Abstract
In this retrospective cohort study, we examined the association between predialysis nephrology care status and emergency department (ED) events among patients with end-stage renal disease. Data pertaining to 76,702 patients who began dialysis treatment between 1999 and 2010 were obtained from the National Health Insurance Research Database of Taiwan (NHIRD). The patients were divided into three groups based on the timing of the first nephrology care visit prior to the initiation of maintenance dialysis, and the frequency of nephrologist visits (i.e., early referral/frequent consultation, early referral/infrequent consultation, late referral). At 1-year post-dialysis initiation, a large number of the patients had experienced at least one all-cause ED visit (58%), infection-related ED visit (17%), or potentially avoidable ED visit (7%). Cox proportional hazard models revealed that patients who received early frequent care faced an 8% lower risk of all-cause ED visit (HR: 0.92; 95% CI: 0.90–0.94), a 24% lower risk of infection-related ED visit (HR: 0.76; 95% CI: 0.73–0.79), and a 24% lower risk of avoidable ED visit (HR: 0.76; 95% CI: 0.71–0.81), compared with patients in the late referral group. With regard to the patients undergoing early infrequent consultations, the only marginally significant association was for infection-related ED visits. Recurrent event analysis revealed generally consistent results. Overall, these findings indicate that continuous nephrology care from early in the predialysis period could reduce the risk of ED utilization in the first year of dialysis treatment.
Keywords: end-stage renal disease (ESRD), chronic kidney disease (CKD), dialysis initiation, quality of care, predialysis nephrology care, early referral, emergency department visits, infection, avoidable emergency department visits
1. Introduction
Researchers around the globe have noted increases in the number of people diagnosed with end-stage renal disease (ESRD) and the number of patients receiving maintenance dialysis treatment [1,2]. Compared to the general population, ESRD patients are generally older individuals with inferior health and multiple comorbidities. As a result, ESRD is associated with high morbidity levels and a high risk of early death [3]. ESRD also imposes high social costs and is a considerable draw on medical resources. A number of researchers have recommended that patients with end-stage of chronic kidney disease (CKD) be referred to specialist nephrology services as soon as possible in order to prepare for long-term dialysis and manage the various risk factors associated with this disease [4]. Early nephrology referral is particularly important for patients with advanced CKD.
Early referral can help to slow the progression to ESRD and diminished renal function [5,6]. It has also been associated with reduced healthcare costs [7], reduced risk of cardiovascular events [8], reduced risk of early technique failure [9], and lower hospitalization and mortality rates [5,10,11,12,13,14]. Patients who receive an early referral tend to enjoy more quality-adjusted life-years (QALYs) [15], superior placement of dialysis access [12,16], and greater freedom in the selection of dialysis modality [17]. However, the association between patterns in predialysis care and visits to the emergency department (ED) has yet to be elucidated.
Patients undergoing dialysis tend to make frequent visits to the emergency department (ED) [18,19], particularly those receiving maintenance hemodialysis [20]. Even after controlling for age and sex, these patients are eight times more likely than those not receiving dialysis to be transferred to an ED [21]. This is a clear indication of the need for strategies and interventions to reduce the health risks facing dialysis patients. One previous study included nephrology care as a prime factor affecting ED use within one year after the initiation of dialysis in patients with ESRD [22]; however, there was no mention of the timing with which nephrology care was implemented.
One recent study reported that the timing of referrals and the frequency of nephrology care strongly influence the healthcare received by patients approaching the ESRD stage [8]. In this study, we employed long-term data in characterizing the association between the timely implementation of continuous predialysis nephrology care and the risk of ED visits within the first year after beginning long-term dialysis. This study was based on the hypothesis that early frequent nephrology care reduces the probability of all-cause, infection-related, and potentially avoidable ED visits among patients diagnosed with ESRD.
2. Methods
2.1. Dataset
The data used in this study was obtained from the National Health Insurance Research Database (NHIRD) of Taiwan, which is maintained by the National Health Research Institute (NHRI). We obtained a random sample of patients who had either received dialysis care, or had a principal diagnosis of acute kidney injury, CKD, or a severe neurological disease during the study period (1997–2011). The random sample included two million patients (random sampling fraction = 71%). The research data included demographic information, diagnoses, treatment procedures, prescriptions, health insurance information, and the type of medical service providers (for outpatients and inpatients). To ensure patient confidentiality, the database includes only de-identified information, and linkages to other databases are prohibited. This study was approved by the institutional review board of the Far Eastern Memorial Hospital (approval number 102167-E), which waived the requirement for informed consent.
2.2. Design and Study Participants
This observational retrospective cohort study enrolled ESRD patients who started receiving long-term dialysis therapy between 1 January 1999 and 31 December 2010. Long-term dialysis was defined as dialysis treatment spanning >90 days, during which the interval between each session was <60 days. We excluded patients with missing information related to date of birth or sex as well as patients aged <20 years at the time of the first dialysis treatment.
Patient tracking was conducted from the initiation of long-term dialysis to the occurrence of an ED event, the receipt of a successful kidney transplant, death, loss to follow-up, or the end of the study period (365 days post-dialysis). The dates of deaths were confirmed by referring to the registry of catastrophic illness or the patient’s discharge status. In cases where the medical care records of a patient were not updated for more than one year, the patient was deemed lost to follow-up.
2.3. Variables
2.3.1. Independent Variables
We adopted the approach outlined by Yang et al. [8] to divide the patients into three groups based on predialysis care patterns (i.e., the time of referral to a specialist and the frequency of consultations with a nephrologist). The groups are described as follows: (1) late nephrology care group: patients who made their first visit to a nephrologist within 6 months prior to the initiation of long-term dialysis; (2) early frequent nephrology care group: patients who made their first visit to a nephrologist more than six months prior to the initiation of long-term dialysis and made at least one visit to a nephrologist every three months; (3) early infrequent nephrology care: patients who made their first visit to a nephrologist more than six months prior to the initiation of long-term dialysis and made visits to a nephrologist at intervals exceeding three months.
2.3.2. Outcome Variables
Analysis was performed to identify all-cause ED visits, infection-related ED visits, and potentially avoidable ED visits within the first year following the initiation of dialysis. ED events were coded using discharge diagnosis codes from the international classification of disease, ninth revision, clinical modification (ICD-9-CM).
Infection-related ED use included ED visits for bacteremia, endocarditis, peritonitis, septicemia, gastrointestinal infection, genitourinary infection, joint or bone infection, pulmonary infection, and soft-tissue infection [23]. The definition and technical specifications of potentially avoidable ED use were obtained from the prevention quality indicators (PQIs) version 6.0 prepared by the agency for healthcare research and quality (AHRQ) [24].
We adopted the AHRQ definitions for overall, acute, and chronic composite PQIs in assessing potentially avoidable ED visits and ED visits related to acute disease and chronic disease conditions. Each composite indicator was aggregated at the patient level. The criteria used to define a potentially avoidable ED visit included the occurrence of any of the following PQIs: bacterial pneumonia, urinary tract infection, dehydration, adult asthma, chronic obstructive pulmonary disease, short- or long-term complications from diabetes mellitus, uncontrolled diabetes mellitus, diabetes mellitus–related lower extremity amputation, hypertension, and heart failure. The criteria used to define acute disease condition included ED visits for dehydration, bacterial pneumonia, urinary tract infection, and dehydration. The criteria used to define chronic disease condition included ED visits for adult asthma, chronic obstructive pulmonary disease, short or long-term complications from diabetes mellitus, uncontrolled diabetes mellitus, diabetes mellitus–related lower extremity amputation, hypertension, and heart failure.
2.4. Covariates
The covariates in this study included the year in which long-term dialysis was initiated and the age, sex, economic status, dialysis modality, and baseline health status of the patients. The baseline health status of the patients was estimated using the occurrence of hospitalization, the number of physician visits, Deyo’s Charlson comorbidity index (CCI), and comorbid conditions within one year prior to the initiation of long-term dialysis.
The NHIRD does not list socioeconomic indicators (e.g., education or personal/household income); therefore, we sought to establish the economic status of the patients based on the level of their insurance premiums. Dialysis modality refers to methods established within three months after the initiation of maintenance dialysis treatment. Outpatient clinic visits were divided into three equal tertiles. CCI and comorbidities were calculated in cases where a diagnosis had been recorded at least three times in the outpatient claim records or at least one time in the inpatient claim records. Comorbid diseases included arrhythmia, cancer, cerebral vascular disease, chronic liver disease, chronic obstructive pulmonary disease, coronary artery disease, dementia, diabetes, dyslipidemia, gout, heart failure, hypertension, osteoporosis, peripheral vascular disease, peptic ulcer disease, psychiatric disorders, and valvular heart disease.
2.5. Statistical Analysis
Prior to conducting the analysis, the distributions of the continuous variables were assessed using the Kolmogorov-Smirnov test. In cases where the data were normally distributed, analysis of variance was used for continuous variables; otherwise, if the data were non-normally distributed, the Kruskal-Wallis test was used to compare between-group differences. The chi-square test was used for categorical variables. The Cox proportional hazard model was used to evaluate the association between patterns in predialysis care and the first ED event in the early dialysis period. Considering that participants could have repeated ED events during follow-up, we employed proportional rates and proportional means regression models for recurrence data [25].
Sensitivity analysis was conducted to determine the composite outcome of an ED event or death. Subgroup analysis was performed according to age, sex, economic status, treatment modality, CCI, and diabetes status to evaluate potential effect modifiers. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient Characteristics and Nephrology Care Status
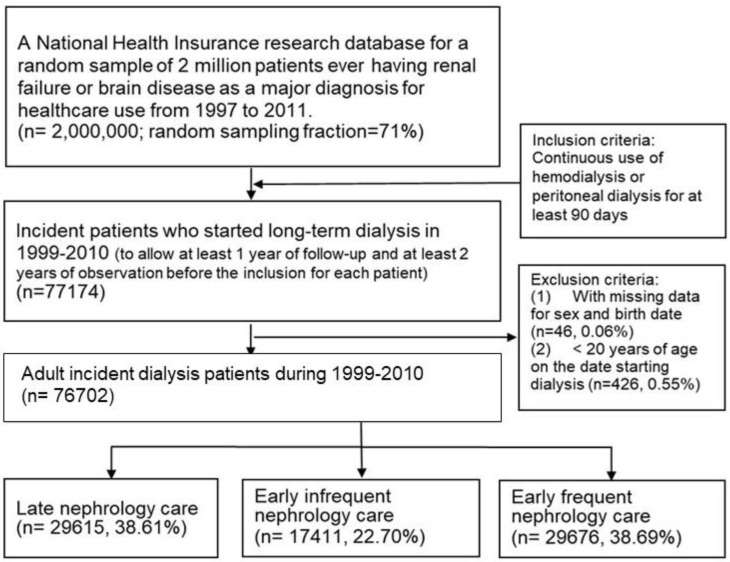
Figure 1 presents a flowchart showing the patient recruitment process. Between 1999 and 2010, a total of 77,174 patients in the database started long-term dialysis; however, 426 patients were excluded due to age and 46 were excluded due to missing data. Among the remaining 76,702 eligible patients, 38.7% received early frequent care, 22.7% received early infrequent care, and 38.6% received late nephrology care.
Figure 1.
Flow chart of cohort establishment.
Compared with the other groups, the early frequent group included patients with a higher average age and higher economic status. Furthermore, most of the patients in the early frequent group were women and underwent peritoneal dialysis. Within one year prior to the initiation of long-term dialysis, patients in the early frequent group were more likely to visit outpatient clinics; however, they were less likely to be admitted to hospital. Patients in the early infrequent care group were more likely to develop comorbid conditions. Baseline characteristics in predialysis referral patterns are listed in Table 1. Baseline characteristics of patients with and without ED visit are shown in Appendix A, Table A1.
Table 1.
Baseline characteristics of patients.
| Characteristics | Nephrology Care Status | p-Value | ||
|---|---|---|---|---|
| Late (n = 29,615, 38.61%) |
Early Infrequent (n = 17,411, 22.70%) |
Early Frequent (n = 29,676, 38.69%) |
||
| Age, mean (SD) | 61.80 (15.23) | 63.19 (14.04) | 63.45 (13.30) | <0.0001 |
| Age, n (%) | ||||
| <65 | 16,115 (54.41) | 8792 (50.50) | 14,870 (50.11) | <0.0001 |
| 65+ | 13,500 (45.59) | 8619 (49.50) | 14,806 (49.89) | |
| Sex, n (%) | ||||
| Male | 15,586 (52.63) | 8660 (49.74) | 13,764 (46.38) | <0.0001 |
| Female | 14,029 (47.37) | 8751 (50.26) | 15,912 (53.62) | |
| Economic status, n (%) | ||||
| Low | 14,550 (49.13) | 8257 (47.42) | 13,260 (44.68) | <0.0001 |
| High | 15,065 (50.87) | 9154 (52.58) | 16,416 (55.32) | |
| Dialysis modality, n (%) | ||||
| Hemodialysis | 27,109 (91.54) | 15,956 (91.64) | 25,799 (86.94) | <0.0001 |
| Peritoneal dialysis | 2506 (8.46) | 1455 (8.36) | 3877 (13.06) | |
| ESRD initiation year, n (%) | ||||
| 1999 | 2375 (8.02) | 1256 (7.21) | 1307 (4.40) | <0.0001 |
| 2000 | 2369 (8.00) | 1239 (7.12) | 1556 (5.24) | |
| 2001 | 2371 (8.01) | 1328 (7.63) | 1892 (6.38) | |
| 2002 | 2446 (8.26) | 1363 (7.83) | 2103 (7.09) | |
| 2003 | 2383 (8.05) | 1536 (8.82) | 2320 (7.82) | |
| 2004 | 2508 (8.47) | 1569 (9.01) | 2199 (7.41) | |
| 2005 | 2528 (8.54) | 1650 (9.48) | 2641 (8.90) | |
| 2006 | 2444 (8.25) | 1557 (8.94) | 2424 (8.17) | |
| 2007 | 2604 (8.79) | 1480 (8.50) | 2881 (9.71) | |
| 2008 | 2597 (8.77) | 1474 (8.47) | 3115 (10.50) | |
| 2009 | 2415 (8.15) | 1489 (8.55) | 3445 (11.61) | |
| 2010 | 2575 (8.69) | 1470 (8.44) | 3793 (12.78) | |
| Prior hospitalization, n (%) | ||||
| Yes | 27,551 (93.03) | 16,208 (93.09) | 25,677 (86.52) | <0.0001 |
| No | 2064 (6.97) | 1203 (6.91) | 3999 (13.48) | |
| Prior physician visits, mean (SD) | 28.77 (20.59) | 35.22 (21.90) | 39.25 (19.82) | <0.0001 |
| Prior physician visits, n (%) | ||||
| Low (0–23) | 13,774 (46.51) | 5765 (33.11) | 5909 (19.91) | <0.0001 |
| Intermediate (24–39) | 8775 (29.63) | 5717 (32.84) | 11,929 (40.20) | |
| High (40+) | 7066 (23.86) | 5929 (34.05) | 11,838 (39.89) | |
| Deyo’s Charlson Comorbidity Index, mean (SD) | 4.16 (2.26) | 4.80 (2.30) | 4.50 (2.13) | <0.0001 |
| Deyo’s Charlson Comorbidity Index, n (%) | ||||
| <4 | 13,119 (44.30) | 5731 (32.92) | 11,397 (38.40) | <0.0001 |
| 4+ | 16,496 (55.70) | 11,680 (67.08) | 18,279 (61.60) | |
| Comorbidity, n (%) | ||||
| Arrhythmia | 2114 (7.14) | 1447 (8.31) | 1931 (6.51) | <0.0001 |
| Cancer | 2199 (7.43) | 1387 (7.97) | 2260 (7.62) | 0.10 |
| Cerebral vascular disease | 3794 (12.81) | 2288 (13.14) | 2753 (9.28) | <0.0001 |
| Chronic liver disease | 2918 (9.85) | 1939 (11.14) | 2870 (9.67) | <0.0001 |
| Chronic obstructive pulmonary disease | 2921 (9.86) | 1921 (11.03) | 2346 (7.91) | <0.0001 |
| Coronary artery disease | 2879 (9.72) | 2081 (11.95) | 2522 (8.50) | <0.0001 |
| Dementia | 1102 (3.72) | 741 (4.26) | 876 (2.95) | <0.0001 |
| Diabetes | 15,121 (51.06) | 9972 (57.27) | 15,647 (52.73) | <0.0001 |
| Dyslipidemia | 4847 (16.37) | 3446 (19.79) | 6495 (21.89) | <0.0001 |
| Gout | 3201 (10.81) | 2508 (14.40) | 4981 (16.78) | <0.0001 |
| Heart failure | 13,508 (45.61) | 9174 (52.69) | 13,140 (44.28) | <0.0001 |
| Hypertension | 19,901 (67.20) | 13,226 (75.96) | 22,538 (75.95) | <0.0001 |
| Osteoporosis | 1406 (4.75) | 1165 (6.69) | 1841 (6.20) | <0.0001 |
| Peptic ulcer disease | 4462 (15.07) | 3208 (18.43) | 4879 (16.44) | <0.0001 |
| Peripheral vascular disease | 833 (2.81) | 492 (2.83) | 724 (2.44) | 0.007 |
| Psychiatric disorder | 1524 (5.15) | 1197 (6.87) | 1936 (6.52) | <0.0001 |
| Vascular heart disease | 1940 (6.55) | 1260 (7.24) | 1565 (5.27) | <0.0001 |
3.2. ED Event Distribution
During follow-up, a large number of the patients experienced at least one all-cause ED visit (58%), infection-related ED visit (17%), or potentially avoidable ED visit (7%). The mean number of ED visits per patient-year were as follows: all-cause ED visits (1.73), infection-related ED visits (0.28), and potentially avoidable ED visits (0.09). Patients who received early referrals and made frequent visits to their physician had the lowest mean number of ED visits, and had a lower rate of frequent ED users (i.e., equal to or more than three times visits to the ED) (Table 2).
Table 2.
Characteristics for ESRD patients and 1-year post-dialysis ED visits.
| All Patients (n = 76,702) |
Nephrology Care Status | ||||
|---|---|---|---|---|---|
| Late (n = 29,615, 38.61%) |
Early Infrequent (n = 17,411, 22.70%) |
Early Frequent (n = 29,676, 38.69%) |
|||
| All-cause | ED visits No. (%) of patients | 44,714 (58.30) |
16,852 (56.90) |
10,462 (60.09) |
17,400 (58.63) |
| Mean No. of ED visits per patient-year | 1.73 | 1.70 | 1.89 | 1.66 | |
| ED visits ≥ 3 times, N (%) | 16,651 (21.71) |
6078 (20.52) |
4172 (23.96) |
6401 (21.57) |
|
| Infection-related | ED visits No. (%) of patients | 13,353 (17.41) |
5314 (17.94) |
3243 (18.63) |
4796 (16.16) |
| Mean No. of ED visits per patient-year | 0.28 | 0.30 | 0.30 | 0.25 | |
| ED visits ≥ 3 times, N (%) | 1405 (1.83) |
580 (1.96) |
334 (1.92) |
491 (1.65) |
|
| Avoidable | ED visits No. (%) of patients | 5352 (6.98) |
2138 (7.22) |
1368 (7.86) |
1846 (6.22) |
| Mean No. of ED visits per patient-year | 0.09 | 0.10 | 0.11 | 0.08 | |
| ED visits ≥ 3 times, N (%) | 204 (0.27) |
95 (0.32) |
55 (0.32) |
54 (0.18) |
|
3.3. Risk of ED Events within 1-Year after Beginning Dialysis
Compared to patients in the late group, the adjusted hazard ratio (HR) of all-cause ED visits was 0.92 for patients receiving early frequent care (95% confidence interval [CI]: 0.90–0.94) and 1.01 for patients receiving early infrequent care (95% CI: 0.99–1.04) (Table 3).
Table 3.
Adjusted risk for 1-year EDs based on predialysis nephrology care status.
| Outcome and Predialysis Nephrology Care Status a | COX Proportional Model | Recurrent Event Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | RR | 95% CI | ||||
| All-cause ED visit | Early frequent | 0.92 | 0.90 | 0.94 | 0.94 | 0.93 | 0.96 |
| Early infrequent | 1.02 | 0.99 | 1.04 | 1.01 | 0.99 | 1.02 | |
| Infection-related ED visit | Early frequent | 0.76 | 0.73 | 0.79 | 0.84 | 0.82 | 0.87 |
| Early infrequent | 0.94 | 0.90 | 0.98 | 0.96 | 0.93 | 0.99 | |
| Avoidable ED visit | Early frequent | 0.76 | 0.71 | 0.81 | 0.79 | 0.75 | 0.84 |
| Early infrequent | 0.95 | 0.89 | 1.02 | 0.97 | 0.91 | 1.03 | |
Notes: Adjusted for ESRD initiation year, age, sex, economic status, dialysis modality, prior hospitalization, prior physician visits, and comorbid conditions (arrhythmia, cancer, cerebral vascular disease, chronic liver disease, chronic obstructive pulmonary disease, coronary artery disease, dementia, diabetes, dyslipidemia, gout, heart failure, hypertension, osteoporosis, peptic ulcer disease, peripheral vascular disease, psychiatric disorder, vascular heart disease). Abbreviations: ED, emergency department; CI, confidence interval; HR, hazard ratio; RR, relative risk. a Reference group is late nephrology care.
After adjustment for covariates, patients receiving early frequent care faced a 24% lower risk of infection-related ED visit (HR: 0.76; 95% CI: 0.73–0.79), whereas patients receiving early infrequent care experienced 6% lower risk of infection-related ED visit (HR: 0.94; 95% CI: 0.90–0.98).
In terms of potentially avoidable ED visits, patients in the early frequent group faced a 24% lower risk for all-condition avoidable ED visits (HR: 0.76; 95% CI: 0.71–0.81) compared to patients in the late group. The effects of early frequent care on acute-condition and chronic-condition avoidable ED visits followed the same trend (data not shown).
As shown in Table 3, our analysis of recurrent ED visits yielded results similar to those above. When death was considered a composite outcome in sensitivity analysis, the influence of early frequent care on first ED events anda recurrent ED events was consistent. Subgroup analysis revealed that younger patients, non-diabetics, patients with lower CCI scores, and those with higher economic status benefited the most from early frequent nephrology care in terms of improved healthcare outcomes (Figure 2 and Figure 3).
Figure 2.
Subgroup analysis of hazard ratio for 1-year ED visit between the early-frequent and late-nephrology-care groups for first event of (a) all-cause, (b) infection-related, and (c) potentially avoidable ED visit. Abbreviations: ED, emergency department; CCI, Deyo’s Charlson Comorbidity Index; DM, diabetes mellitus; HD, hemodialysis; HR, hazard ratio; PD, peritoneal dialysis; Pint, p value for interaction term.
Figure 3.
Subgroup analysis of relative risk for 1-year ED visits between the early-frequent and late-nephrology-care groups for recurrent events of (a) all-cause, (b) infection-related, and (c) potentially avoidable ED visits. Abbreviations: ED, emergency department; CCI, Deyo’s Charlson Comorbidity Index; DM, diabetes mellitus; HD, hemodialysis; HR, hazard ratio; PD, peritoneal dialysis; Pint, p value for interaction term.
4. Discussion
In this study, we investigated the relationship between the timing and continuity of predialysis nephrology care and ED events among patients undergoing maintenance dialysis. This observational cohort study was based on data obtained from a comprehensive nationwide database (NHIRD) of healthcare utilization in Taiwan. Our results revealed that frequent nephrology care for ESRD patients in the early predialysis stage was associated with a lower risk of all-cause, infection-related, and potentially avoidable ED visits within the first year of dialysis (adjusted HR = 0.92, 0.76, and 0.76, respectively). Note that the time of care was not the sole issue. Early but infrequent care was associated with only a marginally lower risk of infection-related ED visit.
We discovered that 58% of the patients in this study were admitted to the ED within one year after beginning dialysis treatment, and the mean number of all-cause ED visits was 1.73 per patient-year during the study period (1999–2011). Two recent population-based studies investigated the incidence of ED visits among patients with ESRD. One U.S. Medicare cohort study reported that after initiating dialysis, 55% of the patients were admitted to ED within one year (2.89 ED visits per patient-year) [22]. Another Canadian cohort study reported 1.5 ED visits per patient-year [21]. It is likely that the high rate of ED visits among dialysis patients can be attributed to urgent or critical clinical situations, such as complications of vascular access or adverse cardiovascular events.
Researchers have previously reported that patients who received early referrals have better control over clinical parameters (e.g., blood pressure and serum levels of albumin, bicarbonate, calcium, cholesterol, hemoglobin, potassium, and phosphate), compared to patients who received referrals after an extended duration [11,13,26]. This is an indication that early referrals make it easier to manage health status and in so doing reduce the likelihood of escalation to urgent status. In this study, we discovered that patients who received referrals early and made frequent visits to their physician were less likely to be hospitalized within one year prior to initiating dialysis, despite the fact that patients in this group tended to be older and presented higher CCI scores. This means that frequent consultations with nephrologists before the disease has a chance to progress enhances the likelihood of a favorable outcome.
Nonetheless, it is important to note that the patient (not the physician) decides whether to visit the ED. This appears to indicate that early nephrological intervention may influence the self-management ability of the patient by enabling more informed decisions. The education of patients in terms of self-care could help them to identify signs of impending clinical difficulties and improve their ability to deal appropriately with important symptoms that might otherwise result in an ED visit. For instance, nephrologists could educate their patients of the dangers of swelling and/or rapid gain weight. Patients should be made aware that under these conditions, they should control their intake of dietary phosphorus, undergo dialysis earlier than scheduled, or perhaps take a diuretic instead of heading directly to the ED. Prior to dialysis, it is important that nephrologists enable the timely preparation of dialysis access in hemodialysis patients [27] in order to reduce the risk of systemic infection [28,29]. Many CKD patients with impaired kidney function take medication at inappropriate dosages [30,31]. Researchers have shown that adverse events associated with the inappropriate use of medications can increase the frequency of ED visits among older adults [32]. Nephrologists are more likely than primary care physicians to focus on appropriate dosing for patients with renal disease [33].
In terms of the initial contact with a nephrologist, the cutoff point between a late referral and early referral has been set at one month [27], three months [9,10,11,13], four months [14,17,34], six months [5,8,35,36], or one year [7,26,37]. Notwithstanding the variations in defining nephrology referral timing, Quaglia et al. mentioned that, “Predialysis nephrology care is a much wider concept than providing the patient with a dialysis access—a crucial but minimal requirement—and consequently demands a longer time (i.e., several years) to produce results.” [38]. In this study, we discovered that a referral six months prior to dialysis does not necessarily reduce the risk of unfavorable outcomes; however, an early referral in conjunction with frequent nephrologist consultations can reduce the risk of ED visits among patients with ESRD. The continuity of nephrology care makes a difference. With continuous care, nephrologists may coordinate the care plan and provide proactive care according to the spectrum of problems encountered in pre-ESRD patients. On the other hand, patients would have more chances to learn how to cope with the health challenges at predialysis stage with the continuous help and support from professionals.
This study has a number of limitations. First, the NHIRD does not list marital status, education, health literacy, family history, health behaviors, or social support. Moreover, we were unable to control for clinical values, such as the results of laboratory tests. Second, our results are susceptible to various forms of bias due to our inability to measure the rate of CKD progression to ESRD. Third, we were unable to determine the triage status of each ED stay, which made it impossible to exclude inappropriate (i.e., nonurgent) ED visits. Additionally, we considered only the timing and frequency of nephrology care prior to maintenance dialysis. We did not explore patient–provider relationships, health education content during the care process, the circulation and/or discussion of medical information between patients and care providers, or medication adherence. It is very likely that these factors could affect the outcomes of nephrology care. Otherwise, a retrospective observational design can only describe associations; i.e., it cannot be used to indicate causation. Further studies are required to validate actual causal relationships. Finally, predialysis management by primary care providers can vary considerably among regions [11]; thus, the generalizability of the study findings should be carefully verified.
To the best of our knowledge, this was the first study to use nationwide data to explore the association between continuity of predialysis nephrology care and ED events among patients with ESRD in the early dialysis period. The need of continuity of nephrology care in the pre-ESRD stage should be addressed. A suitable disease management regime can reduce health risks, improve health care quality for patients undergoing dialysis, and facilitate the efficient use of medical resources. We recommend that health policy makers and health professionals encourage patients approaching the end stage of CKD to seek consultation with a nephrologist in a timely manner, and educate patients concerning the continuity of nephrology care.
5. Conclusions
This study provides further evidence to support the contention that patients undergoing dialysis face a high risk of ED visits, and that timely and frequent consultations with a nephrologist during the predialysis stage is associated with a lower incidence of ED events within the first year of beginning dialysis.
Acknowledgments
The study is based on data provided by National Health Insurance Administration, Ministry of Health and Welfare, and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions shown in this article do not represent those of National Health Research Institutes or National Health Insurance Administration, Ministry of Health and Welfare, Taiwan.
Appendix A
Table A1.
Baseline characteristics of patients with and without ED visit.
| Characteristics | All-Cause ED Visit | p-Value | Infection-Related ED Visit | p-Value | Potentially Avoidable ED Visit | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 31,988, 41.70%) |
Yes (n = 44,714, 58.30%) |
No (n = 63349, 82.59%) |
Yes (n = 13353, 17.41%) |
No (n = 71,350, 93.02%) |
Yes (n = 5352, 6.98%) |
||||
| Age, mean (SD) | 60.90 (14.39) | 64.09 (14.01) | <0.0001 | 61.86 (14.21) | 67.03 (13.67) | <0.0001 | 62.54 (14.29) | 65.58 (13.44) | <0.0001 |
| Age, n (%) | |||||||||
| <65 | 18,418 (57.58) | 21,359 (47.77) | <0.0001 | 34,615 (54.64) | 5162 (38.66) | <0.0001 | 37,412 (52.43) | 2365 (44.19) | <0.0001 |
| 65+ | 13,570 (42.42) | 23,355 (52.23) | 28,734 (45.36) | 8191 (61.34) | 33,938 (47.57) | 2987 (55.81) | |||
| Sex, n (%) | |||||||||
| Male | 16,017 (50.07) | 21,993 (49.19) | 0.02 | 31,562 (49.82) | 6448 (48.29) | 0.001 | 35,438 (49.67) | 2572 (48.06) | 0.02 |
| Female | 15,971 (49.93) | 22,721 (50.81) | 31,787 (50.18) | 6905 (51.71) | 35,912 (50.33) | 2780 (51.94) | |||
| Economic status, n (%) | |||||||||
| Low | 14,568 (45.54) | 21,499 (48.08) | <0.0001 | 29,558 (46.66) | 6509 (48.75) | <0.0001 | 33,385 (46.79) | 2682 (50.11) | <0.0001 |
| High | 17,420 (54.46) | 23,215 (51.92) | 33,791 (53.34) | 6844 (51.25) | 37,965 (53.21) | 2670 (49.89) | |||
| Dialysis modality, n (%) | |||||||||
| Hemodialysis | 28,240 (88.28) | 40,624 (90.85) | <0.0001 | 56,736 (89.56) | 12,128 (90.83) | <0.0001 | 63,886 (89.54) | 4978 (93.01) | <0.0001 |
| Peritoneal dialysis | 3748 (11.72) | 4090 (9.15) | 6613 (10.44) | 1225 (9.17) | 7464(10.46) | 374 (6.99) | |||
| ESRD initiation year, n (%) | |||||||||
| 1999 | 3001 (9.38) | 1937 (4.33) | <0.0001 | 4653 (7.35) | 285 (2.13) | <0.0001 | 4768 (6.68) | 170 (3.18) | <0.0001 |
| 2000 | 3413 (10.67) | 1751 (3.92) | 4776 (7.54) | 388 (2.91) | 4952 (6.94) | 212 (3.96) | |||
| 2001 | 2242 (7.01) | 3349 (7.49) | 4807 (7.59) | 784 (5.87) | 5163 (7.24) | 428 (8.00) | |||
| 2002 | 2277 (7.12) | 3635 (8.13) | 5028 (7.94) | 884 (6.62) | 5468 (7.66) | 444 (8.30) | |||
| 2003 | 3337 (10.43) | 2902 (6.49) | 5459 (8.62) | 780 (5.84) | 5877 (8.24) | 362 (6.76) | |||
| 2004 | 3608 (11.28) | 2668 (5.97) | 5473 (8.64) | 803 (6.01) | 5957 (8.35) | 319 (5.96) | |||
| 2005 | 2424 (7.58) | 4395 (9.83) | 5571 (8.79) | 1248 (9.35) | 6259 (8.77) | 560 (10.46) | |||
| 2006 | 2250 (7.03) | 4175 (9.34) | 5079 (8.02) | 1346 (10.08) | 5910 (8.28) | 515 (9.62) | |||
| 2007 | 2363 (7.39) | 4602 (10.29) | 5467 (8.63) | 1498 (11.22) | 6425 (9.00) | 540 (10.09) | |||
| 2008 | 2280 (7.13) | 4906 (10.97) | 5510 (8.70) | 1676 (12.55) | 6579 (9.22) | 607 (11.34) | |||
| 2009 | 2368 (7.40) | 4981 (11.14) | 5608 (8.85) | 1741 (13.04) | 6737 (9.44) | 612 (11.43) | |||
| 2010 | 2425 (7.58) | 5413 (12.11) | 5918 (9.34) | 1920 (14.38) | 7255 (10.17) | 583 (10.89) | |||
| Prior hospitalization, n (%) | |||||||||
| Yes | 28,088 (87.81) | 41,348 (92.47) | <0.0001 | 56,763 (89.60) | 12,673 (94.91) | <0.0001 | 64,360 (90.20) | 5076 (94.84) | <0.0001 |
| No | 3900 (12.19) | 3366 (7.53) | 6586(10.40) | 680 (5.09) | 6990 (9.80) | 276 (5.16) | |||
| Prior physician visits, mean (SD) | 32.35 (20.21) | 35.67 (21.64) | <0.0001 | 33.77 (20.79) | 36.71 (22.46) | <0.0001 | 33.97 (20.96) | 38.43 (22.75) | <0.0001 |
| Prior physician visits, n (%) | |||||||||
| Low (0–23) | 11,726 (36.66) | 13, 722 (30.69) | <0.0001 | 21,545 (34.01) | 3903 (29.23) | <0.0001 | 24,069 (33.73) | 1379 (25.77) | <0.0001 |
| Intermediate (24–39) | 11,011 (34.42) | 15,410 (34.46) | 21,864 (34.51) | 4557 (34.13) | 24,594 (34.47) | 1827 (34.14) | |||
| High (40+) | 9251 (28.92) | 15,582 (34.85) | 19,940 (31.48) | 4893 (36.64) | 22,687 (31.80) | 2146 (40.10) | |||
| CCI, mean (SD) | 4.08 (2.13) | 4.69 (2.27) | <0.0001 | 4.33 (2.19) | 4.96 (2.36) | <0.0001 | 4.38 (2.22) | 5.22 (2.26) | <0.0001 |
| CCI, n (%) | |||||||||
| <4 | 14,923 (46.65) | 15,324 (34.27) | <0.0001 | 26,275 (41.48) | 3972 (29.75) | <0.0001 | 28,957 (40.58) | 1290 (24.10) | <0.0001 |
| 4+ | 17,065 (53.35) | 29,390 (65.73) | 37,074 (58.52) | 9381 (70.25) | 42,393 (59.42) | 4062 (75.90) | |||
| Comorbidity, n (%) | |||||||||
| Arrhythmia | 2005 (6.27) | 3487 (7.80) | <0.0001 | 4297 (6.78) | 1195 (8.95) | <0.0001 | 4996 (7.00) | 496 (9.27) | <0.0001 |
| Cancer | 2047 (6.40) | 3799 (8.50) | <0.0001 | 4533 (7.16) | 1313 (9.83) | <0.0001 | 5468 (7.66) | 378 (7.06) | 0.11 |
| Cerebral vascular disease | 3168 (9.90) | 5667 (12.67) | <0.0001 | 6626 (10.46) | 2209 (16.54) | <0.0001 | 7964 (11.16) | 871 (16.27) | <0.0001 |
| Chronic liver disease | 2896 (9.05) | 4831 (10.80) | <0.0001 | 6144 (9.70) | 1583 (11.86) | <0.0001 | 7174 (10.05) | 553 (10.33) | 0.51 |
| COPD | 2792 (8.73) | 4396 (9.83) | <0.0001 | 5492 (8.67) | 1696 (12.70) | <0.0001 | 6500 (9.11) | 688 (12.86) | <0.0001 |
| Coronary artery disease | 2466 (7.71) | 5016 (11.22) | <0.0001 | 5769 (9.11) | 1713 (12.83) | <0.0001 | 6714 (9.41) | 768 (14.35) | <0.0001 |
| Dementia | 918 (2.87) | 1801 (4.03) | <0.0001 | 1818 (2.87) | 901 (6.75) | <0.0001 | 2428 (3.40) | 291 (5.44) | <0.0001 |
| Diabetes | 14,830 (46.36) | 25,910 (57.95) | <0.0001 | 32,390 (51.13) | 8350 (62.53) | <0.0001 | 36,844 (51.64) | 3896 (72.80) | <0.0001 |
| Dyslipidemia | 5470 (17.10) | 9318 (20.84) | <0.0001 | 12,106 (19.11) | 2682 (20.09) | 0.009 | 13,537 (18.97) | 1251 (23.37) | <0.0001 |
| Gout | 4233 (13.23) | 6457 (14.44) | <0.0001 | 8751 (13.81) | 1939 (14.52) | 0.03 | 10,044 (14.08) | 646 (12.07) | <0.0001 |
| Heart failure | 13,753 (42.99) | 22,069 (49.36) | <0.0001 | 28,914 (45.64) | 6908 (51.73) | <0.0001 | 32,685 (45.81) | 3137 (58.61) | <0.0001 |
| Hypertension | 22,536 (70.45) | 33,129 (74.09) | <0.0001 | 45758 (72.23) | 9907 (74.19) | <0.0001 | 51,457 (72.12) | 4208 (78.62) | <0.0001 |
| Osteoporosis | 1653 (5.17) | 2759 (6.17) | <0.0001 | 3424 (5.40) | 988 (7.40) | <0.0001 | 4034 (5.65) | 378 (7.06) | <0.0001 |
| Peptic ulcer disease | 4591 (14.35) | 7958 (17.80) | <0.0001 | 9882 (15.60) | 2667 (19.97) | <0.0001 | 11,517 (16.14) | 1032 (19.28) | <0.0001 |
| Peripheral vascular disease | 739 (2.31) | 1310 (2.93) | <0.0001 | 1599 (2.52) | 450 (3.37) | <0.0001 | 1866 (2.62) | 183 (3.42) | 0.0004 |
| Psychiatric disorder | 1578 (4.93) | 3079 (6.89) | <0.0001 | 3603 (5.69) | 1054 (7.89) | <0.0001 | 4191 (5.87) | 466 (8.71) | <0.0001 |
| Vascular heart disease | 1719 (5.37) | 3046 (6.81) | <0.0001 | 3789 (5.98) | 976 (7.31) | <0.0001 | 4315 (6.05) | 450 (8.41) | <0.0001 |
Abbreviations: CCI, Deyo’s Charlson Comorbidity Index; COPD, Chronic obstructive pulmonary disease.
Author Contributions
Conceptualization/Design, Y.-Y.C., L.C., J.-W.H. and J.-Y.Y.; Data analysis and interpretation, Y.-Y.C. and J.-Y.Y.; Project administration, J.-W.H.; Resources, L.C. and J.-W.H.; Supervision: L.C.; Manuscript writing: Y.-Y.C. and J.-Y.Y.; Final approval of manuscript: All authors.
Funding
The study was supported by grants from the Ministry of Science and Technology (MOST-104-2314-B-418-006), and Far Eastern Memorial Hospital (FEMH-104-2314-B-418-006) in Taiwan. The funding source had no role in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wetmore J.B., Collins A.J. Global challenges posed by the growth of end-stage renal disease. Ren. Replace. Ther. 2016;2:15. doi: 10.1186/s41100-016-0021-7. [DOI] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C., Agodoa L.Y., Albertus P., Ayanian J., Balkrishnan R., Bragg-Gresham J., Cao J., Chen J.L., et al. US renal data system 2016 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2017;69:A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philipneri M.D., Rocca Rey L.A., Schnitzler M.A., Abbott K.C., Brennan D.C., Takemoto S.K., Buchanan P.M., Burroughs T.E., Willoughby L.M., Lentine K.L. Delivery patterns of recommended chronic kidney disease care in clinical practice: Administrative claims-based analysis and systematic literature review. Clin. Exp. Nephrol. 2008;12:41–52. doi: 10.1007/s10157-007-0016-3. [DOI] [PubMed] [Google Scholar]
- 4.Tisher C.C., Bastl C.P., Bistrian B.R., Chesney R., Coggins C., Diener-West M., Fanestil D.D., Grantham J., Kunau R., Luke R.G., et al. Morbidity and mortality of renal dialysis: An NIH consensus conference statement. Ann. Intern. Med. 1994;121:62–70. doi: 10.7326/0003-4819-121-1-199407010-00013. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S., Nakata H., Yoshihara F., Kamide K., Horio T., Nakahama H., Kawano Y. Effect of early nephrology referral on the initiation of hemodialysis and survival in patients with chronic kidney disease and cardiovascular diseases. Circ. J. 2007;71:511–516. doi: 10.1253/circj.71.511. [DOI] [PubMed] [Google Scholar]
- 6.Smart N.A., Dieberg G., Ladhani M., Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst. Rev. 2014;6:121. doi: 10.1002/14651858.CD007333.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Lee J., Lee J.P., Park J.I., Hwang J.H., Jang H.M., Choi J.Y., Kim Y.L., Yang C.W., Kang S.W., Kim N.H., et al. Early Nephrology Referral Reduces the Economic Costs among Patients Who Start Renal Replacement Therapy: A Prospective Cohort Study in Korea. PLoS ONE. 2014;9:e99460. doi: 10.1371/journal.pone.0099460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J.Y., Huang J.W., Chen L., Chen Y.Y., Pai M.F., Tung K.T., Peng Y.S., Hung K.Y. Frequency of Early Predialysis Nephrology Care and Postdialysis Cardiovascular Events. Am. J. Kidney Dis. 2017;70:164–172. doi: 10.1053/j.ajkd.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 9.See E.J., Johnson D.W., Hawley C.M., Pascoe E.M., Badve S.V., Boudville N., Clayton P.A., Sud K., Polkinghorne K.R., Borlace M., et al. Risk Predictors and Causes of Technique Failure Within the First Year of Peritoneal Dialysis: An Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) Study. Am. J. Kidney Dis. 2018;72:188–197. doi: 10.1053/j.ajkd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmayer W.C., Owen W.F., Jr., Levin R., Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J. Am. Soc. Nephrol. 2003;14:486–492. doi: 10.1097/01.ASN.0000046047.66958.C3. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S., Jeganathan J.A. Timing of nephrology referral: Influence on mortality and morbidity in chronic kidney disease. Nephrourol. Mon. 2012;4:578. doi: 10.5812/numonthly.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smart N.A., Titus T.T. Outcomes of Early versus Late Nephrology Referral in Chronic Kidney Disease: A Systematic Review. Am. J. Med. 2011;124:1073–1080.e2. doi: 10.1016/j.amjmed.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Dogan E., Erkoc R., Sayarlioglu H., Durmus A., Topal C. Effects of late referral to a nephrologist in patients with chronic renal failure. Nephrology. 2005;10:516–519. doi: 10.1111/j.1440-1797.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 14.Roubicek C., Brunet P., Huiart L., Thirion X., Leonetti F., Dussol B., Jaber K., Andrieu D., Ramananarivo P., Berland Y. Timing of nephrology referral: Influence on mortality and morbidity. Am. J. Kidney Dis. 2000;36:35–41. doi: 10.1053/ajkd.2000.8241. [DOI] [PubMed] [Google Scholar]
- 15.Black C., Sharma P., Scotland G., McCullough K., McGurn D., Robertson L., Fluck N., MacLeod A., McNamee P., Prescott G. Early referral strategies for management of people with markers of renal disease: A systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol. Assess. 2010;14:1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 16.Astor B.C., Eustace J., Powe N., Klag M.J., Sadler J.H., Fink N.E., Coresh J. Timing of nephrologist referral and arteriovenous access use: The CHOICE study. Am. J. Kidney Dis. 2001;38:494–501. doi: 10.1053/ajkd.2001.26833. [DOI] [PubMed] [Google Scholar]
- 17.Stack A.G. Determinants of modality selection among incident US dialysis patients: Results from a national study. J. Am. Soc. Nephrol. 2002;13:1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 18.Miller J.B., Brauer E., Rao H., Wickenheiser K., Dev S., Omino R., Stokes-Buzzelli S. The most frequent ED patients carry insurance and a significant burden of disease. Am. J. Emerg. Med. 2013;31:16–19. doi: 10.1016/j.ajem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Morris J.N., Howard E.P., Steel K., Schreiber R., Fries B.E., Lipsitz L.A., Goldman B. Predicting risk of hospital and emergency department use for home care elderly persons through a secondary analysis of cross-national data. BMC Health Serv. Res. 2014;14:519. doi: 10.1186/s12913-014-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y.C., Hsu H.K., Lai T.S., Chiang W.C., Lin S.L., Chen Y.M., Chen C.C., Chu T.S. Emergency department utilization and resuscitation rate among patients receiving maintenance hemodialysis. J. Med. Assoc. 2019 doi: 10.1016/j.jfma.2019.01.007. in press. [DOI] [PubMed] [Google Scholar]
- 21.Komenda P., Tangri N., Klajncar E., Eng A., Di Nella M., Hiebert B., Strome T., de Faria L.R., Zacharias J.M., Verrelli M., et al. Patterns of emergency department utilization by patients on chronic dialysis: A population-based study. PLoS ONE. 2018;13:e0195323. doi: 10.1371/journal.pone.0195323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovasik B.P., Zhang R., Hockenberry J.M., Schrager J.D., Pastan S.O., Mohan S., Patzer R.E. Emergency department use and hospital admissions among patients with end-stage renal disease in the United States. JAMA Intern. Med. 2016;176:1563–1565. doi: 10.1001/jamainternmed.2016.4975. [DOI] [PubMed] [Google Scholar]
- 23.Dalrymple L.S., Johansen K.L., Chertow G.M., Cheng S.-C., Grimes B., Gold E.B., Kaysen G.A. Infection-related hospitalizations in older patients with ESRD. Am. J. Kidney Dis. 2010;56:522–530. doi: 10.1053/j.ajkd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality (AHRQ) Prevention Quality Indicators Technical Specifications Updates—Version 6.0 (ICD-9), October 2016. [(accessed on 12 April 2018)]; Available online: https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD09_v60.aspx.
- 25.Lin D.Y., Wei L.J., Yang I., Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J. R. Stat. Soc. Ser. B Stat. Methodol. 2000;62:711–730. doi: 10.1111/1467-9868.00259. [DOI] [Google Scholar]
- 26.Kim D.H., Kim M., Kim H., Kim Y.L., Kang S.W., Yang C.W., Kim N.H., Kim Y.S., Lee J.P. Early Referral to a Nephrologist Improved Patient Survival: Prospective Cohort Study for End-Stage Renal Disease in Korea. PLoS ONE. 2013;8:e55323. doi: 10.1371/journal.pone.0055323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt R.J., Domico J.R., Sorkin M.I., Hobbs G. Early referral and its impact on emergent first dialyses, health care costs, and outcome. Am. J. Kidney Dis. 1998;32:278–283. doi: 10.1053/ajkd.1998.v32.pm9708613. [DOI] [PubMed] [Google Scholar]
- 28.Hoen B., Paul-Dauphin A., Hestin D., Kessler M. EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J. Am. Soc. Nephrol. 1998;9:869–876. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 29.Lemaire X., Morena M., Leray-Moragués H., Henriet-Viprey D., Chenine L., Defez-Fougeron C., Canaud B. Analysis of risk factors for catheter-related bacteremia in 2000 permanent dual catheters for hemodialysis. Blood Purif. 2009;28:21–28. doi: 10.1159/000210034. [DOI] [PubMed] [Google Scholar]
- 30.Farag A., Garg A.X., Li L., Jain A.K. Dosing errors in prescribed antibiotics for older persons with CKD: A retrospective time series analysis. Am. J. Kidney Dis. 2014;63:422–428. doi: 10.1053/j.ajkd.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Gallieni M., Cancarini G. Drugs in the elderly with chronic kidney disease: Beware of potentially inappropriate medications. Nephrol. Dial. Transpl. 2015;30:342–344. doi: 10.1093/ndt/gfu191. [DOI] [PubMed] [Google Scholar]
- 32.Budnitz D.S., Shehab N., Kegler S.R., Richards C.L. Medication use leading to emergency department visits for adverse drug events in older adults. Ann. Intern. Med. 2007;147:755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J.X., Nash D.M., McArthur E., Farag A., Garg A.X., Jain A.K. Nephrology comanagement and the quality of antibiotic prescribing in primary care for patients with chronic kidney disease: A retrospective cross-sectional study. Nephrol. Dial. Transpl. 2018 doi: 10.1093/ndt/gfy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazmi W.H., Obrador G.T., Khan S.S., Pereira B.J., Kausz A.T. Late nephrology referral and mortality among patients with end-stage renal disease: A propensity score analysis. Nephrol. Dial. Transpl. 2004;19:1808–1814. doi: 10.1093/ndt/gfg573. [DOI] [PubMed] [Google Scholar]
- 35.Gallego E., López A., Lorenzo I., López E., Llamas F., Illescas M.L., Andrés E., Serrano A., Olivas E., Gómez Roldán C. Influence of early or late referral to nephrologist over morbidity and mortality in hemodialysis. Nefrologia. 2003;23:234–242. [PubMed] [Google Scholar]
- 36.Jungers P., Joly D., Nguyen-Khoa T., Mothu N., Bassilios N., Grünfeld J.P. Continued late referral of patients with chronic kidney disease. Presse Med. 2006;35:17–22. doi: 10.1016/S0755-4982(06)74514-6. [DOI] [PubMed] [Google Scholar]
- 37.Di Napoli A., Valle S., d’Adamo G., Pezzotti P., Chicca S., Pignocco M., Spinelli C., Di Giulio S., Di Lallo D., Predialysis Study Group of Lazio Survey of determinants and effects of timing of referral to a nephrologist: The patient’s point of view. J. Nephrol. 2010;23:603–613. [PubMed] [Google Scholar]
- 38.Quaglia M., Canavese C., Stratta P. Early Nephrology Referral: How Early Is Early Enough? Arch. Intern. Med. 2011;171:2065–2066. doi: 10.1001/archinternmed.2011.585. [DOI] [PubMed] [Google Scholar]