Abstract
Objective:
Previous studies of imaging predictors on acute treatment response in late life depression (LLD) demonstrated that poor response to selective serotonin reuptake inhibitors (SSRI) is associated with pre-treatment low functional connectivity (FC) within executive control network and high FC within default-mode network including the ventromedial prefrontal cortex (vmPFC). However, there is less research in regional resting-state functional activity that explains FC changes related to SSRI response.
Methods:
Thirty-six older MDD patients not currently on antidepressant treatment had a baseline, pre-treatment resting state functional magnetic resonance imaging scan, followed by sertraline treatment for 12 weeks. Depression severity was assessed using the Montgomery-Åsberg Depression Rating Scale (MADRS). Subjects whose MADRS score decreased < 50% from baseline or who discontinued sertraline for any reason were classified as non-responders (n=21). Subjects whose 12-week MADRS score dropped ≥ 50% from baseline were defined as responders (n=15). We conducted the amplitude of low-frequency fluctuation (ALFF) and region of interest (ROI)-to-ROI FC analyses independently. Significance threshold was set at p<0.05 with FDR correction for multiple comparisons.
Results:
Relative to the responder group, the non-responder group showed significantly less ALFF in the dorsomedial prefrontal cortex (dmPFC), and greater ALFF in the vmPFC/subgenual cingulate area. For ROI-to-ROI connectivity, there was significantly greater connectivity between the vmPFC and the cerebellar vermis in the non-responder group.
Conclusion:
Our study highlighted the association of vmPFC resting state activity and connectivity with SSRI response. Future studies are warranted for understanding the role of vmPFC-vermis connectivity in late-life depression.
Keywords: Depression, Neuroimaging, Geriatrics, anti-depressants
Introduction
Major depression occurring in later life, also called late-life depression (LLD), is common in older adults (60 years and older).1 Among community-dwelling elderly, about 15% experience clinically significant depressive symptoms, with 2– 4% suffering from major depression and approximately 10% having minor depression2, 3, 4, 5 LLD patients have a poorer response to antidepressants compared with younger depressed patients, due in part to aging-related brain changes.6
Selective serotonin reuptake inhibitors (SSRIs) are considered first-line antidepressant treatment for LLD.7 SSRIs have good efficacy and better tolerability compared with other antidepressants like tricyclic antidepressants (TCAs), and are associated with significantly lower orthostatic, cognitive, anticholinergic, and cardiovascular adverse effects.8, 9, 10, 11 Despite being generally effective, about 30% of patients fail to respond to SSRIs at adequate doses.12,13 Identifying individuals who may or may not respond to an SSRI before treatment is important for therapeutic planning. Neuroimaging may represent one tool that can inform efforts aimed prediction of response to specific interventions.
Structural and functional magnetic resonance imaging have been used to identify neuroimaging predictors of SSRI response in older depressed patients. A study using diffusion tensor imaging reported that relative to those who achieved remission (N=25), LLD patients who failed to achieve remission (N=23) had lower fractional anisotropy, a measure of structural integrity, in multiple frontal limbic brain areas, including the rostral and dorsal anterior cingulate (ACC), dorsolateral prefrontal cortex (dlPFC), genu of the corpus callosum, white matter adjacent to the hippocampus, multiple posterior cingulate cortex (PCC) regions, and insular white matter.14 A fMRI study using an executive-control task revealed that LLD patients (n=13) showed lower activation in the left dlPFC and weaker dlPFC-ACC connectivity relative to controls (n=13) pre-treatment but had increased activation in the right dlPFC post 12 weeks of paroxetine.15 There were also a few studies using fMRI during resting state. A study on resting state functional connectivity (FC) in 16 LLD and 10 control subjects revealed that at baseline, LLD patients had low FC within executive control network (ECN including dlPFC) and high FC within default-mode network (DMN, such as posterior cingulate-PCC) compared with the control group. Lower ECN at baseline predicted lower remission rate after 12-week treatment with escitalopram.16 Although the study by Andreescu et al 17 did not find changes in ECN, they found increased FC in the left striatum in the treatment-resistant participants relative to treatment-responsive participants, and the PCC-striatum FC increase remained significant even after adjusting for white matter hyperintensity burden. A recent study from the same group longitudinally scanned 33 late-life depression patients five times within 12-week venlafaxine medication. They found that in addition to the changes in the executive control network (ECN, seeded from dorsolateral prefrontal cortex) and default mode network (DMN, seeded from posterior cingulate) over time, non-remitters showed significantly increased eigenvector centrality (EVC, a measure reflecting how a region is densely connected with other regions) in the medial prefrontal cortex (mPFC), and decreased ECV in the inferior frontal cortex (IFG) in relative to remitters.18 Overall, these studies suggest that poor response to SSRI in LLD could be related to reduced dorsal ECN and increased DMN activity/connectivity as well as micro-structural damage in the prefrontal area.
While the above studies characterized structural and functional connectivity changes in SSRI non-responders relative to responders in LLD, very few have directly examined resting-state activity. The amplitude of low-frequency fluctuation (ALFF), which measures the fluctuation strength of low-frequency blood oxygen level-dependent (BOLD) signals, is considered to reflect spontaneous neural activity of the brain. We believe fMRI is more sensitive than structural MRI in monitoring neural changes related to medication and resting state activity (such as ALFF), which is less influenced by task performance than task-related fMRI, is more feasible to be used as potential neuroimaging predictors of medication effect. Examining ALFF during resting state not only can detect regional neural activity changes in SSRI non-responders relative to responders in LLD, but also can allow us to better explain changes of seed-based FC.
Therefore, the present study focused on identifying pre-treatment resting-state fMRI predictors of treatment response in older unmedicated depressed adults using ALFF and seed-based FC. All patients were subsequently treated with sertraline for 12 weeks to identify responders and non-responders. We hypothesized that lower resting activity within the dorsal prefrontal regions (within the ECN) and higher activity within the default-mode network regions (including the ventromedial prefrontal cortex, vmPFC) would be related to poor response to 12-week treatment.
Materials and methods:
Participants:
The study was approved by the institutional review boards of the University of Connecticut Health Center and Hartford Hospital. All subjects were provided information about the study, including a review of the consent form, and then provided written, informed consent to participate. Depressed subjects (N=38) were recruited from a volunteer registry, clinic referrals, newspaper advertisements, and community presentations. Inclusion criteria included age 60 or above, ability to read and write English, Mini-Mental State Exam (MMSE) score 27 or greater, and currently meeting criteria for major depression. Exclusion criteria were current antidepressant treatment, other mental or neurological illness, contraindication for performing fMRI, and contraindication for sertraline treatment. While we did not specifically exclude other classes of psychotropics, we did exclude other major psychiatric disorders. As a result, no subject was on another type of psychotropics at the time of the MRI scan.
Baseline Assessments:
A study psychiatrist interviewed each subject to establish a clinical diagnosis of major depression and then administered the Montgomery-Åsberg Depression Rating Scale (MADRS)19 to assess depression severity.
MRI scanning
After baseline clinical assessment, subjects were scanned with a Skyra 3T scanner (Siemens, Erlangen, Germany) with 32 surface coils at the Olin Neuropsychiatry Research Center at the Institute of Living at Hartford Hospital. Both functional and structural MRIs were acquired axillary paralleled with the anterior commissure-posterior commissure (AC-PC) line. The acquisition parameters for the structural magnetization-prepared rapid gradient-echo (MPRAGE) images were: TR/TE=2200/2.88 ms, flip angle=13°, matrix=220×320×208, voxelsize 0.8×0.8×0.8mm. The seven-minute resting functional images were acquired using a simultaneous multislice echo-planar imaging (EPI) sequence (factor=8) with TR=475ms, TE=30ms, flip angle=60°, matrix=80×80×49, and voxelsize=3×3×3mm. During the fMRI scanning, subjects were instructed to relax with their eyes opened without falling asleep; after scanning, each subject confirmed not falling asleep during scanning.
Intervention
After the imaging scan was performed, all depressed subjects were offered open-label treatment with sertraline for 12 weeks. Individuals taking antidepressants at baseline who otherwise met inclusion criteria, underwent a study-related two-week medication washout with weekly telephone contact to assess clinical status and provide in-person assessments as warranted. Because of its long half-life, individuals taking fluoxetine at baseline were excluded.
Among the 38 subjects, two subjects were excluded because of artifacts in images. Therefore, we report results based on remaining 36 subjects. For sertraline dosing, subjects younger than 80 years old (N= 28) were started on 50 mg daily for two weeks to rule out drug sensitivity and minimize risk of side effects, then were increased to 100 mg daily. The protocol was flexible, allowing for the dose to be increased by 50 mg every two weeks, up to a maximum dose of 200 mg daily. The study physician could start patients on a lower dose based on clinical factors including patient history. Subjects 80 years and older (N= 8) were started on 25 mg and increased to 50 mg daily after two weeks. The dose could be increased by 25–50 mg every two weeks, up to a maximum 200mg daily. For subjects who might experience problems with tolerability, the protocol allowed for the dosing to be reduced to a previous level.
Individuals with difficulty tolerating sertraline, leading to discontinuation, (N= 15) were offered a switch to standard doses of bupropion or desvenlafaxine through the study, and if neither medication was appropriate, the study psychiatrist worked with the subject to identify appropriate antidepressant treatment and those subjects were not included in the study.
We defined responders as a 50% or greater decrease in the MADRS score from baseline.20 Non-responders were defined as failing to achieve a 50% decrease in MADRS from baseline, or discontinuation of the medication before the twelve weeks due to side effects.
Data processing and analysis:
The resting-state fMRI data was preprocessed using the default settings of functional connectivity (CONN) toolbox v17a21 including functional realignment with motion estimation and correction, centering functional data to origin coordinates, functional outlier detection using ART-based identification of outlier scans for scrubbing, functional and structural segmentation, coregistration, normalization, and smoothness using a Gaussian kernel of 8 mm3. After preprocessing, images were then band-pass filtered to 0.008 ~0.09 Hz. Other de-noising steps included regression of the six motion parameters and their first-order derivatives, and regression of white matter and cerebrospinal fluid (CSF) signals. Preprocessing quality assurance was check for each preprocessing step and each subject. No additional subjects (except the two who had artifacts in their images) were excluded using Conn toolbox’s default criteria of excluding subjects with head motion over 2mm.
Amplitude of Low-Frequency Fluctuation (ALFF) and Functional Connectivity (FC) Analysis:
ALFF is a quantitative measure of neural oscillation strength during resting state22, and research has revealed ALFF alterations in several psychiatric disorders.23 We computed whole-brain voxelwise ALFF from each subject’s preprocessed images using the DPABI V2.3 package (http://rfmri.org/dpabi). The amplitudes in subject-level maps were transformed into Z-scores to create standardized subject-level maps. Anatomical images and Z-score maps are then transformed into MNI152 standard space. In parallel, using the regions of interests (ROI) atlas provided by CONN toolbox v17a21, we computed ROI-to-ROI functional connectivity (FC) for each participant. The default atlas provided by CONN toolbox included an atlas of cortical and subcortical areas from the FSL Harvard-Oxford Atlas, as well as cerebellar areas from the AAL atlas; and a few commonly used networks and areas (e.g., DMN included medial prefrontal cortex-MPFC, posterior cingulate-PCC, right lateral parietal cortex-RLP and left parietal cortex -LLP areas). We used atlas-based ROIs instead of using significant clusters from ALFF as ROIs to avoid circular analysis.
Two-sample t tests on baseline ALFF and FCs between sertraline responders and non-responders were computed and effect of age, sex, and baseline MADRS were controlled. Significance level for both analyses was set at p < 0.05 with a False Discovery Rate (FDR) correction for multiple comparisons using the programs of DPABI (statistical analysis for ALFF) and CONN toolbox for functional connectivity.
Results
Demographic characteristics of the sample are shown in table 1. The two groups were of similar age, sex, educational attainment, and baseline MADRS. As expected, the MADRS score decreased significantly (t32=3.58, p<0.001) at the twelve-week follow-up for the responder group (mean MADRS of 3.86) compared with the non-responder group (mean MADRS of 12.1).
Table 1.
Comparisons of the demographics between the Sertraline responder and non-responder groups (two sample t tests on continuous variables and chi-square tests on categorical variables)
| Variable | Responders (N=15) | Non-responders (N=21) | P-Value |
|---|---|---|---|
| Age in years mean (±SD) | 73.2(±6.71) | 73.571(±7.553) | 0.87 |
| Female gender, N (%) | 10(66.6) | 13(61.9) | 0.76 |
| Race | |||
| White, N (%) | 14(93.3%) | 21(100%) | |
| African American, N (%) | 1(6.7%) | 0(0%) | |
| Education, years, mean (±SD) | 14.9(±3.2) | 15.14(±2.3) | 0.82 |
| MMSE† score (±SD) | 29.5(±0.63) | 29.3(±1.35) | 0.46 |
| MADRS≠ score | |||
| Baseline, mean (SD) | 18.67(±5.15) | 20.14(±5.6) | 0.42 |
| At 12 weeks, mean (SD) | 3.86(±3.1) | 12.1(±6.5) | 0.005* |
| Episodes of depression 1, N (%) | 2(13.3) | 5(23.8) | |
| More than 1, N (%) | 8(53.3) | 6(28.5) | |
| Don’t know/NA, N (%) | 3(20%) | 9(42.8) | |
| More than or equal 95, N (%) | 2(13.3) | 1(4.7) | |
Mini mental state examination
Montgomery-Asberg Depression Scale
Indicates a significant statistical difference with P<.05.
Neuroimaging Results
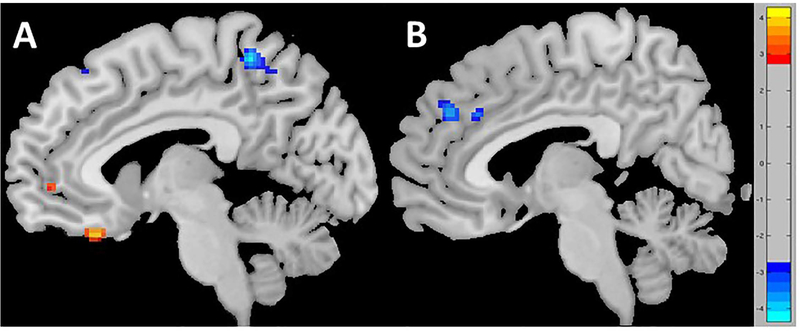
A two-sample t test on ALFF revealed that relative to the responder group, non-responders showed significantly stronger baseline ALFF in the vmPFC/subgenual cingulate area, inferior frontal gyrus area (i.e., IFG/vlPFC), and dorsal anterior cingulate (dACC), as well as significantly lower ALFF in the left and right precuneus, and left precentral and the dorsomedial prefrontal (dmPFC) cortices (Table 2 and Figure 1).
Table 2.
The regions and peak co-ordinates that revealed significantly difference in ALFF between the responder and non-responder groups (in MNI space)
| Contrasts | Regions | Hemi-sphere | Cluster voxelsize | Z score | Peak co-ordinates |
|---|---|---|---|---|---|
| Non-responders > responders | Ventromedial prefrontal cortex (Subgenual cingulate) | Left | 44 | −4.18 | −8, 30, −24 |
| Ventrolateral prefrontal cortex | Left | 56 | −3.36 | −20, 24, −20 | |
| Dorsal anterior cingulate | Left | 17 | −3.21 | −8, 50, 0 | |
| Non-responders < responders | Dorsomedial prefrontal cortex | Medial | 68 | 3.83 | 0, 48, 28 |
| Precuneus | Right | 25 | 3.46 | 14, −48, 64 | |
| Precuneus | Left | 6 | 3.12 | −10, −50, 54 | |
| Frontal eye field | Left | 79 | 4.02 | −36, −2, 53 |
Figure 1.
Significant differences in ALFF of the non-responder relative to responder groups. Relative to responders, non-responders showed A) greater ALFF (red) in the subgenual and ventromedial prefrontal cortex (vmPFC)as well as the dorsomedial prefrontal cortex(dmPFC)/dorsal anterior cingulate cortex(dACC); B) Lower ALFF in the precuneus area (blue).
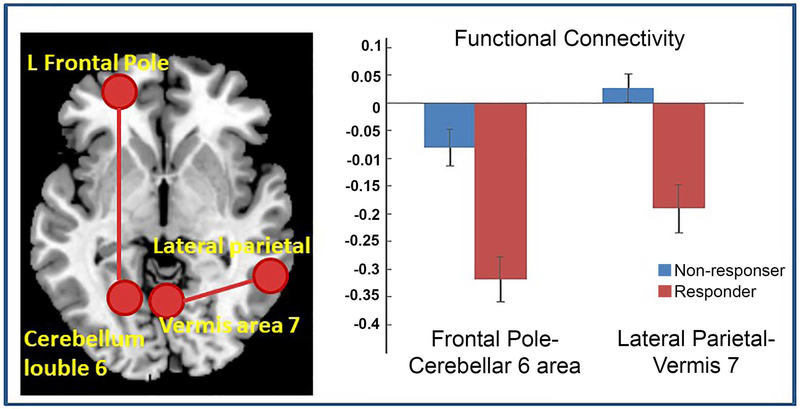
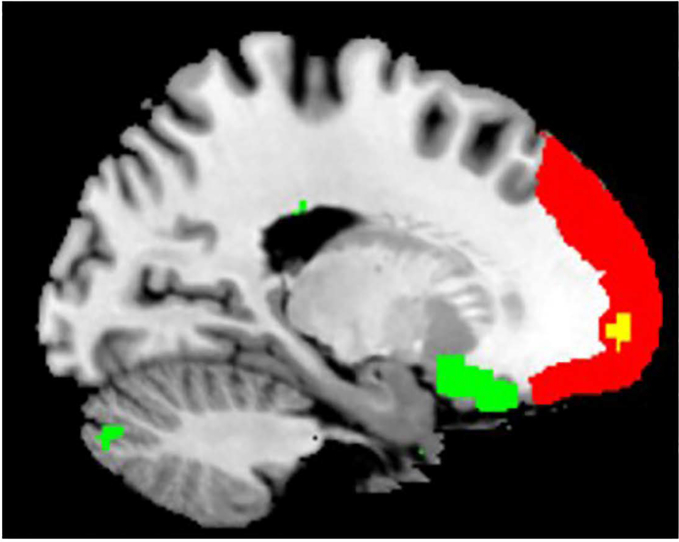
A two-sample t tests on ROI-to-ROI FCs revealed that compared with the responders, non-responders had greater functional connectivity between left frontal pole and cerebellum lobule 6 (t32=4.49, p=0.014, FDR correction), and between the left lateral parietal cortex of the default mode network (DMN) and cerebellar vermis 7 (t32=4.37, p=0.0202, FDR correction, Figure 2). A permutation test with 5000 iterations confirmed these results. Interestingly, there were overlaps in some voxels between the atlas-based left frontal pole and the significant vmPFC cluster identified from the ALFF analysis (Figure 3). Therefore, there were both greater vmPFC ALFF and stronger vmPFC-cerebellum FC in the non-responder group relative to responders. Of note, the frontal pole (vmPFC) and cerebellum lobule 6 were negatively correlated in both groups, with the non-responder group showing a less negative correlation. Similarly, the responder group had negative lateral parietal – vermis correlation, whereas non-responders had a positive (or no) correlation.
Figure 2.
Greater functional connectivity between the left frontal pole seed and cerebellar lobule 6 and between the left lateral parietal seed and vermis 7 in Sertraline non-responder group relative to responder group.
Figure 3.
Illustration of the locations of the frontal pole seed (red) during the ROI-to-ROI analysis and the vmPFC cluster (green) that its ALFF was significantly greater in Sertraline non-responders relative to responders as shown in Figure 1. The voxels in yellow are overlaps between the frontal pole seed and the vmPFC cluster.
Discussion
In our twelve-week prospective study on LLD subjects initially treated with sertraline, we used baseline resting state fMRI to detect activity/connectivity features to predict subsequent treatment response. We found that relative to sertraline responders, non-responders had significantly lower ALFF in the dmPFC, and higher ALFF in the vmPFC/subgenual cingulate area. There was also significantly greater connectivity between the vmPFC and cerebellar vermis in the non-responders relative to responders. In addition to dorsal and ventral PFC, our results also highlighted the importance of the resting connectivity between both the vmPFC and cerebellum in prediction of SSRI responses in late-life depression.
While both dmPFC and vmPFC are parts of the mentalizing network (Gallagher and Frith, 200324; Amodio and Frith, 200625), they seem to play different roles. The dmPFC is more related to executive inhibition32, 33, 34 and decision making, and the vmPFC is more related to self-referential thoughts and rumination26,27,28. Lower dmPFC and higher vmPFC resting activity in Sertraline non-responders relative to responders at baseline suggest that non-responders might have deficits in attention inhibition on self-related rumination. In a sense, our finding of lower ALFF of dmPFC in non-responders is analogue to previous findings that lower ECN activity predicted low remission rate and persistence of depressive symptoms16. The association of vmPFC activation with SSRI response has been frequently found in studies in young or middle aged depressive patients.29,30, 31, 32 Only one study in LLD noted decreased activation of the vmPFC in response to negative words was normalized after 7-month naturalistic treatments.33 Our results that higher vmPFC in the non-remitters relative to remitters is consistent with this finding. Our recent study34 revealed that greater ALFF in the vmPFC and lower ALFF in the dmPFC was associated with higher neuroticism, and neuroticism has also been found less responsive to SSRI.35 Greater ALFF in the vmPFC and lower ALFF in the dmPFC in the non-responder group in our current study also might be related to neuroticism. However, we didn’t find significant difference in neuroticism score between the two groups (t=0.5755, p=0.5687). It might be other psychological factors affecting the results. Therefore, characterizing clinical features associated with resting vmPFC activity at baseline is important for selecting treatment strategy.
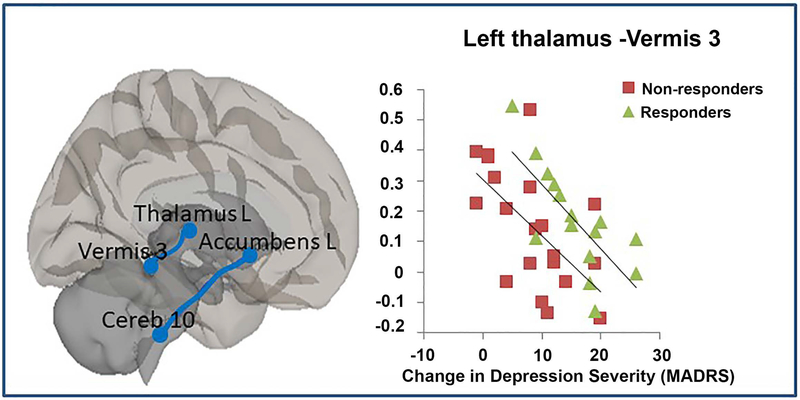
Another feature of our findings is the association of baseline cerebellum connectivity and treatment response in LLD. What is particularly intriguing about our findings is that non-responders not only had greater resting activity in the vmPFC, but also had greater functional connectivity between vmPFC and cerebellar lobule 6. Cerebellar function is heterogeneous and complex and the regional cerebellar functions are not as specific as cerebral cortex. The cerebellar lobule 6 is frequently reported engaging in cognitive tasks, however, it is also involved in emotion perception.36 The cerebellum is one of the key regions that consistently identified mediating responses to antidepressant treatment.37 It not only contributes to motor function, planning, and coordination of movement, but also plays an important role in emotion and cognition.38 Anatomically, cerebellar regions have reciprocal connections with brainstem reticular nuclei39 and limbic and autonomic system regions40,41, 42, as well as rostral and caudal anterior cingulate.43 These connections are anatomical basis for the cerebellum’s involvement in emotion information processing. We previously found the PCC-vermis FC is correlated with depression severity.44 Therefore, it is not surprising that we found the association of greater synchronization between the cerebellum and vmPFC, caudate, and thalamus with poor sertraline treatment response. Similar findings were reported by Yamamura and colleagues45, i.e. greater thalamus activity in treatment resistant patients. We speculate that enhanced cerebellar-striatum/thalamus and vmPFC-cerebellar lobule 6 connectivity might reflect a sub-biotype of depression (such as those with psychomotor retardation or anxiety46 and worth further study in the future. Of note, one study in middle-aged depression showed reduced resting local cerebellar connectivity in antidepressant resistant patients (Guo et al, 2013). Future studies in a large sample to explore biotypes of depression and related clinical features should provide more meaningful information.
The study does have limitations, the most important being small sample size. Another limitation is that we used a treatment protocol that was not fully standardized, given that NBOLD is not a clinical trial. Given the initial dosage difference between individuals <80 years old and those ≥80 years, and aging may impact on response to SSRI treatment as well, ideally we should examine interaction effect of age and SSRI responses on resting activity and connectivity. However, there were only 5 non-responders and 3 responders who were 80 years or older which prevented further data analysis. Future studies of larger sample sizes that incorporate standardized treatment protocols and examine impact of aging are therefore warranted.
Conclusion
Both our finding and prior studies have identified a role (albeit inconsistent in direction) for the vmPFC in association with SSRI treatment response in LLD patients. This neuroimaging marker should be explored in future research as a component of multivariate treatment prediction models in larger sized studies.
Figure 4.
Significant correlations between two functional connectivity pairs (the left nucleus accumbens and cerebellar lobule 10; the left thalamus and vermis 3) and MADRS changing score after 12-week Sertraline treatment.
Key points:
High percentage of antidepressant non-response in LLD patients.
Use of resting fMRI in determining biomarkers for selecting subsequent treatment.
The vmPFC activity and connectivity in association with response to SSRI.
Acknowledgements:
This study was supported by NIH research funds R01 MH096725 and R01MH098301.
Footnotes
Disclosures: None of the authors has any conflict of interests.
References
- 1.Cieri F, Esposito R, Cera N, Pieramico V, Tartaro A, di Giannantonio M. Late-Life Depression: Modifications of Brain Resting State Activity. J Geriatr Psychiatry Neurol. 2017;30(3):140–50. [DOI] [PubMed] [Google Scholar]
- 2.Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–11. [DOI] [PubMed] [Google Scholar]
- 3.Blazer D, Swartz M, Woodbury M, Manton KG, Hughes D, George LK. Depressive symptoms and depressive diagnoses in a community population. Use of a new procedure for analysis of psychiatric classification. Arch Gen Psychiatry. 1988;45(12):1078–84. [DOI] [PubMed] [Google Scholar]
- 4.Katona CL. Approaches to the management of depression in old age. Gerontology. 1994;40 Suppl 1:5–9. [DOI] [PubMed] [Google Scholar]
- 5.Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry. 2000;57(6):601–7. [DOI] [PubMed] [Google Scholar]
- 6.Scocco P, Frank E. Interpersonal psychotherapy as augmentation treatment in depressed elderly responding poorly to antidepressant drugs: a case series. Psychother Psychosom. 2002;71(6):357–61. [DOI] [PubMed] [Google Scholar]
- 7.Preskorn SH. Recent pharmacologic advances in antidepressant therapy for the elderly. Am J Med. 1993;94(5A):2S–12S. [PubMed] [Google Scholar]
- 8.Newhouse PA. Use of serotonin selective reuptake inhibitors in geriatric depression. J Clin Psychiatry. 1996;57 Suppl 5:12–22. [PubMed] [Google Scholar]
- 9.Nelson JC, Kennedy JS, Pollock BG, Laghrissi-Thode F, Narayan M, Nobler MS, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry. 1999;156(7):1024–8. [DOI] [PubMed] [Google Scholar]
- 10.Bondareff W, Alpert M, Friedhoff AJ, Richter EM, Clary CM, Batzar E. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157(5):729–36. [DOI] [PubMed] [Google Scholar]
- 11.Altamura AC, De Novellis F, Guercetti G, Invernizzi G, Percudani M, Montgomery SA. Fluoxetine compared with amitriptyline in elderly depression: a controlled clinical trial. Int J Clin Pharmacol Res. 1989;9(6):391–6. [PubMed] [Google Scholar]
- 12.Karp JF, Whyte EM, Lenze EJ, Dew MA, Begley A, Miller MD, et al. Rescue pharmacotherapy with duloxetine for selective serotonin reuptake inhibitor nonresponders in late-life depression: outcome and tolerability. J Clin Psychiatry. 2008;69(3):457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–44. [DOI] [PubMed] [Google Scholar]
- 15.Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013;214(3):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel MJ, Andreescu C, Price JC, Edelman KL, Reynolds CF 3rd, Aizenstein HJ. Machine learning approaches for integrating clinical and imaging features in late-life depression classification and response prediction. Int J Geriatr Psychiatry. 2015;30(10):1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 20.Lavergne F, Berlin I, Gamma A, Stassen H, Angst J. Onset of improvement and response to mirtazapine in depression: a multicenter naturalistic study of 4771 patients. Neuropsychiatr Dis Treat. 2005;1(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. [DOI] [PubMed] [Google Scholar]
- 22.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339–51. [DOI] [PubMed] [Google Scholar]
- 23.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7(2):77–83. [DOI] [PubMed] [Google Scholar]
- 25.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–77. [DOI] [PubMed] [Google Scholar]
- 26.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Overwalle F Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158(6):899–905. [DOI] [PubMed] [Google Scholar]
- 31.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, 3rd, Craighead WE, Mayberg HS. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76(7):527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–43. [DOI] [PubMed] [Google Scholar]
- 33.Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2008;64(4):349–55. [DOI] [PubMed] [Google Scholar]
- 34.Steffens DC, Wang L, Manning KJ, Pearlson GD. Negative Affectivity, Aging, and Depression: Results From the Neurobiology of Late-Life Depression (NBOLD) Study. Am J Geriatr Psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 35.Manning KJ, Chan G, Steffens DC. Neuroticism Traits Selectively Impact Long Term Illness Course and Cognitive Decline in Late-Life Depression. Am J Geriatr Psychiatry. 2017;25(3):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6(3):254–67. [DOI] [PubMed] [Google Scholar]
- 39.Andrezik JA, Dormer KJ, Foreman RD, Person RJ. Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neuroscience. 1984;11(2):497–507. [DOI] [PubMed] [Google Scholar]
- 40.Dietrichs E, Haines DE. Demonstration of hypothalamo-cerebellar and cerebello-hypothalamic fibres in a prosimian primate (Galago crassicaudatus). Anat Embryol (Berl). 1984;170(3):313–8. [DOI] [PubMed] [Google Scholar]
- 41.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434(2):117–65. [DOI] [PubMed] [Google Scholar]
- 42.Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45(2):268–87. [DOI] [PubMed] [Google Scholar]
- 43.Vilensky JA, van Hoesen GW. Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Res. 1981;205(2):391–5. [DOI] [PubMed] [Google Scholar]
- 44.Alalade E, Denny K, Potter G, Steffens D, Wang L. Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS One. 2011;6(5):e20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamura T, Okamoto Y, Okada G, Takaishi Y, Takamura M, Mantani A, et al. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl Psychiatry. 2016;6:e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]