Abstract
Gram-negative pathogens such as Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa are the leading cause of nosocomial infections throughout the world. One commonality shared among these pathogens is their ubiquitous presence, robust host-colonization and most importantly, resistance to antibiotics. A significant number of two-component systems (TCSs) exist in these pathogens, which are involved in regulation of gene expression in response to environmental signals such as antibiotic exposure. While the development of antimicrobial resistance is a complex phenomenon, it has been shown that TCSs are involved in sensing antibiotics and regulating genes associated with antibiotic resistance. In this review, we aim to interpret current knowledge about the signaling mechanisms of TCSs in these three pathogenic bacteria. We further attempt to answer questions about the role of TCSs in antimicrobial resistance. We will also briefly discuss how specific two-component systems present in K. pneumoniae, A. baumannii, and P. aeruginosa may serve as potential therapeutic targets.
Keywords: two-component regulatory proteins, antimicrobial resistance, biofilms
1. Introduction
Antimicrobial resistance in several infectious pathogens has become a serious public health concern. As per the World Health Organization (WHO), the 21st century may well be called the post-antibiotic era [1]. The incidence of infections caused by multidrug-resistant (MDR) gram-negative bacteria is increasing worldwide [2,3]. The emergence of pan-drug-resistant (PDR) bacteria, which are resistant to all classes of available antimicrobial agents, represents a worrisome endpoint in the fight with bacterial infections [4,5]. Despite the limited reports of isolation of such resistant bacteria, there is a great concern in the medical community, as clinicians are left with very few options for treating patients with PDR bacteria.
Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae are well-known nosocomial pathogens; recent years have seen a worldwide rise in their multi-drug resistant and pan-drug resistant counterparts [6,7,8]. They have been included in the WHO’s list of antibiotic-resistant priority 1 (critical) pathogens [1]. They have also been annotated as being a part of the ESKAPE pathogen group [8,9,10,11] (Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacteriaceae). This acronym is derived from their ability to “escape” from antimicrobial therapy. This acronym has been further modified as ESCAPE pathogens with the ‘C’ referring to Clostridium difficile, and ‘E’ for all Enterobacteriaceae, including E. coli, Proteus spp., and Enterobacter spp. [12]. Studies have documented increasing resistance rates in P. aeruginosa clinical isolates to fluoroquinolones, cephalosporins, and carbapenems [13]. A. baumannii and K. pneumoniae are now being recognized as emerging pathogens in many medical facilities [14,15]. According to the National Nosocomial Infections Surveillance (NNIS) data, the proportion of infections due to Acinetobacter spp. has been steadily increasing, and now accounts for ∽7% of intensive care unit (ICU)-related pneumonias [16]. Infections due to multidrug-resistant A. baumannii have been associated with increased lengths of hospital and ICU stays [14]. K. pneumoniae is also a well-recognized nosocomial pathogen, and an important cause of pneumonia and urinary tract infections in ICU settings [15]. Since the early 1990s, many reports of extended-spectrum β-lactamase (ESBL)-possessing K. pneumoniae have emerged [15]. In surveillance studies, resistance to third-generation cephalosporins amongst K. pneumoniae has reached ∼15–20%, and ciprofloxacin resistance has ranged from ∼10–50% [17,18]. Recently, outbreaks of carbapenemase-producing K. pneumoniae have been reported, threatening the use of this class of antimicrobial agents [17,18].
Even though resistance mechanisms in these organisms have been increasingly explored, limited information is available regarding the role of their sensory mechanisms in resistance. Sensing is the first step for bacterial defense against extrinsic environmental stressors such as antibiotic exposure and potentially plays a role in the evolution of resistance mechanisms. Two-component regulatory systems (TCSs) in bacteria act as key sensory pathways that enable microbes’ adaptation in both the environment as well as the host [19]. Studies have suggested that TCSs may play an important role in the survival and development of antimicrobial resistance [20]. This review aims to explore the connection between TCSs and antimicrobial resistance in pathogenic gram-negative bacteria. We will focus on three key bacteria: P. aeruginosa, K. pneumonia, and A. baumannii, which pose serious threats to human health [21,22,23]. We believe these organisms also share a commonality in terms of the mechanism of antibiotic resistance.
2. Antimicrobial Resistance in Gram-Negative Pathogenic Bacteria
The development and emergence of antimicrobial resistance is a complicated process and yet occur soon after the introduction of novel anti-microbial drugs. Resistance to antibiotics can develop either by spontaneous mutations [24] or by the acquisition of resistant genes [25]. The origin of antibiotic-resistant genes may be present on commensal [26] or environmental bacteria [27]. To fully understand the acquisition and spread of antibiotic resistance among the human bacterial pathogens, these ecosystems should be taken into consideration.
Development of resistance is thought to be an ongoing evolutionary process. A genetic change in the form of mutation often occurs naturally. Such mutations can influence the ability of a cell to grow and survive in the presence of environmental stressors such as antimicrobials [28]. The selection of mutant is dependent on the intensity of selective pressure, the immune status of the host, the size of the pathogen population, the presence of other microorganisms and lastly the geographical topography [28,29]. Often, random mutations of the genes encoding for the antibiotic lytic enzymes give rise to modified catalysts with increasingly extended spectra of resistance [30]. One example is the β-lactamase encoding gene. The β-lactamase encoding genes are ancient and have been isolated in strains from remote environments [31,32]. The plasmid-encoded β-lactamase, TEM, has been shown to be related to a variety of enzyme families, providing hints to its adaptability [33].
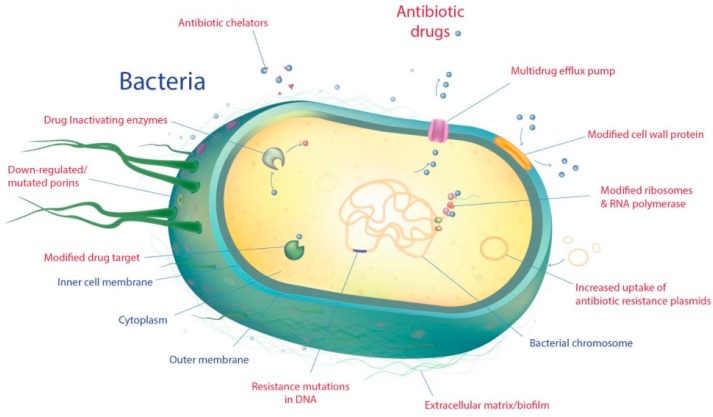
Antimicrobial resistance can develop through several mechanisms. These can be broadly categorized into two main types; intrinsic and acquired. Intrinsic resistance mechanisms include inherent bacterial defenses such as altered outer membrane permeability and hyperactive efflux pumps; whereas, the acquired resistance mechanisms involve horizontal gene transfer and acquisition of genetic elements. Here we will discuss these mechanisms with respect to P. aeruginosa, K. pneumonia, and A. baumannii. Figure 1 highlights the key resistance mechanisms in pathogenic bacteria.
Figure 1.
Key resistance mechanisms in gram-negative pathogenic bacteria.
2.1. Intrinsic Resistance
Intrinsic gene functions leading to naturally resistant phenotypes have given rise to the term ‘resistome’ [34]. Resistome of a microorganism includes all the genes and their products that contribute to its antibiotic resistance [35]. Understanding the resistome is critical as it forms the basis for horizontal gene transfer and the eventual emergence of antimicrobial resistance [35,36]. The search of P. aeruginosa PAO1 genome, for example, reveals several genes encoding enzymes for resistance to chloramphenicol, aminoglycoside, and β-lactam antibiotics [37]. Resistome analysis in multidrug-resistant isolates of K. pneumoniae revealed an average of 11–13 acquired resistance genes along with the extended-spectrum beta-lactamase genes and the AraC-type regulator, which confers resistance to virtually all antimicrobial agents available in clinical practice [36,38,39]. The genome of A. baumannii encodes a variety of different β-lactamases, including metallo-enzymes that confers resistance to carbapenems, as well as resistance-nodulation-cell division (RND)-type multidrug efflux pumps (AdeABC, AdeFGH, and AdeIJK) [40,41,42,43].
The outer membrane (OM) in gram-negative bacteria plays a major role in pathogen-host interaction and forms a selective permeability barrier. OMs, like other biological membranes, are fundamentally built as a bilayer of lipids. As such, lipid bilayers permit little permeability for hydrophilic solutes, including most nutrients and many antibiotics [44]. They contain protein-formed channels, allowing the influx of nutrients and the extrusion of waste products [44]. Porins are one such class of constitutively expressed nonspecific or substrate specific diffusion channel-forming proteins [45]. The properties of these porins are significant for the intrinsic level of antibiotic resistance in gram-negative bacteria. The major outer membrane porin of P. aeruginosa (OprF) transports solutes at least two times slower compared with that of bacteria, such as E. coli [46]. A recent study in K. pneumoniae suggested that porin deficiency is a widespread phenomenon in MDR resistant isolates [47,48,49]. The genome of K. pneumoniae encodes for several key porins, however, OmpK35, OmpK36, and OmpK37 are most widely associated with AMR. Most susceptible clinical isolates of K. pneumoniae express both OmpK35 and OmpK36 porins, while most extended spectrum β-lactamases encoding K. pneumoniae express only the OmpK36 or none [50,51]. Very often loss of one type of porin is compensated by expression of others, adding to the already complex role of porins in antimicrobial resistance. The major A. baumannii porin, OmpA, has been shown to play a crucial role in AMR [52]. OmpA facilitates AMR by extrusion of antibiotics from the periplasmic space through the outer membrane and by interacting with inner membrane efflux systems [52] facilitating surface motility [53] and biofilm formation [54].
Bacterial efflux pumps represent another important mechanism for limiting antibacterial molecules inside the cell or from their targets. Efflux pumps are central to the adaptation and survival of the cell in various environments. These are divided into families such as the RND; major facilitator superfamily (MFS); small multidrug resistance (SMR), and multidrug and toxin extrusion (MATE) family of proteins. Another class of proteins, known as the ATP binding cassette (ABC) transporters, have been found to be present in various pathogenic bacteria and have been shown to be involved in antibiotic resistance. Contribution of efflux systems to clinically important antibiotic resistance has been described in P. aeruginosa (MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM) [55,56,57,58], A. baumannii (AdeABC, AdeFGH, CraA, AmvA, AbeM, and AbeS) [40,43,59,60,61], and K. pneumoniae (TolC, AcrAB, KocC, and KexD) [62,63,64]. Readers are referred to excellent reviews by Yoon et al. [61] Pulzova et al. [49], and Zowalaty et al. [65].
2.2. Acquired Resistance
Modifying enzymes catalyze reactions including acetylation, phosphorylation, and adenylation. The steric hindrance caused decreases the affinity of the drug for its target, resulting in AMR [66,67]. One example is aminoglycoside acetyltransferase (AAC) found in Pseudomonas and Acinetobacter which can inactivate most aminoglycosides including amikacin and gentamicin [68,69]. These enzymes are either acquired through horizontal transfer of plasmids or transposons or through spontaneous mutations which expand the functionality of encoding genes [70]. The other mechanisms are target modification and lipopolysaccharides (LPSs) modification [71]. Where antibiotics affect bacterial cell-wall or target cell division, target modification works by either acquisition of binding proteins that do not affect cell-wall or acquisition of mutations within the RNA polymerase, DNA gyrase or topoisomerase IV, modification of ribosomal proteins, or protection of the target site by another protein altogether [17,67,72].
The other more complex mechanism for evading external stressors such as antimicrobials is the formation of structured communities known as biofilms [73]. Biofilms are comprised of an exopolysaccharide matrix surrounding the bacterial communities, with well-established channels for nutrients and water inflow as well as waste outflow [73]. The exopolysaccharide matrix limits the penetration of antibiotics while the proximity allows for horizontal gene transfer from the persister cells [74,75]. Persister cells are those subgroups that have survived an antimicrobial exposure and can give rise to resistant colonies [75]. Further, cells inside the biofilms grow relatively slowly and present low metabolic activity which is detrimental to the activity of most currently available antibiotics [75]. Interestingly, sub-inhibitory concentrations of aminoglycosides, especially tobramycin, have been shown to induce biofilm formation in P. aeruginosa [76]. Azithromycin has been shown to inhibit the expression of the small RNAs rsmY and rsmZ, a process that depends on the GacA/Rsm signal transduction pathway. GacA/Rsm pathway is known to positively control quorum sensing and reciprocally control biofilm formation in P. aeruginosa [77]. Readers are referred to reviews by Høiby et al. 2010 [70], Philip S. Stewart, 2002 [74], and Ahmed et al. 2018 [78].
Comparative genomic analysis suggests that horizontal gene transfer (HGT) plays a significant role in determining the genetic repertoire of the clinical isolates of pathogenic bacteria [25,79,80]. Genomic diversity is, in part, attributable to the acquisition of genetic material that has integrated into the chromosome at a relatively limited number of sites [72]. Acquired mobile genetic elements (plasmids, insertion sequences, transposons) mobilize the antimicrobial resistance genes and can confer resistance to the major classes of antimicrobials among different bacteria. Resistance to environmental stressors is triggered by contact between bacterial sensing systems and the immediate extrinsic environment. The interaction between the sensing systems within the bacteria and the external environment leads to adaptive physiological changes through modulation of gene expression. Two-component regulatory systems detect physical and chemical changes in the environment and then relay this signal to the cytoplasm, where the modulation of gene expression occurs.
3. Two-Component Regulatory Systems in Gram-Negative Pathogenic Bacteria
TCSs have been known to regulate a wide variety of cellular functions in response to environmental signals such as nutrient limitation, oxygen availability, phosphate limitation or osmolarity, and antimicrobial agents [81,82,83]. For instance, in P. aeruginosa PilG/PilH mediates pili production under yet unknown signals and NarX/NarL is involved in nitrate sensing and respiration, biofilm formation and motility [84]. AlgZ/AlgR mediates alginate production under osmolarity and nitrate signals in mucoid strains [85]. PhoR/PhoB senses inorganic phosphate and is involved in regulating quorum sensing and swarming motility [86]. PfeS/PfeR senses enterobactin mediated iron acquisition [87] while FleS/FleR mediates adhesion and sense mucins [88]. GacS/GacA controls virulence in response to unknown signals and CbrA/CbrB senses various carbon sources and modulates metabolism, virulence and antibiotic resistance [89]. CheA/CheY regulates chemotaxis in response to magnesium [82,83,90].
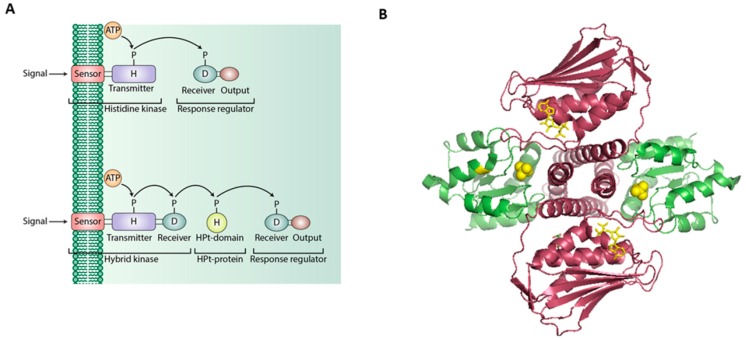
Typically, a TCS consists of a sensor kinase with a conserved histidine residue [H box] also known as histidine kinase [HK] which senses external signals and transfers a phosphate molecule to the response regulator [RR] with a conserved aspartate residue which then mediates cellular response towards the external stimuli (Figure 2). In contrast to the orthodox TCS systems, the phosphorelay system mediated by hybrid histidine kinase (HHK) is also shown.
Figure 2.
Schematic diagram of the functions and domains of sensor kinase and response regulator proteins in TCSs and HHK-mediated phosphorelays. (A) Representation of the classical TCS and phosphorelay signaling systems. (B) Structure of the complex between the entire cytoplasmic portion of Thermotoga maritima class I histidine kinase (magenta) and its cognate, response regulator (green) (PDB entry code 3 DGE) [91].
Bacterial HKs are classified into five types (Type I, II, III, IV, and CheA). Among the five HK family types, Type I and II are found to be genetically related, but type III and IV are not [92]. The likeness between Type I and II is based on the presence of orthodox kinase domains, which contain the N, G1, F, and G2 consensus motifs. Type III and IV HKs possess so-called unorthodox kinase domains in which N1 of the N-box motif is either a glycine (Type III) or a proline (Type IV) residue, the F box is absent, and the G2 motif is truncated. Within the Type I group, three separate subtypes exist: The Type IA group contains 12 HKs, the Type IB group contains the hybrid HKs and the Type IC group contains three HKs, including the nitrogen regulator ntrB. The kinase domain of CheA is characterized by insertions between the N and G1 boxes and the G1 and F boxes. The N-box of CheA contains a histidine residue at the N1 position [92]. Secondary structure analysis of HKs predicts a helix–loop–helix structure important for signal recognition [92].
The HK types found in P. aeruginosa genome were assembled in Figure 3 using the gene tables of the completed genomes listed in the Pseudomonas genome database (http://www.pseudomonas.com). This was compared with the available classification on MiST3 (mistdb.com). The protein sequences were aligned in Phylogeny.fr using MUSCLE for multiple alignments, PhyML for tree building, and TreeDyn for tree rendering [93,94]. Of the total HKs identified in P. aeruginosa, one cluster within the Type IA group (PA1396, 1976, 1992, 3271, and 4936) contains orthodox kinase domains while the H-box motifs containing a non-polar residue at position 4 and a glutamine residue at position 5. This clade of HKs forms a distinct branch within the Type IA group. In addition, a cluster of HKs in the Type IC group possesses the consensus H-box motif HDLNQPL in which the asparagine residue replaces the typical positively charged residue at position 4, but the glutamine residue at position 5 is highly conserved. P. aeruginosa lacks Type II HKs and possesses four CheAs [90]. Two HKs, PA3078, and PA4380, which cannot be assigned to the defined type, are categorized as unclassified. Helix–loop–helix structures are also predicted in the H-box region of the Type III HKs. The H to N distances for each of the HK types are similar to those found in the different HK types of E. coli. Finally, the majority of the HKs are found in operons with cognate RRs [92].
Figure 3.
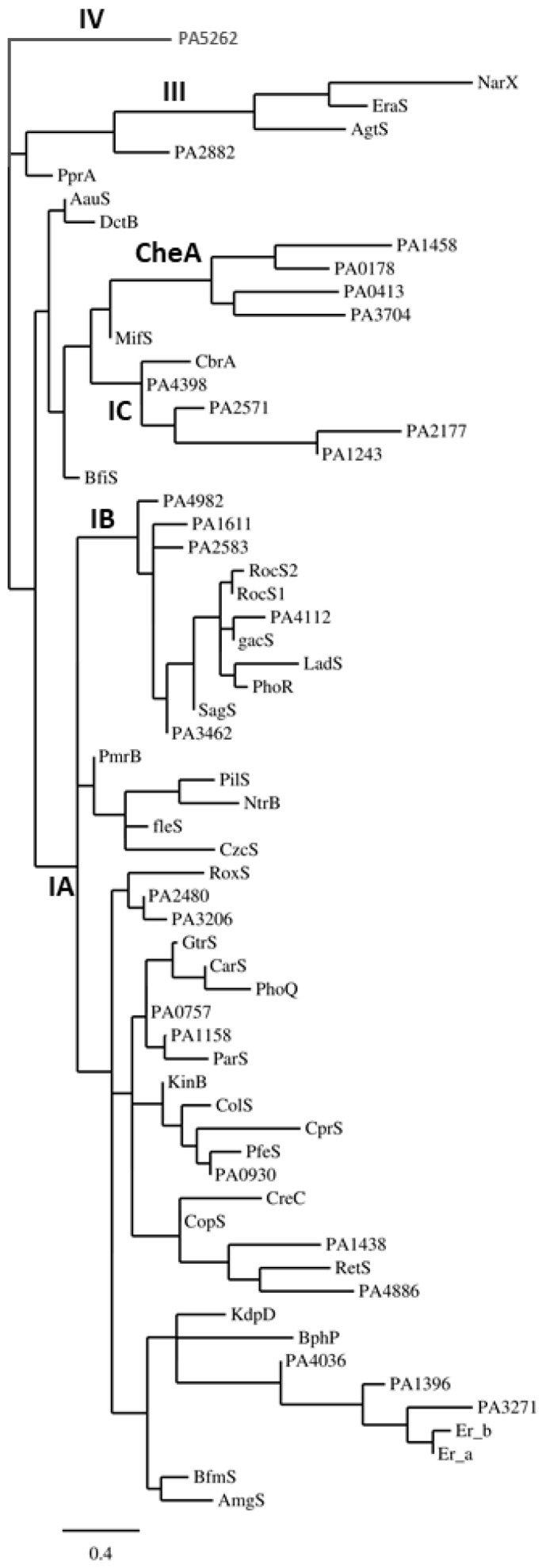
Phylogenetic analysis of the HKs of P. aeruginosa. Known HKs were aligned followed by phylogenetic analysis at Phylogeny.fr [93,94].
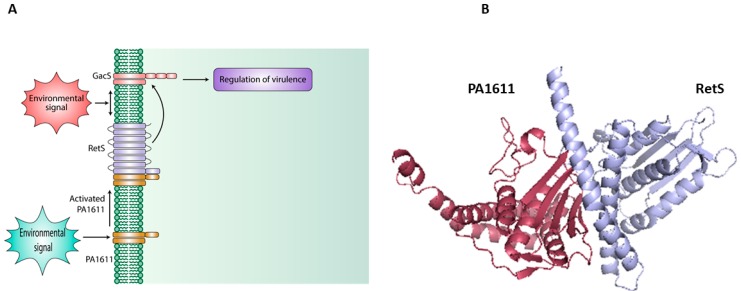
Apart from the classical HK and RR domains, a histidine phosphotransfer protein (Hpt) may be encoded in the same operon. These Hpt proteins are sometimes present either as isolates, as seen in orphan histidine kinase, or along with HHK for phosphotransfer [95]. The division of domains in phosphorelays provides additional checkpoints for phosphorylation and may serve to integrate signals for a collective response as cross-talks are allowed in such systems [95]. Recent studies also suggest a non-phosphotransfer based signaling pathway (Figure 4), in which two HKs can interact directly to elicit a certain response by controlling downstream responses of the non-cognate binding partners [96].
Figure 4.
Schematic diagram of the direct protein-protein interaction mediated signaling using the PA1611-RetS interaction model as an example [96]. (A) Canonical TC Sensor GacS phosphorylates its response regulator GacA to regulate virulence in P. aeruginosa; however, under yet unknown environmental signals, HHK PA1611 is activated and binds to HHK RetS. Under such conditions, GacS is again free to phosphorylate its cognate response regulator and mediate downstream signaling. (B) A docked complex for homology models for PA1611 and RetS showing predicted interacting surfaces.
3.1. One-Component Signaling Systems
One-component systems (OCSs) can be defined as proteins that contain both input and output domains but lack typical histidine kinase and response regulator domains of TCSs. OCSs represent the simplest model for signal transduction by a single protein. One example is RocR in which PAS and HTH are input and output domains respectively. As reported by Ulrich et al., one-component systems were shown to numerically dominate over TCSs in bacteria which was also co-relatable to their genome size [97]. One component signal transduction proteins also demonstrate less conservation within their input and output domain architecture than TCSs [97]. Based on domain architecture, one component systems seem to be the precursors for two-component signaling systems. The structure and abundance of TCSs also suggest that the addition of a histidine kinase might have been an evolutionary step at intercepting external signals [98].
P. aeruginosa PAO1 (genome size 6.2Mbp) encodes for 435 OCSs (mistdb.com) and 130 TCSs (41 HK; 17 HHK; 69 RR; others 5). This is not very different from clinical strains P. aeruginosa 138,244 (widely disseminated and associated with multidrug resistance) (428 OCSs and 134 TCSs), P. aeruginosa 152,504 (rare allele) (466 OCSs, and 145TCSs). However, in contrast, K. pneumonia subsp. pneumoniae strain HS11286 (genome 5.3Mbp) encodes for 387 OCSs and 66 TCSs (27 HK; 5 HHK; 32 RR; Others 2), and A. baumannii (genome 4.3 Mbp) exhibits 235 OCSs and 29 TCSs (11 HK; 3 HHK; 15 RR).In contrast to the one-component systems, the counterparts of two-component signaling systems are highly conserved, and the RRs are more conserved than HKs.
3.2. Hybrid Histidine Kinase [HHK] and Direct-Interaction-Mediated Signaling
Hybrid histidine kinase proteins have, in recent years, become an emerging group of proteins with demonstrated roles in complex signaling mechanisms [96,99,100,101]. P. aeruginosa PAO1 encodes for 15 HHKs while its environmental counterpart P. aeruginosa UCBPP-PA14 has 18. K. pneumoniae subsp. pneumoniae HS11286 encodes for 5 and A. baumannii encodes for 3 HHKs in their respective genomes. HHKs in P. aeruginosa PAO1 includes those involved in a key regulatory pathway regulating biofilm formation and virulence (RetS; GacS; LadS; PA1611) [13,96,100,102]. HHKs in K. pneumoniae include the ArcB (oxygen sensor) [103]; EvgS (capsular polysaccharide) [104]; BarA (carbon metabolism) [105]; RcsC (motility and capsular synthesis) [39]; however, those in A. baumanii (IX87_RS17040, IX87_RS13225, IX87_RS03185) remain largely unexamined. Interestingly, HHKs have been shown to function via both canonical phosphotransfer-mediated signaling as well as by direct protein–protein interaction-mediated signaling one example is the LadS-GacS-RetS-PA1611 system in P. aeruginosa [96,100,101]. Previously, this mechanism has been observed in the PmrB/PmrA TCS in Salmonella [106]. The PmrB/A TCS is required for resistance to acidic environment and antibiotic stresses [106]. PmrD, another regulatory protein from the same operon, binds to and protects the phosphorylated form of PmrA from the phosphatase activity of its cognate sensor, PmrB [106].
In the phosphorelay systems involving HHKs, the Hpt protein serves as the phosphodonor to the terminal response regulator which eventually mediates a cellular response via the output domain. The Hpt protein is also capable of receiving phosphor from HHKs and functioning as an independent protein [107]. An Hpt may serve as a point of signal integration or transmission of signals between two non-cognate TCSs. Although as much as 90% of the TCSs in eukaryotes use hybrid HKs, only 20% of the characterized prokaryote genomes encode hybrid kinases whereas in archaea the number is only 1% [108]. This is explained by the fact that the larger size of a eukaryotic cell necessitates complex signaling and multi-step phosphorelays. With the modular organization in the phosphorelays, phosphorylation at any level may lead to activation of the output domain [109].
Recent studies have highlighted interactions between the HHKs and other auxiliary proteins such as those involved in biofilm formation and regulating efflux pumps in pathogenic bacteria. One classic example is the SagS HHK in P. aeruginosa [110,111]. SagS is expressed during the biofilm development stages and regulates c-di-GMP levels as well as activates MexAB-oprM and MexEF-oprN systems [112,113].
As signaling pathways must act in a combinatorial fashion for normal cellular functioning, there is a possibility of inter-signaling system- information transfer. The direct interaction between TCS proteins is a recently uncovered strategy that the bacteria use to integrate signals other than those detected by a given sensor [114]. These proteins may function as negative or positive regulators of the TCSs involved. Regulatory proteins may also target the response regulators to either protect from dephosphorylation or cause dephosphorylation [115]. Apart from pure phosphotransfer-based signaling, some sensor kinases may also function by either de-phosphorylating the response regulator or protecting the RR from de-phosphorylation. They can perform these functions by binding or sequestering their cognate/non-cognate partners.
Interestingly, more and more reports have emerged suggesting that TCS–TCS direct interaction may also affect signaling states and act as signal transducers for interacting proteins [116,117,118,119,120]. A clear correlation between structural properties, domain interaction, and signaling states is suggested. Though a single TCS may function independently in several different ways, the interaction between TCSs can be described with Boolean operators with the analogy to the neural networks as was first noted in E. coli [121,122]. In E. coli, ArcB, TorS, RcS, and EvgA have been shown to signal through RRs in non-cognate clusters. These recruitment mechanisms are crucial for chemotaxis and sporulation systems [98,123]. In such case, HHKs are a particularly interesting group, which are able to function alone or in conjunction with diverse cognate and non-cognate partners to form signaling complexes [86,124,125,126,127,128]. A multilayer regulation provides an organism greater control over environmental responses. For cells to function as one single unit, the signaling pathways must act in a combinatorial fashion. Thus, apart from sensing the external signal, there is a possibility of inter-signaling system-information transfer, with HHKs being involved in forming signaling complexes [86,124,125] and play a role in multidrug resistance [100,126,127,129].
4. Role of Two-Component Regulatory Systems in Antimicrobial Resistance in Gram- Negative Pathogenic Bacteria
The current knowledge on bacterial genome sequences has made it possible to investigate, identify, and predict two-component regulatory proteins as well as their interacting partners. Two-component regulatory proteins in P. aeruginosa have been widely studied and reviewed. However, the same is not the case for K. pneumoniae and A. baumannii. Table 1 provides a comprehensive listing of available knowledge about various two-component regulatory systems in these three pathogens.
Table 1.
Key TCSs that are reportedly or potentially associated with virulence and/or antibiotics resistance in P. aeruginosa, A. baumannii and K. pneumoniae.
| Name of the Two-Component System | Confirmed or Predicted Function | Reference(s) |
|---|---|---|
| P. aeruginosa | ||
| PhoQ/PhoP | Regulating ABC transporter system; Resistance to antimicrobial peptides, polymyxins, and aminoglycosides; Regulating virulence, swarming motility and biofilm formation; Mg2+ sensing. | [130,131,132,133] |
| PmrA/PmrB | Activated by low Mg2+ and cationic antimicrobial peptides; Resistance against polymyxin B, colistin and other antimicrobial peptides | [131,134,135] |
| CpxA/CpxR | Role in cell envelope stress response; Activates MexAB-OprM efflux pump expression | [136] |
| CprS/CprR | Role in LPS modification and antimicrobial peptide resistance | [137] |
| ParR/ParS | Role in resistance to colistin and polymyxins; Role in quorum sensing, phenazine production, and motility | [137,138] |
| GacS/GacA | Regulating virulence factors; Biofilm formation; Antibiotic resistance; Motility; Iron metabolism; Type III and type VI secretion | [139] |
| PvrS/PvrR | Regulation of the MexAB-OprM efflux pump; Biofilm formation. Controls of fimbrial genes | [140,141,142,143] |
| RcsC-RcsB | Role in biofilm formation and control of fimbrial genes | [140,141] |
| AmgS-AmgR | Involved in aminoglycoside resistance and cell envelope stress response | [144,145,146] |
| PA1611 | Biofilm formation and virulence regulation | [96,129] |
| BfiS/BfiR | Biofilm maturation | [147] |
| HptB/HsbR | Involved in swarming motility and biofilm formation | [148,149] |
| RocS2/RocA2 | Regulation of fimbrial adhesins and antimicrobial resistance | [143,150] |
| ErbR/EraR | Control of biofilm specific antibiotic resistance | [151] |
| TctE/TctD | Controls expression of tricarboxylic acid (TCA) uptake system | [86] |
| PhoR–PhoB | Plays a role in quorum sensing and swarming motility | [86,152] |
| ChpA/PilG/PilH/ChpB | Regulation of the chemosensory pili (Pil–Chp) system, twitching motility and cAMP levels; Regulates virulence genes | [153,154] |
| FimS (AlgZ)/AlgR | Regulation of virulence; Alginate biosynthesis; Motility; Biofilm formation; Cytotoxicity and type III secretion system expression | [155] |
| ColS/ColR | Polymyxin resistance; Virulence and cell adherence | [20] |
| CreC–CreB | Role in catabolism; Swarming and swimming motility; Antibiotic resistance; Biofilm and global gene regulation | [156] |
| PirR–PirS | Iron acquisition | [157] |
| FleS–FleR | Flagellar motility; Adhesion to mucins | [158] |
| PA1396/PA1397 | Plays a role in interspecies signaling; Responds to diffusible signal factor (DSF); Regulates biofilm formation and antibiotic resistance | [159] |
| CzcS–CzcR | Regulates heavy metal resistance; Controls antibiotic resistance and pathogenicity | [160,161] |
| RetS | Regulates virulence; Biofilm formation; Regulates Type III and VI secretion/cytotoxicity | [99,162] |
| LadS | Regulates virulence; Biofilm formation; Type III secretion/cytotoxicity | [99,101] |
| BqsS/BqrR/CarS/CarR | Biofilm formation; Iron sensing; Antibiotic resistance and cationic stress tolerance. Maintains Ca2+ homeostasis; Regulates pyocyanin secretion; Motility. | [163] |
| PfeS–PfeR | Iron acquisition | [87] |
| CopS–CopR | Tolerance to Cu2+, Zn2+; Imipenem resistance | [161] |
| GtrS/GltR | Regulates glucose transport and Type III secretion system | [164,165] |
| WspE–WspR | Regulates biofilm formation, autoaggregation, and cyclic-di-GMP synthesis | [166,167,168] |
| NarX–NarL | Nitrate sensing and respiration; Biofilm formation; Motility | [84] |
| BfmS/BfmR | Biofilm formation/maintenance | [147] |
| PprA–PprB | Regulates outer membrane permeability; Aminoglycoside resistance; Controls virulence including type III secretion system and biofilm formation | [169,170,171] |
| RoxS/RoxR | Confers cyanide tolerance | [172] |
| PilS–PilR | Involved in regulating the expression of the T4P major subunit PilA; Biofilm formation; Motility; Positively regulates the transcription of flagellar regulatory genes | [173] |
| CbrA–CbrB | Metabolic regulation of carbon and nitrogen utilization. Modulates biofilm formation; Cytotoxicity; Motility; Antibiotic resistance | [89] |
| AruS/AruR | Controls the expression of the arginine transaminase pathway | [174,175] |
| NtrB/NtrC | Responds to cellular nitrogen levels and activates nitrogen scavenging genes | [176] |
| DctB/DctD | Controls the expression of C4-dicarboxylate transporters | [177] |
| KinA/AlgB | Regulates alginate biosynthesis; Regulates virulence | [178] |
| MifS/MifR | Role in biofilm formation and metabolism | [179] |
| K. pneumoniae | ||
| CpxA/CpxR | Sensing extracellular pH and membrane composition; Regulating cell envelope protein folding and protein degradation | [180,181] |
| PhoP/PhoQ | Activates pmrHFIJKLM; Responsible for L-amino arabinose synthesis and polymyxin resistance | [182,183,184] |
| PhoR/PhoB | Phosphate assimilation | [180] |
| QseC/QseB | Involved in regulation of the flagella and motility genes | [185] |
| KvgA/KvgS | Involved in tolerating free radical stresses and sensing iron-limiting conditions | [186] |
| KvhA/KvhS | Regulates capsular polysaccharide synthesis | [187,188] |
| PmrA/PmrB | Regulator of genes for lipopolysaccharide modification | [189] |
| RcsC/RcsB | Involved in the capsular polysaccharide biosynthesis; Type III system; Regulates the production of major pilin protein MrkA; Confers resistance to low pH | [190] |
| EnvZ/OmpR | Senses osmotic signals; Regulates the c-di-GMP signaling pathway; Regulates type III fimbriae and biofilm formation | [191,192,193] |
| CusS/CusR | Induced by Copper and regulates the CusCFBARS efflux system; Tolerance to silver | [194,195,196] |
| KdpD/KdpE | Potassium transporter system | [197,198,199] |
| BaeS/BaeR | Regulates Multidrug efflux pump AdeABC; Regulates Modification of lipopolysaccharides | [199,200] |
| ArcB/ArcA | Involved in modulating the expression of genes encoding for proteins with membrane modification functions and TCA cycle enzymes depending upon oxygen levels. | [199,201] |
| NarX/NarL | Role in nitrate and nitrite reductase synthesis | [202,203] |
| UhpB/UhpA | Role in uptake of hexose phosphates | [199,204,205] |
| EvgS/EvgA | Regulates capsular polysaccharide biosynthesis | [206,207] |
| GlnL/GlnG | Role in glutamate metabolism | [208,209] |
| ZraR/ZraS | Zinc-responsive TCS; Activated under high calcium and iron conditions | [210] |
| CitA/CitB | Regulates citrate metabolism under anaerobic conditions | [211,212] |
| CrrA/CrrB | Involved in polymyxin resistance | [213] |
| A. baumannii | ||
| PmrA/PmrB | Regulates genes involved in lipopolysaccharide modification | [214,215] |
| AdeS/AdeR | Regulates genes encoding the AdeABC pump | [59,216] |
| BaeS/BaeR | Stress response under high osmotic conditions | [60,217,218] |
| BfmS/BfmR | Regulates biofilm formation and antibiotic resistance | [219,220,221] |
| GacS/GacA | Regulates genes associated with pili and biofilm development, motility and resistance against host antimicrobial peptides | [222,223] |
| A1S_2811 | Involved in surface motility and biofilm formation | [224] |
| KdpD/KpdE | Regulates potassium transport | [225] |
| GlnL/GlnG | Involved in nitrogen assimilation | [226] |
| PhoR/PhoB | Regulates phosphate assimilation | [227] |
| CusS/CusR | Senses copper ions and upregulates the expression of an RND family efflux pump that removes copper ions from the cell | [228] |
| OmpR/EnvZ | Regulates virulence; Phase variation; Osmotic tolerance | [229] |
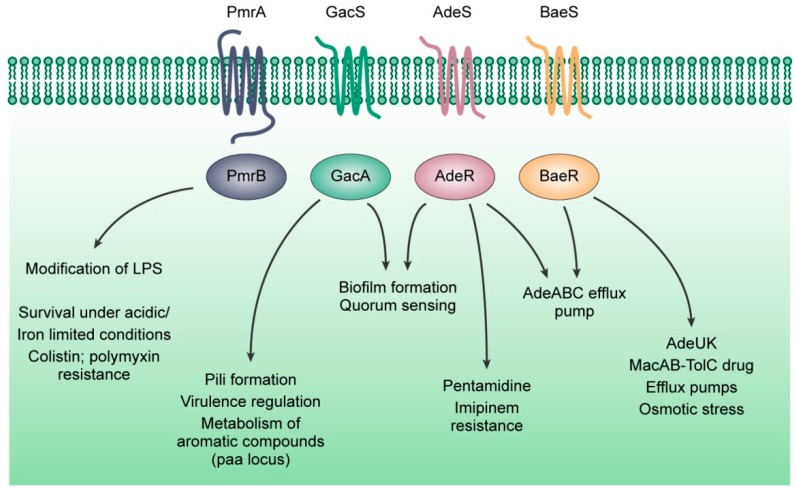
We analyzed two-component regulatory proteins between these three pathogens and found a surprising conservation in the PmrAB, GacSA, AdeRS, and BaeSR, systems. Among the HKs and RRs, we observed a greater degree of conservation in the RRs. We will discuss the available pool of knowledge on these four systems and their role in antimicrobial resistance (Figure 5). We focus only on these four TCSs [217,218] as they exist across the three bacteria under discussion and are related to antimicrobial resistance.
Figure 5.
The known roles of PmrAB, GacSA, AdeRS, and BaeSR, two-component regulatory systems in antimicrobial resistance.
4.1. The PmrAB System
PmrAB TCS was shown to be involved in the LPS modification in P. aeruginosa. The genes pmrB and pmrA encode for the sensor histidine kinase (PmrB) and its cognate response regulator (PmrA), which, once phosphorylated, activates the pmrC and pmrHFIJKLM as well as downstream genes. Genetic targets of PmrAB in P. aeruginosa include the cprA gene required for polymyxin resistance [20] and the pmr genes (PA3552–PA3559) for resistance from antimicrobial peptides [230]. The HK PmrB in P. aeruginosa, K. pneumonia, and A. baumanii showed a BLOSUM similarity score of 29%. PmrA in P. aeruginosa, K. pneumonia, and A. baumanii showed a 43% similarity (BLOSUM). Phylogenetically, PmrA in P. aeruginosa is more closely related to K. pneumoniae as compared to that of A. baumanii. Interestingly, a closer look at the past studies reveals a far more functional similarity.
Mutations in the pmrAB operon (polymyxin resistance) have been linked to enhanced resistance to antimicrobial peptides as well as survival in chronic infections. The PmrAB system (PA4776–PA4777) was first identified in P. aeruginosa clinical isolates with resistance to polymyxins. It was observed that a mutation in the PmrAB locus resulted in resistance to polymyxins and other cationic antimicrobial peptides (CAPs) [134,231,232]. Further PmrAB was shown to have a role in survival and persistence in chronic lung infections. A mutation in the PmrB resulted in an increased ability to survive in a mouse model of chronic respiratory infection as compared to both the wild type as well as those adapted to the mouse lung but lacking the mutation [233]. Recent studies have argued that PmrB mutants were, in fact, more susceptible to antimicrobials, such as ciprofloxacin, colistin, gentamycin, polymyxin B, tobramycin, and tetracycline [234]. The same study also identified at least 216 proteins that were differentially regulated in a PmrB mutant. Interestingly, the PmrB mutant was found to show enhanced resistance to host-derived antimicrobial peptides [234].
To survive environmental stressors, one of the unique mechanisms that the pathogenic bacteria employ is the ability to remodel their outer membranes. This remodeling occurs, mainly at the level of lipid A in the lipopolysaccharide (LPS). The remodeling of lipid A occurs in a PhoPQ-PmrAB dependent manner by palmitoylation or deacylation, or both, by the addition of 4-aminoarabinose (L-Ara4N) or phosphoethanolamine (pEtN) [235]. The addition of L-Ara4N is considered the most effective by decreasing the net negative charge of the membrane to zero [46]. Thus, this would reduce its binding to polymyxins, resulting in resistance. The second, PEtN modification, decreases the net charge from −1.5 to −1 [46].
Similar to that in P. aeruginosa, pmrAB TCS in K. pneumoniae has been shown to regulate lipopolysaccharide modification [236]. In A. baumanii, mutations in PmrAB have been shown to be associated with colistin resistance [237], however, the mechanism is still unclear. It has been suggested that mutations in PmrA or B, or both, could result in lipid modifications [238]. The HK gene pmrB seems to be the more common site for bacterial mutations compared to the RR gene pmrA. Interestingly, the acquisition of colistin resistance was also found to be associated with decreased virulence and fitness [239]. In contrast, recent studies on PmrB mutant in A. baumanii have shown no reduction in virulence or fitness [240]. It has been suggested that PmrB senses acidic pH (pH 5.5), low magnesium levels and iron limitation, and also increases the survival under antimicrobial stress [241,242,243] via yet unknown mechanisms.
4.2. The GacSA System
The GacSA TCS is one of the most widely studied systems in P. aeruginosa. GacS is an HK and GacA is the RR. Phosphorylation of GacS is under the control of hybrid sensor kinases, RetS (PA4856) [99], PA1611 [129], and LadS (PA3974) [101]. These three HHKs are known to bind to GacS under yet unknown environmental stimuli to reciprocally control the acute-chronic disease transition in P. aeruginosa. Once phosphorylated, GacA activates the transcription of two small regulatory RNAs, RsmZ (PA3621.1), and RsmY (PA0527.1) [99]. RsmY/Z control the activation of the RNA-binding protein RsmA (PA0905) [244]. RsmA is known to regulate genes of the Type III secretion system, type IV pili formation and iron homeostasis while repressing QS, Type VI secretion and potentially other transcription factors [245]. The MexEF-OprN pump in P. aeruginosa has been found to remove several antibiotics, Pseudomonas quinolone signal and specific quorum sensing molecules from the cell [246]. RsmA has been shown to control the expression of the MexEF-OprN pump [247]. A rsmA mutant demonstrated results in activated expression of the genes encoding the MexEF-OprN pump [247]. The GacSA system is also involved in antibiotic resistance, through RsmA/RsmZ, to three different families of antibiotics: tobramycin, ciprofloxacin, and tetracycline [248]. Further, biofilms known to be resistant to available antibiotics in P. aeruginosa are affected primarily by the pel and psl operons and broadly by the modulation in intracellular c-di-GMP levels [249]. c-di-GMP is a second messenger shown to promote biofilm formation and antimicrobial resistance in P. aeruginosa [249]. Both the pel and psl operons are post-transcriptionally regulated by the RetS-LadS systems via RsmY/Z [99,100,250]. c-di-GMP exerts a broader control via its effect on a variety of regulatory proteins and RNAs. Previously, it has been shown that c-di-GMP levels, are modulated by the diguanylate cyclase WspR (PA3702), which is involved in the switch between acute and chronic infection phase and is shown to be dependent on RsmY/Z [251]. Small colony variants (SCV) of P. aeruginosa clinical isolates are known to exhibit hyper biofilm formation, hyper pilation and demonstrate enhanced resistance to several antibiotics [252]. Often the SCV phenotype is associated with elevated intracellular levels of c-di-GMP [142]. Studies have also shown that a mutation in the PmrAB system is associated with SCVs and hyperbiofilm phenotypes.
The amgRS operon encodes a membrane stress-responsive TCS found to be linked to intrinsic aminoglycoside resistance in P. aeruginosa. The AmgS is the sensor kinase and the AmgR is the response regulator. AmgRS TCS has been shown to provide resistance against aminoglycoside-related membrane damage [145]. AmgRS was shown to be activated in the presence of aminoglycosides which in turn promoted the mexXY expression [145]. It was observed that while overexpression of the AmgRS system slightly reduced the colony size of wild-type PA14, the SCV formation was enhanced significantly when GacA was overexpressed simultaneously, [232].
Similar to P. aeruginosa, the GacSA system in A. baumannii was shown to be a global regulator of virulence, pili, biofilm formation and resistance to host-derived antimicrobial peptides and motility [222]. It was also shown that GacSA played a key role in attachment to abiotic surfaces, arginine metabolism, and biofilm formation [253]. A GasS mutant of A. baumannii showed decreased virulence towards Candida albicans [254] and was unable to use citrate as the carbon source [255]. The phenylacetic acid (PAA) pathway is crucial to the metabolism of aromatic compounds and environmental pollutants in bacteria. In A. baumanii, the PAA pathway is encoded by the paa operon and it was observed that the deletion of gacS resulted in repression of the entire paa operon [222].
A search for the GacSA system in K. pneumoniae identified BarA with 93% similarity to the GacS HK and UvrY with 100% similarity to the GacA RR in P. aeruginosa. BarA and UvrY correspond to KpST66_3517 and KpST66_0986 in K. pneumoniae genome [256]. These proteins consist of an N-terminal cytosolic domain, a canonical pair of transmembrane regions linked by a periplasmic bridge, a transmitter domain containing a conserved histidine residue, a central receiver domain with a conserved aspartate residue, and a C-terminal phosphotransfer domain with a conserved histidine residue [257]. To date, there have been few studies on this TCS and its role in antimicrobial resistance in K. pneumoniae.
The carbon storage regulation (Csr) system has been shown to have a major impact on regulation of carbon metabolism pathways, motility, and biofilm formation [256]. The Csr system is composed of small regulatory RNAs possessing repeated sequence elements that allow them to interact with multiple copies of the RNA binding proteins, thereby preventing its regulatory interaction with its mRNA targets downstream [256]. UvrY has been shown to activate the expression of the noncoding csrB and csrC RNAs in E. coli [257]. This, in turn, sequesters CsrA and prevents it from activating downstream genes. CsrB is a carbon source utilization system which has been shown to integrate signals from the UvrY-BarA TCS [105]. Functional studies for the UvrY-BarA in Escherichia coli have shown roles in catalase expression [258,259], biofilm formation [256] and quorum sensing [260].
Unlike PmrAB, AdeRS, and BaeSR, GacSA is not a contiguous operon in any of the three pathogens discussed above suggesting that GacSA may be involved in crosstalk between different TCSs. The full extent of its role in these pathogens yet remains to be understood.
4.3. The AdeRS and the BaeSR Systems
The AdeRS two-component regulatory system is composed of the AdeS as a sensor kinase, whereas AdeR is the RR. The AdeRS system is one of the best characterized TCS in A. baumanii. The AdeRS TCS has been shown to be involved in a more global regulation of gene expression in A. baumanii either directly in sensing cell density/growth, or indirectly as in sensing osmolality via the BaeSR system [261].
Efflux pumps are usually regulated by regulatory proteins adjacent to them. There are few reports of efflux pumps regulated by TCSs including the NorA pump in S. aureus [262]. Interestingly, AdeRS has been demonstrated to control the expression of adeABC efflux pump in A. baumanii [59]. AdeABC efflux pump, a three-component system, and a member of the RND family has been shown to play a role in resistance to aminoglycosides, tetracycline, erythromycin, chloramphenicol, trimethoprim, fluoroquinolones, and tigecycline [263]. AdeABC consists of AdeA, the inner membrane fusion protein, AdeB the transmembrane component, and AdeC the outer membrane protein. A. baumanii ATCC 17978 has two adeA genes and one adeB gene, but lacks the adeC [264]. The overexpression of AdeABC is also associated with increased virulence, which probably explains why adeRS mutations are frequently observed in clinical isolates [61,216,265,266]. Recent studies showed that AdeRS, directly or indirectly, regulates 579 genes, most notably those involved in the expression of efflux pumps, biofilm formation and virulence in a Galleria mellonella larvae infection model [267]. Intriguingly, some outcomes of the AdeRS deletion appeared to be strain specific. Further truncations or point mutations within AdeR or S leads to activation of AdeABC efflux system and results in multidrug resistance [268,269].
A search for AdeR homolog in P. aeruginosa resulted in a yet unknown response regulator belonging to OmpR family, containing a DNA-binding response regulator with 91% similarity (WP_033958295.1) and 93% similarity in K. pneumonia (WP_004199992.1). The roles of either are not known yet.
The BaeSR TCS was first discovered in E. coli [270] and Salmonella enterica serovar Typhimurium [217]. The BaeSR TCS consists of an inner-membrane-bound BaeS HK, and a cytoplasmic RR, BaeR. Genome analysis of A. baumannii ATCC 17978 shows that the coding sequences of baeR (A1S_2883) and baeS (A1S_2884) are arranged sequentially, suggesting that the two genes may be co-transcribed as one operon. P. aeruginosa encodes a similar protein to BaeS (Locus: CRQ12647; 96% similarity), whose function remains unknown. Deletion of baeSR in A. baumanii led to a significantly reduced expression of the major efflux pumps, such as AdeABC, AdeIJK, and MacAB-TolC [218], resulting in increased susceptibility to tigecycline. The regulons of AdeRS and BaeSR overlap. This could also mean that BaeSR may function through crosstalk with AdeRS [217,218].
5. Two-Component Regulatory Systems as Potential Drug Targets
Two-component regulatory systems in gram-negative pathogens, though highly complex, may serve as attractive drug targets for a variety of reasons. The first reason is the high degree of structural and functional homology between various TCSs. A compound that is effective against a specific TCS, should plausibly be effective against other bacteria too, [271,272]. Secondly, TCSs regulate diverse, but essential functions in the cells and thus form a very effective target so that an inhibitor would inflict a global effect and not just targeting one pathway. Targeting a central regulatory system can significantly affect cell viability with low risk of development of quick resistance. Thirdly, many antimicrobial resistant genes are regulated either directly or indirectly by TCSs and targeting TCSs forms an excellent adjunct to currently available antimicrobials. Lastly, the possibility of negative side effects with the use of TCS targeting drugs are expected to be minimum as the bacterial histidine based TCS is very different from the eukaryotic serine/threonine based signaling systems. Thus, TCSs serve as an excellent target for drug development for combating microbial infections, including those resistant to currently available antibiotics.
Designing a drug-targeting specific TCSs is, however, complicated. The possible sites of intervention for a TCS are to be determined. Studies in the past have targeted the RR DNA binding [273], autophosphorylation sites [274] as well as ATP-binding domains [275]. Suggested sites for targeting include the site for autophosphorylation, site of interaction for HK-RR, facilitating the dephosphorylation of the HK, and inhibiting binding to the downstream genes. The question remains about the active sites for the known TCSs and whether they are common to all pathogens of one class. Targeting TCSs could be better if the targeted TCSs were conserved across several gram-positive and gram-negative bacterial species. Then there is the question about selectivity and spectrum of activity based on conservation of the active sites. Undoubtedly, further studies are needed before an antimicrobial drug targeting TCS is successfully developed.
6. Conclusions and Perspective
Studies of TCS signaling circuits continue to reveal new layers of complexity for these systems. The conventional notion of the TCSs is frequently being challenged by new findings and our understanding of bacterial signaling through TCSs and other related systems is continuously evolving. The complex connection of bacterial signaling systems and antibiotic resistance are expected to be revealed by further studies and so will the underlying mechanisms.
Autoregulation is an important concept in TCS signaling and has only recently begun to emerge and is well-illustrated by the BvgS system from the BvgS/BvgA system in Bordetella bronchiseptica. It was observed that autoregulation by BvgS modulates the sensitivity of the system to an applied stimulus [276]. Autoregulation can be positive or negative. Positive autoregulation occurs when a response regulator activates transcription of its own gene and the gene encoding its partner histidine kinase. In contrast, negative autoregulation is less common and involves a response regulator that represses its own expression. The other example of autoregulation within TCSs is the PhoQ/PhoP system in E. coli. In the steady state, output was unaffected by autoregulation over a wide range of (stimulus levels) magnesium concentrations. However, when faced with growth-limiting levels of magnesium, the autoregulation of histidine kinase, PhoQ amplified the output. When PhoQ was mutated to incapacitate phosphatase activity, there was strong amplification of autoregulation irrespective of the stimulus conditions [277]. Phosphorylation, though important, is not necessary for autoregulation. In the case of TorR [278] and LuxO [279], the response regulator can repress its own expression, irrespective of its phosphorylation status. Interestingly, autoregulation has also been said to result in a “short term memory” or a “learning behavior.” This means that once a signal has been perceived in the past, the bacteria form a memory of the stimulus and upon subsequent exposures respond faster or more extensively to a signal [280]. In the case where two proteins “cross-communicate” through the expression of an auxiliary protein, also known as two-component “connectors,” autoregulation may influence the system by controlling the relative concentrations of the interacting proteins. Understanding autoregulation is important because often it is mediated by highly conserved proteins and can be acquired by HGT. This is important to developing antimicrobial resistance amongst pathogens and must be considered when identifying viable drug targets.
Cross-talks of the TCSs and information exchange through direct interactions are other intriguing aspects of bacterial signaling mediated by TCSs and related systems. Is there a possibility of physiological cross-talk amongst TCSs in different species given their similarities in domains and functions? Further work is required in P. aeruginosa, A. baumanii, and K. pneumoniae to investigate the role of autoregulation, identify connectors in cross-talk of the TCSs, and its role in antimicrobial resistance. In this increasingly critical time of drug resistance, there is a growing need to understand the signals perceived by TCSs, their complex circuitries, as well as their modular architectures. New methods are to be developed to study TCS activity in vivo to answer questions about specificity and selectivity. In any case, TCSs promise a significant therapeutic target.
Abbreviations
| AAC | Aminoglycoside Acetyltransferase |
| ABC | ATP Binding Cassette |
| AMR | Antimicrobial Resistance |
| CAPs | Cationic Antimicrobial Peptides |
| CDC | Centers for Disease Control and Prevention |
| CF | Cystic Fibrosis |
| CHDL | Carbapenem-Hydrolysing Class D β-Lactamase |
| Csr | Carbon Storage Regulation |
| DSF | Diffusible Signal Factor |
| ESBL | Extended-Spectrum β-lactamase |
| ESCAPE | Enterococcus faecium, Staphylococcus aureus, Clostridium difficile, A. baumannii, P. aeruginosa, and Enterobacteriaceae |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacteriaceae |
| HAP | Histidyl-Aspartyl Phosphorelay |
| HGT | Horizontal Gene Transfer |
| HHK | Hybrid Histidine Kinase |
| HK | Histidine Kinase |
| Hpt | Histidine Phosphotransfer Protein |
| HTH | Helix-turn-helix |
| ICU | Intensive Care Unit |
| L-Ara4N | 4-Amino-4-Deoxy-L-Arabinose |
| LPSs | Lipopolysaccharides |
| MATE | Multidrug and Toxin Extrusion |
| MBL | Metallo β-lactamases |
| MDR | Multidrug-Resistant |
| MFS | Major Facilitator Superfamily |
| NDM-1 | New Delhi Metallo- β-lactamase 1 |
| NNIS | National Nosocomial Infections Surveillance |
| OCSs | One-Component Systems |
| OM | Outer Membrane |
| PAA | Phenylacetic Acid |
| PAS | Period clock protein, Aryl hydrocarbon receptor, and Single-minded protein |
| PDR | Pan-Drug Resistant |
| pEtN | Phosphoethanolamine |
| RND | Resistance-Nodulation-Cell Division |
| RR | Response Regulator |
| SCV | Small Colony Variants |
| SMR | Small Multidrug Resistance |
| TCA | Tricarboxylic Acid |
| TCSs | Two-Component Systems |
| WHO | World Health Organization |
Funding
This research was supported by grants the National Science and Engineering Research Council of Canada (No. 402943-2011 RGPIN), IRT-15R55, and from NSFC (No. 31570131).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization . Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. World Health Organization; Geneva, Switzerland: 2017. p. 12. [Google Scholar]
- 2.Chairat S., Ben Yahia H., Rojo-Bezares B., Saenz Y., Torres C., Ben Slama K. High prevalence of imipenem-resistant and metallo-beta-lactamase-producing Pseudomonas aeruginosa in the Burns Hospital in Tunisia: Detection of a novel class 1 integron. J. Chemother. 2019:1–7. doi: 10.1080/1120009X.2019.1582168. [DOI] [PubMed] [Google Scholar]
- 3.Zhe S., Qianru Z., Liying Z., Zhidong Z., Ling J., He H. Draft genome sequence of a multidrug-resistant beta-lactamase-harboring Bacillus cereusS66, isolated from China. J. Glob. Antimicrob. Resist. 2019 doi: 10.1016/j.jgar.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Goic-Barisic I., Seruga Music M., Kovacic A., Tonkic M., Hrenovic J. Pan drug-resistant environmental Isolate of Acinetobacter baumannii from Croatia. Microb. Drug Resist. 2017;23:494–496. doi: 10.1089/mdr.2016.0229. [DOI] [PubMed] [Google Scholar]
- 5.Li L., Yu T., Ma Y., Yang Z., Wang W., Song X., Shen Y., Guo T., Kong J., Wang M., Xu H. The genetic structures of an Extensively Drug Resistant (XDR) Klebsiella pneumoniae and Its plasmids. Front. Cell Infect. Microbiol. 2018;8:446. doi: 10.3389/fcimb.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C.Y., Jerng J.S., Chen K.Y., Lee L.N., Yu C.J., Hsueh P.R., Yang P.C. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: Clinical features, risk-factors and outcomes. Clin. Microbiol. Infect. 2006;12:63–68. doi: 10.1111/j.1469-0691.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonnevend Á., Ghazawi A., Hashmey R., Haidermota A., Girgis S., Alfaresi M., Omar M., Paterson D.L., Zowawi H.M., Pál T. Multihospital occurrence of pan-resistant Klebsiella pneumoniae sequence type 147 with an ISEcp1-directed blaOXA-181 Insertion in the mgrB gene in the United Arab Emirates. Antimicrob. Agents Chemother. 2017;61:e00418-17. doi: 10.1128/AAC.00418-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsueh P.-R., Teng L.-J., Chen C.-Y., Chen W.-H., Yu C.-J., Ho S.-W., Luh K.-T. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg. Infect. Dis. 2002;8:827–832. doi: 10.3201/eid0805.020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes M., Vira D., Medikonda R., Kumar N. Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: Clinical features, risk factors, and outcome. Graefes Arch. Clin. Exp. Ophthalmol. 2016;254:315–322. doi: 10.1007/s00417-015-3208-7. [DOI] [PubMed] [Google Scholar]
- 11.Ozer E.A., Krapp F., Hauser A.R., Qi C. Case report of an extensively drug-resistant Klebsiella pneumoniae Infection with genomic characterization of the strain and review of similar cases in the United States. Open. Forum Infect. Dis. 2018;5 doi: 10.1093/ofid/ofy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson L.R. Bad bugs, no drugs: No ESCAPE revisited. Clin. Infect. Dis. 2009;49:992–993. doi: 10.1086/605539. [DOI] [PubMed] [Google Scholar]
- 13.Cabot G., Zamorano L., Moya B., Juan C., Navas A., Blazquez J., Oliver A. Evolution of Pseudomonas aeruginosa antimicrobial resistance and fitness under low and high mutation rates. Antimicrob. Agents Chemother. 2016;60:1767–1778. doi: 10.1128/AAC.02676-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsakiridou E., Makris D., Daniil Z., Manoulakas E., Chatzipantazi V., Vlachos O., Xidopoulos G., Charalampidou O., Zakynthinos E. Acinetobacter baumannii infection in prior ICU bed occupants is an independent risk factor for subsequent cases of ventilator-associated pneumonia. BioMed. Res. Int. 2014;2014:193516. doi: 10.1155/2014/193516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin R.M., Bachman M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 2018;8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaynes R., Edwards J.R. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 17.Landman D., Trehan M., Panwar M., Kochar S., Bratu S., Quale J., Doymaz M. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, NY. J. Antimicrob. Chemother. 2007;60:78–82. doi: 10.1093/jac/dkm129. [DOI] [PubMed] [Google Scholar]
- 18.Streit J.M., Jones R.N., Sader H.S., Fritsche T.R. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: Report from the SENTRY Antimicrobial Surveillance Program (North America, 2001) Int. J. Antimicrob. Agents. 2004;24:111–118. doi: 10.1016/j.ijantimicag.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Lingzhi L., Haojie G., Dan G., Hongmei M., Yang L., Mengdie J., Chengkun Z., Xiaohui Z. The role of two-component regulatory system in beta-lactam antibiotics resistance. Microbiol. Res. 2018;215:126–129. doi: 10.1016/j.micres.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Gutu A.D., Sgambati N., Strasbourger P., Brannon M.K., Jacobs M.A., Haugen E., Kaul R.K., Johansen H.K., Hoiby N., Moskowitz S.M. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob. Agents Chemother. 2013;57:2204–2215. doi: 10.1128/AAC.02353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch E.B., Tam V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev. Pharm. Outcomes Res. 2010;10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella E., Ciccozzi M., Lo Presti A., Fogolari M., Azarian T., Prosperi M., Salemi M., Equestre M., Antonelli F., Conti A., et al. Multi-drug resistant Klebsiella pneumoniae strains circulating in hospital setting: Whole-genome sequencing and Bayesian phylogenetic analysis for outbreak investigations. Sci. Rep. 2017;7:3534. doi: 10.1038/s41598-017-03581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mira A., Apalara J., Thyagarajan R., Sengstock D.M., Kaye K.S., Chopra T. Multidrug-resistant Acinetobacter baumannii: An emerging pathogen among older adults in community hospitals and nursing homes. Clin. Infect. Dis. 2010;50:1611–1616. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 24.Martinez J.L., Baquero F. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 2000;44:1771–1777. doi: 10.1128/AAC.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andam C.P., Fournier G.P., Gogarten J.P. Multilevel populations and the evolution of antibiotic resistance through horizontal gene transfer. FEMS Microbiol. Rev. 2011;35:756–767. doi: 10.1111/j.1574-6976.2011.00274.x. [DOI] [PubMed] [Google Scholar]
- 26.Sommer M.O.A., Dantas G., Church G.M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325:1128–1131. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies J.E. Origins, acquisition and dissemination of antibiotic resistance determinants. Ciba Found Symp. 1997;207:15–27. [PubMed] [Google Scholar]
- 28.Blanquart F., Lehtinen S., Lipsitch M., Fraser C. The evolution of antibiotic resistance in a structured host population. J. R. Soc. Interface. 2018;15:20180040. doi: 10.1098/rsif.2018.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J., Lee S., Zhao X., Dong Y., Drlica K., Amin A., Musser J.M., Ramaswamy S., Domagala J. Selection of antibiotic-resistant bacterial mutants: Allelic diversity among fluoroquinolone-resistant mutations. J. Infect. Dis. 2000;182:517–525. doi: 10.1086/315708. [DOI] [PubMed] [Google Scholar]
- 30.Gniadkowski M. Evolution of extended-spectrum beta-lactamases by mutation. Clin. Microbiol. Infect. 2008;14:11–32. doi: 10.1111/j.1469-0691.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 31.Bush K. Past and present perspectives on beta-Lactamases. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutkind G.O., Di Conza J., Power P., Radice M. Beta-lactamase-mediated resistance: A biochemical, epidemiological and genetic overview. Curr. Pharm. Des. 2013;19:164–208. doi: 10.2174/138161213804070320. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj P., Singh N.S., Virdi J.S. Escherichia coli β-Lactamases: What Really Matters. Front. Microbiol. 2016;7:417. doi: 10.3389/fmicb.2016.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez J.L. The antibiotic resistome: Challenge and opportunity for therapeutic intervention. Future Med. Chem. 2012;4:347–359. doi: 10.4155/fmc.12.2. [DOI] [PubMed] [Google Scholar]
- 35.Perry J.A., Wright G.D. The antibiotic resistance “mobilome”: Searching for the link between environment and clinic. Front. Microbiol. 2013;4:138. doi: 10.3389/fmicb.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corona F., Blanco P., Alcalde-Rico M., Hernando-Amado S., Lira F., Bernardini A., Sanchez M.B., Martinez J.L. The analysis of the antibiotic resistome offers new opportunities for therapeutic intervention. Future Med. Chem. 2016;8:1133–1151. doi: 10.4155/fmc-2016-0027. [DOI] [PubMed] [Google Scholar]
- 37.Stover C.K., Pham X.Q., Erwin A.L., Mizoguchi S.D., Warrener P., Hickey M.J., Brinkman F.S., Hufnagle W.O., Kowalik D.J., Lagrou M., et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 38.Veleba M., Higgins P.G., Gonzalez G., Seifert H., Schneiders T. Characterization of RarA, a novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2012;56:4450–4458. doi: 10.1128/AAC.00456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jana B., Cain A.K., Doerrler W.T., Boinett C.J., Fookes M.C., Parkhill J., Guardabassi L. The secondary resistome of multidrug-resistant Klebsiella pneumoniae. Sci. Rep. 2017;7:42483. doi: 10.1038/srep42483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnet S., Courvalin P., Lambert T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 2001;45:3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damier-Piolle L., Magnet S., Bremont S., Lambert T., Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008;52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon E.J., Chabane Y.N., Goussard S., Snesrud E., Courvalin P., De E., Grillot-Courvalin C. Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. mBio. 2015;6:e00309-15. doi: 10.1128/mBio.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coyne S., Courvalin P., Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zgurskaya H.I., Löpez C.A., Gnanakaran S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass It. ACS Infect. Dis. 2015;1:512–522. doi: 10.1021/acsinfecdis.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J. Biol. Chem. 1976;251:2176–2178. [PubMed] [Google Scholar]
- 46.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. MMBR. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise M.G., Horvath E., Young K., Sahm D.F., Kazmierczak K.M. Global survey of Klebsiella pneumoniae major porins from ertapenem non-susceptible isolates lacking carbapenemases. J. Med. Microbiol. 2018;67:289–295. doi: 10.1099/jmm.0.000691. [DOI] [PubMed] [Google Scholar]
- 48.Hong J.H., Clancy C.J., Cheng S., Shields R.K., Chen L., Doi Y., Zhao Y., Perlin D.S., Kreiswirth B.N., Nguyen M.H. Characterization of porin expression in Klebsiella pneumoniae Carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob. Agents Chemother. 2013;57:2147–2153. doi: 10.1128/AAC.02411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulzova L., Navratilova L., Comor L. Alterations in outer membrane permeability favor drug-resistant phenotype of Klebsiella pneumoniae. Microb. Drug Resist. 2017;23:413–420. doi: 10.1089/mdr.2016.0017. [DOI] [PubMed] [Google Scholar]
- 50.Domenech-Sanchez A., Hernandez-Alles S., Martinez-Martinez L., Benedi V.J., Alberti S. Identification and characterization of a new porin gene of Klebsiella pneumoniae: Its role in beta-lactam antibiotic resistance. J. Bacteriol. 1999;181:2726–2732. doi: 10.1128/jb.181.9.2726-2732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez-Martinez L., Pascual A., Conejo Mdel C., Garcia I., Joyanes P., Domenech-Sanchez A., Benedi V.J. Energy-dependent accumulation of norfloxacin and porin expression in clinical isolates of Klebsiella pneumoniae and relationship to extended-spectrum beta-lactamase production. Antimicrob. Agents Chemother. 2002;46:3926–3932. doi: 10.1128/AAC.46.12.3926-3932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smani Y., Fabrega A., Roca I., Sanchez-Encinales V., Vila J., Pachon J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014;58:1806–1808. doi: 10.1128/AAC.02101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemmer K.M., Bonomo R.A., Rather P.N. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaddy J.A., Tomaras A.P., Actis L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009;77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aeschlimann J.R. The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2003;23:916–924. doi: 10.1592/phco.23.7.916.32722. [DOI] [PubMed] [Google Scholar]
- 56.Evans K., Adewoye L., Poole K. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: Identification of MexR binding sites in the mexA-mexR Intergenic Region. J. Bacteriol. 2001;183:807–812. doi: 10.1128/JB.183.3.807-812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun J., Deng Z., Yan A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014;453:254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 58.Yang L., Chen L., Shen L., Surette M., Duan K. Inactivation of MuxABC-OpmB transporter system in Pseudomonas aeruginosa leads to increased ampicillin and carbenicillin resistance and decreased virulence. J. Microbiol. 2011;49:107–114. doi: 10.1007/s12275-011-0186-2. [DOI] [PubMed] [Google Scholar]
- 59.Marchand I., Damier-Piolle L., Courvalin P., Lambert T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 2004;48:3298–3304. doi: 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wieczorek P., Sacha P., Hauschild T., Zorawski M., Krawczyk M., Tryniszewska E. Multidrug resistant Acinetobacter baumannii—The role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem. Cytobiol. 2008;46:257–267. doi: 10.2478/v10042-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 61.Yoon E.J., Balloy V., Fiette L., Chignard M., Courvalin P., Grillot-Courvalin C. Contribution of the Ade resistance-nodulation-cell division-type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. mBio. 2016;7:e00697-16. doi: 10.1128/mBio.00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruzin A., Visalli M.A., Keeney D., Bradford P.A. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2005;49:1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D.W., Onishi M., Kishino T., Matsuo T., Ogawa W., Kuroda T., Tsuchiya T. Properties and expression of a multidrug efflux pump AcrAB-KocC from Klebsiella pneumoniae. Biol. Pharm. Bull. 2008;31:577–582. doi: 10.1248/bpb.31.577. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa W., Onishi M., Ni R., Tsuchiya T., Kuroda T. Functional study of the novel multidrug efflux pump KexD from Klebsiella pneumoniae. Gene. 2012;498:177–182. doi: 10.1016/j.gene.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 65.El Zowalaty M.E., Al Thani A.A., Webster T.J., El Zowalaty A.E., Schweizer H.P., Nasrallah G.K., Marei H.E., Ashour H.M. Pseudomonas aeruginosa: Arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol. 2015;10:1683–1706. doi: 10.2217/fmb.15.48. [DOI] [PubMed] [Google Scholar]
- 66.Van Hoek A.H.A.M., Mevius D., Guerra B., Mullany P., Roberts A.P., Aarts H.J.M. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011;2:203. doi: 10.3389/fmicb.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munita J.M., Arias C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramirez M.S., Tolmasky M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005;57:1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Hoiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Maldonado R.F., Sá-Correia I., Valvano M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lerminiaux N.A., Cameron A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019;65:34–44. doi: 10.1139/cjm-2018-0275. [DOI] [PubMed] [Google Scholar]
- 73.O’Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 74.Stewart P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Zentralbl. Bakteriol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 75.Mah T.F., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Curr. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman L.R., D’Argenio D.A., MacCoss M.J., Zhang Z., Jones R.A., Miller S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 77.Perez-Martinez I., Haas D. Azithromycin inhibits expression of the GacA-dependent small RNAs RsmY and RsmZ in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011;55:3399–3405. doi: 10.1128/AAC.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed M.N., Porse A., Sommer M.O.A., Høiby N., Ciofu O. Evolution of antibiotic resistance in biofilm and planktonic Pseudomonas aeruginosa populations exposed to subinhibitory levels of Ciprofloxacin. Antimicrob. Agents Chemother. 2018;62:e00320-18. doi: 10.1128/AAC.00320-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cordero O.X., Hogeweg P. The impact of long-distance horizontal gene transfer on prokaryotic genome size. Proc. Natl. Acad. Sci. USA. 2009;106:21748–21753. doi: 10.1073/pnas.0907584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Von Wintersdorff C.J.H., Penders J., van Niekerk J.M., Mills N.D., Majumder S., van Alphen L.B., Savelkoul P.H.M., Wolffs P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otto M. Bacterial sensing of antimicrobial peptides. Contrib. Microbiol. Immunol. 2009;16:136–149. doi: 10.1159/000219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodrigue A., Quentin Y., Lazdunski A., Méjean V., Foglino M. Cell signalling by oligosaccharides. Two-component systems in Pseudomonas aeruginosa: Why so many? Curr. Trends Microbiol. 2000;8:498–504. doi: 10.1016/S0966-842X(00)01833-3. [DOI] [PubMed] [Google Scholar]
- 83.Ma S., Wozniak D., Ohman D. Identification of the histidine protein kinase KinB in Pseudomonas aeruginosa and its phosphorylation of the alginate regulator algB. J. Biol. Chem. 1997;272:17952–17960. doi: 10.1074/jbc.272.29.17952. [DOI] [PubMed] [Google Scholar]
- 84.Benkert B., Quack N., Schreiber K., Jaensch L., Jahn D., Schobert M. Nitrate-responsive NarX-NarL represses arginine-mediated induction of the Pseudomonas aeruginosa arginine fermentation arcDABC operon. Microbiolgy (Reading, England) 2008;154:3053–3060. doi: 10.1099/mic.0.2008/018929-0. [DOI] [PubMed] [Google Scholar]
- 85.Kimbara K., Chakrabarty A.M. Control of alginate synthesis in Pseudomonas aeruginosa: Regulation of the algR1 gene. Biochem. Biophys. Res. Commun. 1989;164:601–608. doi: 10.1016/0006-291X(89)91502-7. [DOI] [PubMed] [Google Scholar]
- 86.Bielecki P., Jensen V., Schulze W., Godeke J., Strehmel J., Eckweiler D., Nicolai T., Bielecka A., Wille T., Gerlach R.G., et al. Cross talk between the response regulators PhoB and TctD allows for the integration of diverse environmental signals in Pseudomonas aeruginosa. Nucleic Acids Res. 2015;43:6413–6425. doi: 10.1093/nar/gkv599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dean C.R., Neshat S., Poole K. PfeR, an enterobactin-responsive activator of ferric enterobactin receptor gene expression in Pseudomonas aeruginosa. J. Bacteriol. 1996;178:5361–5369. doi: 10.1128/jb.178.18.5361-5369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ritchings B.W., Almira E.C., Lory S., Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect. Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeung A.T., Bains M., Hancock R.E. The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa. J. Bacteriol. 2011;193:918–931. doi: 10.1128/JB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lukat G.S., Stock J.B. Response regulation in bacterial chemotaxis. J. Cell Biochem. 1993;51:41–46. doi: 10.1002/jcb.240510109. [DOI] [PubMed] [Google Scholar]
- 91.Casino P., Rubio V., Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 92.Kim D., Forst S. Genomic analysis of the histidine kinase family in bacteria and archaea. Microbiology. 2001;147:1197–1212. doi: 10.1099/00221287-147-5-1197. [DOI] [PubMed] [Google Scholar]
- 93.Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dereeper A., Audic S., Claverie J.M., Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010;10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dago A.E., Schug A., Procaccini A., Hoch J.A., Weigt M., Szurmant H. Structural basis of histidine kinase autophosphorylation deduced by integrating genomics, molecular dynamics, and mutagenesis. Proc. Natl. Acad. Sci. USA. 2012;109:E1733–E1742. doi: 10.1073/pnas.1201301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhagirath A.Y., Pydi S.P., Li Y., Lin C., Kong W., Chelikani P., Duan K. Characterization of the direct Interaction between hybrid sensor kinases PA1611 and RetS that controls biofilm formation and the type III secretion system in Pseudomonas aeruginosa. ACS Infect. Dis. 2017;3:162–175. doi: 10.1021/acsinfecdis.6b00153. [DOI] [PubMed] [Google Scholar]
- 97.Ulrich L., Koonin E., Zhulin I. One-component systems dominate signal transduction in prokaryotes. Curr. Trends Microbiol. 2005;13:52–56. doi: 10.1016/j.tim.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koretke K., Lupas A., Warren P., Rosenberg M., Brown J. Evolution of two-component signal transduction. Mol. Biol. Evol. 2000;17:1956–1970. doi: 10.1093/oxfordjournals.molbev.a026297. [DOI] [PubMed] [Google Scholar]
- 99.Goodman A.L., Kulasekara B., Rietsch A., Boyd D., Smith R.S., Lory S. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell. 2004;7:745–754. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 100.Goodman A.L., Merighi M., Hyodo M., Ventre I., Filloux A., Lory S. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chambonnier G., Roux L., Redelberger D., Fadel F., Filloux A., Sivaneson M., de Bentzmann S., Bordi C. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genet. 2016;12:e1006032. doi: 10.1371/journal.pgen.1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitrophanov A., Groisman E. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramos P.I., Custodio M.G., Quispe Saji G.D., Cardoso T., da Silva G.L., Braun G., Martins W.M., Girardello R., de Vasconcelos A.T., Fernandez E., et al. The polymyxin B-induced transcriptomic response of a clinical, multidrug-resistant Klebsiella pneumoniae involves multiple regulatory elements and intracellular targets. BMC Genom. 2016;17:737. doi: 10.1186/s12864-016-3070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Babouee Flury B., Dona V., Buetti N., Furrer H., Endimiani A. First two cases of severe multifocal infections caused by Klebsiella pneumoniae in Switzerland: Characterization of an atypical non-K1/K2-serotype strain causing liver abscess and endocarditis. J. Glob. Antimicrob. Resist. 2017;10:165–170. doi: 10.1016/j.jgar.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 105.Dorman M.J., Feltwell T., Goulding D.A., Parkhill J., Short F.L. The capsule regulatory network of Klebsiella pneumoniae defined by density-TraDISort. mBio. 2018;9:e01863-18. doi: 10.1128/mBio.01863-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merighi M., Carroll-Portillo A., Septer A.N., Bhatiya A., Gunn J.S. Role of Salmonella enterica serovar typhimurium two-component system PreA/PreB in modulating PmrA-regulated gene transcription. J. Bacteriol. 2006;188:141–149. doi: 10.1128/JB.188.1.141-149.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mourey L., Da Re S., Pedelacq J., Tolstykh T., Faurie C., Guillet V., Stock J., Samama J. Crystal structure of the CheA histidine phosphotransfer domain that mediates response regulator phosphorylation in bacterial chemotaxis. J. Biol. Chem. 2001;276:31074–31082. doi: 10.1074/jbc.M101943200. [DOI] [PubMed] [Google Scholar]
- 108.Schaller G.E., Shiu S.H., Armitage J.P. Two-component systems and their co-option for eukaryotic signal transduction. Curr. Biol. 2011;21:R320–R330. doi: 10.1016/j.cub.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 109.Pena-Sandoval G.R., Kwon O., Georgellis D. Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J. Bacteriol. 2005;187:3267–3272. doi: 10.1128/JB.187.9.3267-3272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dingemans J., Al-Feghali R.E., Lau G.W., Sauer K. Controlling chronic Pseudomonas aeruginosa infections by strategically interfering with the sensory function of SagS. Mol. Microbiol. 2019 doi: 10.1111/mmi.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dingemans J., Poudyal B., Sondermann H., Sauer K. The yin and yang of SagS: Distinct residues in the HmsP domain of SagS Independently regulate biofilm formation and biofilm drug tolerance. mSphere. 2018;3:e00192-18. doi: 10.1128/mSphere.00192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pamp S.J., Gjermansen M., Johansen H.K., Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008;68:223–240. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 113.Gupta K., Liao J., Petrova O.E., Cherny K.E., Sauer K. Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa. Mol. Microbiol. 2014;92:488–506. doi: 10.1111/mmi.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gardner S.G., Miller J.B., Dean T., Robinson T., Erickson M., Ridge P.G., McCleary W.R. Genetic analysis, structural modeling, and direct coupling analysis suggest a mechanism for phosphate signaling in Escherichia coli. BMC Genet. 2015;16:S2. doi: 10.1186/1471-2156-16-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mitchell S.L., Ismail A.M., Kenrick S.A., Camilli A. The VieB auxiliary protein negatively regulates the VieSA signal transduction system in Vibrio cholerae. BMC Microbiol. 2015;15:59. doi: 10.1186/s12866-015-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Airola M.V., Watts K.J., Bilwes A.M., Crane B.R. Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure. 2010;18:436–448. doi: 10.1016/j.str.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsing W., Silhavy T.J. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J. Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matamouros S., Hager K.R., Miller S.I. HAMP domain rotation and tilting movements associated with signal transduction in the PhoQ sensor kinase. mBio. 2015;6:e00616-15. doi: 10.1128/mBio.00616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meena N., Kaur H., Mondal A.K. Interactions among HAMP domain repeats act as an osmosensing molecular switch in group III hybrid histidine kinases from fungi. J. Biol. Chem. 2010;285:12121–12132. doi: 10.1074/jbc.M109.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muzamal U., Gomez D., Kapadia F., Golemi-Kotra D. Diversity of two-component systems: Insights into the signal transduction mechanism by the Staphylococcus aureus two-component system GraSR. F1000Res. 2014;3:252. doi: 10.12688/f1000research.5512.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hellingwerf K.J., Postma P.W., Tommassen J., Westerhoff H.V. Signal transduction in bacteria: Phospho-neural network(s) in Escherichia coli? FEMS Microbiol. Rev. 1995;16:309–321. doi: 10.1111/j.1574-6976.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 122.Gronlund A. Networking genetic regulation and neural computation: Directed network topology and its effect on the dynamics. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2004;70:061908. doi: 10.1103/PhysRevE.70.061908. [DOI] [PubMed] [Google Scholar]
- 123.Huynh T.N., Chen L.L., Stewart V. Sensor-response regulator interactions in a cross-regulated signal transduction network. Microbiology. 2015;161:1504–1515. doi: 10.1099/mic.0.000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Firon A., Tazi A., Da Cunha V., Brinster S., Sauvage E., Dramsi S., Golenbock D.T., Glaser P., Poyart C., Trieu-Cuot P. The Abi-domain protein Abx1 interacts with the CovS histidine kinase to control virulence gene expression in group B Streptococcus. PLoS Pathog. 2013;9:e1003179. doi: 10.1371/journal.ppat.1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gendrin C., Lembo A., Whidbey C., Burnside K., Berry J., Ngo L., Banerjee A., Xue L., Arrington J., Doran K.S., Tao W.A., et al. The sensor histidine kinase RgfC affects group B streptococcal virulence factor expression independent of its response regulator RgfA. Infect. Immun. 2015;83:1078–1088. doi: 10.1128/IAI.02738-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He Y.W., Wang C., Zhou L., Song H., Dow J.M., Zhang L.H. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 2006;281:33414–33421. doi: 10.1074/jbc.M606571200. [DOI] [PubMed] [Google Scholar]
- 127.Reisinger S.J., Huntwork S., Viollier P.H., Ryan K.R. DivL performs critical cell cycle functions in Caulobacter crescentus independent of kinase activity. J. Bacteriol. 2007;189:8308–8320. doi: 10.1128/JB.00868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao R., Stock A.M. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kong W., Chen L., Zhao J., Shen T., Surette M., Shen L., Duan K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol. Microbiol. 2013;88:784–797. doi: 10.1111/mmi.12223. [DOI] [PubMed] [Google Scholar]
- 130.Chen L., Duan K. A PhoPQ-regulated ABC transporter system exports Tetracycline in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:3016–3024. doi: 10.1128/AAC.02986-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilton M., Charron-Mazenod L., Moore R., Lewenza S. Extracellular DNA acidifies biofilms and Induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:544–553. doi: 10.1128/AAC.01650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McPhee J.B., Bains M., Winsor G., Lewenza S., Kwasnicka A., Brazas M.D., Brinkman F.S., Hancock R.E. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 2006;188:3995–4006. doi: 10.1128/JB.00053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jochumsen N., Marvig R.L., Damkiaer S., Jensen R.L., Paulander W., Molin S., Jelsbak L., Folkesson A. The evolution of antimicrobial peptide resistance in Pseudomonas aeruginosa is shaped by strong epistatic interactions. Nat. Commun. 2016;7:13002. doi: 10.1038/ncomms13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moskowitz S.M., Ernst R.K., Miller S.I. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 2004;186:575–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barbosa C., Trebosc V., Kemmer C., Rosenstiel P., Beardmore R., Schulenburg H., Jansen G. Alternative evolutionary paths to bacterial antibiotic resistance cause distinct collateral effects. Mol. Biol. Evol. 2017;34:2229–2244. doi: 10.1093/molbev/msx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tian Z.X., Yi X.X., Cho A., O’Gara F., Wang Y.P. CpxR Activates MexAB-OprM Efflux Pump Expression and Enhances Antibiotic Resistance in Both Laboratory and Clinical nalB-Type Isolates of Pseudomonas aeruginosa. PLoS Pathog. 2016;12:e1005932. doi: 10.1371/journal.ppat.1005932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez L., Gooderham W.J., Bains M., McPhee J.B., Wiegand I., Hancock R.E. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob. Agents Chemother. 2010;54:3372–3382. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muller C., Plesiat P., Jeannot K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and beta-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011;55:1211–1221. doi: 10.1128/AAC.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed. Res. Int. 2015;2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mikkelsen H., Ball G., Giraud C., Filloux A. Expression of Pseudomonas aeruginosa cupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS ONE. 2009;4:e6018. doi: 10.1371/journal.pone.0006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mikkelsen H., Hui K., Barraud N., Filloux A. The pathogenicity island encoded PvrSR/RcsCB regulatory network controls biofilm formation and dispersal in Pseudomonas aeruginosa PA14. Mol. Microbiol. 2013;89:450–463. doi: 10.1111/mmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drenkard E., Ausubel F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 143.Sivaneson M., Mikkelsen H., Ventre I., Bordi C., Filloux A. Two-component regulatory systems in Pseudomonas aeruginosa: An intricate network mediating fimbrial and efflux pump gene expression. Mol. Microbiol. 2011;79:1353–1366. doi: 10.1111/j.1365-2958.2010.07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lau C.H., Fraud S., Jones M., Peterson S.N., Poole K. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;57:2243–2251. doi: 10.1128/AAC.00170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lau C.H., Krahn T., Gilmour C., Mullen E., Poole K. AmgRS-mediated envelope stress-inducible expression of the mexXY multidrug efflux operon of Pseudomonas aeruginosa. Microbiologyopen. 2015;4:121–135. doi: 10.1002/mbo3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fruci M., Poole K. Aminoglycoside-inducible expression of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: Involvement of the envelope stress-responsive AmgRS two-component system. PLoS ONE. 2018;13:e0205036. doi: 10.1371/journal.pone.0205036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Petrova O.E., Sauer K. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog. 2009;5:e1000668. doi: 10.1371/journal.ppat.1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hsu J.L., Chen H.C., Peng H.L., Chang H.Y. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 2008;283:9933–9944. doi: 10.1074/jbc.M708836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhuwan M., Lee H.J., Peng H.L., Chang H.Y. Histidine-containing phosphotransfer protein-B (HptB) regulates swarming motility through partner-switching system in Pseudomonas aeruginosa PAO1 strain. J. Biol. Chem. 2012;287:1903–1914. doi: 10.1074/jbc.M111.256586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kulasekara H.D., Ventre I., Kulasekara B.R., Lazdunski A., Filloux A., Lory S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 2005;55:368–380. doi: 10.1111/j.1365-2958.2004.04402.x. [DOI] [PubMed] [Google Scholar]
- 151.Beaudoin T., Zhang L., Hinz A.J., Parr C.J., Mah T.F. The biofilm-specific antibiotic resistance gene ndvB is important for expression of ethanol oxidation genes in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2012;194:3128–3136. doi: 10.1128/JB.06178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blus-Kadosh I., Zilka A., Yerushalmi G., Banin E. The effect of pstS and phoB on quorum sensing and swarming motility in Pseudomonas aeruginosa. PLoS ONE. 2013;8:e74444. doi: 10.1371/journal.pone.0074444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo Y., Zhao K., Baker A.E., Kuchma S.L., Coggan K.A., Wolfgang M.C., Wong G.C., O’Toole G.A. A Hierarchical Cascade of Second Messengers Regulates Pseudomonas aeruginosa Surface Behaviors. mBio. 2015;6:e02456-14. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fulcher N.B., Holliday P.M., Klem E., Cann M.J., Wolfgang M.C. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol. Microbiol. 2010;76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Intile P.J., Diaz M.R., Urbanowski M.L., Wolfgang M.C., Yahr T.L. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J. Bacteriol. 2014;196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zamorano L., Moya B., Juan C., Mulet X., Blazquez J., Oliver A. The Pseudomonas aeruginosa CreBC two-component system plays a major role in the response to beta-lactams, fitness, biofilm growth, and global regulation. Antimicrob. Agents Chemother. 2014;58:5084–5095. doi: 10.1128/AAC.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vasil M.L., Ochsner U.A. The response of Pseudomonas aeruginosa to iron: Genetics, biochemistry and virulence. Mol. Microbiol. 1999;34:399–413. doi: 10.1046/j.1365-2958.1999.01586.x. [DOI] [PubMed] [Google Scholar]
- 158.Dasgupta N., Wolfgang M.C., Goodman A.L., Arora S.K., Jyot J., Lory S., Ramphal R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 159.Ryan R.P., Fouhy Y., Garcia B.F., Watt S.A., Niehaus K., Yang L., Tolker-Nielsen T., Dow J.M. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 2008;68:75–86. doi: 10.1111/j.1365-2958.2008.06132.x. [DOI] [PubMed] [Google Scholar]
- 160.Perron K., Caille O., Rossier C., Van Delden C., Dumas J.L., Kohler T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J. Biol. Chem. 2004;279:8761–8768. doi: 10.1074/jbc.M312080200. [DOI] [PubMed] [Google Scholar]
- 161.Caille O., Rossier C., Perron K. A copper-activated two-component system interacts with zinc and imipenem resistance in Pseudomonas aeruginosa. J. Bacteriol. 2007;189:4561–4568. doi: 10.1128/JB.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laskowski M.A., Osborn E., Kazmierczak B.I. A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol. Microbiol. 2004;54:1090–1103. doi: 10.1111/j.1365-2958.2004.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kreamer N.N., Costa F., Newman D.K. The ferrous iron-responsive BqsRS two-component system activates genes that promote cationic stress tolerance. mBio. 2015;6:e02549. doi: 10.1128/mBio.02549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sage A.E., Proctor W.D., Phibbs P.V., Jr. A two-component response regulator, gltR, is required for glucose transport activity in Pseudomonas aeruginosa PAO1. J. Bacteriol. 1996;178:6064–6066. doi: 10.1128/jb.178.20.6064-6066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wolfgang M.C., Lee V.T., Gilmore M.E., Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell. 2003;4:253–263. doi: 10.1016/S1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 166.Huangyutitham V., Guvener Z.T., Harwood C.S. Subcellular clustering of the phosphorylated WspR response regulator protein stimulates its diguanylate cyclase activity. mBio. 2013;4:e00242-13. doi: 10.1128/mBio.00242-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Borlee B.R., Goldman A.D., Murakami K., Samudrala R., Wozniak D.J., Parsek M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010;75:827–842. doi: 10.1111/j.1365-2958.2009.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hickman J.W., Tifrea D.F., Harwood C.S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Y., Ha U., Zeng L., Jin S. Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2003;47:95–101. doi: 10.1128/AAC.47.1.95-101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giraud C., Bernard C.S., Calderon V., Yang L., Filloux A., Molin S., Fichant G., Bordi C., de Bentzmann S. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 2011;13:666–683. doi: 10.1111/j.1462-2920.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 171.De Bentzmann S., Giraud C., Bernard C.S., Calderon V., Ewald F., Plesiat P., Nguyen C., Grunwald D., Attree I., Jeannot K., et al. Unique biofilm signature, drug susceptibility and decreased virulence in Drosophila through the Pseudomonas aeruginosa two-component system PprAB. PLoS Pathog. 2012;8:e1003052. doi: 10.1371/annotation/5c169544-7d19-40db-9a58-28b2fdf2c82c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hurley B.P., Goodman A.L., Mumy K.L., Murphy P., Lory S., McCormick B.A. The two-component sensor response regulator RoxS/RoxR plays a role in Pseudomonas aeruginosa interactions with airway epithelial cells. Microbes Infect. 2010;12:190–198. doi: 10.1016/j.micinf.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kilmury S.L.N., Burrows L.L. The Pseudomonas aeruginosa PilSR two-component system regulates both twitching and swimming motilities. mBio. 2018;9:e01310-18. doi: 10.1128/mBio.01310-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beckmann C., Brittnacher M., Ernst R., Mayer-Hamblett N., Miller S.I., Burns J.L. Use of phage display to identify potential Pseudomonas aeruginosa gene products relevant to early cystic fibrosis airway infections. Infect. Immun. 2005;73:444–452. doi: 10.1128/IAI.73.1.444-452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Z., Lu C.D. Functional genomics enables identification of genes of the arginine transaminase pathway in Pseudomonas aeruginosa. J. Bacteriol. 2007;189:3945–3953. doi: 10.1128/JB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li W., Lu C.D. Regulation of carbon and nitrogen utilization by CbrAB and NtrBC two-component systems in Pseudomonas aeruginosa. J. Bacteriol. 2007;189:5413–5420. doi: 10.1128/JB.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Valentini M., Storelli N., Lapouge K. Identification of C(4)-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2011;193:4307–4316. doi: 10.1128/JB.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chand N.S., Clatworthy A.E., Hung D.T. The two-component sensor KinB acts as a phosphatase to regulate Pseudomonas aeruginosa Virulence. J. Bacteriol. 2012;194:6537–6547. doi: 10.1128/JB.01168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tatke G., Kumari H., Silva-Herzog E., Ramirez L., Mathee K. Pseudomonas aeruginosa MifS-MifR two-component system is specific for alpha-ketoglutarate utilization. PLoS ONE. 2015;10:e0129629. doi: 10.1371/journal.pone.0129629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Srinivasan V.B., Vaidyanathan V., Mondal A., Rajamohan G. Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH-K2044. PLoS ONE. 2012;7:e33777. doi: 10.1371/journal.pone.0033777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Raivio T.L. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- 182.Cannatelli A., D’Andrea M.M., Giani T., Di Pilato V., Arena F., Ambretti S., Gaibani P., Rossolini G.M. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 2013;57:5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jayol A., Nordmann P., Brink A., Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob. Agents Chemother. 2015;59:2780–2784. doi: 10.1128/AAC.05055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lippa A.M., Goulian M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009;5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sperandio V., Torres A.G., Kaper J.B. Quorum sensing Escherichia coli regulators B and C (QseBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 186.Lai Y.C., Lin G.T., Yang S.L., Chang H.Y., Peng H.L. Identification and characterization of KvgAS, a two-component system in Klebsiella pneumoniae CG43. Fems Microbiol. Lett. 2003;218:121–126. doi: 10.1111/j.1574-6968.2003.tb11507.x. [DOI] [PubMed] [Google Scholar]
- 187.Lin C.T., Huang T.Y., Liang W.C., Peng H.L. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. J. Biochem. 2006;140:429–438. doi: 10.1093/jb/mvj168. [DOI] [PubMed] [Google Scholar]
- 188.Lin C.T., Peng H.L. Regulation of the homologous two-component systems KvgAS and KvhAS in Klebsiella pneumoniae CG43. J. Biochem. 2006;140:639–648. doi: 10.1093/jb/mvj196. [DOI] [PubMed] [Google Scholar]
- 189.Cannatelli A., Di Pilato V., Giani T., Arena F., Ambretti S., Gaibani P., D’Andrea M.M., Rossolini G.M. In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob. Agents Chemother. 2014;58:4399–4403. doi: 10.1128/AAC.02555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Su K., Zhou X., Luo M., Xu X., Liu P., Li X., Xue J., Chen S., Xu W., Li Y., Qiu J. Genome-wide identification of genes regulated by RcsA, RcsB, and RcsAB phosphorelay regulators in Klebsiella pneumoniae NTUH-K2044. Microb. Pathog. 2018;123:36–41. doi: 10.1016/j.micpath.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 191.Nixon B.T., Ronson C.W., Ausubel F.M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc. Natl. Acad. Sci. USA. 1986;83:7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hsing W.H., Russo F.D., Bernd K.K., Silhavy T.J. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J. Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin T.H., Chen Y., Kuo J.T., Lai Y.C., Wu C.C., Huang C.F., Lin C.T. Phosphorylated OmpR Is required for type 3 fimbriae expression in Klebsiella pneumoniae under hypertonic conditions. Front. Microbiol. 2018;9:2405. doi: 10.3389/fmicb.2018.02405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zahid N., Zulfiqiar S., Shakoori A.R. Functional analysis of cus operon promoter of Klebsiella pneumoniae using E. coli lacZ assay. Gene. 2012;495:81–88. doi: 10.1016/j.gene.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 195.Chaturvedi K.S., Henderson J.P. Pathogenic adaptations to host-derived antibacterial copper. Front. Cell. Infect. Microbiol. 2014;4:3. doi: 10.3389/fcimb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hanczvikkel A., Fuzi M., Ungvari E., Toth A. Transmissible silver resistance readily evolves in high-risk clone isolates of Klebsiella pneumoniae. Acta Microbiol. Immunol. Hung. 2018;65:387–403. doi: 10.1556/030.65.2018.031. [DOI] [PubMed] [Google Scholar]
- 197.Goulian M. Two-component signaling circuit structure and properties. Curr. Opin. Microbiol. 2010;13:184–189. doi: 10.1016/j.mib.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pfluger-Grau K., Gorke B. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Curr. Trends Microbiol. 2010;18:205–214. doi: 10.1016/j.tim.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 199.Zhou L., Lei X.H., Bochner B.R., Wanner B.L. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. J. Bacteriol. 2003;185:4956–4972. doi: 10.1128/JB.185.16.4956-4972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nagakubo S., Nishino K., Hirata T., Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 2002;184:4161–4167. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perrenoud A., Sauer U. Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J. Bacteriol. 2005;187:3171–3179. doi: 10.1128/JB.187.9.3171-3179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin J.T., Goldman B.S., Stewart V. Structures of genes nasA and nasB, encoding assimilatory nitrate and nitrite reductases in Klebsiella Pneumoniae M5al. J. Bacteriol. 1993;175:2370–2378. doi: 10.1128/jb.175.8.2370-2378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Moreno-Vivian C., Cabello P., Martinez-Luque M., Blasco R., Castillo F. Prokaryotic nitrate reduction: Molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 1999;181:6573–6584. doi: 10.1128/jb.181.21.6573-6584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Verhamme D.T., Postma P.W., Crielaard W., Hellingwerf K.J. Cooperativity in signal transfer through the Uhp system of Escherichia coli. J. Bacteriol. 2002;184:4205–4210. doi: 10.1128/JB.184.15.4205-4210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Castaneda-Garcia A., Blazquez J., Rodriguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics (Basel) 2013;2:217–236. doi: 10.3390/antibiotics2020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo S.C., Lou Y.C., Rajasekaran M., Chang Y.W., Hsiao C.D., Chen C. Structural basis of a physical blockage mechanism for the interaction of response regulator PmrA with connector protein PmrD from Klebsiella pneumoniae. J. Biol. Chem. 2013;288:25551–25561. doi: 10.1074/jbc.M113.481978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nishino K., Yamaguchi A. EvgA of the two-component signal transduction system modulates production of the YhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 2002;184:2319–2323. doi: 10.1128/JB.184.8.2319-2323.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen Y.M., Backman K., Magasanik B. Characterization of a Gene, glnl, the Product of Which Is Involved in the Regulation of Nitrogen-Utilization in Escherichia coli. J. Bacteriol. 1982;150:214–220. doi: 10.1128/jb.150.1.214-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Claveriemartin F., Magasanik B. Positive and negative effects of DNA bending on activation of transcription from a distant site. J. Mol. Biol. 1992;227:996–1008. doi: 10.1016/0022-2836(92)90516-M. [DOI] [PubMed] [Google Scholar]
- 210.Sallai L., Tucker P.A. Crystal structure of the central and C-terminal domain of the sigma(54)-activator ZraR. J. Struct. Biol. 2005;151:160–170. doi: 10.1016/j.jsb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 211.Scheu P.D., Witan J., Rauschmeier M., Graf S., Liao Y.F., Ebert-Jung A., Basche T., Erker W., Unden G. CitA/CitB two-component system regulating citrate fermentation in Escherichia coli and its relation to the DcuS/DcuR system in vivo. J. Bacteriol. 2012;194:636–645. doi: 10.1128/JB.06345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Meyer M., Dimroth P., Bott M. Catabolite repression of the citrate fermentation genes in Klebsiella pneumoniae: Evidence for involvement of the cyclic AMP receptor protein. J. Bacteriol. 2001;183:5248–5256. doi: 10.1128/JB.183.18.5248-5256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cheng Y.H., Lin T.L., Lin Y.T., Wang J.T. Amino acid substitutions of CrrB responsible for resistance to colistin through CrrC in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016;60:3709–3716. doi: 10.1128/AAC.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lean S.S., Yeo C.C., Suhaili Z., Thong K.L. Comparative genomics of two ST 195 carbapenem-resistant Acinetobacter baumannii with different susceptibility to polymyxin revealed underlying resistance mechanism. Front. Microbiol. 2015;6:1445. doi: 10.3389/fmicb.2015.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jaidane N., Naas T., Mansour W., Radhia B.B., Jerbi S., Boujaafar N., Bouallegue O., Bonnin R.A. Genomic analysis of in vivo acquired resistance to colistin and rifampicin in Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2018;51:266–269. doi: 10.1016/j.ijantimicag.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 216.Richmond G.E., Evans L.P., Anderson M.J., Wand M.E., Bonney L.C., Ivens A., Chua K.L., Webber M.A., Sutton J.M., Peterson M.L., et al. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio. 2016;7:e00430-16. doi: 10.1128/mBio.00430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lin M.F., Lin Y.Y., Yeh H.W., Lan C.Y. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 2014;14:119. doi: 10.1186/1471-2180-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lin M.F., Lin Y.Y., Lan C.Y. The role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in Acinetobacter baumannii. PLoS ONE. 2015;10:e0132843. doi: 10.1371/journal.pone.0132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tomaras A.P., Flagler M.J., Dorsey C.W., Gaddy J.A., Actis L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology. 2008;154:3398–3409. doi: 10.1099/mic.0.2008/019471-0. [DOI] [PubMed] [Google Scholar]
- 220.Liou M.L., Soo P.C., Ling S.R., Kuo H.Y., Tang C.Y., Chang K.C. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J. Microbiol. Immunol. Infect. 2014;47:275–281. doi: 10.1016/j.jmii.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 221.Russo T.A., Manohar A., Beanan J.M., Olson R., MacDonald U., Graham J., Umland T.C. The response regulator BfmR is a potential drug target for Acinetobacter baumannii. mSphere. 2016;1:e00082-16. doi: 10.1128/mSphere.00082-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cerqueira G.M., Kostoulias X., Khoo C., Aibinu I., Qu Y., Traven A., Peleg A.Y. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J. Infect. Dis. 2014;210:46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 223.Bhuiyan M.S., Ellett F., Murray G.L., Kostoulias X., Cerqueira G.M., Schulze K.E., Mahamad Maifiah M.H., Li J., Creek D.J., Lieschke G.J., et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA. 2016;113:9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen R., Lv R., Xiao L., Wang M., Du Z., Tan Y., Cui Y., Yan Y., Luo Y., Yang R., et al. A1S_2811, a CheA/Y-like hybrid two-component regulator from Acinetobacter baumannii ATCC17978, is involved in surface motility and biofilm formation in this bacterium. Microbiologyopen. 2017;6:e00510. doi: 10.1002/mbo3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Samir R., Hussein S.H., Elhosseiny N.M., Khattab M.S., Shawky A.E., Attia A.S. Adaptation to potassium-limitation is essential for Acinetobacter baumannii pneumonia pathogenesis. J. Infect. Dis. 2016;214:2006–2013. doi: 10.1093/infdis/jiw476. [DOI] [PubMed] [Google Scholar]
- 226.Ninfa A.J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gavigan J.A., Marshall L.M., Dobson A.D. Regulation of polyphosphate kinase gene expression in Acinetobacter baumannii 252. Microbiology. 1999;145:2931–2937. doi: 10.1099/00221287-145-10-2931. [DOI] [PubMed] [Google Scholar]
- 228.Williams C.L., Neu H.M., Gilbreath J.J., Michel S.L., Zurawski D.V., Merrell D.S. Copper resistance of the emerging pathogen Acinetobacter baumannii. Appl. Environ. Microbiol. 2016;82:6174–6188. doi: 10.1128/AEM.01813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tipton K.A., Rather P.N. An OmpR-EnvZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii Strain AB5075. J. Bacteriol. 2017;199:e00705-16. doi: 10.1128/JB.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mulcahy H., Charron-Mazenod L., Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.McPhee J.B., Lewenza S., Hancock R.E. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 232.Schniederjans M., Koska M., Häussler S. Transcriptional and mutational profiling of an aminoglycoside-resistant Pseudomonas aeruginosa small-colony variant. Antimicrob. Agents Chemother. 2017;61:e01178-17. doi: 10.1128/AAC.01178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fothergill J.L., Neill D.R., Loman N., Winstanley C., Kadioglu A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 2014;5:4780. doi: 10.1038/ncomms5780. [DOI] [PubMed] [Google Scholar]
- 234.Bricio-Moreno L., Sheridan V.H., Goodhead I., Armstrong S., Wong J.K.L., Waters E.M., Sarsby J., Panagiotou S., Dunn J., Chakraborty A., et al. Evolutionary trade-offs associated with loss of PmrB function in host-adapted Pseudomonas aeruginosa. Nat. Commun. 2018;9:2635. doi: 10.1038/s41467-018-04996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nowicki E.M., O’Brien J.P., Brodbelt J.S., Trent M.S. Extracellular zinc induces phosphoethanolamine addition to Pseudomonas aeruginosa lipid A via the ColRS two-component system. Mol. Microbiol. 2015;97:166–178. doi: 10.1111/mmi.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cheng H.Y., Chen Y.F., Peng H.L. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010;17:60. doi: 10.1186/1423-0127-17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carretero-Ledesma M., García-Quintanilla M., Martín-Peña R., Pulido M.R., Pachón J., McConnell M.J. Phenotypic changes associated with Colistin resistance due to Lipopolysaccharide loss in Acinetobacter baumannii. Virulence. 2018;9:930–942. doi: 10.1080/21505594.2018.1460187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim Y., Bae I.K., Jeong S.H., Yong D., Lee K. In vivo selection of pan-drug resistant Acinetobacter baumannii during antibiotic treatment. Yonsei Med. J. 2015;56:928–934. doi: 10.3349/ymj.2015.56.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lopez-Rojas R., Dominguez-Herrera J., McConnell M.J., Docobo-Perez F., Smani Y., Fernandez-Reyes M., Rivas L., Pachon J. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J. Infect. Dis. 2011;203:545–548. doi: 10.1093/infdis/jiq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Durante-Mangoni E., Del Franco M., Andini R., Bernardo M., Giannouli M., Zarrilli R. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn Microbiol. Infect. Dis. 2015;82:222–226. doi: 10.1016/j.diagmicrobio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 241.Lopez-Rojas R., Garcia-Quintanilla M., Labrador-Herrera G., Pachon J., McConnell M.J. Impaired growth under iron-limiting conditions associated with the acquisition of colistin resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2016;47:473–477. doi: 10.1016/j.ijantimicag.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 242.Wand M.E., Bock L.J., Bonney L.C., Sutton J.M. Retention of virulence following adaptation to colistin in Acinetobacter baumannii reflects the mechanism of resistance. J. Antimicrob. Chemother. 2015;70:2209–2216. doi: 10.1093/jac/dkv097. [DOI] [PubMed] [Google Scholar]
- 243.Da Silva G.J., Domingues S. Interplay between colistin resistance, virulence and fitness in Acinetobacter baumannii. Antibiotics. 2017;6:28. doi: 10.3390/antibiotics6040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Janssen K.H., Diaz M.R., Golden M., Graham J.W., Sanders W., Wolfgang M.C., Yahr T.L. Functional analyses of the RsmY and RsmZ small noncoding regulatory RNAs in Pseudomonas aeruginosa. J. Bacteriol. 2018;200:e00736-17. doi: 10.1128/JB.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Marden J.N., Diaz M.R., Walton W.G., Gode C.J., Betts L., Urbanowski M.L., Redinbo M.R., Yahr T.L., Wolfgang M.C. An unusual CsrA family member operates in series with RsmA to amplify post-transcriptional responses in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2013;110:15055–15060. doi: 10.1073/pnas.1307217110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Llanes C., Köhler T., Patry I., Dehecq B., van Delden C., Plésiat P. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to Ciprofloxacin. Antimicrob. Agents Chemother. 2011;55:5676–5684. doi: 10.1128/AAC.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lamarche M.G., Déziel E. MexEF-OprN efflux pump exports the Pseudomonas Quinolone Signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline) PLoS ONE. 2011;6:e24310. doi: 10.1371/journal.pone.0024310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Linares J.F., Gustafsson I., Baquero F., Martinez J.L. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Valentini M., Filloux A. Biofilms and Cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016;291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ventre I., Goodman A.L., Vallet-Gely I., Vasseur P., Soscia C., Molin S., Bleves S., Lazdunski A., Lory S., Filloux A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA. 2006;103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moscoso J.A., Mikkelsen H., Heeb S., Williams P., Filloux A. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ. Microbiol. 2011;13:3128–3138. doi: 10.1111/j.1462-2920.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 252.Evans T.J. Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis. Future Microbiol. 2015;10:231–239. doi: 10.2217/fmb.14.107. [DOI] [PubMed] [Google Scholar]
- 253.Kentache T., Ben Abdelkrim A., Jouenne T., De E., Hardouin J. Global dynamic proteome study of a pellicle-forming Acinetobacter baumannii strain. Mol. Cell. Proteomics MCP. 2017;16:100–112. doi: 10.1074/mcp.M116.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Peleg A.Y., Tampakakis E., Fuchs B.B., Eliopoulos G.M., Moellering R.C., Jr., Mylonakis E. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:14585–14590. doi: 10.1073/pnas.0805048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dorsey C.W., Tomaras A.P., Actis L.A. Genetic and phenotypic analysis of Acinetobacter baumannii insertion derivatives generated with a transposome system. Appl. Environ. Microbiol. 2002;68:6353–6360. doi: 10.1128/AEM.68.12.6353-6360.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Suzuki K., Wang X., Weilbacher T., Pernestig A.-K., Melefors O., Georgellis D., Babitzke P., Romeo T. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J. Bacteriol. 2002;184:5130–5140. doi: 10.1128/JB.184.18.5130-5140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tomenius H., Pernestig A.K., Mendez-Catala C.F., Georgellis D., Normark S., Melefors O. Genetic and functional characterization of the Escherichia coli BarA-UvrY two-component system: Point mutations in the HAMP linker of the BarA sensor give a dominant-negative phenotype. J. Bacteriol. 2005;187:7317–7324. doi: 10.1128/JB.187.21.7317-7324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mukhopadhyay S., Audia J.P., Roy R.N., Schellhorn H.E. Transcriptional induction of the conserved alternative sigma factor RpoS in Escherichia coli is dependent on BarA, a probable two-component regulator. Mol. Microbiol. 2000;37:371–381. doi: 10.1046/j.1365-2958.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- 259.Mukhopadhyay S., Schellhorn H.E. Identification and characterization of hydrogen peroxide-sensitive mutants of Escherichia coli: Genes that require OxyR for expression. J. Bacteriol. 1997;179:330–338. doi: 10.1128/jb.179.2.330-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Michael B., Smith J.N., Swift S., Heffron F., Ahmer B.M. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 2001;183:5733–5742. doi: 10.1128/JB.183.19.5733-5742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fernando D., Kumar A. Growth phase-dependent expression of RND efflux pump- and outer membrane porin-encoding genes in Acinetobacter baumannii ATCC 19606. J. Antimicrob. Chemother. 2012;67:569–572. doi: 10.1093/jac/dkr519. [DOI] [PubMed] [Google Scholar]
- 262.Costa S.S., Viveiros M., Amaral L., Couto I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013;7:59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ruzin A., Keeney D., Bradford P.A. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 2007;59:1001–1004. doi: 10.1093/jac/dkm058. [DOI] [PubMed] [Google Scholar]
- 264.Vila J., Marti S., Sanchez-Cespedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2007;59:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 265.Sun J.R., Chan M.C., Chang T.Y., Wang W.Y., Chiueh T.S. Overexpression of the adeB gene in clinical isolates of tigecycline-nonsusceptible Acinetobacter baumannii without insertion mutations in adeRS. Antimicrob. Agents Chemother. 2010;54:4934–4938. doi: 10.1128/AAC.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Higgins P.G., Schneiders T., Hamprecht A., Seifert H. In vivo selection of a missense mutation in adeR and conversion of the novel blaOXA-164 gene into blaOXA-58 in carbapenem-resistant Acinetobacter baumannii isolates from a hospitalized patient. Antimicrob. Agents Chemother. 2010;54:5021–5027. doi: 10.1128/AAC.00598-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kröger C., Kary S.C., Schauer K., Cameron A.D.S. Genetic regulation of virulence and antibiotic resistance in Acinetobacter baumannii. Genes. 2016;8:12. doi: 10.3390/genes8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sun J.R., Perng C.L., Chan M.C., Morita Y., Lin J.C., Su C.M., Wang W.Y., Chang T.Y., Chiueh T.S. A truncated AdeS kinase protein generated by ISAba1 insertion correlates with tigecycline resistance in Acinetobacter baumannii. PLoS ONE. 2012;7:e49534. doi: 10.1371/journal.pone.0049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sun J.R., Jeng W.Y., Perng C.L., Yang Y.S., Soo P.C., Chiang Y.S., Chiueh T.S. Single amino acid substitution Gly186Val in AdeS restores tigecycline susceptibility of Acinetobacter baumannii. J. Antimicrob. Chemother. 2016;71:1488–1492. doi: 10.1093/jac/dkw002. [DOI] [PubMed] [Google Scholar]
- 270.Raffa R.G., Raivio T.L. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 2002;45:1599–1611. doi: 10.1046/j.1365-2958.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- 271.Anand P., Chandra N. Characterizing the pocketome of Mycobacterium tuberculosis and application in rationalizing polypharmacological target selection. Sci. Rep. 2014;4:6356. doi: 10.1038/srep06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ramakrishnan G., Chandra N.R., Srinivasan N. Recognizing drug targets using evolutionary information: Implications for repurposing FDA-approved drugs against Mycobacterium tuberculosis H37Rv. Mol. Biosyst. 2015;11:3316–3331. doi: 10.1039/C5MB00476D. [DOI] [PubMed] [Google Scholar]
- 273.Zheng H., Aleiwi B., Ellsworth E., Abramovitch R.B. Inhibition of Mycobacterium tuberculosis DosRST two-component regulatory system signaling by targeting response regulator DNA binding and sensor kinase heme. bioRxivorg. 2018 doi: 10.1101/411793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stephenson K., Yamaguchi Y., Hoch J.A. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 2000;275:38900–38904. doi: 10.1074/jbc.M006633200. [DOI] [PubMed] [Google Scholar]
- 275.Wilke K.E., Francis S., Carlson E.E. Inactivation of multiple bacterial histidine kinases by targeting the ATP-binding domain. ACS Chem. Biol. 2015;10:328–335. doi: 10.1021/cb5008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Williams C., Cotter P. Autoregulation is essential for precise temporal and steady-state regulation by the Bordetella BvgAS phosphorelay. J. Bacteriol. 2007;189:1974–1982. doi: 10.1128/JB.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Miyashiro T., Goulian M. High stimulus unmasks positive feedback in an autoregulated bacterial signaling circuit. Proc. Natl. Acad. Sci. USA. 2008;105:17457–17462. doi: 10.1073/pnas.0807278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ansaldi M., Simon G., Lepelletier M., Mejean V. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 2000;182:961–966. doi: 10.1128/JB.182.4.961-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Svenningsen S., Tu K., Bassler B. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J. 2009;28:429–439. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hoffer S., Westerhoff H., Hellingwerf K., Postma P., Tommassen J. Autoamplification of a two-component regulatory system results in “learning” behavior. J. Bacteriol. 2001;183:4914–4917. doi: 10.1128/JB.183.16.4914-4917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]