Abstract
The Nrf2 signal transduction pathway plays a major role in adaptive responses to oxidative stress and in maintaining adaptive homeostasis, yet Nrf2 signaling undergoes a significant age-dependent decline that is still poorly understood. We used mouse embryonic fibroblasts (MEFs) cultured under hyperoxic conditions of 40% O2, as a model of accelerated ageing. Hyperoxia increased baseline levels of Nrf2 and multiple transcriptional targets (20S Proteasome, Immunoproteasome, Lon protease, NQO1, and HO-1), but resulted in loss of cellular ability to adapt to signaling levels (1.0 μM) of H2O2. In contrast, MEFs cultured at physiologically relevant conditions of 5% O2 exhibited a transient induction of Nrf2 Phase II target genes and stress-protective enzymes (the Lon protease and OXR1) following H2O2 treatment. Importantly, all of these effects have been seen in older cells and organisms. Levels of Two major Nrf2 inhibitors, Bach1 and c-Myc, were strongly elevated by hyperoxia and appeared to exert a ceiling on Nrf2 signaling. Bach1 and c-Myc also increase during ageing and may thus be the mechanism by which adaptive homeostasis is compromised with age.
Keywords: Adaptive homeostasis, Hyperoxia, Nrf2, 20S proteasome, Immunoproteasome, NQO1, Bach1, c-Myc, Lon protease, OXR1
1. Introduction
Cells, tissues, and organisms must continually adapt to ever-changing environmental conditions. One approach that enables successful coping has been described as ‘Adaptive Homeostasis:’ “The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events, or the removal or cessation of such molecules or events.” [1], In practice, this means increasing the transcription and/or translation of stress-protective genes after a suitable signal has been received which, in turn, leads to a temporary or transient increase in stress-resistance until the signal-response is turned off and the homeostatic range of stress-resistance contracts back to baseline levels. Previous work has demonstrated in yeast [2], mammalian cells [[3], [4], [5], [6], [7], [8], [9], [10], [11]] and model organisms [[12], [13], [14], [15], [16], [17]] that short-term, very low and non-damaging (i.e. signaling) doses of oxidants such as hydrogen peroxide (H2O2) are capable of inducing an array of stress-protective cellular defense pathways, necessary for an organism to cope with a subsequent oxidative insult.
Adaptive Homeostasis is sometimes confused with ‘Hormesis’ but significant differences exist. Simply put, Hormesis proposes that a small amount of subcellular damage results in an overcompensation of repair mechanisms, that increase stress-resistance [[18], [19], [20]]. In contrast, Adaptive Homeostasis is not a damage/repair process at all, but rather results from the specific and selective activation of intracellular signal-transduction pathways in response to extremely low and non-damaging levels of signaling agents such as H2O2 [1,21]. Of course, transient adaptation can also occur at much higher levels of ‘signaling agents’ that are actually toxic, but such adaptation is greatly diminished as a result of the accompanying toxicity [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,21]. Instead of a toxicological concept, Adaptive Homeostasis should be considered as a physiological processes in which the elasticity of the homeostatic range is continually utilized to transiently expand and contract our ability to cope with ever changing internal and external environmental conditions in real time.
A key component of the adaptive homeostatic response is the nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which is a crucial transcriptional regulator that binds to nuclear DNA Electrophile Response Elements (EpRE's) [also called Antioxidant Response Elements (AREs)] and is necessary in the activation of Phase II detoxification and stress-protective enzymes. Nrf2-target enzymes include the 20S proteasome [10], NAD(P)H:quinone oxidoreductase 1 (NQO-1) [22], heme oxygenase-1 [23], glutathione S-transferases [24,25] and the two subunits of gamma-glutamylcysteine ligase: GCLC/GCLM [26]. Because the adaptive response is transient, multiple Nrf2-transcriptional competitors ensure negative regulation, including Bach1 [27], which competes with Nrf2 through binding to the EpRE/ARE and inhibiting its transcriptional activity, and c-Myc which binds to Nrf2 and both inhibits transcription and promotes Nrf2 degradation [28]. Similarly, the less well-known Oxidation Resistance 1 gene (OXR-1) is a cell-protective element shown to be induced in neurons by oxidative stress [29]. Deletion of OXR1 in several human cell lines, leads to increased sensitivity to H2O2 induced stress and decreased mtDNA stability [30].
The adaptive response in proteolytic capacity is largely due to the adaptive capacity of the Proteasome and the mitochondrial Lon protease. The Proteasome is the major proteinase responsible for maintaining intracellular protein homeostasis (‘proteostasis’). The Proteasome degrades the bulk of oxidatively damaged proteins in the cytoplasm, nucleus, and endoplasmic reticulum [31]. Whereas, the Lon protease degrades oxidized mitochondrial proteins, including aconitase [32,33]. In response to an appropriate adaptive signal, the Lon protease [13] and both the 20S ‘core’ Proteasome and the Immunoproteasome exhibit large increases in synthesis [34]. Importantly, the 20S Proteasome and the Immunoproteasome are the forms of the enzyme most effective in selectively targeting oxidized proteins [35]. Though of major significance in normal protein turnover, the ubiquitin-ATP-dependent 26S Proteasome is actually very poor at degrading oxidized proteins [36], and is itself, sensitive to oxidative stress. Following an oxidative signal, the 26S Proteasome undergoes a conformational change, wherein the highly oxidant-sensitive 19S regulatory caps are removed, thus ‘freeing’ the 20S Proteasome [31,37,38]. As a result, Nrf2 is no longer degraded, its concentration rapidly increases and, following phosphorylation, it undergoes nuclear translocation. Once in the nucleus, Nrf2 binds to EpRE/ARE elements in the upstream regulatory regions of a large number of stress-protective genes, including the 20S Proteasome subunits [39]. The adaptive increase in the 20S Proteasome has been shown to contribute to increased fitness and stress-resistance, while its loss is associated with decreased survival [34].
We have proposed that the age-related decline in Adaptive Homeostasis is an underlying factor behind many age-related diseases and ailments [14,[40], [41], [42]]. Inability to transiently modulate various protective enzymes, results in a feedforward mechanism, wherein decreased Proteasome activity results in further accumulation of damaged protein aggregates, which further inhibits Proteasome activity [43]. Cytosolic protein aggregates are also transported into the mitochondria [44] where they are degraded by the Lon protease [45]. However, both the Proteasome and the Lon protease exhibit a loss of function with ageing [46,47], which may further contribute to the exponential accumulation of oxidatively damaged proteins in the last third of life [48].
Although the air we breathe typically contains some 21% oxygen, only cells in the upper airways and the eyes experience high O2 concentrations. For cells in most of our tissues and organs physiological ‘normoxia’ is much closer to 3–5% O2. When mammalian cells are cultured under ambient ‘room air’ conditions they attempt to adapt to the toxic conditions and many develop aberrant phenotypes. For example, culturing cells at ambient atmospheric O2 has been shown to significantly alter immune cells response to stimuli [49], decrease survival and proliferation of neurons [50], and affect structure and function of mitochondria [51]. At 21% O2, A549 cells show increased tolerance to CuO nanoparticles, due to elevated expression of antioxidant genes, in comparison with cells cultured at 13% O2 [52]. Post-mitotic cells, chronically exposed to hyperoxic conditions show increased vulnerability to cell death and reduced proteolysis [53].
To further explore the age-related changes in the Nrf2-mediated adaptive homeostatic response, we utilized different oxygen (O2) concentrations as a model for accelerated ageing. Previously, we have reported that culturing mammalian cells at 40% O2 results in an ‘accelerated ageing phenotype’ compared with cells cultured at 21% O2 activity [43,53]. In addition, we have found evidence that growing cells in a 5% O2 environment is even more beneficial than culturing them at 21% O2. In the present study, using Mouse Embryonic Fibroblasts cells (MEFs), an adaptive response was elicited by using a non-damaging signaling dose of H2O2 to assess changes in the amounts of Nrf2, the extent of its cytosol to nuclear translocation, the levels of Nrf2 competitors such as Bach1 and c-Myc, and the levels of expression of Nrf2-target genes. In addition we have examined if a threshold in the Nrf2-mediated adaptive response occurs when cells are exposed to ‘accelerated aging’ by growth at 40% O2 (hyperoxia) versus 21% (atmospheric) or 5% O2 (normoxia). Our evidence indicates that culturing at normoxic conditions promotes the adaptive response, whereas ‘accelerated ageing’ by chronic exposure to hyperoxic conditions not only eliminates the adaptive response, but also shows evidence of a ‘ceiling effect’ indicative of a maximum threshold. Our results also suggest possible mechanisms by which Bach1 and c-Myc may abrogate adaptive homeostasis in ageing.
2. Results
2.1. H2O2 signaling does not induce adaptive homeostasis in MEFs cultured at 40% O2 that appear to be maximally adapted already
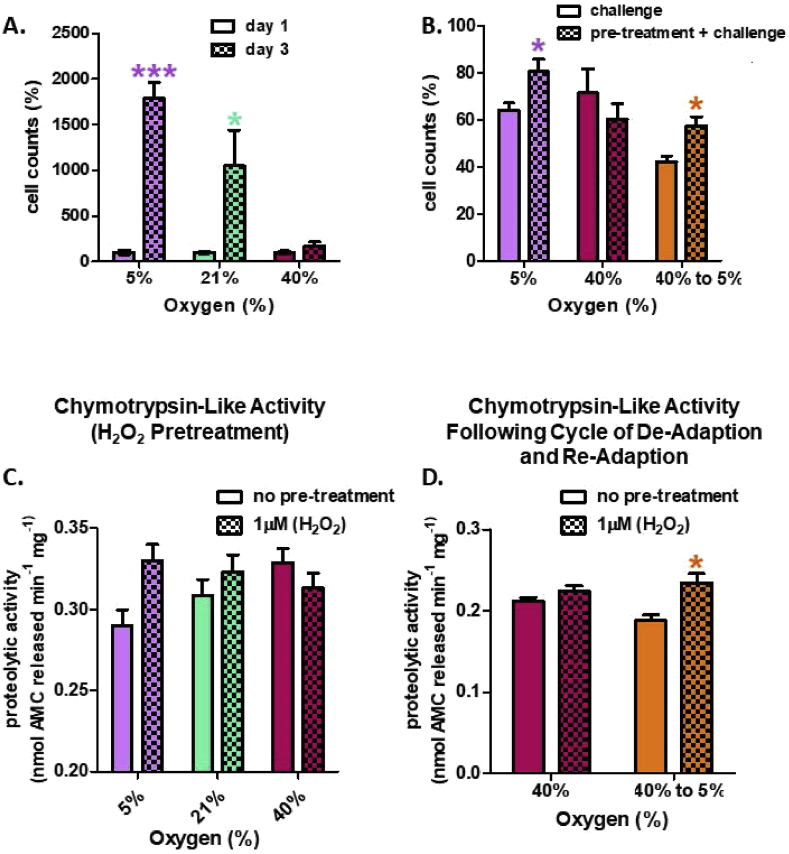
First, we determined the effect of oxygen tension on cell growth. Almost all cell culture studies are conducted at 21% (ambient) oxygen levels, but decreasing the oxygen tension to 5% (physiological) clearly had a very positive effect on cell growth, with an almost 40% increase in cell number after 3 days of growth (Fig. 2A). Growth was clearly attenuated at 40% O2 (hyperoxia), however, compared to either 21% (ambient) or 5% (physiological normoxia) presumably reflecting the increased levels of oxygen-induced stresses (Fig. 2A).
Fig. 2.
Cell Growth and Proteasomal Chymotrypsin-like Activity. (A) Growth rate is attenuated with increasing O2 culturing concentration. Cells were seeded at 1 × 105 in 6-well plates at either 5%, 21%, or 40% O2, with 3 replicates (n = 3) per oxygen concentration. After 3 days of incubation, cells were washed twice with PBS, detached with trypsin, and counted with a hemocytometer. (B) Pretreatment with 1.0 μM H2O2 (per 500,000 cells) results in a protective effect against a subsequent challenge dose of 3.0 mM H2O2 per 500,000 cells, in MEFs cultured at 5% O2 but not in MEFs cultured at 40% O2. Cells were either cultured at control conditions of 5% oxygen (5%), 40% oxygen for 2 weeks (40%), or cultured at 40% oxygen and then transferred back to 5% for 2 weeks to de-adapt the cell lines before assays. Cells were pre-exposed to 1.0 μM H2O2 (per 500,000 cells) in a final volume of 2.0 ml in 6 well plates, for 1 h, or used as controls, and then allowed to recover for 18 h before the challenge dose. The challenge dose was administered for 1 h and cells were allowed to recover for 24 h before cell counts were taken. (C) A signaling treatment of 1.0 μM H2O2 (per 500,000 cells) increases proteolytic capacity in MEF's cultured at 5% O2 but fails to increase proteolytic capacity in MEFs cultured at higher O2 concentrations. An oxidative signaling dose of 1.0 μM H2O2 was administered for 1 h and cells were allowed to recover for 18 h. (D) De-adaptation to hyperoxia allows restoration of H2O2 adaptive responses. Cells cultured at 40% O2 for 28 days were transferred back to 5% O2 for 21 days. Cells were either cultured in standard media or media with 1 μM H2O2 (final concentration) for 1 h and then harvested 18 h after. Chymotrypsin-like activity was measured. Data are expressed as means ± standard errors and statistically significant differences are indicated as * (p < 0.05), ** (p < 0.01), *** (p < 0.001).
To determine the survival advantage of the adaptive response, cells were challenged with a toxic dose of 3.0 mM hydrogen peroxide (H2O2). In cells cultured under physiologically relevant conditions of 5% O2 a pre-exposure to a 1.0 μM signaling dose of H2O2 was shown to be advantageous and increased survival, but had no impact on survival in cells continually cultured under hyperoxic conditions (40% O2) (Fig. 2B, first 4 bars). Cells grown at 21% ambient O2 exhibited a significantly smaller adaptive response that did cells cultured at 5% O2, but still exhibited greater adaptive survival than did cells grown at 40% O2 (not shown).
Studies in yeast [2] and mammalian cells [11,54,55] demonstrate the temporal reversibility of the adaptive response. Here, we explored whether the adaptive response could be reverted in cells continuously cultured under hyperoxic conditions (40% O2). To do this, MEF cells cultured for four weeks at 40% O2 were ‘re-adapted’ by transferring them back to the physiologically relevant condition of 5% O2 and allowing them to acclimate for three weeks Importantly, cells that were transferred from 40% O2 back to 5% O2 recovered their capacity for Adaptive Homeostasis if they were pre-exposed to a 1.0 μM signaling level of H2O2 before being given the 3.0 mM challenge dose of H2O2 (Fig. 2B, last 2 bars). These results indicate that the fitness advantage of the adaptive response can be restored once cells are moved back to 5% O2. Growth was clearly attenuated under 40% O2 compared to physiological normoxia, however, suggesting that under chronic hyperoxia, cells are faced with a chronically elevated level of oxygen-induced stresses (Fig. 2B).
Given the fact that almost all published studies have used cells cultured at 21% (ambient) oxygen levels, and the observation that cell culture at 21% generates results somewhat halfway between those seen at 5% versus 40% O2 (Fig. 2 and numerous other results from our lab.), for the rest of this paper we decided to concentrate on differences between cells grown at 5% versus 40% O2.
2.2. Hyperoxia Increases 20S Proteasome Subunit Levels but abrogates their adaptive responses to H2O2
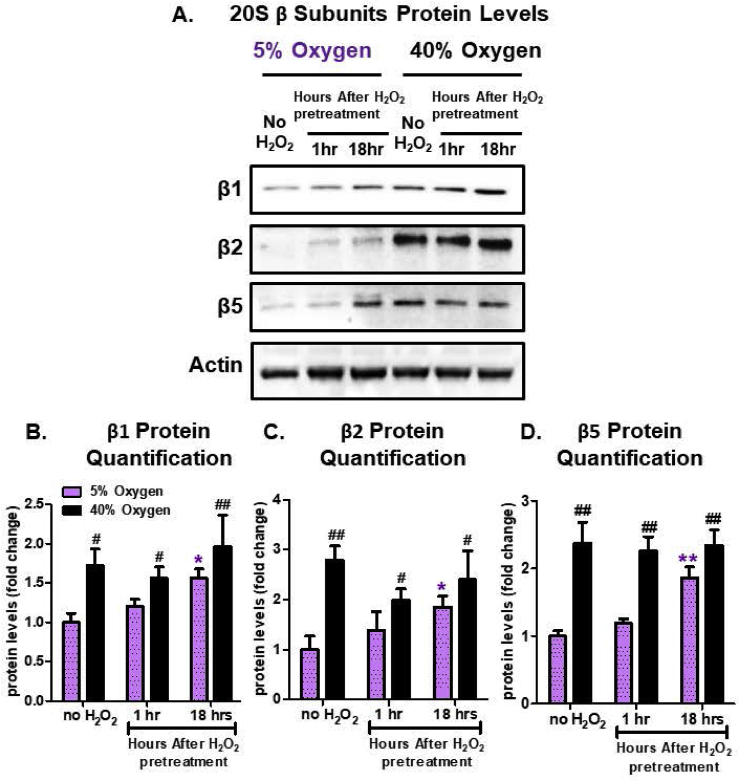
In order to address if chronic hyperoxia triggers a basal increase in stress-protective enzymes, we assessed protein levels of the 20S Proteasome following H2O2 pretreatment in cells cultured either at physiological normoxia (5% O2) or hyperoxia (40% O2). For MEFs cultured at 5% O2, protein quantification revealed that all 20S Proteasome catalytic subunits (β1, β2, and β5) were significantly increased by 18 h following exposure to the 1.0 μM H2O2 signaling dose, whereas 1 h was too soon to see an adaptive response (Fig. 3A). This adaptive response at physiologically relevant conditions is in line with previous studies showing that the adaptive response is largely the result of de novo synthesis of the Proteasome [2,9,10,31]. However, MEFs cultured at 40% O2 had significantly higher basal levels of these catalytic proteasomal subunits regardless of whether they experienced a pre-exposure to H2O2 (Fig. 3A–D). We suggest that these results reflect the absence of further adaptive responses in MEFs cultured at 40% O2.
Fig. 3.
Hyperoxia Increases 20S Proteasome Subunit Levels but Abrogates their Adaptive Responses to H2O2. MEF cells were chronically cultured at 5% or 40% prior to pretreatment. Cells were either pretreated with a non-damaging amount (1.0 μM) of H2O2 in a final volume of 2.0 ml in 6 well plates, for 1 h (or used as controls) and then allowed to recover for either 1 h or 18 h post treatment. All treatments were done in replicates of 6 (n = 6). (A) Amounts of the three proteolytic subunits of the 20S proteasome (β1, β2, β5) were assessed by Western blot and normalized to the actin loading control. MEF cells propagated at 5% showed increased amounts of the three subunits, following H2O2 pretreatment, whereas the MEF cells propagated at 40% showed increased baseline levels, but no further adaptive increase following H2O2 pretreatment. (B) Quantification of the amount of 20S β1 subunit. (C) Quantification of the amount of 20S β2 subunit. (B) Quantification of the amount of 20S β5 subunit. All data are expressed as means ± standard errors. Statistically significant differences in 5% O2 cultured cell lines to the 5% O2 control were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Statistically significant differences in the 40% O2 cultured cell line to the 5% O2 control were indicated by # (p < 0.05), ## (p < 0.01), ### (p < 0.001).
2.3. Hyperoxia Increases immunoproteasome subunit levels but abrogates their adaptive responses to H2O2
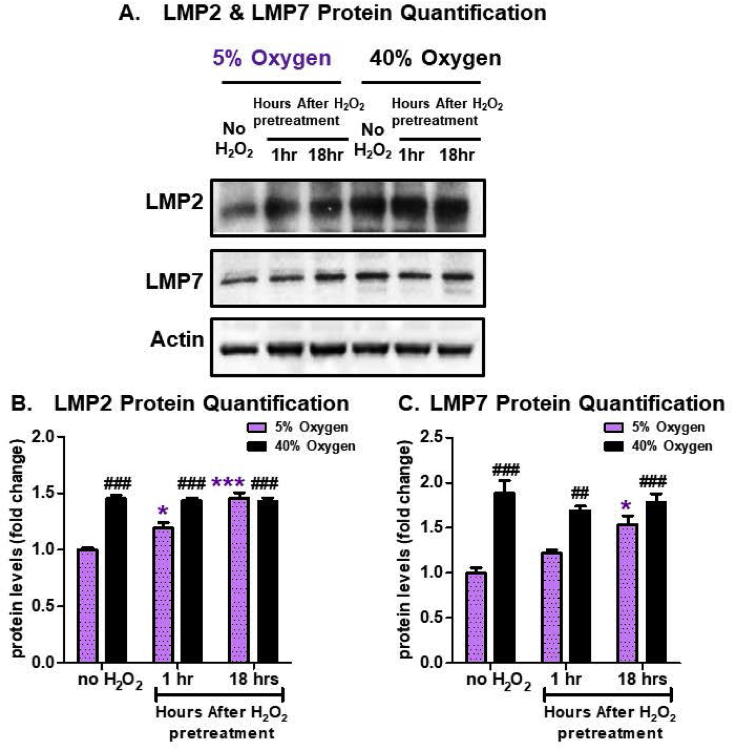
Assessment of Immunoproteasome subunits also revealed an adaptive response to 1.0 μM H2O2 in MEFs cultured at 5% O2, but not at 40% O2. In MEFs cultured at 5% O2, the two subunits, LMP2 and LMP7, respectively were significantly increased 18 h following an adaptive dose of H2O2 (Fig. 4A–C), but in MEFs cultured at 40% O2 the amount of protein was already significantly elevated in control conditions and exhibited no further increase following H2O2 signaling (Fig. 4A–C). These results further suggest a physiological limit to the inducibility of stress-protective enzymes.
Fig. 4.
Hyperoxia Increases Immunoproteasome Proteasome Subunit Levels but Abrogates their Adaptive Responses to H2O2. MEF cells were chronically cultured at 5% or 40% prior to pretreatment. Cells were either not pretreated with H2O2 or pretreated with a non-damaging signaling amount (1.0 μM H2O2 in a final volume of 2.0 ml in 6 well plates) for 1 h and then allowed to recover for either 1 h or 18 h post treatment. All treatments were done in replicates of 6 (n = 6). (A) The levels of the two proteolytic subunits of the immunoproteasome (LMP2 and LMP7) were assessed by Western blot and normalized to the actin loading control. MEF cells propagated at 5% showed increased amounts of both subunits, following H2O2 pretreatment, whereas the MEF cells propagated at 40% exhibited increased baseline levels, but no further adaptive increase following H2O2 pretreatment. (B) Quantification of the amount of the Immunoproteasome LMP2 subunit. (C) Quantification of the amount of the Immunoproteasome LMP7 subunit. (B) Quantification of the amount of the 20S β5 subunit. All data are expressed as means ± standard errors. Statistically significant differences in 5% cultured cell lines to the 5% O2 control were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Statistically significant differences in the 40% O2 cultured cell line to the 5% O2 control were indicated by # (p < 0.05), ## (p < 0.01), ### (p < 0.001).
2.4. Hyperoxia Increases Nrf2, HO1, and NQO1 levels but abrogates their adaptive responses to H2O2
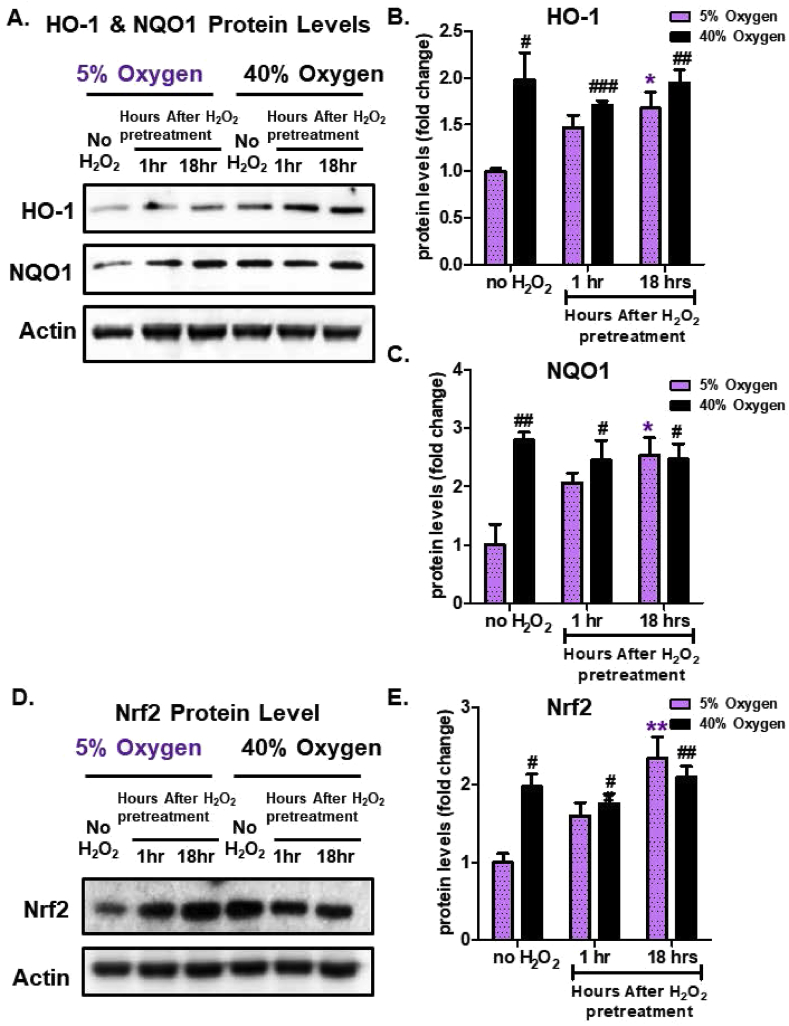
To address whether additional Nrf2-regulated stress-protective enzymes show a difference in induction due to culturing conditions, heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase (quinone 1) (NQO1) protein levels were measured. Cells cultured at physiologically relevant conditions (5% O2), showed an adaptive increase in HO-1 and NQO1 (Fig. 5A–C) 18 h after exposure to a 1.0 μM H2O2 signal. In contrast, cells continually cultured under hyperoxic conditions, exhibited elevated basal HO-1 levels, but could not be induced further following H2O2 signaling (Fig. 5A and B). Similarly, NQO1 levels were induced 18 h after administration of the H2O2 adaptive signaling dose in cells cultured at 5% O2 (Fig. 5A,C). However, chronic hyperoxia (40% O2) resulted in a higher NQO1 basal levels, compared to the baseline seen in cells cultured at physiologically relevant conditions (5% O2) and NQO1 was not further induced following H2O2 signaling: nor did it exceed the adaptive levels seen in cells cultured at 5% O2 (Fig. 5A,C).
Fig. 5.
Hyperoxia Increases Nrf2, HO1, and NQO1 Levels but Abrogates their Adaptive Responses to H2O2. MEF cells were chronically cultured at 5% or 40% O2 prior to pretreatment. Cells were either pretreated with a 1.0 μM non-damaging, signaling level of H2O2 for 1 h in a final volume of 2.0 ml in 6 well plates, and then allowed to recover for either 1 h or 18 h post treatment, or were used as controls. All treatments were done in replicates of 6 (n = 6). (A) Western blot of HO-1 and NQO1, normalized to an actin loading control. (B) Quantification of the amount of HO-1. (C) Quantification of the amount of NQO1. (D) western blot of Nrf2, normalized to an actin loading control. (E) Quantification of the amount of Nrf2, normalized to an actin loading control. MEF cells propagated at 5% exhibited increased amounts of Nrf2 and Nrf2-regulated enzymes (HO-1 and NQO1), following 1.0 μM H2O2 pretreatment, whereas MEF cells propagated at 40% O2 had increased baseline Nrf2 levels, but showed no further increase following H2O2 pretreatment. All data are expressed as means ± standard errors. Statistically significant differences in 5% cultured cell lines to the 5% O2 control were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Statistically significant differences in the 40% O2 cultured cell line to the 5% O2 control were indicated by # (p < 0.05), ## (p < 0.01), ### (p < 0.001).
2.5. Dysregulation of Nrf2 nuclear translocation by growth under chronic hyperoxia
Since Nrf2 is the master transcriptional regulator for many adaptive stress responses, we next explored whether Nrf2 levels were affected by growth under different oxygen conditions. Under physiologically relevant conditions of 5% O2, cells exhibited an increase in Nrf2 levels 18 h after receiving an H2O2 signaling dose (Fig. 5D and E). However, in cells continually cultured under hyperoxic conditions (40% O2), baseline Nrf2 levels were already elevated and remained unaffected by a signaling dose of H2O2 (Fig. 5D and E). This pattern was similar to that seen above for Nrf2 target genes including the Proteasome, Immunoproteasome, HO-1, and NQO1.
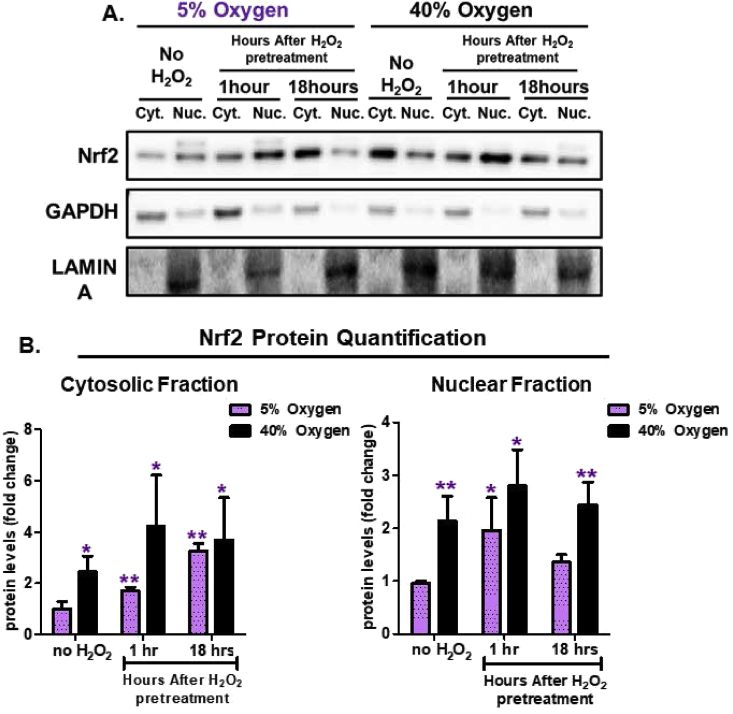
In response to adaptive signaling (such as that initiated by non-damaging 1.0 μM H2O2), Nrf2 escapes degradation by the ATP/ubiquitin-dependent 26S Proteasome, undergoes phosphorylation, and enters the nucleus. Therefore we also assessed temporal differences in nuclear versus cytosolic Nrf2 levels at both 5% and 40% O2 culturing conditions, and following H2O2 signaling. Under physiologically relevant growth conditions of 5% O2, baseline Nrf2 levels in both the cytosol (Fig. 6A and B) and the nucleus (Fig. 6A and B) were relatively low. Within just 1 h of adaptive H2O2 signaling dose, however, there was a significant accumulation of Nrf2 in the nucleus, enabling its activation of target genes. In contrast, 18 h after H2O2 signaling, at which point transcription and translation of Nrf2 target genes was already maximal, Nrf2 nuclear accumulation diminished and cytosolic levels rose (Fig. 6A and B). Cells continually grown under hyperoxic conditions of 40% O2 showed high baseline levels of both cytosolic and nuclear Nrf2 (Fig. 6A and B) compared to cells cultured under 5% O2. Significantly, an H2O2 signaling dose failed to affect either cytosolic or nuclear Nrf2 levels, both of which remained high, in these hyperoxia-cultured cells at either 1hr or 18hr (Fig. 6A and B).
Fig. 6.
Nrf2 Nuclear Translocation is Dysregulated by Chronic Hyperoxia. MEF cells were cultured either at 5% or 40% oxygen prior to pretreatment. Cells were either not pretreated with H2O2 or pretreated with a non-damaging, signaling amount (1.0 μM H2O2 in a final volume of 2.0 ml in 6 well plates) for 1 h and then allowed to recover for either 1 h or 18 h post treatment. Both cytosolic and nuclear cell fractions were isolated. All treatments were done in replicates of 6 (n = 6). (A) Western blot of Nrf2 levels in cytosolic versus nuclear cell fractions at 1-h and 18-h after initial H2O2 pretreatment and normalized either to GAPDH (cytosolic fraction) or LAMIN (nuclear fraction) loading controls. (B) Quantification of Nrf2 levels within cytosolic and nuclear fractions normalized to loading controls. MEF cells cultured at 5%, showed an increasing accumulation of Nrf2, within the cytosolic fraction, at 1-h and 18-h post-pretreatment. In the nuclear fraction, Nrf2 accumulated at 1 h and then decreased back to baseline levels by 18 h after pretreatment. In contrast, MEF cells cultured at 40% oxygen, showed no change in Nrf2 accumulation following H2O2 pretreatment. All data are expressed as means ± standard errors and statistically significant differences were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001).
2.6. Dysregulation of Bach1 and c-Myc Nuclear Translocation by growth under chronic hyperoxia
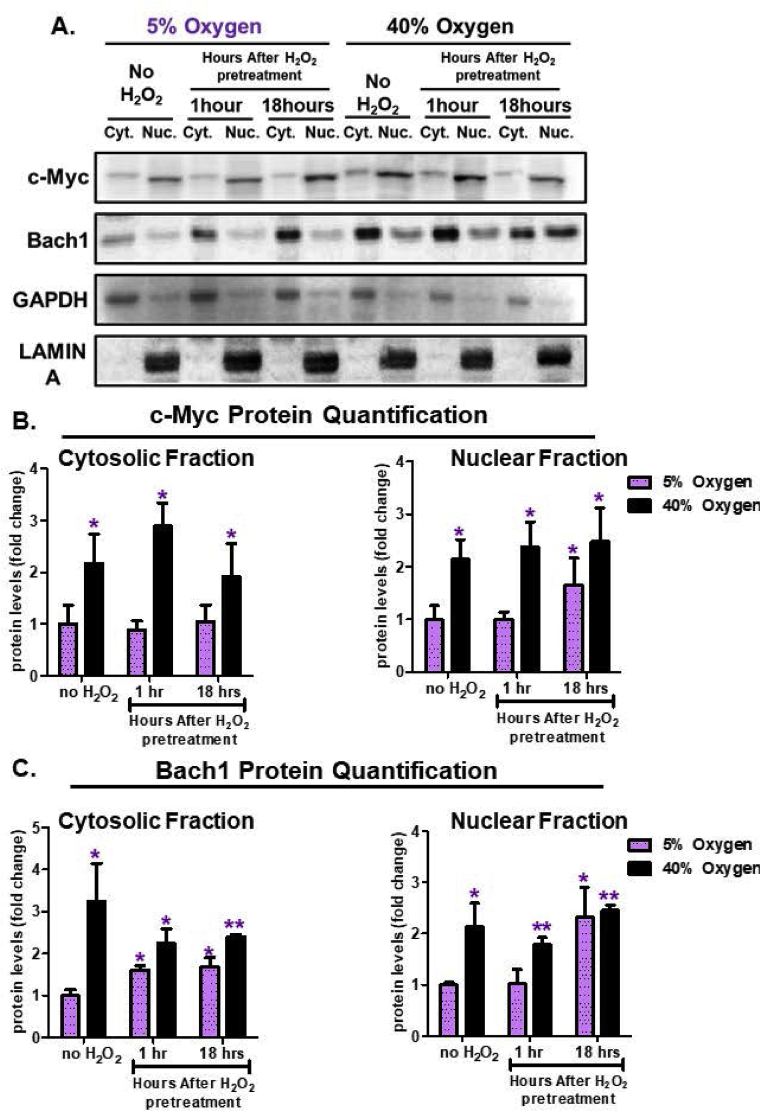
The adaptive homeostatic response is transient. As such, in response to an oxidative signal, cells must be able to temporarily turn ‘on’ the system (i.e. Nrf2 nuclear translocation), and subsequently reverse or turn ‘off’ the system (i.e. Nrf2 nuclear suppression) when no longer necessary. Potential transcriptional enzymes that may serve as the ‘off’ switch are Bach1, which competes with Nrf2 binding to EpRE/ARE [27], and/or c-Myc, which interacts with and inhibits Nrf2 directly, and/or promotes Nrf2 degradation [28]. In our experiments under physiologically relevant growth conditions of 5% O2, cells pretreated with an adaptive signaling dose of H2O2, showed little change in cytosolic or nuclear c-Myc levels, until 18 h after the signal at which point c-Myc nuclear accumulation was evident (Fig. 7A and B). Bach1 exhibited increased cytosolic levels at 1 and 18 h post H2O2 signaling (Fig. 7A,C), but only began to show nuclear accumulation 18 h post H2O2 (Fig. 7A,C). Together, these results suggest that the nuclear accumulation of both c-Myc and Bach1 (18 h post H2O2 signal) lagged some 17 h behind Nrf2 nuclear accumulation (1 h post initial H2O2 signal) allowing ample time for Nrf2-activated gene expression and serving as an effective Nrf2 ‘off’ switch.
Fig. 7.
Bach1 and c-Myc Nuclear Translocation is Dysregulated by Chronic Hyperoxia. MEF cells were cultured either at 5% or 40% oxygen prior to pretreatment. Cells were either not pretreated with H2O2 or pretreated with a non-damaging, signaling level (1.0 μM H2O2 in a final volume of 2.0 ml in 6 well plates) for 1 h and then allowed to recover for either 1 h or 18 h post treatment. Cytosolic and nuclear fractions were isolated. All treatments were done in replicates of 6 (n = 6). (A) The levels of c-Myc and Bach1 were assessed by Western blot in the cytosolic versus nuclear fractions 1-h and 18-h after initial H2O2 pretreatment and normalized either to GAPDH (cytosolic fraction) or LAMIN (nuclear fraction) loading controls. (B) Quantification of c-Myc levels within cytosolic and nuclear fractions. MEF cells cultured at 5% oxygen, showed an increasing accumulation of c-Myc within the nuclear fraction by 18 h post-pretreatment, with no change in the cytosolic fraction. MEF cells cultured at 40% oxygen, showed increased baseline amounts of c-Myc in both nuclear and cytosolic fraction, but no adaptive accumulation in either fraction post-pretreatment. (C) Quantification of Bach1 levels within the cytosolic and nuclear fractions. MEF cells cultured at 5% oxygen, showed an increasing amount of Bach1 at 1 h and 18 h in the cytosolic fraction, whereas, Bach1 accumulated after 18 h post-pretreatment in the nuclear fraction. In contrast, MEF cells propagated at 40% oxygen showed a baseline increase in Bach1 levels in the cytosolic and nuclear fractions, but no difference in cells either not pretreated or pretreated with hydrogen peroxide. All data are expressed as means ± standard errors and Statistically significant differences were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001).
In contrast to the above results of effective H2O2-Nrf2 signaling for Adaptive Homeostasis in cells grown under normoxia, cells cultured under hyperoxic conditions exhibited high baseline levels of nuclear c-Myc (Fig. 7A and B), with no change following H2O2 signaling (Fig. 7A and B). Moreover, c-Myc levels in hyperoxic cells matched those achieved 18 h post H2O2 signaling, suggesting dysregulation of c-Myc temporal inhibition. Additionally, Bach1 exhibited heightened basal cytosolic and nuclear levels, which were further increased in the nucleus 18 h post H2O2 signal (Fig. 7A and B). Thus, cells grown under hyperoxia, which face chronically elevated levels of oxidative stress, showed high baseline levels of Nrf2 transcriptional inhibitors, but were unable to exceed this limit. This may suggest that growth under hyperoxic conditions forces cells to continually combat oxidative insults that result in elevated levels of both Nrf2 and its inhibitors, such that further adaptation is not possible.
2.7. Hyperoxia induces Lon expression but prevents further adaptive homeostasis
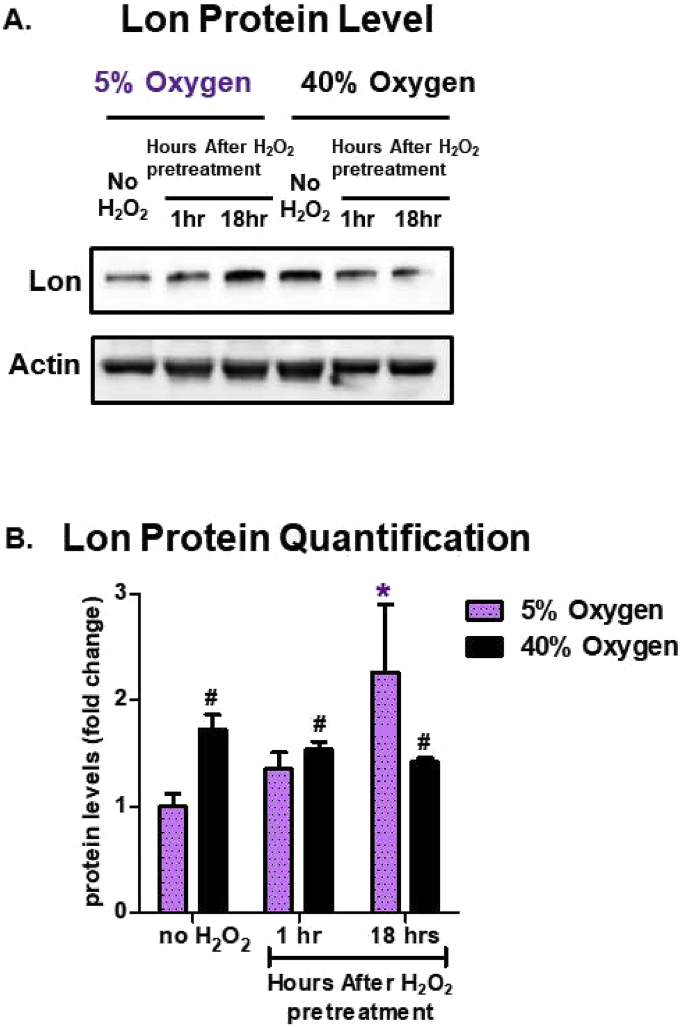
The mitochondrial Lon protease is necessary for clearance of oxidized mitochondrial proteins, such as oxidized-aconitase [32]. Prior work has shown it is highly inducible by multiple forms of stress [13,33,[56], [57], [58], [59], [60]] and is necessary for survival [13,61] and development [62]. Here we sought to address if the stress adaptive induction of Lon changed in response to oxygen concentration. Cells cultured at physiologically relevant levels (5% O2) showed a marked increase in Lon inducibility 18 h post H2O2 signaling dose (Fig. 8A and B). However, cells cultured under chronic hyperoxia (40% O2) exhibited high baseline levels of Lon, that could not be further increased by exposure to H2O2 signaling (Fig. 8A and B).
Fig. 8.
Hyperoxia Increases the Level of the Lon Protease but Abrogates its Adaptive Responses to H2O2. MEF cells were chronically cultured at 5% or 40% prior to pretreatment. Cells were either not pretreated with H2O2 or pretreated with a non-damaging, signaling amount (1 μM H2O2 in a final volume of 2.0 ml in 6 well plates) for 1 h and then allowed to recover for either 1 h or 18 h post treatment. All treatments were done in replicates of 6 (n = 6). (A) The amount of Lon protease protein was assessed by Western blot and normalized to an actin loading control. (B) Quantification of the amount of Lon protein. MEF cells cultured at 5% oxygen showed an increased amount of Lon 18 h after initial pretreatment. All data are expressed as means ± standard errors. Statistically significant differences in 5% cultured cell lines to the 5% O2 control were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Statistically significant differences in the 40% O2 cultured cell line to the 5% O2 control were indicated by # (p < 0.05), ## (p < 0.01), ### (p < 0.001).
2.8. Oxr1 isoforms are sensitive to Chronic High Oxygen Exposure
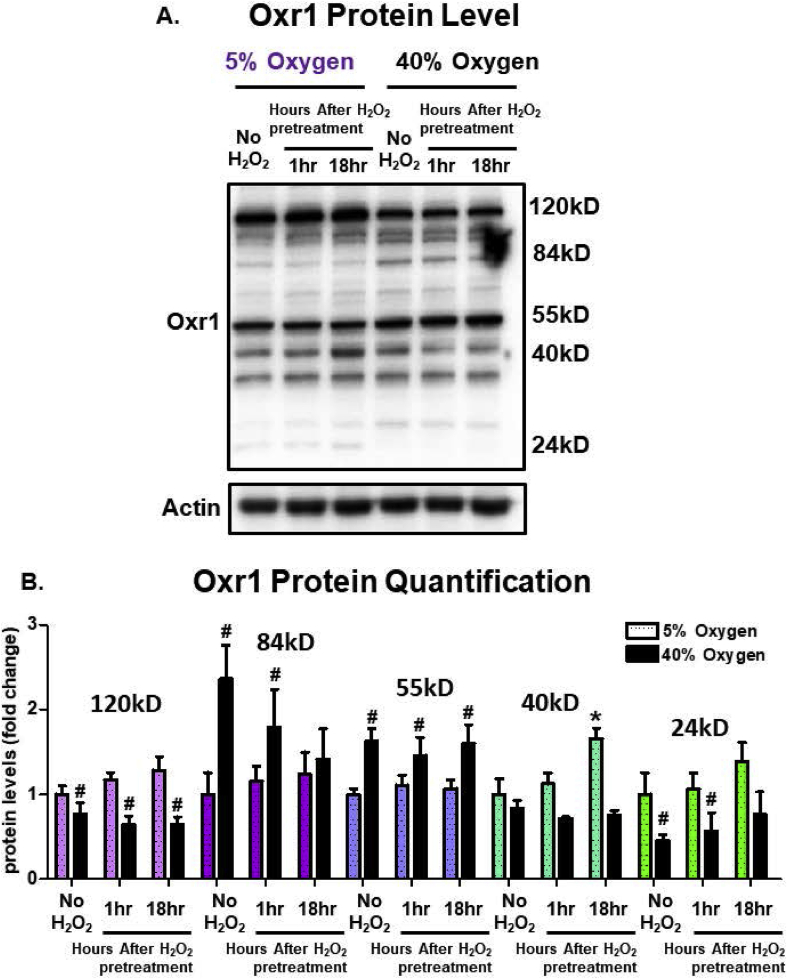
We also explored a relatively novel protein, oxidation resistance 1 (Oxr1), that has been implicated as necessary for oxidative stress protection, perhaps especially in neuronal cells. Originally identified in bacteria to protect against oxidative DNA damage [63], it has been shown to protect against neurodegeneration in mammals [64,65], potentially through its partnership with peroxiredoxin 2 [66]. Multiple isoforms of Oxr1 are found in mammalian cells, with the high molecular weight forms (55kD and greater) primarily located in the cytoplasm, whereas the smaller isoforms (less than 55kD) localize to mitochondria. Here, we show that several Oxr1 isoform levels changed in response to either O2 culturing concentrations, or exposure to H2O2 signaling, or both. Higher levels of the largest Oxr1 isoform, at 120kD, were seen in cells grown at 5% O2 than in cells cultured at 40% O2, but this 120kD isoform was not responsive to H2O2 signaling at either O2 culturing condition (Fig. 9A and B). The 85kD full-length active Oxr1 variant was elevated more than two-fold in response to chronic hyperoxia but H2O2 signaling actually caused a decrease in its levels; H2O2 had no effect on 85kD Oxr1 levels in cells grown at 5% O2 (Fig. 9A and B). Levels of the 55kD Oxr1 isoform exhibited a basal increase at 40% O2, but were unresponsive to an H2O2 signal. The mitochondrial-targeted 40kD Oxr1 isoform was relatively unresponsive to O2 culturing conditions but exhibited a significant increase in levels at 18 h post H2O2 signaling only in cells grown at 5% O2 (Fig. 9A and B). Finally, the 24kD mitochondrial-specific Oxr1 isoform was significantly decreased by culture at 40% O2 and H2O2 signaling had no effect at either 5% or 40% O2 culturing condition. Our results demonstrate a complex interaction of oxygen and H2O2 effects on the levels of cytoplasmic and mitochondrial Oxr1 isoforms that must await further investigation to be fully explained.
Fig. 9.
Cytoplasmic and Mitochondrial Oxr1 Isoforms are Differentially Sensitive to Chronic High Oxygen Exposure. Mouse embryonic fibroblasts (MEF) cells were cultured either at 5% (low) or 40% (high) oxygen for 2 weeks prior to exposing cells to either no pretreatment, or pretreating them with a 1.0 μM non-damaging, signaling amount of hydrogen peroxide in a final volume of 2.0 ml in 6 well plates. Cell lysates were Western blotted and probed with an Oxr1 antibody. All treatments were done in replicates of 6 (n = 6). Culturing at 40% recruited 84kD and 55kD cytoplasmic Oxr1 isoforms and result in a decrease in the 120kD cytoplasmic isoform. The mitochondrial 40kD Oxr1 isoform was the only isoform responsive to an oxidative H2O2 signal following growth at 5% O2, while the 24kD mitochondrial isoform was the only one that was responsive to an oxidative H2O2 signal following growth at 40% O2. All data are expressed as means ± standard errors. Statistically significant differences in 5% cultured cell lines to the 5% O2 control were indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Statistically significant differences in the 40% O2 cultured cell line to the 5% O2 control were indicated by # (p < 0.05), ## (p < 0.01), ### (p < 0.001).
3. Discussion
In this study we have utilized a hyperoxic model of accelerated ageing to study the responsiveness of the Nrf2 signal transduction pathway and its ability to mediate adaptive homeostasis. Our results demonstrate that H2O2 induces a transient induction of the adaptive stress response in cells grown under physiologically relevant conditions of 5% O2. In contrast, cells grown at ambient (∼21%) O2 exhibit a more limited ability to adapt, and cells cultured at a hyperoxic level of 40% O2 fail to exhibit any significant adaptive homeostasis. These findings suggest that chronic exposure to hyperoxic conditions is a means of accelerating the oxidation-related stresses associated with aging and is a useful model that mimicks several aspects of cellular ageing, as previously postulated [43,53]. Many of the observations reported in this paper have now been repeated in human bronchiolar epithelial (HBE) cells but, given the large amount of date already reported here, and the fact that the HBE cells are part of a very different study, the results are not reported in detail here.
The homeostatic adaptive response is the transient expansion of expression of stress-protective enzymes and the activation of damage removal and repair machinery necessary for clearance of dysfunctional enzymes, lipids and organelles; together, all these adaptive responses serve to prevent the accumulation of damaged cellular components, and to increase the chances of survival and vigor in response to toxic situations [67]. As the leading inducer of multiple phase II detoxification and metabolic enzymes, short-term Nrf2 transcriptional activation is responsible for initiating the adaptive homeostatic response to oxidants such as H2O2. Here, we show Nrf2 and its multiple down-stream targets can be robustly increased at physiologically relevant conditions in response to an external stimulus. However, the Nrf2-mediated adaptive increase is lost with the chronic oxidative stress of hyperoxic growth conditions. This finding mirrors earlier work showing a basal rise in Nrf2 levels in chronic diseases (including multiple forms of cancer) [68,69] and with age [14,70,71], accompanied by a loss of Nrf2 inducibility but are no longer inducible. This suggests that a new baseline is created that allows cells, tissues, or organisms to cope with an age-related increase in chronic oxidative stress; if an additional stress is encountered, however, the older organism is unable to further adapt or adjust.
Cells continually cultured under physiologically relevant conditions (5% O2) have excellent growth rates and can increase their proteolytic capacity and stress-resistance when induced with signaling levels of H2O2 that enable cells to flourish and thrive [37,72]. In contrast, cells continually cultured under increasingly high oxygen concentrations (from 21% up to 40%), become vulnerable to damage accumulation, a finding matching earlier work [[73], [74], [75]]. Thus, differences in O2 culturing conditions force cells to chronically elevate stress protective systems in order to protect against cellular damage accumulation. If an additional stress is applied, however (i.e. an oxidative insult with H2O2), cells cultured at high O2 levels are unable to further increase stress-protective systems, resulting in lowered cell growth and poor protein turnover.
Protein clearance is a crucial component of cellular homeostasis, with the Proteasome at the forefront against protein aggregation. The 20S Proteasome and the less-well studied Immunoproteasome (also termed the ‘inducible Proteasome’), both show evidence of increased levels and activity in response to oxidative stress [9,37,76,77]. Basal levels of the 20S Proteasome subunits (β1, β2, β5) and the Immunoproteasome subunits (LMP2 & LMP7), are increased in response to hyperoxic conditions (40% O2). Strikingly, the actual amounts of 20S Proteasome and Immunoproteasome, and the cellular proteolytic capacity, seen under hyperoxia are equal to the maximal levels achieved in cells treated with signaling levels of H2O2 after being cultured under physiological conditions of 5% O2. These findings are similar to studies exploring changes in Proteasome content and activity in response to chronic oxidative stress [78], and with age [12,15,17,[79], [80], [81]]. Because loss of proteostasis only becomes evident in the final third of life [82], these findings may provide important clues to the mechanism(s) of age-dependent loss of adaptive homeostasis. In this regard it is interesting that oxidized proteins are rapidly cleared in early passage fibroblasts, but in high passage number cells there is a marked accumulation of oxidatively modified (often aggregated and cross-linked) proteins and an inability degrade damaged proteins following an oxidative stress such as H2O2 [7], a finding that holds true in higher organisms [15,17,72,79,83,84]. Overall prior work, and now evidence from the present study, further suggests there is a potential ‘threshold’ in the ability of cells to cope with chronic oxidation-related stresses such that any additional perturbation may be the final cellular ‘straw’ that may topple the homeostatic balance.
Further evidence of the negative impact of chronic oxidation -related stresses on adaptive homeostasis comes from other Nrf2-inducible enzymes we studied, including heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), and the mitochondrial Lon protease. In all cases, basal levels rise with hyperoxia, yet the adaptive responses seen in cells grown under physiologically relevant concentrations of 5% O2 are lost with chronic hyperoxia. Importantly, the levels of these enzymes seen under hyperoxic conditions are equal to those seen in H2O2 adapted cells cultured at 5% O2 providing further evidence for a cellular ‘ceiling’ effect. Thus, it appears that chronic elevation of Nrf2 and the Nrf2-mediated response has an upper limit, which once achieved, cannot be further increased. A similar finding was previously reported using human primary endothelial cells that exhibited marked Nrf2-inducibility when cultured under physiological conditions, but that lost inducibility when cultured under ambient air (21% O2) [85]. Together, these findings highlight the vulnerable state of cells, tissues, and organisms subjected to chronic oxidative stress, wherein any additional insult can overwhelm defense systems. This concept agrees well with prior studies suggesting that during cellular senescence [6,7,57,86,87] and in both invertebrate [12,13,[15], [16], [17]] and vertebrate ageing [14,88,89] (all of which are highly oxidant sensitive states) further perturbations to the system can trigger morbidity and accelerated mortality.
The cellular ‘ceiling’ observed for Nrf2 effects impacts the regulation of the Proteasome and other Nrf2-regulated enzymes. This apparent ceiling effect for Nrf2-regulated enzymes was previously demonstrated in ageing [14,90]. Nrf2 is responsible for inducing the adaptive responses of multiple phase II enzymes [91] and the Proteasome [39,92], and Nrf2 signaling is lost in ageing [14,90]. This suggests that during the ageing process, as in chronic hyperoxia, constraints imposed upon Nrf2 may impact its downstream targets. Under physiological conditions, total Nrf2 amounts follow the canonical pattern of short-term elevation (within an hour) in response to an oxidative signal, followed by a return to basal conditions (within 18 h) after the signal [92]. However, under hyperoxic conditions total Nrf2 remains consistently elevated and more importantly, unresponsive to an additional oxidative signal. To further address Nrf2 regulation, cytosolic versus nuclear amounts of Nrf2 were measured and compared. Under physiologically relevant conditions (5% O2) Nrf2 showed a rapid nuclear accumulation (within 1 h) in response to a non-damaging H2O2 signal, enabling it to interact with its target enzymes and effect their transcriptional activation [10]. Nuclear Nrf2 levels subsequently declined, and cytoplasmic Nrf2 levels increased within 18 h of H2O2 treatment. However, under hyperoxic growth conditions, both Nrf2 cytosolic and nuclear basal levels were elevated, suggesting that cells were already undergoing an oxidative stress.
Because Nrf2 is the physiological ‘on switch’ for the adaptive response, cells require a physiological ‘off switch,’ not only for the energetic cost, but maintenance of the homeostatic range [67]. Prior work has suggested that certain Nrf2 transcriptional inhibitors, specifically Bach1 and c-Myc [85,88,90,91] may act as potential physiological regulators. Both Bach1 [27] and c-Myc [93] interfere with Nrf2-regulated transcription. Here we report novel findings of the temporal transition of c-Myc and Bach1 in response to a non-damaging H2O2 signal. Under physiological conditions (5% O2) Bach1 and c-Myc begin to accumulate only 18 h after the initial H2O2 stimulus, suggesting they may help to dislodge Nrf2 from its EpRE/ARE binding sites and/or prevent further nuclear Nrf2 accumulation. However, under hyperoxic growth conditions, both Bach1 and c-Myc exhibited elevated basal cytosolic and nuclear levels. This is important as it suggests temporal dysregulation, wherein the ‘off switch’ is activated too early [94]. It would appear that Nrf2 tries to compete with Bach1 and c-Myc in order to further elevate de novo synthesis of stress protective enzymes but that ultimately, with age or chronic hyperoxia, it fails. Synthesis of stress protective enzymes may be blocked. Dysregulation of Nrf2 signaling may underlie the age dependent decline in proteostasis that occurs despite a basal rise in stress protective enzymes [14,15,17,21,94,95]. Because activation of proteolytic responses is hindered, transient increases in protein damage may not be dealt with and oxidized proteins that would normally be degraded are allowed to accumulate. Thus, changes the balance of Nrf2, Bach1, and c-Myc levels may account for significant dysregulation of stress responses and adaptive homeostasis during chronic hyperoxia and in ageing.
4. Experimental procedures
4.1. Cell culturing
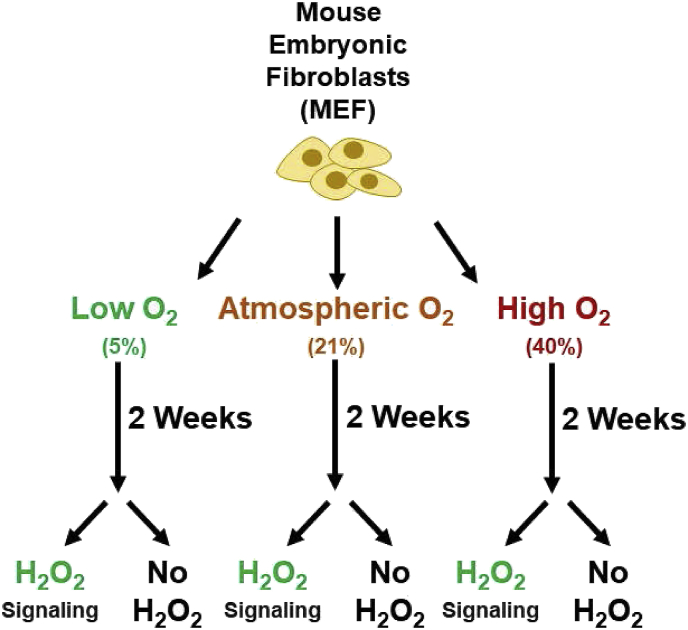
Mouse embryonic fibroblasts (MEFs) were continuously cultured at 5%, 21%, or 40% O2 (with 5% CO2 for all conditions) for approximately two weeks prior to hydrogen peroxide (H2O2) pretreatment (Fig. 1). For re-adaption experiments, subsets of the 40% O2 cells were transferred back to 5% O2 for two weeks to de-adapt the lines. Dulbecco's Modified Eagle's Medium (Cat # 25-500, Genesee, USA) with 10% Fetal Bovine Serum (Cat # 50926-5, Sigma-Aldrich, USA). Antimicrobial, antibiotic-antimycotics were added at a final concentration of 1%, (Cat #: 25-539, Genesee, USA). Media was renewed twice a week.
Fig. 1.
Experimental Design. Mouse embryonic fibroblasts (MEF) cells were cultured at 5% (physiological), 21% (atmospheric), or 40% (hyperoxic) oxygen for 2 weeks prior to either being pre-treated with a signaling dose of 1.0 μM hydrogen peroxide (H2O2), or used as controls.
4.2. Hydrogen peroxide pretreatment
MEFs were seeded at 5 × 105 cells per well in 6-well plates at 2 mL per well and allowed to attach overnight before being pretreated with 1.0 μM H2O2 for 1 h. After pretreatment, cells were washed twice with warmed (37 °C) phosphate-buffered saline (PBS), replaced with fresh complete media and allowed to recover for 18 h before harvesting. MEFs were harvested by scraping cells with cold PBS. Cells were pelleted at 5,000 g for 5 min and then resuspended in proteolysis buffer [50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol (DTT) (pH 7.5)] and lysed by three rounds of freeze-thaw cycles. Samples were then centrifuged at 10,000 g for 10 min and supernatant was collected and protein concentration quantified using a BCA protein assay kit (Cat # 23250, Thermo-Fisher Scientific, USA). Cytoplasmic and nuclear fractions were isolated using NE-PER Nuclear and Cytoplasmic Extraction Kit (Cat # 78833, Thermo-Fisher Scientific, USA).
4.3. Proteolysis assays
Samples were loaded in triplicate at 5 μg cell lysate per well in a 96-well plate with AMC-conjugated substrates. The final volume per well was 100 μL. Samples were run alongside AMC standards to create a standard curve of known AMC concentrations. Fluorescence readings were taken at 10 min intervals using excitation wavelengths of 355 nm and emission of 444 nm for a total of 4 h. For measurements of Chymotrypsin-like activity, Caspase-like activity and Trypsin-like activity, the substrates used were 2 μM N-succinyl-Leu-Leu-Val-Tyr-AMC, Z-Leu-Leu-Glu-AMC and Boc-Leu-Arg-Arg-AMC, respectively. Significant differences were determined using a One-Way ANOVA followed by a Bonferroni corrected post-hoc test.
4.4. Hydrogen peroxide challenge assay
A pre-exposure of 1.0 μM H2O2 per 5 × 105 cells was administered for 1 h followed by an 18-h recovery period for 5%, 21%, and 40% O2 culturing conditions. After the recovery period, a challenge dose of 3.0 mM H2O2 per 5 × 105 cells for 1 h was administered. Cells were allowed to recover for 24 h before cell counts were taken. For cell counts, cells were washed twice with PBS, detached with trypsin (Cat # 25-510, Genesee Scientific, USA), and counted using trypan blue stain (Cat # 15250061, ThermoFisher Scientific, USA) and a hemocytometer.
4.5. Cell counts
MEFs cultured at 5%, 21%, and 40% O2 were seeded at 2 × 104 in 24-well plates with 10 replicates per oxygen concentration. After 4 days of incubation, cells were washed twice with PBS, detached with trypsin (Cat # 25-510, Genesee, USA), and counted using trypan blue stain (Cat # 15250061, ThermoFisher Scientific, USA) and a hemocytometer.
4.6. Protein quantification
Cell lysate was resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane and analyzed by Western blotting. All antibody were purchased from Santa Cruz Biotechnology unless otherwise noted (Supplemental Table 1). The Oxr1 antibody was generously provided by Dr. Peter Oliver. Chemiluminesence (Cat # 32106, Pierce, USA) was visualized on the PXi imaging system (Syngene, USA). Relative band intensity was quantified using ImageJ (National Institute of Health, USA), and normalized to HRP-actin.
4.7. Statistical considerations
One-Way ANOVA was used to test for significant differences between control and treatment groups. Prism 6 was used for completing statistical analyses, with statistical significance reported as follows:
*p < 0.05, **p < 0.01, ***p < 0.001. All data are expressed as means ± standard errors.
Summary
Hyperoxia was used as a model of accelerated ageing that diminished the effectiveness of the Nrf2 signal transduction pathway to cope with stress. Increased levels of Bach1 and c-Myc appear to have inhibited Nrf2 and may explain the abrogated adaptive homeostasis that accompanies ageing.
Author contributions
P.Y. Sun and L.C.D. Pomatto designed and performed experiments, and participated in writing drafts of the paper. K. Yu, S. Gullapalli, C.P. Bwiza, C. Sisliyan, and S. Wong all performed experiments.
H. Zhang and H.J. Forman contributed to the design and interpretation of experiments and participated in revising the manuscript. P.L. Oliver and K.E. Davies produced background work on OXR1, generated OXR1 antibodies used in this study, contributed to the interpretation of experiments, and participated in revising the manuscript. K.J.A. Davies initiated and supervised the project, designed experiments, and participated in writing drafts, and the final version of the paper.
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgements
LCD Pomatto was supported by grant #DGE-1418060 of the USA National Science Foundation and by NIH grant #ES003598 from the National Institute of Environmental Health Sciences of the US National Institutes of Health and is supported by NIH grant #Fi2GM123963 from the National Institute of General Medical Sciences of the National Institutes of Health. KJA Davies was supported by grant #ES003598 from the National Institute of Environmental Health Sciences of the US National Institutes of Health. KJA Davies and PY Sun were supported by grant# AG052374 from the National Institute on Aging of the US National Institutes of Health. HJF Forman and H Zhang were supported by grant #ES023864 from the National Institute of Environmental Health Sciences. K Yu was supported by Rose Hills USC Summer Research Scholarship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101194.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davies K.J. Adaptive homeostasis. Mol. Aspect. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies J.M., Lowry C.V., Davies K.J. Transient adaptation to oxidative stress in yeast. Arch. Biochem. Biophys. 1995;317(1):1–6. doi: 10.1006/abbi.1995.1128. [DOI] [PubMed] [Google Scholar]
- 3.Grune T., Reinheckel T., Davies K. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11(7):526–534. [PubMed] [Google Scholar]
- 4.Grune T., Reinheckel T., Davies K.J. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J. Biol. Chem. 1996;271(26):15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 5.Grune T. Proteolysis in cultured liver epithelial cells during oxidative stress Role of the multicatalytic proteinase complex, proteasome. J. Biol. Chem. 1995;270(5):2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- 6.GRUNE T. Ezrin turnover and cell shape changes catalyzed by proteasome in oxidatively stressed cells. FASEB J. 2002;16(12):1602–1610. doi: 10.1096/fj.02-0015com. [DOI] [PubMed] [Google Scholar]
- 7.Jung T. Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch. Biochem. Biophys. 2009;483(1):127–135. doi: 10.1016/j.abb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Pickering A.M., Davies K.J. Degradation of damaged proteins-the main function of the 20S proteasome. Prog. Mol. Biol. Transl. Sci. 2012;109:227. doi: 10.1016/B978-0-12-397863-9.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering A.M. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010;432(3):585–595. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering A.M. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287(13):10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering A.M. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickering A.M. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, <em>Caenorhabditis elegans</em> and <em>Drosophila melanogaster</em>. J. Exp. Biol. 2013;216(4):543. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomatto L.C. The mitochondrial lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr. Biol. 2017;27(1):1–15. doi: 10.1016/j.cub.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomatto L.C. Aging attenuates redox adaptive homeostasis and proteostasis in female mice exposed to traffic-derived nanoparticles (‘vehicular smog’) Free Radic. Biol. Med. 2018;121:86–97. doi: 10.1016/j.freeradbiomed.2018.04.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pomatto L.C. The age-and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging (Albany NY) 2017;9(4):1153. doi: 10.18632/aging.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pomatto L.C. Sexual dimorphism in oxidant-induced adaptive homeostasis in multiple wild-type D. melanogaster strains. Arch. Biochem. Biophys. 2017;636:57–70. doi: 10.1016/j.abb.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raynes R. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016;72(2):143–151. doi: 10.1093/gerona/glw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabrese E.J. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16(6):693–707. doi: 10.1007/s10522-015-9601-0. [DOI] [PubMed] [Google Scholar]
- 19.Gems D., Partridge L. Stress-response hormesis and aging:“that which does not kill us makes us stronger”. Cell Metabol. 2008;7(3):200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Southam C.M. 1943. Effects of Extract of Western Red-Cedar Heartwood on Certain Wood-Decaying Fungi in Culture. [Google Scholar]
- 21.Pomatto L.C.D., Davies K.J.A. Adaptive Homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018;124:420–430. doi: 10.1016/j.freeradbiomed.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. U. S. A. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin D. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279(10):8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 24.Chanas S.A. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem. J. 2002;365(2):405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thimmulappa R.K. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62(18):5196–5203. [PubMed] [Google Scholar]
- 26.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Dhakshinamoorthy S. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J. Biol. Chem. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 28.Levy S., Forman H.J. C-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62(3):237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver P.L. Oxr1 is essential for protection against oxidative stress-induced neurodegeneration. PLoS Genet. 2011;7(10) doi: 10.1371/journal.pgen.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M. Human OXR1 maintains mitochondrial DNA integrity and counteracts hydrogen peroxide-induced oxidative stress by regulating antioxidant pathways involving p21. Free Radic. Biol. Med. 2014;77:41–48. doi: 10.1016/j.freeradbiomed.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Raynes R., Pomatto L.C., Davies K.J. Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Aspect. Med. 2016;50:41–55. doi: 10.1016/j.mam.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bota D.A., Davies K.J. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4(9):674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 33.Bota D.A., Van Remmen H., Davies K.J. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532(1–2):103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 34.Pickering A.M. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010;432(3):585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickering A.M., Davies K.J. Differential roles of proteasome and immunoproteasome regulators Pa28αβ, Pa28γ and Pa200 in the degradation of oxidized proteins. Arch. Biochem. Biophys. 2012;523(2):181–190. doi: 10.1016/j.abb.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies K.J. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83(3–4):301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 37.Grune T. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Biol. Med. 2011;51(7):1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeg S. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic. Biol. Med. 2016;99:153–166. doi: 10.1016/j.freeradbiomed.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering A.M. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287(13):10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pomatto L.C.D. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging (Albany NY) 2017;9(4):1153–1185. doi: 10.18632/aging.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raynes R. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72(2):143–151. doi: 10.1093/gerona/glw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomeli N., Bota D.A., Davies K.J.A. Diminished stress resistance and defective Adaptive Homeostasis in age-related diseases. Clin. Sci. (Lond.) 2017;131(21):2573–2599. doi: 10.1042/CS20160982. [DOI] [PubMed] [Google Scholar]
- 43.Sitte N. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14(11):1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 44.Ruan L. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature. 2017;543(7645):443–446. doi: 10.1038/nature21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngo J.K. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(11):1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shringarpure R., Davies K.J. Protein turnover by the proteasome in aging and disease. Free Radic. Biol. Med. 2002;32(11):1084–1089. doi: 10.1016/s0891-5849(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 47.Bota D.A., Van Remmen H., Davies K.J. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532(1–2):103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 48.Levine R.L., Stadtman E.R. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36(9):1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 49.Atkuri K.R., Herzenberg L.A. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl. Acad. Sci. U. S. A. 2005;102(10):3756–3759. doi: 10.1073/pnas.0409910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan F.S. Enhanced survival of rat neonatal cerebral cortical neurons at subatmospheric oxygen tensions in vitro. Brain Res. 1986;384(1):199–203. doi: 10.1016/0006-8993(86)91240-0. [DOI] [PubMed] [Google Scholar]
- 51.Tiede L.M. Oxygen matters: tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis. 2011;2:e246. doi: 10.1038/cddis.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A. Quantifying the magnitude of the oxygen artefact inherent in culturing airway cells under atmospheric oxygen versus physiological levels. FEBS Lett. 2016;590(2):258–269. doi: 10.1002/1873-3468.12026. [DOI] [PubMed] [Google Scholar]
- 53.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II—aging of nondividing cells. FASEB J. 2000;14(15):2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- 54.Wiese A.G., Pacifici R.E., Davies K.J. Transient adaptation to oxidative stress in mammalian cells. Arch. Biochem. Biophys. 1995;318(1):231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 55.Santa-Gonzalez G.A. Distinctive adaptive response to repeated exposure to hydrogen peroxide associated with upregulation of DNA repair genes and cell cycle arrest. Redox Biol. 2016;9:124–133. doi: 10.1016/j.redox.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngo J.K., Davies K.J. Mitochondrial Lon protease is a human stress protein. Free Radic. Biol. Med. 2009;46(8):1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ngo J.K. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011;66(11):1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ngo J.K., Pomatto L.C., Davies K.J. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1(1):258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pareek G. Lon protease inactivation in Drosophila causes unfolded protein stress and inhibition of mitochondrial translation. Cell Death Discov. 2018;5(1):51. doi: 10.1038/s41420-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sepuri N.B. Mitochondrial LON protease-dependent degradation of cytochrome c oxidase subunits under hypoxia and myocardial ischemia. Biochim. Biophys. Acta (BBA)Bioenerg. 2017;1858(7):519–528. doi: 10.1016/j.bbabio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erjavec N. Deletion of the mitochondrial Pim1/Lon protease in yeast results in accelerated aging and impairment of the proteasome. Free Radic. Biol. Med. 2013;56:9–16. doi: 10.1016/j.freeradbiomed.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Quirós Pedro M. ATP-dependent lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8(2):542–556. doi: 10.1016/j.celrep.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Volkert M.R., Elliott N.A., Housman D.E. Functional genomics reveals a family of eukaryotic oxidation protection genes. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97(26):14530–14535. doi: 10.1073/pnas.260495897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finelli M.J. Oxidation resistance 1 modulates glycolytic pathways in the cerebellum via an interaction with glucose-6-phosphate isomerase. Mol. Neurobiol. 2018:1–20. doi: 10.1007/s12035-018-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliver P.L. Oxr1 is essential for protection against oxidative stress-induced neurodegeneration. PLoS Genet. 2011;7(10) doi: 10.1371/journal.pgen.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Svistunova D.M. Oxidation resistance 1 regulates post-translational modifications of peroxiredoxin 2 in the cerebellum. Free Radic. Biol. Med. 2019 Jan;130:151–162. doi: 10.1016/j.freeradbiomed.2018.10.447. Epub 2018 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davies K.J.A. Adaptive homeostasis. Mol. Aspect. Med. 2016;49(Supplement C):1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon E.J., Giaccia A. Dual roles of NRF2 in tumor prevention and progression: possible implications in cancer treatment. Free Radic. Biol. Med. 2015;79:292–299. doi: 10.1016/j.freeradbiomed.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menegon S., Columbano A., Giordano S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016;22(7):578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Zhou L. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2018;14:35–40. doi: 10.1016/j.redox.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forman H.J., Zhang J., Zhang H. Aging effects on basal and lipopolysaccharide inducible expression of antioxidant and inflammatory genes in human blood monocytes. Free Radic. Biol. Med. 2018;120:S59. [Google Scholar]
- 72.Breusing N. Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mech. Ageing Dev. 2009;130(11):748–753. doi: 10.1016/j.mad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II—aging of nondividing cells. FASEB J. 2000;14(15):2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- 74.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I—effects of proliferative senescence. FASEB J. 2000;14(15):2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 75.Sitte N. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14(11):1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 76.Grimm S. Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem. J. 2012;448(1):127–139. doi: 10.1042/BJ20120298. [DOI] [PubMed] [Google Scholar]
- 77.Raynes R., Pomatto L.C., Davies K.J. Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Aspect. Med. 2016;50:41–55. doi: 10.1016/j.mam.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. FASEB J. 2000;14(15):2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 79.Pickering A.M., Lehr M., Miller R.A. Lifespan of mice and primates correlates with immunoproteasome expression. J. Clin. Investig. 2015;125(5):2059–2068. doi: 10.1172/JCI80514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mishto M. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer's disease brains. Neurobiol. Aging. 2006;27(1):54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Fernando R. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019 Feb 20;132:58–66. doi: 10.1016/j.freeradbiomed.2018.08.037. Epub 2018 Sep 5. [DOI] [PubMed] [Google Scholar]
- 82.Levine R.L., Stadtman E.R. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36(9):1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 83.Tsakiri E.N. Proteasome dysfunction in D rosophila signals to an N rf2‐dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell. 2013;12(5):802–813. doi: 10.1111/acel.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsakiri E.N. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress. FASEB J. 2013;27(6):2407–2420. doi: 10.1096/fj.12-221408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapple S.J. Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 2016;92:152–162. doi: 10.1016/j.freeradbiomed.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Grune T., Davies K.J. The proteasomal system and HNE-modified proteins. Mol. Aspect. Med. 2003;24(4):195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 87.Merker K., Grune T. Proteolysis of oxidised proteins and cellular senescence. Exp. Gerontol. 2000;35(6):779–786. doi: 10.1016/s0531-5565(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic. Biol. Med. 2012;52(9):2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu S.-F. Ontogeny and aging of Nrf2 pathway genes in livers of rats. Life Sci. 2018;203:99–104. doi: 10.1016/j.lfs.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2018;14(Supplement C):35–40. doi: 10.1016/j.redox.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pickering A.M. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 2013;216(Pt 4):543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levy S., Forman H.J. C‐Myc is a Nrf2‐interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62(3):237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pomatto L.C.D., Sun P.Y., Davies K.J.A. To Adapt or Not to Adapt: Consequences of Declining Adaptive Homeostasis and Proteostasis with Age. Mech. Age. Dev. 2018;177:80–87. doi: 10.1016/j.mad.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pomatto L.C., Davies K.J. The role of declining Adaptive Homeostasis in ageing. J. Physiol. 2017;595(24):7275–7309. doi: 10.1113/JP275072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.