Abstract
Neurotensin and its high-affinity receptor, NTR1, are involved in the growth of various tumors. Few data are available regarding NTR1 expression in normal and tumoral human prostate tissue samples. NTR1 expression was assessed using immunohistochemistry in 12 normal prostate tissues, 11 benign prostatic hyperplasia (BPH), 44 prostate cancers, and 15 related metastatic lymph nodes (one per patient, when available). NTR1-staining was negative in normal prostate and BPH samples. NTR1 was overexpressed in four out of 44 (9.1%) primary tumors. There was no clear association between NTR1 overexpression and age, PSA-values, Gleason score, pT-status, nodal-status, or margin. NTR1 was expressed at a high level of five out of 15 (33.3%) metastatic lymph nodes. NTR1 overexpression was thus more frequent in metastatic lymph nodes than in primary tumors (p = 0.038). In this limited series of samples, NTR1 overexpression was observed in few primary prostate cancers. Upregulation was more frequent in related lymph nodes. The presence of this target in metastatic lymph nodes may open new perspectives for imaging and radionuclide therapy of prostate cancer. Factors driving NTR1 expression in primary prostate cancer and in nodal and distant metastases still need to be characterized.
Keywords: neurotensin receptor-1, NTR1, prostate cancer, neuropeptide
1. Introduction
Prostate cancer is the most frequent cancer in men. It is initially an androgen-dependent disease that may progress to an androgen-independent stage: castration resistant prostate cancer (CRPC) with poor prognosis related to the reactivation of androgen-receptor (AR) transcriptional activity [1,2]. Drugs targeting androgen and their receptors in CRPC have been approved but resistance limits their successes. Alternative pathways for the proliferation of prostate cancer cells have been identified, such as the neurotensin/neurotensin receptors axis [3].
Neurotensin effects are mediated through three receptor subtypes: NTR1 (high-affinity receptor) and NTR2 (low-affinity receptor), both G-protein coupled receptors, and NTR3, a single transmembrane domain. Physiologically, neurotensin is released from endocrine cells (N cells) in response to lipid ingestion and is involved in the stimulation of pancreatic, biliary, and gastric acid secretions; the facilitation of fatty acid absorption; and the regulation of small-bowel motility [4]. Human prostate cancer cells exhibit a growth response to subnanomolar concentrations of neurotensin [3]. After binding to its high-affinity receptor, NTR1, neurotensin stimulates mitosis of cancerous prostatic cells through Src-, MMP-, and PKC-dependent ligand-mediated transactivation of EGFR, which mediate IGF-1R phosphorylation and stimulation of the MAP kinase pathway in a PI3K-dependent manner [5,6,7]. Moreover, the selective NTR1 antagonist SR48692 is able to inhibit the neurotensin-induced growth of prostate cancer cells [6], suggesting the involvement of NTR1 as an oncogenic receptor in prostate cancer. Neurotensin has been showed to mediate neuroendocrine differentiation of prostate cancer [8]. Receptor subtypes involved in this mechanism are still a matter of debate [8,9]. Interestingly, androgen deprivation induces acute neurotensin production, suggesting the involvement of neurotensin and its receptors in androgen-independent prostate cancer and/or neuroendocrine differentiation of prostate cancer [8,9,10]. Non-tumoral cells in the microenvironment producing neurotensin are in the spotlight [8]. To assess the potential applications of neurotensin receptors, NTR1 expression has been analyzed in several prostate cancer cell models with controversial results. NTR1 expression has been highlighted in both malignant and non-malignant cells with the highest expression in malignant cancerous cells [11]. In this study, the authors also demonstrated that malignant cells with a basal phenotype express a higher level of NTR1, and in a benign tissue section, that NTR1 was stained in basal and luminal compartments as well as in stroma [11]. Finally, the authors conclude that NTR1 expression is modulated by the overall differentiation state, which is not directly influenced by androgens [11]. However, expression of NTR1 at the mRNA level does not necessarily translate into similar results at the protein levels [12,13].
In light of these conflicting results and given the paucity of data on human prostate cancer samples, we investigated in this pilot study NTR1 expression in samples of normal human prostate tissues, benign prostatic hyperplasia (BPH), primary prostate cancer, and metastatic lymph nodes.
2. Results
Analysis of Western blots revealed that a single band is visible at the expected NTR1 molecular weight on PC-3 and HT-29 cell lines. LNCaP cells weakly express NTR1 (Figure 1A). Subcellular localization of NTR1 was assessed by immunocytofluorescence. Staining was mainly cytoplasmic and granular, often accompanied with a membranous labelling in positive cell lines (Figure 1B). Immunocytochemistry results revealed that formalin-fixed PC-3 cells express NTR1, whereas formalin-fixed LNCaP cells exhibit weak NTR1 expression. Finally, to assess whether the tumoral environment may modify NTR1 expression, we looked at its expression in a PC-3 xenograft using confocal microscopy. NTR1 expression seems to be higher in xenograft than in cultured cells. Staining was cytoplasmic and granular, often accompanied with a membranous labelling. Figure 1C shows confocal microscopy of the PC-3 xenograft. Study of the NTR1 signal across a single cell confirmed that NTR1 expression is cytoplasmic and accompanied by a membranous labelling.
Figure 1.
Expression profile of NTR1 in prostate cancer cell lines PC-3 and LNCaP and the colonic cell line HT-29 (positive control). (A) Western blot of NTR1 showing high expression in HT-29 and PC-3 cells and weak expression in LNCaP cells. (B) NTR1 immunofluorescence revealing cytoplasmic and membranous labelling in HT-29 and PC-3 cells. (C) Confocal microscopy (40× and 63× magnifications) of a PC-3 xenograft confirming cytoplasmic and membranous labelling.
Normal prostate samples and BPH samples either did not express or showed weak expression of NTR1. Neural cells are often stained for NTR1. As regards primary tumors of prostate cancer cases, NTR1 overexpression (≥10% of stained tumor cells) was seen in 4 of 44 cases (9.1%). Among the other cases, NTR1 staining was either absent (30 cases) or weak (<10% of tumor cells stained). NTR1 staining was granular and mainly cytoplasmic (Figure 2).
Figure 2.
Expression profiles of NTR1 in prostate cancer. (A) No NTR1 expression in Gleason 6 prostate cancer. (B) Strong NTR1 staining in Gleason 8 prostate cancer. Images were obtained with 10× magnification.
Stromal cells did not express NTR1. The tumor presenting the highest NTR1 expression was a poorly differentiated prostatic adenocarcinoma (CK20−, CK7−, PSAp+, p63−, p504s+) without neuroendocrine differentiation (CgA-, synaptophysin-), Gleason 9, node-positive, T3, R0. This patient was 63 years old with a PSA value below 10 ng/mL.
Table 1 shows that none of the studied parameters (age, PSA-values, pT-status, N-status, Gleason score, margin status) were associated significantly with NTR1-expression.
Table 1.
Association between NTR1 expression in primary tumors and clinical and pathological data in 44 patients with prostate cancer.
| Variable | NTR1 Overexpression | p |
|---|---|---|
| Age | 0.622 | |
| ≤64 years (n = 19) | 1/19 | |
| >64 years (n = 25) | 3/25 | |
| PSA value | 1.000 | |
| <10 ng/mL (n = 22) | 2/22 | |
| ≥10 ng/mL (n = 22) | 2/22 | |
| pT-status | 1.000 | |
| pT2 (n = 15) | 1/15 | |
| pT3–T4 (n = 29) | 3/29 | |
| N-status | 0.282 | |
| N0–X (n = 27) | 1/27 | |
| N+ (n = 17) | 3/17 | |
| Gleason score | 0.358 | |
| 5–6 (n = 12) | 1/12 | |
| 7 (n = 12) | 0/12 | |
| 8–9 (n = 20) | 3/20 | |
| Gleason score grouped | 0.316 | |
| 5–7 (n = 24) | 1/24 | |
| 8–9 (n = 20) | 3/20 | |
| Margin status | 0.558 | |
| R0 (n = 33) | 4/33 | |
| R1 (n = 11) | 0/11 |
The rate of NTR1-overexpression in metastatic lymph nodes (33.3%) was higher than in primary tumors (9.1%) (p = 0.038) (Table 2).
Table 2.
NTR1 expression according to the origin of tumor sample.
| NTR1 Overexpression | p | |
|---|---|---|
| Tumor sample | 0.038 | |
| Primary tumor (n = 44) | 4/44 | |
| Metastatic lymph nodes (n = 15 †) | 5/15 |
† Two samples were not included in the analysis due to the lack of available biological material from two of the 17 node-positive patients.
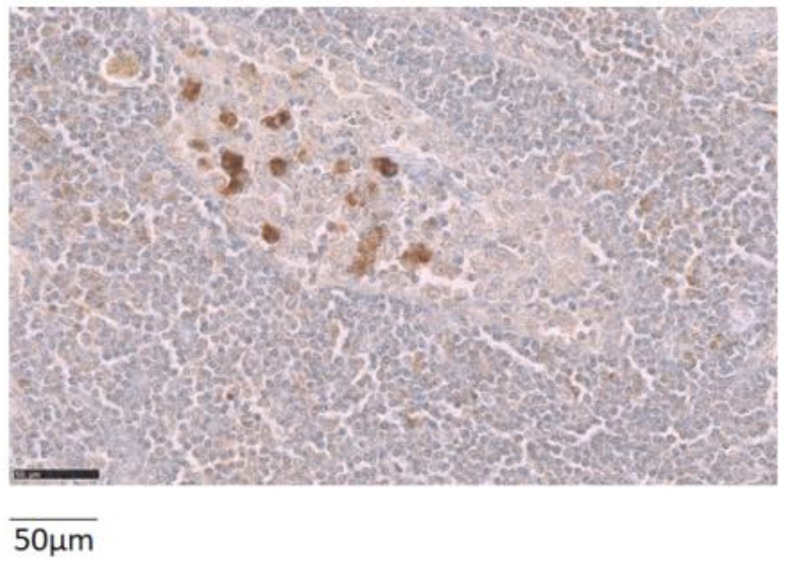
A lymph node metastasis was available for analysis in 15 patients. NTR1 was overexpressed in five of the 15 cases (33.3%) (Figure 3).
Figure 3.
Representative example of the NTR1 expression in a metastatic lymph node. The image was obtained with 40× magnification.
In two out of these five cases, both the primary tumor and nodal metastasis overexpressed NTR1, while in three cases only the lymph node metastasis overexpressed NTR1. In addition, there was only one case where the primary tumor expressed NTR1 while the lymph node did not.
3. Discussion
Data regarding NTR1 expression in prostate cancer are limited mainly to studies on cell lines [11,12,13] with conflicting results. Herein, we confirm the upregulation of NTR1 in the PC-3 model compared to LNCaP cells [10,14]. Whether the absence of AR and/or prostate-specific membrane antigen (PSMA) and/or the differentiation state in PC-3 cells is linked to the overexpression of NTR1 remains to be explored. The NTR1 receptor is mainly cytoplasmic in prostate cancer cells as shown by the granular labelling, as also described in some other malignancies [15,16]. This immunostaining pattern may be judged as non-classical by some experts [17], but an original mechanism by which NTR2 is able to promote cytoplasmic retention of NTR1 has been described [18]. In our hands, PC-3 cells also express the NTR2 subtype (on Western blot, data not shown) as already reported [19]. Moreover, a chronic exposure to the peptide neurotensin is able to activate the NTR1 gene [15]. Under these conditions, NTR1 is accumulated in the perinuclear recycling compartment (cytoplasmic staining) and then addressed to the cell membrane [20,21]. Our granular and cytoplasmic staining of NTR1 in prostate cancer cells is in agreement with the proposed mechanisms.
Then, we assessed the expression of the NTR1 protein by immunohistochemistry in a pilot series of primary prostate cancer cases and lymph nodes metastases if present. Our data show that about 10% of primary prostate cancers overexpressed NTR1. NTR1 expression was not associated with any clinical, biological, or pathological parameters, but the number of samples is quite small. There was a trend for a higher rate of NTR1 expression in patients with a higher Gleason score or from primary prostate cancer from node-positive patients. Preliminary data in the literature incriminate neurotensin and its receptors in prostate cancer with neuroendocrine differentiation [8,10]. In this study, the tumor with the highest NTR1 expression did not exhibit a neuroendocrine profile. A specific study of NTR1 expression in neuroendocrine prostate cancer would be of interest as these tumors may have lost PSMA expression. Even so, such cases account only for a small number of prostate cancers [22]. Loss of PSMA with 18F-FDG-avidity at discordant sites has also been reported in PSMA-targeted radionuclide therapy studies [23]. Moreover, investigation of molecular cross-talk between hormone receptors and/or PSMA and NTR1 would be helpful to better understand the regulation of NTR1 expression in prostate cancer.
Most importantly, the therapy potential offered by targeting NTR1 relies on a better understanding of the NTR1 expression in regional and distant metastases. We were able to study lymph node metastases from 15 patients (one lymph node per case). The rate of NTR1 overexpression is higher in these metastatic tissues (33.3%) than in the overall series of primary tumors (P = 0.038). Moreover, the number of tumoral cells expressing NTR1 in metastatic lymph nodes is also higher than in primary tumors (66.0 ± 31.3% versus 12.5 ± 5%, respectively). Factors driving upregulation of NTR1 in metastatic tissues remain to be elucidated.
NTR1 has been found to be expressed in metastases of other cancers, such as pancreatic cancer and hepatocellular carcinoma [24,25]. It has been suggested that co-expression of NTR1 and its endogenous peptide constitutes an important stimulus promoting cancer invasion and metastases [25,26]. There might also be an implication in prostate cancer. Neurotensin is indeed able to stimulate prostate cancer cell growth, while the selective neurotensin antagonist (SR48692) decreases prostate cancer cell growth [6]. Therefore, pharmacological blockade of NTR1 using antagonists might prove to be useful. It is now necessary to elucidate the place of NTR1 targeting in the current prostate cancer management strategy, notably in high-risk prostate cancer patients. Several radiolabelled probes are under investigation in this setting, such as small molecules targeting PSMA [27]. For future targeted therapy, NTR1 expression should also be assessed on samples of distant metastases from untreated and castration-resistant prostate cancer patients. Although the frequency of NTR1 expression in primary tumors and metastatic lymph nodes appears to be lower than that of PSMA, investigating PSMA-negative (on IHC or PET imaging) prostate tumors would be of particular interest. Therefore, imaging and therapy of prostate cancer patients with the recently developed radiolabelled NTR1 analogues [28,29,30] can offer a complete insight on the role of this approach.
The main limitation of our study is the low number of primary tumors and metastatic lymph nodes studied and validation of these data on a larger series is needed. The prognostic value of NTR1 in prostate cancer still needs to be addressed.
4. Materials and Methods
4.1. Antibody Used
The anti-NTR1 antibody used was a goat polyclonal antibody directed against the human COOH terminus of the receptor (C-20; Santa Cruz Biotechnology, Inc., Heldeiberg, Germany), as used by others [15]. Rules for G-protein coupled receptor immunohistochemical (IHC) studies in human tissues have been established previously [17]. Our methodology was as follows: (i) Western blotting of prostate cancer cell line lysate for antibody specificity, and (ii) immunochemistry of the cell line fixed and embedded with the same material used for the human cancer sample to evaluate the influence of the fixative, subcellular localization of the immunostaining. Finally, our series of human normal and cancerous prostate samples was analyzed regarding NTR1 expression.
4.2. Cell Lines
For all experiments, the human colonic cell line HT-29 was used as a positive control. The human prostate cancer cells LNCaP (lymph node from metastatic prostate adenocarcinoma, AR+, PSMA+) and PC-3 (bone metastasis from high grade prostate cancer; AR−, PSMA−) have been used in this study to explore NTR1 expression in prostate cancer. PC-3 cells were cultured at 37 °C and 5% CO2 in DMEM/F12 (Gibco, Illkirch, France) medium supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. LNCaP and HT-29 cells were cultured at 37 °C and 5% CO2 in RPMI 1640 (Gibco) medium supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were then submitted to Western blotting, immunofluorescence, immunochemistry, and xenografting in nude mice (PC-3 cells).
4.3. Xenograft
All animal experiments/protocols were performed in accordance with the guidelines of the Institute of Nuclear Medicine and Allied Sciences (INMAS) animal ethics committee (Regn. no: 8/GO/a/99/CPCSEA, approval date: 13 November 2011). The animals were euthanized using cervical dislocation and all animal experiments were performed in accordance with the relevant laws (Animals ACT 1986) and the guidelines of the INMAS animal ethics committee (no. INM/DASQA/1AEC/09/15, approval date: 9 September 2015). The institutional animal ethics committee approved the experiments. 5 × 106–6 × 106 of PC-3 cells (100 µL) in 50% Matrigel in PBS v/v were injected to the left flank of nude mice. Tumors were obtained within a period of three to four weeks. The PC-3 tumor was excised and was formalin-fixed and paraffin-embedded for confocal microscopy. Distribution of the NTR1 signal across a single cell was analyzed using Image-J (version 1.50i, NIH, Bethesda, MD, USA).
4.4. Western Blotting
NTR1 expression was investigated by Western blotting. Cell lines were lysed using RIPA buffer. Membrane proteins were denatured by boiling at 85 °C for 5 min using 4× Laemmli sample buffer. Protein samples were submitted to electrophoresis for 60 min at a constant 200 V on SDS polyacrylamid gel and subsequently electroblotted onto PVDF membrane for 90 min at a constant 300 mA current. The membranes were blocked in PBS containing 5% (w/v) BSA for 1 h at room temperature and were incubated overnight at 4 °C with rabbit polyclonal anti human NTR1. The signal was finally recorded using autoradiography films.
4.5. Immunofluorescence
HT-29, LNCaP, and PC-3 cells were grown for two days at 37 °C and 5% CO2, respectively. Non-specific binding sites were blocked in PBS containing 1% BSA and 0.3% Triton X-100 for 90 min. After washing, cells were incubated with the primary antibody anti-human NTR1 (1/500) at room temperature overnight and then with FITC-labelled donkey anti-goat secondary antibody for 90 min. Nuclei were labelled with DAPI. For each experiment, non-specific binding was evaluated by omitting the primary antibody. The cells were imaged using a Leica immunofluorescence microscope and an Olympus camera at 20× magnification.
4.6. Immunochemistry Procedure
NTR1-immunochemistry was carried out as described by Souaze and colleagues [15].
4.7. Human Samples
Forty-four primary prostate cancer samples and fifteen related metastatic lymph nodes were obtained from the tumor bank of the University Hospital of Toulouse. Twelve normal prostate samples and 11 benign prostatic hyperplasia samples from other patients were also examined for comparison. Patient samples were obtained after informed consent in accordance with the Declaration of Helsinki and stored at the “CRB Cancer des Hôpitaux de Toulouse (BB-0033-00014)” collection. According to the French law, CRB Cancer collection has been declared to the Ministry of Higher Education and Research (DC-2008-463) and obtained a transfer agreement (AC-2008-820) after approbation by ethical committees under N°CEBH 2012/40 (approval date: 7 June 2012). Clinical and biological annotations of the samples have been declared to the CNIL (Comité National Informatique et Libertés).
Regarding the 44 cases of prostate cancer, no patient had received hormonal treatment, chemotherapy or radiotherapy prior to surgery. Twenty-five patients were aged 64 years and older; 22 patients had prostate-specific antigen (PSA) serum level ≥10 ng/mL; the Gleason scores were 5 or 6 (3 + 3) in 12 cases, 7 (3 + 4) in six cases, 7 (4 + 3) in six cases, and 8 or 9 in 20 cases; 15 cases were pT2, 28 pT3, and one pT4; 27 were pN0 or pNx; and 17 were pN+. Among the 17 patients with lymph node metastases, a lymph node sample was available for analysis in 15 cases (one node per patient) and constitutes the metastatic lymph node group (two cases were discarded for analysis due to the lack of biological material). NTR1 expression was assessed under a light microscope and was scored according to published procedures [15]. The chosen cut-off value of 10% or more of stained cells as a positivity threshold by Souazé et al. has demonstrated clinical value for another internalized target, Prostate Specific Membrane Antigen (PSMA) [31].
4.8. Statistical Analysis
In order to study associations between NTR1 expression and other parameters, NTR1 data were dichotomized into two groups based on the positivity cut-off reported in literature [15]. We studied associations between NTR1 expression and various clinical, pathological, and biological parameters in cases with prostate cancer (age, serum PSA level, pT status, lymph node status, Gleason score...). Differences between categorized variables were assessed with the χ2 test or Fischer’s exact test as appropriate. All p-values are two-sides. p < 0.05 was considered statistically significant. All data were analyzed using GraphPad Prism v6.01 (GraphPad software, San Diego, CA, USA).
5. Conclusions
In this pilot study, NTR1 was overexpressed in a small percentage of primary prostate cancer, and significantly more often overexpressed in metastatic lymph nodes. Factors driving overexpression of NTR1 in prostate cancers remain to be discovered. The presence of this target in metastatic lymph nodes may open new perspectives for imaging and radionuclide therapy of prostate cancer that deserve further investigation.
Acknowledgments
This work was achieved within the context of the Laboratory of Excellence TRAIL ANR-10-LABX-57. Dr Clément Morgat was awarded the Raman Charpak Fellowship 2014 to perform animal experiments at the Institute of Nuclear Medicine and Allied Sciences (INMAS) New Delhi, India. We warmly thank the staff at INMAS.
Abbreviations
| PSMA | Prostate Specific Membrane Antigen |
| NTR1 | Neurotensin receptor-1 |
| NTR2 | Neurotensin receptor-2 |
| NTR3 | Neurotensin receptor-3 |
| FITC | Fluorescein isothiocyanate |
| DAPI | 4′,6-Diamidino-2-phenylindol |
| IHC | Immunohistochemistry |
| PET | Positron Emission Tomography |
| CK | Cytokeratine |
| BPH | Benign Prostatic Hyperplasia |
| CRPC | Castration Resistant Prostate Cancer |
Author Contributions
Conceptualization, C.M. and E.H.; methodology, C.M., A.C., R.S.,G.M., V.V., P.F., and E.H.; formal analysis, C.M, A.C., and R.S.; resources, G.M. and B.M.; writing—original draft preparation, C.M., A.C., and R.S.; writing—review and editing, C.M. and A.C.; supervision, V.M., P.F., and E.H.; project administration, C.M.; funding acquisition, C.M. and E.H.
Funding
This study was funded by the Laboratory of Excellence TRAIL ANR-10-LABX-57 and by the “Institut National du Cancer, INCa” grant THERACAN 2017-166.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Di Zazzo E., Galasso G., Giovannelli P., Di Donato M., Castoria G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018;8:2. doi: 10.3389/fonc.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Zazzo E., Galasso G., Giovannelli P., Di Donato M., Di Santi A., Cernera G., Rossi V., Abbondanza C., Moncharmont B., Sinisi A.A., et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget. 2015;7:193–208. doi: 10.18632/oncotarget.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehgal I., Powers S., Huntley B., Powis G., Pittelkow M., Maihle N.J. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc. Natl. Acad. Sci. USA. 1994;91:4673–4677. doi: 10.1073/pnas.91.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgat C., Mishra A.K., Varshney R., Allard M., Fernandez P., Hindié E. Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. J. Nucl. Med. 2014;55:1650–1657. doi: 10.2967/jnumed.114.142000. [DOI] [PubMed] [Google Scholar]
- 5.Amorino G.P., Deeble P.D., Parsons S.J. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26:745–756. doi: 10.1038/sj.onc.1209814. [DOI] [PubMed] [Google Scholar]
- 6.DaSilva J.O., Amorino G.P., Casarez E.V., Pemberton B., Parsons S.J. Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling. Prostate. 2013;73:801–812. doi: 10.1002/pros.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan S., Dobner P.R., Carraway R.E. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul. Pept. 2004;120:155–166. doi: 10.1016/j.regpep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S., Tian H., Niu X., Wang J., Li X., Jiang N., Wen S., Chen X., Ren S., Xu C., et al. Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer. Oncogene. 2019 doi: 10.1038/s41388-019-0750-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vias M., Burtt G., Culig Z., Veerakumarasivam A., Neal D.E., Mills I.G. A role for neurotensin in bicalutamide resistant prostate cancer cells. Prostate. 2007;67:190–202. doi: 10.1002/pros.20518. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto K., Kyoda Y., Tanaka T., Maeda T., Kobayashi K., Uchida K., Kitamura H., Hirata K., Tsukamoto T., Masumori N. The potential of neurotensin secreted from neuroendocrine tumor cells to promote gelsolin-mediated invasiveness of prostate adenocarcinoma cells. Lab. Investig. 2015;95:283–295. doi: 10.1038/labinvest.2014.165. [DOI] [PubMed] [Google Scholar]
- 11.Swift S.L., Burns J.E., Maitland N.J. Altered Expression of Neurotensin Receptors Is Associated with the Differentiation State of Prostate Cancer. Cancer Res. 2010;70:347–356. doi: 10.1158/0008-5472.CAN-09-1252. [DOI] [PubMed] [Google Scholar]
- 12.Valerie N.C.K., Casarez E.V., DaSilva J.O., Dunlap-Brown M.E., Parsons S.J., Amorino G.P., Dziegielewski J. Inhibition of Neurotensin Receptor 1 Selectively Sensitizes Prostate Cancer to Ionizing Radiation. Cancer Res. 2011;71:6817–6826. doi: 10.1158/0008-5472.CAN-11-1646. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R.M., Severns V., Brown D.C., Bisoffi M., Sillerud L.O. Prostate Cancer Targeting Motifs: Expression of ανβ3, Neurotensin Receptor 1, Prostate Specific Membrane Antigen, and Prostate Stem Cell Antigen in Human Prostate Cancer Cell Lines and Xenografts. Prostate. 2012;72:523–532. doi: 10.1002/pros.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H., Wang H., Zhang H., Wang M., Giglio B., Ma X., Jiang G., Yuan H., Wu Z., Li Z. Imaging Neurotensin Receptor in Prostate Cancer With 64Cu-Labeled Neurotensin Analogs. Mol. Imaging. 2017;16 doi: 10.1177/1536012117711369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souazé F., Dupouy S., Viardot-Foucault V., Bruyneel E., Attoub S., Gespach C., Gompel A., Forgez P. Expression of Neurotensin and NT1 Receptor in Human Breast Cancer: A Potential Role in Tumor Progression. Cancer Res. 2006;66:6243–6249. doi: 10.1158/0008-5472.CAN-06-0450. [DOI] [PubMed] [Google Scholar]
- 16.Alifano M., Souazé F., Dupouy S., Camilleri-Broët S., Younes M., Ahmed-Zaïd S.-M., Takahashi T., Cancellieri A., Damiani S., Boaron M., et al. Neurotensin Receptor 1 Determines the Outcome of Non–Small Cell Lung Cancer. Clin. Cancer Res. 2010;16:4401–4410. doi: 10.1158/1078-0432.CCR-10-0659. [DOI] [PubMed] [Google Scholar]
- 17.Reubi J.C. Strict rules are needed for validation of G-protein-coupled receptor immunohistochemical studies in human tissues. Endocrine. 2014;47:659–661. doi: 10.1007/s12020-014-0320-0. [DOI] [PubMed] [Google Scholar]
- 18.Hwang J.R., Baek M.W., Sim J., Choi H.-S., Han J.M., Kim Y.L., Hwang J.-I., Kwon H.B., Beaudet N., Sarret P., et al. Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem. Biophys. Res. Commun. 2010;391:1007–1013. doi: 10.1016/j.bbrc.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Maschauer S., Greff C., Einsiedel J., Ott J., Tripal P., Hübner H., Gmeiner P., Prante O. Improved radiosynthesis and preliminary in vivo evaluation of a 18F-labeled glycopeptide–peptoid hybrid for PET imaging of neurotensin receptor 2. Bioorg. Med. Chem. 2015;23:4026–4033. doi: 10.1016/j.bmc.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 20.Souazé F., Viardot-Foucault V., Roullet N., Toy-Miou-Leong M., Gompel A., Bruyneel E., Comperat E., Faux M.C., Mareel M., Rostène W., et al. Neurotensin receptor 1 gene activation by the Tcf/β-catenin pathway is an early event in human colonic adenomas. Carcinogenesis. 2006;27:708–716. doi: 10.1093/carcin/bgi269. [DOI] [PubMed] [Google Scholar]
- 21.Toy-Miou-Leong M., Cortes C.L., Beaudet A., Rostène W., Forgez P. Receptor Trafficking via the Perinuclear Recycling Compartment Accompanied by Cell Division Is Necessary for Permanent Neurotensin Cell Sensitization and Leads to Chronic Mitogen-activated Protein Kinase Activation. J. Biol. Chem. 2004;279:12636–12646. doi: 10.1074/jbc.M303384200. [DOI] [PubMed] [Google Scholar]
- 22.Bakht M.K., Derecichei I., Li Y., Ferraiuolo R.-M., Dunning M., Oh S.W., Hussein A., Youn H., Stringer K.F., Jeong C.W., et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer. 2019;26:131–146. doi: 10.1530/ERC-18-0226. [DOI] [PubMed] [Google Scholar]
- 23.Hofman M.S., Violet J., Hicks R.J., Ferdinandus J., Thang S.P., Akhurst T., Iravani A., Kong G., Ravi Kumar A., Murphy D.G., et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 24.Körner M., Waser B., Strobel O., Büchler M., Reubi J.C. Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. 2015;5:17. doi: 10.1186/s13550-015-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Y., Long X., Zhang L., Chen J., Liu P., Li H., Wei F., Yu W., Ren X., Yu J. NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget. 2016;7:70303–70322. doi: 10.18632/oncotarget.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lange R., Dimoudis N., Weidle U.H. Identification of genes associated with enhanced metastasis of a large cell lung carcinoma cell line. Anticancer Res. 2003;23:187–194. [PubMed] [Google Scholar]
- 27.Muteganya R., Goldman S., Aoun F., Roumeguère T., Albisinni S. Current Imaging Techniques for Lymph Node Staging in Prostate Cancer: A Review. Front. Surg. 2018;5:74. doi: 10.3389/fsurg.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maschauer S., Einsiedel J., Hübner H., Gmeiner P., Prante O. 18F- and 68Ga-Labeled Neurotensin Peptides for PET Imaging of Neurotensin Receptor 1. J. Med. Chem. 2016;59:6480–6492. doi: 10.1021/acs.jmedchem.6b00675. [DOI] [PubMed] [Google Scholar]
- 29.Schulz J., Rohracker M., Stiebler M., Goldschmidt J., Stöber F., Noriega M., Pethe A., Lukas M., Osterkamp F., Reineke U., et al. Proof of Therapeutic Efficacy of a 177Lu-Labeled Neurotensin Receptor 1 Antagonist in a Colon Carcinoma Xenograft Model. J. Nucl. Med. 2017;58:936–941. doi: 10.2967/jnumed.116.185140. [DOI] [PubMed] [Google Scholar]
- 30.Baum R.P., Singh A., Schuchardt C., Kulkarni H.R., Klette I., Wiessalla S., Osterkamp F., Reineke U., Smerling C. 177Lu-3BP-227 for Neurotensin Receptor 1–Targeted Therapy of Metastatic Pancreatic Adenocarcinoma: First Clinical Results. J. Nucl. Med. 2018;59:809–814. doi: 10.2967/jnumed.117.193847. [DOI] [PubMed] [Google Scholar]
- 31.Woythal N., Arsenic R., Kempkensteffen C., Miller K., Janssen J.-C., Huang K., Makowski M.R., Brenner W., Prasad V. Immunohistochemical Validation of PSMA Expression Measured by 68Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018;59:238–243. doi: 10.2967/jnumed.117.195172. [DOI] [PubMed] [Google Scholar]