Abstract
Matricaria chamomilla L. is a popular medicinal herb that is used for healing various diseases and is widely distributed worldwide in temperate climate zones, and even in the subtropical climate of Southern and Western Iran. This study was aimed at comparing the volatile oil constituents, along with antiradical potential and HPLC analysis of methanolic extracts from twelve plant samples growing in Iran. The present research was carried out for the first time on these populations. Among seventeen identified volatile chemicals evaluated by GC/MS and GC/FID, representing 92.73–97.71% of the total oils, α-bisabolone oxide A (45.64–65.41%) was the major constituent, except in case of “Sarableh” as a new chemotype, where (E)- and (Z)-γ-bisabolene (42.76 and 40.08%, respectively) were the predominant components. Oxygenated sesquiterpenes (53.31–74.52%) were the most abundant compounds in the samples excluding “Sarableh” with 91.3% sesquiterpene hydrocarbons. “Sarableh” also exerted the most potent antioxidant capacity with EC50 = 7.76 ± 0.3 µg/mL and 6.51 ± 0.63 mmol TE (Trolox® equivalents)/g. In addition, populations “Lali” and “Bagh Malek” contained the highest amounts of apigenin and luteolin with 1.19 ± 0.01 mg/g and 2.20 ± 0.0 mg/g of plant material, respectively. Our findings depict a clear correlation between phytochemical profiles and antiradical potential of M. chamomilla and geographical factors.
Keywords: Matricaria chamomilla L., Iranian populations, ecological effects, volatile compounds, antiradical capacity
1. Introduction
Matricaria chamomilla L. (syn. Chamomilla recutita L. Rauschert, German chamomile), belonging to the Asteraceae family, is one of the well-known medicinal plant species which has been widely used for centuries. The herb is currently consumed around the world as herbal tea, with more than 1 million cups daily [1,2]. M. chamomilla is used in the treatment of many ailments and disorders; internally to facilitate digestion and as antispasmodic, externally to treat minor wounds. In folk medicine, its use spreads from the relief of various pains such as headaches and toothaches to the facilitation of menstruation [3]. Chamomile essential oil (EO) has been commonly applied in Iranian folk medicine as an anti-inflammatory, antispasmodic, anti-peptic ulcer, antibacterial and antifungal agent [4,5].
Several papers report sesquiterpenes as the most dominant constituent of M. chamomilla EO. Spathulenol (12.50%) [6], α-bisabolol oxide A (7.9–62.1%) [7,8,9,10], α-bisabolol oxide B (25.56%) [10], β-farnesene (52.73%) [11], (E)-β-farnesene (42.59%) [12], β-cubebene (27.8%) [13], trans-β-farnesene [14,15], chamazulene (27.8–31.2%) [16], and α-bisabolol (56.9%) [17] were recently reported as the main EO compounds.
Non-volatile compounds of M. chamomilla have also been investigated. The major phytochemicals comprise polyphenols, particularly the flavonoids apigenin, patuletin, quercetin, luteolin and their glucosides [18,19,20]; among them apigenin is reported as one of the most bioactive phenolics [19,21].
In the present study, EO compositions of twelve Iranian M. chamomilla populations collected from diverse regions were qualitatively and quantitatively assessed. Since the main radical scavengers are phenolics, flavonoids being the major group within phenolics [22], our study was also designed to determine apigenin and luteolin contents, as its main flavonoid aglycons [19,23], by utilizing analytical HPLC. Antiradical capacities of the extracts with DPPH and ORAC assays were also compared. In order to make comparison, the plants were harvested at the same flourishing period. To the best of our knowledge, this is the first report of these populations.
2. Results
2.1. Essential Oil Contents
As shown in Table 1, the studied plant samples were characterized mostly with similar EO yields. Populations “B” (1.03 ± 0.003%) and “BM” (0.78 ± 0.017%) contained the highest and lowest amount of EO, respectively (Table 1). As the plant sample “B” was cultivated in the university’s garden and it was regularly irrigated, the highest EO content can be predicted.
Table 1.
Essential oil constituents and yields of twelve harvested Matricaria chamomilla populations.
| No. | RI A | RT B | Populations | B | I | BM | L | MS | Mo | G | SS | Mu | A | DS | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds C | Amount (%) | |||||||||||||||
| 1 | 1063 | 7.79 | Artemisia ketone | nd | 0.65 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 2 | 1423 | 17.75 | Trans-caryophyllene | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.5 | |
| 3 | 1454 | 18.75 | (E)-β-Farnesene | 8.51 b | 16.68 a | 9.58 a,b | 10.60 a,b | 13.92 a,b | 13.91 a,b | 9.82 a,b | 6.61 b | 10.33 a,b | 15.29 a | 12.37 a,b | 5.25 b | |
| 4 | 1484 | 19.45 | Germacrene D | 1.31 | nd | nd | nd | nd | nd | nd | nd | nd | 0.55 | nd | 2.21 | |
| 5 | 1500 | 20 | Bicyclo-germacrene | 2.01 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 6 | 1506 | 20.21 | (Z)-α-Bisabolene | nd | nd | nd | nd | nd | 0.81 | nd | nd | nd | nd | nd | nd | |
| 7 | 1514 | 20.3 | (Z)-γ-Bisabolene | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 40.08 | |
| 8 | 1529 | 20.7 | (E)-γ-Bisabolene | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 42.76 | |
| 9 | 1561 | 21.65 | (E)-Nerolidol | 1.76 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 10 | 1577 | 22.1 | (+)-Spathulenol | 1.53 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 11 | 1630 | 23.18 | (γ)-Eudesmol | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.78 | |
| 12 | 1656 | 24.16 | α-Bisabolol oxide-B | 21.88 a | 1.55 b,c | 1.52 b,c | 1.59 b,c | 1.39 c | 1.65 b,c | 1.68 b,c | 1.80 b | 1.58 b,c | 1.64 b,c | 1.37 c | nd | |
| 13 | 1685 | 24.56 | α-Bisabolol | nd | nd | nd | nd | 1.32 | 2.86 | 2.07 | nd | nd | nd | nd | nd | |
| 14 | 1693 | 24.9 | α-Bisabolone oxide A | 11.36 d | 47.91 b,c | 53.28 b | 52.14 b,c | 46.98 c | 46.74 c | 45.64 c | 51.87 b,c | 65.41 a | 47.7 b,c | 49.18 b,c | nd | |
| 15 | 1730 | 25.76 | Chamazulene | 19.22 a | 8.29 b,c | 9.74 b | 4.74 e | 8.44 b,c | 6.14 d | 8.02 c | 9.3 b,c | 4.18 e | 2.58 f | 6.06 d | nd | |
| 16 | 1748 | 26.18 | α-Bisabolol oxide A | 16.78 b,c | 14.03 c | 13.93 c | 19.35 b | 16.75 b,c | 19.41 b | 24.02 a | 22.26 a,b | 7.53 d | 20.25 b | 17.22 b,c | nd | |
| 17 | 1890 | 26.31 | (E)-Spiroether | 8.37 a | 7.51 b | 4.70 c | 5.75 b,c | 7.12 a,b | 6.41 b | 6.49 b | 3.73 c | 5.31 b,c | 5.96 b | 7.26 a,b | nd | |
| Total identified compounds % | 92.73 | 96.64 | 92.76 | 94.2 | 95.27 | 96.51 | 97.71 | 95.59 | 94.35 | 94.53 | 93.47 | 93.08 | ||||
| EOs yield % | 1.03 ± 0.003 | 0.84 ± 0.006 | 0.78 ± 0.17 | 0.88 ± 0.012 | 0.94 ± 0.035 | 0.79 ± 0.006 | 0.88 ± 0.021 | 0.91 ± 0.009 | 0.9 ± 0.023 | 0.89 ± 0.006 | 0.83 ± 0.009 | 0.98 ± 0.021 | ||||
A Relative retention index to C8–C24 n-alkanes on HP-5MS column; B Retention times; C Compounds listed in order of elution from HP-5MS column; nd: not detected; the means were compared using Duncan’s comparisons test (p < 0.05); small letters (a, b, c) in each row show the significant difference of related component among various populations.
2.2. Chemical Profiles of Volatile Oils
Seventeen compounds were detected in these twelve populations. The sesquiterpene α-bisabolone oxide A (45.64–65.41%) was the major EO constituent in the samples except “B” and “S”, whereas its concentration was the highest in population “Mu” (Table 1).
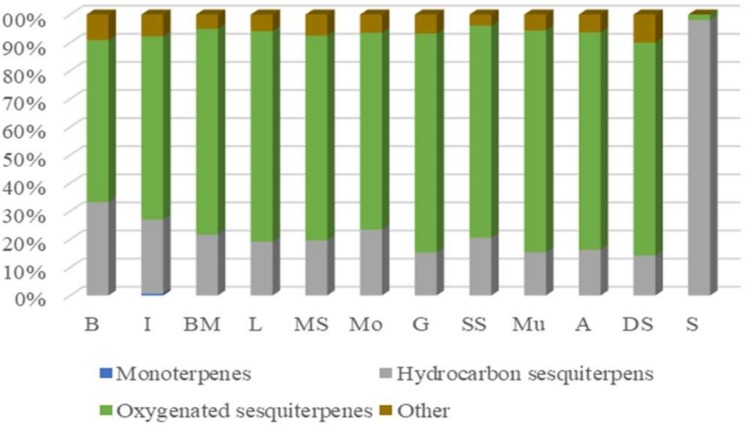
The cultivated sample “B” was rich in α-bisabolol oxide B (21.88%) and chamazulene (19.22%), while the percentages of these compounds were lower under the wild growth conditions. The blue colour of EOs in all samples except “S” represented their chamazulene contents, whereas, “S” due to lack of this compound showed a yellowish green colour. Accordingly, oxygenated sesquiterpenes (53.31–74.52%) were the predominant chemical group of EO constituents in all studied samples, excluding “S”, which was rich in sesquiterpene hydrocarbons (Figure 1).
Figure 1.
Volatile oil components from Matricaria chamomilla populations as a percentage of total identified compounds.
2.3. Apigenin and Luteolin Quantification of Methanolic Extracts
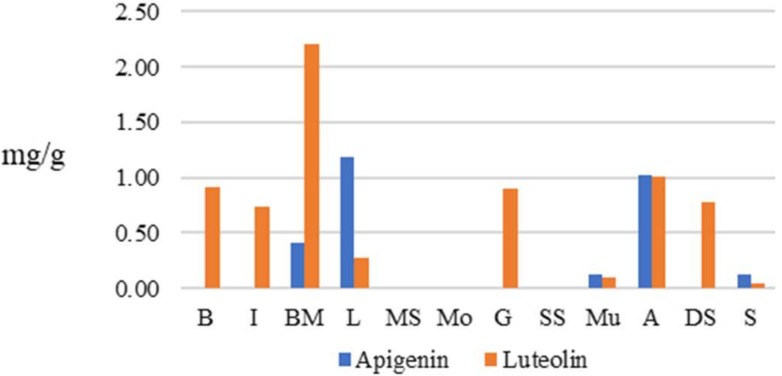
Methanolic extracts of samples “L” and “A” contained the highest amounts of apigenin, with 1.19 ± 0.01 mg/g and 1.02 ± 0.01 mg/g, respectively. Luteolin was present in higher concentrations in “BM” (2.20 ± 0.0 mg/g) and “A” (1.01 ± 0.02 mg/g) extracts (Figure 2).
Figure 2.
Apigenin and luteolin contents of twelve plant samples (mg/g of dry weight) analysed by HPLC.
2.4. Classification of M. Chamomilla Populations
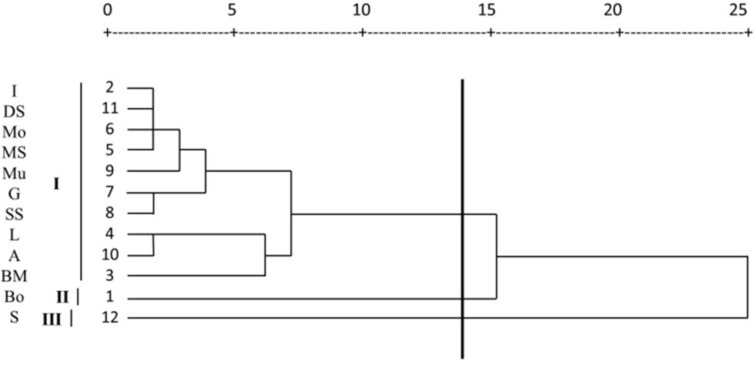
To characterize and identify the different chemotypes of Iranian M. chamomilla populations, their EO compositions and main flavonoids (apigenin and luteolin) were submitted to cluster analysis (CA) and principal component analysis (PCA). As shown in Figure 3 and Figure 4, the dendrograms allowed separating the M. chamomilla populations into three main groups, each representing a distinct chemotype.
Figure 3.
Dendrogram of the Matricaria chamomilla populations resulting from the cluster analysis (based on Euclidean distances) of the volatile oil components. chemotype I (α-bisabolone oxide A and α-bisabolol oxide A), chemotype II (chamazulene and α-bisabolol oxide B), chemotype III ((Z) and (E)-γ-bisabolene).
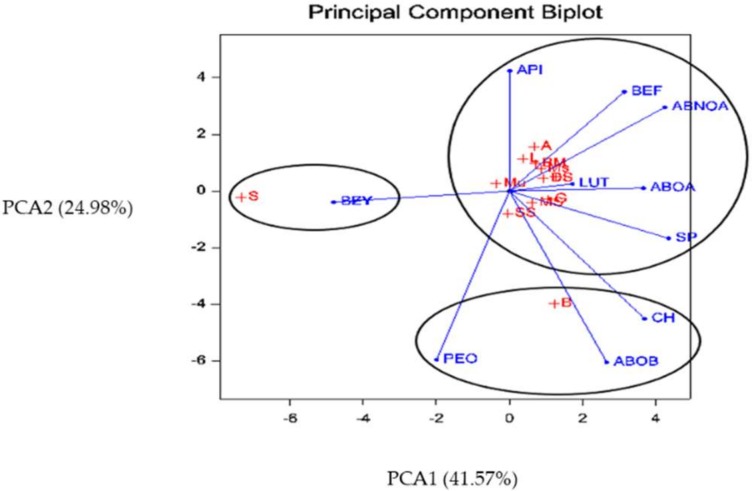
Figure 4.
Principal component analysis (PCA) of the Matricaria chamomilla populations. ABOA: α-bisabolol oxide A, ABOB: α-bisabolol oxide B, PEO: percentage of essential oil, CH: chamazulene, SP: (E)-spiroether, ABNOA: α-bisabolone oxide A, BEF: (E)-β-farnesene, BZY: (Z)-γ-bisabolene, BEY: (E)-γ-bisabolene, LUT: luteolin, API: apigenin.
PCA is a mathematical procedure that transforms several correlated variables into various uncorrelated variables called principal components (PC). PC1, PC2, and PC3 showed the highest variation of phytochemicals among the studied populations. PC1 explained 41.57% of total variation and had a positive correlation with α-bisabolol oxide A, (E)-β-farnesene, α-bisabolone oxide A and (E)-spiroether, and negative correlation with (Z)-γ-bisabolene and (E)-γ-bisabolene. The second PC (PC2), with 24.98% of variance, demonstrated positive correlation with chamazulene, α-bisabolol oxide B, α-bisabolone oxide A and the EO content. Furthermore, PC3 represented positive correlation in the case of apigenin and luteolin, which accounted for 12.02% of the total variance (Table 2).
Table 2.
Eigenvalues, variance and cumulative variance for three principal components.
| Major Phytochemicals | Principal Components | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Chamazulene | 0.377 | 0.881 | 0.023 |
| α-Bisabolol oxide A | 0.760 | 0.126 | 0.114 |
| (E)-β-Farnesene | 0.679 | −0.293 | −0.004 |
| α-Bisabolol oxide B | 0.044 | 0.961 | 0.011 |
| α-Bisabolone oxide A | 0.742 | 0.525 | 0.104 |
| (E)-Spiroether | 0.849 | 0.377 | −0.032 |
| (Z)-γ-Bisabolene | −0.965 | −0.119 | −0.130 |
| (E)-γ-Bisabolene | −0.965 | −0.119 | −0.130 |
| Essential oil content | −0.480 | 0.600 | −0.348 |
| Apigenin | 0.002 | −0.295 | 0.638 |
| Luteolin | 0.160 | 0.235 | 0.857 |
| Eigen values | 4.57 | 2.74 | 1.32 |
| Variance (%) | 41.57 | 24.98 | 12.02 |
| Cumulative variance (%) | 41.57 | 66.55 | 78.57 |
Regarding a significant contribution of phytochemical variation in PC1 and PC2, the scatter plot of PC1 and PC2 was used to specify phytochemical distance. The studied populations were classified in three groups, which confirmed the CA results.
In accordance with the CA, the populations “I”, “DS”, “Mo”, “MS”, “Mu”, “G”, “SS”, “L”, “A” and “BM” were classified into the same category, while, “B” and “S” were grouped into the individual subclasses. The first group possessed α-bisabolone oxide A and α-bisabolol oxide A as the major constituents as well as apigenin and luteolin (chemotype I). The second chemotype (II), was characterized by high amounts of chamazulene and α-bisabolol oxide B. The chemotype (III) was the richest in (Z) and (E)-γ-bisabolene.
2.5. Effect of Environmental Factors on Phytochemicals
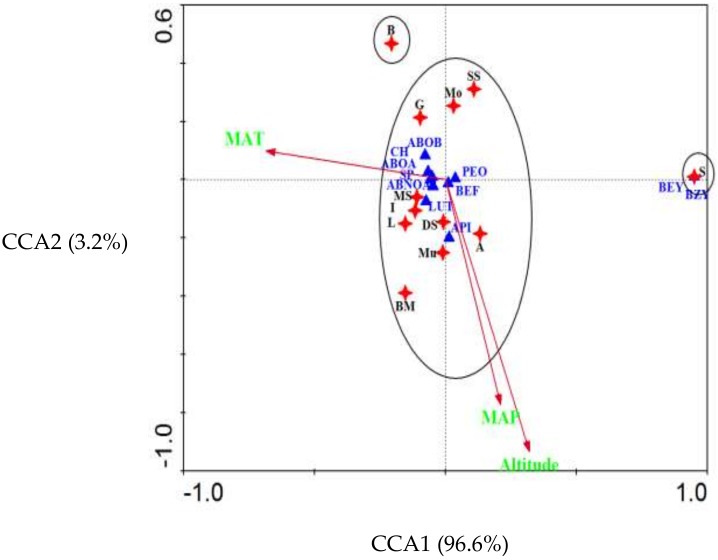
In order to assess the effect of environmental factors on EO components, along with apigenin and luteolin contents, canonical correspondence analysis (CCA) was applied based on a matrix of three environmental factors including altitude, mean annual temperature (MAT), and mean annual precipitation (MAP) [24] and major EO compounds, along with apigenin and luteolin contents (Figure 5).
Figure 5.
Canonical correspondence analysis (CCA) biplot of Matricaria chamomilla populations, linking percentages of the major constituents, collected from different environmental conditions. Populations B: Bodgold, I: Izeh, BM: Bagh Malek, L: Lali, MS: Masjed Soleyman, Mo: Mollasani, SS: Saleh Shahr, Mu: Murmuri, A: Abdanan, DS: Darreh Shahr, and S: Sarableh, MAT: mean annual temperature; MAP: mean annual precipitation; ABOA: α-bisabolol oxide A, ABOB: α-bisabolol oxide B, PEO: essential oil percentage, CH: chamazulene, SP: (E)-spiroether, ABNOA: α-bisabolone oxide A, BEF: (E)-β-farnesene, BZY: (Z)-γ-bisabolene, BEY: (E)-γ-bisabolene, LUT: luteolin, API: apigenin.
According to the CCA, phytochemicals of populations in first group were significantly affected by ecological parameters (altitude, temperature and precipitation) while the main oil composition and flavonoids of “Sarableh” and “Boldgold” were changed by genetic factors. Therefore, the “Sarableh” population can be introduced as a new chemotype.
2.6. Antiradical Activity of the Extracts
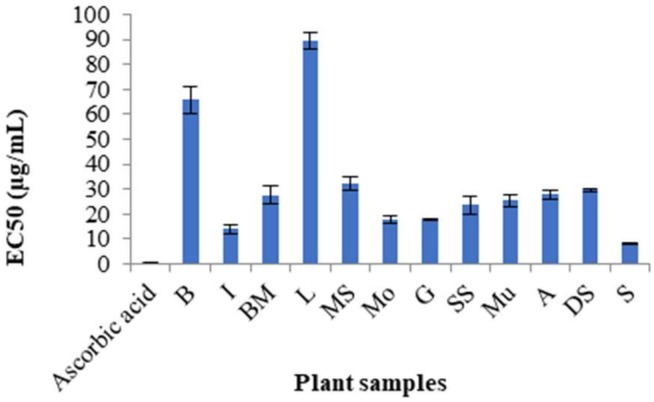
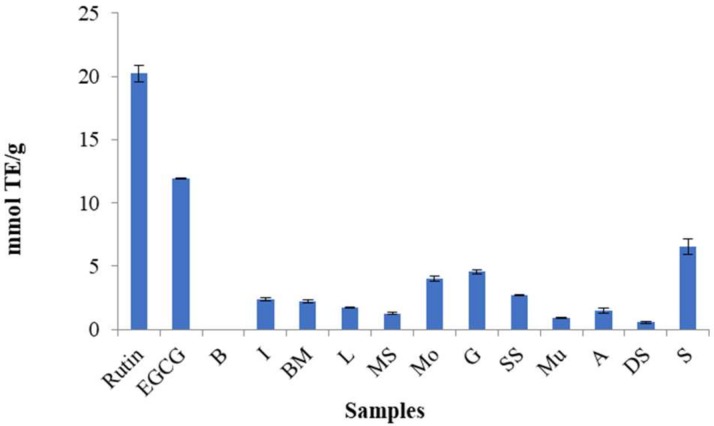
In the evaluation of the antioxidant potential of the twelve selected populations, “S” showed the most significant antiradical capacity, with EC50 = 7.76 ± 0.3 µg/mL and 6.51 ± 0.63 mmol TE/g measured by DPPH and ORAC assays, respectively. However, the extracts showed lower activity compared to ascorbic acid (EC50 = 0.3 ± 0.02 µg/mL) in the DPPH and rutin (20.22 ± 0.63 mmol TE/g) and EGCG (11.97 ± 0.02 mmol TE/g) in the ORAC assay (Figure 6 and Figure 7).
Figure 6.
Antiradical scavenging activity of twelve plant samples of Matricaria chamomilla in the DPPH assay.
Figure 7.
Antiradical capacity of selected Matricaria chamomilla populations in the ORAC assay.
3. Discussion
The results of the present study confirm the variability of EO composition, flavonoid profile and antiradical activity, which are significantly affected by a diversity of ecological conditions.
The abiotic factors (e.g., moisture, topography, temperature, and edaphic factors) highly impact the phytoconstituents variation, and/or biotic factors remarkably influenced the terpene biosynthesis pathways and chemotype profiles [25]. However, the role of other ecological effects such as climatic factors (e.g., humidity, wind, atmospheric gases, and light), physiographic factors (e.g., steepness and sunlight on vegetation and direction of slopes), edaphic factors (the soil attributes), and biotic factors (e.g., the existence of lianas, epiphytes, parasites etc.) may also be considerable in chemotype variations.
In accordance with our findings, oxygenated sesquiterpenes (53.31–74.52%) are the most dominant EO compounds of the selected Matricaria chamomilla L. samples, which corroborates previous reports. The wild populations were significantly different compared with the cultivated sample (Bodgold) in EO composition. α-Bisabolone oxide A was mostly the major EO constituent in the populations.
In the literature, α-bisabolone oxide A was previously identified from M. chamomilla by different groups; for instance, the EO, diethyl ether and dichloromethane fractions of EO contained α-bisabolone oxide A, with 47.7, 57.7 and 50.5%, respectively [8]; whereas the EO of an Estonian M. chamomilla sample was characterized by high bisabolone oxide A content (13.9%) [2]. This compound was the major EO constituent of M. chamomilla grown in Italy (9.2–11.2%) [26], Iran (53.45%) [27], India (20.4 and 8.9%) [28] and Turkey (47.7%) [7]. The evaluation of the daily α-bisabolol oxide A content in M. chamomilla EO revealed that the highest amount can be between 16:00–18:00 (55.41%) [29]. Moreover, this aromatic phytoconstituent was isolated with three extraction methods (7.9, 42.3 and 50.5%) in EO of the species [9].
It is noteworthy, that the sample “Sarableh” (with the highest altitude and least minimum and maximum annual temperatures) as a new chemotype was the richest in two geometric isomers of γ-bisabolene (82.84%), whilst these sesquiterpenes were not markedly detected in the other plant populations. This population also demonstrated the most potent antiradical capacity compared with those samples.
γ-Bisabolene was previously specified as the major characteristic constituent of Pimpinella pruatjan Molk. [30]. EO of Ocimum africanum Lour. was also rich in (E)-γ-bisabolene (2.6–9.5%) [31]. This sesquiterpene was isolated from seeds of Ziziphus jujuba var. inermis. [32]. The main EO compounds of M. chamomilla harvested in Iran were identified as α-bisabolol (7.27%), (Z,Z)-farnesol (39.70–66.00%) [33], (E)-β-farnesene (24.19%) [34] and α-bisabolol oxide A (17.14%) [35]. EOs containing remarkable amounts of γ-bisabolene exhibited anti-inflammatory properties and anti-proliferative activities in human prostate cancer, glioblastoma, lung carcinoma, breast carcinoma, colon adenocarcinoma and human oral squamous cell lines [36,37,38,39].
According to the results, the abundance of luteolin was much higher than the apigenin content. The lack of luteolin and apigenin as the selected flavones was reported in populations “MS” and “Mo”. These samples are most probably rich in other phenolic natural products.
The total phenolic and flavonoid contents of a M. chamomilla extract were 37.51 and 21.72 mg/g of dry weight, respectively; this report was formerly accomplished by using HPLC-DAD [40]. Moreover, chlorogenic acid, apigenin-7-glucoside, rutin, cynaroside, luteolin, apigenin and apigenin-7-glucoside derivatives were previously qualified as the significant phytochemical composition of M. chamomilla extract [23].
As apigenin and luteolin were present in “Sarableh” in low concentrations, the high free radical scavenging capacity of this sample is obviously related to other polyphenolic compounds.
In a similar study, methanol extracts of M. chamomilla yielded from different samples indicated more potent antiradical activity than EOs analysed by the DPPH test, due to polyphenols present in extracts; although, they possessed a moderate effect in comparison with the standards [6].
Furthermore, in vitro antioxidant activities of M. chamomilla extracts, along with its apigenin and apigenin-7-glycoside contents were previously characterized, with IC50 of 18.19 ± 0.96 µg/mL, 2.0 ± 0.1 mg/g and 20.1 ± 0.9 mg/g, respectively [41], confirming the primary importance of apigenin in the antioxidant capacity of M. chamomilla. Moreover, free radical scavenging activity (DPPH assay) of M. chamomilla volatile oil and its major components were formerly recorded in the following order: chamazulene > α-bisabolol oxide A > chamomile EO > (E)-β-farnesene > α-bisabolol [7].
4. Materials and Methods
4.1. Plant Material
M. chamomilla flowers (300 g/sample) were individually collected from different regions of Iran in the flowering period, in spring (April) 2017. The harvested populations “Izeh”, “Bagh Malek”, “Lali”, “Masjed Soleyman”, “Mollasani”, “Gotvand” and “Saleh Shahr” from Khuzestan and “Murmuri”, “Abdanan”, “Darreh Shahr” and “Sarableh” from Ilam province were compared with “Bodgold”, which was cultivated at the botanical garden of Department of Horticultural Sciences, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
The plants were identified by Dr. Mehrangiz Chehrazi affiliated with the same department, and a voucher specimen of each sample was deposited in the herbarium of the department. The voucher’s codes and geographic coordinates including the latitude, longitude, altitude using the Global Positioning System (GPS), along with mean annual temperatures of the studied populations are given in Table 3. The meteorological data (MAP, MAT, MMaxAT, and MMinAT) were collected from September until May 2017 [24]. The flowers were shade dried and finely ground. Each powdered sample was individually well-mixed and subjected to analysis.
Table 3.
Geographic locations and climatic conditions of the studied Matricaria chamomilla populations from Iran.
| Population Name | Voucher’s Code | Abbreviated Name | Altitude (m) | Latitude | Longitude | MAP (mm/year) | MAT (°C) | MMaxAT (°C) | MMinAT (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Bodold (Ahvaz) | KHAU_236 | B | 16 | 31°18′ N | 48°39′ E | 98.20 | 22.62 | 35.77 | 9.47 |
| Izeh | KHAU_237 | I | 428 | 31°57′ N | 48°49′ E | 472.80 | 18.35 | 31.28 | 5.42 |
| Bagh Malek | KHAU_238 | BM | 907 | 31°19′ N | 50°05′ E | 285.90 | 20.28 | 32.17 | 8.4 |
| Lali | KHAU_239 | L | 373 | 32°20′ N | 49°05′ E | 280.60 | 21.26 | 33.57 | 8.95 |
| Masjed Soleyman | KHAU_240 | MS | 250 | 32°02′ N | 49°11′ E | 241.30 | 22.67 | 34.27 | 11.07 |
| Mollasani | KHAU_241 | Mo | 51 | 31°39′ N | 48°57′ E | 100.80 | 22.26 | 36.35 | 8.17 |
| Gotvand | KHAU_242 | G | 70 | 32°14′ N | 48°48′ E | 159.70 | 21.08 | 34.96 | 7.21 |
| Saleh Shahr | KHAU_243 | SS | 65 | 32°04′ N | 48°40′ E | 164.30 | 20.69 | 34.07 | 7.32 |
| Murmuri | KHAU_244 | Mu | 530 | 32°46′ N | 47°37′ E | 213.90 | 23.35 | 35.17 | 11.53 |
| Abdanan | KHAU_245 | A | 740 | 32°55′ N | 47°31′ E | 363.60 | 19.62 | 30.44 | 8.8 |
| Darreh Shahr | KHAU_246 | DS | 629 | 33°05′ N | 47°28′ E | 463.00 | 17.85 | 30.9 | 4.8 |
| Sarableh | KHAU_247 | S | 1037 | 33°47′ N | 46°35′ E | 345.80 | 15.01 | 27.31 | 2.71 |
MAP: mean annual precipitation; MAT: mean annual temperature; MMaxAT: mean maximum anuual temperature; MMinAT: mean minimum anuual temperature.
4.2. Chemicals and Spectrophotometric Measurements
Analytical grade 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azobis-2-methylpropionamidine dihydrochloride (AAPH) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox®) (Sigma-Aldrich, Steinheim, Germany), fluorescein (Fluka Analytical, Buchs, Germany); ascorbic acid, rutin and Na2SO4 (Merck, Darmstadt, Germany); epigallocatechin gallate (EGCG), apigenin (≥99%) and luteolin (≥97%) (Sigma-Aldrich, Germany) were purchased. Furthermore, all solvents of analytical grade were provided by the Merck company (Germany). Spectrophotometric measurements were carried out by a UV-VIS spectrophotometer (FLUOstar Optima, BMG Labtech, Ortenberg, Germany).
4.3. Extraction of Volatile Oils
To extract the EOs, 60 g of each powdered sample was individually subjected to Clevenger apparatus (hydro-distillation method) for 3 h. The obtained EOs were dried over anhydrous sodium sulphate and stored in refrigerator at 4 °C until analysis. Yields of extracted EOs were calculated by Equation (1):
| EO% = (EOs weight)/(dried plants weight) × 100 | (1) |
Diethyl ether was used to elute the whole amount of EOs from the apparatus, and the weighing process was performed after evaporating the solvent.
4.4. Gas Chromatographic Analysis (GC-FID)
In the case of GC-FID analysis, the EOs were analyzed by Shimadzu GC-17A (Kyoto, Japan) equipped with an FID detector and SGE™ BP5 capillary column (Trajan Scientific and Medical, Victoria, Australia) (30 m × 0.25 mm column with a 0.25 μm film thickness). The split mode in GC was a ratio of 1:100. Injector and FID detector temperatures were set at 280 and 300 °C, respectively. The oven temperature was kept at 60 °C for 1 min and then raised to 250 °C at 5.0 °C/min and held for 2 min, while the ambient oven temperature range was +4 to +450 °C. Helium gas was used at a flow rate of 1 mL/min as a carrier gas.
4.5. Gas Chromatography-Mass Spectrometric (GC-MS) Analysis
Analysis of the samples was carried out using an Agilent 7890B gas chromatograph (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a 5977B mass spectrometry detector. The GC instrument was equipped with a split inlet, working in a split ratio of 100:1 mode with a 30 m × 0.25 mm HP-5MS capillary column with a 0.25 μm film thickness and 0.25 μm particle size (temperature range: −60 to +320/340 °C). The injection port temperature was 250 °C. The oven temperature was kept at 60 °C for 1 min and then programmed from 60 °C to 250 °C at 5 °C/min; then, the temperature was kept at 250 °C for 2 min. Helium (99.999%) was used as a carrier gas with a flow rate of 1 mL/min and inlet pressure 35.3 kPa. The mass spectrometer was operated in the electron impact mode at 70 eV, and the inert ion source (HES EI) temperature was set to 350 °C; the temperature of the quadrupole was set at 150 °C, while the MS interface was set to 250 °C. A scan rate of 0.6 s (cycle time: 0.2 s) was applied, covering a mass range from 35 to 600 amu.
4.6. Identification of Essential Oil Components
Most of the compounds were identified using two different analytical approaches: (a) comparison of Kovats indices of n-alkanes (C8–C24) [42] and (b) comparison of mass spectra (using authentic chemicals and Wiley spectral library collection). Identification was considered tentative when based on mass spectral data alone. In GC-FID and GC-MS, data acquisition and analysis were performed using Chrom-cardTM (Scientific Analytical Solutions, Zurich, Switzerland, version DS) and XcaliburTM software (Thermo Fisher Scientific, Waltham, MA, USA, 4.0 Quick Start), respectively.
4.7. Preparation of Solvent Extracts
Five g of each sample was individually extracted with MeOH (3 × 75 mL) in an ultrasonic bath (VWR-USC300D) for 10 min, at 40 °C under power grade 9.
After removing the solvent under reduced pressure at 50 °C (Rotavapor R-114, Büchi), the concentrated extracts were subjected to evaluate the antiradical assays and HPLC analysis.
4.8. HPLC Analysis of Apigenin And Luteolin
Twenty μL of each extract (1 mg/mL) was separately injected into an analytical high-performance liquid chromatography system (HPLC) (Knauer, Berlin, Germany) by using an end capped Eurospher II 100-5 C18, Vertex Plus Column (Knauer, Berlin, Germany) (250 × 4.6 mm with precolumn) with particle size: 5 µm, pore size: 100 Å and temperature 30 °C; coupled to UV detector (Knauer GmbH-Smartline 2600, Berlin, Germany) at a wavelength range 190 to 500 nm (quantification at 330 nm), while MeOH/H2O was applied as the mobile phase with a gradient system, increasing MeOH from 30% to 70% within 40 min, with a flow rate of 1 mL/min, at ambient temperature. Analysis was performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
4.9. Antiradical Capacity
4.9.1. DPPH Assay
Free radical scavenging activity of the plant extracts was assessed by DPPH assay [43]. The measurement was carried out on 96-well microtiter plates. In brief, Microdilution series of samples (1 mg/mL, dissolved in MeOH) were prepared starting with 150 µL. To gain 200 µL of sample, 50 µL of DPPH reagent (100 µM) was added to each sample. The microplate was stored at room temperature under dark conditions. The absorbance was measured after 30 min at 550 nm using the microplate reader. MeOH (HPLC grade) and ascorbic acid (0.01 mg/mL) were used as a blank control and standard, respectively. Antiradical activity was calculated using the following Equation (2):
| I% = [(A0 − A1/A0) × 100] | (2) |
where A0 is the absorbance of the control and A1 is the absorbance of the standard sample. Anti-radical activity of the samples was expressed as EC50 (concentration of the compounds that caused 50% inhibition). EC50 (µg/mL) values were calculated using GraphPad Prism software version 6.05 (GraphPad Software, San Diego, CA, USA). Each sample was measured in triplicate.
4.9.2. ORAC Assay
Twelve extracts were subjected to ORAC assay [44] with slight modifications. Microtiter plates (96-well) were used for measurement of the samples. Briefly, 20 µL of extracts (0.01 mg/mL) was mixed with 60 µL of AAPH (peroxyl free radical generator) (12 mM) and 120 µL of fluorescein solution (70 mM). Then, the fluorescence was measured for 3 h at 1.5-min cycle intervals with the microplate reader. The standard Trolox® was used. Activity of all samples was compared with rutin and EGCG as positive controls. Antioxidant capacities were reported as μmol TE (Trolox® equivalents)/g of dry matter.
4.10. Statistical Analysis
All the experiments were done in triplicate, and the results are expressed as means ± SD. The data were assessed with one-way analysis of variance (ANOVA) using SAS software (version 9.2, SAS Institute Inc., Cary, NC, USA) and GraphPad Prism version 6.05. The means were compared using Duncan’s comparisons test (p < 0.05).
5. Conclusions
According to our preliminary study, the chemo-diversity and antiradical potential of twelve studied Matricaria chamomilla populations were highly affected by a variety of ecological conditions. To acquire the best yield with a good profile of active principles, it is crucial to combine a good genotype with optimal environmental circumstances.
In accordance with our findings, oxygenated sesquiterpenes are the most dominant EO compounds of the selected Matricaria chamomilla samples. Almost all plant populations contained apigenin and luteolin. Since the plant sample “Sarableh” indicated the most potent capacity to scavenge free radicals and its apigenin and luteolin contents were insignificant, its activity undoubtedly refers to the presence of other polyphenolic compounds. Due to high amounts of apigenin and luteolin, populations “Lali” and “Bagh Malek” can be considered as a rich source of these compounds. Our results confirm the effects of growth conditions on the quantity and quality of aromatic phytochemicals. More investigations are required to study the amounts of other polyphenolic compounds in diverse populations and the correlations between secondary metabolite contents, bioactivities and ecological effects.
Acknowledgments
The authors cordially appreciate Mehrangiz Chehrazi (Department of Horticultural Science, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran) for taxonomical identification of plant samples.
Author Contributions
E.P. harvested the plant materials and performed the essential oil extraction. M.M.S. designed the experiments and analyzed the essential oils. E.K. conducted the study. Bioactivity was analyzed by Z.P.Z., J.M. wrote, and J.H. and D.C. revised the manuscript.
Funding
This project was financially supported by the Shahid Chamran University of Ahvaz. Financial support from the National Research, Development and Innovation Office (OTKA K115796), Economic Development and Innovation Operative Programme GINOP-2.3.2-15-2016-00012 and János Bolyai Research Scholarship of the Hungarian Academy of Sciences is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Singleton V.L., Rossi J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965;16:144–158. [Google Scholar]
- 2.Raal A., Kaur H., Orav A., Arak E., Kailas T., Müürisepp M. Content and composition of essential oils in some Asteraceae species. Proc. Est. Acad. Sci. 2011;60:55–63. doi: 10.3176/proc.2011.1.06. [DOI] [Google Scholar]
- 3.Shivananda Nayak B., Sivachandra Raju S., Chalapathi Rao A. Wound healing activity of Matricaria recutita L. extract. J. Wound Care. 2007;16:298–302. doi: 10.12968/jowc.2007.16.7.27061. [DOI] [PubMed] [Google Scholar]
- 4.Sashidhara K., Verma R., Ram P. Essential oil composition of Matricaria recutita L. from the lower region of the Himalayas. Flavour Fragr. J. 2006;21:274–276. doi: 10.1002/ffj.1582. [DOI] [Google Scholar]
- 5.Owlia P., Rasooli I., Saderi H., Aliahmad M. Retardation of biofilm formation with reduced productivity of alginate as a result of Pseudomonas aeruginosa exposure to Matricaria chamomilla essential oil. Pharmacogn. Mag. 2007;3:83–89. [Google Scholar]
- 6.Formisano C., Delfine S., Oliviero F., Carlo G., Rigano D., Senatore F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy) Ind. Crop. Prod. 2014;23:256–263. doi: 10.1016/j.indcrop.2014.09.042. [DOI] [Google Scholar]
- 7.Firat Z., Demirci F., Demirci B. Antioxidant activity of chamomile essential oil and main components. Nat. Volatiles Essent. Oils. 2018;5:11–16. [Google Scholar]
- 8.Göger G., Demirci B., Ilg S., Demirci F. Antimicrobial and toxicity profiles evaluation of the chamomile (Matricaria recutita L.) essential oil combination with standard antimicrobial agents. Ind. Crop. Prod. 2018;120:279–285. doi: 10.1016/j.indcrop.2018.04.024. [DOI] [Google Scholar]
- 9.Homami S.S., Jaimand K., Rezaee M.B., Afzalzadeh R. Comparative studies of different extraction methods of essential oil from Matricaria recutita L. in Iran. J. Chil. Chem. Soc. 2016;61:2982–2984. doi: 10.4067/S0717-97072016000200026. [DOI] [Google Scholar]
- 10.Wesolowska A., Grzeszczuk M., Kulpa D. Propagation method and distillation apparatus type affect essential oil from different parts of Matricaria recutita L. plants. J. Essent. Oil Bear. Plants. 2015;18:179–194. doi: 10.1080/0972060X.2014.895210. [DOI] [Google Scholar]
- 11.Andrade M.A., Azevedo S., Motta F.N., Lucília M., Silva C.L., De Santana J.M., Bastos I.M.D. Essential oils: In vitro activity against Leishmania amazonensis, cytotoxicity and chemical composition. BMC Complement. Altern. Med. 2016;16:1–8. doi: 10.1186/s12906-016-1401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuskin S., Godin B., Leroy P., Capella Q., Wathelet J. Fast gas chromatography characterisation of purified semiochemicals from essential oils of Matricaria chamomilla L. (Asteraceae) and Nepeta cataria L. (Lamiaceae) J. Chromatogr. A. 2009;1216:2768–2775. doi: 10.1016/j.chroma.2008.09.109. [DOI] [PubMed] [Google Scholar]
- 13.Berechet M.D., Manaila E., Stelescu M.D., Craciun G. The composition of essential oils obtained from Achillea millefolium and Matricaria chamomilla L., originary from Romania. Rev. Chim. 2017;68:2787–2795. [Google Scholar]
- 14.Šavikin K., Menković N., Ristić M., Arsić I., Zdunić G., Đorđević S., Ćujić N., Pljevljakušić D. Quality testing of chamomile essential oil Chamomilla recutita (L.) Rausch., variety Banatska in comparison to requirements of the European Pharmacopoeia and ISO standard. Lek. sirovine. 2011;31:55–65. [Google Scholar]
- 15.Soković M., Glamočlija J., Marin P.D., Brkić D., van Griensven L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhoudi R. Chemical constituents and antioxidant properties of Matricaria recutita and Chamaemelum nobile essential oil growing wild in the south west of Iran. J. Essent. Oil Bear. Plants. 2013;16:531–537. doi: 10.1080/0972060X.2013.813219. [DOI] [Google Scholar]
- 17.Ansari M.J., Al-Ghamdi A., Usmani S., Ali Khan K.A., Alqarni A.S., Kaur M., Al-Waili N. In vitro evaluation of the effects of some plant essential oils on Ascosphaera apis, the causative agent of Chalkbrood disease. Saudi J. Biol. Sci. 2017;24:1001–1006. doi: 10.1016/j.sjbs.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choopani R., Sadr S., Kaveh S., Kaveh N., Dehghan S. Pharmacological treatment of catarrh in Iranian traditional medicine. J. Tradit. Chinese Med. Sci. 2015;5:71–74. doi: 10.1016/j.jtcme.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvetanovic A., Svarc-Gajic J., Zekovic Z., Savic S., Vulic J., Maskovic P., Cetkovic G. Comparative analysis of antioxidant, antimicrobiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta. 2015;242:721–732. doi: 10.1007/s00425-015-2308-2. [DOI] [PubMed] [Google Scholar]
- 20.Cushnie T.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaiter L., Bouheroum M., Benayache S., Benayache F., Leon F., Brouard I., Quintana J., Estevez F., Bermejo J. Sesquiterpene lactones and other constituents from Matricaria chamomilla L. Biochem. Syst. Ecol. 2007;35:533–538. doi: 10.1016/j.bse.2007.03.008. [DOI] [Google Scholar]
- 22.Da Silva Pinto M. Tea: A new perspective on health benefits. Food Res. Int. 2013;53:558–567. doi: 10.1016/j.foodres.2013.01.038. [DOI] [Google Scholar]
- 23.Ionita R., Alexandra P., Mihasan M., Lucian D., Hancianu M., Cioanca O., Hritcu L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine. 2018;47:113–120. doi: 10.1016/j.phymed.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 24.Iran Meteorological Organization. [(accessed on 3 January 2019)]. Available online: https://www.irimo.ir/far/index.php.
- 25.Croteau R., Gershenzon J. Genetic Control of Monoterpene Biosynthesis in Mints (Mentha: Lamiaceae) In: Ellis B.E., Kuroki G.W., Stafford H.A., editors. Genetic Engineering of Plant Secondary Metabolism. Plenum Press; New York, NY, USA: 1994. pp. 193–229. [Google Scholar]
- 26.Tirillini B., Pagiotti R., Menghini L., Pintore G. Essential oil composition of ligulate and tubular flowers and receptacle from wild Chamomilla recutita (L.) Rausch. grown in Italy. J. Essent. Oil Res. 2006;18:42–45. doi: 10.1080/10412905.2006.9699381. [DOI] [Google Scholar]
- 27.Rahmati M., Azizi M., Hasanzadeh Khayyat M., Nemati H., Asili J. Yield and oil constituents of chamomile (Matricaria chamomilla L.) flowers depending on nitrogen application, plant density and climate conditions. J. Essent. Oil Bear. Plants. 2011;14:731–741. doi: 10.1080/0972060X.2011.10643996. [DOI] [Google Scholar]
- 28.Das M., Ram G., Singh A., Mallavarapu G.R., Remash S., Ram M., Kumar S. Volatile constituents of different plant parts of Chamomilla recutita L. Rausch grown in the Indo-Gangetic plains. Flavour Fragr. J. 2002;17:9–12. doi: 10.1002/ffj.1035. [DOI] [Google Scholar]
- 29.Salehi A., Hazrati S. How essential oil content and composition fluctuate in German chamomile flowers during the day? J. Essent. Oil Bear. Plants. 2017;20:622–631. doi: 10.1080/0972060X.2017.1351895. [DOI] [Google Scholar]
- 30.Nurcahyanti A.D.R., Nasser I.J., Sporer F., Graf J., Bermawie N., Reichling J., Wink M. Chemical composition of the essential oil from aerial parts of Javanian Pimpinella Pruatjan Molk. and its molecular phylogeny. Diversity. 2016;8:15. doi: 10.3390/d8030015. [DOI] [Google Scholar]
- 31.Padalia R.C., Singh V.R., Bhatt G., Chauhan A., Upadhyay R.K., Verma R.S., Chanotiya C.S. Optimization of harvesting and post-harvest drying methods of Ocimum africanum Lour. for production of quality essential oil. J. Essent. Oil Res. 2018;30:437–443. doi: 10.1080/10412905.2018.1495110. [DOI] [Google Scholar]
- 32.Lee N.K., Shin H.J., Kim W.-S., In G., Han C.K. Studies on the Chemical Constituents from the Seeds of Zizyphus jujuba var. inermis. Nat. Prod. Sci. 2017;23:258–264. doi: 10.20307/nps.2017.23.4.258. [DOI] [Google Scholar]
- 33.Jaimand K., Rezaee M. A study on chemical composition of essential oils of Matricaria chamomilla L. from Tehran, Hammedan and Kazeroon. Iran. J. Med. Aromat. Plants Res. 2003;13:11–24. [Google Scholar]
- 34.Ayoughi F., Barzegar M., Sahari M.A., Naghdibadi H. Chemical compositions of essential oils of Artemisia dracunculus L. and endemic Matricaria chamomilla L. and an evaluation of their antioxidative effects. J. Agric. Sci. Technol. 2011;13:79–88. [Google Scholar]
- 35.Amiri S., Sharafzadeh S. Essential oil components of German chamomile cultivated in Firoozabad, Iran. Orient. J. Chem. 2014;30:365–367. doi: 10.13005/ojc/300151. [DOI] [Google Scholar]
- 36.Farag M.A., Al-Mahdy D.A. Comparative study of the chemical composition and biological activities of Magnolia grandiflora and Magnolia virginiana flower essential oils. Nat. Prod. Res. 2013;27:1091–1097. doi: 10.1080/14786419.2012.696256. [DOI] [PubMed] [Google Scholar]
- 37.Bayala B., Bassole I.H.N., Gnoula C., Nebie R., Yonli A., Morel L., Figueredo G., Nikiema J.B., Lobaccaro J.M.A., Simpore J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylvestre M., Pichette A., Longtin A., Nagau F., Legault J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Jou Y.-J., Chen C.-J., Liu Y.-C., Way T.-D., Lai C.-H., Hua C.-H., Wang C.-Y., Huang S.-H., Kao J.-Y., Lin C.-W. Quantitative phosphoproteomic analysis reveals gamma-bisabolene inducing p53-mediated apoptosis of human oral squamous cell carcinoma via HDAC2 inhibition and ERK1/2 activation. Proteomics. 2015;15:3296–3309. doi: 10.1002/pmic.201400568. [DOI] [PubMed] [Google Scholar]
- 40.Apaza M.J.T. Antibacterial effect against Streptococcus mutans (ATCC 25175) and phenolic compounds profile of chamomile (Matricaria chamomile L.) cultivated in Puno. Rev. Investig. Altoandinas. 2015;17:173–182. doi: 10.18271/ria.2015.110. [DOI] [Google Scholar]
- 41.Vieira Pereira S., Reis R.A.S., Garbuio D.C., Alexandre L., Pedro De Freitas L.A. Dynamic maceration of Matricaria chamomilla inflorescences: Optimal conditions for flavonoids and antioxidant activity. Rev. Bras. Farmacogn. 2018;28:111–117. doi: 10.1016/j.bjp.2017.11.006. [DOI] [Google Scholar]
- 42.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 43.Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 44.Mielnik M.B., Rzeszutek A., Triumf E.C., Egelandsdal B. Antioxidant and other quality properties of reindeer muscle from two different Norwegian regions. Meat Sci. 2011;89:526–532. doi: 10.1016/j.meatsci.2011.05.021. [DOI] [PubMed] [Google Scholar]