874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. https://www.ema.europa.eu/en/medicines/human/referrals/human-papillomavirus-vaccines-cervarix-gardasil-gardasil-9-silgard .
- 82.Esteve M, Loras C, García-Planella E. Inflammatory bowel disease in travelers:choosing the right vaccines and check-ups. World J Gastroenterol. 2011;17:2708–2714. doi: 10.3748/wjg.v17.i22.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.International travel and health:traveller vaccinations. World Health Organization; 2016. [[Accessed January 1, 2019]]. http://www.who.int/ith/updates/20110427/en/ [Google Scholar]
- 84.Ekenberg C, Friis-Møller N, Ulstrup T, Aalykke C. Inadvertent yellow fever vaccination of a patient with Crohn's disease treated with infliximab and methotrexate. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-215403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitaker JA, Franco-Paredes C, del Rio C, Edupuganti S. Rethinking typhoid fever vaccines:implications for travelers and people living in highly endemic areas. J Travel Med. 2009;16:46–52. doi: 10.1111/j.1708-8305.2008.00273.x. [DOI] [PubMed] [Google Scholar]
- 86.Meslin FX. Rabies as a traveler's risk, especially in high-endemicity areas. J Travel Med. 2005;12(Suppl 1):S30–S40. doi: 10.2310/7060.2005.12055. [DOI] [PubMed] [Google Scholar]
- 87.Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease:prevention and diagnosis. Gut. 2008;57:549–558. doi: 10.1136/gut.2006.114660. [DOI] [PubMed] [Google Scholar]
- 88.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B:a historical overview. Vaccine. 2008;26:6266–6273. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 89.Buhl MR, Lindquist L. Japanese encephalitis in travelers:review of cases and seasonal risk. J Travel Med. 2009;16:217–219. doi: 10.1111/j.1708-8305.2009.00333.x. [DOI] [PubMed] [Google Scholar]
- 90.Burchard GD, Caumes E, Connor BA, et al. Expert opinion on vaccination of travelers against Japanese encephalitis. J Travel Med. 2009;16:204–216. doi: 10.1111/j.1708-8305.2009.00330.x. [DOI] [PubMed] [Google Scholar]
- 91.Firbas C, Jilma B. Product review on the JE vaccine IXIARO. Hum Vaccin Immunother. 2015;11:411–420. doi: 10.4161/21645515.2014.983412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease:more than 5 years of follow-up in the TREAT™registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107–112. doi: 10.1016/j.crohns.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 94.Narula N, Yamamura DL, Marshall JK. Should my patient with inflammatory bowel disease on immunosuppressive therapy be vaccinated against influenza virus? Can J Gastroenterol. 2010;24:121–125. doi: 10.1155/2010/375878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim SB, Park SJ, Chung SH, et al. Vaccination and complementary and alternative medicine in patients with inflammatory bowel disease. Intest Res. 2014;12:124–130. doi: 10.5217/ir.2014.12.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pham HV, Hasan I, Udaltsova N, et al. Rates and predictors of vaccinations among inflammatory bowel disease patients receiving anti-tumor necrosis factor agents. Dig Dis Sci. 2018;63:209–217. doi: 10.1007/s10620-017-4716-6. [DOI] [PubMed] [Google Scholar]
- 97.Blewett LA, Johnson PJ, Lee B, Scal PB. When a usual source of care and usual provider matter:adult prevention and screening services. J Gen Intern Med. 2008;23:1354–1360. doi: 10.1007/s11606-008-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ben Musa R, Gampa A, Basu S, et al. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:15358–15366. doi: 10.3748/wjg.v20.i41.15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gandhi RT, Wurcel A, Lee H, et al. Isolated antibody to hepatitis B core antigen in human immunodeficiency virus type-1-infected individuals. Clin Infect Dis. 2003;36:1602–1605. doi: 10.1086/375084. [DOI] [PubMed] [Google Scholar]
- 100.Rahier JF, Ben-Horin S, Chowers Y, et al. European Crohn's and Colitis Organisation (ECCO) European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131:Screening for cervical cancer. Obstet Gynecol. 2012;120:1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 102.Centers for Disease Control and Prevention (CDC) Contraindications and precautions for polio vaccination. [[Accessed January 1, 2019]]. https://www.cdc.gov/vaccines/vpd/polio/hcp/contraindications-precautions.html .
- 103.Staples JE, Gershman M, Fischer M Centers for Disease Control and Prevention (CDC) Yellow fever vaccine:recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 104.Staples JE, Bocchini JA, Jr, Rubin L, Fischer M Centers for Disease Control and Prevention. Yellow fever vaccine booster doses:recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:647–650. [PMC free article] [PubMed] [Google Scholar]
- 105.Petersen BW, Damon IK, Pertowski CA, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep. 2015;64:1–26. [PubMed] [Google Scholar]
- 106.World Health Organization. Immunization, vaccines and biologicals:Contraindications. [[Accessed January 1, 2019]]. http://www.who.int/immunization/policy/contraindications.pdf .
- 107.Jackson BR, Iqbal S, Mahon B Centers for Disease Control and Prevention (CDC) Updated recommendations for the use of typhoid vaccine—Advisory Committee on Immunization Practices, United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:305–308. [PMC free article] [PubMed] [Google Scholar]


