Abstract
Aquaporins (AQPs) are water channel proteins that are essential to life, being expressed in all kingdoms. In humans, there are 13 AQPs, at least one of which is found in every organ system. The structural biology of the AQP family is well-established and many functions for AQPs have been reported in health and disease. AQP expression is linked to numerous pathologies including tumor metastasis, fluid dysregulation, and traumatic injury. The targeted modulation of AQPs therefore presents an opportunity to develop novel treatments for diverse conditions. Various techniques such as video microscopy, light scattering and fluorescence quenching have been used to test putative AQP inhibitors in both AQP-expressing mammalian cells and heterologous expression systems. The inherent variability within these methods has caused discrepancy and many molecules that are inhibitory in one experimental system (such as tetraethylammonium, acetazolamide, and anti-epileptic drugs) have no activity in others. Some heavy metal ions (that would not be suitable for therapeutic use) and the compound, TGN-020, have been shown to inhibit some AQPs. Clinical trials for neuromyelitis optica treatments using anti-AQP4 IgG are in progress. However, these antibodies have no effect on water transport. More research to standardize high-throughput assays is required to identify AQP modulators for which there is an urgent and unmet clinical need.
Keywords: aquaporin, AQP inhibitors, AQP modulators, functional assays, AQP expression, AQPs in disease, AQP structure, TGN-020, heavy metals, small molecule inhibitors
1. Aquaporin Structure
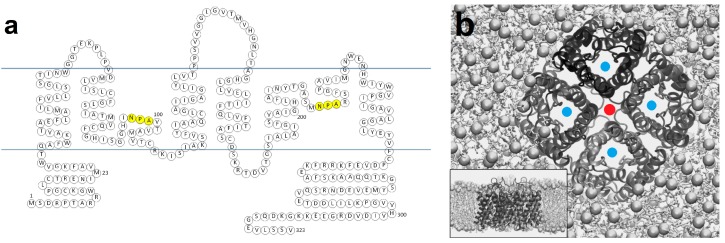
Aquaporins (AQPs) are transmembrane proteins that facilitate the bidirectional transport of water across cell membranes; water flows through AQP pores following an osmotic gradient to support water homeostasis [1,2,3]. A subset of the AQP family, the aquaglyceroporins, also allows the transport of glycerol and other small neutral solutes such as urea and ammonia [4,5,6,7]. These include AQP3, AQP7, AQP9, and AQP10. Currently, there are 13 known mammalian AQPs, each with a structure comprising six membrane-spanning helices (Table 1). The amino- and carboxy-termini of APQs are both intracellular [3,8]. Two opposing, shorter helices do not span the entire membrane (Figure 1), but form the pore itself [3,9,10]. These half helices contain the family’s signature triplet amino acid NPA (Asn-Pro-Ala) motif, as seen in Figure 1a [8,11]. The residues in this motif orient water molecules to prevent protons from entering the channel, as well as acting as the hydrogen bond donors and acceptors that facilitate the passage of water molecules [3]. The narrowest segment of the AQP channel is within the transmembrane region of the pore. For AQP1, it is 2.8 Å in diameter, which is similar to the size of a single water molecule. This feature means water molecules pass through AQPs in single file. A high resolution (0.88Å) X-ray crystal structure of a yeast AQP, Aqy1, revealed that the selectivity filter within the pore contains four positions for water molecules and that it is not possible for these positions to be occupied simultaneously. Instead they travel in a pairwise fashion, filling two of these positions at a time [12].
AQPs form homotetramers within the plasma membrane. Each homotetramer consists of four water channels (Figure 1b) [13,14]. Each water channel is functionally independent. It has been shown that channel pore size can sometimes be variable. When the residues responsible for the widening and narrowing of the channel entrance were in an ‘open’ state, molecular dynamics simulations suggested that the channel entrance was wider in monomeric than tetrameric AQP5, although it is not clear how this might affect water permeability [15]. For AQP4, functional and mutagenesis studies suggested that non-tetrameric AQPs have the same water permeability as those found in tetramers [16]. Although the reason AQPs form tetramers is not fully understood [17,18], it has been shown that non-tetrameric forms of AQP4 do not undergo hypotonicity-induced intracellular trafficking [16].
A controversial topic in AQP biology, related to their homotetrameric nature, has been their conduction of ions and whether this takes place. AQP tetramers contain a potential fifth central pore as shown in Figure 1b, reminiscent of cation channels such as Kir 2.1 [19]. The use of forskolin has been reported to induce cationic permeability in AQP1 [20], however, follow-up experiments by different groups failed to replicate this [21]. Direct binding of cGMP to AQP1 expressed in Xenopus oocytes has been shown to cause an ionic current after cGMP binding to a predicted site found in the AQP1 carboxyl tail [22]. The central pore of AQP1 was also found to be gas permeable using molecular dynamics simulations. However, conduction of O2 and CO2 through AQP1 may only be physiologically relevant in membranes of low gas permeability where there are high concentrations of AQPs [23]. Recently, a review of evidence for the gas permeability of AQPs suggested that that it may be functionally important in some cells, but not in others [24]. Less controversially, AQP6 is permeable to nitrate and chloride ions [25,26,27,28], and AQP8 is permeable to ammonia [29,30,31]. In a high resolution X-ray crystal structure of human AQP5, however, a lipid molecule co-crystallized with the protein, occluding the fifth central pore, suggesting another possible role [32].
Figure 1.
Aquaporin structure: (a) primary sequence of Aquaporin 4 (AQP4), the water channel with the highest recorded water permeability (see Table 1), highlighting the NPA (Asn-Pro-Ala) residues in yellow; (b) tertiary structure of the AQP4 homotetramer showing four water pores (marked with a blue dot) and the ‘fifth pore’ produced by formation of the tetramer (marked with a red dot). Adapted from an image originally published in [33].
2. Aquaporin Function
AQP channels facilitate the movement of water and small uncharged solutes across membranes down a concentration gradient. As such, they are implicated in a myriad of diseases and AQP modulators are thought to be promising therapeutic targets for new drug development [8,14]. In some cases, compounds that modify the transcription and translation of AQPs may also be beneficial such as modifying AQP7 expression as a treatment for obesity [14]. Most of the information currently known about AQPs pertains to AQPs 1, 2, 3, and 4, which are the focus of this review. Table 1 shows the currently-known structural and functional features of AQP family members. AQP4 appears to have the highest known water permeability of the AQPs and its role as the major AQP within the central nervous system (CNS) supports this, as tight control is required to ensure optimal conditions for CNS function. The lowest permeabilities are seen in AQP0 and AQP6. The main function of AQP0 as the major intrinsic protein (MIP) in the eye lens (where it has a structural and adhesive function) means that it does not require a high water permeability. AQP6 is an intracellular AQP, but its main function is not yet known.
Table 1.
A summary of AQPs 0–12 showing the information available on their structure, permeability, location within the body and pathophysiology. The osmotic water permeability coefficient (Pf) relates the magnitude of bulk water flow across a membrane in response to hydrostatic or osmotic pressure gradients to the magnitude of the driving pressure gradient. It depends on both the permeability of individual water channels and the density of channels in the membrane. Pgly is the glycerol permeability coefficient. The single channel osmotic water permeability coefficient (pf) is also given where available.
| AQP | Structural Information Available | Water Permeability (Glycerol Permeability Is Also Given for Aquaglyceroporins) |
Main Locations in Humans | Disease States | References |
|---|---|---|---|---|---|
| AQP0 | PDB ref: 1YMG Resolution: 2.2 Å Species: Bos taurus Method: X-ray diffraction Amino acids: 263 |
Xenopus oocytes—5 ng cRNA Pf: 1.3 × 10−3 cm/s (control with no AQP 0.67 × 10−3 cm/s) pf: 0.25 × 10−14 cm3/s—single channel permeability |
Lens | Mutations in the gene for AQP0 often result in bilateral cataracts | [7,34,35,36] |
| AQP1 | PDB ref: 1FQY Resolution: 3.8 Å Species: Homo sapiens Method: Electron crystallography Amino acids: 269 |
Xenopus oocytes—5 ng cRNA Pf: 19 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 6.0 × 10−14 cm3/s—single channel permeability |
Erythrocytes Choroid plexus Eye epithelium Corneal epithelium Bile duct Capillaries Nephron Airways Skeletal muscle |
Deficiency causes inability to concentrate urine Tumors with AQP1 have increased metastases and invasiveness Altered pain sensation |
[3,7,37,38,39,40] |
| AQP2 | PDB ref: 4NEF Resolution: 2.75 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 242 |
Xenopus oocytes—5 ng cRNA Pf: 10 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 3.3 × 10−14 cm3/s—single channel permeability |
Collecting duct cells | Deficiency causes diabetes insipidus | [7,39,41,42] |
| AQP3 |
No structural data available UniProtKB—O92482 Species: Homo sapiens Amino acids: 292 |
Xenopus oocytes—5 ng cRNA Pf: 8.0 × 10−3 cm/s (control 0.67 × 10c3 cm/s) pf: 2.1 × 10−14 cm3/s—single channel permeability Pgly: 23 × 10−6 cm/s |
Epidermis Collecting duct cells Erythrocytes |
Skin dehydration Psoriasis Tumor invasiveness and metastases |
[7,43,44,45] |
| AQP4 | PDB ref: 3GD8 Resolution: 1.8 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 223 |
Xenopus oocytes—5 ng cRNA Pf: 29 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 24 × 10−14 cm3/s—single channel permeability |
Astrocytes Parietal cells Collecting duct cells Retina |
Cytotoxic edema Vasogenic edema Neuromyelitis optica |
[7,44,46] |
| AQP5 | PDB ref: 3D9S Resolution: 2 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 266 |
Xenopus oocytes—5 ng cRNA Pf: 10 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 5.0 × 10−14 cm3/s—single channel permeability |
Salivary, lacrimal and sweat glands Lung |
Sjögren’s syndrome | [7,32,47] |
| AQP6 |
No structural data available UniProtKB—Q13520 Species: Homo sapiens Amino acids: 282 |
Xenopus oocytes—5–10 ng cRNA Pf: 1.2 × 10−3 cm/s (control 0.53 × 10−3 cm/s) (93 × 10 −4 cm/s after activation with mercury) No single channel data available. |
Collecting duct | Unknown | [48,49] |
| AQP7 |
No structural data available UniProtKB—O14520 Species: Homo sapiens Amino acids: 342 |
Xenopus oocytes—5 ng cRNA Pf: 18.6 × 10−3 cm/s (control 1.7 × 10−3 cm/s) Pgly: 18.9 × 10−6 cm/s No single channel data available |
Adipocytes Testis Proximal kidney tubule |
Adipocyte hypertrophy | [4,8] |
| AQP8 |
No structural data available UniProtKB—O94778 Species: Homo sapiens Amino acids: 261 |
Xenopus oocytes—10 ng cRNA Pf: 22 × 10−3 cm/s (control 0.8 × 10−3 cm/s) pf: 8.2 × 10−14 cm3/s—single channel permeability |
Pancreas Testis |
Unknown | [44,50] |
| AQP9 | No structural data available UniProtKB—O43315 Species: Homo sapiens Amino acids: 295 |
Xenopus oocytes—10 ng cRNA Pf: 12.3 × 10−3 cm/s (control 1.8 × 10−3 cm/s) Pgly: ~10 × 10−6 cm/s (controls: H2O 0 cm/s, AQP4 ~2.5 × 10−6 cm/s) No single channel data available |
Hepatocytes Osteoclasts Astrocytes Leukocytes |
Osteoporosis | [43,51] |
| AQP10 | PDB ref: 6F7H Resolution: 2.3 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 301 |
Xenopus oocytes—10 ng cRNA Pf: ~0.05 × 10−3 cm/s (controls: negative control ~0.02 × 10−3 cm/s, AQP8 ~0.2 × 10−3 cm/s No single channel data available |
Intestinal epithelial cells Adipocytes |
Unknown | [44,52] |
| AQP11 |
No structural data available UniProtKB—Q8NBQ7 Species: Homo sapiens Amino acids: 271 |
Spodoptera frugiperda—Sf9 membrane vesicles containing 1 µg mouse AQP11 protein Pf: 29.8 × 10−3 cm/s (controls: negative control 8.2 × 10−3 cm/s, 246.1 × 10−3 cm/s human AQP1, 197.7 × 10−3 cm/s His-rat AQP4) No single channel data available |
Testis Thymus Kidney Liver |
Polycystic kidneys | [44,53] |
| AQP12 |
No structural data available AQP12A UniProtKB—Q8IXF9 AQP12B UniProtKB—A6NM10 Species: Homo sapiens Amino acids: 295 |
Pancreatic rough endoplasmic reticulum vesicles—600 µg total RNA Pf: 13.0 × 10−3 cm/s (control 15.1 × 10−3 cm/s) non-significant difference No single channel data available |
Pancreatic acinar cells | Unknown | [54,55] |
2.1. AQP1
AQP1 was first identified in red blood cells and is linked to pathologies such as cancer, the inability to concentrate urine, and altered pain sensation [37,40,56]. Expression of AQP1 in some tumor types is correlated with increased angiogenesis, invasion, metastasis, and growth [3,57]. Aggressive melanomas were shown to have higher expression of AQP1. Silencing of the AQP1 gene in a mouse melanoma xenograft model, reduced angiogenesis and metastasis [1]. In addition to this, it was found that matrix metalloproteinase (MMP) 2 was downregulated upon AQP1 knockdown although it was not determined which of AQP1 or MMP2 was important in mediating this effect [1]. AQP1 is also found in the endothelial cells of the lungs and epithelial cells of bile ducts as well as the kidney tubules and in the epithelia of the proximal convoluted tubule and the loop of Henle [10,47]. AQP1-deficient humans are unable to concentrate their urine beyond 450 mOsm compared to the more normal 1000 mOsm and above [8,58]. They are also prone to fluid accumulation in the lungs [47]. Modulating AQP1 may therefore lead to treatments that can aid conditions of excess fluid such as heart failure and cirrhosis [3,10,14]. Another role of AQP1 is pain perception; its deletion can cause a reduction of pain from inflammation or cold providing a possible basis for analgesia [3]. There is also evidence to show AQP1 in the choroid plexus where cerebrospinal fluid is produced [47].
2.2. AQP2
AQP2 is found in the kidneys, specifically within the cells of the collecting duct. Its regulation is one of the best-studied examples of hormone-mediated AQP translocation; following vasopressin (anti-diuretic hormone) release, AQP2-containing vesicles translocate to the plasma membrane facilitating water reabsorption [8,59]. Vasopressin binds to V2 receptors of the principal cells in the renal collecting ducts, activating cAMP and protein kinase (PK) A, which phosphorylates Ser-256 and causes the exocytosis of AQP2-containing vesicles [25,60]. Central diabetes insipidus is the lack of vasopressin and its key symptoms are polyuria with an inability to concentrate urine [61]. Nephrogenic diabetes insipidus (NDI) is usually caused by a problem with the vasopressin receptor on collecting duct cells and is often linked to the AQP2 gene expression [61]. There are also cases of inherited NDI which can be caused by the same vasopressin receptor mutations but also mutations in the AQP2 gene; 19 such mutations have been identified [42,60]. Acquired diabetes insipidus is seen in cases such as long term lithium therapy where AQP2 subcellular localization can become dysregulated resulting in reduced surface localization and therefore loss of AQP2 function [47]. When vasopressin is secreted inappropriately, high levels of water retention and hyponatremia occur [61]. This is also seen in conditions such as congestive heart failure, where there is increased water retention due to an upregulation of vasopressin, and consequently AQP2 expression and translocation to the plasma membrane [60,62]. The exact mechanism by which AQP2 is recycled in the principal collecting duct cells is not yet known, however clathrin-coated pits are thought to have a role in this process [63].
2.3. AQP3
AQP3 is an aquaglyceroporin that allows the transport of glycerol in addition to water [8]. It is found in the epithelia of renal collecting ducts, urinary tract, digestive tract, and respiratory tract [47]. It is also found in the skin and aids skin hydration [13]. The overexpression of AQP3 is linked to skin malignancies such as melanomas [64,65]. AQP3 knock-out (KO) mice have been shown to be resistant to tumor formation in skin cancer models through reduced glycerol transport, resulting in lower ATP formation and reduced cell proliferation [3,14,57,64]. AQP3 has also been shown to transport hydrogen peroxide and this function aids NF-κB signaling. AQP3 KO mice had reduced H2O2 levels, impaired NF-κB activation and a reduced induction of psoriasis [66]. In addition to this, AQP3 has roles within the normal function of bacterial phagocytosis by macrophages and the trafficking of T cells [3,67,68].
2.4. AQP4
AQP4 is the main water channel expressed in the CNS and is found at highest concentration in astrocyte end-feet, which together with blood vessels form the blood–brain barrier (BBB) [13,69]. AQP4 expression is increased in cerebral edema and ischemia. This has been shown to be correlated with the release of inflammatory cytokines [3,13,70,71]. AQP4 is also thought to aid the migration of astrocytes when neuronal injury occurs and glial scars form [72]. AQP4 has been shown to have a link with seizures where the regulation of cell volume by water flow can reduce susceptibility to seizures [73]. For the potassium channel, Kir 4.1, to be active, water flow through AQP4 is required [1,9,71,74,75]. During trauma or ischemia to the brain, there is a state of hypoxia which is then followed by a failure in Na+, K+, and Cl− pumps in the plasma membrane, causing dysregulation of osmolality [56,76,77,78]. This results in an increased intracranial pressure (ICP) and cerebral edema due to water flow, primarily into astrocytes during the cytotoxic phase of cerebral edema [70]. Limited therapeutic options are currently available to control cerebral edema. They are restricted to the administration of hyperosmotic solutions (such as mannitol, glycerol, and sodium chloride), hypothermia, or ultimately a surgical intervention to remove part of the skull to alleviate the uncontrolled rise in ICP [70,79,80]. AQP4 inhibition may be beneficial in cytotoxic edema as AQP4 KO mice were protected from cytotoxic edema. [3,13,14,56,70,81]. Vasogenic edema occurs due the movement of water and plasma proteins across a leaky BBB [9,76]. In models of vasogenic edema, however, AQP4 KO resulted in worse outcomes, due to an inability to remove excess water from brain tissue [3,9,56,70]. Any inhibition of AQP4 function, therefore needs to be acute.
Neuromyelitis optica (NMO) is an autoimmune condition where the CNS is affected by inflammation and demyelination [82]. It is symptomatically similar to multiple sclerosis (MS), but there are some key differences including the fact that some treatments for MS can exacerbate NMO [82]. Most patients with NMO have autoantibodies that target AQP4 in astrocytes and cause complement- and cell-mediated cytotoxicity [83]. The usual treatment for NMO involves steroids and other immunosuppressants such as azathioprine and mycophenolate [82].
2.5. AQPs 0, 5–12
AQP0, also known as MIP, was first identified within the lens. Its role is not only as a water channel (it has a low permeability compared to other family members, Table 1) but also to maintain the transparency and structure of the lens [35]. Mutations in the genes for AQP0 were found to result in congenital cataracts [35,84].
AQP5 was first found within the submandibular glands and is now known to have a role within many secretory glands including salivary, lacrimal, and sweat glands [85]. Binding of acetylcholine to muscarinic M3 receptors is thought to induce the translocation of AQP5 vesicles to the cell surface membrane and problems with this process have been shown to be linked to conditions such as Sjögren’s syndrome [32,86,87].
AQP6 is an intracellular water channel found in vesicles and its water permeability is low [48], as shown in Table 1. These vesicles also contain H+–ATPase and AQP6 has been found to become more active at low pH suggesting that the role of AQP6 is in acid secretion [27,84].
AQP7, an aquaglyceroporin, is found in adipocytes and is thought to have a role in fat metabolism aiding degradation of triglycerides in fasting states [8]. AQP7 KO mice are seen to have higher levels of fat than wild type controls and this is thought to be a therapeutic target for obesity [13]. However, in the case of an individual with a homozygous nonfunctional AQP7 mutation, body mass index, cholesterol, and glucose levels were all normal, with only a reduced increase in glycerol levels during exercise compared to controls [88].
AQP8 has been identified in the brain, small intestine, testis, and the salivary glands [49]. Its function was disputed, but it is now known that AQP8 transports ammonia, as well as water, and that the transport of ammonia may be its primary function, maintaining acid-base equilibrium [30].
AQP9, an aquaglyceroporin, is found in hepatocytes and its function is thought to be significant during periods of exercise or prolonged fasting affecting glucose production [85]. It is found in osteoclasts and is thought to have a role in osteoporosis [89]. It is also found in astrocytes and leukocytes [43,49]. AQP9 and AQP7 are also known to mediate the uptake of arsenic compounds, which are used in some cancer treatments [44].
Little is known about the remaining AQPs. AQP10, an aquaglyceroporin, is found in intestinal epithelial cells and in adipocytes [43,47,90]. AQP11 is an intracellular aquaporin but its function remains unclear [44]. AQP11 contains a cysteine residue instead of an alanine in its first NPA motif and in the second motif, it has a leucine instead of an arginine [91]. It is found to be expressed in the testis, brain, kidneys, and liver [43]. AQP11 KO mice have been found to die soon after birth due to polycystic kidney disease [92]. This is thought to be due to AQP11 being required for correct glycosylation of polycystin-1 and -2 [93]. AQP12 is expressed in pancreatic acinar cells and in AQP12 KO models, those that develop pancreatitis develop a much more severe pathology [54,55].
3. Aquaporin Assays
Water permeability can be assayed in various ways. Measuring volume change due to osmotic gradients is one indirect approach. Epithelial assays are based on using epithelial tissues placed on permeable supports and adding solutes to cause the fluid in a capillary tube to move. Transepithelial flux is measured via this change and fluorescent dyes can be used to evaluate permeability [94,95]. Transepithelial permeability results from water flow that has occurred both cellularly and paracellularly, whereas water flux through AQPs occurs only cellularly, which is a limitation of this as an approach to deducing AQP function.
Osmotic swelling techniques are by far the most common methods used for assaying volume changes and Xenopus oocytes are often used as they have a very low intrinsic water permeability [96]. Osmotic gradients are used and oocytes expressing AQPs change volume when these gradients are altered [97]. While the use of Xenopus oocytes is very common, there is substantial variability between oocyte preparations, meaning data may vary between laboratories even using the same materials [94,98].
Microscopy techniques are also used to measure permeability via a fluorophore that is taken up by cells. Esterases within the cell produce the active fluorophore and the rate of change of fluorophore concentration can then be detected using a plate-reader or fluorescence microscope after an osmotic gradient has been applied [99]. The limitation of this technique is that the fluorescence is relative to the dimensions of the cell monolayer where thinner cells produce a larger relative change in fluorescence than cell-lines that have a thicker monolayer. This can affect reproducibility in the same cell-line if confluence is varied between assays. It is also dependent on the amount of fluorophore that is taken up by the cells, which can sometimes be pumped out, producing variability in the data [100].
Stopped-flow spectroscopy involves the use of suspensions of cells, vesicles, or proteoliposomes which are mixed with solutes to produce a change in volume. The difference in light-scattering after this volume change can then be used to calculate the volume change [99]. Limitations of stopped-flow spectroscopy techniques include the dead time and mixing ability of the instrument used [101,102].
Computational methods involve the use of high-resolution structures and molecular dynamics simulations, which can provide new insights into AQP structure and function at atomic resolution. However, high-quality structures are not always available and simulations can sometimes produce data that are different from experimental data [94,103]. A summary of these techniques is shown in Table 2.
Table 2.
Various assay methods used in the measurement of AQP function and the validation of AQP inhibitors. Their benefits and limitations are shown.
| Assay Type | Assay Principle | Benefits | Limitations | References |
|---|---|---|---|---|
| Epithelial | Epithelial cells are placed on supports, solutes are added and transepithelial flux is measured by the height of the fluid in capillary tubes | Assists AQP identification and characterization in tissues Individual membrane permeability is calculated |
Not very robust Measures paracellular as well as cellular water flow |
[94,95] |
| Osmotic Swelling | Osmotic gradients are used to cause flux of solutes and water in cells endogenously-expressing or transfected with AQP genes; cell volume changes are measured | Well-established technique Oocytes have low intrinsic water permeability Other cells can be used Inward and outward gradients possible |
Preparation techniques vary causing discrepancies in results between laboratories Not very reproducible Variability between oocytes |
[94,96,98] |
| Microscopy | Most often fluorescent dyes are used that permeate the plasma membrane and are cleaved by esterases. As osmotic shock occurs, the fluorescence is altered, which is measured and used to calculate cell volume changes | Linear relationship produced between fluorophore and cell volume changes More accurate than counterparts Very sensitive Robust |
Thickness of cell-line monolayer can produce variability in results Fluorophore may not always stay within the cell depending on cell-line |
[94,99,104] |
| Stopped-Flow Spectroscopy | Suspensions of cells or vesicles are mixed with various osmotic solutions and flux of water causes volume changes, which result in altered light scattering. The linear relationship between optical properties and cell volume allows permeability to be calculated; alternatively fluorophores can be used. | Fast kinetics measured Can use cells, vesicles and proteoliposomes Linear relationship between optical properties and volume changes |
Variability in dead time and mixing ability can cause problems with reproducibility | [94,99,101] |
| Computational Methods | High resolution atomic structures of AQPs are used and molecular dynamics simulations allow in silico measurement of water permeability | Provides new insights into AQP structure and function | High-quality structures not always available May disagree with experimental data |
[94,103] |
4. Aquaporin Inhibitors
Most of the molecules that are currently under investigation as AQP inhibitors target AQPs 1, 2, 3, or 4. There are many patents, clinical trials, and studies on AQP up-regulators, modulators, and inhibitors. For the purposes of this review, only AQP inhibitors are considered (Table 3).
Table 3.
Currently-available AQP inhibitors, their structures, the AQPs they inhibit (species are highlighted as h—human, m—mouse, and r—rat) and the conditions under which they were assayed.
| Inhibitor | Conditions | AQPs Inhibited | Structure | References |
|---|---|---|---|---|
| Tetraethyl-ammonium |
Xenopus oocytes 100 µM |
hAQP1 |
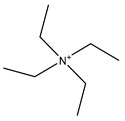
|
[105,106] |
| Phloretin |
Xenopus oocytes 0.1 mM Proteoliposomes 0.5 mM |
rAQP9 hAQP3 |
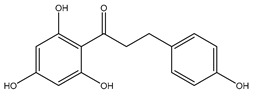
|
[107,108] |
| Mercury chloride |
Xenopus oocytes 1 mM |
hAQP1 |
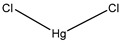
|
[96,109] |
| AuPhen | Erythrocytes 50 µM Adipocytes 15 µM |
hAQP3 hAQP7 |
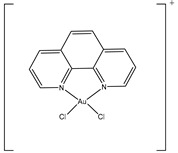
|
[110,111] |
| Silver nitrate | Erythrocytes 10 µM |
hAQP3 |
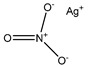
|
[112] |
| Copper sulfate | Swan 71 cells 100 µM |
hAQP3 |
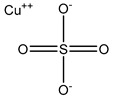
|
[113] |
| Nickel chloride | Human bronchial epithelium cells 1 mM |
hAQP3 |
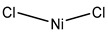
|
[114] |
| Furosemide |
Xenopus oocytes 10 µM |
hAQP1 |
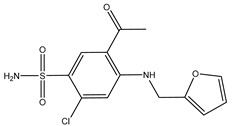
|
[115] |
| Bumetanide |
Xenopus oocytes 100 µM |
rAQP4 |
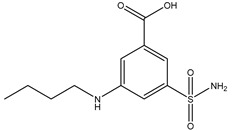
|
[116] |
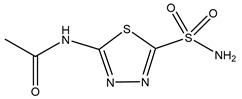
| N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide |
Xenopus oocytes 20 µM |
hAQP4-M23 |
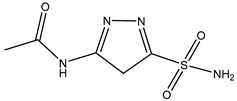
|
[117] |
| IMD-0354 | Mice 0.76 mg/kg |
mAQP4-M23 |
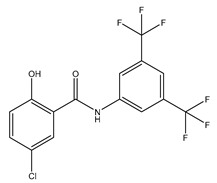
|
[118] |
| Acetazolamide | HEK293 cells 10 µM |
rAQP1 rAQP4 |
|
[119] |
| TGN-020 | C57/BL6 male mice 200 mg/kg (23–28 g) |
mAQP4 |
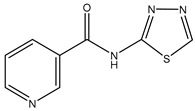
|
[81] |
| Topiramate |
Xenopus oocytes 20 µM |
rAQP4-M23 |
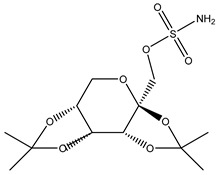
|
[120] |
| DFP00173 | Human erythrocytes 25 µM |
hAQP3 |
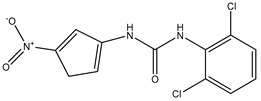
|
[121] |
| Z433927330 | Human erythrocytes 25 µM |
mAQP7 |
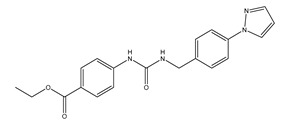
|
[121] |
Several molecules have been suggested as inhibitors of various AQPs over the past few years. Of these, most are small molecules (Table 3). The similarity found between AQPs and ion channels has caused interest in ion channel modulators as possible AQP inhibitors [43]. Arylsulfonamides such as acetazolamide are molecules that have attracted attention as potential AQP inhibitors [122]. Anti-epileptic drugs (AEDs) have also been suggested to have an AQP modulating function [70]. However, they have yet to be proven as definitive AQP inhibitors. A novel molecule named TGN-020 has shown great promise very recently as an inhibitor in mouse models [81]. Micro RNA (miRNA) and small interfering RNA (siRNA) have also been used to modulate AQPs [70]. Mercury chloride binds to a pore-lining cysteine residue found on several (but not all) AQPs and blocks the channel [3]. Mercury and its compounds is known to be toxic due to their non-specificity which causes many off-target effects [11]. Other heavy metal compounds (such as silver and gold compounds) have been investigated as inhibitors, but the challenge is finding a molecule with a side-effect profile that is tolerable. Currently-known inhibitors are discussed by type in the sections below.
4.1. Small Molecule Inhibitors
Tetraethylammonium (TEA) is a small molecule inhibitor that was first tested due to perceived similarities between AQPs and ion channels. Using human AQP1 expressed in Xenopus oocytes, a maximum single dose of 10 mM TEA caused a reduction in water permeability of 33% [105]. Another study using 4 µM and 100 µM TEA showed an inhibition of 44% for Xenopus oocytes expressing AQP1, with no differences at the two concentrations; TEA also inhibited AQP2 and AQP4, with maximal inhibition of 40% and 57%, respectively, at 100 µM TEA [123]. Further testing of TEA in Xenopus oocytes or in native AQP1-expressing erythrocytes has failed to show inhibition [3,124]. TEA is thought to act by binding to a tyrosine residue located on the extracellular end of transmembrane helix 5 [123].
Acetazolamide is a carbonic anhydrase inhibitor used in glaucoma to reduce aqueous humor production and hence intraocular pressure [125]. It has been shown to be a reversible inhibitor of AQP1 and AQP4 (Table 3). It has inhibitory effects on Xenopus oocytes expressing human AQP4 in a dose-dependent manner with maximal inhibition of 85% at the highest dose of 20 µM [126]. A reduction in the water permeability of rat AQP4 (assayed in proteoliposomes) to 46% was observed at a maximum dose of 1.25 mM acetazolamide [127]. In HEK293 cells expressing rat AQP1, there was a 39% reduction in water permeability with 100 µM acetazolamide compared to untreated rat AQP1 (using GFP fluorescence to measure swelling) [119]. However, acetazolamide appears to be unable to inhibit endogenous AQPs in human cells [10,124].
Anti-epileptic drugs (AEDs) have also been suggested as possible AQP inhibitors [120]. Their anti-epileptic action has been hypothesized take place through modulation of AQPs. Many AEDs such as topiramate, zonisamide, and lamotrigine are known to have a similar inhibitory effect to acetazolamide on carbonic anhydrase enzyme as well as an in silico predicted binding site on AQP4 that is similar to that hypothesized for acetazolamide [120]. AEDs had an inhibitory effect on AQP4 in Xenopus oocytes [120], however, this could not be reproduced in rat thyroid epithelial cells [122]. AEDs are thought to possibly have an inhibitory effect on AQP1, AQP4, and AQP5 [128]. However due to their unconfirmed mechanisms of action and relatively non-specific action, there is no conclusive evidence showing that AEDs are effective and safe AQP inhibitors [10,122].
TGN-020 was shown in mouse models to significantly reduce AQP4-mediated edema following ischemia, but the molecule was administered before injury [81]. There was an approximately 10% reduction in brain volume [81]. Therapeutic administration must necessarily occur after a stroke, although a prophylactic treatment could be considered if the side effect profile of TGN-020 was acceptable. TGN-020 has since been tested on the same middle cerebral artery occlusion (MCAO) model and administered post-injury in mice. In a study using mouse models at a dose of 100 mg/kg, TGN-020 was administered 15 minutes post-ischemic injury and treated animals had better motor scores and less AQP4 expression around blood vessels when compared to untreated controls [129]. The half-maximal inhibitory concentration (IC50) of TGN-020 was 3µM in Xenopus oocytes expressing human AQP4, but there is no evidence to show that TGN-020 is AQP4-specific [130].
Phloretin is a small molecule that acts as a non-specific aquaglyceroporin inhibitor. It has also been shown to inhibit the urea transporter, UT-A1, found in the kidney [131]. It has been speculated that the same mechanism underpinning urea inhibition is responsible for inhibition of the aquaglyceroporins, AQP3 and AQP9 [107,132]. Then 100 µM of phloretin was used to inhibit AQP9 expressed in Xenopus oocytes, resulting in an 86% inhibition [107]. AQP3 glycerol permeability in proteoliposomes was inhibited by 500 µM phloretin, producing 83% inhibition. Phloretin had no effect on control proteoliposomes which had a Pgly of ~2.8 × 10−6 cm/s [108].
A recent study identified new inhibitors of the aquaglyceroporins, AQP3 and AQP7. The compound DFP00173 was able to inhibit the glycerol permeability of human erythrocytes with an IC50 of ~0.2 µM. Compound Z433927330 reduced glycerol permeability with an IC50 of ~0.6 µM. In a Chinese hamster ovary cell-line, compound DFP00173 was able to inhibit mouse AQP3 with an IC50 of ~0.1 µM and was selective for AQP3 over AQP7 and AQP9. Compound Z433927330 was able to inhibit mouse AQP7 in the same cell-line with an IC50 of ~0.2 µM and with selectivity for AQP7 over AQP3 and AQP9; IC50s for this compound for mouse AQP3 and AQP9 were ~0.7 µM and ~1.1 µM, respectively [121].
4.2. Heavy Metal Ion Inhibitors
Heavy metal compounds have been used in cytotoxic treatments for many years, with the use of the platinum compound cisplatin being notable in cancer therapy. Many AQPs have been correlated with cancers, their presence influencing disease severity, increased local invasion, and the occurrence of metastasis [57]. AQP1 overexpression has been identified in brain, breast, lung, renal, cervical, ovarian, and colorectal cancers [133]. AQP3 overexpression has been found in skin, stomach, renal, liver, colorectal, lung, and cervical cancers [134]. AQP3 is highly expressed in skin carcinoma cells. The uptake of glycerol through AQP3 is thought to aid the growth of these cancer cells [57]. AQP4 overexpression is found in brain, lung, and thyroid cancers [135]. AQP5 overexpression has been found in breast, cervical, colorectal, liver, lung, pancreatic, ovarian, and esophageal cancers [136]. AQP7 and AQP8 overexpression are found in thyroid and cervical cancer, respectively [133]. AQP9 overexpression has been found in brain, liver, and ovarian cancers [136].
Nickel chloride has been shown to inhibit AQP3 overexpressed in human bronchial epithelium cells. Water permeability was reduced to approximately 60% in cells treated with 1 mM NiCl2 compared to non-transfected control cells [114]. Mutational studies showed that the extracellular loop residues, Trp-128, Ser-152, and His-241, were all required for the observed inhibitory effect, but that further studies are required to confirm similar effects in other AQP3-expressing tissues such as the kidney [114]. Copper ions also inhibit AQP3 function and attempts to reduce the toxicity of copper compounds have been addressed by using nanoparticles for delivery [57]. Copper sulfate has been identified as an AQP3 inhibitor at 100 µM [113]. It is believed that copper ions act by binding the same three residues on the extracellular loops as nickel ions [137]. This inhibition mechanism is different from that of mercury ions, which bind to Cys-40 in AQP3 [137,138]. Copper compounds have been shown to specifically inhibit AQP3 and not AQP4, AQP5, or AQP1 [137], while other AQPs remain to be tested. Gold-based compounds have also been used as AQP3 inhibitors and have been shown to be effective. AQP3 is found in erythrocytes, alongside AQP1, however its role is mostly for glycerol rather than both water and glycerol permeability [110]. Erythrocytes showed a 90% decrease in glycerol permeability following treatment with 50 µM AuPhen. The binding of this gold compound is thought to be via Cys-40 and inhibition can be almost completely reversed by the addition of the reducing agent, 2-mercaptoethanol [110]. AuPhen and other Au(III) compounds such as Auterpy were also compared with copper and platinum compounds as AQP3 inhibitors [111,139]. Due to their effective inhibition of glycerol transport in AQP3, they were tested on AQP7, another aquaglyceroporin. Initial results have demonstrated that 15 µM AuPhen inhibited water and glycerol transport through AQP7 [111]: there was a reduction in water permeability (63%) as well as glycerol permeability (79%). Adipocytes overexpressing hAQP7 were used and permeability was measured using loading with 5 µM of calcein-AM and hyperosmotic shock. In silico docking studies suggest that Auphen binds AQP7 through an interaction with Met-47 [140]. Silver has also been used as a possible AQP inhibitor and is found to produce a rapid and irreversible inhibition of AQP1 in erythrocytes [112]. It has been shown to be a much more potent inhibitor of AQP1 in erythrocytes than mercury, [112]. Silver nitrate and silver sulfadiazine were tested as inhibitors to prevent shrinking of human erythrocytes in hyperosmotic solutions. A dose-response curve was used to calculate an IC50 of 3.9 µm and 1.24 µm and a 60% and 75% inhibition for silver nitrate and silver sulfadiazine, respectively. Gold and silver are thought to bind to sulfhydryl groups on cysteine residues of AQPs, but the complete mechanisms are not yet understood [110,111,112].
5. Antibody Treatments
An anti-AQP4 monoclonal antibody was developed as a potential treatment for NMO. “Aquaporumab” competitively binds to AQP4 in astrocytes and displaces AQP4-IgG, reducing NMO lesions in mouse models [82,83]. This is yet to be tested in humans, although in vivo studies have been promising [141].
6. Patents and Clinical Trials
A search of the European Patent Office archive in February 2019 returned 484 AQP-related patents or patent applications. These documents protect various techniques and methods such as AQP upregulation and modulation, detection methods, mRNA expression, as well as siRNA silencing techniques. Approximately 40 of these patents are for inhibitors of AQPs or NMO treatments.
US patent 2008/0194513 A1 identifies the proliferation of blood vessels within the eyes as a factor in sight loss in several ocular conditions. Interfering RNAs were therefore used to silence AQP4 mRNA expression in order to reduce ocular neovascularization. Administration of siRNA at 1–10 nM provided over 70% inhibition of AQP4 expression in Madin-Darby canine kidney (MDCK) cells transfected with human AQP4 compared to controls [142]. A similar patent was filed by the same inventors using similar techniques for the inhibition of AQP1 expression for ocular conditions [143].
US patent 7,659,312 B2 identifies various AQP4 inhibitors to be used in cerebral edema and other water-related disorders. Twenty-one compounds are described that are similar in structure to the loop diuretics, furosemide, and bumetanide. A total of 15 compounds had inhibitory effects on AQP4 when tested in Xenopus oocytes transfected with AQP4-M23. 20 µM inhibitor compounds were used and experiments were completed twice. Aryl sulphonamide-like compounds and bi-aryl compounds were identified as inhibitors. Docking energies were also analyzed using AQP4 monomers built from electron diffraction structures; compounds with higher inhibition often had better docking energies.
Phenylbenzamide-based compounds are patented inhibitors of AQP2 and AQP4 for the treatment of AQP-related conditions such as edema, ischemic stroke, epilepsy, and meningitis. Phenylbenzamides such as niclosamide, a compound usually used for treatment of helminth infections, is known to have an inhibitory effect on NF-kB and IKK-β. The compound IMD-0354 was used to inhibit IKK-β in myocardial ischemia models [144]. Compound 3 has a similar structure to IMD-0354 but the two trifluromethyl groups are replaced by chloride. Compound 3 is claimed to be an inhibitor of AQP2, AQP4-M1, and AQP4-M23 with over 60% inhibition. It was at very poor inhibitor of AQP1 and AQP5. Compound 1 (IMD-0354) inhibited cerebral edema formation in a murine water toxicity model. Treatment with 0.76 mg/kg produced an 11.2% reduction, while 7.6 mg/kg showed a 15.9% reduction in edema. Following MCAO in mice, there was a 29.4% increase in survival 24 h post-injury [118]. A subsequent patent described prodrug salts of these compounds [145].
Ghrelin is a 28 amino acid peptide that is secreted by gastric mucosa cells and is a hormone that regulates hunger. It is known to also have other anti-inflammatory properties thought to be protective in neuronal injury [146]. Traumatic brain injury (TBI) causes acute, elevated ICP and brain damage. Repeated mild brain injuries (mBI), such as concussions, can cause long-term injury to the brain and increased risk of neurodegeneration with aging. Treatments for TBI and mBI are very limited. Administration of ghrelin reduced the levels of reactive oxygen species in neurons and volume loss in mice post-injury [147]. The administration of ghrelin at doses 1.5–1000 times native levels provided therapeutic effects lasting from 30 minutes up to 24 h. Ghrelin was also able to reduce oxidative bursts post mBI [148]. Ghrelin has been shown to reduce levels of brain water after acute hypoxia in rats, as well as the permeability of blood vessels. It is thought to do this through reduction in TNF-α levels together with other anti-inflammatory effects on IL1, IL6, and NF-κB [149]. In another study, levels of AQP4, serum S100B and vascular permeability were quantified to show disruption of the BBB post-TBI in mice. Treatment with ghrelin was able to reduce these levels and help prevent BBB damage post-TBI [150].
A possible NMO treatment centers on a peptide corresponding to AQP4 loop C. T cells reactive to this peptide from AQP4 -/- mice produced a pathological response reminiscent of NMO when injected into AQP4 +/+ mice. T cells are found in NMO lesions and are thought to be required for the production of anti-AQP4 IgG. It was noted that immunosuppression therapy would be required before treatment takes place [151].
US patent 2015/0224108 A1 presents techniques to create prodrugs and derivatives of the loop diuretics, bumetanide and furosemide, as AQP inhibitors and to aid their administration to the site of action [152].
The use of phloretin as an AQP9 inhibitor was shown to reduce osteoclast levels. This inhibition would aid treatment in conditions such as osteoporosis, where excessive bone resorption takes place. AQP9 expression is higher in osteoclasts than other cells; 50 µM phloretin inhibited AQP9 expression, causing a reduction in size and number of osteoclasts but not their precursors [153].
A search of the US National Library of Medicines Clinical Trials archive in February 2019 returned 61 clinical trials involving AQP-based treatments. Several of these trials examine urinary AQP2 levels and the conditions under which they are altered. Several trials also examine NMO, MS, and the therapeutic use of AQP4-IgGs. A search of the University Hospital Medical Information Network (UMIN) - Clinical Trials Registry in February 2019 returned two current clinical trials taking place using anti-AQP4 IgGs. There are currently no clinical trials taking place in the UK using AQP inhibitors or anti-AQP4 IgGs according to the UK Clinical Trials Gateway.
7. Discussion
Finding inhibitors for AQP water channels has been considerably more challenging than first thought. The identification of molecules that inhibit water transport through specific AQP isoforms is not a simple proposition for drug discovery on account of the high level of structural conservation within the AQP family (Table 1). The small diameter of all AQP-pores together with the chemical properties of the pore-lining amino acid side-chains mean that hydrophilic compounds are unlikely to enter and block them.
No definitive, small molecule AQP inhibitors have been identified to date that could be used therapeutically. Heavy metal compounds are certainly effective AQP inhibitors, but their non-specificity and toxicity substantially limit their therapeutic potential. Using liposomes to administer heavy metal compounds would reduce off-target effects, but more research is required. The most promising AQP inhibitor with clinical potential to date is TGN-020, which has not yet shown any side effects in vivo, but is yet to be tested in human clinical trials. Notably, antibodies used for NMO treatment have no effect on water permeability. There is a clear need for AQP modulators, particularly in conditions such as TBI where, in the early stages, AQP4 inhibition is required to manage cytotoxic edema but, thereafter, upregulation of AQP4 would aid clearance of vasogenic edema.
The lack of reliable in vitro phenotypic assays suitable for screening and validating the pharmacological regulation of AQP function (Table 2), means that pharmaceutical companies have been unable to meet the challenge of developing small molecules that block the AQP pore. For example, molecules discovered using Xenopus oocytes consistently fail to show efficacy in mammalian cells and vice versa. Screening programs for AQP inhibitors should therefore include multiple assays that allow more robust and reproducible readouts.
8. Conclusions
AQPs are expressed ubiquitously and have implications in myriad human diseases. The progress of identifying AQP inhibitors has been slow and as research continues, AQPs are increasingly implicated in more diseases, highlighting the clear and urgent need for AQP modulators. More research is needed to find effective AQP inhibitors. Current screening processes are extremely variable. Approaches to standardize assays would be beneficial in identifying promising molecules for future study.
Abbreviations
| AQP | aquaporin |
| NPA | Asn-Pro-Ala |
| Å | Ångstrom |
| CNS | central nervous system |
| MIP | major intrinsic protein |
| RNA | ribonucleic acid |
| cRNA | complementary ribonucleic acid |
| MMP | matrix metalloproteinase |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| NDI | nephrogenic diabetes insipidus |
| KO | knock-out |
| BBB | blood–brain barrier |
| ICP | intracranial pressure |
| NMO | neuromyelitis optica |
| MS | multiple sclerosis |
| AEDs | anti-epileptic drugs |
| miRNA | micro ribonucleic acid |
| siRNA | small interfering ribonucleic acid |
| TEA | tetraethylammonium |
| µM | micromolar |
| HEK293 | human embryonic kidney 293 |
| MCAO | middle cerebral artery occlusion |
| IC50 | half maximal inhibitory concentration |
| NiCl2 | nickel chloride |
| mM | millimolar |
| GFP | green fluorescent protein |
| hAQP | human aquaporin |
| calcein-AM | calcein acetoxymethyl |
| AgNO3 | silver nitrate |
| AgSul | silver sulfadiazine |
| IgG | immunoglobulin G |
| mRNA | messenger ribonucleic acid |
| qPCR | quantitative polymerase chain reaction |
| ELISA | enzyme-linked immunosorbent assay |
| nM | nanomolar |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activate B cells |
| IKK-β | inhibitor of nuclear factor kappa-B |
| 24h | 24 hours |
| TBI | traumatic brain injury |
| mBI | mild brain injury |
| TNF-α | tumor necrosis factor alpha |
| IL1 | interleukin 1 |
| IL6 | interleukin 6 |
| UMIN | University Hospital Medical Information Network |
Funding
This work was supported by a UK Biotechnology and Biological Sciences Research Council International Partnering Award (BB/P025927/1) to PK, ACC, and RMB. MA is supported by a studentship co-funded by Aston University and the UK Engineering and Physical Sciences Research Council (EP/R512889/1) to RMB. PK is the recipient of an Aston University 50th Anniversary Prize Fellowship. MMS was supported by HCED grant number GD-13-13 (M. Salman).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Simone L., Gargano C.D., Pisani F., Cibelli A., Mola M.G., Frigeri A., Svelto M., Nicchia G.P. Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma. J. Cell. Mol. Med. 2018;22:904–912. doi: 10.1111/jcmm.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu J., Zhang Y.Z., Chen H., Guo Z. MicroRNA-488 inhibits proliferation, invasion and EMT in osteosarcoma cell lines by targeting aquaporin 3. Int. J. Oncol. 2018;53:1493–1504. doi: 10.3892/ijo.2018.4483. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Verkman A.S., Anderson M.O., Papadopoulos M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014;13:259. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishibashi K., Kuwahara M., Gu Y., Kageyama Y., Tohsaka A., Suzuki F., Marumo F., Sasaki S. Cloning and Functional Expression of a New Water Channel Abundantly Expressed in the Testis Permeable to Water, Glycerol, and Urea. J. Biol. Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 5.Geyer R.R., Musa-Aziz R., Qin X., Boron W.F. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am. J. Physiol.-Cell Physiol. 2013;304:C985–C994. doi: 10.1152/ajpcell.00033.2013. [DOI] [PubMed] [Google Scholar]
- 6.Litman T., Søgaard R., Zeuthen T. Ammonia and Urea Permeability of Mammalian Aquaporins. In: Beitz E., editor. Aquaporins. Springer; Berlin/Heidelberg, Germany: 2009. pp. 327–358. [DOI] [PubMed] [Google Scholar]
- 7.Yang B., Verkman A.S. Water and Glycerol Permeabilities of Aquaporins 1–5 and MIP Determined Quantitatively by Expression of Epitope-tagged Constructs inXenopus Oocytes. J. Biol. Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 8.Agre P., Kozono D. Aquaporin water channels: Molecular mechanisms for human diseases1. FEBS Lett. 2003;555:72–78. doi: 10.1016/S0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 9.Verkman A.S., Smith A.J., Phuan P.-w., Tradtrantip L., Anderson M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets. 2017;21:1161–1170. doi: 10.1080/14728222.2017.1398236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B., Kim J.K., Verkman A.S. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006;580:6679–6684. doi: 10.1016/j.febslet.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle N.A. Aquaporins as targets for drug discovery. Drug Discov. Today. 2005;10:485–493. doi: 10.1016/S1359-6446(05)03390-8. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson U.K., Fischer G., Friemann R., Enkavi G., Tajkhorshid E., Neutze R. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science. 2013;340:1346–1349. doi: 10.1126/science.1234306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos M.C., Verkman A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007;22:778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tradtrantip L., Jin B.-J., Yao X., Anderson M.O., Verkman A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017;969:239–250. doi: 10.1007/978-94-024-1057-0_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janosi L., Ceccarelli M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS ONE. 2013;8:e59897. doi: 10.1371/journal.pone.0059897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchen P., Conner M.T., Bill R.M., Conner A.C. Structural Determinants of Oligomerization of the Aquaporin-4 Channel. J. Biol. Chem. 2016;291:6858–6871. doi: 10.1074/jbc.M115.694729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verkman A.S., Mitra A.K. Structure and function of aquaporin water channels. Am. J. Physiol.-Ren. Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 18.Gonen T., Walz T. The structure of aquaporins. Quart. Rev. Biophys. 2006;39:361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- 19.Yool A.J., Weinstein A.M. New Roles for Old Holes: Ion Channel Function in Aquaporin-1. Physiology. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- 20.Yool A.J., Stamer W.D., Regan J.W. Forskolin Stimulation of Water and Cation Permeability in Aquaporin1 Water Channels. Science. 1996;273:1216–1218. doi: 10.1126/science.273.5279.1216. [DOI] [PubMed] [Google Scholar]
- 21.Agre P., Lee M.D., Devidas S., Guggino W.B. Aquaporins and Ion Conductance. Science. 1997;275:1490–1492. doi: 10.1126/science.275.5305.1490. [DOI] [PubMed] [Google Scholar]
- 22.Anthony T.L., Brooks H.L., Boassa D., Leonov S., Yanochko G.M., Regan J.W., Yool A.J. Cloned Human Aquaporin-1 Is a Cyclic GMP-Gated Ion Channel. Mol. Pharmacol. 2000;57:576–588. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Cohen J., Boron W.F., Schulten K., Tajkhorshid E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J. Struct. Biol. 2007;157:534–544. doi: 10.1016/j.jsb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Endeward V., Arias-Hidalgo M., Al-Samir S., Gros G. CO₂ Permeability of Biological Membranes and Role of CO₂ Channels. Membranes. 2017;7:61. doi: 10.3390/membranes7040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes D., Agasse A., Thiébaud P., Delrot S., Gerós H., Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta (BBA) Biomembr. 2009;1788:1213–1228. doi: 10.1016/j.bbamem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda M., Beitz E., Kozono D., Guggino W.B., Agre P., Yasui M. Characterization of Aquaporin-6 as a Nitrate Channel in Mammalian Cells: REQUIREMENT OF PORE-LINING RESIDUE THREONINE 63. J. Biol. Chem. 2002;277:39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 27.Yasui M., Kwon T.-H., Knepper M.A., Nielsen S., Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazama A., Kozono D., Guggino W.B., Agre P., Yasui M. Ion Permeation of AQP6 Water Channel Protein: SINGLE-CHANNEL RECORDINGS AFTER Hg2+ACTIVATION. J. Biol. Chem. 2002;277:29224–29230. doi: 10.1074/jbc.M204258200. [DOI] [PubMed] [Google Scholar]
- 29.Krane C.M., Goldstein D.L. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm. Genome. 2007;18:452–462. doi: 10.1007/s00335-007-9041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saparov S.M., Liu K., Agre P., Pohl P. Fast and Selective Ammonia Transport by Aquaporin-8. J. Biol. Chem. 2007;282:5296–5301. doi: 10.1074/jbc.M609343200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soria L.R., Fanelli E., Altamura N., Svelto M., Marinelli R.A., Calamita G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem. Biophys. Res. Commun. 2010;393:217–221. doi: 10.1016/j.bbrc.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 32.Horsefield R., Nordén K., Fellert M., Backmark A., Törnroth-Horsefield S., Terwisscha van Scheltinga A.C., Kvassman J., Kjellbom P., Johanson U., Neutze R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA. 2008;105:13327–13332. doi: 10.1073/pnas.0801466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitchen P., Day R.E., Salman M.M., Conner M.T., Bill R.M., Conner A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta (BBA) 2015;1850:2410–2421. doi: 10.1016/j.bbagen.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Han B.-G., Guliaev A.B., Walian P.J., Jap B.K. Water Transport in AQP0 Aquaporin: Molecular Dynamics Studies. J. Mol. Biol. 2006;360:285–296. doi: 10.1016/j.jmb.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Chepelinsky A.B. Structural Function of MIP/Aquaporin 0 in the Eye Lens; Genetic Defects Lead to Congenital Inherited Cataracts. In: Beitz E., editor. Aquaporins. Springer; Berlin/Heidelberg, Germany: 2009. pp. 265–297. [DOI] [PubMed] [Google Scholar]
- 36.Harries W.E., Akhavan D., Miercke L.J., Khademi S., Stroud R.M. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc. Natl. Acad. Sci. USA. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen S., Smith B.L., Christensen E.I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agre P. Aquaporin water channels (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2004;43:4278–4290. doi: 10.1002/anie.200460804. [DOI] [PubMed] [Google Scholar]
- 39.Noda Y., Sohara E., Ohta E., Sasaki S. Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 2010;6:168–178. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 40.Murata K., Mitsuoka K., Hirai T., Walz T., Agre P., Heymann J.B., Engel A., Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 41.Frick A., Eriksson U.K., de Mattia F., Öberg F., Hedfalk K., Neutze R., de Grip W.J., Deen P.M.T., Törnroth-Horsefield S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA. 2014;111:6305–6310. doi: 10.1073/pnas.1321406111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvanese L., D’Auria G., Vangone A., Falcigno L., Oliva R. Structural Basis for Mutations of Human Aquaporins Associated to Genetic Diseases. Int. J. Mol. Sci. 2018;19:1577. doi: 10.3390/ijms19061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeyaseelan K., Sepramaniam S., Armugam A., Wintour E.M. Aquaporins: A promising target for drug development. Expert Opin. Ther. Targets. 2006;10:889–909. doi: 10.1517/14728222.10.6.889. [DOI] [PubMed] [Google Scholar]
- 44.Ishibashi K., Hara S., Kondo S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009;13:107–117. doi: 10.1007/s10157-008-0118-6. [DOI] [PubMed] [Google Scholar]
- 45.Soler D.C., Young A.E., Griffith A.D., Fu P.f., Cooper K.D., McCormick T.S., Popkin D.L. Overexpression of AQP3 and AQP10 in the skin exacerbates psoriasiform acanthosis. Exp. Dermatol. 2017;26:949–951. doi: 10.1111/exd.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho J.D., Yeh R., Sandstrom A., Chorny I., Harries W.E.C., Robbins R.A., Miercke L.J.W., Stroud R.M. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc. Natl. Acad. Sci. USA. 2009;106:7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takata K., Matsuzaki T., Tajika Y. Aquaporins: Water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 2004;39:1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Yasui M., Hazama A., Kwon T.-H., Nielsen S., Guggino W.B., Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402:184. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 49.Ishibashi K., Sasaki S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 1997;1:247–253. doi: 10.1007/BF02480636. [DOI] [PubMed] [Google Scholar]
- 50.Ma T., Yang B., Verkman A.S. Cloning of a Novel Water and Urea-Permeable Aquaporin from Mouse Expressed Strongly in Colon, Placenta, Liver, and Heart. Biochem. Biophys. Res. Commun. 1997;240:324–328. doi: 10.1006/bbrc.1997.7664. [DOI] [PubMed] [Google Scholar]
- 51.Ishibashi K., Kuwahara M., Gu Y., Tanaka Y., Marumo F., Sasaki S. Cloning and Functional Expression of a New Aquaporin (AQP9) Abundantly Expressed in the Peripheral Leukocytes Permeable to Water and Urea, but Not to Glycerol. Biochem. Biophys. Res. Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- 52.Hatakeyama S., Yoshida Y., Tani T., Koyama Y., Nihei K., Ohshiro K., Kamiie J.-I., Yaoita E., Suda T., Hatakeyama K., et al. Cloning of a New Aquaporin (AQP10) Abundantly Expressed in Duodenum and Jejunum. Biochem. Biophys. Res. Commun. 2001;287:814–819. doi: 10.1006/bbrc.2001.5661. [DOI] [PubMed] [Google Scholar]
- 53.Yakata K., Tani K., Fujiyoshi Y. Water permeability and characterization of aquaporin-11. J. Struct. Biol. 2011;174:315–320. doi: 10.1016/j.jsb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Ohta E., Itoh T., Nemoto T., Kumagai J., Ko S.B.H., Ishibashi K., Ohno M., Uchida K., Ohta A., Sohara E., et al. Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am. J. Physiol.-Cell Physiol. 2009;297:C1368–C1378. doi: 10.1152/ajpcell.00117.2009. [DOI] [PubMed] [Google Scholar]
- 55.Itoh T., Rai T., Kuwahara M., Ko S.B., Uchida S., Sasaki S., Ishibashi K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005;330:832–838. doi: 10.1016/j.bbrc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 56.Nakano T., Nishigami C., Irie K., Shigemori Y., Sano K., Yamashita Y., Myose T., Tominaga K., Matsuo K., Nakamura Y., et al. Goreisan Prevents Brain Edema after Cerebral Ischemic Stroke by Inhibiting Aquaporin 4 Upregulation in Mice. J. Stroke Cerebrovasc. Dis. 2018;27:758–763. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Nave M., Castro R.E., Rodrigues C.M., Casini A., Soveral G., Gaspar M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine. 2016;11:1817–1830. doi: 10.2217/nnm-2016-0086. [DOI] [PubMed] [Google Scholar]
- 58.King L.S., Choi M., Fernandez P.C., Cartron J.-P., Agre P. Defective Urinary Concentrating Ability Due to a Complete Deficiency of Aquaporin-1. N. Engl. J. Med. 2001;345:175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- 59.Kitchen P., Conner A.C. Control of the Aquaporin-4 Channel Water Permeability by Structural Dynamics of Aromatic/Arginine Selectivity Filter Residues. Biochemistry. 2015;54:6753–6755. doi: 10.1021/acs.biochem.5b01053. [DOI] [PubMed] [Google Scholar]
- 60.Deen P.M.T., van Balkom B.W.M., Kamsteeg E.-J. Routing of the aquaporin-2 water channel in health and disease. Eur. J. Cell Biol. 2000;79:523–530. doi: 10.1078/0171-9335-00075. [DOI] [PubMed] [Google Scholar]
- 61.Judith Radin M., Yu M.-J., Stoedkilde L., Lance Miller R., Hoffert J.D., Frokiaer J., Pisitkun T., Knepper M.A. Aquaporin-2 regulation in health and disease. Vet. Clin. Pathol. 2012;41:455–470. doi: 10.1111/j.1939-165x.2012.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrier R.W., Cadnapaphornchai M.A. Renal aquaporin water channels: From molecules to human disease. Prog. Biophys. Mol. Biol. 2003;81:117–131. doi: 10.1016/S0079-6107(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 63.Brown D. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol.-Ren. Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 64.Hara-Chikuma M., Verkman A.S. Prevention of Skin Tumorigenesis and Impairment of Epidermal Cell Proliferation by Targeted Aquaporin-3 Gene Disruption. Mol. Cell. Biol. 2008;28:326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hara-Chikuma M., Verkman A.S. Roles of Aquaporin-3 in the Epidermis. J. Investig. Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 66.Hara-Chikuma M., Satooka H., Watanabe S., Honda T., Miyachi Y., Watanabe T., Verkman A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015;6:7454. doi: 10.1038/ncomms8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu N., Feng X., He C., Gao H., Yang L., Ma Q., Guo L., Qiao Y., Yang H., Ma T. Defective macrophage function in aquaporin-3 deficiency. Faseb J. 2011;25:4233–4239. doi: 10.1096/fj.11-182808. [DOI] [PubMed] [Google Scholar]
- 68.Hara-Chikuma M., Chikuma S., Sugiyama Y., Kabashima K., Verkman A.S., Inoue S., Miyachi Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012;209:1743–1752. doi: 10.1084/jem.20112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jullienne A., Fukuda A.M., Ichkova A., Nishiyama N., Aussudre J., Obenaus A., Badaut J. Modulating the water channel AQP4 alters miRNA expression, astrocyte connectivity and water diffusion in the rodent brain. Sci. Rep. 2018;8:4186. doi: 10.1038/s41598-018-22268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang G., Yang G.-Y. Aquaporin-4: A Potential Therapeutic Target for Cerebral Edema. Int. J. Mol. Sci. 2016;17:1413. doi: 10.3390/ijms17101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang B., Li W., Jin H., Nie X., Shen H., Li E., Wang W. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir. Physiol. Neurobiol. 2018;255:50–57. doi: 10.1016/j.resp.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Verkman A.S., Phuan P.-W., Asavapanumas N., Tradtrantip L. Biology of AQP4 and anti-AQP4 antibody: Therapeutic implications for NMO. Brain Pathol. 2013;23:684–695. doi: 10.1111/bpa.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salman M.M., Sheilabi M.A., Bhattacharyya D., Kitchen P., Conner A.C., Bill R.M., Woodroofe M.N., Conner M.T., Princivalle A.P. Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur. J. Neurosci. 2017;46:2121–2132. doi: 10.1111/ejn.13652. [DOI] [PubMed] [Google Scholar]
- 74.Binder D.K., Yao X., Zador Z., Sick T.J., Verkman A.S., Manley G.T. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- 75.Amiry-Moghaddam M., Williamson A., Palomba M., Eid T., de Lanerolle N.C., Nagelhus E.A., Adams M.E., Froehner S.C., Agre P., Ottersen O.P. Delayed K+ clearance associated with aquaporin-4 mislocalization: Phenotypic defects in brains of α-syntrophin-null mice. Proc. Natl. Acad. Sci. USA. 2003;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg G.A. Ischemic brain edema. Prog. Cardiovasc. Dis. 1999;42:209–216. doi: 10.1016/S0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 77.Simard J.M., Kent T.A., Chen M., Tarasov K.V., Gerzanich V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salman M.M., Kitchen P., Woodroofe M.N., Bill R.M., Conner A.C., Heath P.R., Conner M.T. Transcriptome Analysis of Gene Expression Provides New Insights into the Effect of Mild Therapeutic Hypothermia on Primary Human Cortical Astrocytes Cultured under Hypoxia. Front. Cell. Neurosci. 2017;11:386. doi: 10.3389/fncel.2017.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yenari M.A., Han H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012;13:267. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 80.Salman M.M., Kitchen P., Woodroofe M.N., Brown J.E., Bill R.M., Conner A.C., Conner M.T. Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. Eur. J. Neurosci. 2017;46:2542–2547. doi: 10.1111/ejn.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igarashi H., Huber V.J., Tsujita M., Nakada T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011;32:113–116. doi: 10.1007/s10072-010-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papadopoulos M.C., Bennett J.L., Verkman A.S. Treatment of neuromyelitis optica: State-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014;10:493. doi: 10.1038/nrneurol.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tradtrantip L., Zhang H., Saadoun S., Phuan P.-W., Lam C., Papadopoulos M.C., Bennett J.L., Verkman A.S. Anti–Aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agre P., King L.S., Yasui M., Guggino W.B., Ottersen O.P., Fujiyoshi Y., Engel A., Nielsen S. Aquaporin water channels--from atomic structure to clinical medicine. J. Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Day R.E., Kitchen P., Owen D.S., Bland C., Marshall L., Conner A.C., Bill R.M., Conner M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta (BBA) 2014;1840:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 86.Tada J., Sawa T., Yamanaka N., Shono M., Akamatsu T., Tsumura K., Parvin M.N., Kanamori N., Hosoi K. Involvement of Vesicle–Cytoskeleton Interaction in AQP5 Trafficking in AQP5-Gene-Transfected HSG Cells. Biochem. Biophys. Res. Commun. 1999;266:443–447. doi: 10.1006/bbrc.1999.1828. [DOI] [PubMed] [Google Scholar]
- 87.Ishikawa Y., Eguchi T., Skowronski M.T., Ishida H. Acetylcholine Acts on M3Muscarinic Receptors and Induces the Translocation of Aquaporin5 Water Channel via Cytosolic Ca2+Elevation in Rat Parotid Glands. Biochem. Biophys. Res. Commun. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 88.Kondo H., Shimomura I., Kishida K., Kuriyama H., Makino Y., Nishizawa H., Matsuda M., Maeda N., Nagaretani H., Kihara S., et al. Human aquaporin adipose (AQPap) gene. Eur. J. Biochem. 2002;269:1814–1826. doi: 10.1046/j.1432-1033.2002.02821.x. [DOI] [PubMed] [Google Scholar]
- 89.Soveral G., Casini A. Aquaporin modulators: A patent review (2010–2015) Expert Opin. Ther. Patents. 2017;27:49–62. doi: 10.1080/13543776.2017.1236085. [DOI] [PubMed] [Google Scholar]
- 90.Laforenza U., Scaffino M.F., Gastaldi G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE. 2013;8:e54474. doi: 10.1371/journal.pone.0054474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gorelick D.A., Praetorius J., Tsunenari T., Nielsen S., Agre P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006;7:14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morishita Y., Matsuzaki T., Hara-chikuma M., Andoo A., Shimono M., Matsuki A., Kobayashi K., Ikeda M., Yamamoto T., Verkman A., et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inoue Y., Sohara E., Kobayashi K., Chiga M., Rai T., Ishibashi K., Horie S., Su X., Zhou J., Sasaki S., et al. Aberrant Glycosylation and Localization of Polycystin-1 Cause Polycystic Kidney in an AQP11 Knockout Model. J. Am. Soc. Nephrol. 2014;25:2789–2799. doi: 10.1681/ASN.2013060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madeira A., Moura T.F., Soveral G. Detecting Aquaporin Function and Regulation. Front. Chem. 2016;4:3. doi: 10.3389/fchem.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dorr R.A., Kierbel A., Vera J., Parisi M. A new data-acquisition system for the measurement of the net water flux across epithelia. Comput. Methods Progr. Biomed. 1997;53:9–14. doi: 10.1016/S0169-2607(96)01801-9. [DOI] [PubMed] [Google Scholar]
- 96.Preston G.M., Carroll T.P., Guggino W.B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 97.Kitchen P., Day R.E., Taylor L.H., Salman M.M., Bill R.M., Conner M.T., Conner A.C. Identification and Molecular Mechanisms of the Rapid Tonicity-induced Relocalization of the Aquaporin 4 Channel. J. Biol. Chem. 2015;290:16873–16881. doi: 10.1074/jbc.M115.646034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patil R.V., Xu S., van Hoek A.N., Rusinko A., Feng Z., May J., Hellberg M., Sharif N.A., Wax M.B., Irigoyen M., et al. Rapid Identification of Novel Inhibitors of the Human Aquaporin-1 Water Channel. Chem. Biol. Drug Des. 2016;87:794–805. doi: 10.1111/cbdd.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mola M.G., Nicchia G.P., Svelto M., Spray D.C., Frigeri A. Automated cell-based assay for screening of aquaporin inhibitors. Anal. Chem. 2009;81:8219–8229. doi: 10.1021/ac901526k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zelenina M., Brismar H. Osmotic water permeability measurements using confocal laser scanning microscopy. Eur. Biophys. J. 2000;29:165–171. doi: 10.1007/PL00006645. [DOI] [PubMed] [Google Scholar]
- 101.Dickson P.N., Margerum D.W. Extension of accessible first-order rate constants and accurate dead-time determinations for stopped-flow spectroscopy. Anal. Chem. 1986;58:3153–3158. doi: 10.1021/ac00127a052. [DOI] [Google Scholar]
- 102.Keifer P.A. Flow techniques in NMR spectroscopy. In: Webb G.A., editor. Annual Reports on NMR Spectroscopy. Volume 62. Academic Press; Cambridge, MA, USA: 2007. pp. 1–47. [Google Scholar]
- 103.Hub J.S., Grubmüller H., de Groot B.L. Dynamics and Energetics of Permeation Through Aquaporins. What Do We Learn from Molecular Dynamics Simulations? In: Beitz E., editor. Aquaporins. Springer; Berlin/Heidelberg, Germany: 2009. pp. 57–76. [DOI] [PubMed] [Google Scholar]
- 104.Madeira A., Camps M., Zorzano A., Moura T.F., Soveral G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE. 2013;8:e83442. doi: 10.1371/journal.pone.0083442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brooks H.L., Regan J.W., Yool A.J. Inhibition of Aquaporin-1 Water Permeability by Tetraethylammonium: Involvement of the Loop E Pore Region. Mol. Pharmacol. 2000;57:1021–1026. [PubMed] [Google Scholar]
- 106.Müller E.M., Hub J.S., Grubmüller H., de Groot B.L. Is TEA an inhibitor for human Aquaporin-1? Pflug. Archiv Eur. J. Physiol. 2008;456:663–669. doi: 10.1007/s00424-007-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsukaguchi H., Shayakul C., Berger U.V., Mackenzie B., Devidas S., Guggino W.B., van Hoek A.N., Hediger M.A. Molecular Characterization of a Broad Selectivity Neutral Solute Channel. J. Biol. Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- 108.Müller-Lucks A., Gena P., Frascaria D., Altamura N., Svelto M., Beitz E., Calamita G. Preparative scale production and functional reconstitution of a human aquaglyceroporin (AQP3) using a cell free expression system. New Biotechnol. 2013;30:545–551. doi: 10.1016/j.nbt.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Preston G.M., Jung J.S., Guggino W.B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- 110.Martins A.P., Marrone A., Ciancetta A., Galán Cobo A., Echevarría M., Moura T.F., Re N., Casini A., Soveral G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE. 2012;7:e37435. doi: 10.1371/journal.pone.0037435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Almeida A., Soveral G., Casini A. Gold compounds as aquaporin inhibitors: New opportunities for therapy and imaging. MedChemComm. 2014;5:1444–1453. doi: 10.1039/C4MD00265B. [DOI] [Google Scholar]
- 112.Niemietz C.M., Tyerman S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002;531:443–447. doi: 10.1016/S0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- 113.Alejandra R., Natalia S., Alicia E.D. The blocking of aquaporin-3 (AQP3) impairs extravillous trophoblast cell migration. Biochem. Biophys. Res. Commun. 2018;499:227–232. doi: 10.1016/j.bbrc.2018.03.133. [DOI] [PubMed] [Google Scholar]
- 114.Zelenina M., Bondar A.A., Zelenin S., Aperia A. Nickel and extracellular acidification inhibit the water permeability of human aquaporin-3 in lung epithelial cells. J. Biol. Chem. 2003;278:30037–30043. doi: 10.1074/jbc.M302206200. [DOI] [PubMed] [Google Scholar]
- 115.Ozu M., Dorr R.A., Teresa Politi M., Parisi M., Toriano R. Water flux through human aquaporin 1: Inhibition by intracellular furosemide and maximal response with high osmotic gradients. Eur. Biophys. J. 2011;40:737–746. doi: 10.1007/s00249-011-0687-2. [DOI] [PubMed] [Google Scholar]
- 116.Migliati E., Meurice N., DuBois P., Fang J.S., Somasekharan S., Beckett E., Flynn G., Yool A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009;76:105–112. doi: 10.1124/mol.108.053744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsutomu Nakada V.H. Inhibitors of Aquaporin 4, Methods and Uses Thereof. 7,659,312. U.S. Patent. 2010 Feb 9;
- 118.Pelletier M.F., Farr G.W., Mcguirk P.R., Hall C.H., Boron W.F. Methods of Treating Cerebral Edema. US9573885B2. U.S. Patent. 2017 Feb 21;
- 119.Gao J., Wang X., Chang Y., Zhang J., Song Q., Yu H., Li X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006;350:165–170. doi: 10.1016/j.ab.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Huber V.J., Tsujita M., Kwee I.L., Nakada T. Inhibition of Aquaporin 4 by antiepileptic drugs. Bioorg. Med. Chem. 2009;17:418–424. doi: 10.1016/j.bmc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 121.Sonntag Y., Gena P., Maggio A., Singh T., Artner I., Oklinski M.K., Johanson U., Kjellbom P., Nieland J.D., Nielsen S., et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019 doi: 10.1074/jbc.RA118.006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang B., Zhang H., Verkman A.S. Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides. Bioorg. Med. Chem. 2008;16:7489–7493. doi: 10.1016/j.bmc.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Detmers F.J.M., de Groot B.L., Müller E.M., Hinton A., Konings I.B.M., Sze M., Flitsch S.L., Grubmüller H., Deen P.M.T. Quaternary Ammonium Compounds as Water Channel Blockers: SPECIFICITY, POTENCY, AND SITE OF ACTION. J. Biol. Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- 124.Søgaard R., Zeuthen T. Test of blockers of AQP1 water permeability by a high-resolution method: No effects of tetraethylammonium ions or acetazolamide. Pflüg. Arch. Eur. J. Physiol. 2008;456:285–292. doi: 10.1007/s00424-007-0392-2. [DOI] [PubMed] [Google Scholar]
- 125.Pastorekova S., Parkkila S., Pastorek J., Supuran C.T. Review Article. J. Enzym. Inhib. Med. Chem. 2004;19:199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]
- 126.Huber V.J., Tsujita M., Yamazaki M., Sakimura K., Nakada T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007;17:1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 127.Tanimura Y., Hiroaki Y., Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J. Struct. Biol. 2009;166:16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Shank R.P., Maryanoff B.E. Molecular Pharmacodynamics, Clinical Therapeutics, and Pharmacokinetics of Topiramate. CNS Neurosci. Ther. 2008;14:120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pirici I., Balsanu T.A., Bogdan C., Margaritescu C., Divan T., Vitalie V., Mogoanta L., Pirici D., Carare R.O., Muresanu D.F. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. Int. J. Mol. Sci. 2017;19:46. doi: 10.3390/ijms19010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huber V.J., Tsujita M., Nakada T. Aquaporins in drug discovery and pharmacotherapy. Mol. Asp. Med. 2012;33:691–703. doi: 10.1016/j.mam.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 131.Esteva-Font C., Phuan P.-W., Anderson M.O., Verkman A.S. A Small Molecule Screen Identifies Selective Inhibitors of Urea Transporter UT-A. Chem. Biol. 2013;20:1235–1244. doi: 10.1016/j.chembiol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ishibashi K., Sasaki S., Fushimi K., Uchida S., Kuwahara M., Saito H., Furukawa T., Nakajima K., Yamaguchi Y., Gojobori T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA. 1994;91:6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dajani S., Saripalli A., Sharma-Walia N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget. 2018;9:36392–36405. doi: 10.18632/oncotarget.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J., Feng L., Zhu Z., Zheng M., Wang D., Chen Z., Sun H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015;13:96. doi: 10.1186/s12967-015-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Papadopoulos M.C., Saadoun S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta (BBA) Biomembr. 2015;1848:2576–2583. doi: 10.1016/j.bbamem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 136.Aikman B., de Almeida A., Meier-Menches S.M., Casini A. Aquaporins in cancer development: Opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metall. Integr. Biometal Sci. 2018;10:696–712. doi: 10.1039/C8MT00072G. [DOI] [PubMed] [Google Scholar]
- 137.Zelenina M., Tritto S., Bondar A.A., Zelenin S., Aperia A. Copper Inhibits the Water and Glycerol Permeability of Aquaporin-3. J. Biol. Chem. 2004;279:51939–51943. doi: 10.1074/jbc.M407645200. [DOI] [PubMed] [Google Scholar]
- 138.Spinello A., de Almeida A., Casini A., Barone G. The inhibition of glycerol permeation through aquaglyceroporin-3 induced by mercury(II): A molecular dynamics study. J. Inorg. Biochem. 2016;160:78–84. doi: 10.1016/j.jinorgbio.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 139.Martins A.P., Ciancetta A., de Almeida A., Marrone A., Re N., Soveral G., Casini A. Aquaporin Inhibition by Gold(III) Compounds: New Insights. ChemMedChem. 2013;8:1086–1092. doi: 10.1002/cmdc.201300107. [DOI] [PubMed] [Google Scholar]
- 140.Madeira A., de Almeida A., de Graaf C., Camps M., Zorzano A., Moura T.F., Casini A., Soveral G. A Gold Coordination Compound as a Chemical Probe to Unravel Aquaporin-7 Function. ChemBioChem. 2014;15:1487–1494. doi: 10.1002/cbic.201402103. [DOI] [PubMed] [Google Scholar]
- 141.Jha R.M., Kochanek P.M., Simard J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019;145:230–246. doi: 10.1016/j.neuropharm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patil R.V., Chatterton J.E., Sharif N.A., Wax M.B. RNAi-Mediated Inhibition of Aquaporin 4 for Treatment of Ocular Neovascularization. US20080214486A1. U.S. Patent. 2008 Jul 31;
- 143.Chatterton J.E., Patil R.V., Sharif N.A., Clark A.F., Wax M.B. RNAi-Mediated Inhibition of Aquaporin 1 for Treatment of Iop-Related Conditions. US20080171719A1. U.S. Patent. 2008 Sep 4;
- 144.Haraguchi G., Suzuki J.-I., Kakuta T., Maejima Y., Onai Y., Isobe M., Itai A., Fukasawa H., Muto S. Inhibition of IκB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Pelletier M.F., Mcguirk P.R., Farr G.W., Zamboni R., Colucci J., Zaghdane H. Prodrug Salts. US9827253B2. U.S. Patent. 2017 Nov 28;
- 146.Baatar D., Patel K., Taub D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011;340:44–58. doi: 10.1016/j.mce.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 147.Lopez N.E., Lindsay G., Karina L.R., Mary H.A., Putnam J., Eliceiri B., Coimbra R., Bansal V. Ghrelin decreases motor deficits after traumatic brain injury. J. Surg. Res. 2014;187:230–236. doi: 10.1016/j.jss.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 148.Bansal V. Methods of Treating Mild Brain Injury. US10105416B2. U.S. Patent. 2018 Oct 23;
- 149.Hossienzadeh F., Babri S., Alipour M.R., Ebrahimi H., Mohaddes G. Effect of ghrelin on brain edema induced by acute and chronic systemic hypoxia. Neurosci. Lett. 2013;534:47–51. doi: 10.1016/j.neulet.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 150.Lopez N.E., Krzyzaniak M.J., Blow C., Putnam J., Ortiz-Pomales Y., Hageny A.-M., Eliceiri B., Coimbra R., Bansal V. Ghrelin Prevents Disruption of the Blood–Brain Barrier after Traumatic Brain Injury. J. Neurotrauma. 2012;29:385–393. doi: 10.1089/neu.2011.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levy M. Highly Soluble Aquaporin-4 Extracellular Loop c Peptide Immunization for Treatment of Neuromyelitis Optica. US20170080063A1. U.S. Patent. 2017 Mar 23;
- 152.Flynn G.A., Yool A.J., Migliati E.R., Ritter L.S. Aquaporin Modulators and Methods of Using Them for the Treatment of Edema and Fluid Imbalance. US20150224108A1. U.S. Patent. 2015 Aug 13;
- 153.Bar-shavit Z., Aharon R. Modulation of Osteoclast Differentiation. US20150065427A1. U.S. Patent. 2015 Mar 5;