Abstract
BACKGROUND AND OBJECTIVES:
There is interest in applying genomic sequencing (GS) to newborns’ clinical care. Here we explore parents’ and clinicians’ attitudes toward and perceptions of the risks, benefits, and utility of newborn GS compared with newborn screening (NBS) prior to receiving study results.
METHODS:
The BabySeq Project is a randomized controlled trial used to explore the impact of integrating GS into the clinical care of newborns. Parents (n = 493) of enrolled infants (n = 309) and clinicians (n = 144) completed a baseline survey at enrollment. We examined between-group differences in perceived utility and attitudes toward NBS and GS. Open-ended responses about risks and benefits of each technology were categorized by theme.
RESULTS:
The majority of parents (71%) and clinicians (51%) agreed that there are health benefits of GS, although parents and clinicians agreed more that there are risks associated with GS (35%, 70%) than with NBS (19%, 39%; all P < .05), Parents perceived more benefit and less risk of GS than did clinicians. Clinicians endorsed concerns about privacy and discrimination related to genomic information more strongly than did parents, and parents anticipated benefits of GS that clinicians did not.
CONCLUSIONS:
Parents and clinicians are less confident in GS than NBS, but parents perceive a more favorable risk/benefit ratio of GS than do clinicians. Clinicians should be aware that parents’ optimism may stem from their perceived benefits beyond clinical utility.
Nearly all infants born in the United States receive state-mandated newborn screening (NBS) at birth. Fifty years after being implemented to screen newborns for phenylketonuria, allowing early intervention to avoid resulting disabilities, NBS programs have expanded to now screen for >30 conditions that present in the newborn period for which there is early treatment or management.1,2 The program is largely hailed as a success and is credited with improving the health of newborns by preventing or mitigating the symptoms of certain conditions.3,4
Continued technological advancements in genomic sequencing (GS), alongside its increased affordability, have generated growing interest in applying GS to the clinical care of newborns.5,6 Some have even suggested that every infant will be sequenced at birth in the not-too-distant future.7,8 Others have noted the inevitability of introducing GS into or alongside current NBS programs.9,10 GS of newborns has the potential to provide comprehensive information of clinical and personal utility to clinicians and families.6,11–13 Advocates of GS expect its utility to surpass that of NBS because GS could shorten the time to diagnose diseases14 by interrogating thousands of disease-causing variants at once15 and detecting conditions that NBS currently cannot.11 Additionally, GS can identify the risk of future disease thus creating opportunities for early interventions to prevent or prepare for future health problems.2,4,15 Critics of GS remind us that despite its promise, GS is unlikely to replace all screening, because biochemical tests like tandem mass spectrometry are superior at detecting some disorders.9 Limitations of GS also include our incomplete knowledge about control elements and the identification of variants of uncertain significance.
Despite the potential for GS to enrich current NBS programs, the increased amount of information about an individual that can be generated and the implications of this information for family members’ own health or family planning decisions has led to considerable debate.2,4,15 For example, the ethical implications of the ability of GS to identify risk for later-onset disorders in newborns raises questions about the impact on a child’s right to an open future.4,16–20 Professional guidelines urge caution with regard to the use of GS in children, advise against returning information associated with adult-onset conditions to parents of children,16,21,22 recommend testing only individual or targeted panels of genes when such testing will suffice,21 and generally discourage sequencing healthy children.21,22 In addition to the potential for ambiguity in the interpretation of results,23 the psychological and psychosocial impact of receiving GS information on families is as of yet unknown.24
Research has revealed that many parents express hypothetical interest in receiving their newborn’s GS information,12,25 yet little is known about the perspectives of those who might actually receive GS information about their newborns or those who provide medical care for them. In this article, we explore attitudes toward and perceptions of the risks, benefits, and utility of GS compared with standard NBS among clinicians and parents participating in a randomized controlled trial of GS in newborns.
METHODS
Study Design and Participants
The BabySeq Project is a randomized clinical trial exploring the medical, behavioral, and economic impact of integrating GS into the clinical care of newborns. The study design has been described in detail elsewhere.26 To summarize, we are enrolling newborns, their parents, and their clinicians into 2 cohorts: a healthy newborn cohort from Brigham and Women’s Hospital (BWH) Well Baby Nursery and an ICU newborn cohort from ICUs at BWH, Boston Children’s Hospital, and Massachusetts General Hospital. Within each cohort, half of the families are randomly assigned to modified standard of care (standard NBS report and an in-depth family history report), and half are randomly assigned to modified standard of care plus a type of GS (whole-exome sequencing). Families in the GS arm receive a genome report with results about monogenic and recessive carrier variants in genes associated with conditions that present or for which there are interventions during the childhood period, as well as a set of highly penetrant, actionable adult-onset conditions and pharmacogenomics variants. For newborn participants who have or develop a clinical presentation that may have a genetic etiology, families may receive results from an indication-focused sequence analysis. Genome reports are uploaded into the newborn’s medical record at the associated hospital and sent to clinicians involved in their care. The Partners Human Research Committee, which serves as the review board for BWH, Boston Children’s Hospital Office of Clinical Investigations, and Baylor College of Medicine’s Institutional Review Board approved this study.
The infant’s parents and clinicians, including primary care physicians and pediatric subspecialists, complete multiple surveys throughout the study. In this report, we focus on parents’ and clinicians’ baseline attitudes toward and perceptions of the benefits, risks, and utility of GS compared with NBS. Both groups completed baseline surveys after enrollment into the project before they knew their randomization status and before any results were returned.
Measures
Ten items were used to assess parents’ and clinicians’ attitudes about obtaining informed consent versus mandating testing, perceptions of the risks and benefits, and concerns about privacy and discrimination of NBS and GS. Response options to these questions were on a 5-point Likert scale anchored by “strongly disagree” to “strongly agree.” Parents were asked to rate the importance of having each type of information (NBS and GS) about their newborn now and in 10 years from now on a 5-point scale anchored by “not at all important” (1) and “very important” (5) to measure their perceived utility. To evaluate clinicians’ perceived utility, they were asked to rate the usefulness of each type of technology at this time and in 10 years for identifying conditions in their patients, managing their patients’ care, and predicting their patients’ future risk of disease. Responses were on a 10-point scale anchored by “not at all useful” (1) to “extremely useful” (10).
Data Analysis
Descriptive statistics were calculated to characterize parents’ and clinicians’ demographic characteristics and responses to survey items. We compared attitudes toward NBS versus GS among parents and clinicians using χ2 and Wilcoxon signed rank tests. Paired t tests were used to assess whether there were differences in perceived current and future utility (defined as 10 years from now) of NBS versus GS among parents and clinicians. Wilcoxon signed rank tests were used to explore differences between parents’ and clinicians’ perceived current and future utility of each technology (NBS versus GS). To achieve this, we first calculated the difference in the mean current and future utility scores for each technology of parents and clinicians. All statistical calculations were performed using SPSS statistical software (IBM SPSS Statistics, IBM Corporation).
Parents and clinicians who agreed or strongly agreed that there were risks or benefits of each technology were then asked to specify those risks and benefits via open-ended responses. We analyzed these open-ended responses qualitatively using thematic content analysis. We identified themes of responses using an inductive approach, with which 3 coders, who were overseen by a fourth coder with qualitative expertise (S.P.), coded all responses independently and then discussed any discrepancies until consensus was reached.
RESULTS
Participant Characteristics
We enrolled 493 parents of 309 newborns and 144 clinicians. Parents’ and clinicians’ characteristics are presented in Tables 1 and 2. Parents who completed surveys were 50% women, the majority were college graduates or higher (88%), and 68% were non-Hispanic white. The majority of clinicians were white (82%), and 90% had no specific genetics training.
TABLE 1.
Parent Characteristics
| Characteristic, n (%) Unless Otherwise Noted | N = 493 |
|---|---|
| Agea | |
| Mean in y (SD) | 35.9 (±4.8) |
| Sex | |
| Male | 247 (50%) |
| Female | 246 (50%) |
| Race and/or ethnicity | |
| Hispanic or Latino | 32 (6%) |
| Non-Hispanic white | 335 (68%) |
| Non-Hispanic otherb | 126 (26%) |
| Education | |
| Did not graduate from college | 59 (12%) |
| College graduate or higher | 434 (88%) |
| Annual household incomec | |
| ≤$99 999 | 97 (20%) |
| ≥$100 000 | 386 (80%) |
n = 476.
Non-Hispanic other includes African American, Asian American, and other.
n = 483.
TABLE 2.
Clinician Characteristics
| Characteristic, n (%) Unless Otherwise Noted | N = 148 |
|---|---|
| Agea | |
| Mean in y (SD) | 46.0 (±13.8) |
| Sex | |
| Male | 50 (35%) |
| Female | 98 (66%) |
| Race and/or ethnicityb | |
| Hispanic or Latino | 5 (4%) |
| Non-Hispanic white | 118 (82%) |
| Non-Hispanic otherc | 21 (15%) |
| Years in practiced | |
| 1–10 | 35 (25%) |
| 11–20 | 53 (37%) |
| ≥21 | 51 (37%) |
| Genetics traininge | |
| No | 127 (90%) |
| Yes | 14 (10%) |
| Clinician type | |
| Neonatologist | 53 (36%) |
| Pediatrician | 92 (62%) |
| Clinician otherf | 3 (2%) |
n = 133.
n = 144.
Non-Hispanic other includes African American, Asian American, and other.
n = 139.
n = 141.
Clinician other includes cardiologist and family medicine practitioner.
Attitudes and Perceived Risks and Benefits
Summary statistics of parents’ and clinicians’ attitudes toward NBS and GS are presented in Table 3. The vast majority of parents (93%) and all clinicians (100%) agreed that all newborns should receive NBS, but only 33% of parents and 8% of clinicians felt that all newborns should receive GS (P < .001). Parents agreed more than clinicians that parental informed consent should be required for NBS (57%, 31%, respectively; χ2 P ≤ .001), but parents and clinicians felt similarly that parental informed consent should be required for GS (86%, 93%; χ2 P = .058).
TABLE 3.
Parents’ and Clinicians’ Attitudes
| Parents’ Attitudes | Clinicians’ Attitudes | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Disagree, % | Neither Agree nor Disagree, % | Agree, % | N | Disagree, % | Neither Agree nor Disagree, % | Agree, % | |
| Every newborn should receive | ||||||||
| NBS | 494 | 1 | 6 | 93 | 146 | — | — | 100 |
| GS | 494 | 10 | 57 | 33 | 146 | 42 | 50 | 8 |
| Parental informed consent should be required for | ||||||||
| NBS | 493 | 22 | 21 | 57 | 147 | 54 | 15 | 31 |
| GS | 492 | 3 | 11 | 86 | 147 | 1 | 5 | 93 |
| There are health benefits associated with | ||||||||
| NBS | 493 | 2 | 16 | 82 | 145 | 1 | 2 | 97 |
| GS | 494 | 2 | 27 | 71 | 147 | 5 | 44 | 51 |
| There are risks associated with | ||||||||
| NBS | 490 | 50 | 31 | 19 | 144 | 48 | 13 | 39 |
| GS | 493 | 28 | 37 | 35 | 148 | 4 | 26 | 70 |
| Concerned about the privacy of child’s genomic Informationa,b | 493 | 17 | 18 | 65 | 145 | 4 | 13 | 83 |
| Concerned child will be discriminated against on the basis of genomic informationc,d | 490 | 22 | 25 | 53 | 147 | 8 | 20 | 72 |
Response options were on a scale ranging from strongly disagree (1) to strongly agree (5). Scores 1 and 2 were combined to create the disagree category. Scores 4 and 5 were combined to create the agree category. —, not applicable.
Parent survey question: I am concerned about the privacy of my child’s genomic information.
Clinician survey question: My patients’ parents should be concerned about the privacy of their child’s genomic information.
Parent survey question: I am concerned that my child will be discriminated against on the basis of his or her genomic information.
Clinician survey question: My patients’ parents should be concerned that their child will be discriminated against on the basis of his or her genomic information.
These attitudes are in line with their views toward the risks and benefits of these technologies, as both groups agreed more that there are health benefits associated with NBS (82% of parents, 97% of clinicians) than with GS (71%, 51%; all P < .05), although parents had a more favorable view of the health benefits of GS than clinicians (χ2 P < .001). Likewise, parents and clinicians agreed more that there are risks associated with GS (35%, 70%) than with NBS (19%, 39%; all P < .05), although clinicians agreed significantly more than parents that there are risks of both technologies (GS χ2 P < .001; NBS χ2 P < .001).
Seventy-two percent of clinicians agreed that parents should be concerned about discrimination based on their child’s genomic information, but only 53% of parents reported being concerned about discrimination (χ2 P < .001). Likewise, 83% of clinicians agreed that parents should be concerned about the privacy of their child’s genomic information, whereas only 65% of parents expressed concern about it (χ2 P < .001). In open-ended responses (Table 4), parents and clinicians both cited the potential for discrimination as more of a risk of GS than of NBS. They also cited the potential to receive uncertain or unwanted results, such as results associated with untreatable or later-onset disorders, as more of a risk of GS than of NBS, with clinicians in particular noting the risks associated with the potential for uncertain results with GS (eg, variants of unknown significance, issues of penetrance). Both parents and clinicians identified psychological distress as a risk of both NBS and GS, describing the possibility of parents feeling anxious or depressed in response to results from either technology.
TABLE 4.
Themes of Benefits and Risks in Parents’ and Clinicians’ Open-ended Responses
| Theme | Definition |
|---|---|
| Benefits | |
| Ability to prepare | Awareness of health problems that may arise can aid in preparation and planning, exclusive of clinical intervention |
| Diagnosis and/or identification of risk and early intervention | Early diagnosis, identification of risk for diseases; prevention, early treatment, and intervention |
| Family planning and testing Value of information | Carrier status, family testing Knowledge for its own sake; “knowledge is power” |
| Risks | |
| Analytic validity | Test sensitivity, false-positives, and false-negatives |
| Uncertain results | Issues of penetrance, variants of unknown significance |
| Impact on family | Family members treating child differently on the basis of results |
| Privacy and discrimination | Risks of privacy of results; child could face genetic and insurance discrimination |
| Psychological distress | Anxiety, worry, stress for patient or family |
| Unwanted results | Receiving unwanted or too much information; receiving results associated with later-onset conditions |
In terms of benefit, parents and clinicians saw diagnosis and/or identification of risk and early intervention as the main benefits of both NBS and GS, citing the potential to mitigate or prevent symptoms associated with a broad range of disorders, although the potential to identify the risk of future conditions was a much stronger theme in responses about GS than about NBS. Other benefits identified through open-ended responses included family planning and testing, the ability to prepare for health problems that may arise (exclusive of clinical intervention, including mental preparation or parental education), and valuing information solely for the sake of knowledge (ie, “knowledge is power”), although the ability to prepare and the value of information were largely absent from clinicians’ responses.
Perceived Utility
As shown in Fig 1, parents rated the importance of having NBS information higher than having GS information now but rated the 2 types of information as equally important 10 years from now. When we examined the differences between parents’ current and future utility perceptions, we found they thought that GS information would be significantly more important in 10 years compared with now (P < .001) and that the importance of having NBS information would decrease over the next 10 years (P < .001).
FIGURE 1.
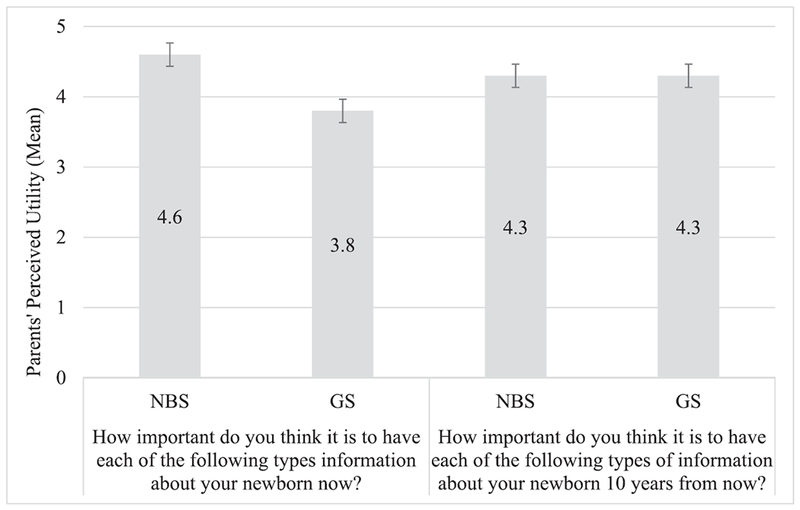
Parents’ perceived utility. Parents’ perceived utility is measured on an importance scale that ranges from 1 to 5 in which 1 indicates no importance and 5 indicates high importance. Error bars represent SEs.
Clinicians similarly responded that NBS was more useful than GS at this time for identifying conditions in their patients (Table 5; P <.001) and managing their patients’ care (P < .001) but felt NBS and GS were equally useful at this time for predicting their patients’ future risk of disease (P > .05). In terms of the future utility of these technologies, clinicians indicated that NBS will still be more useful than GS for identifying conditions in their patients (P < .05) but felt that GS will be more useful than NBS for predicting their patients’ future risk of disease (P < .001). We also examined differences between clinicians’ perception of current and future utility and found that they similarly expect GS to be more useful in 10 years compared with now (all 3 purposes P < .001). Although clinicians responded that NBS would increase in utility over time for managing their patients’ care and predicting their patients’ future risk of disease, they anticipated the utility of GS to increase more than the utility of NBS over the next 10 years for all 3 purposes (Wilcoxon signed rank test P < .001).
TABLE 5.
Clinicians’ Perceived Use
| How Useful Do You Think NBS Is at This Time | How Useful Do You Think NBS Will Be in 10 y | How Useful Do You Think GS Is at This Time | How Useful Do You Think GS Will Be in 10 y | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| For identifying conditions in your patients | 148 | 8.9 (±1.4) | 148 | 8.9 (±1.5) | 148 | 6.1 (±1.9) | 146 | 8.4 (±1.6) |
| For managing your patients’ care | 148 | 7.8 (±2.4) | 147 | 8.2 (±2.1) | 147 | 5.6 (±2.2) | 146 | 7.8 (±1.9) |
| For predicting your patients’ future risk of disease | 146 | 6.3 (±2.9) | 148 | 7.4 (±2.5) | 146 | 6.2 (±2.1) | 147 | 8.3 (±1.6) |
Clinicians’ perceived utility is measured on a usefulness scale that ranges from 1 to 10 in which 1 indicates no usefulness and 10 indicates high usefulness.
We found no differences in any attitude items between types of clinicians (neonatologists versus pediatricians), nor between cohorts of parents (Well Baby Nursery versus ICU).
DISCUSSION
Overall, parents and clinicians viewed NBS more favorably than GS. Parents and clinicians were less confident in GS compared with NBS, but the majority of both parents and clinicians agreed that there are health benefits of newborn GS. Parents perceived more benefit and less risk of GS than did clinicians, with clinicians viewing the difference in risks and benefits between the 2 technologies as more disparate than parents did. Clinicians may be viewing GS as riskier on the basis of their concerns about privacy issues and discrimination related to genomic information, which they endorsed more strongly than parents. As such, parents seem to perceive a more favorable benefit/risk ratio of GS than clinicians.
Parents’ and clinicians’ open-ended responses describing the risks and benefits of GS provide some additional insight into the way they perceive GS. Although both parents and clinicians most often identified psychological distress as a risk of GS, clinicians also often cited the uncertainty associated with genomic information and many times linked that to parents’ distress, suggesting that GS could cause unnecessary parental distress by causing parents to worry about health problems that may never arise or that the child is not actually at risk for developing. Clinicians also more often identified the possibility of receiving unwanted results, such as results associated with later-onset conditions. These concerns recall the potential harms of GS noted in the literature, such as impinging on the child’s right to an open future4,16–20 or disrupting family dynamics, like parent-child bonding.24
Parents also anticipated benefits that clinicians did not, including the ability to prepare and the benefit of knowledge for its own sake. These results echo other studies that have revealed that parents find their children’s genomic information useful for a broad variety of reasons, including psychological and pragmatic reasons,13,27 which traditionally fall outside of the realm of what is considered clinical utility, because these benefits do not lead to a change of clinical management.28
Professional guidelines and recommendations about sequencing in children emphasize that the benefits of the test should be weighed against the risks. The definition of utility of genomic technologies, however, is currently an area of debate, with some arguing that a broader spectrum of utility should be recognized with regard to genomic technologies29,30 and others emphasizing that consumers’ and patients’ perception of use does not necessarily mean the information actually has utility.31 Our results reveal that both parents and clinicians identify benefits of GS that are not typically covered under the umbrella of what is considered “clinical utility,” such as family planning and testing, the intrinsic value of information, and the ability to prepare for the future. Additionally, parents and clinicians view the risks and benefits of GS differently. Underscored in these findings is the importance of further exploring the utility of genomic technologies during the newborn period, broadly defined. In addition, clinicians should be aware that parents might have a more optimistic view of GS and should be prepared to discuss with parents the risks, benefits, and goals of using GS to help set realistic expectations.
Our results should be considered within the limitations of our study. First, we are reporting on the perspectives of parents who have chosen to participate in the BabySeq Project. As such, it is unsurprising that they hold a relatively optimistic view of GS; parents who declined to participate may have a less favorable attitude. In addition, the majority of respondents were highly educated and white. Thus, these views may not be generalizable to a larger population of parents. Additionally, we have reported attitudes toward these 2 technologies at baseline, and although a small number of parents of ICU infants may have already received NBS results, most families had not, and none had yet received their study results, nor had any clinicians received any study results for their patients. Follow-up surveys are used to query parents’ and clinicians’ attitudes toward these 2 technologies again after they have received results (3 months postdisclosure for parents and end of study for clinicians); analysis of these surveys will be used to determine whether receiving study results impacts their attitudes. Future analyses will also explore whether there is concordance between parents’ attitudes, because disagreements in perspectives could affect clinical practice. Finally, parents who considered enrollment in the study participated in an ~1-hour education and consent session with a genetic counselor, during which they were educated about the risks and benefits of GS, which may have had an effect on their attitudes.
CONCLUSIONS
As we continue to introduce GS into the clinical care of newborns, there is a need to understand parents’ and clinicians’ attitudes toward and perceived utility of GS at this life stage. Understanding how parents and clinicians perceive GS, especially in comparison with the successful and longstanding NBS program in the United States, can provide insight into how parents and clinicians may view the integration of GS and how they may respond to GS results. Our results reveal that parents and clinicians are less confident in GS than NBS, but parents are more optimistic about GS than are clinicians. Clinicians should be aware that parents’ optimism may stem from a broader range of perceived benefits that are outside of what is generally considered clinical benefit, and as such, they may need to help set realistic expectations for GS information. Further research into the utility of GS information for infants and their families will help clinicians and parents weigh the risks and benefits of using GS in the newborn period.
Supplementary Material
ACKNOWLEDGMENTS
We thank the families and clinicians for their participation in the BabySeq Project.
FINANCIAL DISCLOSURE: Dr Green receives compensation for speaking or consultation from AIA, GenePeeks, Helix, Ohana, Prudential, and Veritas; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The BabySeq Project is supported by grant U19 HD077671 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Human Genome Research Institute of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- BWH
Brigham and Women’s Hospital
- GS
genomic sequencing
- NBS
newborn screening
Footnotes
POTENTIAL CONFLICT OF INTEREST: Dr Green is a cofounder, advisor, and equity holder in Genome Medical, Inc; the other authors have indicated they have no potential conflicts of interest to disclose.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02422511).
REFERENCES
- 1.King JS, Smith ME. Whole-genome screening of newborns? The constitutional boundaries of state newborn screening programs. Pediatrics. 2016;137(suppl 1):S8–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botkin JR. Ethical issues in pediatric genetic testing and screening. Curr Opin Pediatr. 2016;28(6):700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serving the family from birth to the medical home. A report from the Newborn Screening Task Force convened in Washington DC, May 10-11, 1999. Pediatrics. 2000;106(2 pt 2):383–427 [PubMed] [Google Scholar]
- 4.Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Sénécal K, Vears DF; Global Alliance for Genomics and Health Regulatory and Ethics Working Group Paediatric Task Team. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics. 2017;10(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders CJ, Miller NA, Soden SE, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4(154):154ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark Z, Tan TY, Chong B, et al. ; Melbourne Genomics Health Alliance. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18(11)4090–1096 [DOI] [PubMed] [Google Scholar]
- 7.Collins FS. Francis Collins says medicine in the future will be tailored to your genes. Wall Street Journal. 2014. Available at: https://www.wsj.com/articles/francis-collins-says-medicine-in-the-future-will-be-tailored-to-your-genes-1404763139. Accessed October 31, 2017 [Google Scholar]
- 8.Check E Scientists to sequence genomes of hundreds of newborns. Nature News Blog. 2013. Available at: http://blogs.nature.com/news/2013/09/scientists-to-sequence-hundreds-of-newborns-genomes.html. Accessed October 31, 2017 [Google Scholar]
- 9.Tarini BA, Goldenberg AJ. Ethical issues with newborn screening in the genomics era. Annu Rev Genomics Hum Genet. 2012;13:381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raho JA. The changing moral focus of newborn screening: an ethical analysis by the president’s council on . bioethics. 2008. Available at: https://repository.library.georgetown.edu/bitstream/handle/10822/559379/thechangingmoralfocusofnewbornscreening-appendix-josephraho.pdf?sequence=1. Accessed October 31, 2017 [Google Scholar]
- 11.Bodian DL, Klein E, Iyer RK, et al. Utility of whole-genome sequencing for detection of newborn screening disorders in a population cohort of 1,696 neonates. Genet Med. 2016;18(3):221–230 [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg AJ, Dodson DS, Davis MM, Tarini BA. Parents’ interest in whole-genome sequencing of newborns. Genet Med. 2014;16(1) :78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek J, Slashinski MJ, Robinson JO, et al. Parental perspectives on whole-exome sequencing in pediatric cancer: a typology of perceived utility. JCO Precis Oncol. 2017;1:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willig LK, Petrikin JE, Smith LD, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3 (5) :377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrikin JE, Willig LK, Smith LD, Kingsmore SF. Rapid whole genome sequencing and precision neonatology. Semin Perinatol, 2015;39(8) :623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee on Bioethics. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001. ;107(6):1451–1455 [DOI] [PubMed] [Google Scholar]
- 17.Bredenoord AL, de Vries MC, van Delden JJ. Next-generation sequencing: does the next generation still have a right to an open future? Nat Rev Genet. 2013;14(5) :306. [DOI] [PubMed] [Google Scholar]
- 18.Bredenoord AL, de Vries MC, van Delden H. The right to an open future concerning genetic information. Am JBioeth. 2014; 14(3):21–23 [DOI] [PubMed] [Google Scholar]
- 19.Committee on Bioethics; Committee on Genetics; American College of Medical Genetics; Genomics Social; Ethical; Legal Issues Committee. Ethical and policy issues in genetic testing and screening of children. Pediatrics. 2013;131 (3):620–622 [DOI] [PubMed] [Google Scholar]
- 20.Green RC, Berg JS, Grody WW, et al. ; American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7) :565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botkin JR, Belmont JW, Berg JS, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97 (1):6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Society for Eluman Genetics. Report on the genetic testing of children. 2010. Available at: www.bsgm.org.uk/media/678741/gtoc_booklet_final_new.pdf. Accessed October 31, 2017
- 23.Manolio TA, Fowler DM, Starita LM, et al. Bedside back to bench: building bridges between basic and clinical genomic research. Cell. 2017;169(11:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel LA, Pereira S, McGuire AL. Potential psychosocial risks of sequencing newborns. Pediatrics. 2016;137(suppl 1):S24–S29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waisbren SE, Bäck DK, Liu C, et al. Parents are interested in newborn genomic testing during the early postpartum period. Genet Med. 2015;17 (6):501–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holm IA, Agrawal PB, Ceyhan-Birsoy O, et al. The BabySeq project: implementing genomic sequencing in newborns. BMC Pediatrics. 2018;18(2251: 10.1186/s12887-018-1200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiff M, Giarelli E, Bernhardt BA, et al. Parents’ perceptions of the usefulness of chromosomal microarray analysis for children with autism spectrum disorders. J Autism Dev Disord. 2015;45 (10):3262–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosse SD, Khoury MJ. What is the clinical utility of genetic testing? Genet Med. 2006;8 (7):448–450 [DOI] [PubMed] [Google Scholar]
- 29.ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2015;17 (6) :505–507 [DOI] [PubMed] [Google Scholar]
- 30.Botkin JR, Teutsch SM, Kaye Cl, et al. ; EGAPP Working Group. Outcomes of interest in evidence-based evaluations of genetic tests. Genet Med. 2010;12(4) :228–235 [DOI] [PubMed] [Google Scholar]
- 31.Bunnik EM, Janssens AC, Schermer MH. Personal utility in genomic testing: is there such a thing? J Med Ethics. 2015;41 (4):322–326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


