Abstract
Background
Although antifungals are available and usually used against fungal infections, multidrug-resistant (MDR) fungal pathogens are a growing problem for public health. Moreover, fungal infections have become more prevalent nowadays due to the increasing number of people living with immunodeficiency. Thus, previously rarely-isolated and/or unidentified fungal species including MDR yeast and moulds have emerged around the world. Recent works indicate that polymyxin antibiotics (polymyxin B and colistin) have potential antifungal proprieties. Therefore, investigating the in vitro activity of these molecules against clinical multidrug-resistant yeast and moulds could be very useful.
Methods
In this study, a total of 11 MDR yeast and filamentous fungal strains commonly reported in clinical settings were tested against polymyxin antibiotics. These include strains belonging to the Candida, Cryptococcus and Rhodotorula yeast genera, along with others belonging to the Aspergillus, Fusarium, Scedosporium, Lichtheimia and Rhizopus mould genera. The fungicidal or fungistatic action of colistin against clinical yeast strains was determined by the time-kill study. Further, a checkerboard assay for its combination with antifungal agents, usually used in clinical practices (amphotericin B, itraconazole, voriconazole), was carried out against multi-drug resistant fungal strains.
Results
Polymyxin B and colistin exhibited an antifungal activity against all MDR fungal strains tested with MICs ranging from 16 to 128 μg/ml, except for the Aspergillus species. In addition, colistin has a fungicidal action against yeast species, with minimum fungicidal concentrations ranging from 2 to 4 times MICs. It induces damage to the MDR Candida albicans membrane. A synergistic activity of colistin-amphotericin B and colistin-itraconazole associations against Candida albicans and Lichtheimia corymbifera strains, respectively, and colistin-fluconazole association against Rhodotorula mucilaginosa, was demonstrated using a checkerboard microdilution assay.
Conclusion
colistin could be proposed, in clinical practice, in association with other antifungals, to treat life-threatening fungal infections caused by MDR yeasts or moulds.
Keywords: Polymyxin antibiotics, MDR-fungi, Repurposing-drug, Candida albicans, Molds
Background
Invasive fungal diseases treatment is challenged by the restricted number of available antifungal drugs; with only four different classes of antifungals being available to treat a large number of fungal-associated diseases [1]. (i) Polyenes are the first antifungals available in clinical practice with two drugs mainly used: amphotericin B and nystatin. (ii) Azole antifungals, such as fluconazole, itraconazole, voriconazole, posaconazole and isavuconazole, are the most common drugs used in different clinical situations; azole drugs display a large spectrum of activity against both yeast and filamentous fungi. (iii) Pyrimidine analogues, including 5-flucytosine, are used in combination with other antifungals to treat yeast infections but have little action against most moulds. (iv) Finally, echinocandins, the newest class of antifungals include caspofungin, micafungin and anidulafungin that display fungicidal activity against ascomycetes yeast species [2].
In addition to this limited therapeutic arsenal, there has been a dramatic and worldwide increase in the incidence of fungal infections [3]. In fact, along with the main mycosis agents, such as Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans [4], other life-threatening and emerging pathogens, including not previously well-identified/characterized species and opportunistic multidrug-resistant (MDR) ones, are increasingly reported. These include Candida auris, Scedosporium/Lomentospora spp., Fusarium spp. and Mucorales [2]. Indeed, several factors can explain this increasing incidence of fungal infections; the increasing number of patients with immunodeficiency (ex. HIV, cancer and transplant patients), the ageing of the population [5] and improved detection and diagnostic methods [2]. However, the severity of such fungal infections varies depending on the site of infection (superficial or deep-seated) and the immune status of the concerned patients. One major characteristic of these emerging fungal pathogens is their highly-resistant profile to antifungal drugs. Therefore, these disseminated infections caused by MDR yeasts and moulds are difficult to treat [6], leading thus to a significant increase of morbidity and mortality, in immunocompromised patients but also in healthy individuals [7]. Antifungal resistance can be intrinsic, called primary resistance, or acquired, also called secondary resistance. Many resistance mechanisms have been described, such as biofilm formation (especially in Candida albicans), failure of intracellular drug accumulation or drug target alterations [8]. During the past decade, genome plasticity of human fungal pathogens has been strongly associated with their ability to acquire resistance to antifungals [9]. That is why many studies suggest to use other pharmacological classes and re-purposing old drugs either as a single antifungal agent or in combination with known antifungal drugs [10].
In this regard, antimicrobial peptides (AMPs) have received attention as prospective compounds for a further discovery of new antimycotics. More than 2700 antimicrobial peptides have been identified and the number is growing [11]. Among the AMPs commonly used in therapeutic practices, there are polymyxins which are cyclic, positively charged peptides, obtained naturally from Gram-positive bacteria, such as Paenibacillus polymyxa. Among polymyxin molecules described, two have been used in clinical settings: polymyxin B (PMB) and polymyxin E (colistin) [12]. This class of antibiotics has been discarded in the early 1980s because of their neuro- and nephro-toxicity. However, polymyxins were recently reintroduced in the antimicrobial therapy as a last option to treat infections caused by multidrug-resistant Gram-negative bacteria [13]. In addition to its antibacterial action, polymyxins were shown in the early 1970s to have antifungal activity against various Candida species, including Candida tropicalis with polymyxin E Minimum inhibitory concentrations (MICs) ranging from 30 to 75 μg/ml [14]. More recently, polymyxin sensitivity of life-threatening moulds, such as Fusarium and Rhizopus species, has been described [15, 16]. This study aimed to test the in vitro activity of polymyxin against the most common clinical MDR yeasts and moulds and to assess colistin activity, the mechanism of action and the synergy of colistin-antifungals associations that could be used in the treatment of invasive fungal infections.
Methods
Fungal isolates
In this study, eleven clinical fungi recovered at the University hospital of Marseille were used. Four yeasts belonging to Candida, Cryptococcus and Rhodotorula species and seven moulds belonging to Fusarium, Scedosporium, Lichtheimia, Rhizopus and Aspergillus species were tested (Table 1). Isolates were identified using Matrix-Assisted Laser Desorption/Ionisation mass spectrometry (MALDI-TOF MS) [17] and microscopic methods for the filamentous fungi species. These strains were isolated from different clinical samples including blood culture, cerebrospinal fluid, nails, bronchial aspiration and ocular samples (Table 1). Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, Aspergillus fumigatus ATCC 204205, Aspergillus flavus ATCC 204304, Escherichia coli LH1 [18], Escherichia coli 1R4 [19] and Klebsiella pneumoniae 853 [20] were used as susceptibility testing quality controls.
Table 1.
Phenotypic profiles and Colistin, PMB MICs of fungal strains tested in this study
| Clinical samples | MICs (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anid | Mica | Caspo | Flu | 5-Fc | Posa | Itra | Vorico | AB | Isavu | Ct | PMB | ||
| Candida krusei ATCC 6258 | / | 0.06 | 0.12 | 0.25 | 32 | 8 | 0.25 | 0.12 | 0.25 | 1 | ND | 64 | 32 |
| Candida parapsilosis ATCC 22019 | / | 0.5 | 0.5 | 0.12 | 1 | 0.12 | 0.03 | 0.008 | 0.03 | 0.5 | ND | 64 | 16 |
| Rhodotorula mucilaginosa | Endobucal | > 8 | > 8 | > 8 | 128 | 0.06 | 1 | 0.5 | 2 | 1 | ND | 32 | 16 |
| Cryptococcus neoformans | Blood | > 8 | > 8 | > 8 | 2 | 1 | 0.03 | 0.12 | 0.03 | 0.5 | ND | 32 | 16 |
| Candida albicans H5 | CSF | 0.015 | 0.03 | 0.05 | 16 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | ND | 128 | 128 |
| Candida albicans H6 | Nails | 0.06 | 0.03 | 0.12 | > 256 | 0.12 | > 8 | 32 | 256 | 1 | ND | 128 | 64 |
| Aspergillus fumigatus ATCC 204205 | / | ND | ND | ND | ND | ND | 0.008 | 0.015 | 0.12 | 2 | ND | > 256 | > 256 |
| Aspergillus flavus ATCC 204304 | / | ND | ND | ND | ND | ND | 0.06 | 0.06 | 1 | 4 | ND | > 256 | > 256 |
| Aspergillus calidoustus | Bronchial aspiration | ND | ND | ND | ND | ND | 1 | 6 | 4 | 4 | 0.25 | 256 | 128 |
| Fusarium oxysporumY5 | Nails | ND | ND | ND | ND | ND | > 32 | > 32 | 2 | 4 | > 32 | 64 | 16 |
| Fusarium solani Y6 | Ocular sample | ND | ND | ND | ND | ND | > 32 | > 32 | > 32 | > 32 | > 32 | 64 | 16 |
| Rhizopus oryzae Y9 | Sinus biopsy | ND | ND | ND | ND | ND | > 32 | > 32 | > 32 | > 32 | > 32 | 128 | 64 |
| Lomentospora prolificans Y8 | Blood | ND | ND | ND | ND | ND | > 32 | 64 | > 32 | > 32 | > 32 | 32 | 16 |
| Scedosporium apiospermum F2 | Bronchial aspiration | ND | ND | ND | ND | ND | 2 | 0.75 | > 32 | 4 | ND | 16 | 16 |
| Lichtheimia corymbifera ST87 | Eyes | ND | ND | ND | ND | ND | 2 | 2 | > 32 | 2 | 2 | 32 | 32 |
Anid - Anidulafungin; Mica - Micafungin; Casp - Caspofungin; Flu - Fluconazole; 5-Fc - 5-Flurocytosin; Pos - Posaconazol, Itra - Itraconazole; Vori - Voriconazole; AB - Amphotericin B; Isavu – Isavuconazole; Ct – Colistin; PMB- Polymyxin B; MIC - Minimum Inhibitory Concentration; ND: Not Done
Phenotypic profiles determination
Antifungal susceptibility testing was performed using two different methods: E-test (BioMérieux, Marcy l’Etoile, France) and commercial broth microdilution plates; Sensititre®YeastOne® (Thermo Fisher Scientific, Schwerte, Germany). The MICs obtained for each antifungal tested against yeasts and moulds (Table 1) was compared to the breakpoints provided by the manufacturers or to the epidemiological cutoff as previously described [21] in order to assess the susceptibility of each strain to the different antifungal agents tested.
Polymyxin susceptibility testing
Colistin and PMB MICs were performed using the broth microdilution method as outlined by the Clinical and Laboratory Standards Institute (CLSI) (M38-A, Vol. 22, NO. 16 for filamentous fungi and M27-A2, Vol. 22, NO. 15 for yeasts). Serial colistin and PMB (Sigma Aldrich, St Louis, France) dilutions ranging from 0.5 to 256 μg/ml were prepared in RPMI-1640 (Sigma Aldrich, St Louis, France) with glutamine and without bicarbonate medium buffered to pH 7.0 with MOPS (Sigma Aldrich, St Louis, France) buffer. Fungal inoculums were prepared in the test medium and adjusted to 0.5 MacFarland. A 1:100 dilution followed by a 1:20 dilution were performed on yeast strains to obtain a final inoculum of 0.5 to 2.5 × 103 CFU/mL, whereas only a 1:50 dilution was done for moulds with a final inoculum of approximately 0.4 to 5 × 104 CFU/mL. It is important to mention that fresh conidia of filamentous fungi were obtained after approximately 7 days of incubation at 35 °C on potato dextrose agar. Then, 100 μl of the fungal/bacterial inoculum was added into each colistin or PMB-containing wells. Plates were incubated at 37 °C for 24 h (Candida spp., bacterial strains) or 48 h (Cryptococcus) and at 35 °C for 48 h (filamentous fungi and Rhodothorula mucilaginosa). The susceptibility to polymyxin antibiotics was assessed on the basis of visual observation of growth or inhibition of the isolate in the culture media. The Resazurin (Sigma Aldrich, St Louis, France) was used to indicate the growth of any microorganism by a culture medium colour shift from blue to pink. Then, the inhibition rate was calculated, after measuring the optical density (OD) value by using a plate reader spectrophotometer (Multiskan spectrum, Thermo Scientific, France), as follows: % of fungal growth inhibition = (OD of untreated well - OD of tested well)*100 / (OD of untreated well – OD of blank well); where a blank well contains only the RPMI medium (i.e. without any fungal strain and without any antibiotic agent), an untreated well contains a given strain in the RPMI medium without any antibiotic agents and a tested well contains both a given strain in the RPMI medium and a given antibiotic agent. Thus, the growth inhibition rate is close to 100% when the concentration of an antibiotic reaches to the MIC as the OD of the tested well is quasi-equal to the OD of the blank well.
Colistin time-kill experiment and minimum fungicidal concentration (MFC) determination
Colistin time-kill study was performed, as previously described [22]. 9 ml of the fungal suspension was adjusted to a 0.5 McFarland turbidity. One ml of the adjusted fungal suspension was added to 9 ml of either RPMI-1640 medium, as a control, or to a solution of growth medium supplemented with an appropriate concentration of antibiotic solution. The colistin concentrations in the resulting solutions were 0.5, 1, 2, 4, 8, 16, and 32 times the MICs for the tested isolates. Then, the tubes were incubated at 37 °C on an orbital shaker. At 0, 6, 12, 24, 36, and 48 h following the introduction of the tested isolate into the solutions tubes, 100 μl aliquots were taken from each test solution. Different serial dilutions were performed on these aliquots, and a 10 μl aliquot from each dilution was streaked on Sabouraud agar plates (Biomérieux, France) and incubated for approximately 24 h for colony count determination. Then, the Minimum fungicidal concentration (MFC) was determined as the lowest antibiotic concentration leading to no significant growth or less than three colonies on Sabouraud agar plates in comparison to the growth control. The experimental data was analyzed using the GraphPad Prism 5.3 software (GraphPad Inc., San Diego, CA, USA) to obtain time-kill curves.
Study of the mechanism of action of colistin on Candida albicans species
We used propidium iodide (PI), a membrane-impermeable DNA stain, to demonstrate the eventual fungicidal activity of colistin by its ability to induce cell membrane damages [23]. Colistin-treated and untreated cells were suspended in PBS and stained with 5 μg/ml of PI for 20 min in the dark at room temperature. PI fluorescence was examined under a fluorescence microscope at λex = 535 nm and λem = 617 nm.
Colistin / antifungals association checkerboard testing
Checkerboard broth microdilution method was used to test the synergy of colistin with three antifungal agents commonly used in clinical settings; amphotericin B, fluconazole and itraconazole (Sigma Aldrich, St Louis, France). Firstly, MICs of individual agents were determined because the range of concentration of drugs to test the associations was established according to these MICs. Eight doubling dilutions of the two agents being tested (i.e. colistin and antifungals) were prepared in the susceptibility testing medium RPMI-1640 (1MIC, ½ MIC, ¼ MIC, 1/8 MIC, 1/16 MIC, 1/32 MIC, 1/64 MIC, 1/128 MIC). 50 μl of each agent was added in wells of a microtiter plate to provide a total of 64 drug combinations. Additional rows were used to determine the MIC of each antimicrobial agent alone by adding 100 ml of each agent. The fungal inoculum was prepared according to the CLSI standard protocol and 100 μl was added to each well. The plates were incubated under optimal growth conditions for yeasts and filamentous moulds. The results were analysed and MIC100 were determined visually and by optical density measurements on a microplates reader (Multiskan Spectrum, Thermo Scientific), based on a reduction in absorbance compared to the free drug control wells. Then, we calculated the Fractional Inhibitory Concentrations (FICs), where FIC1 (Colistin) = MIC of colistin in the combination/MIC of colistin alone and FIC2 (antifungal drug) = MIC of antifungal drug in the combination/MIC of antifungal drug alone. The fractional inhibitory index (FIX) is the sum of FIC1 and FIC2 and was interpreted as follows: if the FIX is ≤0.5, then there is synergy between the tested antimicrobials; if it is > 0.5 but ≤1 then there is additivity between the tested antimicrobials; if it is > 1 but ≤4, there is indifference between the tested antimicrobials, and if the FIX is > 4, that means that there is an antagonism between the tested antimicrobials.
Results
Resistance phenotypic profiles of the emerging fungal pathogens used in this study
The MICs obtained with the different antifungal classes tested against the 4 Candida spp. and Aspergillus spp. quality control strains corresponded to the recommended 24 h and 48 h - MICs limits of microbroth dilution method outlined in the CLSI protocol. This confirms the adequateness of the method used to determine susceptibility profiles for all isolates. C. albicans H6 was resistant to all azole antifungal agents tested while C. albicans H5 was only resistant to fluconazole (Table 1). Both strains remained sensitive to echinocandins and amphotericin B antifungals. Rhodotorula mucilaginosa was resistant in vitro to fluconazole with MIC = 128 μg/ml and to all echinocandin agents. Cryptococcus neoformans was also resistant to echinocandin class (Table 1).
All filamentous fungi tested were resistant to amphotericin B with a high MIC for Rhizopus oryzae and Scedosporium species (> 32 μg/ml). Fusarium, Scedosporium and Rhizopus species were also resistant to azoles (voriconazole, itraconazole and posaconazole) usually used in clinical settings (Table 1). The pyrimidine analogue 5-fluorocytosine and echinocandin agents were not tested against moulds because of their relatively poor activity against filamentous fungi.
Polymyxins exhibited in vitro, antifungal activity against MDR yeasts and filamentous fungi
Susceptibility testing showed clear endpoints with a 100% growth inhibition of the MDR strains tested. The MICs of Escherichia coli LH1 (MIC = 8 μg/ml) (16), Escherichia coli 1R4 (MIC = 8 μg/ml) (17) and Klebsiella pneumoniae 853 (MIC = 64 μg/ml) [20] were within the quality control ranges.
The polymyxins MICs against the fungi strains ranged from 16 to 128 μg/ml (Table 1), except for Aspergillus fumigatus and A. flavus strains, which appeared to be not sensitive to both colistin and PMB, with MICs ≥256 μg/ml. Among the 11 clinical strains tested, 4 isolates including Lichtheimia corymbifera, Lomentospora prolificans and Scedosporium apiospermum exhibited the highest susceptibility to polymyxin molecules, with MICs ranging from 16 μg/ml to 32 μg/ml; in contrast Aspergillus calidoustus exhibited the lowest susceptibility to colistin and PMB, with MICs of 256 μg/ml and 128 μg/ml, respectively.
Colistin presents fungicidal activity against clinical MDR yeasts
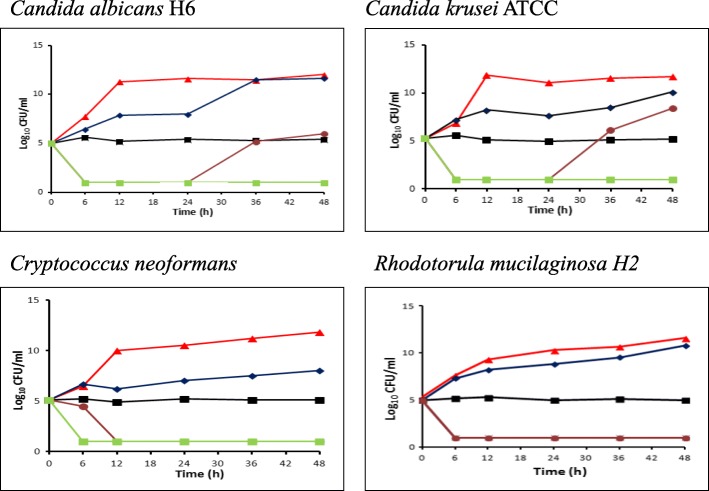
The colistin MICs ranged from 16 to 128 μg/ml for the yeast isolates. As shown on Fig. 1, no inhibitory effect was observed at colistin concentrations equal to 0.5X MICs and the curves were nearly identical to those found in the controls for all species tested. At colistin concentrations equal to 1X MICs, fungistatic effect was observed (Fig. 1). In contrast, colistin fungicidal activities against C. albicans, C. neoformans and R. mucilaginosa were noted, no later than 12th hour of incubation, with MFC ≥ to two-fold of its MIC values (Fig. 1). For C. albicans strains, the MFC was 256 μg/ml, but regrowth was observed at the 36th hour of incubation. The latter phenomenon was not observed in Cryptococcus neoformans or in Rhodotorula mucilaginosa species.
Fig. 1.
Time-kill kinetics of colistin against four fungal strains (C. albicans, C. krusei, C. neoformans and R. mucilaginosa). The colistin concentrations used are as following: (▲) control (no colistin added), (♦) 0.5X MIC, (■) 1X MIC,(●) 2X MIC, (■) 4X MIC
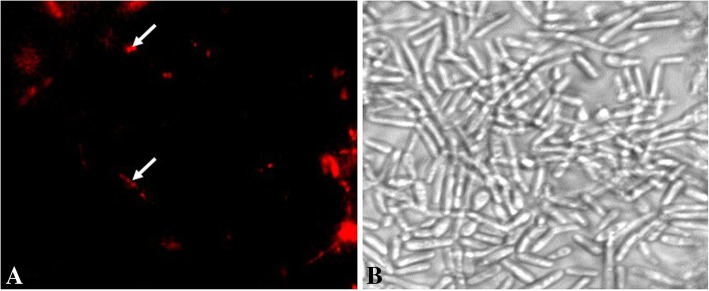
Moreover, a red fluorescence was observed by fluorescence microscopy (Fig. 2) indicating the presence of interaction between PI and nuclear DNA of C. albicans H6 treated with a colistin concentration equal to MFC. This observation allowed us to conclude that colistin can induce cell membrane damage which provide further evidence that it can lead to cell death, confirming its fungicidal activity.
Fig. 2.
Fluorescence microscopy of Candida albicans H6 after treatment with 5 μg/ml of propidium iodide. a: fluorescence image of cells treated with 256 μg/ml (2X MIC) of colistin for 24 h. b: Brightfield image of cells treated with colistin for 24 h. Scale bar: 2 μm
Synergistic activity of colistin with itraconazole, amphotericin B and fluconazole
Based on checkerboard association testing (Fig. 3), colistin-itraconazole and colistin-amphotericin B exhibited a synergistic activity against MDR C. albicans and the mucoralean Lichtheimia corymbifera. We noted that itraconazole MIC decreased from 32 μg/ml to 2 μg/ml (for C. albicans H6), with a FIX = 0.5, and from 2 μg/ml to 0.5 μg/ml (for L. corymbifera), with a FIX = 0.2 when combined with colistin (64 μg/ml and 0.5 μg/ml, respectively) (Table 2). The MIC of amphotericin B decreased from 2 μg/ml to 0.5 μg/ml (for L. corymbifera) and from 1 μg/ml to 0.5 μg/ml (for C. albicans H6) when combined with colistin (0.5 μg/ml and 1 μg/ml, respectively) (Table 2). Interestingly, colistin acted in synergy with fluconazole against R. mucilaginosa but not against Candida albicans H5 (Table 2). Although the colistin MICs decreased from 128 μg/ml to 1 μg/ml when combined with fluconazole (16 μg/ml), no synergy against C. albicans H5 was observed with a FIX = 1 (Table 2).
Fig. 3.
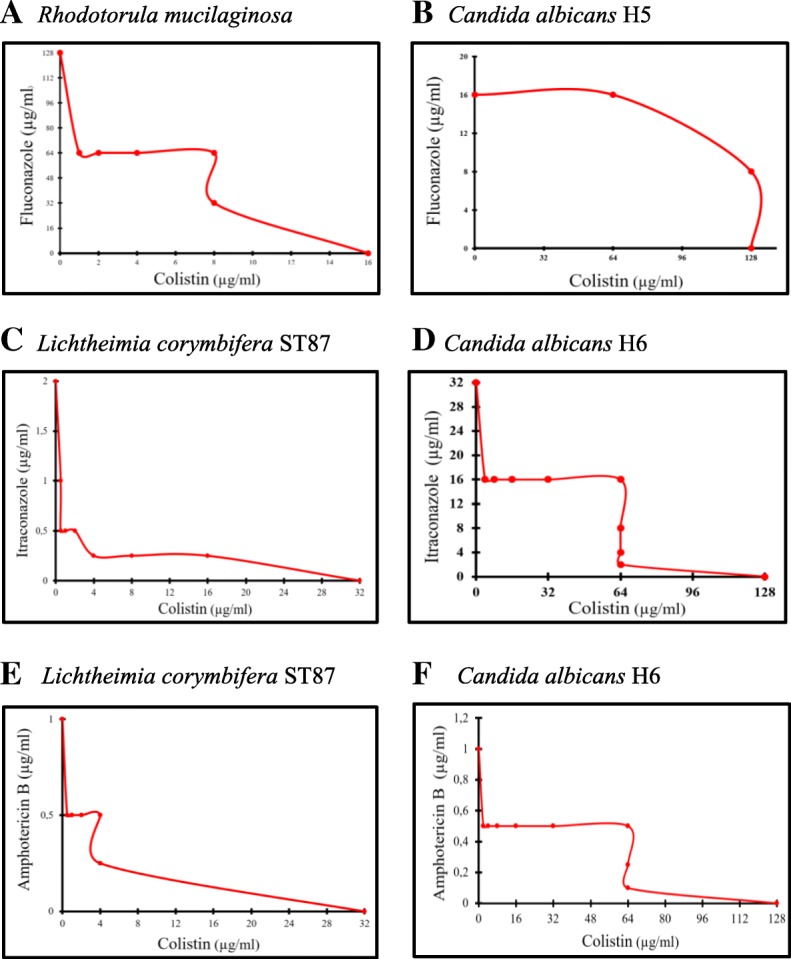
Plots of the checkerboard assays for the combinations of colistin with 3 antifungals (fluconazole, itraconazole and amphotericin b). Each dot presents the MICs of colistin (x-axis) and the antifungal agent (y-axis) used in the combination against R. mucilaginosa (a), C. albicans H5 (b), L. corymbifera (c and e) and C. albicans H6 (d and f)
Table 2.
MIC and FIX values of colistin in combination with antifungals from the checkerboard assay
| Strains tested | Agents in combination | MIC alone (μg/ml) | MIC in the combination (μg/ml) | FIC | FIX | Outcome |
|---|---|---|---|---|---|---|
| Rhodotorula mucilaginosa | Colistin | 16 | 1 | 0.06 | 0.5 | Synergy |
| Fluconazole | 128 | 64 | 0.5 | |||
| Candida albicans H5 | Colistin | 128 | 1 | 0.007 | 1 | Additivity |
| Fluconazole | 16 | 16 | 1 | |||
| Candida albicans H6 | Colistin | 128 | 64 | 0.5 | 0.5 | Synergy |
| Itraconazole | 32 | 2 | 0.06 | |||
| Colistin | 128 | 1 | 0.007 | 0.5 | Synergy | |
| Amphotericin B | 1 | 0.5 | 0.5 | |||
| Lichtheimia corymbifera ST87 | Colistin | 32 | 0.5 | 0.01 | 0.2 | Synergy |
| Itraconazole | 2 | 0.5 | 0.25 | |||
| Colistin | 32 | 0.5 | 0.01 | 0.2 | Synergy | |
| Amphotericin B | 2 | 0.5 | 0.25 |
FIC; Fractional Inhibitory concentration = MIC of the agent in the combination/MIC of the agent alone. FIX; Fractional Inhibitory Index is the sum of the FICs of the agents in the combination
Discussion
Our study demonstrated that polymyxin antibiotics have, aside from their antibactericidal activity, an antifungal activity, especially against multidrug-resistant Candida, Rhodotorula and Cryptococcus yeast isolates, but also against resistant filamentous fungi, such as Scedosporium, Rhizopus and Lichtheimia species. The MICs obtained against Candida spp. ranged between 64 and 128 μg/ml for colistin and mainly between 16 and 64 μg/ml for PMB. The latter is in concordance with PMB MICs already reported by Zeidler et al and Zhai et al studies [7, 24] confirming the validity of our results and the reproducibility of the used technique. Although we obtained a MIC of PMB equal to 16 μg/ml against C. neoformans, a lower MIC (MIC = 8 μg/ml) against this species has been previously described [24]. This could possibly be explained by the resistance phenotype of the strain tested in our study. Moreover, colistin has not been shown, in the early 1970s, to be an effective molecule against three strains belonging to the genus Rhodotorula [14]. However, to the best of our knowledge, neither colistin MICs against C. neoformans nor PMB MICs against R. mucilaginosa have been reported elsewhere.
On the other hand, the filamentous fungi isolates tested in this study constitute the most common emerging cause of human mould infections with an increase being reported from various geographical sites [2], including particularly Aspergillus and Fusarium spp. Here, colistin and PMB MICs ranging from 16 to 64 μg/ml have been observed against the F. oxysporum and F. Solani strains which are resistant to almost all azole antifungals and amphotericin B. The same range of MICs has been reported in previous study where 12 Fusarium spp. were tested against PMB but not against colistin [16]. The absence of colistin and PMB efficacy against several Aspergillus spp. has been reported in various studies with MICs > 256 μg/ml [24, 25] which are similar to our results. Despite that Aspergillus spp. are remaining the first cause of mould infections, mucormycosis is increasingly reported in immune-compromised patients and is associated with an elevated rate of mortality (40–70%) even under an appropriate therapy [26]. Among mucoralean pathogens, Rhizopus is the main frequently identified genus in human infections. In our study, colistin and PMB MICs against R. oryzae are one to two folds higher than those reported in earlier studies [15, 24]. Indeed, in Ben-Ami et al study, the colistin MICs against the fourteen clinical Rhizopus spp. tested were variables and ranged between 16 and 32 μg/ml, but MICs of antifungal agents were not mentioned [15]. So the discordance of MIC results between the Ben-Ami et al study and our work could be explained by the eventual high resistance level of our strain to azole agents which can be due to the over expression of efflux-pumps and/or other mechanisms [27]. It is important to mention that PMB MIC against R. oryzae was equal to 64 μg/ml in this study compared to 32 μg/ml obtained by Zhai et al study [24].
Finally, among the pertinent emerging fungal pathogens shown by several studies, Scedosporium and Lomentospora spp. are often notified [28]. They can induce a broad range of diseases; from colonisation in cystic fibrosis patients (for Scedosporium spp.) to disseminated severe infections in immuno-compromised hosts (for Lomentospora prolificans). Although, the colistin MIC against S. apiospermum strain tested here is within the colistin MICs range previously described by Schemuth et al, the colistin MIC obtained for L. prolificans was higher than that described in this previous study (32 μg/ml versus 12 μg/ml) [29]. Nevertheless, it is worthy to note that MICs90 were used by Schemuth et al [29] whereas MICs100 were used in our study. Finally, to the best of our knowledge, colistin and PMB activities against Lichtheimia corymbifera have not been previously reported.
In human studies, a single dose of 75 to 150 mg of colistin produced bioactive serum colistin concentrations ranging from 6 to 18 μg/ml; higher serum colistin concentrations (13 to 32 μg/ml) were measured during the prolonged therapy of patients with cystic fibrosis [15]. Therefore, the obtained MICs of colistin and PMB are difficult to be achieved with IV administration, mainly due to their renal and neurological toxicities and the risk of frequent selection of bacterial resistant strains.
However, the efficacy of polymyxin molecules on a large number of MDR fungi can be considered advantageous to treat bacterial and fungal co-infections that occur frequently in immunocompromised patients [30] and cystic fibrosis (CF) patients. Chronic bacterial and fungal colonization of the respiratory tract secretions is the main cause of morbidity and mortality in CF patients. Therefore, it would be helpful to use a treatment that is active on both bacteria and fungi in this context. It is worthy to note that, in clinical practice, colistin is administered by inhalation in CF patients as prophylaxis and also as a treatment against Pseudomonas aeruginosa infection [31]. In addition, aerosolised colistin treatment, is used in ventilator-associated pneumonia (VAP) cases caused by MDR bacteria in intensive care unit setting [32]. Interestingly, in a recent in vivo study, Landersdorfer et al [33] observed high epithelial lining fluid and low plasma colistin concentrations following the administration of only a pulmonary dose through jet nebulization, confirming a benefit of the local administration of colistin in comparison to its IV treatment [34]. Moreover, a prospective study conducted on 18 patients with chronic lung disease showed that nebulized colistin is effective and improves the quality of life, without presenting side effects and without selecting colistin-resistant isolates in treated patients [35]. So high-dose nebulized colistin could be proposed against pulmonary life-threatening MDR fungi, without increasing colistin plasma concentration, and thus avoiding colistin’s toxicity.
Similar to CF cases, the use of polymyxin antibiotics can improve the poor prognosis of fungal keratitis, due to the emergence of MDR fungal pathogens, particularly Fusarium spp. [36], and to the limited ocular penetration of antifungals [37]. Notably, PMB can be formulated for ophthalmic use [16], which is described as a highly effective drug on bacterial corneal ulcerations [38]. Moreover, the use of such antimicrobial agent constitutes a potential alternative treatment that may improve the outcome in some critical infections caused by MDR fungi, such as the recent MDR Fusarium keratitis-case report in a 46-year-old man who was still declining even the maximal therapeutic support and therapeutic keratoplasty [36].
Several approaches could be used to overcome the development of antifungal resistance in the treatment of fungal diseases. Aside from the discovery of new effective agents, one realistic alternative option would be to enhance the activity of existing agents. Combination therapies exploit the chances for better efficacy, decreased toxicity and reduced development of drug resistance [39]. A previous study demonstrated an in vitro synergy between colistin and echinocandins in several pathogenic yeasts, namely C. albicans, C. glabrata, C. tropicalis, C. parapsilosis and C. krusei, as well as in fluconazole-resistant C. albicans strains [7].
To the best of our knowledge, no previous studies have tested the activity of colistin in combination with other antifungal agents against fungi of the genera Rhodotorula and Lichtheimia.
A high decrease of colistin’s MICs was observed when it was combined with azoles (with fluconazole against R. mucilaginosa and with itraconazole against either C. albicans or L. corymbifera, Table 2 and Fig. 3). It is well known that the main mechanism of action of azoles is the inhibition of enzymes that transform lanosterol into ergosterol, a major lipid of the fungal membrane. This inhibition alters both the permeability and fluidity of fungal membrane [40]. On the other hand, polymyxins are well known for weakening the outer membrane in Gram-negative bacteria and the disruption of its permeability leading to a leakage of intracellular components [41]. Therefore, and as supported by PI staining results (Fig. 2), it is likely that antifungal azoles ease the polymyxins’ action and add a potential damage to the fungal membrane which results in a synergistic potency of the combined drugs. Moreover, colistin MIC values significantly decreased from 128 μg/ml to 1 μg/ml and from 32 μg/ml to 0.5 μg/ml against C. albicans and L. corymbifera respectively when it was associated with amphotericin B. The association of the fungal membrane permeabilization induced by amphotericin B via ion channel formation [42] with the probable membrane damage occurred by colistin could explain the decrease of MICs and the synergistic effect between colistin and amphotericin B.
Thus, despite the elevated MICs of colistin found in our work against multidrug-resistant yeast and moulds, the use of colistin, in combination with other antifungal agents, remains an excellent way to avoid the development of fungal resistance and to decrease the antifungal effective concentration usually used in clinical settings [16, 22].
Colistin is one of many AMPs already used in clinical settings [11]. So, in addition to the colistin-antifungal combination evaluated in this study, other AMPs could further be tested to potentiate the antifungal activity of existing antifungal compounds. For example, Wakabayashi et al, previously described the synergistic effect of lactoferin, a human antimicrobial peptide, with clotrimazole against C. albicans [43]. Moreover, lactoferin induced an important decrease of all azoles’ MICs tested against azole-resistant Candida spp. [43]. Consequently, natural or synthetic AMPs, have been identified as an original therapeutic alternative that could be investigated by medical researchers and pharmaceutical companies. Using the same approach which was used herein, another AMP, less toxic than polymyxins such as bacitracin or gramicidin analogues, could be tested as monotherapy or in association with antifungals against MDR fungi.
Conclusion
Our findings demonstrate that polymyxins display a broad-spectrum activity against common MDR fungi especially those which are difficult to manage in clinical settings. Unfortunately, polymyxins’ MICs against these MDR strains are higher than those that could clinically be used in human therapy, thus the use of such high toxicity-associated concentration of polymyxins presents the major limitation of their application in clinical mycology practice. However, colistin seems to induce C. albicans membrane damages and to act in synergy with either itraconazole or amphotericin B (each also acting on the fungal membrane). We therefore suggest that colistin (at a ‘safe’ reduced dose) can be used in combination with currently available antifungal drugs, as a last resort option, against life-threatening MDR fungi.
Acknowledgements
The author thanks Magdalen Lardière and CookieTrad for English reviewing.
Funding
This work was supported by the French Government under the « Investissements d’avenir » (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03).
This work was supported by the Région Provence-Alpes-Côte-d’Azur and European funding FEDER PRIMI.
Availability of data and materials
All data are available in Table 1.
Abbreviations
- AMPs
Antimicrobial peptides
- CF
Cystic fibrosis
- CLSI
Clinical and Laboratory Standards Institute
- FIC
Fractional inhibitory concentration
- FIX
Fractional inhibitory index
- MDR
Multidrug resistant
- MFC
Minimal Fungicidal Concentration
- MIC
Minimum inhibitory concentration
- PBS
Phosphate-buffered saline
- PI
Propidium iodide
- PMB
Polymyxin B
Authors’ contributions
JMR and FB were involved in the conception and design of the study. HY performed the experiments. HY, SR, JMR and FB analysed and interpreted the data. HY and FB co-wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hanane Yousfi, Email: hanane.yousf@gmail.com.
Stéphane Ranque, Email: stephane.ranque@ap-hm.fr.
Jean-Marc Rolain, Email: jean-marc.rolain@univ-amu.fr.
Fadi Bittar, Email: fadi.bittar@univ-amu.fr.
References
- 1.Morio F, Jensen RH, Le PP, Arendrup MC. Molecular basis of antifungal drug resistance in yeasts. Int J Antimicrob Agents. 2017;50:599–606. doi: 10.1016/j.ijantimicag.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Geddes-McAlister Jennifer, Shapiro Rebecca S. New pathogens, new tricks: emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Annals of the New York Academy of Sciences. 2018;1435(1):57–78. doi: 10.1111/nyas.13739. [DOI] [PubMed] [Google Scholar]
- 3.Dannaoui E. Antifungal resistance in Mucorales. Int J Antimicrob Agents. 2017;50:617–621. doi: 10.1016/j.ijantimicag.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Alcazar-fuoli L, Mellado E. Current status of antifungal resistance and its impact on clinical practice. Br J Haematol. 2014;166:471–484. doi: 10.1111/bjh.12896. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman CA. Fungal infections in older adults. Clin Infect Dis. 2001;33:550–555. doi: 10.1086/322685. [DOI] [PubMed] [Google Scholar]
- 6.Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis. 2008;46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 7.Zeidler U, Bougnoux ME, Lupan A, Helynck O, Doyen A, Garcia Z, et al. Synergy of the antibiotic colistin with echinocandin antifungals in candida species. J Antimicrob Chemother. 2013;68:1285–1296. doi: 10.1093/jac/dks538. [DOI] [PubMed] [Google Scholar]
- 8.Silva Luís A. Vale. Antifungals: From Genomics to Resistance and the Development of Novel Agents. 2015. Molecular Mechanisms of Resistance of Candida spp. to Membrane-targeting Antifungals; pp. 1–26. [Google Scholar]
- 9.Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryot Cell. 2007;6:1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyclit Lucie, Baron Sophie A., Yousfi Hanane, Rolain Jean-Marc. Zidovudine: A salvage therapy for mcr-1 plasmid-mediated colistin-resistant bacterial infections? International Journal of Antimicrobial Agents. 2018;52(1):11–13. doi: 10.1016/j.ijantimicag.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Bondaryk Małgorzata, Staniszewska Monika, Zielińska Paulina, Urbańczyk-Lipkowska Zofia. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. Journal of Fungi. 2017;3(3):46. doi: 10.3390/jof3030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olaitan A, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:1–18. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholls MWN. Polymyxin sensitivity of Candida tropicalis. J Med Microbiol. 1970;3:529–538. doi: 10.1099/00222615-3-3-529. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Ami R, Lewis RE, Tarrand J, Leventakos K, Kontoyiannis DP. Antifungal activity of colistin against Mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob Agents Chemother. 2010;54:484–490. doi: 10.1128/AAC.00956-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu LH, Wang HF, Sun PL, Hu FR, Chen YL. The antibiotic polymyxin B exhibits novel antifungal activity against Fusarium species. Int J Antimicrob Agents. 2017;49:740–748. doi: 10.1016/j.ijantimicag.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Cassagne C, Normand AC, L’Ollivier C, Ranque S, Piarroux R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses. 2016;59:678–690. doi: 10.1111/myc.12506. [DOI] [PubMed] [Google Scholar]
- 18.Olaitan A, Chabou S, Okdah L, Morand S, Rolain JM. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis. 2016;16:147. doi: 10.1016/S1473-3099(15)00540-X. [DOI] [PubMed] [Google Scholar]
- 19.Leangapichart T, Gautret P, Brouqui P, Memish ZA, Raoult D, Rolain JM. Acquisition of mcr-1 plasmid-mediated colistin resistance in Escherichia coli and Klebsiella pneumoniae during hajj 2013 and 2014. Antimicrob Agents Chemother. 2016;60:6998–6999. doi: 10.1128/AAC.01486-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalaoui Rym, Bakour Sofiane, Livnat Kashat, Assous Marc Victor, Diene Seydina M., Rolain Jean-Marc. Spread of Carbapenem and Colistin-Resistant Klebsiella pneumoniae ST512 Clinical Isolates in Israel: A Cause for Vigilance. Microbial Drug Resistance. 2019;25(1):63–71. doi: 10.1089/mdr.2018.0014. [DOI] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff A, Arthington-Skaggs B, Iqbal N, Ellis D, Pfaller MA, Messer S, et al. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J Clin Microbiol. 2007;45:1811–1820. doi: 10.1128/JCM.00134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankey G, Ashcraft D, Kahn H, Ismail A. Time-kill assay and etest evaluation for synergy with polymyxin B and fluconazole against Candida glabrata. Antimicrob Agents Chemother. 2014;58:5795–5800. doi: 10.1128/AAC.03035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwolek-Mirek M, Zadrag-Tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014;14:1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- 24.Zhai B, Zhou H, Yang L, Zhang J, Jung K, Giam CZ, et al. Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J Antimicrob Chemother. 2010;65:931–938. doi: 10.1093/jac/dkq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short G, Rennison C, Gould K, Fisher A. Activity of Colistin against filamentous Fungi isolated from lung transplant recipients. J Hear Lung Transplant. 2010;29:S185–S186. doi: 10.1016/j.healun.2009.11.589. [DOI] [Google Scholar]
- 26.Buchanan WL, Schaufele RL, Knudsen TA, Sein T, Zaoutis TE, Chiou CC, et al. Epidemiology and outcome of Zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller MA. Antifungal drug resistance : mechanisms , epidemiology , and consequences for treatment. Am J Med. 2012;125:S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Pellon A, Ramirez-Garcia A, Buldain I, Antoran A, Martin-Souto L, Rementeria A, et al. Pathobiology of Lomentospora prolificans: could this species serve as a model of primary antifungal resistance? Int J Antimicrob Agents. 2018;51:10–15. doi: 10.1016/j.ijantimicag.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Schemuth H, Dittmer S, Lackner M, Sedlacek L, Hamprecht A, Steinmann E, et al. In vitro activity of colistin as single agent and in combination with antifungals against filamentous fungi occurring in patients with cystic fibrosis. Mycoses. 2013;56:297–303. doi: 10.1111/myc.12022. [DOI] [PubMed] [Google Scholar]
- 30.Udeani TKC, Moses J, Uzoechina A, Okwori AEJ, Okwosa CN. Microbial aetiologic agents associated with pneumonia in immunocompromised hosts. African J Infect Dis. 2010;4:1–6. doi: 10.4314/ajid.v4i1.55084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elborn JS, Hodson M, Bertram C. Implementation of European standards of care for cystic fibrosis - control and treatment of infection. J Cyst Fibros. 2009;8:211–217. doi: 10.1016/j.jcf.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Ben Lakhal S. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care Springer Paris. 2016;6:1-11. [DOI] [PMC free article] [PubMed]
- 33.Landersdorfer Cornelia B, Nguyen T-H, Linh Thuy Lieu GN, Bischof RJ, Meeusen EN, Li J, Nation RL, et al. Substantial targeting advantage achieved by pulmonary Administration of Colistin Methanesulfonate in a large. Antimicrob Agents Chemother. 2017;61:1–15. doi: 10.1128/AAC.01934-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carillo C, Pecoraro Y, Anile M, Poggi C, Oliva A, Amore D, et al. Colistin-based treatment of multidrug-resistant gram-negative bacterial pulmonary infections after lung transplantation. Transplant Proc. 2019;51:202–205. doi: 10.1016/j.transproceed.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 35.Steinfort DP, Steinfort C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern Med J. 2007;37:495–498. doi: 10.1111/j.1445-5994.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 36.Sara S, Sharpe K, Morris S. Multidrug-resistant Fusarium keratitis: diagnosis and treatment considerations. BMJ Case Rep. 2016;3. 10.1136/bcr-2016-215401. [DOI] [PMC free article] [PubMed]
- 37.Maharana P, Sharma N, Nagpal R, Jhanji V, Das S, Vajpayee R. Recent advances in diagnosis and management of mycotic keratitis. Indian J Ophthalmol. 2016;64:346. doi: 10.4103/0301-4738.185592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosscha MI, Van Dissel JT, Kuijper EJ, Swart W, Jager MJ. The efficacy and safety of topical polymyxin B, neomycin and gramicidin for treatment of presumed bacterial corneal ulceration. Br J Ophthalmol. 2004;88:25–28. doi: 10.1136/bjo.88.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3:1–11. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.François IEJA, Cammue BPA, Borgers M, Ausma J, Dispersyn GD, Thevissen K. Azoles: mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Antiinfect Agents Med Chem. 2006;5:3–13. doi: 10.2174/187152106774755554. [DOI] [Google Scholar]
- 41.Trimble MJ, Mlynarcik P, Kolar M, Hancock REW. Polymyxin: alternative mechanisms of action. Cold Spring Harb Perspect Med. 2016;6:1–22. doi: 10.1101/cshperspect.a025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci. 2012;109:2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakabayashi H, Abe S, Teraguchi S, Hayasawa H, Yamaguchi H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin- related compounds. Antimicrob Agents Chemother. 1998;42:1587–1591. doi: 10.1128/AAC.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in Table 1.