Abstract
In this work, we explore the current knowledge about the phytochemistry and in vitro and in vivo evaluations of the extracts and, where appropriate, the main active components characterized and isolated from the Allamanda cathartica. Of the 15 Allamanda species, most phytochemical, pharmacological, and toxicological studies have focused on A. cathartica. These plants are used for the treatment of various health disorders. Numerous phytochemical investigations of plants from the A. cathartica have shown the presence of hydrocarbons, alcohols, esters, ethers, aldehydes, ketones, fatty acids, phospholipids, volatile compounds, phenolic compounds, flavonoids, alkaloids, steroids, terpenes, lactones, and carbohydrates. Various studies have confirmed that extracts and active substances isolated from the A. cathartica have multiple pharmacological activities. The species A. cathartica has emerged as a source of traditional medicine used for human health. Further studies on the phytochemical, pharmacological, and toxicological properties and their mechanisms of action, safety, and efficacy in the species of A. cathartica is recommended.
Keywords: Allamanda cathartica, phytochemistry, pharmacology, toxicology and biotechnology
1. Introduction
The plant Allamanda is a very widespread group throughout the world. It belongs to the family Apocynaceae and, according to the “The Plant List,” contains approximately 15 species (A. augustifolia, A. blanchetti, A. caccicola, A. cathartica, A. doniana, A. laevis, A. martii, A nobilis, A. oenotherifolia, A. polyantha, A. puberula, A. schottii, A. setulosa, A. thevetifolia, and A. weberbaueri) [1]. The objective of this work is to present complete information about the current research on the distribution, phytochemistry, pharmacology, toxicity, and biotechnology of Allamanda cathartica; to identify its therapeutic potential; and to direct future research opportunities. The most relevant data were searched using the keyword “Allamanda cathartica” in “Google Scholar”, “PubMed”, “ScienceDirect”, “Scopus”, “Taylor & Francis”, “Web of Science”, and “Wiley”. The taxonomy was validated using the “The Plant List”.
2. Ethnobotany
2.1. Botanical Characterization
The genus Allamanda is endemic to South America [2]. The genus is named after the Swiss botanist Jean Frédéric-François Louis Allamand, who collected seeds in Suriname and sent them to Carlos Linnaeus to be named in 1771 [3]. A. cathartica plants are robust shrubs growing up to 6 m tall. The leaves are elliptical to obovate, opposite, or in whorls. The flowers are yellow and trumpet-shaped, with corolla tubes. The flowers are similar in size to the leaves. The fruits are capsules with spins, and the seeds are compressed and winged. The shrubs, with their beautiful yellow flowers, are popular ornamentals [4]. The species flowers grow all year round, and fruits grow from April to July and in October. In botanical texts, A. cathartica is reported to have a wide global distribution in warm climates (Figure 1) [2]. Based on these data, a more exhaustive analysis of the scientific literature was performed.
Figure 1.
Allamanda cathartica.
2.2. Distribution
A. cathartica plants are distributed in tropical and subtropical areas of many countries, including the United States, México, Belize, Honduras, Nicaragua, Costa Rica, Panama, Venezuela, Bolivia, Ecuador, Guyana, French Guyana, Paraguay, Peru [2], Guatemala [5], El Salvador [6], Puerto Rico [7], Trinidad and Tobago [8], Surinam [9], Cuba [10], Martinique [11], Colombia [12], Brazil [3], Hawaii [13], India [14], the Andaman islands [15], Bangladesh [16], Pakistan [17], Malaysia [18], Indonesia [19], The Philippines [20], Thailand [21], Singapore [22], Hong Kong [14], Myanmar [11], Nepal, Sri Lanka [23], China [24], Australia [25], Kuwait [26], Ghana [18], the Republic of Mauritius [27], Cameroon, Madagascar [2], Nigeria [28] Zimbabwe [29], and France [20].
2.3. Synonyms
Synonyms of Allamanda cathartica include Echites verticillata Sessé and Moç, Orelia grandiflora Aublet, Allamanda grandiflora (Aublet) Poiret in Lam, and Allamanda hendersonii W. Bull ex Dombrain [30], as well as Allamanda schotti (Pohl) [31]. In the various countries where Allamanda is found, other popular names have been attributed to it.
The following are synonyms: (in Australia) Allamanda [25]; (in Bangladesh) Allamanda [32], Allokananda [23], and Fok Kaia [33]; (in Brazil) Buiussu, Carolina [34], Alamanda, Cipó-de-leite, Dedal-de-dama, Alamanda-amarela, Alamanda-de-flor-grande, Guissú, Quatro-patacas-amarelas [35], Golden trumpet, Yellow Bell, and Buttercup flower [30]; (in Cuba) Flor de barbero, Barbero loco, Flor de mantequilla, Jazmín de la tierra [10], and Jazmín de Cuba [36]; (in El Salvador) San José [6,37]; (in France) Jasmin dÁmarilla [20]; (in French Guiana) Orélie de la Guyana [20]; (in Guatemala) Amanda, Butter cup, and Campana [5]; (in Hawaii) Lani-ali’I and Allamanda [13]; (in India) Jaharisontakka, Pilikaner, Pivikanher [20], Almanda, golden trump vine, [38], Haldhia phool [39], Ghonta phool [40], and Golden trumpet [41]; (in Indonesia) Bunga Terompet [16]; (in Malaysia) Jamaican sunset [42]; (in Mexico) Berta, Cuernos de chivo, Chicliyo [2], and San José [6,37]; (in Nigeria) Allamonda, Yellow allamanda, Golden trumpet [43], Nkutu [44], and Ako-dodo [45]; and (in Thailand) Golden trumpet [21].
2.4. Traditional Medical Use
In traditional medicine, A. cathartica is indicated for various treatments in many parts of the world: as an antifungal (United States, Caribe [3], and Bangladesh [23]), antiviral (United States and Caribbean [3]), anticancer (Malaysia [46]), and cathartic (India [20] and Bangladesh [23]) or to treat colic (India [47]) or diabetes (India [48]). It is also used as a diuretic and an emetic (India [38]); for the treatment of fever (India [39] and Brazil [34]), hydragogue ascites (India [20] and Bangladesh [23]), hypertension (the Philippines [49] and Bangladesh [23]); to improve blood circulation (Indonesia [16]); and to reduce inflammation (Nigeria [43]). It is also used to treat jaundice (Suriname [8], Brazil [34], and Malaysia [46]), laxative (India [38], Suriname [8], and Nigeria [44]), and Malaria (Nigeria [45], Suriname, [8], Philipphines [20], Malaysia [46], and Brazil [34]). The milky sap is used for lead colic (Mexico and El Salvador [36]), parasitosis (Brazil [34]), rheumatism (Bangladesh [33]), scabies and lice elimination (Brazil [34]), snake bites (Bangladesh [23], Colombia [12], and India [20]), and splenomegaly (Suriname [8] and Brazil [34]). The plant parts used most frequently, in decreasing order, are the leaves, stem bark, flowers, roots, stem, sap, seeds, and branches.
3. Phytochemistry
The chemical constituents of A. cathartica have been extensively studied since 1954 [14]. Preliminary chemical studies showed the presence of alkaloids [13], anthraquinones [50], anthocyanins [51], carbohydrates [52], carotenoids [21], coumarin [53], flavonoids [54], glycosides [28], hydrocarbon [52], lignin [51], lipids [50,52], phenolic compounds [54], quinones [53], saponins [28,54], steroids [54], tannins [28,54], and terpenes [53,54] from various extracts, mainly leaves, flowers, stems, stem bark, roots, and shoots.
Only these groups of chemical compounds have been isolated and identified, and no anthraquinones, anthocyanins, coumarin, quinones, or lignins have been found. The Marvin program was used to draw the structures of organic chemical compounds [55].
In an analysis of the inorganic composition by atomic absorption spectrophotometry from flowers, the following elements were detected at the following concentrations: Fe (12.21 ± 0.038 µg/g), Mn (1.338 ± 0.049 µg/g), Ni (0.593 ± 0.014 µg/g), Cu (0.348 ± 0.006 µg/g), Cr (0.181 ± 0.032 µg/g), Pb (0.104 ± 0.024 µg/g), and Co (0.089 ± 0.010 µg/g) [56].
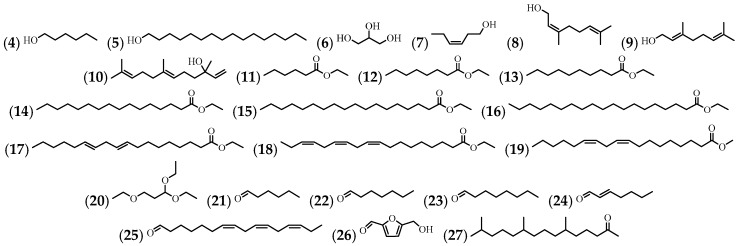
3.1. Hydrocarbons
The presence of 3 hydrocarbons has been confirmed in A. cathartica flowers (Table 1 and Figure 2).
Table 1.
The hydrocarbons from A. cathartica.
Figure 2.
The structures of the hydrocarbons from A. cathartica.
3.2. Alcohol, Ester, Ether, Aldehyde, and Ketone
Seven alcohol compounds were identified, as well as 9 esters, 1 ether, 6 aldehydes, and 1 ketone in various extracts of flowers, leaves, and stems (Table 2 and Figure 3).
Table 2.
The alcohols, esters, ethers, aldehydes, and ketones from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (4) | 1-Hexanol | Flowers | [10] |
| (5) | 1-Hexadecanol | Flowers | [10] |
| (6) | Glycerin | Leaves and stem | [57] |
| (7) | (Z)-3-Hexenol | Flowers | [10] |
| (8) | Nerol | Flowers | [35] |
| (9) | Geraniol | Flowers | [35] |
| (10) | (E)-Nerolidol | Flowers | [35] |
| (11) | Hexanoic acid, ethyl ester | Leaves and stem | [57] |
| (12) | Octanoic acid, ethyl ester | Leaves and stem | [57] |
| (13) | Decanoic acid, ethyl ester | Leaves and stem | [57] |
| (14) | Hexadecanoic acid, ethyl ester | Leaves and stem | [57] |
| (15) | Octadecanoic acid, ethyl ester | Leaves and stem | [57] |
| (16) | Nonadecanoic acid, ethyl ester | Leaves | [57] |
| (17) | 9,12-Octadecadienoic acid, ethyl ester | Leaves and stem | [57] |
| (18) | 9,12,15-octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | Leaves and stem | [43,57] |
| (19) | Methyl linoleate | Flowers | [10] |
| (20) | Propane, 1,1,3-triethoxy- | Leaves and stem | [57] |
| (21) | Hexanal | Flowers | [10] |
| (22) | Heptanal | Flowers | [10] |
| (23) | Octanal | Flowers | [10] |
| (24) | (E)-2-Heptenal | Flowers | [10] |
| (25) | Cis,cis,cis-7,10,13-hexadecatrienal | Leaves | [57] |
| (26) | 2-furancarboxaldehyde, 5-(hydroxymethyl)- | Stem | [57] |
| (27) | 6,10,14-Trimethyl-2-pentadecanone | Flowers | [10] |
Figure 3.
The structures of the alcohols, esters, ethers, aldehydes, and ketones from A. cathartica.
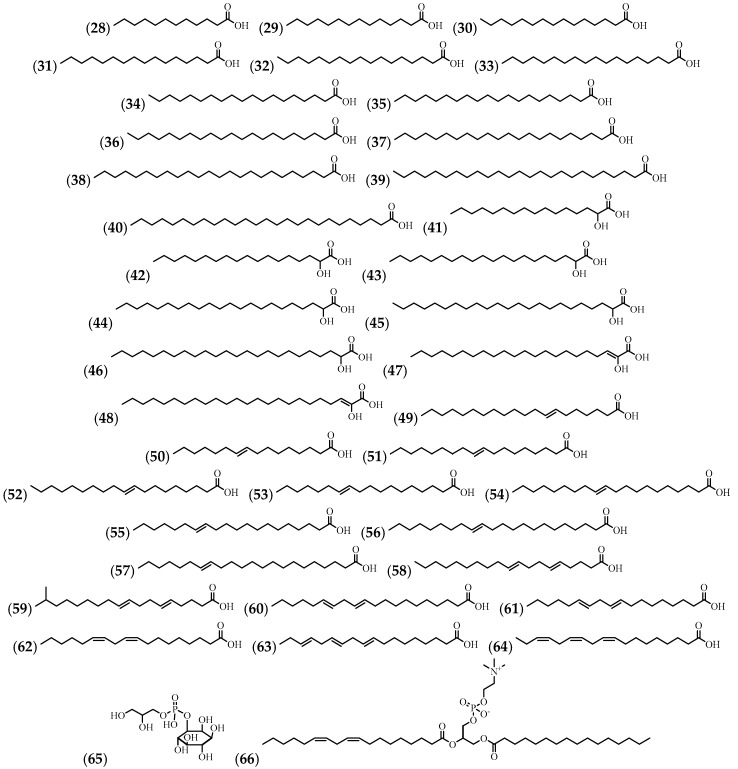
3.3. Fatty Acids and Phospholipids
A fatty acid composition analysis resulted in the identification of 37 compounds and a compound of very unusual structure (59). Two phospholipids were also identified. The flowers, leaves, and stems were used for the isolation of these compounds (Table 3 and Figure 4).
Table 3.
The fatty acids and phospholipids from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (28) | Dodecanoic acid | Flowers, leaves, and stem | [52,57] |
| (29) | Tetradecanoic acid | Flowers, leaves, and stem | [7,52,57] |
| (30) | Pentadecanoic acid | Leaves and flowers | [7,57] |
| (31) | Hexadecanoic acid | Flowers, leaves, and stem | [7,43,52,57] |
| (32) | Heptadecanoic acid | Flowers | [7] |
| (33) | Octadecanoic acid | Flowers and leaves | [7,52] |
| (34) | Nonadecanoic acid | Flowers | [7] |
| (35) | Eicosanoic acid | Flowers and leaves | [7,52] |
| (36) | Heneicosanoic acid | Flowers | [7] |
| (37) | Docosanoic acid | Flowers | [7] |
| (38) | Tetracosanoic acid | Flowers | [7] |
| (39) | Pentacosanoic acid | Flowers | [7] |
| (40) | Hexacosanoic acid | Flowers | [7] |
| (41) | 2-Hydroxyhexadecanoic acid | Flowers | [7] |
| (42) | 2-Hydroxyoctadecanoic acid | Flowers | [7] |
| (43) | 2-Hydroxyeicosanoic acid | Flowers | [7] |
| (44) | 2-Hydroxydocosanoic acid | Flowers | [7] |
| (45) | 2-Hydroxytricosanoic acid | Flowers | [7] |
| (46) | 2-Hydroxytetracosanoic acid | Flowers | [7] |
| (47) | 2-Hydroxydocosenoic acid | Flowers | [7] |
| (48) | 2-Hydroxytetracosenoic acid | Flowers | [7] |
| (49) | 7-Eicosenoic acid | Flowers | [7] |
| (50) | 9-Hexadecenoic acid | Flowers | [7] |
| (51) | 9-Octadecenoic acid | Flowers, leaves, and stem | [7,52,57] |
| (52) | 9-Nonadecenoic acid | Flowers | [7] |
| (53) | 11-Octadecenoic acid | Flowers | [7] |
| (54) | 11-Eicosenoic acid | Flowers | [7] |
| (55) | 13-Eicosenoic acid | Flowers | [7] |
| (56) | 13-Docosenoic acid | Flowers | [7] |
| (57) | 15-Docosenoic acid | Flowers | [7] |
| (58) | 5,9-Nonadecadienoic acid | Flowers | [7] |
| (59) | 17-Methyl-5,9-octadecadienoic acid * | Flowers | [7] |
| (60) | 11,14-Eicosadienoic acid | Flowers | [7] |
| (61) | 9,12-Octadecadienoic acid | Flowers and leaves | [7,52] |
| (62) | 9,12-Octadecadienoic acid (Z,Z)- | Stem | [57] |
| (63) | 9,12,15-Octadecatrienoic acid | Flowers | [7] |
| (64) | 9,12,15-Octadecatrienoic acid (Z,Z,Z)- | Leaves and Stem | [44,57] |
| (65) | Phosphatidylinositol | Flowers | [7] |
| (66) | Phosphatidycholine | Flowers | [7] |
Note: * Not reported in nature.
Figure 4.
The structures of the fatty acids and phospholipids from A. cathartica.
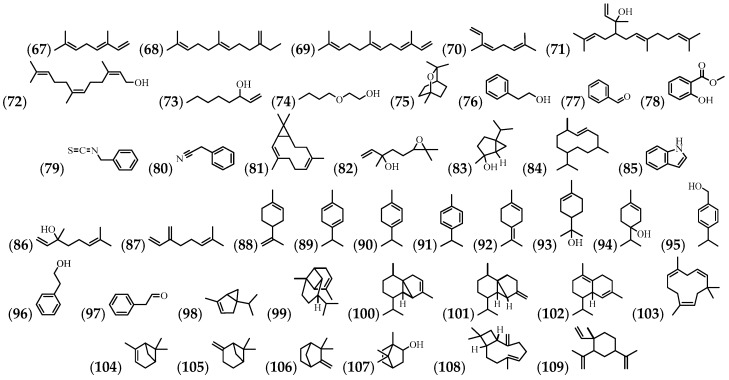
3.4. Volatile Compounds
A total of 43 volatile compounds have also been identified, mostly in flowers and leaves (Table 4 and Figure 5).
Table 4.
The volatile compounds from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (67) | (E)-β-ocineme | Flowers | [10] |
| (68) | (E)-β-Farnesene | Flowers | [10] |
| (69) | (E,E)-α-Farnesene | Flowers | [10] |
| (70) | (Z)-β-ocimene | Flowers | [10] |
| (71) | (E,E)-Geranyl linaool | Flowers | [10] |
| (72) | (Z,Z)-Farnesol | Flowers | [10] |
| (73) | 1-Octen-3-ol | Flowers | [10] |
| (74) | 2-Butooxyethanol | Flowers | [10] |
| (75) | 1,8-cineole | Flowers | [10] |
| (76) | 2-Phenylethanol | Flowers | [10] |
| (77) | Benzaldehyde | Flowers | [10] |
| (78) | Benzoic acid, 2-hydroxy-, methyl ester | Leaves | [57] |
| (79) | Benzyl isothiocyanate | Flowers | [35] |
| (80) | Phenylacetonitrile | Flowers | [35] |
| (81) | Bicyclogermacrene | Flowers | [35] |
| (82) | Trans-Linalool oxide | Flowers | [35] |
| (83) | Cis-sabinehydrate | Flowers | [10] |
| (84) | Germacrene D | Flowers | [35] |
| (85) | Indole | Flowers | [10] |
| (86) | Linalool | Flowers | [35] |
| (87) | Myrcene | Flowers | [10] |
| (88) | Limonene | Flowers | [10] |
| (89) | γ-Terpinene | Flowers | [10] |
| (90) | α-Terpinene | Flowers | [10] |
| (91) | p-cyneme | Flowers | [10] |
| (92) | Terpinolene | Flowers | [10] |
| (93) | α-Terpineol | Flowers | [10,35] |
| (94) | Terpinen-4-ol | Flowers | [10] |
| (95) | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | N.R. | [58] |
| (96) | Cumin alcohol | Flowers | [35] |
| (97) | Phenylacetaldehyde | Flowers | [10,35] |
| (98) | α-Thujene | Flowers | [10] |
| (99) | α-Copaene | Flowers | [35] |
| (100) | α-Cubebene | Flowers | [35] |
| (101) | β-Cubebene | Flowers | [35] |
| (102) | δ-Cadinene | Flowers | [35] |
| (103) | α-Humulene | Flowers | [35] |
| (104) | α-Pinene | Flowers | [10] |
| (105) | β-Pinene | Flowers | [10] |
| (106) | Camphene | Flowers | [10] |
| (107) | Isoborneol | Flowers | [10] |
| (108) | β-Caryophyllene | Flowers | [10,35] |
| (109) | β-Elemene | Flowers | [35] |
Note: N.R. = Not reported.
Figure 5.
The structures of the volatile compounds from A. cathartica.
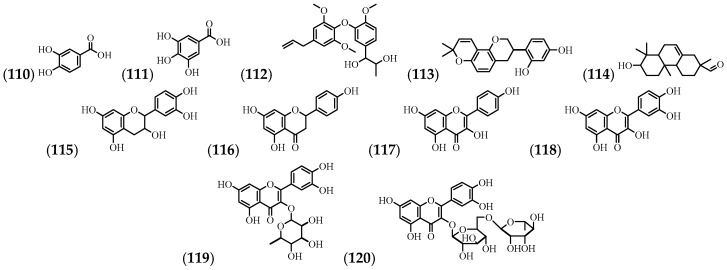
3.5. Phenolic Compounds and Flavonoids
From the flowers and stems, 5 phenolic compounds and 6 flavonoids have been identified (Table 5 and Figure 6).
Table 5.
The phenolic compounds and flavonoids from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (110) | Protocatechuic acid | Flowers | [24] |
| (111) | Gallic acid | Flowers | [24] |
| (112) | 1-(3-(4-Allyl-2,6-dimethoxyphenoxy)-4-methoxyphenyl)propane-1,2,diol | Stem | [59] |
| (113) | Glabridin | Stem | [59] |
| (114) | 2-phenanthrenecarboxaldehyde, 1,2,3,4,4a,4b,5,6,7,8,8a,9-dodecahydro-7-hydroxy-2,4b,8,8-tetramethyl- | Leaves and stem | [57] |
| (115) | Epicatechin | Flowers | [24] |
| (116) | Naringenin | Stem | [59] |
| (117) | Kaempferol | Stem | [59] |
| (118) | Quercetin | Flowers | [60] |
| (119) | Quercitrin | Flowers | [60] |
| (120) | Rutin | Flowers | [61] |
Figure 6.
The structures of the phenolic compounds and flavonoids from A. cathartica.
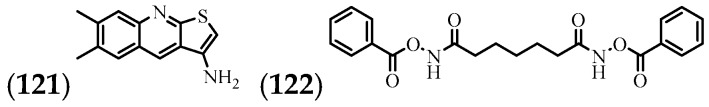
3.6. Alkaloids
Two alkaloids present in the stems are the only ones reported in the literature [38] (Table 6 and Figure 7).
Table 6.
The alkaloids from A. cathartica.
Figure 7.
The structures of the alkaloids from A. cathartica.
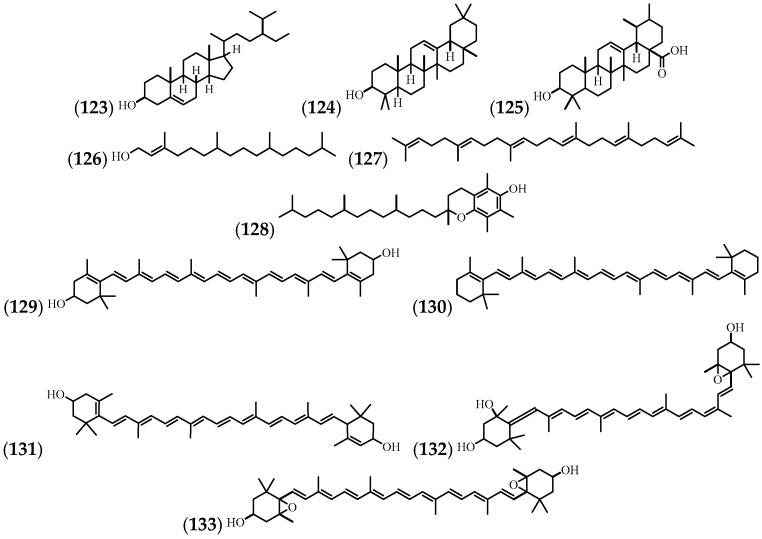
3.7. Steroids and Terpenes
Carotenoids are terpene compounds. They can be yellow, orange, or red in pigment, and they are widely distributed in nature. In plants, they play an important role in photosynthesis and in the colouring of flowers and fruits [62]. A. cathartica carotenoids have been found in flowers, leaves, and stems (Table 7 and Figure 8).
Table 7.
The steroids and terpenes from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (123) | β-sitosterol | Leaves and stem | [63] |
| (124) | β-Amyrin | Leaves and stem | [63] |
| (125) | Ursolic acid | Leaves and stem | [14,63] |
| (126) | Phytol | Flowers, leaves, and stem | [10,57] |
| (127) | Squalene | Leaves | [57] |
| (128) | Vitamine E | Leaves | [57] |
| (129) | Zeaxanthin | Flowers | [21] |
| (130) | b-Carotene | Flowers | [21] |
| (131) | Lutein | Flowers | [21] |
| (132) | Neoxanthin | Flowers | [21] |
| (133) | Violaxanthin | Flowers | [21] |
Figure 8.
The structures of the steroids and terpenes from A. cathartica.
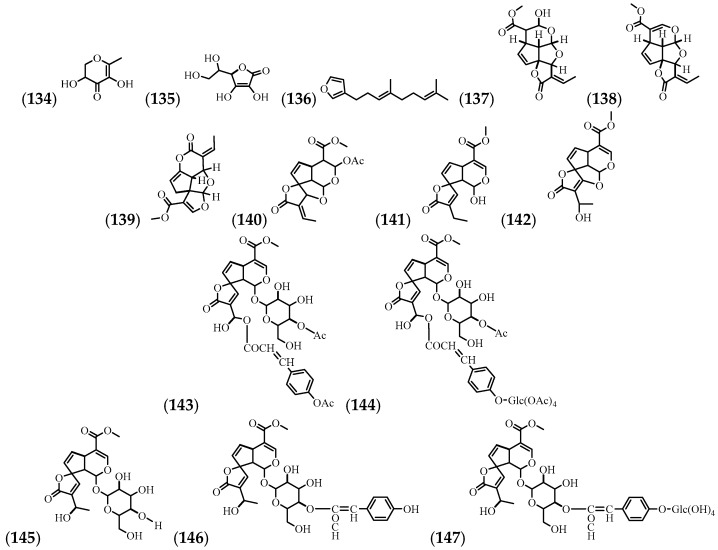
3.8. Lactones
The mechanisms for recovering compound (145) from ethanol and ethyl acetate extracts have been established, with ethanol showing the greatest yield [64]. The most commonly used plant parts for the isolation and identification of compounds are flowers, roots, leaves, root bark, and bark (inner part) (Table 8 and Figure 9).
Table 8.
The lactones from A. cathartica.
| No. | Compound’s Name | Parts Used | Reference |
|---|---|---|---|
| (134) | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | Leaves and stem | [57] |
| (135) | Vitamine C | Leaves | [14] |
| (136) | Dendrolasin | Flowers | [35] |
| (137) | Allamandin | Root bark | [65] |
| (138) | Plumericin | Leaves, root, stem, leaves, flowers, bark, and root bark | [9,18,65,66] |
| (139) | Isoplumericin | Leaves, root, root bark, stem, and bark | [9,18,65,66] |
| (140) | Acetylallamandin | Root bark | [65] |
| (141) | Allamdin | Root bark | [65] |
| (142) | Allamandicin | Root bark | [65] |
| (143) | Penta-acetylplumieride coumarate | Root | [66] |
| (144) | Octa-acetylplumieride coumarate | Root | [66] |
| (145) | Plumieride | Root, stem, leaves, flowers, bark, and bark (inner part) | [18] |
| (146) | Plumieride coumarate | Root, stem, leaves, flowers, bark, and bark (inner part) | [18,66] |
| (147) | Plumieride coumarate glucoside | Root, stem, leaves, flowers, bark, and bark (inner part) | [18,66] |
Figure 9.
The structures of the lactones from A. cathartica.
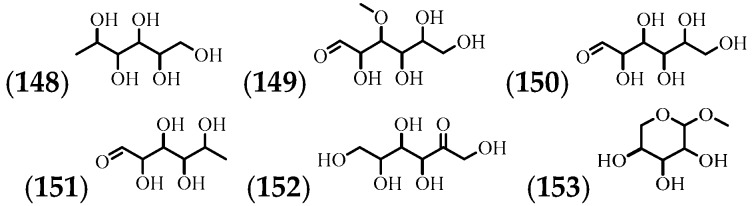
3.9. Carbohydrates
The presence of 6 carbohydrates in the leaves, stems, and nectar has been shown (Table 9 and Figure 10).
Table 9.
The carbohydrates from A. cathartica.
Figure 10.
The structures of the carbohydrates from A. cathartica.
4. Pharmacological Activity
A. cathartica has been reported in traditional medicine, and the first biological and pharmacological studies were documented in 1943 [68]. A more general view of the pharmacological investigations on various crude extracts and isolated chemical compounds of the species are described below.
4.1. Analgesic
In a previous study conducted in our laboratory, it was observed that the ethanol extract from the aerial parts of A. cathartica showed an analgesic activity in the murine model.
4.2. Anti-Inflammatory
The inhibition of haemolysis in human erythrocytes by an aqueous fraction from a methanol extract was evaluated, with rates of 69.49 ± 0.49% compared to the positive control acetyl salicylic acid (0.1 mg/mL), which showed a 72.79% inhibition [69]. In another study, the compound (119) obtained from fresh A. cathartica flowers was evaluated for anti-inflammatory activity using an in vitro haemolytic membrane stabilization study. The effect of inflammation was studied using erythrocytes exposed to a hypotonic solution. The results indicated that the obtained compound showed a membrane stabilizing activity, which was highest with 75 µg [70]. In an in vivo model, the compound (145) from a flower ethanol extract was evaluated for activity against ulcerative colitis induced by dextran sulfate sodium (DSS) in female mice. As a standard control, 5-Amino-Salicylic Acid was used, and the mice were administered either compound at the same dose (100 mg/kg/day for 7 days). Treatment with the (145) compound resulted in less shortening of the colon, improved histological damage, and less mucin depletion of the intestinal mucosa compared to the group only treated with the vehicle [71].
4.3. Antidepressant
The antidepressant activity of the compound (145) was evaluated in Swiss Webster female mice (0.5, 1, and 2 μg/kg i.p). Doses of 1 and 2 μg/kg showed a significant difference p < 0.001 with respect to the negative control. Imipramide (20 mg/kg i.p.) was used as a positive control [61].
4.4. Antidiabetic
Aqueous extracts from the aerial parts of A. cathartica (400 mg/kg for 28 days) reduced blood glucose levels in diabetic rats with streptozotocin, compared to glibenclamide (5 mg/kg) as a standard, with a statistical significance p < 0.001 [48].
4.5. Antihyperlipidaemic
An ethanolic flower extract of A. cathartica (100, 150, and 300 mg/kg, p.o.) and the compound (145) (0.5, 1, and 2 mg/kg, i.p.) decreased the total and High Density Lipoprotein (HDL) cholesterol levels, with significant differences of p < 0.001 and p < 0.05, respectively, in female Swiss Webster mice at the two highest doses tested [61].
4.6. Antifertility
The oral administration of aqueous leaf extracts of A. cathartica (150 mg/kg/day for 14, 28, and 42 days) induced infertility and changes in various male reproductive endpoints in Parkes strain mice. Histologically, the testes from the extract-treated mice showed nonuniform degenerative changes in the seminiferous. The treatment also had adverse effects on motility, viability, morphology, and the number of spermatozoa in the cauda epididymides. The fertility of the extract-treated males was also suppressed [72]. The oral administration of (145) (15 mg/rat/day for 60 days) in male Wistar rats significantly reduced the weight of the testes, epididymides, seminal vesicles, and prostate compared to the negative controls, and the mobility of the sperm and Sertoli cells also decreased significantly and without systemic side effects. The number of mature Leydig cells was decreased, and a complete suppression of fertility was observed. The content of protein and sialic acid in the testes, epididymides, seminal vesicle, and prostate, as well as the glycogen content of the testes and fructose in the seminal vesicles were reduced. However, testicular cholesterol was elevated [73].
4.7. Wound Healing
Aqueous leaf extracts of A. cathartica (150 mg/kg/day for 14 days) promoted the wound healing activity in Sprague–Dawley rats. Compared to the controls, treated rats had higher rates of wound contraction, decreased periods of epithelialisation, a higher skin breaking strength, a significantly higher weight of the granulation tissue, and more hydroxyproline content. Histological studies of the granulation tissue in treated rats showed less inflammatory cells and increased collagen formation [8].
4.8. Thrombolysis
A. cathartica leaves were extracted with methanol and subsequently partitioned with hexane, carbon tetrachloride, chloroform, and water. The thrombolytic activity of the resulting preparation was evaluated in vitro with the concentration of extract at 0.1 mg/100 μL. As a positive control, streptokinase was used. All extracts showed thrombolytic activity with respect to the negative control with a significant difference of p < 0.001. The chloroform-partitioned extract presented the highest rate of clot lysis (34.51%) [30].
4.9. Purgative Effect
The purgative effect of the aqueous leaf extract of A. cathartica was evaluated at different doses (20, 40, 80, 160, and 320 mg/kg orally). As a positive control, the Senna extract was used under the same conditions and the saline solution was used as a negative control; the extract showed a dose-dependent effect [28].
4.10. Tyrosinase
The tyrosinase inhibitory activity of the methanol stem powder extracts of A. cathartica was examined, and compound (113) was identified as having the highest inhibitory activity against tyrosinase (IC50: 2.93 μM), which was 15 times stronger than the kojic acid used as a positive control (IC50: 43.7 μM) [59].
4.11. Amylase
In leaves extracted with ethanol 50% (v/v), Allotides were identified as being proline-rich and having an α-amylase inhibitory activity [22].
4.12. Antiviral
Through an in silico method, it was determined that some compounds present in A. cathartica have an antiviral activity against human hepatitis B viral capsid protein [58]. The antirabic activity of methanol and aqueous extracts of leaves was evaluated; however, the extracts did not inhibit the rabies virus at the concentrations evaluated [31].
4.13. Antimicrobial
The methods most commonly used to evaluate antimicrobial activity are carried out by plaque, disk, and dilution methods. Table 10 describes the different studies carried out with extracts obtained from different parts of A. cathartica.
Table 10.
The effect of A. cathartica extract on a microorganism.
| Microorganism | Used Part | Extract/Fraction | Reference |
|---|---|---|---|
| Gram Positive | |||
| Agrobacterium tumefaciens | Flowers and leaves | Bound and free flavonoids, steroids, and alkaloids | [74] |
| Bacillus cereus | Leaves | TCM | [75] |
| EtOAc | [69] | ||
| MeOH, PE, TCM, EtOAc, and Dia-Ion | [76] | ||
| Bacillus megaterium | Leaves | TCM | [75] |
| EtOAc | [69] | ||
| Bacillus subtilis | Flowers and Leaves | Bound and free flavonoids and steroids | [74] |
| Leaves | TCM | [75] | |
| Water * | [77] | ||
| Sarcina lutea | Leaves | TCM | [75] |
| Staphylococcus aureus | Flowers | Water * | [78] |
| MeOH 90% | [79] | ||
| Flowers and leaves | Free flavonoids, alkaloids, bound flavonoids, and steroids | [74] | |
| Leaves | MeOH, PE, TCM, EtOAc, and Dia-Ion | [76] | |
| TCM | [77] | ||
| Root | MeOH, EtOAc, and PE | [80] | |
| All plant | N.E. | [68] | |
| Staphylococcus aureus ** | Leaves | MeOH, EtOH, EtOAc, TCM, and PE | [81] |
| Streptococcus pneumonia | Root | MeOH, EtOAc | [80] |
| Gram Negative | |||
| Acinetobacter baumannii ** | Flowers | EtOH | [82] |
| Acinetobacter sp ** | Leaves | MeOH, EtOH, EtOAc, Water, and PE | [81] |
| Bacillus subtillis | Leaves | Bound flavonoids | [74] |
| Escherichia coli | Flowers | Water * | [78] |
| Flowers and leaves | Bound flavonoids and steroids | [74] | |
| Flowers | MeOH 90% | [79] | |
| Leaves | TCM | [75] | |
| MeOH, PE, TCM, EtOAc, and Dia-Ion | [76] | ||
| Root | EtOAc | [80] | |
| Escherichia coli ** | Leaves | Water and PE | [81] |
| Water | [32] | ||
| Klebsiella pneumoniae | Root | MeOH and EtOAc | [80] |
| Flowers | Water * | [78] | |
| Flowers and leaves | Bound and free flavonoids | [74] | |
| Leaves | Water * | [77] | |
| Klebsiella pneumoniae ** | Leaves | Water | [32] |
| Proteus mirabilis ** | Leaves | Water | [32] |
| Proteus sp ** | Leaves | PE | [81] |
| Proteus vulgaris | Leaves | MeOH, PE, TCM, EtOAc, and Dia-Ion | [76] |
| Pseudomonas aeruginosa | Leaves | TCM | [75] |
| Water * | [77] | ||
| Pseudomonas aeruginosa ** | Leaves | Water | [32] |
| MeOH, EtOAc, TCM, and PE | [81] | ||
| Salmonella paratyphi | Leaves | TCM | [75] |
| EtOAc | [69] | ||
| Salmonella typhi | Leaves | TCM | [75] |
| EtOAc | [69] | ||
| Salmonella typhimurium | Flowers | Water * | [78] |
| Shigella boydii | Leaves | TCM | [75] |
| Shigella dysenteriae | Leaves | TCM | [75] |
| Vibrio mimicus | Leaves | TCM | [75] |
| Vibrio parahemolyticus | Leaves | TCM | [75] |
| Fungi | |||
| Aspergillus flavus | Leave and Flowers | MeOH | [83] |
| Aspergillus flavus | Leaves | MeOH:Water (2:1 v/v) | [84] |
| Water * | [77] | ||
| Aspergillus niger | Leaves | TCM | [75] |
| Water * | [77] | ||
| Candida albicans | Leaves | EtOH 99.8% | [85] |
| TCM | [75] | ||
| MeOH | [34] | ||
| Leave and Flowers | MeOH | [83] | |
| Flowers | MeOH 90% | [79] | |
| Candida albicans ** | Leaves | EtOH | [81] |
| Carvularia lunata | Leaves | PE and TCM | [40] |
| Epidermophyton floccosum | Leaves | MeOH | [86] |
| Microsporum gypseum | Leaves | MeOH | [86] |
| Pityrosporum ovale | Leaves | EtOH 99.8% | [85] |
| Sacharomyces cerevaceae | Leaves | TCM | [75] |
| Plant Fungi | |||
| Colletotrichum gloeosporioides | Leaves | TCM | [42] |
| Colletotrichum lidemuthianum | Leaves | PE and TCM | [40] |
| Curvularia luunata | Leaves | Water * | [77] |
| Fusarium oxysporum | Leaves | PE and TCM | [40] |
| MeOH, EtOH, EtOAc, and EtOH 50% | [87] | ||
| Fusarium oxysporum f.sp. capsici | Leave | MeOH | [16] |
| Phomopsis vexans | Leaves | MeOH, EtOH, EtOAc, and EtOH 50% | [87] |
| Phytophthora capsici | Leaves | MeOH, EtOH, EtOAc, and EtOH 50% | [87] |
| Rhizopus arrhizus | Leaves | Water * | [77] |
| Rhizotonia solani | Leaves | MeOH, EtOH, EtOAc, and EtOH 50% | [87] |
| Sclerotium rolsfsii | Leaves | MeOH, EtOH, EtOAc, and EtOH 50% | [87] |
Note: * Used with silver nanoparticles (AgNPs), ** Clinical isolates, TCM = Chloroform, PE = Petroleum ether, MeOH = Methanol, EtOH = Ethanol, and EtOAc = Ethyl acetate.
4.14. Antimalarial
In an in vivo model in albino rats, the antimalarial activity of a leaf ethanol extract from A. cathartica was evaluated at different doses (50, 100, and 200 mg/mL). As a positive control, the compound (128) was used (200 mg/kg), and the extract showed an effect similar to (128) that was dose-dependent [88].
4.15. Nematicide
Bark methanol extracts were evaluated on Bursaphelenchus xylophilus (pinewood nematode), where a minimum effective dose (MED) of 5 mg/cotton ball was found [19]. Fractions of hexane extracts of the leaves and stem from A. cathartica were evaluated in vitro for nematicidal activity at 0.06, 0.1, and 0.2 mg/mL against juvenile larvae of Meloidogyne incognita. The extract showed a nematicidal activity from the first hours of exposure with a rate of 16.87% [89].
4.16. Pesticidal
Aqueous extracts of leaves and flowers from A. cathartica showed pesticidal properties against Oligonychus coffeae [90]. Extractions using petroleum ether, chloroform, and methanol showed pesticidal effects on Tribolium castaneum exposed for 24, 48, and 72 h. The LD50 values at these time points were 684,376, 319,028, and 225,205 μg/cm2 for petroleum ether; 34,289.35, 4,308,567, and 804,082 μg/cm2 for chloroform; and 445,092.10, 38,709.10, and 9,906.21 μg/cm2 for methanol, respectively [76].
4.17. Antihaemorrhagic
Extracts of 96% ethanol made from the leaves, branches, and stems of A. cathartica were evaluated for an in vitro haemorrhagic neutralization activity using the blood of a Swiss Webster mouse with 10 μg Bothrops atrox venom, and the results obtained showed a neutralization of 72 ± 8%. However, it was not clear if the parts of the plant were evaluated together or separately [12].
4.18. Cytotoxicity
The methanolic extract and subsequent fractions (methanol, chloroform, hexane, and carbon tetrachloride) from A. cathartica leaves were evaluated for their toxic effects on brine shrimp. The chloroform-, hexane-, and carbon tetrachloride-soluble fractions showed a significant cytotoxic activity against nauplii brine shrimp, with LC50 values of 1.45, 5.00, and 5.24 μg/mL, respectively [30]. The methanol and aqueous extracts of leaves at concentrations of 10, 5, 2.5, 1.25, and 0.6 mg/mL did not show a cytotoxic activity on BHK-21 cells [31]. In another study of methanol extracts from leaves, an IC50 of 85 μg/mL was found for P388 leukaemia cells [86]. The use of silver nanoparticles (AgNO3) with aqueous latex extracts of A. cathartica showed a dose-dependent effect against human mononuclear blood cells [91]. The methanol, ethyl acetate, petroleum ether, and chloroform extracts from leaves of A. cathartica showed LD50 values of 111.61, 131.14, 332.42, and 47.86 μg/mL, respectively, against Artemia salina [76]. Compounds (142), (139), and (138) obtained from 95% ethanol leaf extracts showed a significant tumour suppression in vitro against human nasopharnyx carcinoma (KB) cells with an LD50 of 2.1, 2.6, and 2.7 μg/mL, respectively [65].
4.19. Antioxidants
The antioxidant activity of A. cathartica was evaluated in vitro using the FRAP and TEAC methods with Methanol:Acetic acid:Water extracts (50:3.7:46.3 v/v/v) as well as the water-soluble and fat-soluble fraction from flowers, which showed antioxidant activities via FRAP of 18.95 ± 0.34 and 4.56 ± 0.11 μmol Fe (II)/g, respectively. By the TEAC method, the antioxidant activity was 7.35 ± 0.26 and 1.46 ± 0.21 μmol Trolox/g, respectively [24]. The ethanol extracts from the leaves had an antioxidant activity (based on the DPPH method) that was dose-dependent at concentrations of 0.5, 1, 2, and 5 mg/mL [92]. The methanol extracts from the flowers showed an antioxidant activity by the DPPH method at a concentration of 0.6 mg/mL [93]. Different plant parts were analysed for their antioxidant activity in vitro where it was higher in shoot > root > leaves > flowers. The relative peroxidase and superoxide dismutase (SOD) activities were in the order of root > shoot > leaves > flowers [17]. The relative in vitro antioxidant activity of various leaf extracts of A. cathartica was in the following order: butylated hydroxyl toluene (BHT) > Dia-Ion resin Absorbed > Chloroform > Ethyl acetate (EtOAc) > Methanol (MeOH) > Petroleum ether (PE) [76]. The carbon tetrachloride fraction from a methanol extract from the leaves had an IC50 of 47.5 ± 0.11 μg/mL in the DPPH model [69]. In the study of isolated compounds, (145) (100 mg/kg orally) administered to female Swiss mice significantly decreased the levels of lipid hydroperoxides (LOOH) and reduced the glutathione (GSH) levels and SOD activity, whereas the catalase (CAT) activity remained unchanged compared with the untreated group. The standard drug 5-ASA reduced the LOOH content and increased the SOD activity compared to the vehicle (VEH) group, whereas treatment with (145) promoted a complete improvement of the oxidative unbalance, restoring all the parameters [71]. In an in vivo model using albino rats, the antioxidant activity of the ethanol extract of leaves (50, 100, and 200 mg/mL) was evaluated, and as a positive control, the compound (128) was used (200 mg/kg), showing a significant increase in TBARS, with a decrease in GSH and CAT levels [88].
5. Toxicity
A. cathartica is reported to be a venomous plant due to the presence of a cardiotoxic glycoside [25]. All parts of the plant cause dermatitis [29]. It has been reported that the leaves and sap produce persistent diarrhoea with high consumption rates. Also, skin irritation has been reported, but the responsible compounds have not been identified [3]. Studies have been carried out on the cytotoxicity and genotoxicity of hexane extracts of leaves of A. cathartica. It was demonstrated that a concentration of 315 mg/mL is cytotoxic to lymphocytes with a 79% cellular viability. In HeLa cells, an IC50 of 13.5 mg/mL was found. These results showed a genotoxicity (p < 0.01) for both cell types, which led the authors to suggest that A. cathartica not be used as a medicinal plant [94]. However, it is necessary to standardize the HPLC samples for at least one compound present in the plant. In the evaluation of acute toxicity (i.p.) in mice, it was observed that the LD50 was 1320 ± 15 mg/kg [28]. The oral administration of 2 mg/kg of ethanolic extract of flowers and the compound (145) in Swiss Webster mice administered as a single dose and evaluated at 14 days showed no toxic effects, no changes in biochemical or haematological parameters, and no genotoxic effects [61]. The toxicological evaluation of the petroleum ether extract of leaves in albino mice showed no toxicity at doses of 100 to 1000 mg/kg p.o. for 72 h [81].
6. Biotechnological Use
The effects of 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (BAP) on the induction of callus from leaf and stem explants were investigated. The regeneration of plants from the nodal explants was achieved. The explants were cultured in a Murashige and Skoog (MS) medium, supplemented with different concentrations of 2,4-D (0.5 and 1.0 mg/L) or in combinations of 2,4-D (0.5, 1.0, and 1.5 mg/L) with BAP (0.5, 1.0, and 1.5 mg/L). In the study of plant regeneration, the nodal explants were cultivated in an MS medium supplemented with BAP at 1.0, 3.0, or 5.0 mg/L for the multiplication of shoots. The MS basal medium was used as a control and was also used for the elongation of the shoots. All cultures were incubated under a photoperiod of 16 h of light and 8 h of darkness. For callus induction, the explants of leaves and stems grown at 1.0 mg/L of 2,4-D and 1.0 mg/L of BAP gave the best callus response (100%). For the multiplication of shoots, the MS medium supplemented with 5 mg/L of BAP gave the best response (100%) with multiple buds formed [46].
7. Conclusions
This review details the ethnomedical, phytochemical, pharmacological, toxicological, and biotechnological uses of A. cathartica. Although there have been several studies on the pharmacological activity of A. cathartica, the potential of this plant is as an analgesic, anti-inflammatory, antidepressant, antidiabetic, antihyperlipedaemic, antifertility agent, wound healing, trombolytic, purgative, tyrosine, amylase, antimicrobial, antimalarial, nematicide, antioxidant, etc. agent.
Author Contributions
All authors contributed to this work, prepared the manuscript, and approved this version of the article.
Funding
This work was supported by Secretaría de Educación Publica (SEP-PROMEP) and Consejo Nacional de Ciencia y Tecnología (CONACyT), México under number ON.551–6/18–7513.
Conflicts of Interest
The authors declare that they have no conflict of interest. The funding sponsors contributed the scholarship payment and had no role in the study design, collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.The Plants List Allamanda [Internet] [(accessed on 15 February 2019)];2019 Available online: http://www.theplantlist.org./tpl1.1/search?q=Allamanda.
- 2.Monroy-Ortiz C., Monroy R. Las Plantas, Compañeras de Siempre: La Experiencia en Morelos. UAEM, Centro de Investigaciones Biológicas de la CONABIO CONANP; Cuernavaca, Mexico: 2006. 582p [Google Scholar]
- 3.David W.N. Poisonous Plants and Animals of Florida and the Caribbean. Sing Cheong Print Co Ltd.; Hong Kong, China: 1997. p. 138. [Google Scholar]
- 4.Wong S.K., Lim Y.Y., Chan E.W.C. Botany, uses, phytochemistry and pharmacology of selected Apocynaceae species: A review. Pharmacogn. Commun. 2013;3:2. [Google Scholar]
- 5.Morales J. La familia Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana Nueva Ser. 2009;47:140–184. [Google Scholar]
- 6.Morales J. Estudios en las Apocynaceae Neotropicales XXVIII: La familia Apocynaceae (Apocynoideae, Rauvolfioideae) de El Salvador, Centroamérica. Darwiniana Nueva Ser. 2006;44:453–489. [Google Scholar]
- 7.Carballeira N.M., Cruz C. 5,9-Nonadecadienoic acids in Malvaviscus arboreus and Allamanda cathartica. Phytochemistry. 1998;49:1253–1256. doi: 10.1016/S0031-9422(98)00111-3. [DOI] [Google Scholar]
- 8.Nayak S., Nalabothu P., Sandiford S., Bhogadi V., Adogwa A. Evaluation of wound healing activity of Allamanda cathartica L. and Laurus nobilis L. extracts on rats. BMC Complement. Altern. Med. 2006;6:12. doi: 10.1186/1472-6882-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Kader M.S., Wisse J., Evans R., van der Werff H., Kingston D.G. Bioactive iridoids and a new lignan from Allamanda cathartica and Himatanthus fallax from the Suriname rainforest. J. Nat. Prod. 1997;60:1294–1297. doi: 10.1021/np970253e. [DOI] [PubMed] [Google Scholar]
- 10.Báez D., Pino J.A., Morales D. Scent composition from flowers of Allamanda cathartica L. from Cuba. J Essent. Oil Bear Plants. 2012;15:12–14. [Google Scholar]
- 11.Warrell D.A. Researching nature’s venoms and poisons. Trans. R. Soc. Trop. Med Hyg. 2009;103:860–866. doi: 10.1016/j.trstmh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Otero R., Nunez V., Barona J., Fonnegra R., Jimenez S.L., Osorio R.G., Saldarriaga M., Diaz A. Snakebites and ethnobotany in the northwest region of Colombia. Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol. 2000;73:233–241. doi: 10.1016/S0378-8741(00)00321-4. [DOI] [PubMed] [Google Scholar]
- 13.Swanholm C.E., St John H., Scheuer P.J. A survey for alkaloids in Hawaiian plants. I. Pac. Sci. 1959;8:295–300. [Google Scholar]
- 14.Arthur H.R., Hui W.H. Products from some plants of Hong Kong. J. Chem. Soc. 1954:2782–2784. doi: 10.1039/jr9540002782. [DOI] [Google Scholar]
- 15.Manogaran S., Sulochana N. Extraction and characterization of Allamanda cathartica. Asian J. Chem. 2005;17:1955. [Google Scholar]
- 16.Suprapta D.N., Khalimi K. Anti-fungal activities of selected tropical plants from Bali Island. Phytopharmacology. 2012;2:265–270. [Google Scholar]
- 17.Hameed A., Nawaz G., Gulzar T. Chemical composition, antioxidant activities and protein profiling of different parts of Allamanda cathartica. Nat. Prod. Res. 2014;28:2066–2071. doi: 10.1080/14786419.2014.923997. [DOI] [PubMed] [Google Scholar]
- 18.Coppen J.J.W., Cobb A.L. The occurrence of iridoids in Plumeria and Allamanda. Phytochemistry. 1983;22:125–128. doi: 10.1016/S0031-9422(00)80071-0. [DOI] [Google Scholar]
- 19.Alen Y., Nakajima S., Nitoda T., Baba N., Kanzaki H., Kawazu K. Antinematodal activity of some tropical rainforest plants against the pinewood nematode, Bursaphelenchus xylophilus. Z. Naturforsch C. 2000;55:295–299. doi: 10.1515/znc-2000-3-425. [DOI] [PubMed] [Google Scholar]
- 20.Datta S.K., Datta P.C. Pharmacognosy of Allamanda bark drugs. Int. J. Crude Drug. Res. 1982;20:43–52. doi: 10.3109/13880208209083286. [DOI] [Google Scholar]
- 21.Tinoi J., Rakariyatham N., Deming R.L. Determination of major carotenoid constituents in petal extracts of eight selected flowering plants in the north of Thailand. Chiang Mai J. Sci. 2006;33:327–334. [Google Scholar]
- 22.Nguyen P.Q.T., Luu T.T., Bai Y., Nguyen G.K.T., Pervushin K., Tam J.P. Allotides: Proline-rich cystine knot alpha-amylase inhibitors from Allamanda cathartica. J. Nat. Prod. 2015;78:695–704. doi: 10.1021/np500866c. [DOI] [PubMed] [Google Scholar]
- 23.Mahbubur Rahman A.H.M., Akter M. Taxonomy and traditional medicinal uses of apocynaceae (Dogbane) family of Rajshahi district, Bangladesh. Int. J. Bot. Stud. 2016;1:5–13. [Google Scholar]
- 24.Li A.-N., Li S., Li H.-B., Xu D.-P., Xu X.-R., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods. 2014;6:319–330. doi: 10.1016/j.jff.2013.10.022. [DOI] [Google Scholar]
- 25.Radford D.J., Cheung K., Urech R., Gollogly J.R., Duffy P. Immunological detection of cardiac glycosides in plants. Aust. Vet. J. 1994;71:236–238. doi: 10.1111/j.1751-0813.1994.tb03418.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhat N.R., Suleiman M.K., Abdal M. Selection of crops for sustainable utilization of land and water resources in Kuwait. World J. Agric. Sci. 2009;5:201–206. [Google Scholar]
- 27.Gurib-Fakim A., Gueho J., Sewraj-Bissoondoyal M. The medicinal plants of Mauritius—Part 1. Int. J. Pharmacogn. 1997;35:237–254. doi: 10.1076/phbi.35.4.237.13313. [DOI] [Google Scholar]
- 28.Akah P.A., Offiah V.N. Gastrointestinal effects of Allamanda cathartica leaf extracts. Int. J. Pharmacogn. 1992;30:213–217. doi: 10.3109/13880209209054001. [DOI] [Google Scholar]
- 29.Maroyi A. Garden Plants in Zimbabwe: Their ethnomedicinal uses and reported toxicity. Ethnobot. Res. Appl. 2012;10:45–57. doi: 10.17348/era.10.0.045-057. [DOI] [Google Scholar]
- 30.Sarker R., Sharmin T., Chowdhury S.R., Islam F. Thrombolytic activity and preliminary cytotoxicity of five different fractions of methanol extract of Allamanda cathartica leaf. J. Appl. Pharm. Sci. 2012;2:129–132. doi: 10.7324/JAPS.2012.2717. [DOI] [Google Scholar]
- 31.Mehta S., Roy S., Chowdhary A. Use of rapid fluorescent focus inhibition test (RFFIT) for in vitro evaluation of anti-rabies activity. Virus Dis. 2017;28:127–132. doi: 10.1007/s13337-017-0371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashrafuzzaman M., Ali H., Liza L.N., Zinnah K.M.A. Antimicrobial activity of some medicinal plants against multi drug resistant human pathogens. Adv. Biosci. Bioeng. 2013;1:1–24. [Google Scholar]
- 33.Haque M.M., Choudhury M.S., Hossain M.S., Haque M.A., Seraj S., Rahmatullah M. Ethnographic information and medicinal formulations of a Mro community of Gazalia Union in the Bandarbans district of Bangladesh. Am. Eur. J. Sustain. Agric. 2012;6:162–171. [Google Scholar]
- 34.Scio E., Mendes R.F., Motta E.V.S., Bellozi P.M.Q., Aragão D.M.O., Mello J., Fabri R.L., Moreira J.R., de Assis I.V.L., Bouzada M.L.M. Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health. InTech; London, UK: 2012. Antimicrobial and Antioxidant Activities of Some Plant Extracts. [Google Scholar]
- 35.Maia J.G.S., das Zoghbi M.G.B., Andrade E.H.A., Carreira L.M.M. Volatiles from Flowers of Thevetia peruviana (Pers.) K. Schum. and Allamanda cathartics Linn. (Apocynaceae) J. Essent. Oil Res. 2000;1:322–324. doi: 10.1080/10412905.2000.9699526. [DOI] [Google Scholar]
- 36.Hirschhorn H.H. Botanical remedies of south and central America, and the Caribbean: An archival analysis. Part I. J. Ethnopharmacol. 1981;4:129–158. doi: 10.1016/0378-8741(81)90032-5. [DOI] [PubMed] [Google Scholar]
- 37.Hirschhorn H.H. Botanical remedies of South and Central America, and the Caribbean: An archival analysis. Part II. Conclusion. J. Ethnopharmacol. 1982;5:163–180. doi: 10.1016/0378-8741(82)90041-1. [DOI] [PubMed] [Google Scholar]
- 38.Bharath Kumar R., Asha S., Babu B.S. A note on phytodiversity and phytochemistry of important plant species of Vignan University Campus, Vadlamudi, Andhra Pradesh. Int. J. Pharm. Bio-Sci. 2014;5:373–386. [Google Scholar]
- 39.Dutta M.L. Plants used as ethnomedicine by the Thengal Kacharies of Assam, India. Asian J. Plant Sci. Res. 2017;7:7–8. [Google Scholar]
- 40.Singha I.M., Unni B.G., Kakoty Y., Das J., Wann S.B., Singh L., Kalita M.C. Evaluation of in vitro antifungal activity of medicinal plants against phytopathogenic fungi. Arch. Phytopathol. Plant Prot. 2011;44:1033–1040. doi: 10.1080/03235401003672913. [DOI] [Google Scholar]
- 41.Joshi S.C., Sharma A., Chaturvedi M. Antifertility potential of some medicinal plants in males: An overview. Int. J. Pharm. Pharm. Sci. 2011;3:204–217. [Google Scholar]
- 42.Haron F.F., Sijam K., Omar D., Rahmani M. Bioassay-guided isolation of antifungal plumericin from Allamanda species (Apocynaceae) J. Biol. Sci. 2013;13:158–162. [Google Scholar]
- 43.Fasola T.R., Iyamah P.C. The use of ethnobotanicals in the management of inflammation in Nigeria: A review. Int. J. Environ. 2015;4:1–18. doi: 10.3126/ije.v4i2.12620. [DOI] [Google Scholar]
- 44.Nwambie A.I., Akah P.A. Preliminary studies on some Nigerian herbal purgative recipes. Int. J. Pharmacogn. 1993;31:278–282. doi: 10.3109/13880209309082953. [DOI] [Google Scholar]
- 45.Iyamah P.C., Idu M. Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J. Ethnopharmacol. 2015;173:287–302. doi: 10.1016/j.jep.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Wong K.F., Taha R.M. The effect of 2,4-dichlorophenoxyacetic acid and 6-benzylaminopurine on callus induction and plant regeneration of Allamanda cathartica—A valuable medicinal plant. Res. J. Biotecnol. 2012;7:75. [Google Scholar]
- 47.Pawar K.P., Bhitre M.J., Kalamkar P.V., Kale M.K. Pharmacognostical studies on leaves of Allamanda cathartica with detail physicochemical and phytochemical evaluation. Res. J. Pharmacogn. Phytochem. 2015;7:69. doi: 10.5958/0975-4385.2015.00013.8. [DOI] [Google Scholar]
- 48.Chaithra Amin B., Satish S., Abhishek N., Ajay Kumar K. An investigation on anti-diabetic activity in aqueous extract of aerial parts of Allamanda cathartica Linn in streptozotocin induced diabetic rats. Int. J. Pharm. Chem. Res. 2017;3:242–247. [Google Scholar]
- 49.Blasco F.A., De Guzman G.Q., Alejandro G.J.D. A survey of ethnomedicinal plants in Surigao del Sur Mountain Range, Philippines. Int. J. Pure Appl. Biosci. 2014;2:166–172. [Google Scholar]
- 50.Essiett U.A., Udo E. Comparative phytochemical screening and nutritional potentials of the stems, leaves and flowers of Allamanda cathartica (Apocynaceae) Int. J. Sci. Technol. 2015;4:248–253. [Google Scholar]
- 51.Savithramma N., Linga Rao M., Suhrulatha D. Qualitative and quantification analysis of phytochemicals from leaf aqueous extract of Allamanda cathartica L. and Terminalia paniculata Roth. Int. J. 2013;1:821–825. [Google Scholar]
- 52.Augustus G.D.P.S., Seiler G.J. Phytochemicals of selected plant species of the Apocynaceae and Asclepiadaceae from Western Ghats, Tamil Nadu, India. Biomass Bioenergy. 2011;35:3012–3017. doi: 10.1016/j.biombioe.2011.03.043. [DOI] [Google Scholar]
- 53.Joselin J., Brintha T.S.S., Florence A.R., Jeeva S. Screening of select ornamental flowers of the family Apocynaceae for phytochemical constituents. Asian Pac. J. Trop. Dis. 2012;2:S260–S264. doi: 10.1016/S2222-1808(12)60162-5. [DOI] [Google Scholar]
- 54.Mukherjee K., Ray L.N. Phytochemical screening of some Indian medicinal plant species part II. Int. J. Crude Drug Res. 1986;24:187–205. doi: 10.3109/13880208609060898. [DOI] [Google Scholar]
- 55.Marvin MarvinSketch. [(accessed on 18 April 2018)]; Available online: http://www.chemaxon.com.
- 56.Rizvi M.A., Yasmeen K., Ali S.A., Iqbal G. Detection of trace elements in medicinal flowers of Pakistan. Int. J. Adv. Res. 2014;2:195–203. [Google Scholar]
- 57.Prabhadevi V., Sahaya S.S., Johnson M., Venkatramani B., Janakiraman N. Phytochemical studies on Allamanda cathartica L. using GC–MS. Asian Pac. J. Trop. Biomed. 2012;2(Suppl. 2):S550–S554. doi: 10.1016/S2221-1691(12)60272-X. [DOI] [Google Scholar]
- 58.Mathew S., Sreekumar S., Biju C.K. Identification of lead compounds against human hepatitis B viral capsid protein in three medicinal plants through in silico method. IOSR J. Pharm. Biol. Sci. 2016;11:1–6. [Google Scholar]
- 59.Yamauchi K., Mitsunaga T., Batubara I. Isolation, identification and tyrosinase inhibitory activities of the extractives from Allamanda cathartica. Nat. Resour. 2011;2:167. doi: 10.4236/nr.2011.23022. [DOI] [Google Scholar]
- 60.Hema K., Sukumar D. Isolation and phytochemical studies of quercetin and quercetin 3-O-rhamnoside. Int. J. Pharm. Bio-Sci. 2013;4:519–524. [Google Scholar]
- 61.Bonomini T.J., Holzmann I., Thiesen L.C., Fratoni E., Muller A.F.F., Lucinda-Silva R.M., Yunes R.A., Malheiros A., Gonçalves A.E., Dalmagro A.P. Neuropharmacological and acute toxicological evaluation of ethanolic extract of Allamanda cathartica L. flowers and plumieride. Regul. Toxicol. Pharmacol. 2017;91:9–19. doi: 10.1016/j.yrtph.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Ohmiya A. Diversity of carotenoid composition in flower petals. Jpn. Agric. Res. Q. 2011;45:163–171. doi: 10.6090/jarq.45.163. [DOI] [Google Scholar]
- 63.Gupta N.C., Singh B., Bhakuni D.S. Steroids and triterpenes from Alangium lamarckii, Allamanda cathartica, Abrus precatorius and Holoptelea integrifolia. Phytochemistry. 1969;8:791–792. doi: 10.1016/S0031-9422(00)85857-4. [DOI] [Google Scholar]
- 64.Bonomini T.J., Góes J.A., Machado M.D., da Silva R.M.L., Malheiros A. Development and optimization of a microwave-assisted extraction of plumieride from Allamanda cathartica L. Flowers. Quim. Nova. 2018;41:36–42. doi: 10.21577/0100-4042.20170153. [DOI] [Google Scholar]
- 65.Kupchan S.M., Dessertine A.L., Blaylock B.T., Bryan R.F. Isolation and structural elucidation of allamandin, an antileukemic iridoid lactone from Allamanda cathartica. J. Org. Chem. 1974;39:2477–2482. doi: 10.1021/jo00931a001. [DOI] [PubMed] [Google Scholar]
- 66.Coppen J.J.W. Iridoids with algicidal properties from Allamanda cathartica. Phytochemistry. 1983;22:179–182. doi: 10.1016/S0031-9422(00)80083-7. [DOI] [Google Scholar]
- 67.Thomas V. Structure and biology of floral nectary in Allamanda cathartica L. (Apocynaceae) Feddes Repert. 1992;103:357–361. doi: 10.1002/fedr.19921030512. [DOI] [Google Scholar]
- 68.Osborn E.M. On the occurrence of antibacterial substances in green plants. Br. J. Exp. Pathol. 1943;24:227. [Google Scholar]
- 69.Sarker R., Sharmin T., Islam F., Chowdhury S.R. In vitro antioxidant, total phenolic, membrane stabilizing and antimicrobial activity of Allamanda cathartica L.: A medicinal plant of Bangladesh. J. Med. Plants Res. 2014;8:63–67. [Google Scholar]
- 70.Hema K. In vitro anti-inflammatory activity of quercitrin isolated from Allamanda cathartica. Int. J. Pharm. Bio-Sci. 2014;5:440–445. [Google Scholar]
- 71.Boeing T., de Souza P., Bonomini T.J., Mariano L.N.B., Somensi L.B., Lucinda R.M., Malheiros A., da Silva L.M., Andrade S.F. Antioxidant and anti-inflammatory effect of plumieride in dextran sulfate sodium-induced colitis in mice. Biomed. Pharmacother. 2018;99:697–703. doi: 10.1016/j.biopha.2018.01.142. [DOI] [PubMed] [Google Scholar]
- 72.Singh A., Singh S.K. Reversible antifertility effect of aqueous leaf extract of Allamanda cathartica L. in male laboratory mice. Andrologia. 2008;40:337–345. doi: 10.1111/j.1439-0272.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- 73.Gupta R.S., Bhatnager A.K., Joshi Y.C., Sharma R., Sharma A. Effects of plumieride, an iridoid on spermatogenesis in male albino rats. Phytomedicine. 2004;11:169–174. doi: 10.1078/0944-7113-00346. [DOI] [PubMed] [Google Scholar]
- 74.Fartyal M., Kumar P. Evaluation of antimicrobial efficacy of alkaloids, flavonoids and steroids of Allamanda cathartica Linn. against some pathogenic bacteria. Int. J. Adv. Pharm. Biol. Chem. 2016;5:303–313. [Google Scholar]
- 75.Islam M.R., Ahamed R., Rahman M.O., Akbar M.A., Al-Amin M., Alam K.D., Lyzu F. In vitro antimicrobial activities of four medicinally important plants in Bangladesh. Eur. J. Sci. Res. 2010;39(Suppl. 2):199–206. [Google Scholar]
- 76.Mannan M.A., Alam M.S., Mustari F., Kudrat-E-Zahan M., Ali R., Haque A.H., Zaman S., Talukder D. In vitro antioxidant, antimicrobial, insecticidal and cytotoxic activities of the medicinal plants: Allamanda cathartica and Mimusops elengi. Eur. J. Med. Plants. 2017;20:1–12. doi: 10.9734/EJMP/2017/35730. [DOI] [Google Scholar]
- 77.Rao M.L., Bhumi G., Savithramma N. Green synthesis of silver nanoparticles by Allamanda cathartica L. leaf extract and evaluation for antimicrobial activity. Int. J. Pharm. Sci. Nanotechnol. 2013;6:2260–2268. [Google Scholar]
- 78.Karunakaran G., Jagathambal M., Gusev A., Kolesnikov E., Mandal A.R., Kuznetsov D. Allamanda cathartica flower’s aqueous extract-mediated green synthesis of silver nanoparticles with excellent antioxidant and antibacterial potential for biomedical application. MRS Commun. 2016;6:41–46. doi: 10.1557/mrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hema K., Krishnaveni R. Antibacterial and antifungal activities of Allamanda cathartica linn. Int. J. Pharm. Bio-Sci. 2014;5:588–593. [Google Scholar]
- 80.Okwubie L., Senior C.C. Evaluation of the antimicrobial activity of the crude root extracts of Allamanda cathartica L (Apocynaceae) Pharm. Innov. J. 2017;6:88–92. [Google Scholar]
- 81.Rajamanickam K., Sudha S.S. In vitro antimicrobial activity and in vivo toxicity of Moringa oleifera and Allamanda cathartica against multiple drug resistant clinical pathogens. Int. J. Pharm. Bio-Sci. 2013;4:768–775. [Google Scholar]
- 82.Chusri S., Siriyong T., Na-Phatthalung P., Voravuthikunchai S.P. Synergistic effects of ethnomedicinal plants of Apocynaceae family and antibiotics against clinical isolates of Acinetobacter baumannii. Asian Pac. J. Trop. Med. 2014;7:456–461. doi: 10.1016/S1995-7645(14)60074-2. [DOI] [PubMed] [Google Scholar]
- 83.Fartyal M. Allamanda cathartica linn.: Extraction and pharmaceutical evaluation of various extracts of leaves and flowers. Int. J. Curr. Pharm. Res. 2016;8:28–32. [Google Scholar]
- 84.Shukla R., Singh P., Prakash B., Dubey N.K. Antifungal, aflatoxin inhibitory and free radical-scavenging activities of some medicinal plants extracts. J. Food Qual. 2012;35:182–189. doi: 10.1111/j.1745-4557.2012.00441.x. [DOI] [Google Scholar]
- 85.Arundhina E. Aktivitas, Ekstrak Etanol daun Alamanda (Allamanda cathartica L.) Sebagai Antijamur Terhadap Candida Albicans dan Pityrosporum ovale Secara in vitro. [(accessed on 25 March 2019)];J. Teknobiol. Available online: http://e-journal.uajy.ac.id/6530/1/jurnal%20BL01139.pdf.
- 86.Tiwari T.N., Pandey V.B., Dubey N.K. Plumieride from Allamanda cathartica as an antidermatophytic agent. Phyther. Res. 2002;16:393–394. doi: 10.1002/ptr.967. [DOI] [PubMed] [Google Scholar]
- 87.Mone M., Saieed A.U., Dastogeer K.M.G., Ali M.A., Meah M.B. Plumieride from Allamanda cathartica as an inhibitory compound to plant pathogenic fungi. Arch. Phytopathol. Plant Prot. 2014;47:1311–1326. doi: 10.1080/03235408.2013.840103. [DOI] [Google Scholar]
- 88.Conrad O.A., Dike I.P., Agbara U. In vivo antioxidant assessment of two antimalarial plants—Allamanda cathartica and Bixa orellana. Asian Pac. J. Trop. Biomed. 2013;3:388–394. doi: 10.1016/S2221-1691(13)60082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fabiyi O.A., Olatunji G.A., Omoyele A.A. Nematicidal and quantitative phytochemical analysis of the chromatographic fractions from the leaf and stem of Allamanda cathartica (L) Ethiop. J. Environ. Stud. Manag. 2014;7:253–257. doi: 10.4314/ejesm.v7i3.4. [DOI] [Google Scholar]
- 90.Radhakrishnan B., Prabhakaran P. Biocidal activity of certain indigenous plant extracts against red spider mite, Oligonychus coffeae (Nietner) infesting tea. J. Biopestic. 2014;7:29. [Google Scholar]
- 91.Das Nelaturi P., Sriramaia N.H., Nagaraj S., Kotakadi V.S., Kutty M., Veeran A.V., Kiranmayee P. An in vitro cytotoxic and genotoxic properties of Allamanda cathartica L. latex green NPs on human peripheral blood mononuclear cells. Nano Biomed. Eng. 2017;9:314–323. [Google Scholar]
- 92.Omonhinmin C.A., Dike I.P., Rotimi S.O. Phytochemical, cytotoxicity and antioxidant activities of five anti-malaria plants. Res. J. Med. Plant. 2015;9:181–189. doi: 10.3923/rjmp.2015.81.89. [DOI] [Google Scholar]
- 93.Victor O.N., Emeka A.G., Chukwuka A.J., Victor A.O., Simeon E.I., Victor A.C., Patience O.N. Preliminary in vitro assessment of some phytochemical constituents and radical scavenging activity of methanol extracts of five flowers varieties. Annu. Res. Rev. Biol. 2015;5:357. doi: 10.9734/ARRB/2015/11188. [DOI] [Google Scholar]
- 94.Chaveeracha A., Taneeb T., Patarapadungkitb N., Khamwachirapithakb P., Sudmoonb R. Cytotoxicity and genotoxicity of Allamanda and Plumeria species. Sci. Asia. 2016;42:375–381. doi: 10.2306/scienceasia1513-1874.2016.42.375. [DOI] [Google Scholar]