Abstract
The Little Bighorn River is the primary source of water for water treatment plants serving the local Crow Agency population, and has special significance in the spiritual and ceremonial life of the Crow tribe. Unfortunately, the watershed suffers from impaired water quality, with high counts of fecal coliform bacteria routinely measured during run-off events. A metagenomic analysis was carried out to identify potential pathogens in the river water. The Oxford Nanopore MinION platform was used to sequence DNA in near real time to identify both uncultured and a coliform-enriched culture of microbes collected from a popular summer swimming area of the Little Bighorn River. Sequences were analyzed using CosmosID bioinformatics and, in agreement with previous studies, enterohemorrhagic and enteropathogenic Escherichia coli and other E. coli pathotypes were identified. Noteworthy was detection and identification of enteroaggregative E. coli O104:H4 and Vibrio cholerae serotype O1 El Tor, however, cholera toxin genes were not identified. Other pathogenic microbes, as well as virulence genes and antimicrobial resistance markers, were also identified and characterized by metagenomic analyses. It is concluded that metagenomics provides a useful and potentially routine tool for identifying in an in-depth manner microbial contamination of waterways and, thereby, protecting public health.
Keywords: metagenomics, pathogen detection, waterborne disease
1. Introduction
Water is essential for human life and productivity, yet both water quality and security are increasingly under threat globally [1,2,3]. Unfortunately, while wealthy countries are able to afford effective water treatment, poorer nations are severely hampered by a lack of resources for safe water to protect public health. In both cases, sources of contaminants entering waterways are not sufficiently addressed [2].
Communities within the Crow Nation in south central Montana have been aware of deteriorating water quality of the Little Bighorn River for many years [4]. Concerned members of the Crow Nation founded the Crow Environmental Health Steering Committee, the mission of which is to research and mitigate issues concerning environmental public health, improve community health and raise awareness among the tribal population. The Steering Committee and tribal elders have also voiced concern about the effects of climate change on water quality and public health [5,6,7]. Concern about a link between climate change and public health is warranted given that studies have indicated that warmer temperatures may promote an expanded range of distribution of vector-borne infectious diseases and extended seasons of transmission [8,9,10].
The Steering Committee has worked closely with faculty and students at the local Little Big Horn College (LBHC) and Montana State University (MSU), who have conducted water quality studies in accord with priorities established by the Crow tribe. A major focus has been to identify and characterize bacterial, chemical, heavy metal, and radionuclide contaminants in domestic well, recreational, and source waters [11,12,13,14,15]. Findings have informed the local public and environmental health efforts, and supported funding procurement to improve sewage and water treatment facilities along a portion of the Little Bighorn River that serves as source water for the community [12,16].
Among the various concerns addressed, Hamner et al. [17] conducted a source tracking study of run-off from a large concentrated animal feed operation (CAFO) located in the headwaters area of the Little Bighorn River. Results of the study demonstrated the presence of several Escherichia coli human disease-associated serotypes immediately downstream of the CAFO drainage that matched serotypes in cattle manure from the CAFO. As part of the study, enterohemorrhagic and enteropathogenic E. coli (EHEC and EPEC) serotypes were also identified in a popular swim hole of the Little Bighorn River, a summer-time recreational site for children in the town of Crow Agency, the administrative center of the Crow tribe. Fecal contamination and run-off from the CAFO and dozens of smaller ranching operations, as well as leakage from septic systems from homes bordering the river banks, contribute to pollution of the Little Bighorn River and many smaller tributary streams within the watershed.
Recent developments in whole genome sequencing (WGS) technology make it feasible to use DNA sequencing for disease diagnostics and public health surveillance of pathogens [18,19]. Development of portable sequencing platforms, such as the Oxford Nanopore Technologies MinION device, allow for rapid sequencing of whole genomes [20], which facilitates metagenomic sequencing to be accomplished in remote locations [21]. Portability can be especially useful during disease outbreaks where laboratory resources are limited, and when coupled with real-time analysis, can facilitate prompt epidemiological study and public health response to epidemic outbreaks [19,22].
Metagenomic sequencing has been used to study the epidemiology of a variety of infectious disease agents. In one study, a metagenomic approach proved more accurate than conventional genotyping in analyzing an outbreak of tuberculosis, namely by improving identification of single nucleotide polymorphisms and assignment of genome clusters (factors related to the evolution of the outbreak strain) and tracing the spread of the outbreak [23].
Metagenomics currently is used to describe microbial populations in water and sediment to understand community structure and the role of microorganisms in ecological processes [18,24,25,26]. Metagenomics has also been used to examine water quality to protect public health [27,28]. Traditional methods for monitoring water quality focus on fecal coliform counts, but methods employing metagenomics provide additional functional and genomic information for species and strains of microbial pathogens. In addition, markers of the potential for antibiotic resistance, and the presence of virulence genes can also be identified in recreational and source waters [27,28].
Rather than target identification of a pre-selected group of pathogenic microbes or virulence genes by traditional culture, microscopy, immunoassay, or PCR-based methods [29], metagenomics employing next generation sequencing allows accurate identification and characterization of all microorganisms within samples for which genomic data are archived. Further, DNA sequence-based identification and characterization can now be done for microorganisms not easily cultured in a diagnostic setting, and can be used to identify multiple pathogens present in a poly-microbial infection or in water bodies.
2. Materials and Methods
Water samples were collected from the Crow Fair swim hole of the Little Bighorn River, Crow Agency, Montana, on July 16, 2017. The swim hole is located at latitude/longitude of 45o36′1”N, 107o27′12”W, and is a popular summer recreational site used by children and adults of the population of ca. 1,600 residents of Crow Agency. During sampling, several children were observed swimming 100 meters upstream of the sampling site. Four samples were collected at ten-minute intervals over the course of 30 minutes, then pooled. Samples were transported on ice to Montana State University for processing by two different methods. First, 100 mL aliquots from each of the four consecutive samplings were pooled (400 mL total) and filtered using 47 mm, 0.45 μm filters to collect particulates. Filters were processed using the PowerWater DNA isolation kit (Qiagen). (Technical notes: The PowerWater kit was chosen in part due to its incorporation of reagents to remove inhibitors that may interfere with downstream PCR and DNA sequencing reactions. Choice of 0.45 μm filters as opposed to 0.22 μm filters was due to the turbidity of the water samples. We followed the manufacturer’s recommendation to use 0.45 μm filters for turbid water samples to reduce clogging and allow a greater volume of water to be filtered than would be possible with the more restrictive 0.22 μm filters. We readily acknowledge that this choice may have reduced the variety of bacteria detected, since during the initial stages of filtering, smaller bacteria would be lost in the larger 0.45 μm pores, but also are aware that as the filters clogged, many smaller cells should have been captured.) Modifications to the PowerWater kit protocol were made as follows: Filters were placed in a 5 mL PowerWater kit tube, and 1.5 mL of PowerWater kit buffer (instead of 1 mL per manufacturer’s instruction) and Metapolyzyme (20 μL of a 10.0 mg mL−1 sterile PBS pH7.5, Sigma #MAC4L) were included in the lysis step to enhance digestion of extracellular material and release of DNA. (Note: In our experience, use of 1.5 mL of the first PowerWater kit buffer was found to increase the yield of DNA compared to using only 1 mL.) The tube was vortexed for ca. 60 s (minimizing fragmentation) and incubated overnight at 37 °C, with periodic rotation and agitation. After overnight incubation, DNA extraction was continued, following PowerWater kit manufacturer’s instructions.
A separate DNA extraction procedure was carried out to harvest DNA for the detection of coliform bacteria. To begin, three technical replicates of ca. 50 mL river water were filtered under vacuum and the filters placed on m-Coliblue24 plates [30] and incubated overnight at 37 °C for coliform counts. Following the manufacturer’s protocol, membrane filters of 0.45 μm pore diameter were used [30] for the m-ColiBlue24 assay. It is noteworthy that consistency was maintained for both filtration procedures, in that 0.45 μm pore filters were also used for filtering the larger 400 mL volume of river water for the uncultured, non-selective procedure described above. After the resulting blue E. coli colonies on the m-Coliblue24 plates were enumerated, one filter yielding colony growth was selected for DNA extraction, using the PowerWater kit and the amended protocol described above. A second filter with colony growth was lifted with forceps, replica plated on CHROMagarO157 agar (CHROMagar), and incubated overnight at 37 °C. The appearance of mauve-colored colonies indicated putative EHEC.
DNA sequencing was accomplished using the MinION sequencing platform (Oxford Nanopore) and the 1D Ligation Sequencing Kit (Nanopore kit SQK-LSK108), following the manufacturer’s instructions. Resulting fast5 data files were basecalled using Albacore Version 1.2.6 after which fastq sequence processing and analyses were performed using CosmosID (www.cosmosid.com) and MG-RAST [31] software. The metagenomic data is available in MG-RAST (mg-rast.org, MG-RAST ID numbers mgm4778816.3 and mgm4778817.3).
Because of the heightened degree of public health concern regarding E. coli serotypes O157:H7 and O104:H4, and V. cholerae O1 El tor, coverage plots and completion estimates were also generated as an additional indication of confidence in identifying these potential pathogens. GraphMap [32] was used for mapping and coverage calculations for these sequences.
DNA preparations from both river water (without selective growth) and m-ColiBlue24 selection samples were examined by PCR for the presence of eae and Stx genes that are indicative of EHEC and EPEC as previously described [17,33]. Presence of eae is characteristic of both EHEC and EPEC; on the other hand, EHEC contains Stx genes while EPEC lacks Stx genes.
3. Results
3.1. Metagenomic Sequences Generated from DNA Prepared Directly from River Water without Selective Growth on m-ColiBLue243
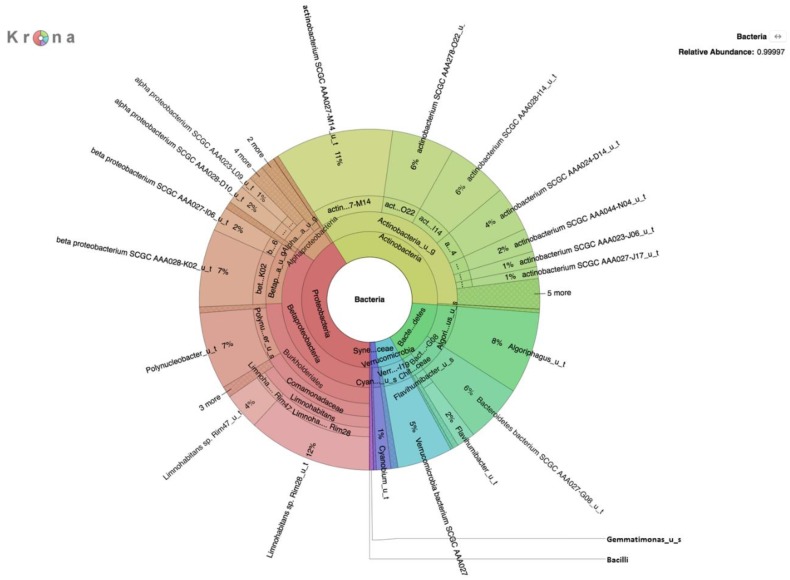
Environmental DNA from microorganisms collected by filtration of water from the Little Big Horn River was subjected to shotgun sequencing using the Oxford Nanopore MinION platform. Sequencing generated 397,884 reads comprising ~1.1 Gbp with an average read length of 2760 bp. CosmosID analysis of the DNA sequences indicated the presence of both Eukarya and Bacteria in the river water community. These included: Eukaryotic protists (Table 1), fungi (Table 2), and bacteria (Table 3 and Figure 1). Several eukaryotic genera that are of potential concern to human health, including Acanthamoeba, Leishmania, Candida, and Rhizomucor, were identified in the analyses (Table 1 and Table 2). Bacteria of concern to human health, Acidovorax and Aeromonas salmonicida, were also identified (Table 3). Limnohabitans was the dominant genera in the filtered river biomass, followed by Actinobacterium, a genus that includes important members of a healthy gut microbiome.
Table 1.
List of eukaryotic microbial genus and species, some of which are pathogenic, identified in the Little Bighorn River metagenome (DNA prepared without selection). WBD (waterborne disease) organisms have a known association with human disease.
| Eukaryotic Genus | Eukaryotic Species | Number of Reads | Disease Association/WBD Organisms |
|---|---|---|---|
| Acanthamoeba | Acanthamoeba polyphaga; Acanthamoeba palestinensis; Acanthamoeba quina; Acanthamoeba castellanii; Acanthamoeba healyi | 55 | Infections of eye, skin, and central nervous system [34] |
| Dictyostelium | Dictyostelium fasciculatum | 39 | |
| Guillardia | Guillardia theta | 6 | |
| Leishmania | Leishmania major; Leishmania donovani; Leishmania arabica; Leishmania infantum; Leishmania turanica; Leishmania aethiopica | 78 | Infections of skin and internal organs [35] |
| Oxytricha | Oxytricha trifallax | 109 | |
| Physarum | Physarum polycephalum | 24 | |
| Salpingoeca | Salpingoeca rosetta | 23 | |
| Symbiodinium | Symbiodinium minutum | 11 |
Table 2.
List of fungal genera and species, some of which are known pathogens, identified as present in the Little Bighorn River metagenome (DNA prepared without selection).
| Fungal Genera | Fungal Species | Number of Reads | Disease Association/WBD Organisms |
|---|---|---|---|
| Amauroascus | Amauroascus niger | 292 | |
| Candida | Candida albicans; Candida dubliniensis | 37 | Infections of the digestive system and vagina; invasive candidiasis [36] |
| Chrysosporium | Chrysosporium queenslandicum | 198 | |
| Drechmeria | Drechmeria coniospora | 5 | |
| Magnaporthe | Magnaporthe oryzae | 5 | |
| Melampsora | Melampsora pinitorqua | 40 | |
| Orpinomyces | Orpinomyces sp | 61 | |
| Pleosporales | Pleosporales sp | 4 | |
| Rhizomucor | Rhizomucor variabilis | 10 | Opportunistic infections [37] |
| Rhizophagus | Rhizophagus irregularis | 108 | |
| Saccharomyces | Saccharomyces cerevisiae | 1 | |
| Trichoderma | Trichoderma longibrachiatum | 3 | |
| Ustilaginoidea | Ustilaginoidea virens | 1 | |
| Verticillium | Verticillium alfalfae | 1 |
Table 3.
Bacterial community identified in the Little Bighorn River metagenome (DNA prepared without selection).
| Bacterial Strain | Number of Reads | Disease Association |
|---|---|---|
| Acidovorax_sp_JHL_3 | 113 | Sepsis [38]; catheter-associated bloodstream infection [39] |
| actinobacterium_SCGC_AAA023_J06 | 197 | |
| actinobacterium_SCGC_AAA024_D14 | 503 | |
| actinobacterium_SCGC_AAA027_M14 | 672 | |
| actinobacterium_SCGC_AAA028_I14 | 580 | |
| actinobacterium_SCGC_AAA044_N04 | 590 | |
| actinobacterium_SCGC_AAA278_O22 | 811 | |
| Aeromonas_salmonicida_subsp_salmonicida_A449 | 3 | Fish pathogen [40]; isolated from human blood [41]; endophthalmitis [42] |
| Bacteroidetes_bacterium_SCGC_AAA027_G08 | 306 | |
| beta_proteobacterium_CB | 105 | |
| beta_proteobacterium_SCGC_AAA027_I06 | 295 | |
| beta_proteobacterium_SCGC_AAA027_K21 | 13 | |
| beta_proteobacterium_SCGC_AAA028_K02 | 52 | |
| Curvibacter_lanceolatus_ATCC_14669 | 173 | |
| Exiguobacterium_acetylicum_DSM_20416 | 2 | |
| Jonesia_denitrificans_DSM_20603 | 2 | |
| Limnohabitans_sp_Rim28 | 2616 | |
| Verrucomicrobia_bacterium_SCGC_AAA027_I19 | 141 |
Figure 1.
Krona plot of bacteria identified in the Little Bighorn River metagenome (DNA prepared without selection).
3.2. Metagenomic Analysis of DNA Prepared from Filter after Selective Growth on m-ColiBlue24 Medium
The average concentration of E. coli was 66 colony forming units (CFU) per 100 mL water that was detected on filters incubated overnight on m-ColiBlue24 medium. This concentration is well below the limit of 126 CFU per 100 mL established by the EPA [43] for recreational water to be considered safe for swimming.
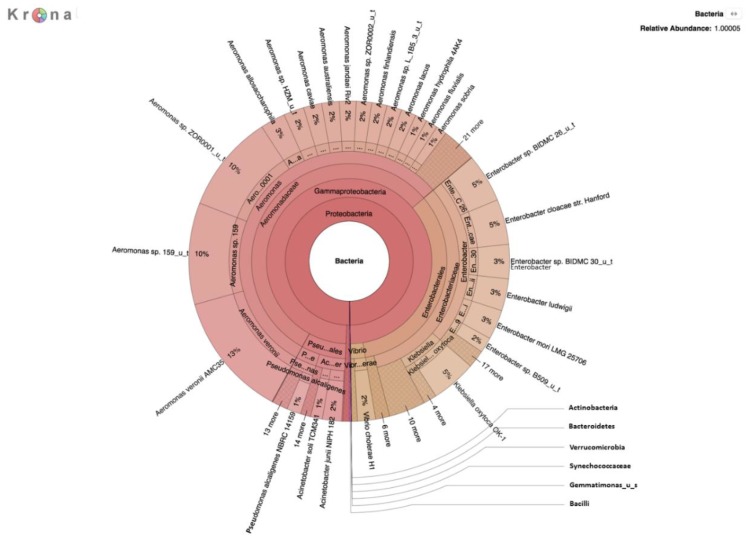
DNA was prepared and sequenced from colonies grown overnight on the filters with an m-ColiBlue24 selection. A total of ~1.6 Gbp of data was generated, comprised of 1,261,165 sequence reads with an average length of 1260 bp. Several bacterial species, some of which are important human pathogens, were detected that had not been identified in the native river water metagenome (Table 4 and Figure 2). In addition to numerous opportunistic pathogens, strains of toxigenic E. coli, Shigella spp., and Vibrio cholerae were also detected.
Table 4.
Bacterial species identified by metagenomic analysis of DNA prepared after selective growth on m-ColiBlue24 medium.
| Bacterial Strain | Number of Reads | Disease Association/WBD Organisms |
|---|---|---|
| Acinetobacter soli NIPH 2899 | 1431 | Bacteremia [44] |
| Acinetobacter junii CIP 64 5 | 1430 | Septicemia [45] |
| Aeromonas veronii AMC34 | 82,139 | Diarrhea [46] |
| Aeromonas allosaccharophila strain CECT 4199 | 60,447 | |
| Aeromonas sp 4287D | 46,956 | |
| Aeromonas australiensis strain CECT 8023 | 46,643 | |
| Aeromonas fluvialis strain LMG 24681 | 30,325 | |
| Aeromonas sobria strain CECT 4245 | 22,042 | |
| Aeromonas sp AE122 | 20,496 | |
| Aeromonas jandaei Riv2 | 19,893 | |
| Aeromonas hydrophila subsp hydrophila ATCC 7966 | 6444 | |
| Aeromonas caviae strain FDA MicroDB 78 | 5495 | |
| Citrobacter braakii strain GTA CB04 | 1010 | Bacteremia [47] |
| Cronobacter dublinensis subsp dublinensis LMG 23823 | 1389 | Opportunistic neonatal infection [48] |
| Enterobacter ludwigii strain EN 119 | 28,696 | |
| Enterobacter mori LMG 25706 | 7371 | |
| Enterobacter asburiae L1 | 3698 | Opportunistic wound infection [49] |
| Escherichia coli O104:H4 str 2011C 3493 | 4390 | Diarrhea, hemolytic uremic syndrome [50] |
| Escherichia coli str K 12 substr MG1655 strain K 12 | 2895 | |
| Escherichia coli O157:H7 str Sakai | 1250 | Diarrhea, hemolytic uremic syndrome [51] |
| Escherichia coli UMN026 | 963 | Urinary tract infection [52] |
| Klebsiella sp BRL6 2 | 6060 | Nosocomial infections, hemorrhagic colitis, pneumonia, urinary tract infections [53,54] |
| Klebsiella oxytoca HKOPL1 | 1982 | |
| Klebsiella pneumoniae strain FDA MicroDB 64 | 928 | |
| Leclercia adecarboxylata ATCC 23216 NBRC 102595 | 1166 | Bacteremia, soft tissue infection [55] |
| Pseudomonas alcaligenes NBRC 14159 | 3819 | Opportunistic infections [56] |
| Shigella flexneri 2a str 301 | 1397 | Shigellosis (diarrhea) [57] |
| Shigella sonnei | 1137 | |
| Shigella boydii Sb227 | 1124 | |
| Shigella boydii 5216 82 | 863 | |
| Vibrio cholerae O1 biovar El Tor str N16961 | 1388 | Cholera [58] |
| Vibrio albensis VL426 | 1161 |
Figure 2.
Krona plot of bacteria identified by metagenomic analysis of DNA prepared after selective growth on m-ColiBlue24 medium.
A number of bacteriophages (Table 5), antimicrobial resistance (AMR) gene markers (Table 6), and virulence genes (Table 7) were identified from the metagenomic analysis after m-ColiBlue24 selection. Several of these bacteriophages, AMR markers, and virulence genes are relevant to human health.
Table 5.
Bacteriophages detected in the metagenomic analysis of DNA from filtered water sample after growth on selective m-ColiBlue24 medium.
| Bacteriophage | Number of Reads | Gene Function and Disease Association |
|---|---|---|
| Aeromonas_phage_phiO18P | 54 | |
| Enterobacteria_phage_cdtI | 39 | |
| Enterobacteria_phage_Fels_2 | 8 | |
| Enterobacteria_phage_fiAA91_ss | 29 | |
| Enterobacteria_phage_HK629 | 49 | |
| Enterobacteria_phage_HK630 | 4 | |
| Enterobacteria_phage_HK633 | 2 | |
| Enterobacteria_phage_lambda | 8 | |
| Enterobacteria_phage_mEp043_c_1 | 7 | |
| Enterobacteria_phage_mEp213 | 64 | |
| Enterobacteria_phage_mEp460 | 33 | |
| Enterobacteria_phage_P1 | 57 | |
| Enterobacteria_phage_P2 | 20 | |
| Enterobacteria_phage_P88 | 38 | |
| Enterobacteria_phage_phiV10 | 9 | |
| Enterobacteria_phage_YYZ_2008 | 18 | |
| Salmonella_phage_RE_2010 | 18 | |
| Salmonella_phage_SSU5 | 15 | |
| Shigella_phage_SfII | 20 | O-antigen modification, enhancing antigen variation and resistance to host defense [59] |
| Shigella_phage_SfIV | 37 | O-antigen modification, enhancing antigen variation and resistance to host defense [60] |
| Stx2_converting_phage_1717 | 21 | Encodes Shiga toxin, a virulence factor inhibiting protein synthesis in infected cells; cytotoxic [61] |
| Yersinia_phage_L_413C | 22 |
Table 6.
Antibiotic resistance markers identified by CosmosID analysis of DNA prepared from filtered water sample after selective growth on m-ColiBlue24 medium.
| Resistance | Gene Name | Number of Reads |
|---|---|---|
| Ampicillin | ampS | 26 |
| Antibiotic Efflux | mexB | 43 |
| acrD | 37 | |
| acrB | 30 | |
| mdtF | 22 | |
| acrF | 18 | |
| mdtC | 13 | |
| mdtG | 10 | |
| Beta-lactamase | blaCEPH-A3 | 25 |
| cphA4 | 20 | |
| pbp2 | 15 | |
| cphA1 | 13 | |
| blaOXA-12 | 12 | |
| cphA7 | 10 | |
| Fluoroquinolone | oqxB | 19 |
| emrR | 10 | |
| Polymyxin Resistance | pmrC | 16 |
| arnA | 12 |
Table 7.
Virulence genes identified by analysis of the metagenome derived from DNA prepared after selective growth on m-ColiBlue24 medium.
| Virulence Gene | Number of Reads | Gene Function and Disease Association |
|---|---|---|
| E. coli GENE f17G | 108 | Fimbrial adhesin; diarrhea [62] |
| E. coli GENE gad | 627 | Homeostasis and acid resistance [63] |
| E. coli GENE iss | 78 | Increased serum survival; extraintestinal infection [64] |
| Pasteurella multocida GENE tetH | 80 | Tetracycline resistance; bacteria cause a variety of diseases in mammals and birds, and opportunistic infections in humans [65,66] |
| Pasteurella multocida GENE tetR | 29 | |
| Vibrio cholerae GENE vasH | 83 | Type VI secretion system; promotes competitive advantage by killing other cell types, and fosters horizontal gene transfer to enhance the evolution of virulence and antibiotic resistance [67] |
| Vibrio cholerae GENE vgrG-3 | 111 | |
| Vibrio cholerae Gene VCA0107 | 19 | |
| Vibrio cholerae Gene VCA0109 | 48 | |
| Vibrio cholerae Gene VCA111 | 51 | |
| Vibrio cholerae Gene VCA0121 | 121 | |
| Yersinia pestis GENE ybtE | 59 | Yersiniabactin iron acquisition system, to obtain iron from the host during infection [68] |
| Yersinia pestis GENE ybtQ | 132 | |
| Yersinia pestis GENE ybtX | 101 |
The eae and Stx genes were both undetected in DNA prepared from unenriched river water, whereas these genes were both detected in DNA isolated from a filter cultured on selective m-ColiBlue24 medium (data from PCR not shown). The presence of Stx2 converting phage sequences was also indicated by the metagenomics analysis (Table 5). Colonies grown on m-ColiBlue24 media and replica plated onto CHROMagarO157 media gave rise to scattered, small spots of mauve growth, indicating the presence of EHEC bacteria.
Five markers of antimicrobial resistance (AMR) at 18 different gene loci were identified in the metagenomic analysis of the m-ColiBlue24 selection sample (Table 6). These markers are related to efflux of antibiotics, resistance to the beta-lactam class of antibiotics, as well as resistance to ampicillin, fluoroquinolones, and polymyxins.
Several virulence genes that contribute to the ability of microbes to cause disease were identified (Table 7). These genes code for virulence factors related to attachment, acid resistance, enhanced serum survival, the competitive advantage against other microbes, and iron acquisition capability.
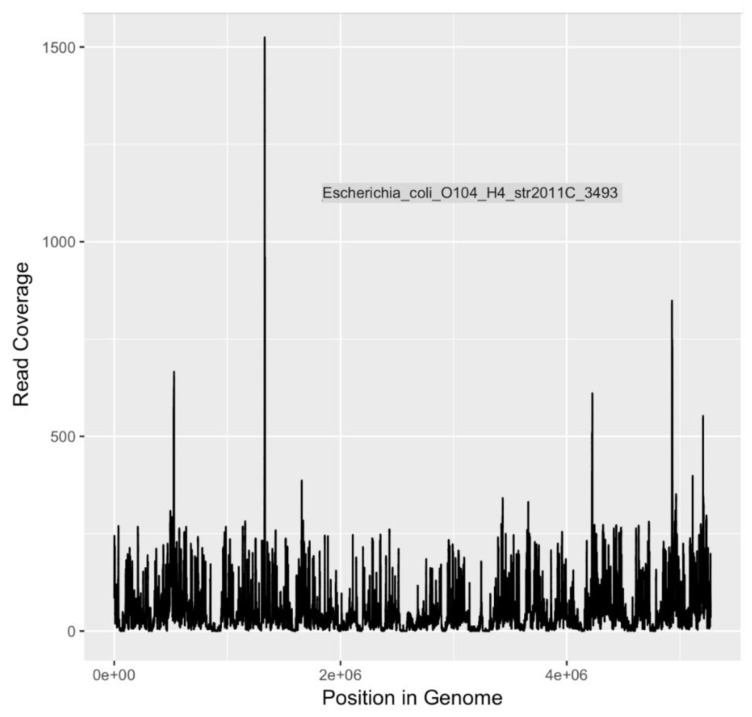
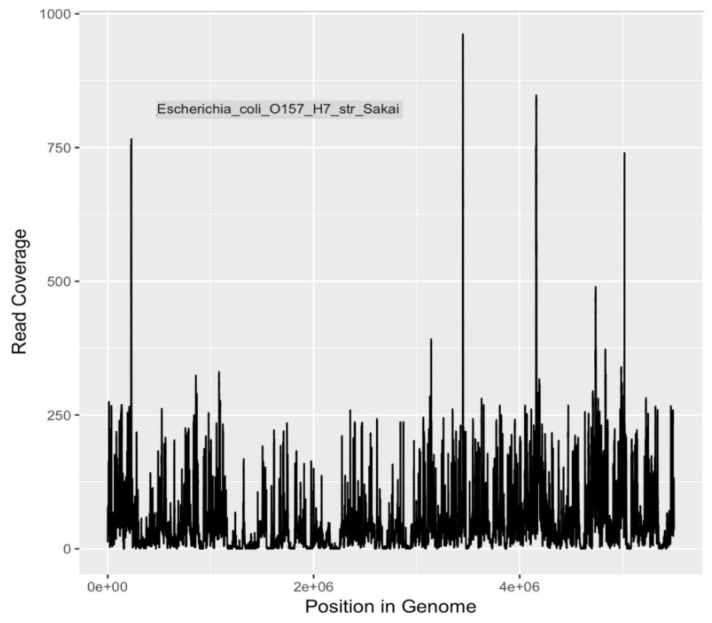
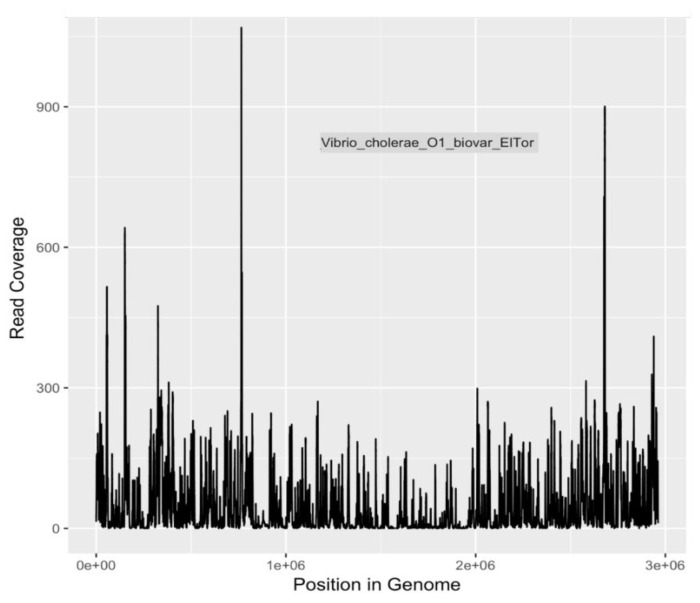
Calculations of DNA sequencing coverage and depth of coverage were made by mapping reads to the genomes of three pathogens of major public health significance. Reads were mapped to E. coli O104:H4, E. coli O157:H7 Sakai, and V. cholerae O1 El Tor with 96%, 95% and 93% coverage (completion) of genomes, respectively (Table 8 and Figure 3, Figure 4 and Figure 5). The depth of coverage was 52×, 50×, and 36×, respectively, for the three genomes (Table 8). Based on genome reporting standards proposed by Bowers et al. [69], these genomic coverages would meet the criterion for high quality metagenome-assembled genomes for these three species. Given that the sequences were mapped to reference genomes with high fidelity, there are unlikely to be multiple, heterogeneous populations for each species. Consequently, these pathogenic populations were present in the river water, and were detectable after selection and enrichment on m-ColiBlue24 media.
Table 8.
Sequencing coverage calculations for E. coli O104:H4, E. coli O157:H7, and V. cholerae O1 biovar Eltor.
| Bacterial Species | Number of Bases Covered | Reference Genome Length | % Coverage along Reference Genome | Depth of Coverage (mean) | Depth of Coverage (st dev) |
|---|---|---|---|---|---|
| E. coli O104:H4 | 5,226,510 | 5,437,407 | 96.3 | 52.2 | 61.8 |
| E. coli O157:H7 | 5,332,768 | 5,594,477 | 95.3 | 50.2 | 60.7 |
| V. cholerae O1 biovar ElTor | 3,770,896 | 4,033,464 | 93.5 | 35.8 | 58.3 |
Figure 3.
Sequencing coverage plot for E. coli O104:H4.
Figure 4.
Sequencing coverage plot for E. coli O157:H7.
Figure 5.
Sequencing coverage plot for V. cholerae O1 El Tor.
4. Discussion
This study describes a metagenomic analysis of water samples collected from a popular swimming site along the Little Big Horn River during the summer of 2017. This work was predicated on previous detection and identification of EHEC and EPEC bacteria in water samples collected from the Little Bighorn River [17] and ongoing concerns of the local community related to water quality and safety. Initial metagenomic analysis of total DNA isolated from filtered river water indicated the presence of species and strains of typical freshwater microorganisms, including both culturable and non-culturable microorganisms. Distinguishing between culturable and non-culturable microbial strains is important, since a study of freshwater lake bacteria estimated approximately only 0.25% of the total bacterial population was culturable [70]. Indeed, most bacterial populations in these environments are viable but not culturable (VBNC) using standard bacteriological culture methods [71,72,73]. A variety of both naturally occurring and potentially pathogenic bacterial species have been shown to enter the VBNC state in response to environmental stress, reducing detection of a significant percentage of a population with relevance to public health in environmental surveillance.
A second metagenomic analysis was also performed using DNA prepared from a filtered water sample after incubation on m-ColiBlue24 medium overnight to allow for selection of coliforms and related species. This two-pronged approach was taken to enhance detection and identification of pathogens, the growth of which may be inhibited by other river bacteria, and therefore not previously recognized in earlier studies targeting detection of coliforms in the river water [17].
Metagenomic analysis of DNA extracted from filters without growth on selective medium revealed a rich diversity of microorganisms, the predominant species of which are presented in Table 1, Table 2 and Table 3. The absence of E. coli in DNA prepared without enrichment on a selective medium was not surprising given the relatively small number of reads and because enrichment on selective media yielded only 66 CFU/100 mL of E. coli in overnight culture on m-ColiBlue24 medium. This medium has been approved by the EPA [30,74] as a sensitive method for detecting and monitoring fecal coliform (E. coli) bacteria in fresh water, where a count of 126 CFU/100 mL (calculated as geometric mean for samples collected over a 30-day period) for E. coli is the maximum permissible limit for recreational waters [43]. Lack of detection of E. coli by metagenomic analysis without selective growth is attributed to the overwhelming abundance and diversity of non-E. coli microorganisms that were present. The proportion of E. coli present in the water samples was representatively small in comparison to the high microbial load on selective media, evidenced by results of the analysis of the m-ColiBlue24-derived metagenome, revealing Gammaproteobacteria and coliform bacteria in significant abundance. Our choice of 0.45 μm pore diameter membrane filters was based on following the manufacture’s protocol [30] for the EPA-approved m-ColiBlue24 method, as well as water sample turbidity. We acknowledge that use of this pore size instead of a smaller pore diameter filter could have resulted in our missing smaller sized microorganisms of public health significance. However, species and strains (Table 4) that were identified, including many serotypes of diarrheagenic bacteria, such as EHEC O157:H7, were also identified in an earlier study [17].
DNA sequences indicative of E. coli serotype O104:H4 and V. cholerae O1 El Tor, both human pathogens of significant interest, were identified (Table 4). Of particular concern, E. coli O104:H4 is an emerging pathogen that first received widespread attention in 2011 as the causative agent of the largest outbreak of Shiga toxin-related disease [75] recorded to date [50]. In Germany and surrounding areas, an O104:H4 outbreak strain caused 3,842 cases of illness, including 18 deaths. Among those stricken, 855 people developed hemolytic uremic syndrome (HUS), leading to an additional 35 deaths [50]. The disease-associated O104:H4 outbreak strain is a novel variant of enteroaggregative E. coli (EAEC) that acquired the Shiga toxin gene that is characteristic of EHEC.
Detection of V. cholerae sequences in the Little Bighorn River is not surprising. V. cholerae, the causative agent of cholera, is an aquatic bacterium with world-wide distribution [76], that may be due to globalization and may indicate changing human demographics. Recently, V. cholerae caused an outbreak of disease in Haiti that had not been seen in 100 years [77,78]. Several virulence genes have been reported as essential for these bacteria to cause an outbreak of cholera, especially including the ctxA and ctxB genes encoding cholera toxin and carried by the bacteriophage CTXφ. This bacteriophage was not detected in this study (Table 5). However, a cluster of genes (VCA0107, VCA0109, VCA0111, VCA0121, vgrG-3, and vasH; see Table 7) associated with the type VI secretion system (T6SS), an important virulence factor of many Gram-negative pathogenic bacteria, including V. cholerae [79], were detected. In the related species V. proteolyticus, the T6SS includes cytotoxic effectors that target both prokaryotic and eukaryotic cells [80]. In V. cholerae, the T6SS has been shown to kill other bacterial species, releasing DNA that in turn can be taken up in the process of horizontal gene transfer (HGT) by naturally competent Vibrio bacteria [67]. Genes taken up by HGT may enhance the antibiotic resistance and virulence potential of Vibrio cells, highlighting the evolutionary potential of pathogenic bacteria in natural environments to become more virulent.
Of relevance to human health, bacteriophage-encoded genes that enhance the pathogenicity of host bacteria were also detected. Two types of Shigella-specific phage, SfII and SfIV, allow for O antigen modification and increased antigen variation [59,60]. The Stx2 converting phage of E. coli O157:H7 and other related Shiga toxigenic E. coli (STEC) encodes the Stx2 protein, an important virulence factor causing lysis of host cells and contributing to hemolytic uremic syndrome [61].
Detection of several AMR markers in the m-ColiBlue24 metagenome (Table 6) is relevant as the worldwide spread of antibiotic resistance is increasingly recognized as a major public health threat, compromising treatment of a variety of infectious diseases [81]. Widespread use of antibiotics in human and veterinary medicine has contributed to an increasing pool of bacteria harboring AMR genes and these bacteria, in turn, are now widely distributed in agricultural products, animals, humans, and the environment [82].
The metagenomic analyses presented in this study indicate that a variety of potential human disease-related pathogens and AMR markers were present and detectable in water samples collected from the Little Bighorn River during the summer of 2017. The presence of gene markers for E. coli O157:H7 (Table 4 and Table 5), a human pathogen of significant concern, is in agreement with earlier findings of Hamner [17]. Presence of Shiga toxin gene markers indicated by both PCR (data not shown) and metagenomic analysis, as well as mauve-colored colony growth on ChromagarO157 medium, a differential/selective medium and indicative test for O157:H7, provide both genetic and phenotypic evidence for continued presence of E. coli O157:H7 bacteria in the Little Bighorn River. As it is understood that the major reservoir of O157:H7 bacteria is cattle and other ruminants [83], livestock ranching operations along the length of the Little Bighorn River, including a large concentrated animal feed operation close to the headwaters of the river, provide likely sources of this contamination to the watershed.
Penicillin derivatives are widely used in animal husbandry and hence ampicillin and beta-lactamase resistance might be expected to coincide with the presence of animal-associated pathogens [84]. However, tetracyclines tend to be more broadly used, and the absence of any tetracycline resistance gene markers would suggest further work is needed to identify sources of contamination. It is not currently known which antibiotics are primarily used in the Little Bighorn watershed.
Animal experiments with the E. coli O157:H7 bacteria or other potential pathogens identified in the present study were not conducted. Therefore, it is unclear whether isolates from the Little Bighorn River are capable of causing disease. Nevertheless, the presence of E. coli O157:H7 bacteria detected in the river consistently and over several years, along with identification of other known pathogens, is of concern. Consequently, the potential for horizontal gene transfer based on detection of AMR genes and evolution of pathogens with enhanced pathogenic potential and spread of AMR cannot be ignored [85].
The metagenomics analyses carried out in this study yielded results that strongly suggest further metagenomic analysis should be conducted, using both longitudinal and seasonal study designs to provide statistically significant data to inform public health efforts.
The Crow Environmental Steering Committee has endorsed continued study, with a focus on both the Crow Fair swim hole site of the present study and upstream sites to determine the extent and potential sources of microbial contamination. The staff of the Crow Water Quality Project continue to educate the community on water quality and environmental health issues. Since the local tribal college is a two-year institution with limited facilities and resources, our use of the portable and relatively affordable MinION sequencing platform may serve as a proof of concept for introducing students at smaller tribal colleges to DNA sequencing technology as a means of monitoring water quality. Use of the MinION system may be applicable to the study of genomics in a teaching and research setting where the cost of other more expensive sequencing technologies is prohibitive.
5. Conclusions
Waterborne disease continues to threaten human health worldwide. Many regulatory agencies employ coliform testing of water as an indication of the extent of fecal contamination and disease risk. Even when the concentration of coliform bacteria is within an acceptable level, this method does not identify specific microbes that may be pathogenic at a very low dose-of-infectivity. In this study, we test the feasibility of using a highly portable DNA sequencing device, that may in the future be readily deployed for routine monitoring of water quality outside of research laboratory settings, for detection and metagenomic analysis of waterborne disease pathogens present in a river affected by fecal contamination from cattle ranching and leaking sewage systems. We demonstrate that even at an “acceptable” level of fecal coliform bacteria deemed to be safe for human recreational use of a river, seemingly rare and unexpected (for rural Montana) pathogens, such as E. coli O104:H4 and V. cholerae, as well as pathogens with a low dose-of-infectivity on the order of 1-10 cells, e.g., E. coli O157:H7, can be detected using metagenomic analysis.
As portable DNA sequencing devices continue to be refined and made more affordable, and as metagenomics software and analysis are fully integrated with these sequencing platforms, it can be envisioned that real time surveillance for water borne pathogens, virulence genes, and AMR gene markers will be incorporated into environmental monitoring to protect human health. The present study serves as a proof of concept of the utility of such an approach, by demonstrating the ability to detect not only pathogenic microorganisms, but also virulence and AMR genes. Use of traditional methods to screen for pathogens and phenotypic traits requires a targeted approach to test for specific agents and genes, and may require weeks or months to complete. Integrated DNA sequencing and metagenomic analysis, on the other hand, can be performed in real time, requiring only hours or days to complete an assessment for waterborne pathogens.
Acknowledgments
We thank Daniel R. Colman, Kerry Williamson, Tatsuya Akiyama, and Eric S. Boyd for critical reading of the manuscript. Principal investigator Timothy Ford and first author Steve Hamner, on behalf of the entire research team and all coauthors, wish to sincerely thank all members of the Crow Environmental Health Steering Committee for sharing their insights and understanding of the issue of pollution of the Little Bighorn River. It has been a privilege felt by all the coauthors to be invited to conduct this research as guests of the Crow community. The research could not have been conducted without the Steering Committee’s approval and blessing. The Steering Committee has furthermore proved invaluable in guiding the crafting and wording of this research report, to produce a document that will most benefit the Steering Committee and Crow community in moving forward to address pollution in the Little Bighorn River watershed. Steve Hamner acknowledges Kirti Tsenshab Rinpoche for his encouragement to conduct microbiology studies benefiting human and public health.
Author Contributions
Conceptualization, S.H. and T.E.F.; methodology, S.H., B.L.B., N.A.H., R.R.C., and T.E.F.; software, N.A.H. and R.R.C.; validation, S.H., B.L.B., N.A.H., M.J.F., J.D., M.J.E., R.R.C., and T.E.F.; formal analysis, S.H., B.L.B., N.A.H., R.R.C., and T.E.F.; investigation, S.H.; resources, S.H., N.A.H., M.J.F., R.R.C., and T.E.F.; data curation, S.H., B.L.B., and N.A.H.; writing—original draft preparation, S.H. and T.E.F.; writing—review and editing, S.H., B.L.B., N.A.H., M.J.F., J.D., M.J.E., R.R.C., and T.E.F.; visualization, S.H., B.L.B., N.A.H., M.J.F., J.D., M.J.E., R.R.C., and T.E.F.; supervision, S.H. and T.E.F.; project administration, S.H. and T.E.F..; funding acquisition, T.E.F.
Funding
This research was funded in part by Uniting for Health Innovation, an “independent, nonprofit organization that unites government, industry, and local communities in the Americas to advance innovation in public health.” Partial support for Steve Hamner’s efforts was provided by Jane A. Dubitzky.
Conflicts of Interest
Bonnie L. Brown is enrolled in the Oxford Nanopore MAP and has received free materials for past research (but not for the present research). Rita R. Colwell is the Founder and Chair of CosmosID. Nur A. Hasan is the Chief Science Officer at CosmosID. Sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.UDP Beyond Scarcity: Power, Poverty and the Global Water Crisis. [(accessed on 22 February 2019)]; Available online: http://www.undp.org/content/dam/undp/library/corporate/HDR/2006%20Global%20HDR/HDR-2006-Beyond%20scarcity-Power-poverty-and-the-global-water-crisis.pdf.
- 2.Vörösmarty C.J., McIntyre P.B., Gessneer M.O., Dudgeon D., Prusevich A., Green P., Glidden S., Bunn S.E., Sullivan C.A., Reidy Liermann C., et al. Global threats to human water security and river biodiversity. Nature. 2010;467:551–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 3.Ford T.E., Hamner S. A perspective on the global pandemic of waterborne disease. Microb. Ecol. 2015;76:2–8. doi: 10.1007/s00248-015-0629-0. [DOI] [PubMed] [Google Scholar]
- 4.Cummins C., Doyle J., Kindness L., Lefthand M.J., Bear Don’t Walk U.J., Bends A.L., Broadaway S.C., Camper A.K., Fitch R., Ford T.E., et al. Community-based participatory research in Indian country: Improving health through water quality research and awareness. Family & Community Health. 2010;33:166–174. doi: 10.1097/FCH.0b013e3181e4bcd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle J.T., Redsteer M.H., Eggers M.J. Exploring effects of climate change on Northern Plains American Indian health. Climatic Change. 2013;120:643–655. doi: 10.1007/s10584-013-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McOliver C.A., Camper A.K., Doyle J.T., Eggers M.J., Ford T.E., Lila M.A., Berner J., Campbell L., Donatuto J. Community-based research as a mechanism to reduce environmental health disparities in American Indian and Alaska Native communities. Int. J. Environ. Res. Public Health. 2015;12:4076–4100. doi: 10.3390/ijerph120404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montana Climate Assessment 2017 Montana Climate Assessment. [(accessed on 22 February 2019)]; Available online: http://montanaclimate.org/sites/default/files/thumbnails/image/2017-Montana-Climate-Assessment-lr.pdf.
- 8.Epstein P.R. Climate change and emerging infectious diseases. Microb. Infect. 2001;3:747–754. doi: 10.1016/S1286-4579(01)01429-0. [DOI] [PubMed] [Google Scholar]
- 9.Ostfeld R.S., Brunner J.L. Climate change and Ixodes tick-borne diseases of humans. Philos. Trans. R. Soc. Lond. Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza J.C., Suk J.E. Vector-borne disease and climate change: A European perspective. FEMS Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggers M.J. Ph.D. Thesis. Montana State University; Bozeman, MT, USA: May, 2014. Community Based Risk Assessment of Exposure to Waterborne Contaminants on the Crow Reservation, Montana. [Google Scholar]
- 12.United States Environmental Protection Agency Final Report: Community Based Risk Assessment of Exposure to Contaminants via Water Sources on the Crow Reservation in Montana. [(accessed on 25 February 2019)]; Available online: https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/9136/report/F.
- 13.Eggers M.J., Moore-Nall A.L., Doyle J.T., Lefthand M.J., Young S.L., Bends A.L., Crow Environmental Health Steering Committee. Camper A.K. Potential health risks from uranium in home well water: An investigation by the Apsaalooke (Crow) tribal research group. Geosciences. 2015;5:67–94. doi: 10.3390/geosciences5010067. [DOI] [Google Scholar]
- 14.Richards C.L., Broadaway S.C., Eggers M.J., Doyle J.T., Pyle B.H., Camper A.K., Ford T.E. Detection of pathogenic and non-pathogenic bacteria in drinking water and associated biofilms on the Crow Reservation, Montana, USA. Microbiol. Ecol. 2015 doi: 10.1007/s00248-015-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggers M.J., Doyle J.T., Lefthand M.J., Young S.L., Moore-Nall A.L., Kindness L., Other Medicine R., Ford T.E., Dietrich E., Parker A.E., et al. Community engaged cumulative risk assessment of exposure to inorganic well water contaminants, Crow Reservation, Montana. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle J.T., Kindness L., Realbird J., Eggers M.J., Camper A.K. Challenges and opportunities for tribal waters: Addressing disparities in safe public drinking water on the Crow Reservation in Montana, USA. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamner S., Broadaway S.C., Berg E., Stettner S., Pyle B.H., Big Man N., Old Elk J., Eggers M.J., Doyle J., Kindness L., et al. Detection and source tracking of Escherichia coli, harboring intimin and Shiga toxin genes, isolated from the Little Bighorn River, Montana. Intl. J. Environ. Health Res. 2013 doi: 10.1080/09603123.2013.835030. [DOI] [PubMed] [Google Scholar]
- 18.Brown B.L., LePrell R.V., Franklin R.B., Rivera M.C., Cabral F.M., Eaves H.L., Gardiakos V., Keegan K.P., King T.L. Metagenomic analysis of planktonic microbial consortia from a non-tidal urban-impacted segment of James River. Standards Genomic Sci. 2015;10 doi: 10.1186/s40793-015-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardy J.L., Loman N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 2017 doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain M., Tyson J.R., Loose M., Ip C.L.C., Eccles D.A., O’Grady J., Malla S., Leggett R.M., Wallerman O., Jansen H.J., et al. MinION Analysis and Reference Consortium: Phase 2 data release and analysis of R9.0 chemistry. F1000Research. 2017;6 doi: 10.12688/f1000research.11354.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menegon M., Cantaloni C., Rodriquez-Prieto A., Cantomo C., Abdelfattah A., Rossato M., Bernardi M., Xumerie L., Loader S., Delledonne M. On site DNA barcoding by nanopore sequencing. PLoS ONE. 2017;12:e0184741. doi: 10.1371/journal.pone.0184741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kafetzopoulou L.E., Pullan S.T., Lemey P., Suchard M.A., Ehichioya D.U., Pahlmann M., Thielebein A., Hinzmann J., Oestereich L., Wozniak D.M., et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science. 2019;363:74–77. doi: 10.1126/science.aau9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roetzer A., Diei R., Kohl T.A., Rückert C., Nübel U., Blom J., Wirth T., Jaenicke S., Schuback S., Rüsch-Gerdes S., et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: A longitudinal molecular epidemiological study. PLoS Med. 2013;10:e1001387. doi: 10.1371/journal.pmed.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghai R., Rodriguez-Valera F., McMahon K.D., Toyama D., Rinke R., Souza de Oliveira T.C., Garcia J.W., Pellon de Miranda F., Henrique- Silva F. Metagenomics of the water column in the pristine upper course of the Amazon River. PLoS ONE. 2011;6:e23785. doi: 10.1371/journal.pone.0023785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rossum T., Peabody M.A., Uyaguari-Diaz M.I., Cronin K.I., Chan M., Slobodan J.R., Nesbitt M.J., Suttle C.A., Hsiao W.W.L., Tang P.K.C., et al. Year-long metagenomics study of river microbiomes across land use and water quality. Front. Microbiol. 2015;6:1405. doi: 10.3389/fmicb.2015.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abia A.L.K., Alisoltani A., Keshri J., Ubomba-Jaswa E. Metagenomic analysis of the bacterial communities and their functional profiles in water and sediments of the Apies River, South Africa, as a function of land use. Science Total Environ. 2018;616:326–334. doi: 10.1016/j.scitotenv.2017.10.322. [DOI] [PubMed] [Google Scholar]
- 27.Jumat M.R., Hasan N.A., Subramaniam P., Heberling C., Colwell R.R., Hong P.-Y. Membrane bioreactor-based wastewater treatment plant in Saudi Arabia: Reduction of viral diversity, load, and infectious capacity. Water. 2017;9:534. doi: 10.3390/w9070534. [DOI] [Google Scholar]
- 28.Leddy M.B., Hasan N.A., Subramaniam P., Heberling C., Cotruvo J., Colwell R.R. Characterization of microbial signatures from advanced treated wastewater biofilms. J. American Water Works Assoc. 2017;109:E503–E512. doi: 10.5942/jawwa.2017.109.0116. [DOI] [Google Scholar]
- 29.Miller R.R., Montoya V., Gardy J.L., Patrick D.M., Tang P. Metagenomics for pathogen detection in public health. Genome Med. 2013;5 doi: 10.1186/gm485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HACH Coliforms, Total and E. coli. [(accessed on 25 February 2019)]; Method 10029. Membrane Filtration. Available online: www.hach.com/asset-get.download-en.jsa?id=7639984023.
- 31.Meyer F., Paarmann D., D’Souza M., Olson R., Glass E.M., Kubal M., Paczian T., Rodriquez A., Stevens R., Wilke A., et al. The metagenomics RAST server—A public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sović I., Šikić A., Fenlon S.N., Chen S., Nagarajan N. Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nature Commun. 2016;7 doi: 10.1038/ncomms11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty S., Deokule J.S., Garg P., Bhattacharya S.K., Nandy R.K., Balakrish Nair G., Yamasaki S., Takeda Y., Ramamurthy T. Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholera O1 and O139 in Ahmedabad, India. J. Clin. Microbiol. 2001;39:3241–3246. doi: 10.1128/JCM.39.9.3241-3246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC Parasites—Acanthamoeba—Granulomatous Amebic Encephalitis (GAE); Keratitis. [(accessed on 25 February 2019)]; Available online: https://www.cdc.gov/parasites/acanthamoeba/index.html.
- 35.CDC Leishmaniasis. [(accessed on 25 February 2019)]; Available online: https://www.cdc.gov/dpdx/leishmaniasis/index.html.
- 36.CDC Candidiasis. [(accessed on 25 February 2019)]; Available online: https://www.cdc.gov/fungal/diseases/candidiasis/
- 37.Patil A.B., Chandramohan K., Shivaprakash M.R., Nadgir S.D., Lakshminarayana S.A. Rhizomucor variabilis: A rare causative agent of primary cutaneous zygomycosis. Indian J. Med. Microbiol. 2013;31:302–305. doi: 10.4103/0255-0857.115662. [DOI] [PubMed] [Google Scholar]
- 38.Shetty A., Barnes R.A., Healy B., Groves P. A case of sepsis caused by Acidovorax. J. Infection. 2005;51:e-171–e-172. doi: 10.1016/j.jinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Orsborne C., Hardy A., Isalska B., Williams S.G., Muldoon E.G. Acidovorax oryzae catheter-associated bloodstream infection. J. Clin. Microbiol. 2014;52:4421–4424. doi: 10.1128/JCM.00657-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reith M.E., Singh R.K., Curtis B., Boyd J.M., Bouevitch A., Kimball J., Munholland J., Murphy C., Sarty D., Williams J., et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: Insights into the evolution of a fish pathogen. BMC Genomics. 2008;9 doi: 10.1186/1471-2164-9-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tewari R., Dudeja M., Nandy S., Das A.K. Isolation of Aeromonas salmonicida from human blood sample: A case report. J. Clin. Diagn. Res. 2014;8:139–140. doi: 10.7860/JCDR/2014/6883.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varshney A., Das M., Chaudhary P., Kumari R., Yadav K. Aeromonas Salmonicida as a causative agent for postoperative endophthalmitis. Middle East Afr. J. Opthalmol. 2017;24:213–215. doi: 10.4103/meajo.MEAJO_238_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United States Environmental Protection Agency Recreational Water Quality Criteria. for Bacteria. [(accessed on 25 February 2019)]; Available online: https://www.epa.gov/sites/production/files/2015-10/documents/rwqc2012.pdf.
- 44.Endo S., Yano H., Kanamori H., Inomata S., Aoyagi T., Hatta M., Gu Y., Tokuda K., Kitagawa M., Kaku M. High frequency of Acinetobacter soli among Acinetobacter isolates causing bacteremia at a tertiary hospital in Japan. J. Clin. Microbiol. 2014;52:911–915. doi: 10.1128/JCM.03009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linde H.-J., Hahn J., Holler E., Reischl U., Lehn N. Septicemia due to Acinetobacter junii. J. Clin. Microbiol. 2002;40:2696–2697. doi: 10.1128/JCM.40.7.2696-2697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vila J., Ruiz J., Gallardo F., Vargas M., Soler L., Figueras M.J., Gascon J. Aeromonas spp. and traveler’s diarrhea: Clinical features and antimicrobial resistance. Emerg. Infect. Dis. 2003;9:552–555. doi: 10.3201/eid0905.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirai J., Uechi K., Hagihara M., Sakanashi D., Kinjo T., Haranaga S., Fujita J. Bacteremia due to Citrobacter braakii: A case report and literature review. J. Infect. Chemother. 2016;22:819–821. doi: 10.1016/j.jiac.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Hunter C., Bean J. Cronobacter: an emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J. Perinatology. 2013;33:581–585. doi: 10.1038/jp.2013.26. [DOI] [PubMed] [Google Scholar]
- 49.Koth K., Boniface J., Chance E.A., Hanes M.C. Enterobacter asburiae and Aeromonas hydrophila: Soft tissue infection requiring debridement. Orthopedics. 2012;3:e996–e999. doi: 10.3928/01477447-20120525-52. [DOI] [PubMed] [Google Scholar]
- 50.Muniesa M., Hammerl J.A., Hertwig S., Appel B., Brüssow H. Shiga toxin-producing Escherichia coli O104:H4: A new challenge for microbiology. Appl. Environ. Microbiol. 2012;78:4065–4073. doi: 10.1128/AEM.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rangel J.M., Sparling P.H., Crowe C., Griffin P.M., Swerdlow D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.UniProt Proteomes—Escherichia coli O17:K52:H18 (Strain UMN026/ExPEC) [(accessed on 25 February 2019)]; Available online: http://www.uniprot.org/proteomes/UP000007097.
- 53.Herzog K.A.T., Schneditz G., Leitner E., Feireri G., Hoffmann K.M., Zollner-Schwetz I., Krause R., Gorkiewicz G., Zechner E.L., Högenauer C. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J. Clin. Microbiol. 2014;52:1607–1616. doi: 10.1128/JCM.03373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C.R., Lee J.H., Park K.S., Jeon J.H., Kim Y.B., Cha C.J., Jeong B.C., Lee S.H. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: Epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Mauri A., Chiarinotti D., Andreoni S., Molinari G.L., Conti N., De Leo M. Leclercia adecarboxylata and catheter-related bacteraemia: Review of the literature and outcome with regard to catheters and patients. J. Med. Microbiol. 2013;62:1620–1623. doi: 10.1099/jmm.0.059535-0. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M., Suzuki S., Matsui M., Hiraki Y., Kawano F., Shibayama K. Genome sequence of a strain of human pathogenic bacterium Pseudomonas alcaligenes that caused bloodstream infection. Genome Announc. 2013;1 doi: 10.1128/genomeA.00919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CDC Shigella—Shigellosis. [(accessed on 25 February 2019)]; Available online: https://www.cdc.gov/shigella.
- 58.CDC Cholera. [(accessed on 25 February 2019)]; Available online: https://wwwnc.cdc.gov/travel/diseases/cholera.
- 59.Sun Q., Knirel Y.A., Wang J., Luo X., Senchenkova S.N., Lan R., Shashkov A.S., Xu J. Serotype-converting bacteriophage SFII encodes an acyltransferase protein that mediates 6-O-acetylation of GlcNAc in Shigella flexneri O-antigens, vonferring on the host a novel O-antigen epitope. J. Bacteriol. 2014;196:3656–3666. doi: 10.1128/JB.02009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jakhetia R., Talukder K.A., Verma N.K. Isolation, characterization and comparative genomics of bacteriophage SfIV, a novel serotype converting phage from Shigella flexneri. BMC Genomics. 2013;14 doi: 10.1186/1471-2164-14-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melton-Celsa A.R. Shiga toxin (Stx) classification, structure and function. Microbiol. Spectr. 2014;2 doi: 10.1128/microbiolspec.EHEC-0024-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saarela S., Westerlund-Wikström B., Rhen M., Korhonen T.K. The GafD protein of the G (F17) fimbrial complex confers adhesiveness of Escherichia coli to laminin. Infect. Immun. 1996;64:2857–2860. doi: 10.1128/iai.64.7.2857-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith D.K., Kassam T., Singh B., Elliott J.F. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J. Bacteriol. 1992;174:5820–5826. doi: 10.1128/jb.174.18.5820-5826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson T.J., Wannemuehler Y.M., Nolan L.K. Evolution of the iss gene in Escherichia coli. Appl. Environ. Microbiol. 2008;74:2360–2369. doi: 10.1128/AEM.02634-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen L.M., McMurry L.M., Levy S.B., Hirsh D.C. A new tetracycline determinant, Tet H, from Pasteurella multocida specifying active efflux of tetracycline. Antimicro. Agents Chemother. 1993;37:2699–2705. doi: 10.1128/AAC.37.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson B.A., Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013;26:631–655. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borgeaud S., Metzger L.C., Scrignari T., Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 68.Sebbane F., Jarrett C., Gardner D., Long D., Hinnebusch J. Role of the Yersinia pestis yersiniabactin iron acquisition system in the incidence of flea-borne plague. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowers R.M., Kyrpides N.C., Stepanauskas R., Harmon-Smith M., Doud D., Reddy T.B.K., Schulz F., Jarett J., Rivers A.R., Eloe-Fadrosh E.A., et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nature Biotech. 2017;33:725–731. doi: 10.1038/nbt.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones J.G. The effect of environmental factors on estimated viable and total populations of planktonic bacteria in lakes and environmental enclosures. Freshwater Biol. 1977;7:67–91. doi: 10.1111/j.1365-2427.1977.tb01659.x. [DOI] [Google Scholar]
- 71.Xu H., Roberts N., Singleton F.L., Attwell R.W., Grimes D.J., Colwell R.R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbiol. Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- 72.Li L., Mendis N., Triqui H., Oliver J.D., Faucher S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding T., Suo Y., Xiang Q., Zhao X., Chen S., Ye X., Liu D. Significance of viable but nonculturable Escherichia coli: Induction, detection, and control. J. Microbiol. Biotechnol. 2017;27:417–426. doi: 10.4014/jmb.1609.09063. [DOI] [PubMed] [Google Scholar]
- 74.Unites States Environmental Protection Agency Analytical Methods Approved for Compliance Monitoring under the Revised Total Coliform Rule. [(accessed on 25 February 2019)]; Available online: https://www.epa.gov/sites/production/files/2017-02/documents/rtcr_approved_methods.pdf.
- 75.Paton J.C., Paton A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998;11:450–479. doi: 10.1128/CMR.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ali M., Nelson A.R., Lopez A.L., Sack D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015 doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alam M.T., Weppelmann T.A., Weber C.D., Johnson J.A., Rashid M.H., Birch C.S., Brumbach B.A., Madsen Beau de Rochars V.E., Glenn J., Ali A. Monitoring water sources for environmental reservoirs of toxigenic Vibrio cholerae O1, Haiti. Emerg. Infect. Dis. 2014;20:356–363. doi: 10.3201/eid2003.131293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasan N.A., Choi S.Y., Eppinger M., Clark P.W., Chen A., Alam M., Haley B.J., Taviani E., Hine E., Su Q., et al. Genomic diversity of 2010 Haitian cholera outbreak strains. PNAS. 2012;109 doi: 10.1073/pnas.1207359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyata S.T., Kitaoka M., Brooks T.M., McAuley S.B., Pukatzki S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. PLoS Pathog. 2011;9 doi: 10.1128/IAI.01266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray A., Schwartz N., de Souza Santos M., Zhang J., Orth K., Salomon D. Type VI secretion system MIX-effectors carry both antibacterial and anti-eukaryotic activities. EMBO Rep. 2017;18:1978–1990. doi: 10.15252/embr.201744226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO Antimicrobial Resistance: Global Report on Surveillance. [(accessed on 25 February 2019)];2014 Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/
- 82.Thanner S., Drissner D., Walsh F. Antimicrobial resistance in agriculture. mBio. 2016;7 doi: 10.1128/mBio.02227-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferens W.A., Hovde C.J. Escherichia coli O157:H7: animal reservoir and source of human infection. Foodborne Pathog. Dis. 2011;8:465–487. doi: 10.1089/fpd.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Economou V., Gousia P. Agricultural and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015;8:49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gyles C., Boerlin P. Horizontally transferred genetic elements and their role in pathogenesis of bacterial disease. Vet. Pathol. 2014;51:328–340. doi: 10.1177/0300985813511131. [DOI] [PubMed] [Google Scholar]