Abstract
Acute lung injury (ALI) is a life-threatening syndrome characterized by acute and severe hypoxemic respiratory failure. Visfatin, which is known as an obesity-related cytokine with pro-inflammatory activities, plays a role in regulation of inflammatory cytokines. The mechanisms of ALI remain unclear in critically ill patients. Survival in ALI patients appear to be influenced by the stress generated by mechanical ventilation and by ALI-associated factors that initiate the inflammatory response. The objective for this study was to understand the mechanisms of how visfatin regulates inflammatory cytokines and promotes ALI. The expression of visfatin was evaluated in ALI patients and mouse sepsis models. Moreover, the underlying mechanisms were investigated using human bronchial epithelial cell lines, BEAS-2B and NL-20. An increase of serum visfatin was discovered in ALI patients compared to normal controls. Results from hematoxylin and eosin (H&E) and immunohistochemistry staining also showed that visfatin protein was upregulated in mouse sepsis models. Moreover, lipopolysaccharide (LPS) induced visfatin expression, activated the STAT3/NFκB pathway, and increased the expression of pro-inflammatory cytokines, including IL1-β, IL-6, and TNF-α in human bronchial epithelial cell lines NL-20 and BEAS-2B. Co-treatment of visfatin inhibitor FK866 reversed the activation of the STAT3/NFκB pathway and the increase of pro-inflammatory cytokines induced by LPS. Our study provides new evidence for the involvement of visfatin and down-stream events in acute lung injury. Further studies are required to confirm whether the anti-visfatin approaches can improve ALI patient survival by alleviating the pro-inflammatory process.
Keywords: acute lung injury, visfatin, FK866
1. Introduction
Acute respiratory distress syndrome (ARDS) is a severe form of acute lung injury (ALI) that is characterized by severe lung tissue inflammation, reduced gas exchange, and, finally, acute hypoxemic respiratory failure [1,2]. In addition, ALI/ARDS is associated with a high mortality rate, between 20% and 50% [3,4,5], and is triggered by various factors such as bacteria and virus infection, major trauma, massive blood transfusion, pneumonia, and sepsis [6,7,8]. Nevertheless, the detailed mechanism of ALI/ARDS remains poorly understood. While the advances in the restrictive fluid management, lung protection strategies, and antibiotics treatment of infection of ALI and ARDS have improved the outcomes, there is still no effective treatment for ALI/ARDS [9,10,11,12]. Lipopolysaccharide (LPS), a component of the outer membrane of bacteria, provokes strong immune reactions in animals through complement activation and has been widely used to study the mechanisms of ALI [13,14]. Colon ascendens stent peritonitis (CASP) procedure, by creating an intestinal leakage of feces and resulting in diffuse peritonitis and polymicrobial sepsis, is also a frequently used mouse model for ALI [13]. Moreover, recent studies showed that ALI/ARDS increased the release of proinflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukin (IL)-1β, IL-6, and IL-8 from immune cells [15,16], and increased the permeability of the alveolar–capillary barrier [8,17]. These phenomena indicate that acute inflammation plays an important role in the ALI/ARDS process. Hence, developing more effective strategies to inhibit inflammatory responses and identifying new diagnostic and therapeutic targets are critical for the improvement of patient outcomes.
Visfatin, also known as pre-B-cell colony-enhancing factor (PBEF) and nicotinamide phosphoribosyltransferase (Nampt), is a 52-kDa molecule first identified as a cytokine involved in B-cell maturation [18]. It is highly expressed in bone marrow, liver, and muscle, and is secreted by a variety of cell types, including lymphocytes, neutrophils, macrophages, and epithelial cells [18,19,20,21]. Visfatin protein has several biologic functions according to its intracellular or extracellular localization [18,22,23]. In the intracellular compartment, visfatin acts as nicotinamide phosphoribosyltransferase (Nampt), which is involved in the mammalian salvage pathway of NAD synthesis [24,25]. Visfatin has been reported as a new inflammatory cytokine that can increase the production of other inflammatory cytokines, such as IL-8, IL-6, IL-1β, and TNF-α in human monocytes [26,27,28]. Furthermore, visfatin is involved in several inflammatory diseases, including psoriasis, rheumatoid arthritis, atherosclerosis, inflammatory bowel diseases, and ALI in sepsis patients [26,29,30,31,32].
A previous study reported that visfatin is highly expressed in ALI of dog, mouse, and human, and two specific visfatin single nucleotide polymorphisms are associated with higher risk of ALI [33]. However, the detailed mechanisms of how visfatin regulates inflammatory cytokines and promotes ALI remain poorly understood. Herein, we report that the elevated visfatin levels led to decreased mouse survival in two ALI mouse models (LPS and CASP), which was rescued by visfatin inhibitor FK866. In addition, in vitro studies show that visfatin stimulates the expression of inflammatory cytokines through STAT3 and NFκB pathways.
2. Results
2.1. Visfatin Correlates with Poor Survival and Inflammatory Cytokines in ALI Patients
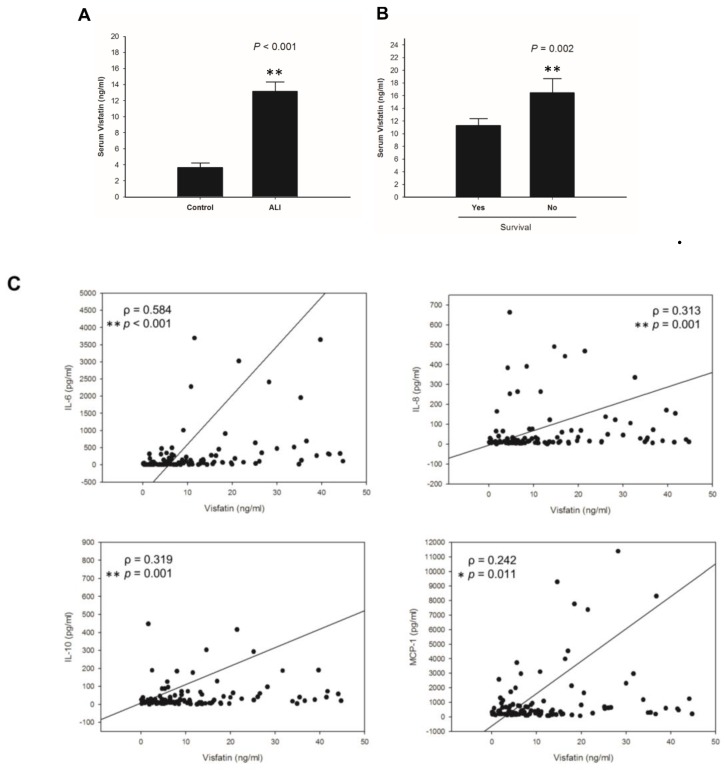
Acute lung injury (ALI), defined as an inflammatory disease in the lung, is mostly caused by over-secretion of pro-inflammatory cytokines [1,34]. It is present with complex autocrine and paracrine inter-relationship of cytokines and pro-inflammatory mediators that initiate and amplify the inflammatory activity. In this study, we first determined the protein levels of visfatin in ALI patients. Between 2013 and 2014, 892 consecutive patients with sepsis were enrolled in the emergency department of Kaohsiung Medical University Hospital. ALI was diagnosed in 110 septic patients. Of these patients, 25 died and 85 survived. Serum visfatin levels were significantly higher in ALI patients, in comparison to normal controls (Figure 1A). Moreover, the survival rate of ALI patients was negatively correlated with visfatin levels (Figure 1B). Since visfatin regulates pro-inflammatory activities through release of inflammatory cytokines, including interleukin1-β (IL1-β, interleukin-6(IL-6), and tumor necrosis factor-α (TNF-α [28,35], we studied the correlation of visfatin with pro-inflammatory cytokines in ALI patients. As shown in Figure 1C, the elevated serum visfatin levels were correlated with IL-6, IL-8, IL-10, and monocyte chemotactic protein-1 (MCP-1) in the ALI patients.
Figure 1.
Upregulation of serum visfatin in acute lung injury (ALI) patients. (A) Serum visfatin levels in ALI patients (n = 110) and normal controls (n = 32). (B) Correlation of patient survival with serum visfatin levels. (C) Spearman’s correlation of serum visfatin with IL-6, IL-8, IL-10, and MCP-1 in ALI patients. Results are mean ± SD. *, p value < 0.05. **, p value < 0.01.
2.2. Visfatin Expression is Upregulated in Both LPS and CASP-Induced ALI Mice, While Visfatin Inhibitor FK866 Attenuated the ALI Phenotypes
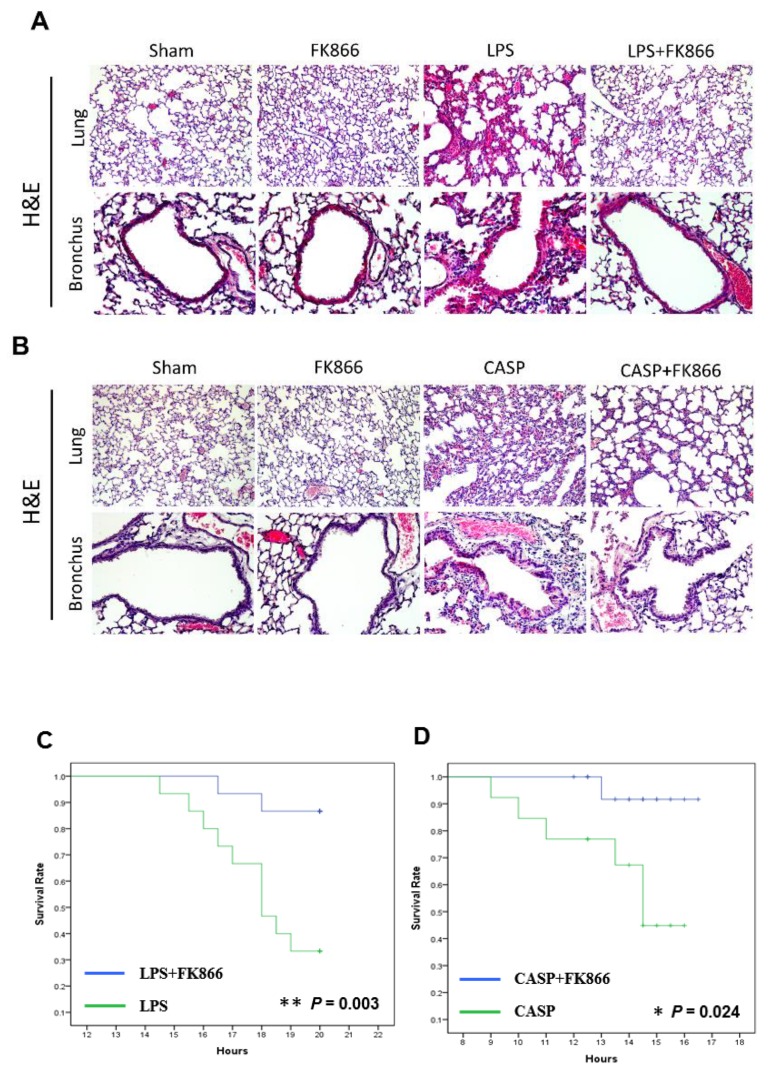
Since serum visfatin was elevated in ALI patients, we further evaluated the in vivo activity of visfatin in mouse models of ALI, including LPS model and CASP model [36,37]. After sacrifice of the mice with LPS or CASP treatment, we performed histological examination by H&E staining of the sections from lung and bronchus tissues, which showed an increase of damage in lung and bronchus tissues, accompanied by septal thickening and interstitial infiltration of neutrophils and lymphocytes (Figure 2A,B). FK866, a visfatin inhibitor, has been indicated as a novel therapeutic compound that attenuates acute lung injury via modulation of innate immune functions [38,39]. In this study, co-treatment of FK866 successfully attenuated the LPS and CASP-induced acute injury in lung and bronchus tissues (Figure 2A,B).
Figure 2.
FK866, a visfatin inhibitor, decreased acute lung injury and increased survival in LPS and CASP ALI mouse models. (A,B) Histology of lung tissues in mice treated with LPS alone, CASP alone, or co-treatment with FK866, determined by H&E staining. Sham mice show normal lung tissue and LPS- or CASP-treated mice show injuries in lung tissue, including fluid-filled alveoli, septal thickening, and interstitial lymphocytic and neutrophilic infiltration. (100× amplification). (C,D) Survival rate in mice treated with LPS alone, CASP alone (n = 15), or co-treatment with FK866 (n = 15) in ALI mouse model. *, p value < 0.05. **, p value < 0.01.
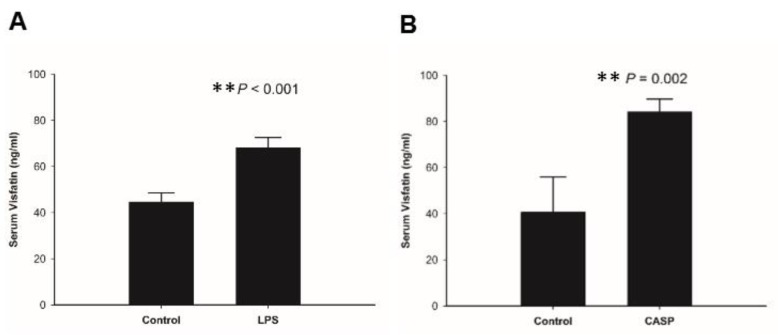
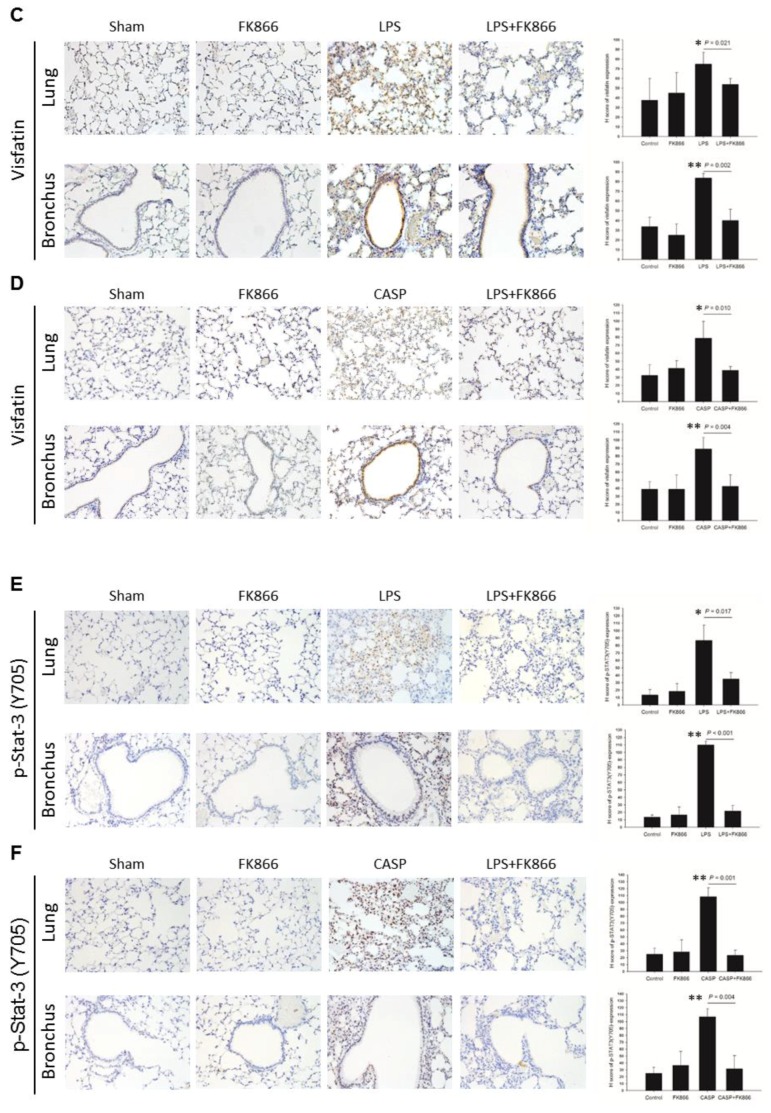
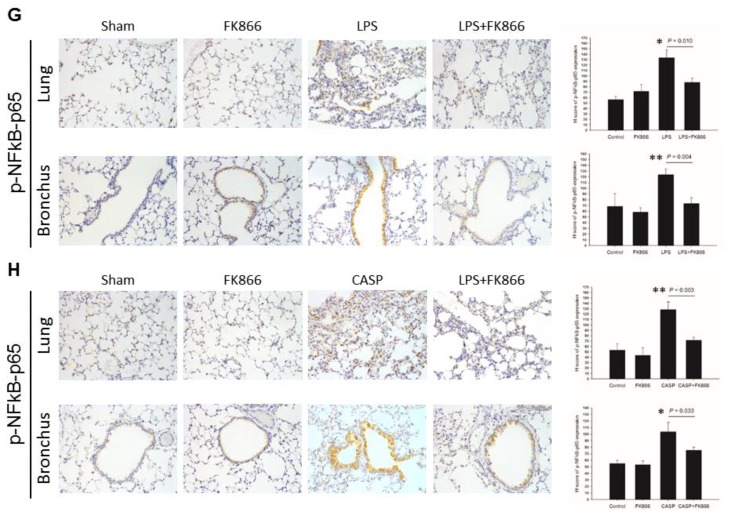
We also analyzed whether FK866 can rescue mouse death caused by treatment of LPS and CASP. Mice with FK866 co-treatment had higher survival rate than those without FK866 treatment in the LPS model (Figure 2C) and the CASP model (Figure 2D). We further investigated the in vivo regulation of visfatin protein in ALI mouse models. Serum visfatin levels were elevated in both LPS and CASP-treated mice, in comparison to mock-treated mice (Figure 3A,B). Signal transducer and activator of transcription 3 (STAT3) acts as a mediator and biomarker in endothelial activation [40]. Previous studies indicated that visfatin promotes endothelial angiogenesis by activating STAT3, characterized by increased tyrosine phosphorylation of residue 705 (Y705), nuclear translocation, and DNA binding activity in human endothelial cells [41,42,43]. Visfatin was reported to cause endothelial dysfunction by increasing inflammatory and adhesion molecule expression through the upregulation of NF-κB activity [44]. Furthermore, the results of immunohistochemistry demonstrated that LPS and CASP treatment dramatically increased visfatin, and p-STAT3(Y705) and p-NF-κB-p65 protein expression in lung and bronchial tissues, while FK866 co-treatment reversed the elevated visfatin, and p-STAT3(Y705) and p-NF-κB-p65 expression caused by LPS or CASP treatment (Figure 3C–H).
Figure 3.
LPS and CASP treatment in mice enhanced visfatin expression in circulation and in lung tissues. (A,B) Serum visfatin levels in sham mice and LPS- and CASP-treated mice. (C,D) Visfatin, (E,F) p-STAT3(Y705), and (G,H) p-NF-κB-p65 expression in lung tissues in sham mice and LPS- and CASP-treated mice, determined by immunohistochemistry. The representative results are from three separate experiments. Results are mean ± SD (n = 3). *, p value < 0.05. **, p value < 0.01. (100× amplification).
2.3. LPS-Upregulated Visfatin Expression in Human Bronchial Epithelial Cells Mediates the Activation of the STAT3/NF-κB Pathway
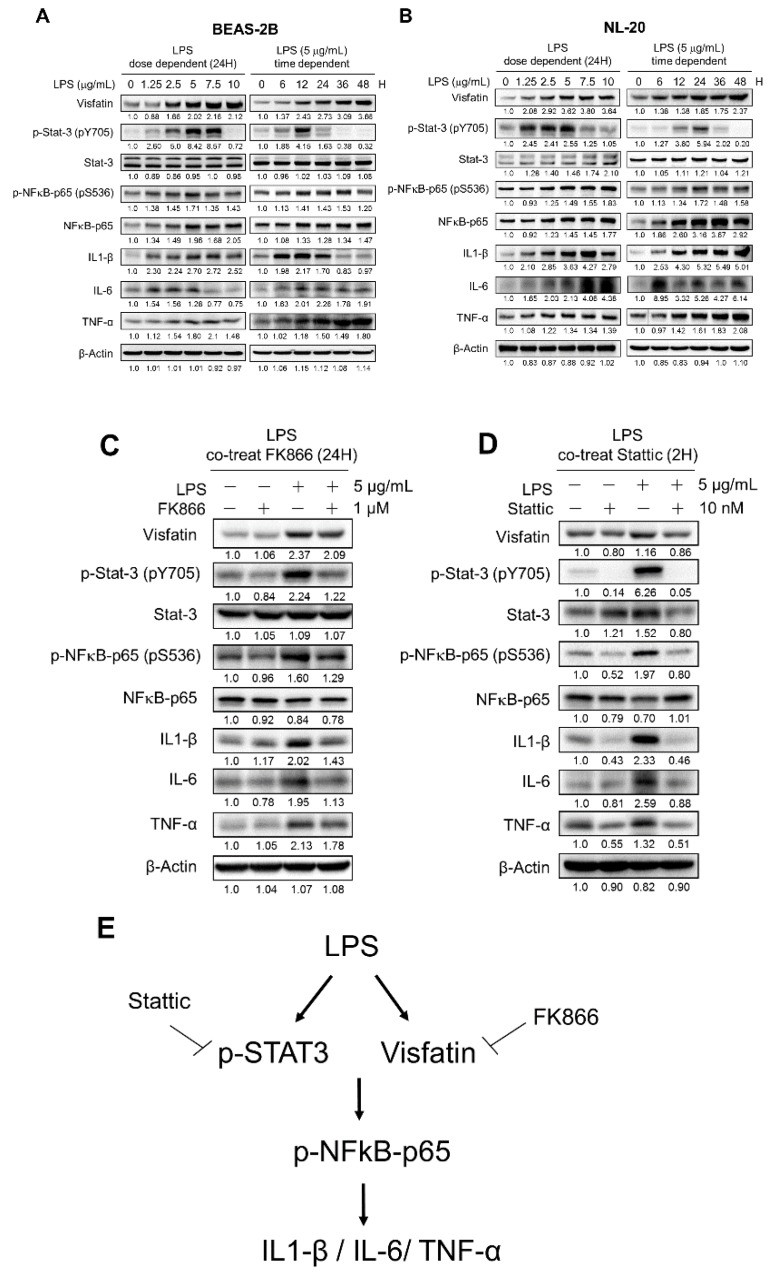
Given the results that visfatin was elevated in both ALI patients and LPS and CASP mouse models, we further investigated the underlying mechanisms using human bronchial epithelial cell lines BEAS-2B and NL-20. After LPS treatment, visfatin expression increased in dose- and time-dependent manners (Figure 4A,B).
Figure 4.
Visfatin increased the expression of pro-inflammatory cytokines through STAT/NF-κB pathway in human bronchial epithelial cells. (A,B) Dose- and time-dependent effects of LPS on the expression of visfatin, STAT3, NFκB, and pro-inflammatory cytokines in human bronchial epithelial cell lines BEAS-2B and NL-20. (C) Co-treatment of FK866 and LPS on the expression of visfatin, STAT3, NFκB, and pro-inflammatory cytokines. (D) Co-treatment of STAT3 inhibitor static and LPS on the expression of visfatin, STAT3, NFκB, and pro-inflammatory cytokines. (E) Schematic representation of the LPS-induced signaling pathway in human bronchial epithelial cells.
To gain insight into the signal pathways involved in the regulation of visfatin in ALI, the activity of STAT3 and NF-kB in bronchial epithelial cells was examined. Western blot result showed that, upon LPS treatment, the expression of p-STAT3(Y705) was upregulated in dose- and time-dependent manners (Figure 4A,B). We further tested whether visfatin regulates the NF-κB activity in bronchial epithelial cells. As expected, the expression of S536-phosphorylated/activated p65 (a subunit of NF-κB) was increased upon LPS treatment (Figure 4A,B). Furthermore, the increased visfatin expression upon LPS treatment was correlated with the elevated expression of inflammatory cytokines, including IL-1β, TNF-α, and IL-6 (Figure 4A,B).
To further confirm that visfatin indeed activates STAT3 and NF-κB, we co-treated FK866 with LPS in BEAS-2B. Co-treatment of FK866, which decreased visfatin expression, also led to a decrease in activation of STAT3 and NF-κB, as well as a decrease in their downstream effectors, including IL1-β, IL-6, and TNF-α (Figure 4C). Next, we tested whether STAT3 regulates NF-κB activity by co-treatment of static, a STAT3 inhibitor, with LPS. As shown in Figure 4D, co-treatment of static and LPS decreased the expression of p-NFκB-p65 and downstream inflammatory cytokines, including IL1-β, IL-6, and TNF-α (Figure 4D). Together, these results support that LPS upregulates visfatin expression and leads to an activated STAT3/NF-κB pathway and down-stream inflammatory cytokine, like IL1-β, IL-6, and TNF-α.
3. Discussion
The pathophysiological mechanisms of acute lung injury are complex and not well understood yet. In this study, we applied LPS and CASP mouse models and a LPS cell model to address the molecular mechanisms behind ALI. Our data clearly demonstrated that visfatin induction activated the STAT3/NFκB pathway and downstream inflammatory cytokines in LPS and CASP-induced ALI. Besides, we provide evidence that inhibition of visfatin activity by a pharmacologic approach (FK866) reverses the inflammatory process caused by LPS and CASP, and improves survival in LPS and CASP mouse models. A schematic representation for LPS-induced signaling pathway in human bronchial epithelial cells was shown in Figure 4E.
In this study, we observed that serum visfatin levels were positively correlated with the serum levels of pro-inflammatory cytokines IL-6, IL-8, IL-10, and MCP-1, not IL1-β or TNF-α, in ALI patients. However, LPS treatment promoted the expression of visfatin and pro-inflammatory cytokines IL1-β, IL-6, and TNF-α in human bronchial epithelial cell lines (Figure 4A,B). While we do not have the answer for this discrepancy in the patterns of pro-inflammatory cytokines in vivo and in vitro, it might be caused by the difference between the complex clinical manifestations in ALI and the over-simplified LPS and CASP models. Another possibility is the involvement of other pathways, than visfatin, in the induction of pro-inflammatory cytokines in ALI patients. For example, NF-κB transcriptional activities and inflammatory lung injury are induced by visfatin via its direct binding to Toll–like receptor 4 (TLR4) and; therefore, visfatin can serve as an innate immunity molecule capable of activating TLR4 in the absence of LPS, which delineates a novel dimension to the induction of lung inflammatory responses by non-infectious mechanisms [45].
In our bronchial epithelial cell model for LPS, we observed the sequential changes in visfatin elevation, STAT/NF-κB activation, and the increase of pro-inflammatory cytokines, which were reversed by co-treatment of FK866 (Figure 4). Moreover, FK866 treatment alleviated the inflammatory changes in the lung tissues and improved the survival in the LPS- and CASP-treated mice (Figure 2 and Figure 3). A recent report also showed that FK866 treatment protects intestinal integrity and reduces bacteremia via the NF-κB-mediated pathway [38]. A NAMPT inhibitor analog, meta-carborane-butyl-3-(3-pyridinyl)-2E-propenamide (MC-PPEA, MC4), was synthesized by replacing the benzoylpiperidine moiety in FK866 with meta-carborane [46]. MC4 also showed inhibitory effects on cecal ligation and puncture (CLP)-induced mortality, TNFα levels, pulmonary myeloperoxidase activity, alveolar injury, and IL-6 and IL-1β messenger RNA levels [47]. Taken together, we discovered that LPS or CASP induced visfatin protein expression and ensured STAT3 or NF-κB activation and induced downstream inflammatory cytokines expression, and this was further confirmed in the cell model (Figure 4).
In conclusion, our study provides in vitro and in vivo evidence for the involvement of visfatin and down-stream events in the pathogenic changes in acute lung injury. The clinical application of FK866 in ALI patients, especially those with elevated visfatin locally and systemically, should be explored to confirm whether the anti-visfatin approaches can improve the inflammatory changes and survival in ALI patients.
4. Materials and Methods
4.1. Patient Enrollment
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital. Patients with sepsis and visiting the Emergency Department of Kaohsiung Medical University Hospital were sequentially enrolled between 2013 and 2014. Patients received treatments and examinations according to emergency physicians’ evaluation. After the patients agreed and provided informed consent, 10 mL of blood were collected for cytokine analysis. Serum was separated by centrifugation at 1600× g for 20 min at 4 °C, divided in aliquots and immediately frozen (−80 °C) until further analysis. Clinical information including the patients’ age, gender, microbiological culture results, laboratory data at the Emergency Department, and diagnosis were evaluated by clinicians and recorded by a research nurse. Patients meeting criteria for both sepsis [48] and ALI [49] were enrolled. Thirty-two adult healthy persons, who came to the hospital for their routine healthy examination, were enrolled as a control group.
4.2. Reagents
Antibody against p-Stat-3(pS727)(sc-8001) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against p-Stat-3(pY705)(#9145), p-NFκB-p65(pS536)(#8242), and NFκB -p65(#3033) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against Stat-3(GTX15523), IL1-β(GTX22105), TNF-α(GTX110520), and IL-6 (GTX110527) were purchased from GeneTex, Inc. (Irvine, CA, USA). Antibody against visfatin(ab58640) was purchased from Abcam plc. (Cambridge, UK). Antibody against β-actin(A5411) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). LHC-9 medium was purchased from Invitrogen (Carlsbad, CA, USA). FK866, lipopolysaccharide, hematoxylin, and eosin were purchased from Sigma-Aldrich (St Louis, MO, USA).
4.3. Cell Culture
The human bronchial epithelial cell lines, NL-20 (BCRC) and BEAS-2B (ATCC), were cultured in LHC-9 medium (Invitrogen, NY, USA) supplemented with 5% fetal bovine serum, 100 U/mL penicillin, and 100 pg/mL streptomycin (Invitrogen) in a humidified incubator with 5% CO2 at 37 °C.
4.4. Animal Model of Abdominal Sepsis
Eight-week-old BALB/C mice were purchased from Lasco, Taiwan. The technique used for induction of colon ascendens stent peritonitis (CASP) was performed as reported recently [50]. A 16-gauge venous catheter was prepared by creating a notch at a distance of 2 mm from the orifice. One millimeter beyond, the catheter was cut following laparotomy. The colon ascendens was exteriorized and a 7/0 ethilon thread (Ethicon, Nordersted, Germany) was stitched through the antimesenteric portion of the colon ascendens, about 10 mm distal to the ileocecal valve. The 16-gauge venous catheter was punctured antimesenterically through the colonic wall into the intestinal lumen, directly proximal of a pre-tied knot, fixed, and the second puncture was performed directly proximal of the stent, again strictly antimesenterically. Then, the inner needle was removed and the stent was cut at the prepared site. To ensure proper intraluminal positioning of the stent, stool was milked from the cecum into the colon ascendens, until a small drop appeared (1 mm in diameter). Fluid resuscitation of animals was performed by flushing 0.5 mL sterile saline solution into the peritoneal cavity before closure of the abdominal wall. After operation, mice were intraperitoneally injected with or without 20 mg/kg of FK866 in 100 µL normal saline every 3 h.
For the LPS experiment, 20 mg/kg of LPS and/or 20 mg/kg of FK866 were injected intraperitoneally. The animal study was approved by the Institutional Animal Care and Use Committee (IACUC No.104056) of Kaohsiung Medical University.
4.5. Immunoblotting Analysis
The whole cell extracts were collected by lysing cells in the buffer containing 50 mM HEPES, 6 mM EDTA, and 1% Triton X-100 supplemented with phosphatase inhibitor (Sigma, St. Louis, MO, USA) and complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Total protein was separated by centrifugation at 12000× g for 20 min at 4 °C. The protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA), with bovine serum albumin as a standard. Equal amounts of proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Pall Corporation, East Hills, NY, USA). The chemiluminescent signal was captured by a ChemiDocTM XRS+ System (Bio-Rad Laboratories, Hercules, CA, USA) and quantified with Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA).
4.6. Enzyme Immunoassay
Serum visfatin levels for mouse and human were measured in duplicate by a visfatin-specific enzyme immunoassay kit, RayBiotech (Norcross, GA, USA) and BioVendor (Brno, Czech Republic), respectively, according to the manufacturer’s instructions.
4.7. Immunohistochemistry
Immunohistochemical staining for visfatin and IL-6 was performed on an automated Bond-Max instrument (Leica Microsystems, Wetzlar, Germany). Slides carrying tissue sections cut from formalin-fixed and paraffin-embedded tissue blocks were dried for 1 h at 60 °C. These slides were then covered by Bond Universal Covertiles and placed into the Bond-Max instrument. All subsequent steps were performed by the automated instrument as follows: (1) Deparaffinization of tissues on the slides by rinsing with Bond Dewax Solution at 72 °C; (2) heat-induced epitope retrieval (antigen unmasking) with Bond Epitope Retrieval Solution 1 for 20 min at 100 °C; (3) peroxide block placement on the slides for 5 min at room temperature; (4) incubation with rabbit antibody at a dilution of 1:200 for 30 min at room temperature; (5) Bond Polymer placement on the slides for 8 min at room temperature; (6) color development with DAB (3,3’-diaminobenzidine tetrahydrochloride) as a chromogen for 5 min at room temperature; and (7) hematoxylin counter-staining for 5 min, followed by mounting of the slides and then examination by light microscopy. The pictures were captured by a Nikon E-800M microscope (Tokyo, Japan). For quantification, the staining of visfatin, p-STAT, and NFκB was scored using the method of histochemical score (H-score) [51], which was calculated as the product of percentage of stained cells and intensity of staining
4.8. Statistical Analysis
Data were given as mean ± SD and all experiments were repeated at least three times independently. All statistical analyses were performed using the SPSS 20.0 statistical software for PC (SPSS, Chicago, IL, USA) and p values less than 0.05 were considered statistically significant. Statistical analysis between the different groups was performed using two-sided Student’s t-test. Spearman’s correlation coefficient was used to determine the relationship between variables. One-way ANOVA, combined with Tukey’s multiple-comparison test, was used to evaluate the statistical significance of differences between three or more groups.
Acknowledgments
All sources of funding of the study should be disclosed. Please clearly indicate grants that you have received in support of your research work. Clearly state if you received funds for covering the costs to publish in open access.
Abbreviations
| ALI | Acute lung injury |
| H&E | Hematoxylin and eosin |
| LPS | Lipopolysaccharide |
| ARDS | Acute respiratory distress syndrome |
| PBEF | Pre-B-cell colony-enhancing factor |
| Nampt | Nicotinamide phosphoribosyltransferase |
| MCP-1 | Monocyte chemotactic protein-1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| CASP | Colon ascendens stent peritonitis |
| TLR4 | Toll–like receptor 4 |
Author Contributions
Conceptualization, Y.-C.L., C.-Y.L., and S.-S.F.Y.; methodology, C.-Y.L., Y.-H.C., W.-C.C., Y.-Y.W., Y.-H.S., and S.-S.F.Y.; software, Y.-C.L. and S.-S.F.Y.; validation, C.H. and S.-S.F.Y.; formal analysis, Y.-C.L., W.-C.C., Y.-Y.W., Y.-H.S., and S.-S.F.Y.; investigation, Y.-C.L., C.-Y.L., W.-C.C., Y.-Y.W., S.C.-S.H., and S.-S.F.Y.; resources, W.-C.C. and Y.-H.S.; writing—original draft preparation, Y.-C.L., C.-Y.L., S.C.-S.H., and S.-S.F.Y.; writing—review and editing, C.-Y.L., C.H., S.C.-S.H., and S.-S.F.Y.; visualization, S.-S.F.Y.; supervision, Y.-H.C., C.H., and S.-S.F.Y.
Funding
This research was financially supported by the grants from Ministry of Science and Technology (107-2314-B-037-120, 107-2314-B-037 -097 -MY2), Kaohsiung Medical University Hospital (KMUH106-R41), Kaohsiung Medical University (KMU-DK107005, KMU-DK108005), and the “Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B)” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Matthay M.A., Zimmerman G.A. Acute lung injury and the acute respiratory distress syndrome: Four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortelliti M.P., Manning H.L. Acute respiratory distress syndrome. Am. Fam. Phys. 2002;65:1823–1830. [PubMed] [Google Scholar]
- 3.Matthay M.A., Zimmerman G.A., Esmon C., Bhattacharya J., Coller B., Doerschuk C.M., Floros J., Gimbrone M.A., Jr., Hoffman E., Hubmayr R.D., et al. Future research directions in acute lung injury: Summary of a National Heart, Lung, and Blood Institute working group. Am. J. Respir. Crit. Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. New Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Goss C.H., Brower R.G., Hudson L.D., Rubenfeld G.D., Network A. Incidence of acute lung injury in the United States. Crit. Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 6.Vasudevan A., Lodha R., Kabra S.K. Acute lung injury and acute respiratory distress syndrome. Indian J. Pediatrics. 2004;71:743–750. doi: 10.1007/BF02730667. [DOI] [PubMed] [Google Scholar]
- 7.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. New Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 9.Luh S.P., Chiang C.H. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): The mechanism, present strategies and future perspectives of therapies. J. Zhejiang Univ. Sci. B. 2007;8:60–69. doi: 10.1631/jzus.2007.B0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson E.R., Matthay M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calfee C.S., Matthay M.A. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz J.V., Brower R., Calfee C.S., Matthay M.A. Therapeutic strategies for severe acute lung injury. Crit. Care Med. 2010;38:1644–1650. doi: 10.1097/CCM.0b013e3181e795ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrera G., Landoni V., Martire-Greco D., Chiarella P., Meiss R., Gomez S.A., Alves-Rosa F., Rearte B., Isturiz M., Palermo M.S., et al. Model of polymicrobial peritonitis that induces the proinflammatory and immunosuppressive phases of sepsis. Infect. Immun. 2011;79:1280–1288. doi: 10.1128/IAI.01127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feterowski C., Emmanuilidis K., Miethke T., Gerauer K., Rump M., Ulm K., Holzmann B., Weighardt H. Effects of functional Toll-like receptor-4 mutations on the immune response to human and experimental sepsis. Immunology. 2003;109:426–431. doi: 10.1046/j.1365-2567.2003.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons P.E. Mediators and mechanisms of acute lung injury. Clin. Chest Med. 2000;21:467–476. doi: 10.1016/s0272-5231(05)70159-3. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia M., Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 17.Herold S., Gabrielli N.M., Vadasz I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am. J. Physiology. Lung Cell. Mol. Physiol. 2013;305:L665–L681. doi: 10.1152/ajplung.00232.2013. [DOI] [PubMed] [Google Scholar]
- 18.Samal B., Sun Y., Stearns G., Xie C., Suggs S., McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Liu P., Cepeda J., Fang D., Easley R.B., Simon B.A., Zhang L.Q., Ye S.Q. Augmentation of Pulmonary Epithelial Cell IL-8 Expression and Permeability by Pre-B-cell Colony Enhancing Factor. J. Inflamm. 2008;5:15. doi: 10.1186/1476-9255-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitani T., Okuno S., Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 21.Jia S.H., Li Y., Parodo J., Kapus A., Fan L., Rotstein O.D., Marshall J.C. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Investig. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Z., Lei H., Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013;24:433–442. doi: 10.1016/j.cytogfr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonoli S.S., Shivprasad S., Prasad C.V., Patil A.B., Desai P.B., Somannavar M.S. Visfatin—A review. Eur. Rev. Med Pharmacol. Sci. 2011;15:9–14. [PubMed] [Google Scholar]
- 24.Revollo J.R., Grimm A.A., Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 25.Rongvaux A., Shea R.J., Mulks M.H., Gigot D., Urbain J., Leo O., Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:113.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Ognjanovic S., Bryant-Greenwood G.D. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am. J. Obstet. Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara A., Matsuda M., Nishizawa M., Segawa K., Tanaka M., Kishimoto K., Matsuki Y., Murakami M., Ichisaka T., Murakami H., et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 28.Moschen A.R., Kaser A., Enrich B., Mosheimer B., Theurl M., Niederegger H., Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 29.Ye S.Q., Zhang L.Q., Adyshev D., Usatyuk P.V., Garcia A.N., Lavoie T.L., Verin A.D., Natarajan V., Garcia J.G. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc. Res. 2005;70:142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Nowell M.A., Richards P.J., Fielding C.A., Ognjanovic S., Topley N., Williams A.S., Bryant-Greenwood G., Jones S.A. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: Implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 31.Dahl T.B., Yndestad A., Skjelland M., Oie E., Dahl A., Michelsen A., Damas J.K., Tunheim S.H., Ueland T., Smith C., et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 32.Hong S.B., Huang Y., Moreno-Vinasco L., Sammani S., Moitra J., Barnard J.W., Ma S.F., Mirzapoiazova T., Evenoski C., Reeves R.R., et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye S.Q., Simon B.A., Maloney J.P., Zambelli-Weiner A., Gao L., Grant A., Easley R.B., McVerry B.J., Tuder R.M., Standiford T., et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am. J. Respir. Crit. Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 34.Manicone A.M. Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev. Clin. Immunol. 2009;5:63–75. doi: 10.1586/177666X.5.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M.H., Tsai C.H., Huang Y.L., Fong Y.C., Tang C.H. Visfatin Promotes IL-6 and TNF-alpha Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018;19:190. doi: 10.3390/ijms19010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustig M.K., Bac V.H., Pavlovic D., Maier S., Grundling M., Grisk O., Wendt M., Heidecke C.D., Lehmann C. Colon ascendens stent peritonitis—A model of sepsis adopted to the rat: Physiological, microcirculatory and laboratory changes. Shock. 2007;28:59–64. doi: 10.1097/SHK.0b013e31802e454f. [DOI] [PubMed] [Google Scholar]
- 37.Traeger T., Koerner P., Kessler W., Cziupka K., Diedrich S., Busemann A., Heidecke C.D., Maier S. Colon ascendens stent peritonitis (CASP)—A standardized model for polymicrobial abdominal sepsis. J. Vis. Exp. JoVE. 2010 doi: 10.3791/2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda A., Yang W.L., Jacob A., Aziz M., Matsuo S., Matsutani T., Uchida E., Wang P. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-kappaB pathway. Ann. Surg. 2014;259:1007–1017. doi: 10.1097/SLA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 39.Schuster S., Penke M., Gorski T., Gebhardt R., Weiss T.S., Kiess W., Garten A. FK866-induced NAMPT inhibition activates AMPK and downregulates mTOR signaling in hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 2015;458:334–340. doi: 10.1016/j.bbrc.2015.01.111. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.Y., Bae Y.H., Bae M.K., Kim S.R., Park H.J., Wee H.J., Bae S.K. Visfatin through STAT3 activation enhances IL-6 expression that promotes endothelial angiogenesis. Biochim. Biophys. Acta. 2009;1793:1759–1767. doi: 10.1016/j.bbamcr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Bi T.Q., Che X.M. Nampt/PBEF/visfatin and cancer. Cancer Biol. Ther. 2010;10:119–125. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- 42.Hung A.C., Lo S., Hou M.F., Lee Y.C., Tsai C.H., Chen Y.Y., Liu W., Su Y.H., Lo Y.H., Wang C.H., et al. Extracellular Visfatin-Promoted Malignant Behavior in Breast Cancer Is Mediated Through c-Abl and STAT3 Activation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:4478–4490. doi: 10.1158/1078-0432.CCR-15-2704. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Zhang Y., Dorweiler B., Cui D., Wang T., Woo C.W., Brunkan C.S., Wolberger C., Imai S., Tabas I. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 2008;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oita R.C., Ferdinando D., Wilson S., Bunce C., Mazzatti D.J. Visfatin induces oxidative stress in differentiated C2C12 myotubes in an Akt- and MAPK-independent, NFkB-dependent manner. Pflug. Arch. Eur. J. Physiol. 2010;459:619–630. doi: 10.1007/s00424-009-0752-1. [DOI] [PubMed] [Google Scholar]
- 45.Camp S.M., Ceco E., Evenoski C.L., Danilov S.M., Zhou T., Chiang E.T., Moreno-Vinasco L., Mapes B., Zhao J., Gursoy G., et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFkappaB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015;5:13135. doi: 10.1038/srep13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee M.W., Jr., Sevryugina Y.V., Khan A., Ye S.Q. Carboranes increase the potency of small molecule inhibitors of nicotinamide phosphoribosyltranferase. J. Med. Chem. 2012;55:7290–7294. doi: 10.1021/jm300740t. [DOI] [PubMed] [Google Scholar]
- 47.Huang P., Lee M.W., Jr., Sadrerafi K., Heruth D.P., Zhang L.Q., Maulik D., Ye S.Q. MC-PPEA as a new and more potent inhibitor of CLP-induced sepsis and pulmonary inflammation than FK866. Drug Des. Dev. Ther. 2017;11:629–641. doi: 10.2147/DDDT.S125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubenfeld G.D., Herridge M.S. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L., Tian Y., Yang J., Li J., Tang H., Wang Y. Colon Ascendens Stent Peritonitis (CASP) Induces Excessive Inflammation and Systemic Metabolic Dysfunction in a Septic Rat Model. J. Proteome Res. 2018;17:680–688. doi: 10.1021/acs.jproteome.7b00730. [DOI] [PubMed] [Google Scholar]
- 51.Debaugnies F., Mahadeb B., Ferster A., Meuleman N., Rozen L., Demulder A., Corazza F. Performances of the H-Score for Diagnosis of Hemophagocytic Lymphohistiocytosis in Adult and Pediatric Patients. Am. J. Clin. Pathol. 2016;145:862–870. doi: 10.1093/ajcp/aqw076. [DOI] [PubMed] [Google Scholar]