Abstract
Several congenital and acquired conditions may result in severe narrowing of the urethra in men, which represent an ongoing surgical challenge and a significant burden on both health and quality of life. In the field of urethral reconstruction, tissue engineering has emerged as a promising alternative to overcome some of the limitations associated with autologous tissue grafts. In this direction, preclinical as well as clinical studies, have shown that degradable scaffolds are able to restore the normal urethral architecture, supporting neo-vascularization and stratification of the tissue. While a wide variety of degradable biomaterials are under scrutiny, such as decellularized matrices, natural, and synthetic polymers, the search for scaffold materials that could fulfill the clinical performance requirements continues. In this article, we discuss the design requirements of the scaffold that appear to be crucial to better resemble the structural, physical, and biological properties of the native urethra and are expected to support an adequate recovery of the urethral function. In this context, we review the biological performance of the degradable polymers currently applied for urethral reconstruction and outline the perspectives on novel functional polymers, which could find application in the design of customized urethral constructs.
Keywords: urethral strictures, urethral tissue engineering, biodegradable polymers, acellular matrix, smart polymers
1. Introduction
The male urethra can be affected by several primary, as well as secondary conditions, including hypospadias, fistulas, trauma, and malignancy [1]. In children, hypospadias is a common congenital malformation of the external genitalia, where shortage of the urethra is a major concern [2]. This disorder occurs in about 1 out of every 200 live male births and its surgical management remains challenging, especially in the severe forms where the patients suffer from post-operative complications [3,4]. In adult males, urethral injuries can take place at any part of the urethra distal to the bladder neck, as the result of different pathologies, including inflammatory, traumatic, or iatrogenic, with an incidence of 5000 new cases annually in the USA alone [5,6]. While male urethral conditions represent a significant medical and surgical burden, female urethral disorders are less demanding due to different etiology and anatomical constraints. Thus, management of female urethral disorders will remain outside the scope of the current review.
Overall, disorders of the male urethra may lead to a breach of the continuity of the urethral mucosa, which results in extravasation of urine, with the possibility of subsequent inflammation and, ultimately, stricture formation. The surgical management of urethral strictures differs according to the severity and length of the affected segment. While the basic approach for short segmental defects comprises resection and end-to-end anastomosis, long defects require open urethroplasty using autologous tissue grafts. Buccal mucosa is currently the most widely used graft material for urethroplasty [7,8,9]. However, limited amount of autologous tissue and donor site morbidity remain as clinical obstacles. Patients undergoing buccal tissue harvest are at risk of suffering scarring and contracture at the donor site, persistent pain, numbness, or parotid duct injury. The overall rate of oral complications has been shown to be between 3% to 4% [10]. Furthermore, buccal grafts are associated with higher costs of urethroplasty and a longer hospital stay [11].
Urethral tissue engineering has recently emerged as a viable alternative to overcome the issues associated with autologous grafts [12]. In addition to eliminating the need of harvesting autologous tissue, tissue engineered solutions are expected to become “off-the-shelf” products in the future [13]. Tissue engineering may involve scaffolds alone, which exploit the body’s natural regeneration ability, or cell-seeded scaffolds, which are used as a graft substitute. In any case, urethral tissue engineering approaches rely on biodegradable scaffold materials, which aim to recapitulate the structural, mechanical, and biological properties of the native urethra, to ultimately restore normal urethral functionality. For instance, it is desirable to provide proper mechanical compliance, to allow elastic deformation during erection of the penis, and barrier function to avoid urine leakage to the underlying tissues [14,15].
The aim of this article is to present the rationale for different designs for urethral replacement, as well as the limitations of each design choice. Given the inability of grafts and non-degradable polymers to meet the clinical performance requirements, this review will focus on the ability of degradable polymeric scaffolds to mimic the native urethral structure, in an effort to restore complete urethral function. Toward this purpose, we initially describe the properties of the male urethra that dictate design requirements for a completely regenerated structure. Then, we review the preclinical and clinical results obtained with current biodegradable biomaterials, discussing their advantages and limitations. Finally, we discuss the perspectives on novel functional polymers, which may find application in the field of urethral tissue engineering.
2. Structural and Functional Properties of the Male Urethra
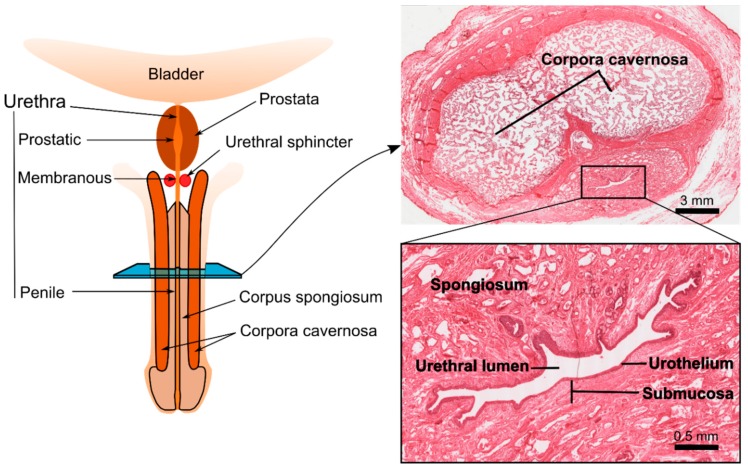
The male urethra is a distensible tubular structure connecting the urinary bladder to the meatus, which mainly serves as a conduit to eliminate the urine. As shown in the diagram of Figure 1, the male urethra can be divided into three segments. The most proximal segment, the prostatic urethra, passes through the prostate and constitutes the widest portion. The middle segment, the membranous urethra, is the shortest and is surrounded by the external urethral sphincter. When the sphincter is closed, the intraluminal pressure on the membranous urethra can reach up to 120 cm H2O. The most distal and longest portion is the penile urethra, which is surrounded by the corpus spongiosum, a richly vascularized erectile tissue that provides support to the urethra. Histologically, the urethra is composed from three different layers: an inner mucosal epithelial lining, a submucosa comprising of fibroblasts, and a muscular layer (Figure 1). The urothelial lining of the urethra appears to be essentially different from that of the bladder [16]. This can be observed by change of the epithelium from transitional (in the bladder), to pseudostratified (in the penile urethra), to stratified squamous at interface with the skin. The muscular layer is mainly composed of smooth muscle cells. The striated component extends from the base of the bladder to the membranous urethra, where it forms the external urethral sphincter for active continence. Each cell layer contains a complex extracellular matrix (ECM) structure, mainly composed of collagen I and III, glycosaminoglycans (GAGs), and elastin [17,18]. Mechanically, while collagen gives strength and structural support, elastin provides reversible extensibility [19]. While the detailed histological composition of the urethra has been thoroughly described in female animals, which are relevant models for continence research the histology of the male urethra, which can potentially be translated into urethral tissue engineering studies, has not been sufficiently explored yet [20,21,22,23,24].
Figure 1.
Diagram showing the general anatomical features of the human penis with a cross section at the level of the penile urethra showing the different anatomical structures. The histological examination at the level of the cross section shows the two corpora cavernosa and the urethra with its three main layers: epithelia, submucosa and spongiosum (histological images reprinted with permission from Ref. [25]). © 2019 Regents of the University of Michigan.
Apart from its signaling role, to maintain the cellular homeostasis, the ECM plays an essential role in defining the mechanical compliance of the urethra, which should be able to dilate its lumen during micturition and stretch considerably during erections [26,27]. During urination, the urinary bladder’s pressure increases up to 50–60 cm H2O and expels the urine with a flow rate about 20–30 mL/s. One of the major points not considered frequently in producing urethral constructs is the maintenance of enough compliance of the urethra during micturition, in order to decrease patient discomfort and protect the upper urinary tracts. Different studies investigated different variables affecting urethral compliance in a clinical background [28,29,30]. Normal urethral compliance is dependent on the optimal passive viscoelastic properties of the urethral wall in order to keep the urination process under low pressure so as not to harm the urinary bladder, kidneys, and cause infections. Different therapeutic and surgical conditions of the urethra can affect these parameters and this was objectively demonstrated through in vivo experiments using different animal models [31]. We hypothetically assume that the same effects can occur when implanting tissue-engineered urethral constructs, and can be significantly ameliorated with appropriate degradability of the scaffold and subsequent healthy tissue regeneration. It is worth noting that differences in stiffness/compliance between the implanted segment (and subsequent regrown engineered neourethra and adjacent native urethra) determines the range of mechanical mismatch that still would not meet the clinical performance requirements. This reflects the inability of the compensatory capacity of the normal urethra to the noncompliant/stiff new urethral segment. This can be elicited from the clinical/functional perspective, as it is known that even a very short fibrotic segment can have as significant clinical burden as long segments. This is clear from the pathological aspects of posterior urethral valves in male children, which are the most common reason of renal transplantation before puberty. Similar outcomes are commonly observed with urethral strictures in adults, in which very short strictures can affect urinary function and represent a risk for renal complications [32].
Urethral biomechanical parameters (tension–strain relation) can explain the relation between the structure and function of the urethra. Studies have shown that the urethra possesses a nonlinear cross-sectional area pressure, meaning that the tissue is fairly deformable at low pressure (facilitating voiding), but less deformable at higher stress levels (protective against over distention) [33]. It has also been shown that abnormal urethral narrowing or obstruction, as well as post-operative complications and recurrence of pathology, can be reliably screened by uroflowmeter measurements [33,34]. Uroflowmetry is considered a sensitive noninvasive functional modality for the assessment of the lower urinary tract dynamics and could be used for better assessment of the functional outcomes. However, this structure–function relationship has not been sufficiently investigated in previous preclinical urethral tissue engineering experiments.
Adding to the complexity of the picture, in hypospadias and urethral surgery, additional biomechanical and clinical problems may arise [34,35,36]. Another aspect which is not frequently taken into account, particularly in pediatric patients, is that the urethra doubles its length during puberty. This should be a main consideration during scaffold synthesis in order to have resulting healthy tissue that have this extent of growth [37]. Urethral graft contracture often occurs following prostatectomy, hypospadias repair, cystoscopy, and urethral cauterization. Several tissue engineering approaches were examined to manage these strictures that included using tissue-engineered buccal mucosa [38]. Another approach has been exploiting the anti-inflammatory properties of stem cells, such as human bone marrow-derived mesenchymal stem cells (MSC) combined with CD34+ hematopoietic stem/progenitor cells [39].
Another important point that has not received too much attention in the design of scaffolds for urological tissue engineering is the avoidance of urine leak, even when the urine is known to be highly cytotoxic. In the normal urethra, urothelium barrier function is maintained by three structures: uroplakin proteins in the apical cell membrane, tight junctions situated between the superficial umbrella cells, and urothelial GAGs and proteoglycans, covering the umbrella cells [40]. Formation and regeneration of the urothelium are critical to prevent urethral stricture formation. In addition, an intact urothelium prevents bladder detrusor muscle over-activity [41], inflammation, and fibrosis. When the protective uroplakin barrier is dysfunctional, as it is during the early stages of urothelium regeneration, cytotoxic agents bind spontaneously to the anionic milieu of inner layers of the bladder mucosa. Thus, deep penetrating urine is believed to exert a significant impact on the viability of cells within the scaffolds [42]. Since urine has a harmful effect on the cellular components of a tissue engineered urethra, it is important that the scaffold has adequate impermeability since it might partially act as an isolation barrier. It is worth noting that leak here is not meant for fluid but for the cytotoxic components [43]. In urethroplasty, the avoidance of urine leak is supported by catheterization of the patient within the first week after graft implantation, until healthy regenerated urothelial tissue is able to provide the adequate barrier function.
3. Polymeric Biomaterials in Urethral Tissue Engineering
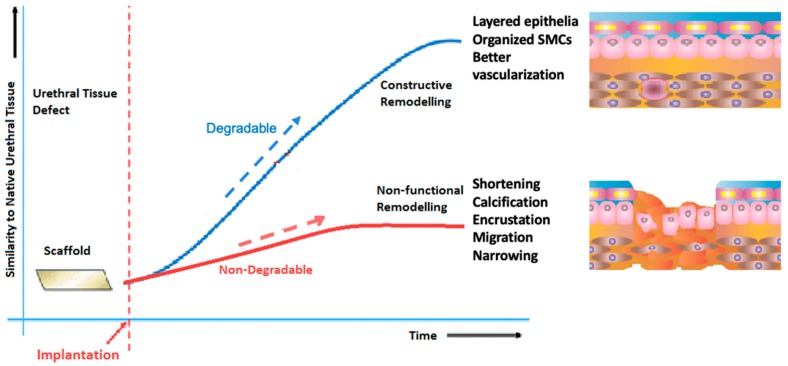
Initially, non-degradable materials were assessed for urinary tract reconstruction, but they resulted into several complications such as calcification, fistulae [44,45], chronic hematuria, stone formation, and development of significant contractures up to 50% of their initial lengths [46]. While non-degradable urinary scaffolds have resulted in failure of the growth of seeded cells, the addition of collagen has resulted in significant cell growth and differentiation [47]. On the other hand, degradable biopolymers are intended to permit tissue ingrowth, while the scaffold is resorbed over time, in a controlled manner (Figure 2). Physiological metabolic processes typically remove the degradation byproducts [48]. As a result, it is important to investigate the biodegradation characteristics of the scaffolds in order to achieve a successful tissue engineered replacement [49]. The incorporation of cells within the scaffold appears to improve the biocompatibility, as the cells contribute to the remodeling process through synthesis of ECM components, which are essential for the long-term survival of the implanted construct.
Figure 2.
Schematic representation of the advantages of scaffold degradability with regards to restoration of “healthier” tissue engineered urethral replacement. Non-degradable biocompatible scaffolds have lead to several undesirable outcomes like shortenig of the implant, calcification, and narrowing of the urethra, collectively kown as “non-functional remodeling”. On the other hand, resulting regrown tissues following implantation of optimized degradable constructs has lead to “constructive remodeling” with the resulting tissues closer to the native normal urethra.
The appropriate synchronization between the biodegradation of the scaffold and cellular component growth is crucial for the success of a tissue-engineered implant. The scaffold should tolerate the mechanical forces sustained during handling, suturing, and normal patient activities [50]. Biodegradable scaffolds in general have a variable biodegradation between days to several months. The timeline to allow proper function and regeneration is one of the most important variables in the design of degradable tissue engineered constructs. This actually has been explained recently in a vascular tissue engineering model of constructs, where the in vivo degradation process goes through consecutive controlled phases [51]. There is an initial “stenting phase” for merely the transfer of intraluminal fluids with minimum acute recoil, sufficient radial strength, and conformability. This is to be followed by a “restoration stage” as a result of the return of in-scaffold contractility in response to physiological (or pharmacological) stimuli, until lastly resorption occurs. Because the mechanical properties of absorbable scaffolds are designed to change over time, there are distinctive concerns for bench testing, including pretest acquisition, handling before and through testing, and time-dependent evaluations. As mentioned above, the first phase of the absorbable urethral scaffolds is to maintain lumen patency. Therefore, the essential attributes are growth capability, recoil, radial strength, and fatigue behavior. Throughout the restoration phase, the scaffold transforms from an active support of the urethra to a passive implant with a discontinuous structure. This transition is monitored by evaluating the modification in radial strength over time furthermore due to the fatigue behavior.
Degradable polymers for urethral tissue engineering have been typically obtained from natural sources or by synthesis. Natural biomaterials include acellular matrices obtained from cadaveric or animal organs via enzymatic, physical, or chemical decellularization methods, or natural polymers, such as silk fibroin (SF). In general, natural biomaterials possess integrin-binding peptide sequences and surface topography that can facilitate cellular growth and differentiation, and accelerate the process of angiogenesis [52]. Synthetic polymers, on the other hand, constitute the biggest subset biodegradable materials in use today. The most commonly used polymers include linear polyesters, poly-lactic acid (PLA), poly-glycolic acid (PGA), poly lactic-co-glycolide (PLGA), copolymers, and polycaprolactone (PCL) [53]. Some of the advantages of synthetic polymers are that they display reproducible mechanical characteristics, degrade fast, and are frequently less expensive than biologic scaffolds. However, these synthetic polymers have limited biocompatibility because they lack specific molecular elements for interaction with cells and proteins, usually requiring surface treatments to promote cell attachment [54]. Comprehensive reviews of the different biomaterials used in urethral tissue engineering are available elsewhere [12,13,55,56]. While these approaches, in general, have a goal to mimic the properties of the urethra to recover complete function, they have not been able to achieve this goal. It is, however, worth noting that it might be possible to restore the functionality of the urethra to an acceptable level without necessarily duplicating its native structure. In the following section we will focus on the preclinical results obtained using biodegradable scaffolds obtained from natural sources.
4. In Vivo Performance of Biodegradable Scaffolds for Urethral Reconstruction
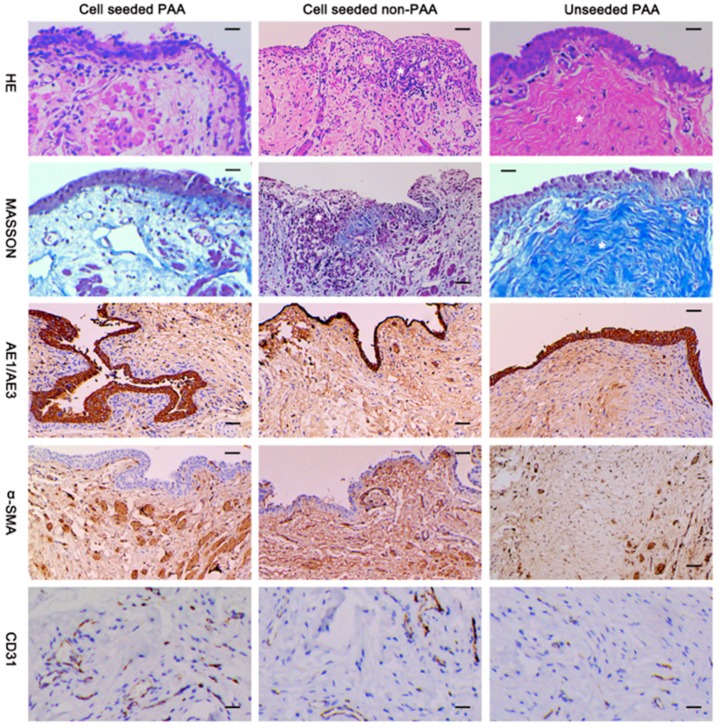
Acellular matrices, such as small intestinal submucosa (SIS) and bladder acellular matrix (BAM), are by far the most frequent scaffold type assessed in vivo for urethral tissue repair [57]. SIS is obtained from porcine small intestines, where the mucosal, serosal, and muscular layers are mechanically removed from the inner and outer surfaces of the intestinal wall to leave a 0.1 mm collagen-rich membrane, mostly formed of the submucosa [58,59]. In vivo animal experiments revealed that, when used for urethral replacement, SIS permitted rapid cellular growth and significant angiogenesis, often comparable to skin and mucosal grafts [60,61,62,63]. Moreover, SIS biodegrades totally in about four to eight weeks, and its degradation products are eliminated in the urine [64]. SIS has an extra benefit over other biomaterials regarding its high tendency to stretch under force and low tendency to break [65]. SIS showed favorable results when used for corporal body grafting to correct severe grades of chordee [66,67]. It was also been shown that seeding SIS with appropriate cells brings a better outcome in a rabbit model of urethroplasty (Figure 3) [68]. It is believed that extended periods of urinary diversion is important when using SIS for urethral replacement to lower the risk of urine extravasation and reduce early irritation [69]. Another option is to line it with another material having higher hydrophobicity in a multilayer manner in order to lower urine leakage. There are a number of natural material systems that have come close to restoring structure to near the native tissue. These systems, however, have still not met all the needed design constraints. The next section will describe some new materials that will help to better restore the original structure and function. Despite their evident advantages, SIS had less favorable results when used in clinical experiments, with the infection being the biggest limitation [70,71]. Furthermore, the regenerative potential of SIS depends on the age of the donor and the area of the intestine from which the SIS matrices are derived. These limitations have prevented SIS from being considered as an ideal scaffold to aid in effective urethral tissue engineering [72].
Figure 3.
Histological and immunohistochemical assessment of urethral reconstruction in animals using cell-loaded, decellularized SIS scaffolds (Cell seeded PAA) showing regrowth of layers of transitional epithelium, more smooth muscle, and vessels in contrast to the cell loaded but non-decellularized (Cell seeded non-PAA) and non-loaded, decellularized scaffolds (Unseeded PAA). Scale bar = 20 μm. (reproduced with permission from [68]). © 2016 Zhang et al.
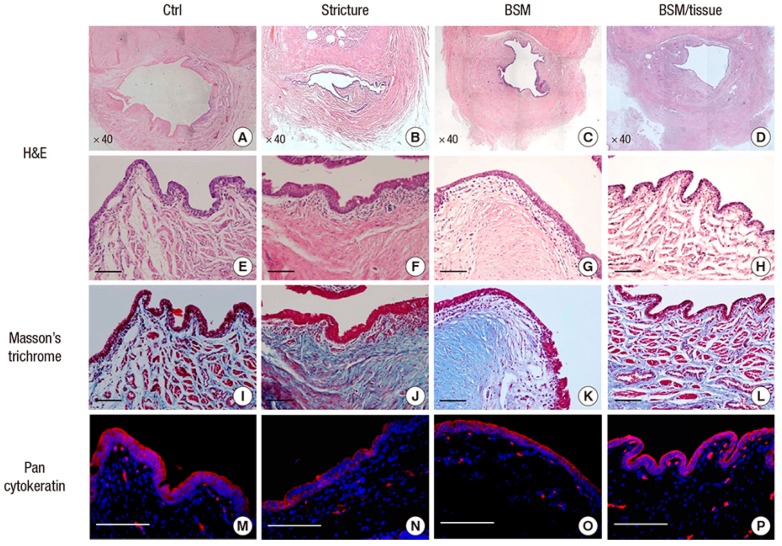
BAM is another example of a decellularized matrix, which has shown to successfully support regeneration of the urethra in vivo [44,73,74,75,76]. Using BAM for urethroplasty in a rabbit model, implantation of cell-loaded scaffolds demonstrated a normal urethral architecture by four weeks and the neourethra could be hardly differentiated from native urethra both grossly and histologically after six months [77]. Chun et al. also showed in his experiments that ventral onlay urethroplasty using an acellular bladder submucosa matrix (BSM) scaffold combined with an autologous urethral tissue graft represents a viable option for urethral replacement (Figure 4) [73]. However, residual immunogenic components have been detected within BAM despite the stringent decellularization process [78], and further improvement in processing may be necessary for BAM to achieve successful tissue engineering. Furthermore, decellularized matrices present other limitations such as batch-to-batch variability, and may cause inflammatory reactions due to residual DNA, RNA, and xeno-antigens, which could lead to graft rejection and functional failure.
Figure 4.
Histological and immunohistochemical analysis of urethra specimens in three groups: stricture model (one month following excision of a longitudinal strip of the urethra), ventral onlay urethroplasty repair using an acellular Bladder Submucosa scaffold (BSM), and repair using both BSM and autologous urethral tissue. It was shown that using an acellular BSM scaffold combined with an autologous urethral tissue graft resulted in a complete epithelialization, compact muscular layers, and progressive infiltration by vessels in the regenerated urethra. In contrast, the BSM grafts alone revealed keratinized epithelium, abundant collagenous fibrous connective tissue, and were devoid of bundles of circular smooth muscle. Low (A–D) and high-magnification (E–H) H&E images, Masson’s Trichrome images (I–L), and Pan-cytokeratin immunofluorescent images (M–P) for each of the three groups and the control (reproduced with permission from [73]).© 2015 The Korean Academy of Medical Sciences
SF is a natural polymer derived from Bombyx mori cocoons that holds promise for urethral tissue engineering applications [79,80]. Traditionally, SF has been utilized as sutures because of their excellent tensile and elasticity features, in contrast to several natural and synthetic biomaterials [81,82]. SF is enzymatically degradable polymers and the degradation by-products are peptides and amino acids [83]. In comparison to SIS, SF has been shown to have less immunogenic and inflammatory responses suggesting higher levels of biocompatibility in contrast to conventional urologic biomaterials [84,85]. It was also shown that bi-layer SF scaffold for onlay urethroplasty in a rabbit model is capable of promoting similar degrees of tissue regeneration as compared to traditional SIS matrices, but with reduced immunogenicity (Figure 5) [86]. Xie et al. showed that cell-seeded SF grafts used for urethroplasty were functioning with no stricture all through the study duration (six months) [79].
Figure 5.
Histological analysis of urethral tissue regrowth 3 months following implantation of silk fibroin based scaffold (first and second columns). Scale bars = 3 mm. Inset: residual silk fibroin matrix fragment (S). Scale bars = 100 mm. Dashed brackets represent areas of scaffold implantation or control urethrotomy (third column). Global tissue regeneration bracketed in second column. Scale bars = 600 mm. (*) = aggregate of mononuclear cells indicative of chronic inflammation (fourth and fifth columns). Magnified de novo smooth muscle (SM) and epithelial (EP) tissue formation displayed in third column. (H&E) hematoxylin and eosin, (MTS) Masson trichrome stain. Scale bars = 200 mm. It shows that both silk fibroin and SIS scaffolds promoted similar extents of smooth muscle and epithelial tissue regeneration throughout the original defect sites with prominent contractile protein. (reproduced with permission from [86]). © 2014 Chung et al.
5. Future Directions: Hybrid and Smart Polymers
As it was previously shown, non-degradable materials did not meet the clinical performance requirements. Despite the moderate success of degradable scaffolds in preclinical studies, the transition from bench-to-bedside remains a challenge. There are, however, some novel approaches and materials being investigated that might be able to meet the clinical performance requirements, alone or in combination with some of the other systems presented here. Natural and synthetic materials can be combined to produce hybrid biomaterials with desired properties for tissue engineering applications. Such properties include mechanical strength, porosity and cell affinity to attract cells, biocompatibility, and biodegradability for enabling replacement of ECM produced by resident cells. Current efforts in biodegradable polymer synthesis are centered on determination of appropriate biomaterials and synthesizing these materials with tailored properties for specific applications.
Another important advancement within the present decade is the emergence of a fourth generation of “smart” biomimetic materials. These materials respond reversibly to temperature, ionic strength, pH, or light [87,88]. The responses of these polymers may include gelation, reversible adsorption on a surface, collapse of a hydrogel, and alteration between hydrophilic and hydrophobic states. Moreover, these materials can be loaded with signaling molecules like ECM components and growth factors. The delivery could be triggered using external stimuli (e.g. pH, temperature, and light) or could be done simultaneously as a result of programmable biodegradation of scaffolds [89]. Smart biomaterials have been investigated in drug delivery and medical devices application and more recently in tissue engineering applications [90]. In a broader aspect, smart biomaterials can be designed by incorporating peptide and/or protein into the polymer network, enabling the creation of a 3D scaffold for tissue engineering application [91].
Thermo-responsive polymers are the largest class of smart polymers. These are characterized with a reversibly alterable phase (or volume) transition that occurs in response to a change in temperature. The solubility of thermo-responsive polymers in aqueous solutions depend on the temperature. Above lower critical solution temperature (LCST) polymer chains are precipitated making it hydrophobic, and below LCST, polymer chains are completely hydrated, making it hydrophilic [92]. The most investigated temperature-responsive polymer with LCST in water is the poly(N-isopropylacrylamide) (PNIPAM). The LCST of PNIPAM was found to be around 32 °C, close to the human body temperature, making the polymer particularly valuable for biological applications [93]. Therefore, by changing the temperature of PNIPAM solution in water, its solubility can be changed from hydrophobic to hydrophilic and vice versa. These characteristics found application in cell sheet engineering for which cell attachment and detachment to or from material surfaces were needed. By avoiding injurious enzymes for cell detachment, cell sheets with ECM proteins could be produced [92,94]. This method might be particularly useful for producing a three-layered urethra composed of different cell monolayers (i.e. epithelial, fibroblast, and smooth muscle) [95]. In this direction, it has been shown that viable urothelial cell sheets could be advantageously obtained by using a temperature-responsive cell culture method [95].
Shape-memory polymers (SMPs) are another class of smart biomaterials. SMPs have been experimented in various vascular and bone tissue engineering/regenerative medicine studies [96,97,98]. For that reason, it has been relevant to explore the concept in response to urinary stimuli. These polymers exist in an original macroscopic shape, and temporarily stay in another shape until going back to their original shape upon exposure to a stimulus, usually heat [99]. For urethral applications, their capacity to stretch during erection and recoil during detumescence should be explored as well. Smart acellular collagen–heparin scaffolds with growth factors (GFs) were shown to support bladder tissue regeneration in a large animal model of diseased bladder [100]. Given the layered nature of urethral tissue and the need to have different cell types in a peculiar spatial distribution close to the native urethral tissue, stress-induced rolling membrane (SIRM) was shown to have the ability to potentially be able to be studied for this purpose [101].
Another subset of smart polymers that has shown promise for engineering electrically active tissues is the group of electroconductive polymers. Electroactive polyurethane polymers have shown great potential in bladder tissue engineering applications, when the aim is to regenerate muscular components and innervation [102,103,104]. Electroconductive polymers may be instrumental when applying electrical or magnetic stimulation strategies for advanced maturation of the urethral tissue engineered constructs [105]. Another potential method to enhance host–scaffold interaction is the creation of smart scaffolds, which can controllably release trophic factors to optimize host reactions toward the biomaterial [103].
Although several experiments have succeeded in regenerating the different urethral tissue layers (i.e., epithelial and muscular layers) [13,55,106] not much attention has been paid to the surrounding corpus spongiosum, which has been shown to be important for successful urethral surgical repair [107,108,109]. Therefore, layered scaffolds can potentially have the ability to regenerate different host tissues that resemble the normal histological architecture of the missing tissues. The incorporation of a smooth muscle cell layer has been suggested to be necessary to obtain optimal cellular grafting results [72].
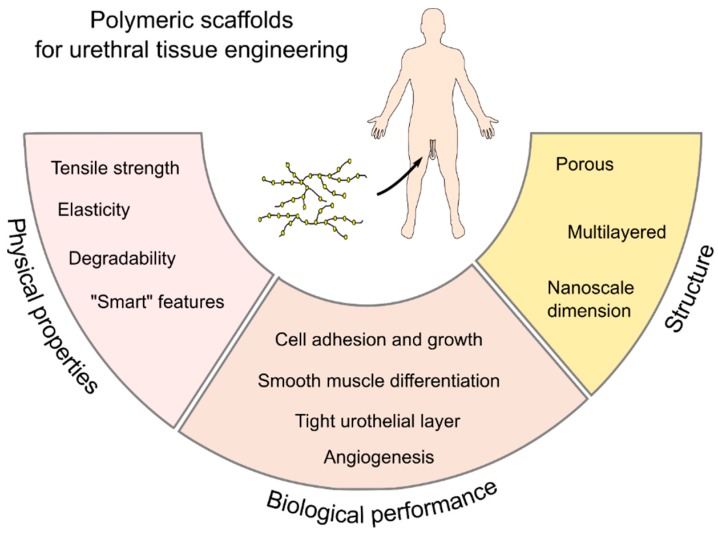
In the future, advances in understanding the physical and chemical factors responsible for urothelial and smooth muscle cell proliferation, migration, differentiation, and function may be exploited in the development of smart biomaterials, in which biologically active agents are incorporated into acellular natural or synthetic matrices. Not only interactions between urothelium and stroma, but also bi-directional interactions between tissue-engineered grafts and the host tissue environment, are important factors in achieving better outcomes in tissue remodeling. This concept has been termed “dynamic reciprocity” [110]. With a further understanding of the biological complexity and the physicochemical nature of native tissue, the appropriate design of new smart biomaterials will be possible by active interdisciplinary collaboration, bringing together polymer chemists, biomaterials scientists, tissue engineers, and clinicians (Figure 6).
Figure 6.
Desirable properties of polymeric scaffolds for urethral tissue engineering. These design constraints appear to be fundamental to better resemble the physical, biological, and structural properties of the native urethra and are expected to support an adequate recovery of the urethral function.
6. Conclusions
The performance of the current treatments for long urethral strictures remains suboptimal due to the shortage of tissue sources, the significant donor site morbidity, or the inability to completely restore the structure and function of the host. While non-degradable polymers have not met the clinical performance requirements because of the potential risk of migration, encrustation, and ultimate narrowing, tissue-engineered solutions based on biodegradable polymers have emerged as potential alternatives for successful recovery, and therefore function, of the urethral structure. Biological scaffolds have shown utility to repair urethral defects, but they display relative mechanical inferiority and their degradability cannot be easily adjusted. On the other hand, synthetic scaffolds have highly controllable chemical, mechanical, and structural characteristics, but proper reproduction of the native extracellular environment remains a challenge.
This suggests that combinations of natural and synthetic polymers could provide synergy with a controllable degradation rate, mechanical compliance, and supportive of formation of different tissue layers and vascularization. It is important to stress that a successful urethral scaffold should maintain clinical performance (e.g. mechanical function) following implantation, and synchronize with the preclinical performance requirements (e.g. degradation rate, ability to duplicate structure, and integrate into the host). This can potentially be achieved by controlled degradation matching regrowth of healthy tissues. Functional and smart polymeric scaffolds have the potential to better mimic the native tissue and may help tissue engineered solutions to meet the pre-clinical and clinical performance requirements in the future.
Acknowledgments
The publication of the article was funded by the Qatar National Library.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lazzeri M., Sansalone S., Guazzoni G., Barbagli G. Incidence, Causes, and Complications of Urethral Stricture Disease. Eur. Urol. Suppl. 2016;15:2–6. doi: 10.1016/j.eursup.2015.10.002. [DOI] [Google Scholar]
- 2.Keays M.A., Dave S. Current hypospadias management: Diagnosis, surgical management, and long-term patient-centred outcomes. Can. Urol. Assoc. J. 2017;11:S48–S53. doi: 10.5489/cuaj.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapple C., Andrich D., Atala A., Barbagli G., Cavalcanti A., Kulkarni S., Mangera A., Nakajima Y. SIU/ICUD Consultation on Urethral Strictures: The Management of Anterior Urethral Stricture Disease Using Substitution Urethroplasty. Urology. 2014;83:S31–S47. doi: 10.1016/j.urology.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Snodgrass W., Bush N. Primary hypospadias repair techniques: A review of the evidence. Urol. Ann. 2016;8:403–408. doi: 10.4103/0974-7796.192097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton A.S., Morey A.F., Aviles R., Garcia C.R. Anterior urethral strictures: Etiology and characteristics. Urology. 2005;65:1055–1058. doi: 10.1016/j.urology.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Mundy A.R., Andrich D.E. Urethral strictures. BJU Int. 2011;107:6–26. doi: 10.1111/j.1464-410X.2010.09800.x. [DOI] [PubMed] [Google Scholar]
- 7.Caldamone A.A., Edstrom L.E., Koyle M.A., Rabinowitz R., Hulbert W.C. Buccal mucosal grafts for urethral reconstruction. Urology. 1998;51:15–19. doi: 10.1016/S0090-4295(98)00088-0. [DOI] [PubMed] [Google Scholar]
- 8.Barbagli G., Palminteri E., Guazzoni G., Montorsi F., Turini D., Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: Are results affected by the surgical technique? J. Urol. 2005;174:955–958. doi: 10.1097/01.ju.0000169422.46721.d7. [DOI] [PubMed] [Google Scholar]
- 9.Ding J., Li Q., Li S., Li F., Zhou C., Zhou Y., Hu J., Xie L., Cao Y., Zhang S. Ten years’ experience for hypospadias repair: Combined buccal mucosa graft and local flap for urethral reconstruction. Urol. Int. 2014;93:454–459. doi: 10.1159/000360796. [DOI] [PubMed] [Google Scholar]
- 10.Markiewicz M.R., DeSantis J.L., Margarone J.E., Pogrel M.A., Chuang S.-K. Morbidity Associated with Oral Mucosa Harvest for Urological Reconstruction: An Overview. J. Oral Maxillofac. Surg. 2008;66:739–744. doi: 10.1016/j.joms.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Harris C.R., Osterberg E.C., Sanford T., Alwaal A., Gaither T.W., McAninch J.W., McCulloch C.E., Breyer B.N. National Variation in Urethroplasty Cost and Predictors of Extreme Cost: A Cost Analysis with Policy Implications. Urology. 2016;94:246–254. doi: 10.1016/j.urology.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas T.O., Mahdi E., Hasan A., AlAnsari A., Pennisi C.P. Current Status of Tissue Engineering in the Management of Severe Hypospadias. Front. Pediatr. 2018;5:283. doi: 10.3389/fped.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Kemp V., De Graaf P., Fledderus J.O., Bosch J.L.H.R., De Kort L.M.O. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS ONE. 2015;10:e0118653. doi: 10.1371/journal.pone.0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng C., Xu Y.-M., Fu Q., Zhu W.-D., Cui L., Chen J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. Part A. 2010;94A:317–325. doi: 10.1002/jbm.a.32729. [DOI] [PubMed] [Google Scholar]
- 15.Beiko D.T., Knudsen B.E., Watterson J.D., Cadieux P.A., Reid G., Denstedt J.D. Urinary tract biomaterials. J. Urol. 2004;171:2438–2444. doi: 10.1097/01.ju.0000125001.56045.6c. [DOI] [PubMed] [Google Scholar]
- 16.de Graaf P., van der Linde E.M., Rosier P.F.W.M., Izeta A., Sievert K.-D., Bosch J.L.H.R., de Kort L.M.O. Systematic Review to Compare Urothelium Differentiation with Urethral Epithelium Differentiation in Fetal Development, as a Basis for Tissue Engineering of the Male Urethra. Tissue Eng. Part B Rev. 2017;23:257–267. doi: 10.1089/ten.teb.2016.0352. [DOI] [PubMed] [Google Scholar]
- 17.Da Silva E.A., Sampaio F.J.B., Ortiz V., Cardoso L.E.M. Regional differences in the extracellular matrix of the human spongy urethra as evidenced by the composition of glycosaminoglycans. J. Urol. 2002;167:2183–2187. doi: 10.1016/S0022-5347(05)65125-7. [DOI] [PubMed] [Google Scholar]
- 18.De Graaf P., Ramadan R., Linssen E.C., Staller N.A., Hendrickx A.P.A., Pigot G.L.S., Meuleman E.J.H., Bouman M., Özer M., Bosch J.L.H.R., et al. The multilayered structure of the human corpus spongiosum. Histol. Histopathol. 2018;33:1335–1345. doi: 10.14670/HH-18-022. [DOI] [PubMed] [Google Scholar]
- 19.Coenen A.M.J., Bernaerts K.V., Harings J.A.W., Jockenhoevel S., Ghazanfari S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018;79:60–82. doi: 10.1016/j.actbio.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Augsburger H.R. Elastic fibre system of the female canine urethra. Histochemical identification of elastic, elaunin and oxytalan fibres. Anat. Histol. Embryol. 1997;26:297–302. doi: 10.1111/j.1439-0264.1997.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim R.J., Kerns J.M., Liu S., Nagel T., Zaszczurynski P., Lin D.L., Damaser M.S. Striated muscle and nerve fascicle distribution in the female raturethral sphincter. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007;290:145–154. doi: 10.1002/ar.20420. [DOI] [PubMed] [Google Scholar]
- 22.Lim S.H., Wang T.-J., Tseng G.-F., Lee Y.F., Huang Y.-S., Chen J.-R., Cheng C.-L. The Distribution of Muscles Fibers and Their Types in the Female Rat Urethra: Cytoarchitecture and Three-Dimensional Reconstruction. Anat. Rec. 2013;296:1640–1649. doi: 10.1002/ar.22740. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski R.J., Prantil R.L., Fraser M.O., Chancellor M.B., de Groat W.C., Huard J., Vorp D.A. Development of an experimental system for the study of urethral biomechanical function. Am. J. Physiol. Physiol. 2004;286:F225–F232. doi: 10.1152/ajprenal.00126.2003. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe H., Yamamoto T.Y. Autonomic innervation of the muscles in the wall of the bladder and proximal urethra of male rats. J. Anat. 1979;128:873–886. [PMC free article] [PubMed] [Google Scholar]
- 25.Male Reproductive System. [(accessed on 20 December 2018)]; Available online: http://histology.medicine.umich.edu/slides/male-reproductive-system.
- 26.Shirozu H., Koyanagi T., Takashima T., Horimoto N., Akazawa K., Nakano H. Penile tumescence in the human fetus at term—A preliminary report. Early Hum. Dev. 1995;41:159–166. doi: 10.1016/0378-3782(95)01618-D. [DOI] [PubMed] [Google Scholar]
- 27.Ohel G., Haddad S., Samueloff A. Fetal Urine Production and Micturition and Fetal Behavioral State. Am. J. Perinatol. 1995;12:91–92. doi: 10.1055/s-2007-994412. [DOI] [PubMed] [Google Scholar]
- 28.Walter J.S., Wheeler J.S., Morgan C., Zaszczurynski P., Plishka M. Measurement of total urethral compliance in females with stress incontinence. Neurourol. Urodyn. 1993;12:273–276. doi: 10.1002/nau.1930120311. [DOI] [PubMed] [Google Scholar]
- 29.Mijailovich S.M., Sullivan M.P., Yalla S.V., Venegas J.G. Effect of urethral compliance on the steady state p-Q relationships assessed with a mechanical analog of the male lower urinary tract. Neurourol. Urodyn. 2007;26:234–246. doi: 10.1002/nau.20302. [DOI] [PubMed] [Google Scholar]
- 30.Thind P., Lose G., Colstrup H. How to measure urethral elastance in a simple way. Elastance: Definition, determination and implications. Urol. Res. 1991;19:241–244. doi: 10.1007/BF00305303. [DOI] [PubMed] [Google Scholar]
- 31.Yalla S.V., Burros H.M. Conduit and regional compliance of female canine urethra. Urology. 1974;4:155–161. doi: 10.1016/0090-4295(74)90325-2. [DOI] [PubMed] [Google Scholar]
- 32.Husmann D.A., Rathbun S.R. Long-Term Followup of Visual Internal Urethrotomy for Management of Short (Less Than 1 Cm) Penile Urethral Strictures Following Hypospadias Repair. J. Urol. 2006;176:1738–1741. doi: 10.1016/S0022-5347(06)00617-3. [DOI] [PubMed] [Google Scholar]
- 33.Lalla M., Gregersen H., Olsen L.H., Jørgensen T.M. In Vivo Biomechanical Assessment of Anterior Rabbit Urethra After Repair of Surgically Created Hypospadias. J. Urol. 2010;184:675–682. doi: 10.1016/j.juro.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 34.Hammouda H.M., El-Ghoneimi A., Bagli D.J., McLorie G.A., Khoury A.E. Tubularized Incised Plate Repair: Functional Outcome After Intermediate Followup. J. Urol. 2003;169:331–333. doi: 10.1016/S0022-5347(05)64120-1. [DOI] [PubMed] [Google Scholar]
- 35.Eassa W., Brzezinski A., Capolicchio J.P., Jednak R., El-Sherbiny M. How do asymptomatic toilet-trained children void following tubularized incised-plate hypospadias repair? Can. Urol. Assoc. J. 2012;6:238–242. doi: 10.5489/cuaj.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalla M., Riis C., Jørgensen C.S., Danielsen C.C., Jørgensen T.M. A biomechanical, histological and biochemical study in an experimental rabbit hypospadias repair model using scanning acoustic microscopy. J. Pediatr. Urol. 2011;7:404–411. doi: 10.1016/j.jpurol.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Gabrich P.N., Vasconcelos J.S.P., Damião R., Silva E.A. Penile anthropometry in Brazilian children and adolescents. J. Pediatr. 2007;83:441–446. doi: 10.1590/S0021-75572007000600008. [DOI] [PubMed] [Google Scholar]
- 38.Simsek A., Aldamanhori R., Chapple C.R., MacNeil S. Overcoming scarring in the urethra: Challenges for tissue engineering. Asian J. Urol. 2018;5:69–77. doi: 10.1016/j.ajur.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J.S., Bury M.I., Fuller N.J., Sturm R.M., Ahmad N., Sharma A.K. Bone Marrow Stem/Progenitor Cells Attenuate the Inflammatory Milieu Following Substitution Urethroplasty. Sci. Rep. 2016;6:35638. doi: 10.1038/srep35638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birder L., Andersson K.-E. Urothelial Signaling. Physiol. Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akkad T., Pelzer A.E., Mitterberger M., Rehder P., Leonhartsberger N., Bartsch G., Strasser H. Influence of intravesical potassium on pelvic floor activity in women with recurrent urinary tract infections: Comparative urodynamics might lead to enhanced detection of dysfunctional voiding. BJU Int. 2007;100:1071–1074. doi: 10.1111/j.1464-410X.2007.07047.x. [DOI] [PubMed] [Google Scholar]
- 42.Rajasekaran M., Stein P., Parsons C.L. Toxic factors in human urine that injure urothelium. Int. J. Urol. 2006;13:409–414. doi: 10.1111/j.1442-2042.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 43.Adamowicz J., Kloskowski T., Tworkiewicz J., Pokrywczyńska M., Drewa T. Urine Is a Highly Cytotoxic Agent: Does It Influence Stem Cell Therapies in Urology? Transplant. Proc. 2012;44:1439–1441. doi: 10.1016/j.transproceed.2012.01.128. [DOI] [PubMed] [Google Scholar]
- 44.Li H., Xu Y., Xie H., Li C., Song L., Feng C., Zhang Q., Xie M., Wang Y., Lv X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A. 2014;20:774–784. doi: 10.1089/ten.tea.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro-Filho L.A., Sievert K.-D. Acellular matrix in urethral reconstruction. Adv. Drug Deliv. Rev. 2015;82–83:38–46. doi: 10.1016/j.addr.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Denstedt J., Atala A. Biomaterials and Tissue Engineering in Urology. Woodhead Publishing; Sawston, UK: 2009. [Google Scholar]
- 47.Bisson I., Hilborn J., Wurm F., Meyrat B., Frey P. Human urothelial cells grown on collagen adsorbed to surface-modified polymers. Urology. 2002;60:176–180. doi: 10.1016/S0090-4295(02)01642-4. [DOI] [PubMed] [Google Scholar]
- 48.Azevedo H.S., Reis R.L. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate. CRC Press; Boca Raton, FL, USA: 2005. [Google Scholar]
- 49.Babensee J.E., Anderson J.M., McIntire L.V., Mikos A.G. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998;33:111–139. doi: 10.1016/S0169-409X(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 50.Knecht S., Erggelet C., Endres M., Sittinger M., Kaps C., Stüssi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007;83:50–57. doi: 10.1002/jbm.b.30765. [DOI] [PubMed] [Google Scholar]
- 51.Perkins L.E.L.E.L., Kossuth M.B.B., Fox J.C.C., Rapoza R.J.J. Paving the way to a bioresorbable technology: Development of the absorb BRS program. Catheter. Cardiovasc. Interv. 2016;88:1–9. doi: 10.1002/ccd.26811. [DOI] [PubMed] [Google Scholar]
- 52.Moon J.J., West J.L. Vascularization of engineered tissues: Approaches to promote angio-genesis in biomaterials. Curr. Top. Med. Chem. 2008;8:300–310. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nooeaid P., Salih V., Beier J.P., Boccaccini A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012;16:2247–2270. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmagnola I., Ranzato E., Chiono V. Functional 3D Tissue Engineering Scaffolds. Elsevier; Amsterdam, The Netherlands: 2018. Scaffold functionalization to support a tissue biocompatibility; pp. 255–277. [Google Scholar]
- 55.Versteegden L.R.M., de Jonge P.K.J.D., IntHout J., van Kuppevelt T.H., Oosterwijk E., Feitz W.F.J., de Vries R.B.M., Daamen W.F. Tissue Engineering of the Urethra: A Systematic Review and Meta-analysis of Preclinical and Clinical Studies. Eur. Urol. 2017;72:594–606. doi: 10.1016/j.eururo.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Qi N., Li W., Tian H. A Systematic Review of Animal and Clinical Studies on the Use of Scaffolds for Urethral Repair. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016;36:111–117. doi: 10.1007/s11596-016-1551-5. [DOI] [PubMed] [Google Scholar]
- 57.Davis N.F., Cunnane E.M., O’Brien F.J., Mulvihill J.J., Walsh M.T. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon. 2018;16:55–65. doi: 10.1016/j.surge.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Badylak S.F., Lantz G.C., Coffey A., Geddes L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Kropp B.P., Moore P., Cowan R., Furness P.D., Kolligian M.E., Frey P., Cheng E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000;164:928–934. doi: 10.1016/S0022-5347(05)67220-5. discussion 934–935. [DOI] [PubMed] [Google Scholar]
- 60.Fiala R., Vidlar A., Vrtal R., Belej K., Student V. Porcine Small Intestinal Submucosa Graft for Repair of Anterior Urethral Strictures. Eur. Urol. 2007;51:1702–1708. doi: 10.1016/j.eururo.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 61.Palminteri E., Berdondini E., Colombo F., Austoni E. Small Intestinal Submucosa (SIS) Graft Urethroplasty: Short-term Results. Eur. Urol. 2007;51:1695–1701. doi: 10.1016/j.eururo.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Palminteri E., Berdondini E., Fusco F., De Nunzio C., Salonia A. Long-term Results of Small Intestinal Submucosa Graft in Bulbar Urethral Reconstruction. Urology. 2012;79:695–701. doi: 10.1016/j.urology.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 63.Campodonico F., Benelli R., Michelazzi A., Ognio E., Toncini C., Maffezzini M. Bladder Cell Culture on Small Intestinal Submucosa as Bioscaffold: Experimental Study on Engineered Urothelial Grafts. Eur. Urol. 2004;46:531–537. doi: 10.1016/j.eururo.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Badylak S.F., Kropp B., McPherson T., Liang H., Snyder P.W. Small Intestinal Submucosa: A Rapidly Resorbed Bioscaffold for Augmentation Cystoplasty in a Dog Model. Tissue Eng. 1998;4:379–387. doi: 10.1089/ten.1998.4.379. [DOI] [PubMed] [Google Scholar]
- 65.Kubricht W.S., Williams B.J., Eastham J.A., Venable D.D. Tensile strength of cadaveric fascia lata compared to small intestinal submucosa using suture pull through analysis. J. Urol. 2001;165:486–490. doi: 10.1097/00005392-200102000-00031. [DOI] [PubMed] [Google Scholar]
- 66.Weiser A.C., Franco I., Herz D.B., Silver R.I., Reda E.F. Single Layered Small Intestinal Submucosa in the Repair of Severe Chordee and Complicated Hypospadias. J. Urol. 2003;170:1593–1595. doi: 10.1097/01.ju.0000083863.01634.e1. [DOI] [PubMed] [Google Scholar]
- 67.Hayn M.H., Bellinger M.F., Schneck F.X. Small Intestine Submucosa as a Corporal Body Graft in the Repair of Severe Chordee. Urology. 2009;73:277–279. doi: 10.1016/j.urology.2008.08.489. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L., Du A., Li J., Pan M., Han W., Xiao Y. Development of a cell-seeded modified small intestinal submucosa for urethroplasty. Heliyon. 2016;2:e00087. doi: 10.1016/j.heliyon.2016.e00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoo J.J., Meng J., Oberpenning F., Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–225. doi: 10.1016/S0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 70.Orabi H., Safwat A.S., Shahat A., Hammouda H.M. The use of small intestinal submucosa graft for hypospadias repair: Pilot study. Arab J. Urol. 2013;11:415–420. doi: 10.1016/j.aju.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hauser S., Bastian P.J., Fechner G., Müller S.C. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology. 2006;68:263–266. doi: 10.1016/j.urology.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 72.Nuininga J.E., van Moerkerk H., Hanssen A., Hulsbergen C.A., Oosterwijk-Wakka J., Oosterwijk E., de Gier R.P.E., Schalken J.A., van Kuppevelt T., Feitz W.F.J. Rabbit urethra replacement with a defined biomatrix or small intestinal submucosa. Eur. Urol. 2003;44:266–271. doi: 10.1016/S0302-2838(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 73.Chun S.Y., Kim B.S., Kwon S.Y., Park S.I., Song P.H., Yoo E.S., Kim B.W., Kwon T.G., Kim H.T. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 2015;30:301–307. doi: 10.3346/jkms.2015.30.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J.-W., Xie M.-K., Zhang Y., Wei G.-J., Li X., Li H.-B., Wang J.-H., Zhu W.-D., Li C., Xu Y.-M., et al. Reconstruction of Penile Urethra With the 3-Dimensional Porous Bladder Acellular Matrix in a Rabbit Model. Urology. 2014;84:1499–1505. doi: 10.1016/j.urology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 75.Li C., Xu Y.-M., Song L.-J., Fu Q., Cui L., Yin S. Urethral Reconstruction Using Oral Keratinocyte Seeded Bladder Acellular Matrix Grafts. J. Urol. 2008;180:1538–1542. doi: 10.1016/j.juro.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Atala A., Guzman L., Retik A.B. A novel inert collagen matrix for hypospadias repair. J. Urol. 1999;162:1148–1151. doi: 10.1016/S0022-5347(01)68105-9. [DOI] [PubMed] [Google Scholar]
- 77.Gu G.-L., Xia S.-J., Zhang J., Liu G.-H., Yan L., Xu Z.-H., Zhu Y.-J. Tubularized Urethral Replacement Using Tissue-Engineered Peritoneum-Like Tissue in a Rabbit Model. Urol. Int. 2012;89:358–364. doi: 10.1159/000339745. [DOI] [PubMed] [Google Scholar]
- 78.Roth C.C., Kropp B.P. Recent advances in urologic tissue engineering. Curr. Urol. Rep. 2009;10:119–125. doi: 10.1007/s11934-009-0022-y. [DOI] [PubMed] [Google Scholar]
- 79.Xie M., Song L., Wang J., Fan S., Zhang Y., Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013;184:774–781. doi: 10.1016/j.jss.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 80.Xie M., Xu Y., Song L., Wang J., Lv X., Zhang Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 2014;188:1–7. doi: 10.1016/j.jss.2013.11.1102. [DOI] [PubMed] [Google Scholar]
- 81.Hudson S.M. Silk polymers: Material science and biotechnology, D. Kaplan, W. Adams, B. Farmer and C. Viney, eds. American Chemical Society, ACS Symposium Series 544 (1944). 370 pp. Polym. Adv. Technol. 1995;6:717. doi: 10.1002/pat.1995.220061107. [DOI] [Google Scholar]
- 82.Cunniff P.M., Fossey S.A., Auerbach M.A., Song J.W., Kaplan D.L., Adams W.W., Eby R.K., Mahoney D., Vezie D.L. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994;5:401–410. doi: 10.1002/pat.1994.220050801. [DOI] [Google Scholar]
- 83.Wang Y., Rudym D.D., Walsh A., Abrahamsen L., Kim H.-J., Kim H.S., Kirker-Head C., Kaplan D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Altman G.H., Diaz F., Jakuba C., Calabro T., Horan R.L., Chen J., Lu H., Richmond J., Kaplan D.L. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 85.Panilaitis B., Altman G.H., Chen J., Jin H.J., Karageorgiou V., Kaplan D.L. Macrophage responses to silk. Biomaterials. 2003;24:3079–3085. doi: 10.1016/S0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 86.Chung Y.G., Tu D., Franck D., Gil E.S., Algarrahi K., Adam R.M., Kaplan D.L., Estrada C.R., Mauney J.R. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS ONE. 2014;9:e91592. doi: 10.1371/journal.pone.0091592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribeiro C., Sencadas V., Correia D.M., Lanceros-Méndez S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces. 2015;136:46–55. doi: 10.1016/j.colsurfb.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 88.Aguilar M.R., San Román J. Smart Polymers and their Applications. Elsevier; Amsterdam, The Netherlands: 2014. Introduction to smart polymers and their applications; pp. 1–11. [Google Scholar]
- 89.Knipe J.M., Peppas N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014;1:57–65. doi: 10.1093/rb/rbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowalski P.S., Bhattacharya C., Afewerki S., Langer R. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018;4:3809–3817. doi: 10.1021/acsbiomaterials.8b00889. [DOI] [PubMed] [Google Scholar]
- 91.Anderson D.G., Burdick J.A., Langer R. MATERIALS SCIENCE: Smart Biomaterials. Science. 2004;305:1923–1924. doi: 10.1126/science.1099987. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y.-J., Matsunaga Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B. 2017;5:4307–4321. doi: 10.1039/C7TB00157F. [DOI] [PubMed] [Google Scholar]
- 93.Jochum F.D., Theato P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013;42:7468–7483. doi: 10.1039/C2CS35191A. [DOI] [PubMed] [Google Scholar]
- 94.Tsuda Y., Shimizu T., Yamato M., Kikuchi A., Sasagawa T., Sekiya S., Kobayashi J., Chen G., Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Shiroyanagi Y., Yamato M., Yamazaki Y., Toma H., Okano T. Transplantable Urothelial Cell Sheets Harvested Noninvasively from Temperature-Responsive Culture Surfaces by Reducing Temperature. Tissue Eng. 2003;9:1005–1012. doi: 10.1089/107632703322495646. [DOI] [PubMed] [Google Scholar]
- 96.Rickert D., Moses M.A., Lendlein A., Kelch S., Franke R.-P. The importance of angiogenesis in the interaction between polymeric biomaterials and surrounding tissue. Clin. Hemorheol. Microcirc. 2003;28:175–181. [PubMed] [Google Scholar]
- 97.Correia C.O., Leite Á.J., Mano J.F. Chitosan/bioactive glass nanoparticles scaffolds with shape memory properties. Carbohydr. Polym. 2015;123:39–45. doi: 10.1016/j.carbpol.2014.12.076. [DOI] [PubMed] [Google Scholar]
- 98.Tzoneva R., Seifert B., Behl M., Lendlein A. Elastic multiblock copolymers for vascular regeneration: Protein adsorption and hemocompatibility. Clin. Hemorheol. Microcirc. 2012;52:337–348. doi: 10.3233/CH-2012-1609. [DOI] [PubMed] [Google Scholar]
- 99.Hardy J.G., Palma M., Wind S.J., Biggs M.J. Responsive Biomaterials: Advances in Materials Based on Shape-Memory Polymers. Adv. Mater. 2016;28:5717–5724. doi: 10.1002/adma.201505417. [DOI] [PubMed] [Google Scholar]
- 100.Roelofs L.A.J., Oosterwijk E., Kortmann B.B.M., Daamen W.F., Tiemessen D.M., Brouwer K.M., Eggink A.J., Crevels A.J., Wijnen R.M.H., van Kuppevelt T.H., et al. Bladder Regeneration Using a Smart Acellular Collagen Scaffold with Growth Factors VEGF, FGF2 and HB-EGF. Tissue Eng. Part A. 2016;22:83–92. doi: 10.1089/ten.tea.2015.0096. [DOI] [PubMed] [Google Scholar]
- 101.Yuan B., Jin Y., Sun Y., Wang D., Sun J., Wang Z., Zhang W., Jiang X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 2012;24:890–896. doi: 10.1002/adma.201104589. [DOI] [PubMed] [Google Scholar]
- 102.Smolar J., Salemi S., Horst M., Sulser T., Eberli D. Stem Cells in Functional Bladder Engineering. Transfus. Med. Hemother. 2016;43:328–335. doi: 10.1159/000447977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hardy J.G., Cornelison R.C., Sukhavasi R.C., Saballos R.J., Vu P., Kaplan D.L., Schmidt C.E. Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering. Bioengineering. 2015;2:15–34. doi: 10.3390/bioengineering2010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y., Wang L., Guo B., Shao Y., Ma P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18–31. doi: 10.1016/j.biomaterials.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 105.StÖlting M.N.L., Arnold A.S., Haralampieva D., Handschin C., Sulser T., Eberli D. Magnetic stimulation supports muscle and nerve regeneration after trauma in mice. Muscle Nerve. 2016;53:598–607. doi: 10.1002/mus.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orabi H., Bouhout S., Morissette A., Rousseau A., Chabaud S., Bolduc S. Tissue engineering of urinary bladder and urethra: Advances from bench to patients. Sci. World J. 2013;2013:154564. doi: 10.1155/2013/154564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dodat H., Landry J.-L., Szwarc C., Culem S., Murat F.-J., Dubois R. Spongioplasty and separation of the corpora cavernosa for hypospadias repair. BJU Int. 2003;91:528–531. doi: 10.1046/j.1464-410X.2003.04110.x. [DOI] [PubMed] [Google Scholar]
- 108.Bhat A., Sabharwal K., Bhat M., Saran R., Singla M., Kumar V. Outcome of tubularized incised plate urethroplasty with spongioplasty alone as additional tissue cover: A prospective study. Indian J. Urol. 2014;30:392–397. doi: 10.4103/0970-1591.134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beaudoin S., Delaage P.-H., Bargy F. Anatomical basis of surgical repair of hypospadias by spongioplasty. Surg. Radiol. Anat. 2000;22:139–141. doi: 10.1007/s00276-000-0139-7. [DOI] [PubMed] [Google Scholar]
- 110.Mauney J.R., Adam R.M. Dynamic reciprocity in cell–scaffold interactions. Adv. Drug Deliv. Rev. 2015;82–83:77–85. doi: 10.1016/j.addr.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]