Graphical abstract
Keywords: Myocarditis, Left ventricular wall thickness, Strain
Highlights
-
•
Different types of myocardium infiltration can lead to similar manifestations.
-
•
Differentiating CA is important as it often carries dismal prognosis if not treated.
-
•
EM due to ibrutinib mimicked CA.
-
•
Ibrutinib discontinuation led to functional and structural normalization.
Introduction
Differential diagnosis of increased left ventricular (LV) wall thickness includes hypertensive heart disease, aortic valve stenosis, hypertrophic cardiomyopathy, and infiltrative cardiomyopathies such as cardiac amyloidosis (CA), all of them chronic conditions. Differentiating CA from other causes of increased LV wall thickness is important because it often carries a dismal prognosis if not treated.1 Although other conditions may mimic CA,2 recent advances in imaging such as myocardial strain, cardiac magnetic resonance imaging (MRI), and nuclear imaging have proved very helpful in discriminating CA from other causes of increased LV wall thickness. Nonetheless, despite sensitivities and specificities of findings such as apical sparing in myocardial strain, late gadolinium enhancement, and short inversion time in T1 mapping on cardiac MRI, as well as radiotracer accumulation on nuclear imaging, often a tissue diagnosis is necessary to confirm the diagnosis of CA.2
As mentioned before, an increase in LV wall thickness is not necessarily due to chronic disease, as acute processes such as ischemia with reperfusion and inflammation can lead to myocardial swelling. Here, we describe a case of acute myocarditis that presented with increased LV wall thickness and demonstrated various imaging features mimicking infiltrative cardiomyopathy.
Case Presentation
A 59-year-old man was seen in the emergency department following an episode of syncope 4 months after starting oral chemotherapy with ibrutinib for chronic lymphocytic leukemia. The syncope occurred in his bathroom and was not preceded by a prodrome. The patient had had a similar episode a few days before, but at that time he did not seek medical attention. The patient denied chest pain and any other cardiac symptoms.
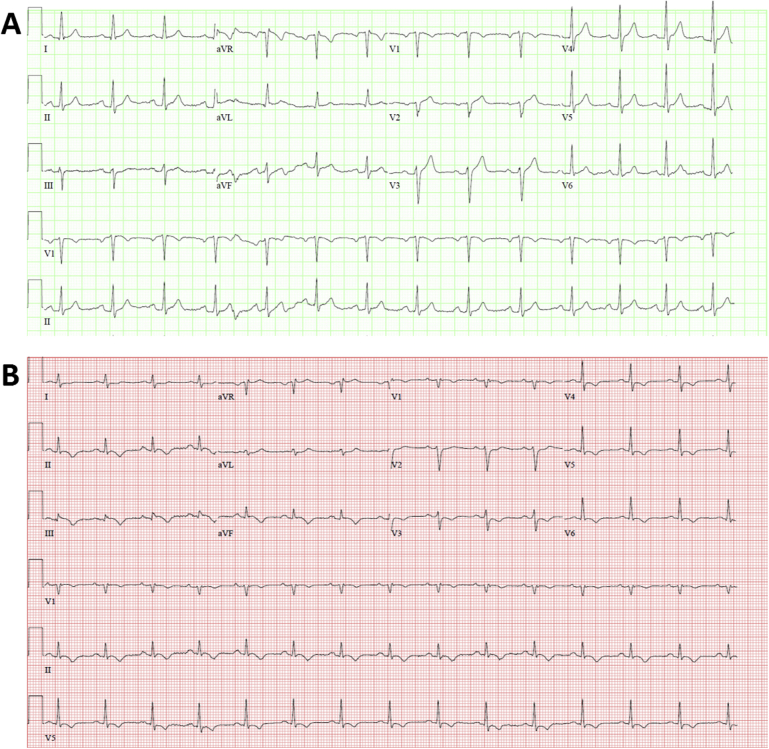
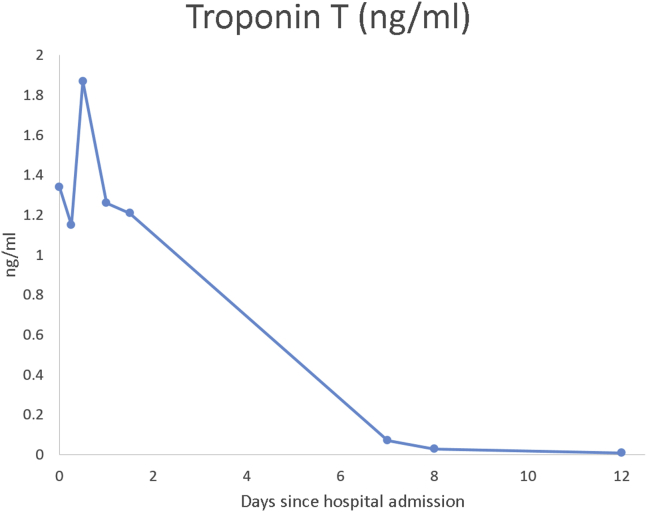
Upon admission to the emergency department, physical examination revealed facial abrasions from the fall, a heart rate of 86 beats/min, and blood pressure of 132/79 mm Hg. Electrocardiography (ECG) showed lower voltage in the limb leads, compared with prior findings from 3 months before, and T-wave inversions in the inferior and lateral leads with questionable ST-segment depressions in lead V3 suggestive of ischemia (Figure 1). Laboratory results revealed elevated levels of troponin T (1.340 ng/mL; reference range, <0.029 ng/mL) with a significant change over time (Figure 2), creatine kinase–MB (23.9 ng/mL; reference range, <10.3 ng/mL), and d-dimer (5,880 ng/mL; reference range, <500 ng/mL).
Figure 1.
(A) Baseline ECG performed 3 months before the day of admission with no abnormal findings. (B) ECG performed on the day of admission showing lower voltage in the limb leads compared with prior ECG from 3 months before and T-wave inversions in the inferior and lateral leads, with questionable ST-segment depressions in lead V3 suggestive of ischemia.
Figure 2.
Troponin T trend since the day of admission. Elevated values with positive delta on the first 2 days in the hospital. Normal value after 7 days, maintained during follow-up.
Despite d-dimer elevation, computed tomographic angiography did not reveal pulmonary embolus or coronary calcification but demonstrated a small pericardial effusion. The patient was admitted to the hospital for further evaluation.
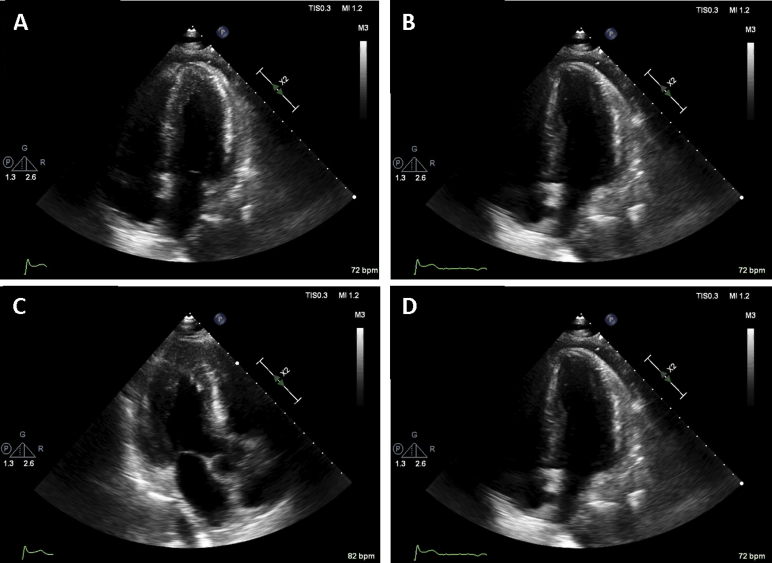
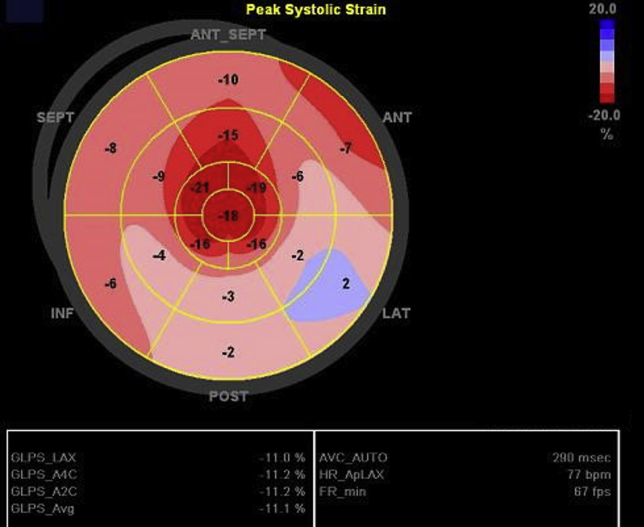
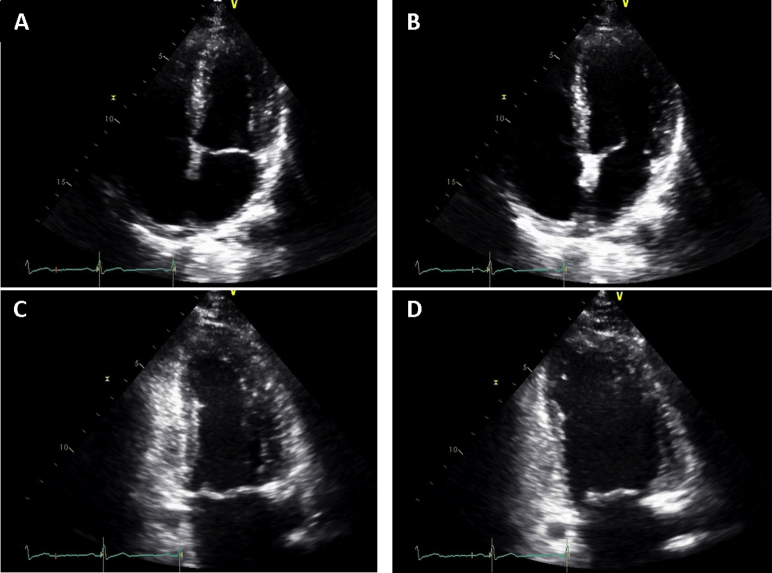
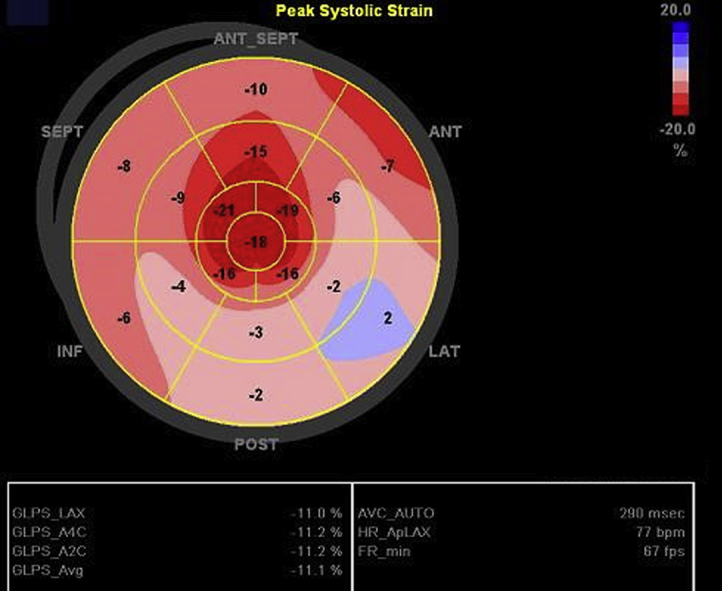
Point-of-care echocardiography on the day of admission showed diffuse thickening of the LV walls without regional wall motion abnormalities (Video 1). In addition, same-day standard transthoracic echocardiography showed a decreased LV ejection fraction of 48%, mildly decreased right ventricular systolic function, and a small circumferential pericardial effusion (Figure 3, Video 2), with relative sparing of longitudinal strain in the apical compared with the mid and basal segments, and an average global longitudinal strain of −11.1% (Figure 4). On standard transthoracic echocardiography (TTE), the interventricular septal and posterior wall thicknesses were 2.3 and 2.2 cm, respectively. These findings were in striking contrast to those on echocardiography performed 3 months prior that showed a normal LVEF and normal interventricular septal and posterior wall thicknesses of 1.0 cm (Figure 5, Video 3).
Figure 3.
TTE on admission: (A) apical four-chamber view during systole, (B) apical four-chamber view during diastole, (C) apical three-chamber view during systole, and (D) apical three-chamber view during diastole. Finding of mild concentric LV hypertrophy with mildly decreased ejection fraction of 48%, grade I LV diastolic dysfunction, mildly decreased right ventricular systolic function, and a small circumferential pericardial effusion.
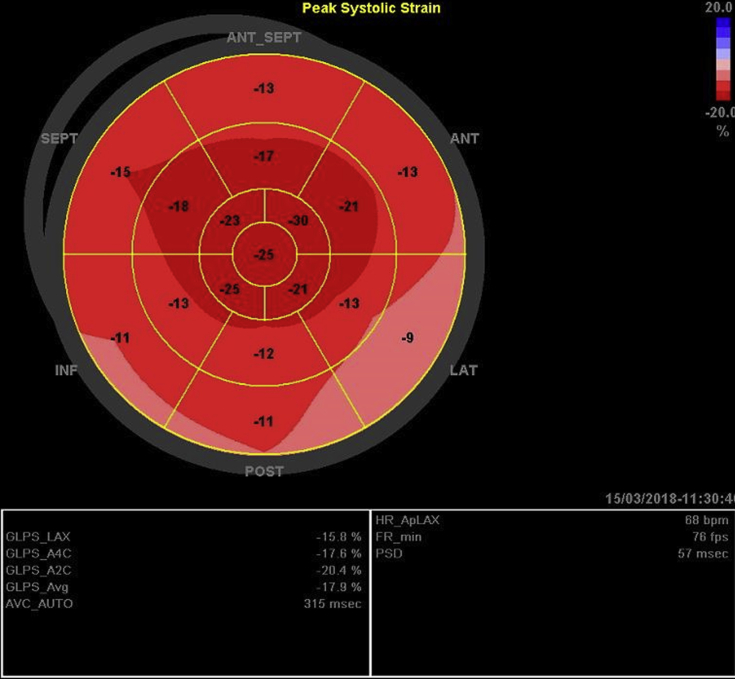
Figure 4.
Global longitudinal strain (GLS) bull's-eye diagram. Finding of apical sparing with an average GLS of −11%. ANT, Anterior; ANT_SEPT, anteroseptal; INF, inferior; LAT, lateral; POST, posterior; SEPT, septal.
Figure 5.
Baseline TTE 3 months before admission: (A) apical four-chamber view during systole, (B) apical four-chamber view during diastole, (C) apical two-chamber view during systole, and (D) apical two-chamber view during diastole. Normal ventricular function and wall thickness.
Same-day right and left coronary angiography was performed to assess for potential ischemic heart disease and hemodynamics. Both LV and right ventricular filling pressures were elevated, with a mean right atrial pressure of 19 mm Hg, mean pulmonary capillary wedge pressure of 19 mm Hg, right ventricular systolic and end-diastolic pressures of 45 and 18 mm Hg, respectively, and a cardiac index of 2.5 L/min/m2 (by the Fick method). Coronary angiography revealed only 40% stenosis in an inferior branch of a large obtuse marginal branch.
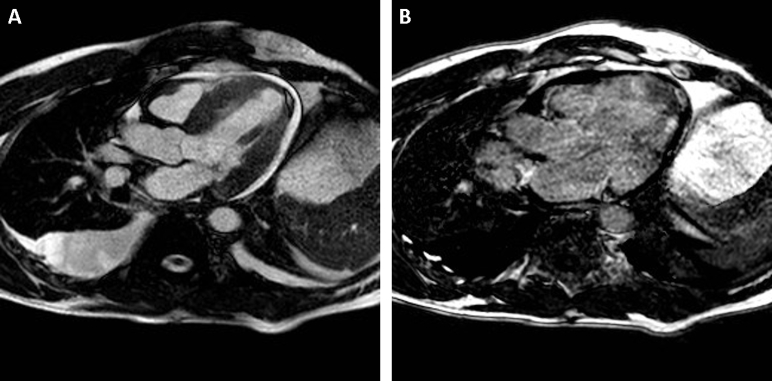
To further elucidate the etiology of the increased LV wall thickness, a contrast-enhanced cardiac magnetic imaging study was performed the next day that again showed diffuse thickening of the left ventricle with septal predominance and small pericardial and pleural effusion (Figure 6A) with mildly depressed systolic function (LVEF 47%). Scar images were characterized by difficulty in obtaining satisfactory nulling of LV myocardium, with suggestion of diffuse areas of nonischemic delayed enhancement. Additionally, inversion time scout images were notable for myocardium nulling at approximate the same inversion time as blood pool, indicating abnormal gadolinium contrast kinetics (Figure 6B, Video 4). The overall findings were reported as suggestive of CA.
Figure 6.
Cardiac MRI (A,B) in four-chamber views, with thickened left ventricle and small pericardial effusion (bright-blood image, A) and poor differentiation between blood pool and myocardium on delayed postcontrast image (B).
Although imaging findings suggested CA, a condition that usually has a chronic and insidious evolution, the acute development of LV hypertrophy (given prior echocardiographic findings) and the presence of cardiac biomarkers suggested other possible causes such as myocarditis. Because of discrepancy between what appeared to be an acute process and the findings suggestive of CA, an endomyocardial biopsy was performed on the third day of hospitalization. The pathology report described an abundant inflammatory infiltrate consistent of mostly eosinophils with rare mononuclear cells predominantly present through the perimysial space of the cardiac muscle fascicles surrounding the small arterioles, without signs of infiltrative disease. The description and location of the infiltrates were suggestive of a hypersensitivity eosinophilic myocarditis (EM) process. Of note, the highest eosinophil count was slightly elevated at 0.6 k/μL (reference range, <0.46 k/μL).
Treatment with intravenous methylprednisolone 50 mg twice daily was initiated, and ibrutinib was discontinued as the results of workup studies regarding oncologic or infectious causes were negative, and an adverse drug reaction was suspected.
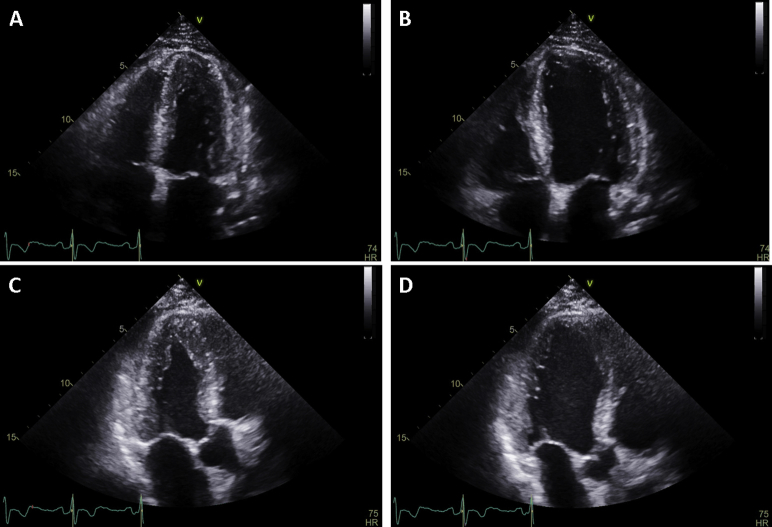
On the eighth day of hospitalization and 3 days after the start of steroid therapy, repeat TTE showed improvement in LV wall thickness and normalization of LV function (LVEF 64%; Figure 7, Video 5). Additionally, the apical sparing pattern seen on global longitudinal strain upon admission was transient in nature and was not appreciated on discharge TTE, which now revealed an average global longitudinal strain of −18% (Figure 8). Despite not being able to determine whether EM was related to ibrutinib, the chemotherapy regimen was changed to obinutuzumab, and the patient was discharged with a tapering dose of steroids.
Figure 7.
TTE before discharge: (A) apical four-chamber view during systole, (B) apical four-chamber view during diastole, (C) apical three-chamber view during systole, and (D) apical three-chamber view during diastole. Finding of improvement in LV wall thickness and normalization of LV function (LVEF 64%).
Figure 8.
Global longitudinal strain (GLS) bull's-eye diagram upon discharge. Resolution of apical sparing pattern with an average GLS of −18%. ANT, Anterior; ANT_SEPT, anteroseptal; INF, inferior; LAT, lateral; POST, posterior; SEPT, septal.
TTE and cardiology visits were scheduled 1 week, 1 month, and 6 months after discharge. On follow-up echocardiography performed 1 and 6 months after discharge, findings returned to baseline. It was determined by the primary hematologist-oncologist to discontinue ibrutinib permanently.
Discussion
This case shows rapid, dramatic, but transient changes in myocardial structure and function that can occur in hyperacute EM. In addition, these changes resemble findings that can be seen in CA, including decreased voltage on ECG, apical sparing on echocardiography, and markedly abnormal gadolinium kinetics with compromised scar imaging on MRI. Finally, removal of the suspected offending agent (ibrutinib) and the initiating steroid therapy led to rapid improvement in LV structure and function.
This patient presented with the main symptoms of syncope and elevated troponin, and with new electrocardiographic findings suggestive of lateral ischemia, without any chest pain. Ischemic etiology was ruled out with coronary angiography, and multimodality imaging including TTE and MRI was performed with findings mimicking those seen with CA. Nonetheless, the rapid onset of the myocardial structural and functional changes was unusual, and an endomyocardial biopsy revealed a diagnosis of EM mimicking CA.
EM is a rare cause of myocardial inflammation, with a large number of differential diagnoses varying from hypersensitivity to autoimmune reactions and infections. Additionally, EM has been associated with various medications. For instance, clozapine-induced EM occurs within the first months of exposure and occasionally progresses to chronic cardiomyopathy.3 However, in many cases, workup is not conclusive, and therefore the cause remains indeterminate. Clinical presentation can also vary from an underdiagnosed and ignored minimally symptomatic inflammation to a high mortality–associated acute form, like the one presented in our case.4, 5
Literature regarding EM is scarce, consisting mostly of case reports, with the largest case series including only eight patients.5 A recent review found that in published cases of EM, the median age was 41 years, and the most common clinical presentation was dyspnea and chest pain. Only 4% presented with syncope as in the present case. ECG at presentation most commonly revealed nonspecific abnormalities of the ST-T segment. Troponin and peripheral eosinophils were elevated in 95.7% and 75.9% of cases, respectively. Echocardiographic findings included a reduced median LVEF of 35% and pericardial effusion in 34.1% of cases, both characteristics found in the present case.5
Most notable in this case is the marked degree of myocardial infiltration causing changes and the striking similarities with noninvasive findings in CA. For instance, amyloid deposition in the extracellular space generates an increase in electrically inactive mass in the myocardium. This results in lower voltage detected on the ECG and a higher incidence of atrial and ventricular arrhythmias.2 Similarly, in this case, the 3-month difference between baseline and admission ECG showed decreased voltage in the limb leads.
On echocardiography, CA often shows marked thickening of ventricular walls leading to diastolic dysfunction, with a pattern of apical sparing of otherwise decreased global longitudinal strain. Previous studies revealed that a relative regional strain ratio > 1 is 93% sensitive and 82% specific for the differentiation of CA from other causes of chronic LV hypertrophy, such as hypertrophic cardiomyopathy and aortic stenosis.6 In this patient, increased LV thickness, diastolic dysfunction, apical sparing on global longitudinal strain, and a strain ratio of 1.2 were seen, all findings highly suggestive of CA.
Finally, characteristic MRI findings of CA include abnormal gadolinium kinetics, as well as difficulty nulling myocardium relative to blood pool, with areas of delayed enhancement in nonischemic pattern.7 A study by Austin et al.8 demonstrated that delayed hyperenhancement on cardiac MRI had sensitivity and specificity of 88% and 90%, respectively.8 All of these features were present in our patient. The similarity of findings in our patient with EM and patients with CA suggest that infiltration of the myocardium in itself, independent of whether it is caused by inflammatory cells or by amyloid, can lead to similar imaging and ECG manifestations.
Inhibitors of tyrosine kinase, such as ibrutinib, have been known to cause heart failure, cardiomyopathy, conduction abnormalities, QT prolongation, acute coronary syndromes, myocardial injury, arterial thrombosis, and hypertension. However, the most common manifestation of cardiotoxicity with tyrosine kinase inhibitors is systolic dysfunction or cardiomyopathy with resultant heart failure.9 The cardiac toxicity of ibrutinib relative to other tyrosine kinase inhibitors is unknown. Interestingly, a recent report described a case of new-onset cardiomyopathy in a 78-year-old man on ibrutinib therapy for relapsing mantle cell lymphoma. The patient presented after 4 months of ibrutinib therapy with an abnormal LVEF, mild global hypokinesis, and mild concentric LV hypertrophy associated with ventricular tachyarrhythmia. As a consequence ibrutinib was suspended, and repeat echocardiography 6 months later showed a recovery of LV function with only mild residual LV dilation.10 Although that case lacked histopathologic diagnosis, the clinical presentation, imaging findings, and clinical course are similar to those presented in our patient.
Conclusion
We present a case of hyperacute EM with acute onset of structural and functional changes in the setting of ibrutinib therapy. Multiple noninvasive diagnostic findings of EM in this patient mimicked CA, and an eventual endomyocardial biopsy revealed the correct diagnosis. The change in chemotherapeutic regimen led to rapid regression of structural changes with concomitant ventricular function normalization that persisted on subsequent follow-up. Further studies are required to determine the causative role of ibrutinib in EM.
Footnotes
Conflicts of interest: The authors report no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.11.001.
Supplementary Data
Apical four-chamber and apical three-chamber view of the point-of-care echocardiogram obtained on the day of admission showing diffuse thickening of LV walls without regional wall motion abnormalities.
Apical four-chamber and apical three-chamber view of the standard transthoracic echocardiogram obtained on the day of admission showing concentric LV hypertrophy with mildly decreased ejection fraction of 48%, grade I LV diastolic dysfunction, mildly decreased right ventricular systolic function, and a small circumferential pericardial effusion.
Apical four-chamber and apical two-chamber view of the standard transthoracic echocardiogram obtained 3 months before admission showing normal ventricular function and wall thickness.
Cardiac MRI showing inversion time scout images with myocardium nulling at approximate the same inversion time as blood pool, indicating abnormal gadolinium contrast kinetics.
Apical four-chamber and apical three-chamber view of the standard transthoracic echocardiogram obtained on the day of discharge showing improvement in LV wall thickness and normalization of LV function (LVEF 64%).
References
- 1.Bejar D., Colombo P.C., Latif F., Yuzefpolskaya M. Infiltrative cardiomyopathies. Clin Med Insights Cardiol. 2015;9(suppl 2):29–38. doi: 10.4137/CMC.S19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhogal S., Ladia V., Sitwala P., Cook E., Bajaj K., Ramu V. Cardiac amyloidosis: an updated review with emphasis on diagnosis and future directions. Curr Probl Cardiol. 2018;43:10–34. doi: 10.1016/j.cpcardiol.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Curto M., Girardi N., Lionetto L., Ciavarella G.M., Ferracuti S., Baldessarini R.J. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18:68. doi: 10.1007/s11920-016-0704-3. [DOI] [PubMed] [Google Scholar]
- 4.Cheung C.C., Constantine M., Ahmadi A., Shiau C., Chen L.Y.C. Eosinophilic myocarditis. Am J Med Sci. 2017;354:486–492. doi: 10.1016/j.amjms.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Brambatti M., Matassini M.V., Adler E.D., Klingel K., Camici P.G., Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Phelan D., Collier P., Thavendiranathan P., Popović Z.B., Hanna M., Plana J.C. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 7.Allderdice C., Marcu C., Kabirdas D. Intracardiac thrombus in leukemia: role of cardiac magnetic resonance imaging in eosinophilic myocarditis. CASE (Phila) 2018;2:114–117. doi: 10.1016/j.case.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin B.A., Tang W.H., Rodriguez E.R., Tan C., Flamm S.D., Taylor D.O. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2:1369–1377. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Chen M.H., Kerkelä R., Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace N., Wong E., Cooper D., Chao H. A case of new-onset cardiomyopathy and ventricular tachycardia in a patient receiving ibrutinib for relapsed mantle cell lymphoma. Clin Case Rep. 2016;4:1120–1121. doi: 10.1002/ccr3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber and apical three-chamber view of the point-of-care echocardiogram obtained on the day of admission showing diffuse thickening of LV walls without regional wall motion abnormalities.
Apical four-chamber and apical three-chamber view of the standard transthoracic echocardiogram obtained on the day of admission showing concentric LV hypertrophy with mildly decreased ejection fraction of 48%, grade I LV diastolic dysfunction, mildly decreased right ventricular systolic function, and a small circumferential pericardial effusion.
Apical four-chamber and apical two-chamber view of the standard transthoracic echocardiogram obtained 3 months before admission showing normal ventricular function and wall thickness.
Cardiac MRI showing inversion time scout images with myocardium nulling at approximate the same inversion time as blood pool, indicating abnormal gadolinium contrast kinetics.
Apical four-chamber and apical three-chamber view of the standard transthoracic echocardiogram obtained on the day of discharge showing improvement in LV wall thickness and normalization of LV function (LVEF 64%).