Abstract
Obesity in older adults is a growing public health problem, yet the appropriate treatment remains controversial partly due to evidence that weight loss reduces bone mass and may increase fracture risk. The purpose of this review is to summarize the research to date on the effects of diet-induced weight loss on bone health in obese (body mass index 30 kg/m2 and above) older (aged 65 years or older) adults. Observational studies have shown that weight loss in this population decreases total hip bone mineral density and increases the risk of frailty fractures (composite of proximal femur, pelvis, and proximal humerus fractures). Randomized controlled trials have largely confirmed these earlier observations but have also shown that exercise, particularly progressive resistance training, can attenuate or even alleviate this bone loss. Further research incorporating outcomes concerning bone quality and mass are needed to identify the optimal exercise and nutritional regimens to counteract the bone loss.
Keywords: older adults, obesity, weight loss, bone, bone mineral density, bone markers, fracture, review
1. The Public Health Problem of Obesity in Older Adults
The number of older adults (≥65 years) in the United States is expected to more than double between 2010 and 2050 from 40.8 million to 88.5 million. Over a third of older adults today are obese (Body Mass Index [BMI] ≥ 30 kg/m2) and trends suggest that the prevalence of obesity in this rapidly growing age group is increasing as well.1 For older adults, obesity is not only associated with a number of serious medical conditions, but also exacerbates the age-related decline in physical function causing frailty, decreases quality of life, and increases nursing home admissions.2 Despite this, there are concerns that weight loss in this obese older population may be harmful due to the loss of bone mass which can lead to an increased risk of fractures.3;4 In 2013, the AHA/ACC/TOS guidelines for the management of obesity stated “the overall safety of weight loss interventions for patients aged 65 and older remains controversial” and “…there is a need for further research to understand the most appropriate strategies and prescriptions for weight loss for some key populations including older adults”.5
2. Effect of Obesity on Age-related Loss of Bone Mass and Fracture Risk
Bone is constantly undergoing the process of remodeling, which is mediated by the tightly regulated actions of bone forming osteoblasts and bone resorbing osteoclasts. Ninety percent of peak bone mass is typically achieved by age 20 and the potential for increasing bone mass remains while bone formation and resorption are closely matched. Starting around the age of 30 there is a progressive shift towards favoring of bone resorption over bone formation.6 Cross-sectional studies looking at age-related changes in areal bone mineral density (BMD) as assessed by dual-energy x-ray absorptiometry (DXA) have noted increasing loss of bone mass with age.7 Volumetric BMD and bone geometry assessed by high resolution peripheral quantitative computed tomography (HRpQCT) in addition to estimated bone strength assessed by finite element analysis (FEA) have also been shown to worsen with age.8–9 Multiple mechanisms including secondary hyperparathyroidism, decreased physical activity with age, and the accumulation of bone marrow fat associated with aging have been implicated with estrogen deficiency associated with menopause in women being the most well studied.6;10–12
The positive association between BMI and BMD at the spine and total hip has been verified in a number of epidemiological studies.13–15 Obesity is thought to decrease fracture risk primarily by increasing mechanical loading on the bone due to an increase in overall body mass. Obesity may also provide additional site specific benefits to fracture risk beyond increased BMD such as increased adiposity at the thigh providing a “cushioning effect” in the event of a fall onto the hip.16–17
Despite these potential benefits, more recent data within the last decade suggest that the BMD in the obese does not correlate well with overall fracture risk.18–22 Increasing BMI is known to lower reproducibility and may also artificially increase BMD measurement at the spine in obese subjects.18 Obesity has a site-dependent effect on fracture risk with decreases in hip, pelvic, and wrist fractures, but increases in ankle, upper arm, and humerus fractures.19 Once corrected for BMD, one meta-analysis found that obesity was associated with an overall increase in osteoporotic fractures (hazard ratio 1.16; 95% confidence interval, 1.09–1.23).21 Epidemiological studies have also corroborated this finding by noting that the majority of fractures actually occur in this population as well.22–23
Obesity is thought to contribute to an increased risk of fracture via a number of separate mechanisms. Obesity is associated with an increased risk of falls as well as a higher risk of greater activities of daily living disability after fall.24–25 Increased central adiposity leads to an increased level of systemic inflammation whereby various inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) increase bone resorption and negatively impact multiple markers of bone quality (e.g. higher cortical porosity, decreased trabecular bone volume fraction, and lower trabecular stiffness).26–28 Obesity and increased adiposity is associated with a decrease in serum 25-hydroxyvitamin D levels.29–30 Parathyroid hormone levels have also been shown to correlate with increased adiposity, though it is not clear if this is solely related to secondary hyperparathyroidism from decreased 25-hydroxyvitamin D or if other mechanisms are involved.31–32
3. Effect of Weight Loss on Bone Mass and Fracture Risk in Older Adults
Observational studies focusing on the effect of weight loss on bone in older adults have shown that weight loss, both intentional and unintentional, is associated with decreases in BMD at the hip as well as an increase in frailty fractures (a composite of proximal femur, pelvis, and proximal humerus fractures).33–35 In the Study of Osteoporosis Fractures, older women who had weight loss of at least 5% from baseline had a 35% greater decline total hip BMD per year relative to weight stable women and a two-fold increase in the risk of subsequent hip fracture after an average follow-up duration of 6.6 years.33 Risk was increased regardless of baseline BMI, even in the overweight and obese population that engaged in voluntary weight loss. Women with involuntary weight loss were at the highest risk of frailty fracture.34 Similar findings were also found in older men in the Osteoporotic Fractures in Men (MrOS) Study where the adjusted average rate of change in total hip BMD was 0.1%/year in men with weight gain, −0.3%/year in weight stable men versus −1.4%/year in men with weight loss. This trend held even in the obese population undergoing voluntary weight loss, who had an average change in total hip BMD of −1.7%/year.35
Randomized controlled trials (RCTs) involving older adults with obesity undergoing voluntary weight loss have provided additional insights.36–45 Table 1 summarizes the findings of 9 RCTs published between 2000–2017 (identified by literature search of Index Medicus between 2000 and 2017, a search of journals that focus on geriatrics or obesity, and a search of references listed in relevant research and review articles) meeting the following specific inclusion criteria: (1) subjects with a minimum age of ≥60 years and mean age of ≥65 years, (2) subjects with a minimum BMI ≥ 27 kg/m2 and mean BMI ≥ 30 kg/m2, (3) a weight loss duration of at least 5 months, and (4) at least one study outcome focusing on BMD outcomes at the hip or the lumbar spine (clinically relevant sites of osteoporotic fractures). One additional study was also included despite not meeting the above criteria since the RCT reported on fracture outcomes data.45
Table 1:
Summary of Intervention Studies Examining the Effect of Weight Loss on Bone Mineral Density and Metabolism in Older adults with obesity
| Reference | Study Design & Interventions | Sample | Summary of Bone Related Outcomes |
|---|---|---|---|
| Chao et al. (2000) | RCT: 12 months; 2 groups: C vs WL; Ca/Vitamin D supplemented | n=67 women; Age ≥60; BMI ≥27.3 | Weight (−4.3% WL vs −1.1% C †); L2-L4 BMD (+0.9 ± 4.1% WL vs −0.7 ± 4.1% C); Femoral Neck BMD (−1.4 ± 3.0% WL vs −0.8 ± 2.9% C). Markers: No differences between groups, but both groups had significant rises in OC. |
| Villareal et al. (2008) | RCT: 12 months; 2 groups: C vs WL+Ex; Ca/Vitamin D supplemented | n=27 sedentary women and men with mild to moderate frailty; Age ≥65; BMI ≥30 | Weight (−10% WL+Ex vs +1% C †); L1-L4 BMD (+0.9 ± 3.1% WL+Ex vs +1.3 ± 5.8% C); Total Hip BMD (−2.4 ± 2.5% WL+Ex vs +0.1 ± 2.1% C *); Trochanter BMD (−3.3 ± 3.1 WL+Ex vs +0.2 ± 3.3% C *); Intertrochanter BMD (−2.7 ± 3.0% WL+Ex vs +0.3 ± 2.7% C *). Markers: OC and CTX increased from baseline at 6 and 12 months in WL+Ex, but were only significantly different from control at 6 months. |
| Santanasto et al. (2011) | RCT: 6 months; 2 groups: Ex vs WL+Ex; Ca/Vitamin D supplemented | n=36 sedentary women and men; Age ≥60; BMI ≥28 | Weight (−5.5% WL+Ex vs −1.2% Ex †); Total Hip BMD (−0.2 ± 3.4% WL+Ex vs +0.5 ± 1.5% Ex) |
| Shah et al. (2011) | RCT: 12 months; 4 groups: C vs Ex vs WL vs WL+Ex; Ca/Vitamin D supplemented | n=107 sedentary men and women with mild to moderate frailty; Age ≥65; BMI ≥30 | Weight (−9.6% WL and −9.4% WL+Ex vs −0.2% C †); L1-L4 BMD (+1.1 ± 3.0% WL vs +0.8 ± 2.8% WL+Ex vs +0.4 ± 2.8% C); Total Hip BMD (−2.6 ± 2.5% WL vs −1.1 ± 2.6% WL+Ex †); Femoral Neck BMD (−2.3 ± 2.5% WL vs −0.9 ± 4.8% WL+Ex vs −0.1 ± 3.1% C); Trochanter BMD (−2.3 ± 2.5% WL vs −1.1 ± 2.6% WL+Ex vs −0.4 ± 2.3% C). Markers: OC & CTX increased in WL, decreased in Ex, and stable in WL+Ex, C †. |
| Armamento-Villareal et al. (2012) | See Shah et al. (2011) | See Shah et al. (2011) | Weight (See Shah et al. [2011]). Serum Sclerostin levels significantly increased from baseline in the WL group at 6 and 12 months but was unchanged in Ex, WL+Ex, and C groups. |
| Waters et al. (2013) | Follow-up of WL+Ex from Shah et al. (2011) at 6, 12, and 30 months; Ca/Vitamin D supplemented | See Shah et al. (2011) | Weight (−9.9% at 6 months, −11.2% at 12 months, −6.9% at 30 months); L1−L4 BMD no significant changes; Total Hip BMD (−1.4 ± 2.5% at 6 months, −1.9 ± 2.5% at 12 months, −4.5 ± 2.4% at 30 months) |
| Beavers et al. (2017) | Two 5 month RCTs; 2 groups: WL+AT vs WL+RT; Ca/Vitamin D supplemented | n=123 sedentary men and women; Age ≥65; BMI ≥27 | Weight (−8.2% WL+AT vs −5.7% WL+RT); L1−L4 BMD (+1.0 ± 0.5% WL+AT vs +1.2 ± 0.5% WL+RT); Total Hip BMD (−0.7 ± 0.2% WL+AT vs +0.3 ± 0.2% WL+RT *); Femoral Neck BMD (−0.7 ± 0.6% WL+AT vs +1.2 ± 0.6% WL+RT *) |
| Villareal et al. (2017) | RCT: 6 months; 4 groups: C vs WL+AT vs WL+RT vs WL+CT; Ca/Vitamin D supplemented | n=160 sedentary men and women with mild to moderate frailty; Age ≥65; BMI ≥30 | Weight (−9.3% WL+AT and −8.4% WL+RT and −8.6% WL+CT vs −0.9% C †); L1-L4 BMD (+0.2 ± 3.4% WL+AT vs +0.7 ± 3.4% WL+RT vs +0.7 ± 2.7% WL+CT vs +0.9 ± 3.4% C); Total Hip BMD (−2.7 ± 2.5% WL+AT vs −0.6 ± 2.5% WL+RT *) Total Hip BMD (−2.7 ± 2.5% WL+AT vs +0.2 ± 2.5% C †) |
| Kelleher et al. (2017) | RCT: 22 weeks; 2 groups: WL vs WL+Vest | n=37 sedentary men and women; Age ≥65; BMI ≥30 | Weight (−12% WL vs −11% WL+Vest); L1-L4 BMD (+2.0 ± 4.5% WL vs +1.2 ± 2.7% WL+Vest); Total Hip BMD (−1.9 ± 2.1% WL vs −0.6 ± 2.2% WL+Vest); Femoral Neck BMD (− 1.2 ± 3.7% WL vs −1.5 ± 3.6% WL+Vest); Markers: No significant differences between groups. |
p<0.05 for the comparison between stated groups
p<0.001 for the comparison between stated groups
C = control group
WL = weight loss only group
WL+Ex = weight loss plus exercise training group
WL+AT = weight loss plus aerobic training group
WL+RT = weight loss plus progressive resistance training group
WL+CT = weight loss plus combined training group
WL+Vest = weight loss plus weighted vest group
RCT = randomized controlled trial
BMI = body mass index (in units of kg/m2)
BMD = bone mineral density
NTX = N-terminal telopeptide
BSAP = bone specific alkaline phosphatase
OC = osteocalcin
CTX = C-terminal telopeptide
P1NP = N-terminal propeptide of type 1 collagen
RCTs involving older adults with obesity have shown that voluntary weight loss without concomitant exercise training decreases BMD at the total hip.36;39 These findings are consistent with previously mentioned observational studies as well as meta-analysis of weight loss trials not limited to the aging population.35;46 In a one year RCT published in 2011 by Shah et al, there was a significant decrease in BMD at the total hip in the weight loss group (−2.6%) compared to the weight stable control group (−0.6%).39 Serum C-terminal telopeptide (CTX) and osteocalcin were also elevated in the weight loss group compared to control. The one year RCT by Chao et al (2000) also found a decrease in total hip BMD (−1.4%) and an increase in osteocalcin in the weight loss group; however, no difference was found compared to control since the control group also lost significant total hip BMD.36 Lack of calcium supplementation may have contributed to this as participants averaged only 800 mg/day of calcium intake based on dietary and medication questionnaires, which is below the daily recommended value by the USPSTF.47 Vitamin D levels were also not assessed whereas in most of the other RCTs reviewed, both calcium and vitamin D supplementation was provided to all study participants.37–44
All RCTs that have been conducted on the older obese population have found no changes in lumbar spine BMD related to weight loss with or without associated exercise training.36–39;42–44 These results are consistent with a prior meta-analysis of weight loss trials which have concluded that BMD at the lumbar spine is unaffected by weight loss.46
RCTs have shown that diet-induced weight loss combined with exercise training (lifestyle therapy) will attenuate, but not completely alleviate, the loss of total hip BMD associated with weight loss in older obese patients. In Villareal et al (2008), obese subjects undergoing lifestyle intervention had greater loss of total hip BMD compared to control (−2.4% vs +0.1%).37 The 2011 study by Shah et al found that despite achieving similar degrees of weight loss in the diet-only (−9.6%) and diet-exercise (−9.4%) groups, the diet-only group had a significantly greater loss of total hip BMD as compared to the lifestyle intervention group (−2.4% vs −1.1%).39 Relative to the control group, CTX and osteocalcin increased in the diet group, but remained unchanged in the diet-exercise group. Santanasto et al (2011) conducted a 6 month study where older adults with obesity were randomized to either exercise or diet-exercise groups.38 The diet-exercise group was able to achieve modest weight loss (−5.5%) whereas the exercise group was weight stable (−1.2%). Total hip BMD at 6 months did not significantly differ between groups; however, this may be due to the smaller difference in weight loss between groups (4.3%) seen as most other RCTs saw at least an 8% difference between dieting and weight stable groups.37;39–45
Progressive resistance exercise training (RT) is more effective than either aerobic exercise training (AT) or a combination of the two (CT) at attenuating loss of BMD at the total hip. In 2017, Beavers et al published the combined results of two separately conducted 5 month RCTs focusing on the differing effects of AT versus RT in conjunction with diet-induced weight loss in overweight and older adults with obesity.43 After adjusting for multiple factors including age, BMI, and degree of weight loss, the study found that BMD at femoral neck (+1.2%) and total hip (+0.2%) were unchanged in the RT group while BMD at the femoral neck (−0.7%) and total hip (−0.7%) were reduced in the AT group. The question of AT or RT in dieting was further expanded upon in a 6 month RCT published in 2017 by Villareal et al.44 Older adults with obesity were randomized to control or diet-induced weight loss in conjunction with AT, RT, or CT. All exercise groups achieve a similar degree of weight loss (−9%) while weight remained stable in the control group. Loss of BMD at the total hip was greatest in AT (−2.7%), reduced in CT (−1.1%), and stable in RT (−0.6%) and control (+0.2%).
The decrease in total hip BMD associated with weight loss may correspond to an increased risk of frailty fractures in older adults with obesity. In 2017, Johnson et al reported on incidence of fractures amongst study participants within the Look AHEAD (Action for Health in Diabetes) trial, where 5,145 volunteers with type 2 diabetes mellitus (DM) between ages 45–76 were randomized to diabetes support and education intervention (DSE) or intensive lifestyle intervention (ILI) with a goal of ≥7% weight loss achieved through diet and increased physical activity.45 No significant differences were noted in the number of incident fractures or hip fractures between groups after a median follow-up time of 11.3 years; however, a significant 39% increased risk of frailty fractures was noted in the ILI group when compared to DSE group. This occurred despite previously documented improvements in fitness and physical activity as well as a decreased incidence of self-reported falls in the ILI group compared to the DSE group.45;48 The finding is also consistent with the initial observations seen in the Study of Osteoporosis Fractures.33;34
Older adults with obesity who experience bone loss associated with voluntary weight loss do not see a reciprocal improvement in bone mass with weight regain.41 In 2013, Waters et al published the results of extended follow-up on 16 volunteers who participated in the exercise-diet group of the previously described one year RCT by Shah et al.40 During the initial 12 months of intervention, the volunteers lost significant weight (−11.2%); however, a portion of the initial weight loss was regained such that only a net change of −6.9% from baseline was noted at 30 month follow-up. While an initial loss of BMD at the total hip was noted at 12 months (−1.9%), further losses were noted at 30 months (−4.5%) despite significant weight regain during the period (Figure 1). These findings are consistent with prior findings most notably postmenopausal women.49–51
Fig. 1.
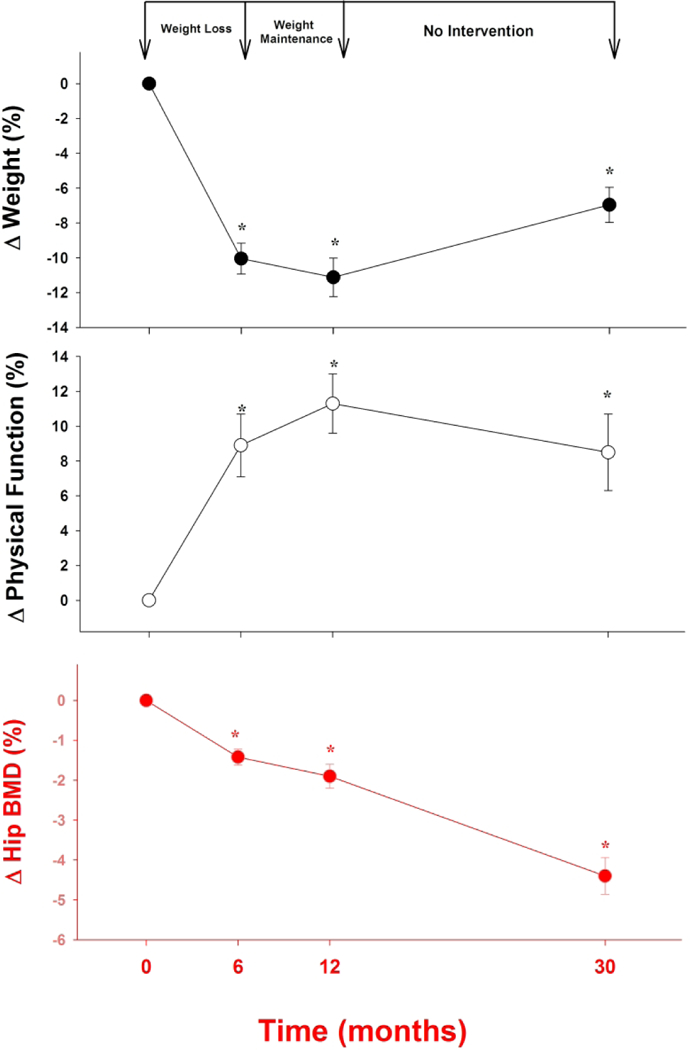
Long-Term Weight Loss with Bone Mineral Density Loss in Obese Older Adults. *p<0.05 compared to baseline (adapted from Waters et al J Nutr Health Aging 17:3–17; 2013)
4. Mechanisms for Bone Loss
Inadequate calcium intake is strongly associated with an increased risk of osteoporosis and fracture.52 Likewise, calcium supplementation has also been shown to reduce the rate of bone loss in osteoporotic patients and decrease fracture risk as well.53 Correction of vitamin D deficiency is a vital as the primary function of vitamin D in calcium balance is to increase the absorption of calcium in the intestine. Unfortunately, age is associated with a decreased absorption of calcium due to intestinal resistance to calcitriol as well as a reduction in expression of intestinal calcium transporters.54 Obesity appears to increase intestinal absorption of calcium, though the exact mechanisms are unknown.55 Further complicating matters, weight loss through caloric restriction itself is associated with decreased intestinal absorption of calcium in a manner independent from the effects of vitamin D.56 Altogether, these findings suggest that negative alterations in calcium balance during weight loss may play a critical role in bone loss that is even further exacerbated in the older populations attempting to lose weight.
Bone and muscle interact closely in both an anatomical and chemical fashion.57 Mechanical loading of muscle stimulates bone formation by inhibiting osteocyte secretion of sclerostin, which is an inhibitor of the Wnt/Lrp5 signaling pathway vital to osteoblast differentiation.58 In Shah et al, multivariate analysis found that changes in lean body mass was the strongest independent predictor of changes in total hip BMD followed by serum osteocalcin and 1-repetition maximum strength.39 A follow-up study on the same intervention groups later demonstrated that change in thigh muscle volume assessed by magnetic resonance imaging (MRI) was also an independent predictor of total hip BMD.40 Limiting muscle loss in the setting of voluntary weight loss will likely play a crucial role in the optimization of treatment of older adults with obesity as failure to do so may simultaneously predispose them to osteoporosis, sarcopenia, and increasing frailty.
Bone mass and bone quality are both adversely affected by increasing inflammation.26–28 Obesity itself is associated with a chronic state of inflammation and adipose tissue in obese patients is known to express higher levels of TNF-α, IL-6, C-reactive protein (CRP), and leptin. TNF-α and IL-6 are key players in osteoclast differentiation and chronic inflammation leads to increased bone resorption.59 Visceral adipose tissue (VAT) is particularly pro-inflammatory and increasing VAT has been associated with lower trabecular bone volume, lower bone formation rate, lower stiffness, and higher cortical porosity – all suggesting decreased bone quality.28 VAT has also been associated with decreasing levels of IGF-1, which is anabolic to the osteoblast.26 As age is already associated with increasing inflammation, obesity is likely exacerbating the negative effects of inflammation on bone health in older adults with obesity.60
5. Measures to Counter Bone Loss
Adequate supplementation of calcium and vitamin D remain at the core of treatment in all aging individuals; however, routine supplementation of calcium may be especially important in the setting of voluntary weight loss.56 Additionally, the fact that calcium absorption in the intestine is strongly affected by the amount of dietary intake of fat should be taken into consideration when developing nutrition plans and assessing patient food diaries.55
Exercise training, particularly RT, provides multiple benefits to bone health in the setting of diet-induced weight loss. Supervised RT has been shown to counteract the expected loss of total hip BMD associated with weight loss in a randomized trial involving older adults with obesity.44 Increases in mechanical loading suppress osteocyte secretion of sclerostin, which is typically increased in the setting of weight loss but negated by concurrent exercise training.61 Exercise acutely increases circulating levels of IGF-1, which promotes osteoblast differentiation.26 Lastly, exercise training improves muscle quality as well as reduces the loss of muscle mass associated with weight loss. Retention of lean body mass through exercise training additionally aids in reducing fracture risk through decreased risk of falls from improved physical function and balance.51
Related to the retention of muscle mass associated with exercise is the question of optimal protein intake for the obese older adult undergoing lifestyle therapy. Aging skeletal muscle has a decreased sensitivity and responsiveness to essential amino acids and likely requires a higher protein intake in order to achieve muscle protein synthesis.62 A review of the effects of protein intake in adults age ≥50 years old suggests that higher protein diets help to preserve lean mass while increasing loss of fat mass during weight loss interventions.63 One study in overweight and obese postmenopausal women (50–70 years old) reported that increasing protein intake from 60 to 86 grams/day attenuated loss of areal BMD at the hip as well as volumetric BMD of the tibia.64 The current recommendation by the PROT-AGE study group is to target at least an average daily intake of 1.2 grams of protein per kilogram of body weight each day while undergoing active exercise training barring contraindications such as renal dysfunction; however, the optimal average daily intake is still unknown.65
6. Conclusions and Future Directions
In the older adult population, the prevalence of obesity is anticipated to grow substantially in the coming years due to an increase in the aging population and in the prevalence of obesity itself.1 Arising from concerns regarding loss of bone mass and an increased risk of frailty fractures associated with both voluntary and involuntary weight loss in the elderly, at present time, there remains no consensus regarding the overall net benefit of diet-induced weight loss in older adults with obesity.5;33 While obesity was once considered protective of bone health, evidence now suggests obesity has a much more complex relationship with bone and is actually associated with an increased risk of ankle, upper arm, and humerus fractures.22 Diet-induced weight loss in older adults results in significant loss of total hip BMD, but not lumbar spine BMD.36–39 This loss of BMD does indeed appear to correspond to an increased risk of frailty fractures.45 Concurrent exercise training attenuates the loss of BMD associated with weight loss and progressive resistance training appears to be particularly effective at attenuating or even alleviating bone loss.39–40;43–44
Further research is needed to determine the optimal exercise regimen to best offset the loss of bone mass associated with weight loss while also taking into consideration the additional benefits that other exercise modalities have on comorbidities common the older adults with obesity. In addition, there remain significant gaps in the literature surrounding the optimal daily intake of protein in the specific population of older adults.
Substantial research is also needed to address the effects of lifestyle intervention on bone quality. Prior studies have heavily relied on areal BMD assessment by DXA which is well known to have decreased reproducibility with higher BMI subjects and cannot assess markers of bone quality.18 Newer imaging modalities including HRpQCT of the distal radius and tibia provides both volumetric BMD as well as multiple markers of bone quality.66 FEA of images obtained via HRpQCT of the distal radius and tibia, MRI of the hip, and/or CT of the lumbar spine can estimate bone strength while taking into account both bone density and quality.67 Microindentation testing can directly measure the material properties of bone, an important component of bone quality.68 Furthermore, currently the Look AHEAD trial is the only study providing incident fracture data and additional trials assessing fracture risk are needed.45
Acknowledgements
This work was supported by the following grants: CX000906, DK109950A, and 1-14-LLY-38 as well as with resources at the Michael E. DeBakey VA Medical Center. The contents of this work do not represent the view of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.National Center for Health Statistics. Health, United States, 2016: with chartbook on long-term trends in health National Center for Health Statistics Website. https://www.cdc.gov/nchs/data/hus/hus16.pdf#058. Published May 2017. Accessed March 25, 2018. [PubMed]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82:923–934. doi: 10.1038/oby.2005.228 [DOI] [PubMed] [Google Scholar]
- 3.Schafer AL. Decline in bone mass during weight loss: a cause for concern? J Bone Miner Res 2016;31:36–39. doi: 10.1002/jbmr.2754 [DOI] [PubMed] [Google Scholar]
- 4.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging 2008. Aug-Sep; 12(7):487–491. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014. July 1;1;63(25 Pt B):3029–3030. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskeletal Dis 2012. April;4(2):61–76. doi: 10.1177/1759720X11430858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla S, Riggs B. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am 2005. December;34(4):1015–30, xi. doi: 10.1016/j.ecl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Riggs B, Melton LJ, Robb R, et al. A population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 2004. December;19(12):1945–54. doi: 10.1359/JBMR.040916 [DOI] [PubMed] [Google Scholar]
- 9.Shanbhogue VV, Brixen K, Hansen S. Age- and Sex-Related Changes in Bone Microarchitecture and Estimated Strength: A Three-Year Prospective Study Using HRpQCT. J Bone Miner Res 2016. August;31(8):1541–9. doi: 10.1002/jbmr.2817 [DOI] [PubMed] [Google Scholar]
- 10.Gallagher JC, Tella SH. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 2014. July;142:155–70. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justesen J, Stenderup K, Ebbesen E, Mosekilde E, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001;2:165–171. [DOI] [PubMed] [Google Scholar]
- 12.Kennel K, Riggs R, Achenbach S. Role of parathyroid hormone in mediating age-related changes in bone resorption in men. Osteoporos Int 2003. August;14(8):631–6. doi: 10.1007/s00198-003-1417-0. [DOI] [PubMed] [Google Scholar]
- 13.Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord 1996. November; 20(10):1027–1032. [PubMed] [Google Scholar]
- 14.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 1993. May;8(5):567–73. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 15.Maimoun L, Mura T, Leprieur E, Avignon A, Mariano-Goulart D, Sultan A. Impact of obesity on bone mass throughout adult life: Influence of gender and severity of obesity. Bone 2016. September;90:23–30. doi: 10.1016/j.bone.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Robinovitch SN, McMahon TA, Hayes WC. Force attenuation in trochanteric soft tissues during impact from a fall. J Orthop Res 1995. November;13(6):956–962. doi: 10.1002/jor.1100130621. [DOI] [PubMed] [Google Scholar]
- 17.Bouxsein ML, Szulc P, Munoz F, Thrall E, Sornay-Rendu E, Delmas PD. Contribution of trochanteric soft tissues to fall force estimates, the factor of risk, and prediction of hip fracture risk. J Bone Miner Res 2007. June;22(6):825–31. doi: 10.1359/jbmr.070309. [DOI] [PubMed] [Google Scholar]
- 18.Yu EW, Bouxsein ML, Roy AE, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res 2014. March;29(3):542–50. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caffarelli C, Alessi C, Nuti R, Gonnelli S. Divergent effects of obesity on fragility fractures. Clin Interv Aging 2014. September 24;9:1629–36. doi: 10.2147/CIA.S64625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii S, Cauley JA, Greendale GA, et al. Pleiotropic effects of obesity on fracture risk: the Study of Women’s Health Across the Nation. J Bone Miner Res 2014. December;29(12):2561–70. doi: 10.1002/jbmr.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014. January;29(1):223–33. doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- 22.Compston JE, Watt NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011. November;124(11):1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res 2012. January;27(1):1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 24.Himes CL, Reynolds SL. Effect of obesity on falls, injury, and disability. J Am Geriatr Soc 2012. January;60(1):124–9. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Waclawczyk A, Hartfield D, et al. Analysis of fall injuries by body mass index. South Med J 2014. May;107(5):294–300. doi: 10.1097/SMJ.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 26.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011. June 15;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr 2015. February;101(2):337–43. doi: 10.3945/ajcn.113.081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen A, Dempster DW, Recker RR, et al. Abdominal Fat Is Associated With Lower Bone Formation and Inferior Bone Quality in Healthy Premenopausal Women: A Transiliac Bone Biopsy Study. J Clin Endocrinol Metab 2013. June;98(6):2562–72. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 2015. April;16(4):341–9. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 30.Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc 2015. May;74(2):115–24. doi: 10.1017/S0029665114001578. [DOI] [PubMed] [Google Scholar]
- 31.Grethen E, McClintock R, Gupta CE, et al. Vitamin D and hyperparathyroidism in obesity. J Clin Endocrinol Metab 2011. May;96(5):1320–6. doi: 10.1210/jc.2010-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adam MA, Untch BR, Danko ME, et al. Severe obesity is associated with symptomatic presentation, higher parathyroid hormone levels, and increased gland weight in primary hyperparathyroidism. J Clin Endocrinol Metab 2010. November;95(11):4917–24. doi: 10.1210/jc.2010-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003. December;51(12):1740–7. [DOI] [PubMed] [Google Scholar]
- 34.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1997. April 28;157(8):857–63. [PubMed] [Google Scholar]
- 35.Ensrud KE, Fullman RL, Barrett-Connor E, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab 2005. April;90(4):1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 36.Chao D, Espeland MA, Farmer D, et al. Effect of voluntary weight loss on bone mineral density in older overweight women. JAGS 2000. July;48:753–759. [DOI] [PubMed] [Google Scholar]
- 37.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in older adults with obesity: a one-year randomized controlled trial. J Clin Endocrinol Metab 2008. June;93(6):2181–7. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately older adults with obesity: a randomized clinical trial. J Obes 2011;2011 pii: 516576. doi: 10.1155/2011/516576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in older adults with obesity prevents increase in bone turnover and attenuates decrease in hip BMD induced by weight loss despite decline in bone-active hormones. J Bone Miner Res 2011. December;26(12):2851–9. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armamento-Villareal R, Aguirre L, Napoli N, et al. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, older adults with obesity. Osteoporos Int 2014. February;25(2):551–8. doi: 10.1007/s00198-013-2450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters DL, Vawter R, Qualls C, et al. Long-term maintenance of weight loss after lifestyle intervention in frail older adults with obesity. J Nutr Health Aging 2013. January;17(1):3–7. doi: 10.1007/s12603-012-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelleher JL, Beavers DP, Henderson RM, et al. Weighted vest use during dietary weight loss on bone health in older adults with obesity. J Osteoporos Phys Act 2017;5(4). pii: 210. doi: 10.4172/2329-9509.1000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beavers KM, Beavers DP, Martin SB, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci 2017. October 12;72(11):1582–1585. doi: 10.1093/gerona/glx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting older adults with obesity. N Engl J Med 2017 2017. May 18;376(20):1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson KC, Bray GA, Cheskin LJ, et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the Look AHEAD randomized clinical trial. J Bone Miner Res 2017. November;32(11):2278–2287. doi: 10.1002/jbmr.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zibellini J, Seimon RV, Lee CM, et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res 2015. December;30(12):2168–78. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 47.Moyer VA. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventative Services Task Force recommendation statement. Ann Intern Med 2013. May 7;158(9):691–6. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 48.Rejeski WJ, Bray GA, Chen SH, et al. Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci 2015. March;70(3):345–53. doi: 10.1093/gerona/glu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA. Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause 2014. May;21(5):501–8. doi: 10.1097/GME.0b013e3182a76fd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villalon KL, Gozansky WS, Van Pelt RE, et al. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 2011. December;19(12):2345–50. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pines A Weight loss, weight regain and bone health. Climacteric 2012. August;15(4):317–9. doi: 10.3109/13697137.2012.667975. [DOI] [PubMed] [Google Scholar]
- 52.Gennair C Calcium and vitamin D nutrition and bone disease of the elderly. Public Health Nutr 2001. April;4(2B):547–559. [DOI] [PubMed] [Google Scholar]
- 53.Meunier P Prevention of hip fractures by correcting calcium and vitamin D insufficiencies in elderly people. Scand J Rheumatol Suppl 1996;103:75–8; discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 54.Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 2011. December 5;347(1–2):25–9. doi: 10.1016/j.mce.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Brolin RE, Taich L. Hormonal and dietary influences on true fractional calcium absorption in women: role of obesity. Osteoporos Int 2012. November;23(11):2607–14. doi: 10.1007/s00198-012-1901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapses SA, Sukumar D, Schneider SH, et al. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr 2013. March;97(3):637–45. doi: 10.3945/ajcn.112.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirschfield HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017. October;28(10):2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 58.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/Sclerostin. J Biol Chem 2008. February 29;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 59.Aguirre L, Napoli N, Waters DL, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in older obese adults. J Clin Endocrinol Metab 2014. September;99(9):3290–7. doi: 10.1210/jc.2013-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age (Dordr) 2013. June;35(3):563–72. doi: 10.1007/s11357-012-9381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in older adults with obesity increasing serum sclerostin and impairs hip geometry but both are prevented by exercise training 2012. May;27(5):1215–21. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005. March;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 63.Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev 2016. March;74(3):210–24. doi: 10.1093/nutrit/nuv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukumar D, Ambia-Sobhan H, Zurfluh R, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized controlled trial. J Bone Miner Res 2011. June;26(6):1339–48. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013. August;14(8):542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 66.Burghardt AJ, Link TM, Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 2011. August;469(8):2179–93. doi: 10.1007/s11999-010-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Rietbergen B, Ito K. A survey of micro-finite element analysis for clinical assessment of bone strength: the first decade. J Biomech 2015. March 18;48(5):832–41. doi: 10.1016/j.jbiomech.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Diez-Perez A, Guerri R, Nogues X et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 2010;25:1877–1885. doi: 10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]


