Abstract
Endocannabinoid (eCB)-binding receptors can be modulated by several ligands and membrane environment, yet the effect of glycosylation remains to be assessed. In this study, we used human neuroblastoma SH-SY5Y cells to interrogate whether expression, cellular localization, and activity of eCB-binding receptors may depend on N-linked glycosylation. Following treatment with tunicamycin (a specific inhibitor of N-linked glycosylation) at the non-cytotoxic dose of 1 µg/mL, mRNA, protein levels and localization of eCB-binding receptors, as well as N-acetylglucosamine (GlcNAc) residues, were evaluated in SH-SY5Y cells by means of quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR), fluorescence-activated cell sorting (FACS), and confocal microscopy, respectively. In addition, the activity of type-1 and type-2 cannabinoid receptors (CB1 and CB2) was assessed by means of rapid binding assays. Significant changes in gene and protein expression were found upon tunicamycin treatment for CB1 and CB2, as well as for GPR55 receptors, but not for transient receptor potential vanilloid 1 (TRPV1). Deglycosylation experiments with N-glycosidase-F and immunoblot of cell membranes derived from SH-SY5Y cells confirmed the presence of one glycosylated form in CB1 (70 kDa), that was reduced by tunicamycin. Morphological studies demonstrated the co-localization of CB1 with GlcNAc residues, and showed that tunicamycin reduced CB1 membrane expression with a marked nuclear localization, as confirmed by immunoblotting. Cleavage of the carbohydrate side chain did not modify CB receptor binding affinity. Overall, these results support N-linked glycosylation as an unprecedented post-translational modification that may modulate eCB-binding receptors’ expression and localization, in particular for CB1.
Keywords: SH-SY5Y cells, endocannabinoid-binding receptors, tunicamycin, N-acetylglucosamine
1. Introduction
Type-1 (CB1) and type-2 (CB2) cannabinoid receptors belong to the seven-transmembrane G protein-coupled receptors (GPCRs) family [1]. They are pivotal components of the endocannabinoid system [2], through which endocannabinoids (eCBs) exert many of their effects both centrally [3] and peripherally [4]. Accumulated evidence suggests the presence of additional receptor targets for eCBs on the cell surface, such as the GPR55 receptor [5], and the transient receptor potential vanilloid 1 (TRPV1) ion channel [6]. The activation of these receptors by eCBs triggers several pathways that control distinct physiologic processes [3,4], as well as a wide range of neurodegenerative and neuroinflammatory disorders such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis [7,8].
Most, but not all, GPCRs are known to be N-linked glycoproteins with heterogeneous oligosaccharides, e.g., D2, D3 dopamine receptors [9], β1-adrenergic receptors [10], nicotinic acetylcholine receptors [11], and A2 adenosine receptors [12]. Of note, available data on GPCRs have shown that glycosylation is a post-translational modification influencing receptor expression and processing, ligand binding, and/or coupling to second messengers [9,13].
In the case of tumor cells, glycosylation is dramatically altered during disease progression due to changes in the expression levels or activity of glycosyltransferases and glycosidases [14,15,16]. Several reports have shown a crucial role for N-linked carbohydrates also in cell-cycle progression and cell viability [17]. These modifications have been associated with enhanced malignancy, and thus they could profoundly impact the modulation of tumor growth [18]. For instance, neuroblastoma, which accounts for 10% of childhood cancers, exhibits aberrant cell-surface glycosylation patterns [19]. Specific glycosylation inhibitors are widely used to interrogate the role of glycosylation in various biological processes, including protein folding and conformation, oligomerization, sorting, cell-cell interactions, and targeting of proteins to sub- or extra-cellular locations [17]. In this context, tunicamycin acts as a specific inhibitor of N-linked glycosylation, and blocks the first step of glycoprotein synthesis, i.e., the UDP-N-acetylglucosamine-dolichol phosphate N-acetylglucosamine-1-phosphate transferase (GPT); thus, it arrests the synthesis of all N-linked glycoproteins [20]. Consequently, there will be an accumulation of misfolded or unfolded glycoproteins in the endoplasmic reticulum (ER), leading to ER stress. Indeed, it has been shown that in many cell types, ER stress can be induced by treating cells with low concentrations of tunicamycin [21]. Instead, at higher concentrations, tunicamycin promotes prostate cancer cell death by activating the mammalian target of rapamycin complex-1 (mTORC1)-dependent pathway [22]. On the other hand, the process of glycosylation is extremely sensitive and can be inhibited by small amounts of tunicamycin, as reported in mouse podocytes and human embryonic kidney (HEK-293) cells [23]. Recently, tunicamycin has been shown to impair also phosphorylation of Anaplastic Lymphoma Kinase (ALK), thus disrupting pro-survival signaling in neuroblastoma cells [24]. In this investigation, the effect of tunicamycin on the major eCB-binding receptors (namely, CB1, CB2, GPR55, and TRPV1) was interrogated in human neuroblastoma SH-SY5Y cells, which indeed express all of them [25]. The aim was to ascertain whether glycosylation can regulate their expression and subcellular distribution, and therefore signal transduction thereof.
2. Results
2.1. Effects of Tunicamycin on mRNA and Protein Expression of eCB-Binding Receptors
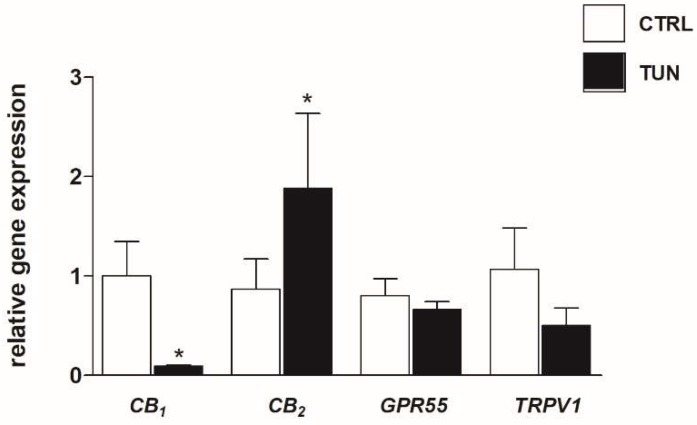
The mRNA expression of CB1, CB2, GPR55, and TRPV1 was evaluated in SH-SY5Y cells upon treatment for 24 h with tunicamycin at the non-cytotoxic dose of 1 µg/mL, by means of qRT-PCR (Figure 1). Tunicamycin concentration was chosen after investigating, by Trypan blue exclusion test, the effects of various doses on cell viability (data not shown). As shown in Figure 1, CB1 mRNA expression decreased significantly (p < 0.05 vs. control) following tunicamycin treatment. In contrast, CB2 mRNA levels were significantly increased (p < 0.05 vs. control) after exposure to tunicamycin (Figure 1), whereas GPR55 expression was unaffected and TRPV1 expression showed a trend towards decrease, though not statistically significant (Figure 1).
Figure 1.
Effect of tunicamycin on mRNA expression of endocannabinoid (eCB)-binding receptors. SH-SY5Y cells were treated for 24 h with 1 µg/mL tunicamycin (TUN). Data are presented as means ± SEM (n = 3). [* p < 0.05 vs. control cells (CTRL)].
Then, the effect of tunicamycin on eCB-binding receptor protein expression was assessed in SH-SY5Y cells under the same experimental conditions through FACS analysis. The intracellular quantitation of eCB-binding receptors, calculated as mean fluorescence intensity values (MFI), revealed a significant decrease of CB1 and GPR55 in cells exposed to 1 µg/mL tunicamycin (p < 0.001 for CB1; p < 0.05 for GPR55) (Table 1). Instead, CB2 and TRPV1 expression slightly increased after tunicamycin treatment (Table 1). In addition, to further corroborate the efficacy of tunicamycin, SH-SY5Y cells were challenged with an anti-biotin-WGA antibody that specifically binds to GlcNAc. As expected, tunicamycin-treated samples showed a significant decrease in WGA expression (p < 0.001 vs. control) (Table 1).
Table 1.
Mean fluorescent intensity (MFI) values of SH-SY5Y cells expressing eCB-binding receptors, and GlcNAc residues. * Denotes p < 0.001; ** p < 0.05.
| Experimental Group | CB1 | CB2 | GPR55 | TRPV1 | GlcNAc |
|---|---|---|---|---|---|
| CTRL | 70.60 ± 2.12 | 16.70 ± 0.50 | 30.00 ± 0.90 | 19.30 ± 0.60 | 146.00 ± 4.38 |
| TUN | 55.80 ± 1.67 * | 20.40 ± 0.61 | 22.60 ± 0.68 ** | 20.40 ± 0.61 | 131.40 ± 3.94 * |
2.2. Assessment of N-linked Glycosylation after Tunicamycin Treatment
All eCB-binding receptors under investigation (CB1, CB2, GPR55 and TRPV1) showed potential N-glycosylation sites, as documented by their sequence analysis through the UNIPROT database (Table 2). Incidentally, such a post-translational modification (PTM) is characterized by a glycosidic bond between GlcNac and a nitrogen atom (usually N4) of an Asn residue in a consensus sequence Asn-X-Ser/Thr (where X is any aminoacid except Pro)—more rarely Asn-X-Cys. In particular, CB1 presents three putative glycosylated Asn residues, all located in the N-terminal region, whereas CB2 and TRPV1 receptors have only one putative glycosylation site, and GPR55 has two (Table 2).
Table 2.
Potential N-glycosylation sites detected by sequence analysis of human eCB-binding receptors in the Uniprot database (www.uniprot.org).
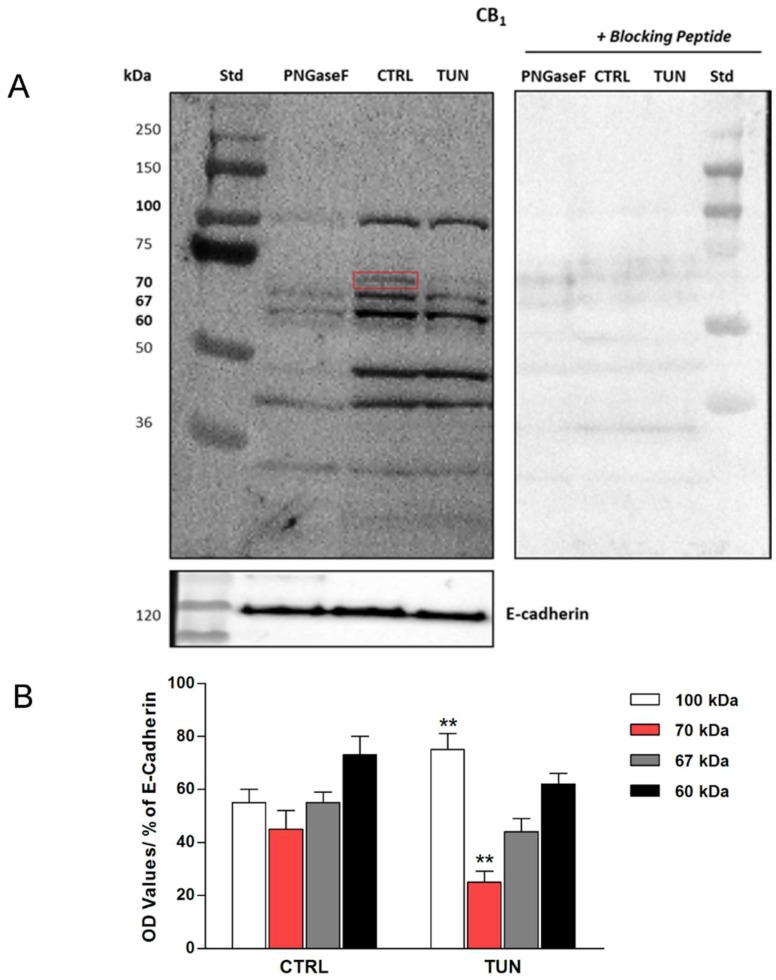
CB1 was the most affected eCB-binding receptor by tunicamycin treatment, both at mRNA and protein levels, and had a greater extent of glycosylation compared to other receptors. Thus, its electrophoretic mobility in plasma membranes isolated from SH-SY5Y cells, after treatment with tunicamycin and PNGaseF (which removes all N-linked sugars) was evaluated by means of Western blot analysis. First, to ascertain the specificity of anti-CB1 antibody, antigen preabsorption experiments were carried out with the corresponding blocking peptide. In particular, the latter erased different immunoreactive bands (at 60 kDa, 67 kDa, 70 kDa, and 100 kDa) which could correspond to CB1 forms with different glycosylation motifs, and to a dimeric form of the receptor (Figure 2A). Upon treatment of the cells with tunicamycin, the immunoreactive band at 70 kDa almost disappeared, suggesting receptor deglycosylation (Figure 2A). In particular, densitometric analysis of immunoreactive bands revealed a significant ~55% reduction of the 70 kDa band in tunicamycin-treated samples compared to the control samples (p < 0.01 vs. control) (Figure 2B). This finding was confirmed by PNGase F treatment, which also erased the 70 kDa band (Figure 2A). Moderate reductions (i.e., ~20% and ~15%) of the intensity of both 67 kDa, and 60 kDa bands were shown in tunicamycin-treated samples compared to controls (Figure 2B), whereas the band at 100 kDa appeared to be more significantly expressed (~40%) in the same samples than in controls (p < 0.01 vs. control) (Figure 2B). Incidentally, no changes were detected by Western blot for CB2, GPR55, and TRPV1 (data not shown).
Figure 2.
Representative Western blot of tunicamycin effect on cannabinoid receptor 1 (CB1) protein expression. SH-SY5Y cells were treated for 24 h with tunicamycin (TUN 1 µg/mL) and with PNGase F (20 µL of 500 U/mL solution), as indicated. Controls with specific blocking peptides are also shown. The red square indicates the immunoreactive band at 70 kDa, erased by tunicamycin and PNGase F treatments (A). Densitometric analysis of CB1 immunoreactive bands (100 kDa, 70 kDa, 67 kDa and 60 kDa) normalized to E-cadherin content. Data are presented as means ± SEM (n = 3) [** p < 0.01 vs. CTRL] (B).
2.3. Localization of eCB-Binding Receptors in SH-SY5Y Cells upon Tunicamycin Treatment
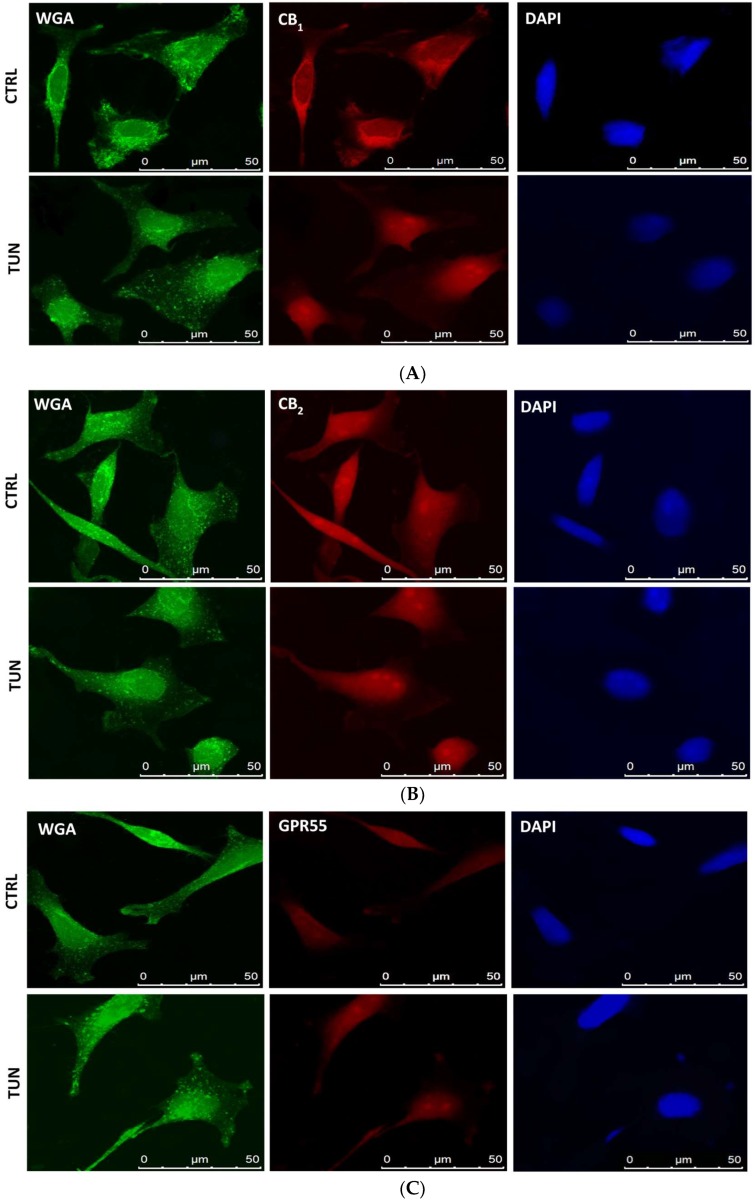
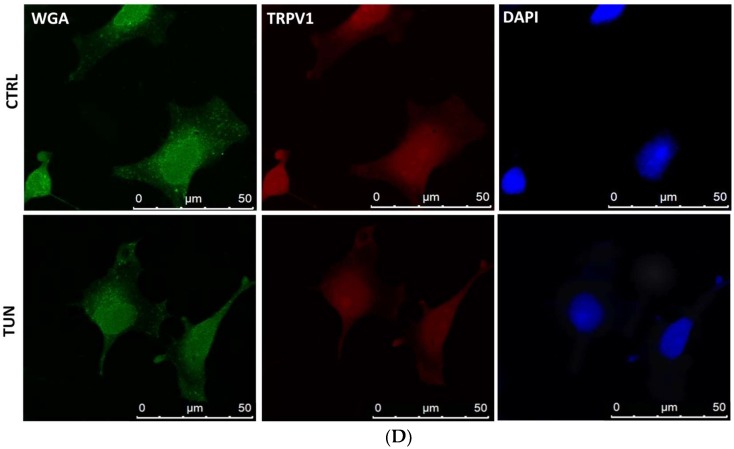
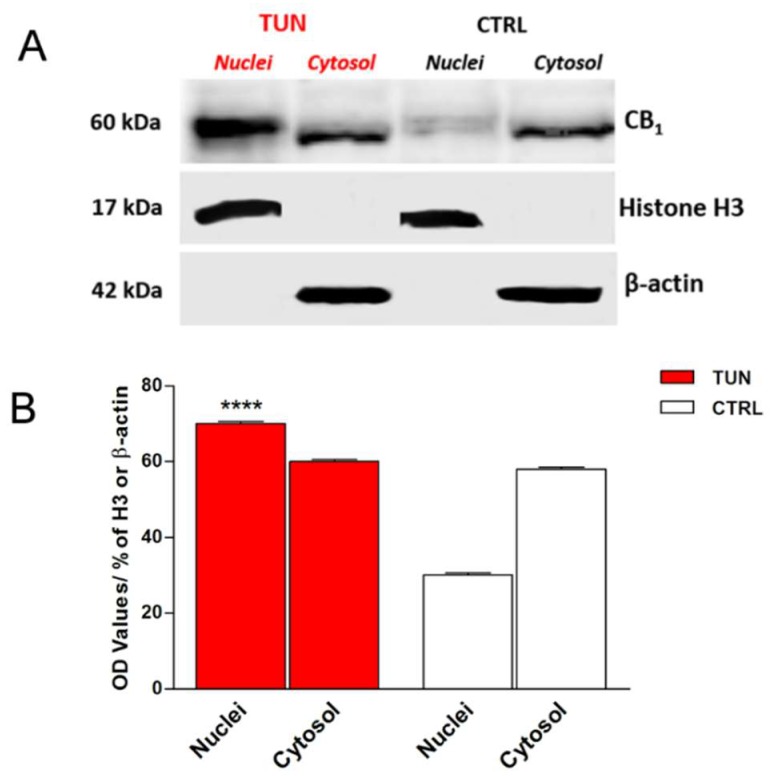
In order to demonstrate that some of eCB-binding receptors were indeed glycosylated, their co-localization with GlcNAc residues was assayed in the SH-SY5Y cells by means of confocal analysis. Subcellular localization of CB1 revealed a diffuse fluorescence throughout the cytoplasm and membranes, and overlapped with biotin-WGA staining (Figure 3A). After tunicamycin treatment, CB1 immunostaining was predominant in the nucleus with sparse co-localization with WGA (Figure 3A). To confirm these data, cell fractionation followed by immunoblotting experiments was performed in SH-SY5Y cells upon tunicamycin treatment. CB1 was found to be more abundant in the nucleus than in the cytoplasm of treated cells (Figure 4A), and consistently densitometric analysis showed a significant 40% higher CB1 immunoreactive band in the nuclei of treated cells than in control cells (p < 0.0001 vs. control) (Figure 4B). Instead, CB2 staining was almost completely cytosolic, with scarce or absent association to the plasma membrane (Figure 3B). No co-localization with WGA was observed for CB2 and GPR55, both under control conditions and upon tunicamycin treatment (Figure 3B,C). Finally, a faint overlap between TRPV1 and WGA staining was detected on cell membranes of controls and tunicamycin-treated cells (Figure 3D).
Figure 3.
Co-localization of eCB-binding receptors with N-acetylglucosamine (GlcNAc). Representative triple immunofluorescence staining of GlcNAc (green), eCB-binding receptors (red), and DAPI (blue) in SH-SY5Y cells. SH-SY5Y cells were incubated with a biotinylated lectin (WGA) to visualize total GlcNAc, and with specific antibodies [anti-CB1 (A), anti-CB2 (B), anti-GPR55 (C), and anti-TRPV1 (D)] for eCB-binding receptors; then with Alexa Fluor 488-labelled streptavidine for GlcNAc residues, with anti-rabbit IgG labeled with Alexa Fluor 595 for eCB-binding receptors and with DAPI for DNA. Scale bars = 50 μm.
Figure 4.
CB1 immunostaining in the nuclear and cytoplasmatic fractions of SH-SY5Y cells, treated or not for 24 h with tunicamycin (TUN 1 µg/mL). Histone H3 and β-actin were used as loading controls for nuclear and cytoplasmatic fractions (A). Densitometric analysis of CB1 immunoreactive band normalized to histone H3 and β-actin. Data are presented as means ± SEM (n = 3) [**** p < 0.0001 vs. nuclei CTRL] (B).
2.4. Effects of Tunicamycin on CB ligand Binding
In order to determine whether CB receptors were functionally affected by tunicamycin, a pan-CB ligand binding assay was performed in SH-SY5Y cells treated for 24 h with 1 µg/mL tunicamycin. Binding of the synthetic cannabinoid [3H]CP55940, a CB1 and CB2 agonist [26] was substantially unchanged in tunicamycin-treated cells with respect to controls (Table 3), suggesting that tunicamycin treatment did not affect CB receptor function in SH-SY5Y cells.
Table 3.
CB1 and CB2 binding activity of control SH-SY5Y cells, and of cells treated with tunicamycin (TUN 1 µg/mL) for 24 h.
| Experimental Group | CB Binding Activity (pmol/mg of Protein) |
|---|---|
| CTRL | 17.40 ± 1.79 |
| TUN | 19.87 ± 0.36 |
Data are presented as means ± SEM (n = 3).
3. Discussion
Glycosylation is well-known to regulate surface expression of GPCRs, like β1 adrenergic receptor [10] and D2 receptor [9]. Based on in silico data, also eCBs receptors have putative N-glycosylation sites [27,28], yet at present, it remains unclear whether the latter sites are indeed glycosylated in real life. Human CB1 and CB2 receptors appear to differ in the number and distribution of their potential N-glycosylation sites. In the N-terminal region, CB2 has only one potential N-glycosylation site, whereas CB1 has three of them. Two potentially glycosylated Asn residues are conserved in rat and mouse species (Asn 77 and Asn 83) [28]. Moreover, the human CB1 sequence has two splice variants (hCB1a and hCB1b) that differ at their N-terminus. In particular, hCB1b shows a deletion of 33 amino acids that includes Asn 77 and Asn 83, and remarkably this variant has been shown to play a role in metabolic regulation [29]. Recently, two splice variants resembling those of the human receptor were discovered also in the mouse CB1-encoding gene CNR1 [30]. Here, the lack of N-glycosylation sites was found to strongly reduce glycosylation level and mitogen-activated protein kinase (MAPK) activity upon CB1 agonist-induced stimulation [30]. In this context, it should be recalled that preliminary data on tunicamycin treatment of CB1 failed to show any efficacy on the inhibition of downstream cyclic AMP accumulation in cultured mouse neuroblastoma N18TG2 cells, suggesting that glycosylation was not engaged in this CB1-dependent signaling pathway [31]. Yet, in the same investigation, the authors cautioned that no agonist binding data were obtained to correlate receptor activation with signal transduction thereof [31]. On the other hand, tunicamycin has already been shown to modify CB2 protein expression profiles in methylotrophic yeast Pichia pastoriis, where a glycosylation site at the N-terminus of the receptor was demonstrated, and the carbohydrate portion accounted for ~3 kDa [32]. As for TRPV1, the presence of N-glycosylation in rats was first shown in 2001 [33], and shortly after, its functional role was reported [34]. Indeed, it was found that N-glycosylation may affect basic functional characteristics of TRPV1, representing a major determinant of capsaicin-evoked desensitization and ionic permeability [34,35]. Conversely, little information (if any) is available on the presence of N-glycosylation sites in GPR55, though sequence analysis via the UNIPROT database strongly supports it at the N-terminus as a PTM of the expressed protein. Up to date the question of whether N-glycosylation sites are essential for eCB-binding receptors function remains largely unanswered. At any rate, our results seem to suggest that glycosylation exerts distinct effects on different eCB-binding receptors, extending previous studies on other GPCRs [10,36,37]. Interestingly, removal of the sugar moiety led to a decreased expression of CB1, resembling previous data on rat EP3 prostaglandin receptor, human AT1 angiotensin-II receptor, human 5-HT5A serotonin receptor, human B2 bradykinin receptor, human TXA2 thromboxane receptor, and human D5 dopamine receptor [38,39,40,41,42]. In the case of rat EP3β-subtype PGE2 receptor, glycosylation appeared to be essential also for an efficient translocation to the plasma membrane [43]. It seems noteworthy that tunicamycin is a commonly used ER stressor that induces the unfolded protein response (UPR) by activating specific ER protein signaling, which in turn leads to inflammatory processes [44]. In this context, CB2 is known to have protective actions in different chronic inflammatory diseases [45]. Therefore, here it might be speculated that the increased gene CB2 expression induced by tunicamicyn may be a compensatory response that cooperates with UPR in re-establishing cellular homeostasis. However, this merely speculative hypothesis remains to be ascertained in independent studies. Notably, tunicamycin has increased expression of GPR55 receptor, whereas no effect was evident on TRPV1, as demonstrated by flow cytometry and quantified as mean fluorescence intensity values (MFI).
Remarkably, treatment with tunicamycin has revealed the presence of at least one glycosylated form in CB1 but not in the other eCB-binding receptors under investigation (data not shown), as demonstrated by the shift of the immunoreactive band at 70 kDa. This is supported by the fact that the extracellular amino-terminal part of CB1 contains three consensus sequences (Asn77, Asn83, and Asn112) that suitable for N-glycosylation. On the other hand, PNGase F treatment seems to confirm the presence of glycosylated forms in CB1, because the molecular weight of this receptor decreased when deglycosylated. In addition, WGA is a useful tool for detecting glyconjugates on cell membrane [46]. Co-localization of this molecule with CB1 confirmed the presence of sugar residues on CB1. Interestingly, tunicamycin seemed to affect also the cellular distribution of CB1, but not of the other eCB-binding receptors analyzed. Indeed, CB1 was found to be more localized to the nucleus in tunicamycin-treated cells than in controls. This finding is in line with a recent study, showing that glycosylation is important for cytoplasmic retention of estrogen receptor GPR30 [47].
On a final note, here we demonstrated that tunicamycin has no effect on CB1/CB2 binding activity, although it can be anticipated that independent site-directed mutational studies are deemed necessary to further our understanding of the functional significance of N-glycosylated residues in these two cannabinoid receptors.
In conclusion, this study supports the concept that, although all major eCB-binding receptors could be potentially glycosylated in human neuroblastoma cells, the role of such a post-transcriptional modification (PTM) differs from receptor to receptor. In the case of CB1, glycosylation appears necessary for normal receptor expression and localization. Therefore, it should be added to other PTMs recently reported to regulate CB1, such as palmitoylation of its cysteine 415 [48], and interaction with membrane cholesterol [49]. Incidentally, so far it has been shown that only N-glycosylated isoforms of Neurotensin receptor-1 (NTSR-1), a GPCR that has been identified as a mediator of cancer progression, are able to localize with membrane structured microdomains by palmitoylation for efficient mitogenic signaling [50]. It will be interesting to further investigate whether in CB1 there is a similar interdependence between palmitoylation and glycosylation, also in the light of the development of novel therapeutic strategies to combat CB1-dependent diseases in humans [51,52].
4. Materials and Methods
4.1. Materials
Dulbecco’s modified Eagle’s medium, fetal calf serum were from Gibco (Life Technologies, Grand Island, NY, USA). Biotinylated labeled wheat germ agglutinin (WGA), bisbenzimide Hoechst 33,258 (H33258), tunicamycin, protease inhibitor, peptide N-glycosidase (PNGase F) from Elizabethkingia miricola and all the other reagents were from Sigma-Aldrich Co (St. Louis, MO, USA). Streptavidine conjugate labeled with Alexa Fluor 488 was from Molecular Probes (Eugene, Oregon, USA). Antibody antirabbit IgG conjugate labeled with Alexa Fluor 595 was purchased from Life Technologies (Life Technologies, Grand Island, NY, USA).
4.2. Cell Culture
Human neuroblastoma SH-SY5Y cells were grown in Dulbecco’s modified Eagle’s medium, supplemented with 15% inactivated fetal bovine serum, 2 mM l-glutamine, 100 units/mL penicillin/streptomycin, 1 mM sodium pyruvate, 1 mM Hepes, and 1 mM nonessential amino acids [25]. Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere at a density of 2 × 105 cells/mL and were treated with different amounts (1, 2 or 3 µg/mL) of tunicamycin, or with vehicle (DMSO, CTRL), for 24 h. Cells were counted and viability was determined by Trypan blue exclusion assay [25].
4.3. Quantitative Real Time-Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Analysis
Total mRNA was extracted from SH-SY5Y cells by using TRIzol (Life technologies, Grand Island, NY, USA) according to the manufacturer instructions. Quantification of total mRNA samples was assessed by using Thermo Scientific NanoDrop 2000c UV-Vis spectrophotometer at 260 nm (Waltham, MA, USA). cDNA was synthetized from 1 µg of total RNA of each sample by using the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). The relative abundance was assessed by RT quantitative PCR (RT-qPCR) using SensiFASTTM SYBR Lo-ROX kit (Bioline, London, UK) to by adjusting the manufacturer instruction final volume of 15 µL on a 7500 Fast Real-time PCR System (Life Technologies, Grand Island, NY, USA). To provide precise quantification of the initial target in each PCR reaction, the amplification plot was examined, as well as the point of early log phase of product accumulation defined by assigning a fluorescence threshold above background, defined as the threshold cycle number or Ct. The relative expression of different amplicons was calculated by the delta–delta Ct (DDCt) method and converted to relative expression ratio (2-DDCt) for statistical analysis [53]. All data were normalized to the endogenous reference genes β-actin, GAPDH, and 18S rRNA combined. The primers used for PCR amplification are reported in Table 4.
Table 4.
List of primer sequences used for qRT-PCR analysis.
| Human Gene | Forward (5′→3′) | Reverse (3′→5′) |
|---|---|---|
| CB1 | CCTTTTGCTGCCTAAATCCAC | CCACTGCTCAAACATCTGAC |
| CB2 | TCAACCCTGTCATCTATGCTC | AGTCAGTCCCAACACTCATC |
| GPR55 | ATCTACATGATCAACCTGGC | ATGAAGCAGATGGTGAAGACGC |
| TRPV1 | TCACCTACATCCTCCTGCTC | AAGTTCTTCCAGTGTCTGCC |
| β-Actin | TTCTACAATGAGCTGCGTG | AGAGGCGTACAGGGATAGCA |
| GAPDH | GATTCCACCCATGGCAAATTC | TGGGATTTCCATTGATGACAAG |
| 18S rRNA | CGCCGCTAGAGGTGAAATTCT | CGAACCTCCGACTTTCGTTCT |
4.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
Control and treated SH-SY5Y cells (1 × 106/mL) were collected, washed twice with ice-cold PBS, fixed in 4% paraformaldehyde in Phosphate-buffered saline (PBS), and permeabilized with a blocking solution solution (80 mM PIPES, 0.5% BSA, 5 mM EGTA, 1 mM MgCl2, 50 mM NH4Cl, 0.05% saponin, 0.02% (w/v) and NaN3, pH 6.8). N-acetylglucosamine (GlcNAc) and eCB-binding receptors were visualized by incubating cells with biotinylated lectin (WGA 20 µg/mL, 1 h), and with anti-CB1 (1:200), anti-CB2 (1:200), anti-GPR55 (1:200) and anti-TRPV1 (1:100) specific antibodies. After three washes with PBS, biotinylated lectin was localized with streptavidine conjugate labeled with Alexa Fluor 488, and eCB-binding receptors were identified using rabbit IgG conjugate labeled with Alexa Fluor 595. Cell fluorescence distribution was analyzed by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA, USA), equipped with a CELLQuest Software Program. One thousand cells from each sample were computed, and mean fluorescence intensity (MFI) was calculated.
4.5. Cellular Fractionation
Subcellular fractionation was performed as reported [54]. Briefly, cell lysates were obtained by homogenization of the samples (control and treated SH-SY5Y cells) in ice-cold buffer (50 mM Tri/HCl pH 7.4) with the addition of 5 mM MgCl2, 250 mM sucrose and protease inhibitor cocktail and were homogenized at 10.000 rpm with RW 16 Basic homogeniser with a Teflon pestle (IKA-Werke GmbH & Co. KG, Staufen im Breisgau, Germany). Then, they were centrifuged at 800× g for 20 min at 4 °C. The pellet was used for the isolation of the nuclei, while the supernatant was used to isolate the cytosolic fraction. The pellet was washed with the same buffer and then centrifuged at 500× g for another 20 min. Cytosolic fraction was extracted by centrifugation of the supernatant at 14,000× g for 30 min at 4 °C. Nuclei were suspended in ice-cold buffer (20 mM Hepes pH 7.9 with the addition of 1.5 mM MgCl2, 0.5 M NaCl, 0.2 mM EDTA, 20% glycerol, and 1% Triton-X-100 and protease inhibitor cocktail) and sonicated twice (amplitude 40, cycle 1) in dry ice. The number of proteins was determined by the Bio-Rad Protein assay (Bio-Rad Laboratories, Hemel Hempstead, UK).
4.6. Protein Deglycosylation Assay and Immunoblotting
Cell lysates were obtained by homogenization of the samples (control and treated SH-SY5Y cells) in a PBS ice-cold buffer with the addition of 1 mM MgCl2, 1 mM CaCl2, and 1 mM DTT and were sonicated twice (amplitude 40, cycle 1) in dry ice. Next, they were centrifuged at 1000× g for 10 min at 4 °C, and the supernatant was collected and centrifuged at 20,000× g for 30 min at 4 °C. The pellet (cell membranes) was recovered and resuspended in 50 mM Tris/HCl buffer pH7.5. The amount of proteins was determined by the Bio-Rad Protein assay (Bio-Rad Laboratories, Hemel Hempstead, UK). Control Samples for deglycosylation studies were treated with 20 μL PNGase F (500 U/mL) of N-glycosidase F in 60 mM sodium phosphate pH 7.5, 0.35% SDS, 70 mM DTT and 1% Triton x-100. The reaction mixture was incubated for 18 h at 37 °C and was stopped by cooling at 4 °C before the addition of the sample buffer for immunoblotting analysis. Equal amounts of total extracts (30 μg of protein) were electrophoresed on 10% or 12% acrylamide gels, then gels were electroblotted overnight onto 0.45 µm nitrocellulose membranes (Biorad Laboratories, CA, USA). Membranes, nuclei, and cytoplasmatic fractions were saturated with a solution of 3% BSA, then were incubated with anti-CB1 (1:200) (Cayman Chemicals, Ann Arbor, MI, USA, item n. 101500) and with polyclonal antibodies for Histone H3 (PA5-16183, ThermoFisher Scientific, Rockford, USA, 1:20,000), cadherin 1 (ABIN1440031, Antibodies-online GmbH, Germany, 1:500), and monoclonal antibody for β-actin (8457, Cell Signaling Technology, Leiden, The Netherlands, 1:1000) for nuclei, membrane, and cytosolic fractions respectively. To block the formation of the antibody/protein complex, the blocking peptides for CB1 (Cayman Chemicals, Ann Arbor, MI, USA, item n. 301500) was preincubated in a 1:1 ratio (v/v) with the corresponding antibody for 1 h at room temperature, then it was diluted to the final working antibody concentration. Goat anti-rabbit-HRP (1:10,000, Thermo Fisher Scientific, MA, USA) was used as secondary antibody. Detection was performed by using the West Dura Chemiluminescence System (Pierce, Rockford, IL, USA). Blots were developed using the LiteAblot Plus Enhanced Chemiluminescent substrate (Euroclone S.p.A, Milano, Italy). The intensity of the immunoreactive bands was quantified by densitometric analysis through the ImageJ software (NIH, Bethesda, MD, USA).
4.7. Confocal Microscopy
Control and treated SH-SY5Y cells (2 × 105/mL) were cultured for 24 h and then fixed in 4% paraformaldehyde. Immunofluorescence staining was performed using a biotinylated lectin (WGA) 20 µg/mL diluted in blocking solution for 1 h to visualize total N-acetylglucosamine and with specific antibodies for eCB-binding receptors [1:200 for CB1, CB2 and GPR55 (Cayman Chemicals, Ann Arbor, MI, USA, item n. 101500, n. 101550, n. 10224), 1:100 for TRPV1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, sc-12498)] in blocking solution for 1 h. Cells were washed with PBS and incubated with streptavidine labeled with Alexa Fluor 488 for N-acetylglucosamine residues and with anti-rabbit IgG labelled with Alexa Fluor 595 in order to identify all eCB receptors. Cells were washed again with PBS and counterstained with H33258 (0.5 µg/mL) for 5 min at RT. After three more washes, samples were resuspended in a MOWIOL solution, placed on a slide, and examined at a Leica TCS SP5 II DMI6000 confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with HCX plan apo 63¥ (numerical aperture 1.4) oil immersion objective. Fluorescent images were derived from the maximum projection of optical serial sections (step size 0.29 μM) using the LAS AF software (2.6.0.7266, Leica Microsystems). For presentation purposes, LAS AF pictures were exported in TIFF format and processed with Adobe Photoshop CS5 (Mountain View, CA, USA) for adjustments of brightness and contrast.
4.8. Receptor Binding Assay on Adherent Living Cells
Control and treated SH-SY5Y cells (1.5 × 105/well) were cultured in a 12-well cell culture plate. After tunicamycin treatment for 24 h, each well was washed twice with 1 mL of PBS and treated with 500 μL of incubation buffer (50 mM Tris–HCl, 5 mM MgCl2, 1 mM CaCl2, 0.2% BSA, pH 7.4), preheated to 37 °C, in the presence of 1 μM “cold” CP55.940 and incubate for 15 min at 37 °C. Then, 2.5 nM [3H]CP55.940 was added and incubated for 1 h in an incubator set at 37 °C. After incubation, the buffer was carefully removed and cells were washed again with ice-cold washing buffer (50 mM Tris–HCl, 500 mM NaCl, 0.1% BSA, pH 7.4). Then, 500 μL of 0.5 M NaOH was added to each well, and cells were pipetted up and down several times to lyse them. The resuspension was then transferred to a 10 mL scintillation vial with liquid scintillation cocktail, and immediately read radioactivity in a scintillation β-counter (Tri-Carb 2810 TR, Perkin Elmer, Waltham, MA, USA) [55].
4.9. Statistical Analysis
Data are reported as means ± S.E.M or S.D. of at least three independent experiments, each performed in duplicate. Data were analyzed by the Prism 5 program (GraphPad Software, La Jolla, CA, USA), using unpaired t-test and one-way or two-way analysis of variance (ANOVA) followed by Tukey test or Bonferroni post hoc analysis, as appropriate. A level of p < 0.05 was considered statistically significant.
Acknowledgments
The authors wish to thank Benedetta Cinque (University of L’Aquila) for her expert assistance with preliminary FACS analyses, and Filomena Fezza (Tor Vergata University of Rome) for her support with receptor binding assay.
Author Contributions
A.C., A.R.L. and G.R. performed morphological analyses; C.B.A., G.R. and C.R. performed immunochemical analyses and receptor binding assay, and analysed the data; A.S. performed bioinformatics analysis; D.T. performed qRT-PCR analysis; G.D. supervised morphological experiments; M.M. conceived the experimental design, supervised the project and revised the manuscript. All authors contributed to draft the paper.
Funding
This research was funded by the Italian Ministry of Education, University and Research (MIUR), under competitive grant PRIN 2015 to M.M.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds tunicamycin and PNGase F are available from the authors.
References
- 1.Pertwee R.G. Endocannabinoids and Their Pharmacological Actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Fezza F., Bari M., Florio R., Talamonti E., Feole M., Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–17106. doi: 10.3390/molecules191117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maccarrone M., Guzmán M., Mackie K., Doherty P., Harkany T. Programming of neural cells by (endo)cannabinoids: From physiological rules to emerging therapies. Nat. Rev. Neurosci. 2014;15:786–801. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo V., De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- 7.Maccarrone M., Battista N., Centonze D. The endocannabinoid pathway in Huntington’s disease: A comparison with other neurodegenerative diseases. Prog. Neurobiol. 2007;81:349–379. doi: 10.1016/j.pneurobio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Bisogno T., Di Marzo V. Cannabinoid receptors and endocannabinoids: Role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets. 2010;9:564–573. doi: 10.2174/187152710793361568. [DOI] [PubMed] [Google Scholar]
- 9.Min C., Zheng M., Zhang X., Guo S., Kwon K.J., Shin C.Y., Kim H.S., Cheon S.H., Kim K.M. N-linked Glycosylation on the N-terminus of the dopamine D2 and D3 receptors determines receptor association with specific microdomains in the plasma membrane. Biochim. Biophys. Acta. 2015;1853:41–51. doi: 10.1016/j.bbamcr.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 10.He J., Xu J., Castleberry A.M., Lau A.G., Hall R.A. Glycosylation of beta(1)-adrenergic receptors regulates receptor surface expression and dimerization. Biochem. Biophys. Res. Commun. 2002;297:565–572. doi: 10.1016/S0006-291X(02)02259-3. [DOI] [PubMed] [Google Scholar]
- 11.Wanamaker C.P., Green W.N. N-linked glycosylation is required for nicotinic receptor assembly but not for subunit associations with calnexin. J. Biol. Chem. 2005;280:33800–33810. doi: 10.1074/jbc.M501813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrington W.W., Jacobson K.A., Stiles G.L. Glycoprotein nature of the A2-adenosine receptor binding subunit. Mol. Pharmacol. 1990;38:177–183. [PMC free article] [PubMed] [Google Scholar]
- 13.Soto A.G., Smith T.H., Chen B., Bhattacharya S., Cordova I.C., Kenakin T., Vaidehi N., Trejo J. N-linked glycosylation of protease-activated receptor-1 at extracellular loop 2 regulates G-protein signaling bias. Proc. Natl. Acad. Sci. USA. 2015;112:E3600–E3608. doi: 10.1073/pnas.1508838112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta. 1999;1473:67–95. doi: 10.1016/S0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 15.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 16.Packer N.H., von der Lieth C.W., Aoki-Kinoshita K.F., Lebrilla C.B., Paulson J.C., Raman R., Rudd P., Sasisekharan R., Taniguchi N., York W.S. Frontiers in glycomics: Bioinformatics and biomarkers in disease. An Nih white paper prepared from discussions by the focus groups at a workshop on the Nih campus, Bethesda, MD (September 11–13, 2006) Proteomics. 2008;8:8–20. doi: 10.1002/pmic.200700917. [DOI] [PubMed] [Google Scholar]
- 17.Helenius A., Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 18.Hauselmann I., Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berois N., Osinga E. Glycobiology of neuroblastoma: Impact on tumor behavior, prognosis, and therapeutic stratergies. Front. Oncol. 2014;4:114. doi: 10.3389/fonc.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esko J.D., Bertozzi C.R. Chemical tools for inhibiting glycosylation. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor; New York, NY, USA: 2008. pp. 707–708. [Google Scholar]
- 21.Oslowski C.M., Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/b978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guha P., Kaptan E., Gade P., Kalvakolanu D.V., Ahmed H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget. 2017;15:4068191–4068207. doi: 10.18632/oncotarget.19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan K., Khoshnoodi J., Ruotsalainen V., Tryggvason K.J. N-linked glycosylation is critical for the plasma membrane localization of nephrin. Am. Soc. Nephrol. 2002;13:1385–1389. doi: 10.1097/01.ASN.0000013297.11876.5B. [DOI] [PubMed] [Google Scholar]
- 24.Del Grosso F., De Mariano M., Passoni L., Luksch R., Tonini G.P., Longo L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer. 2011;11:525. doi: 10.1186/1471-2407-11-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquariello N., Catanzaro G., Marzano V., Amadio D., Barcaroli D., Oddi S., Federici G., Urbani A., Finazzi Agrò A., Maccarrone M. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem. 2009;23:29413–29426. doi: 10.1074/jbc.M109.044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertwee R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onaivi E.S., Chakrabarti A., Chaudhuri G. Cannabinoid receptor genes. Prog. Neurobiol. 1996;48:275–305. doi: 10.1016/0301-0082(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 28.Onaivi E.S., Leonard C.M., Ishiguro H., Zhang P.W., Lin Z., Akinshola B.E., Uhl G.R. Endocannabinoids and cannabinoid receptor genetics. Prog. Neurobiol. 2002;66:307–344. doi: 10.1016/S0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 29.González-Mariscal I., Krzysik-Walker S.M., Doyle M.E., Liu Q.R., Cimbro R., Santa-Cruz Calvo S., Ghosh S., Cieśla Ł., Moaddel R., Carlson O.D., et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruehle S., Wager-Miller J., Straiker A., Farnsworth J., Murphy M.N., Loch S., Monory K., Mackie K., Lutz B. Discovery and characterization of two novel CB1 receptor splice variants with modified N-termini in mouse. J. Neurochem. 2017;142:521–533. doi: 10.1111/jnc.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howlett A.C., Champion-Dorow T.M., McMahon L.L., Westlake T.M. The cannabinoid receptor: Biochemical and cellular properties in neuroblastoma cells. Pharmacol. Biochem. Behav. 1991;40:565–569. doi: 10.1016/0091-3057(91)90364-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R., Kim T.K., Qiao Z.H., Cai J., Pierce W.M., Jr., Song Z.H. Biochemical and mass spectrometric characterization of the human CB2 cannabinoid receptor expressed in Pichia pastoris—Importance of correct processing of the N-terminus. Protein Expr. Purif. 2007;55:225–235. doi: 10.1016/j.pep.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Kedei N., Szabo T., Lile J.D., Treanor J.J., Olah Z., Iadarola M.J., Blumberg P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 34.Wirkner K., Hognestad H., Jahnel R., Hucho F., Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16:997–1001. doi: 10.1097/00001756-200506210-00023. [DOI] [PubMed] [Google Scholar]
- 35.Veldhuis N.A., Lew M.J., Abogadie F.C., Poole D.P., Jennings E.A., Ivanusic J.J., Eilers H., Bunnett N.W., McIntyre P. N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J. Biol. Chem. 2012;287:21765–21772. doi: 10.1074/jbc.M112.342022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Free R.B., Hazelwood L.A., Cabrera D.M., Spalding H.N., Namkung Y., Rankin M.L., Sibley D.R. D1 and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J. Biol. Chem. 2007;282:21285–21300. doi: 10.1074/jbc.M701555200. [DOI] [PubMed] [Google Scholar]
- 37.Russo D., Chazenbalk G.D., Nagayama Y., Wadsworth H.L., Rapoport B. Site-directed mutagenesis of the human thyrotropin receptor: Role of asparagine-linked oligosaccharides in the expression of a functional receptor. Mol. Endocrinol. 1991;5:29–33. doi: 10.1210/mend-5-1-29. [DOI] [PubMed] [Google Scholar]
- 38.Chiang N., Tai H.H. The role of N-glycosylation of human thromboxane A2 receptor in ligand binding. Arch. Biochem. Biophys. 1998;352:207–213. doi: 10.1006/abbi.1998.0620. [DOI] [PubMed] [Google Scholar]
- 39.Karpa K.D., Lidow M.S., Pickering M.T., Levenson R., Bergson C. N-linked glycosylation is required for plasma membranelocalization of D5, but not D1, dopamine receptors in transfectedmammalian cells. Mol. Pharmacol. 1999;56:1071–1078. doi: 10.1124/mol.56.5.1071. [DOI] [PubMed] [Google Scholar]
- 40.Michineau S., Muller L., Pizard A., Alhenc-Gélas F., Rajerison R.M. N-linked glycosylation of the human bradykinin B2 receptor is required for optimal cell-surface expression and coupling. Biol. Chem. 2004;385:49–57. doi: 10.1515/BC.2004.007. [DOI] [PubMed] [Google Scholar]
- 41.Lanctot P.M., Leclerc P.C., Clément M., Auger-Messier M., Escher E., Leduc R., Guillemette G. Importance of N-glycosylation positioning for cell-surface expression, targeting, affinity and quality control of the human AT1 receptor. Biochem. J. 2005;390:367–376. doi: 10.1042/BJ20050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutton A.C., Massoura A.N., Dover T.J., Andrews N.A., Barnes N.M. Identification and functional significance of N-glycosylation of the 5-ht5A receptor. Neurochem. Int. 2008;52:419–425. doi: 10.1016/j.neuint.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Böer U., Neuschäfer-Rube F., Möller U., Püschel G.P. Requirement of N-glycosylation of the prostaglandin E2 receptor EP3beta for correct sorting to the plasma membrane but not for correct folding. Biochem. J. 2000;15:839–847. doi: 10.1042/bj3500839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotamisligil G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. (Lond.) 2008;32:S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacher P., Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi H., Yamashita Y. Role of N-glycosylation in cell surface expression and protection against proteolysis of the intestinal anion exchanger SLC26A3. Am. J. Physiol. Cell Physiol. 2012;302:C781–C795. doi: 10.1152/ajpcell.00165.2011. [DOI] [PubMed] [Google Scholar]
- 47.Pupo M., Bodmer A., Berto M., Maggiolini M., Dietrich P.Y., Picard D. A genetic polymorphism repurposes the G-protein coupled and membrane-associated estrogen receptor GPER to a transcription factor-like molecule promoting paracrine signaling between stroma and breast carcinoma cells. Oncotarget. 2017;8:46728–46744. doi: 10.18632/oncotarget.18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oddi S., Dainese E., Sandiford S., Fezza F., Lanuti M., Chiurchiù V., Totaro A., Catanzaro G., Barcaroli D., De Laurenzi V., et al. Effects of palmitoylation of Cys(415) in helix 8 of the CB(1) cannabinoid receptor on membrane localization and signalling. Br. J. Pharmacol. 2012;165:2635–2651. doi: 10.1111/j.1476-5381.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oddi S., Dainese E., Fezza F., Lanuti M., Barcaroli D., De Laurenzi V., Centonze D., Maccarrone M. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J. Neurochem. 2011;116:858–865. doi: 10.1111/j.1471-4159.2010.07041.x. [DOI] [PubMed] [Google Scholar]
- 50.Heakal Y., Woll M.P., Fox T., Seaton K., Levenson R., Kester M. Neurotensin receptor-1 inducible palmitoylation is required for efficient receptor-mediated mitogenic-signaling within structured membrane microdomains. Cancer Biol. Ther. 2011;12:427–435. doi: 10.4161/cbt.12.5.15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araque A., Castillo P.E., Manzoni O.J., Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donvito G., Nass S.R., Wilkerson J.L., Curry Z.A., Schurman L.D., Kinsey S.G., Lichtman A.H. The endogenous cannabinoid system: A budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43:52–79. doi: 10.1038/npp.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Dimauro I., Pearson T., Caporossi D., Jackson M.J. A simple protocol for the subcellular fractionation of skeletal muscle and cells and tissue. BMC Res. Notes. 2012;5:513. doi: 10.1186/1756-0500-5-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catani V.M., Gasperi V. Assay of CB1 receptor binding. Methods Mol. Biol. 2016;1412:41–55. doi: 10.1007/978-1-4939-3539-0_5. [DOI] [PubMed] [Google Scholar]