Abstract
Although remarkable advances have been made in the treatment of rheumatoid arthritis (RA), novel therapeutic options with different mechanisms of action and fewer side effects have been expected. Recent studies have demonstrated that bone-resorbing osteoclasts are critically involved in the bone destruction associated with RA. Denosumab, a human antibody against receptor activator of nuclear factor-kappa B ligand (RANKL), efficiently suppressed the progression of bone erosion in patients with RA by suppressing osteoclast differentiation and activation in several clinical studies, although it had no effect on inflammation or cartilage destruction. Denosumab, in combination with anti-rheumatic drugs, is considered a pivotal therapeutic option for the prevention of bone destruction in RA.
Keywords: Denosumab, osteoclast, RANKL, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is an inflammatory disorder of unknown etiology, characterized by chronic inflammation of the synovial joints through autoimmune mechanisms 1, 2. Remarkable progress in the treatment of RA has been achieved during the last 20 years. Methotrexate (MTX) has been considered an anchor drug and is used as the first-line therapeutic modality for RA. MTX monotherapy was reported to achieve repair of severely damaged joints (radiographic healing) by suppressing joint inflammation 3. The emergence of biological agents like tumor necrosis factor (TNF) inhibitors has had a remarkable impact on therapeutic strategies for RA and greatly improved disease control in patients with RA. In addition, two targeted synthetic disease-modifying anti-rheumatic drugs—tofacitinib and baricitinib—that target Janus kinases have recently been introduced and showed equal efficacy to biologics in the treatment of RA. Despite the excellent effects of these novel therapeutic agents, there are still several limitations in their treatment of RA. First, and most importantly, almost all currently available anti-rheumatic drugs basically suppress the immunological function of patients and thus are inevitably associated with a wide range of immunosuppression-related side effects such as infections. Furthermore, the effects of these drugs are not perfect, and a fair number of patients do not respond well to treatment. Therefore, novel therapeutic options with different mechanisms of action and fewer side effects have long been expected.
Involvement of osteoclasts in bone destruction associated with rheumatoid arthritis
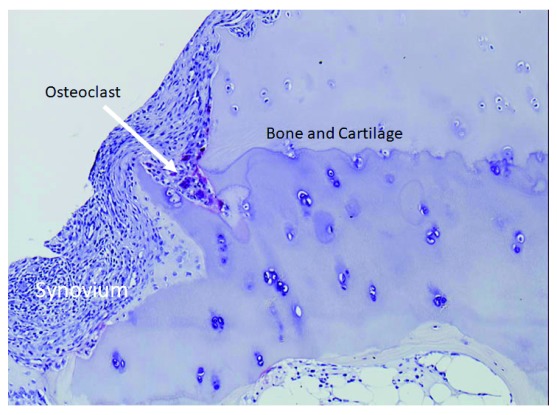
Osteoclasts are multinucleated giant cells primarily responsible for bone resorption. They originate from hematopoietic stem cells and differentiate from monocyte/macrophage-lineage precursor cells. Previous studies showed that osteoclasts are critically involved in not only physiological bone metabolism but also pathologic bone destruction such as that observed in RA, osteoporosis, and cancer bone metastasis 4. In particular, the primary role of osteoclasts in the bone destruction associated with RA has attracted a great deal of attention. Multinucleated giant cells with characteristics of osteoclasts are frequently observed at the interface between the inflammatory synovium and the eroded bone of patients with RA 5 ( Figure 1), and Gravallese et al. 6 reported that these multinucleated cells expressed osteoclast-specific genes like tartrate-resistant acid phosphatase and calcitonin receptor. We previously reported that differentiation to multinucleated osteoclasts was induced when synovial fibroblasts from patients with RA were co-cultured with peripheral blood mononuclear cells in the presence of 1α,25-dihydroxyvitamin D 3 [1α,25(OH) 2D 3] and macrophage colony-stimulating factor (M-CSF) 7.
Figure 1. Bone erosion in rheumatoid arthritis.
The proliferating synovium stimulates osteoclast differentiation, and many osteoclasts are observed at the synovium–bone interface (arrow).
The critical role of osteoclasts in bone destruction associated with RA was confirmed by findings in a rare human case. An inherited disorder characterized by increased bone mineral density (BMD), osteopetrosis arises from a defect in osteoclast differentiation or activation 8. We described a patient whose type II autosomal dominant osteopetrosis was diagnosed in his youth and demonstrated markedly reduced osteoclast activity 9. The patient coincidentally developed RA but showed a slow progression of bone erosion despite severe inflammation and rapid progression of cartilage destruction 9. These results suggest that the normal activity of osteoclasts is required for the bone destruction in RA.
Regulation of osteoclast development by RANKL–RANK pathways
Receptor activator of nuclear factor-kappa B ligand (RANKL) belongs to the TNF superfamily. RANKL was originally identified as an activated T cell–producing factor that modulates dendritic cell survival 10, 11, and many subsequent studies elucidated its essential role in osteoclast biology. RANKL expression is induced in osteoblastic cells and bone marrow stromal cells in response to calciotropic factors such as 1α,25(OH) 2D 3 and parathyroid hormone, and combined treatment of hematopoietic cells with M-CSF and a soluble form of RANKL induced osteoclast differentiation in vitro 4, 12. The receptor for RANKL, RANK, is expressed in monocyte/macrophage-lineage osteoclast precursor cells, and osteoprotegerin (OPG), a member of the TNF receptor superfamily, binds to RANKL and competitively suppresses its binding to RANK. The physiological importance of the RANKL–RANK–OPG axis was confirmed by mouse genetic studies. Targeted disruption of Rankl or Rank, as well as overexpression of Opg, induced osteopetrosis in mice through a defect in osteoclast differentiation, whereas deletion of the Opg gene or overexpression of Rankl led to a marked reduction in bone mass, mimicking osteoporosis 13. Furthermore, several human genetic disorders were shown to be associated with the RANKL–RANK–OPG axis. Patients with RANKL or RANK gene mutations developed a distinct subgroup of recessive osteopetrosis diseases with very few osteoclasts in their skeletal tissues 14. In contrast, familial expansile osteolysis, early-onset Paget’s disease of bone, and expansile skeletal hyperphosphatasia were associated with gene mutations that caused enhancement of RANKL–RANK pathways and thus increased bone resorption 14.
Osteoclasts as a therapeutic target in rheumatoid arthritis
Since osteoclasts are critically involved in the bone destruction associated with RA, osteoclasts are considered a therapeutic target in RA. Bisphosphonates, stable analogues of pyrophosphate, are representative anti-osteoporosis medicines with strong anti-catabolic activity, and several studies have been conducted to investigate their effects in RA. Ralston et al. 15 analyzed the effect of aminohydroxypropylidene bisphosphonate (APD) in patients with RA. APD treatment did not ameliorate radiographic progression even though biochemical markers of increased bone resorption were significantly suppressed in the APD group. Eggelmeijer et al. 16 reported that pamidronate treatment increased BMD in patients but did not ameliorate joint damage or disease activity. More recently, the effect of zoledronic acid (ZA) was analyzed in both animal models and patients with RA. Herrak et al. 17 and Sims et al. 18 almost simultaneously reported that ZA suppressed bone destruction in human TNF-α transgenic mice and collagen-induced arthritis rats by inhibiting osteoclastic bone resorption, respectively. Moreover, preliminary evidence for a structural benefit of ZA in early-stage RA was reported by Jarrett et al. 19, whose findings suggest that, when started in the early stage of RA, osteoclast-targeting therapy is effective. However, the clinical evidence is limited.
RANKL as a therapeutic target in rheumatoid arthritis
Several groups, including ours, reported an increased expression of RANKL in the synovial tissues of patients with RA 20– 22. Later, Hashizume et al. reported that the expression of RANKL was induced in response to interleukin-6 (IL-6) signaling and that TNF-α, IL-17, or IL-1β stimulated the production of IL-6 in synovial fibroblasts, indicating the involvement of inflammatory cytokines in RANKL production in synovial fibroblasts 23. The essential role of RANKL–RANK pathways in arthritic bone destruction was confirmed in a series of animal experiments. OPG treatment ameliorated arthritic bone destruction in adjuvant arthritis rats 24 and markedly reduced bone erosion in RANKL-deficient mice with serum transfer-induced arthritis 25. In addition, systemic bone loss, as well as local bone erosion, was ameliorated by OPG injection combined with an anti-TNF-α antibody in TNF-α transgenic mice 26.
Denosumab is a fully human IgG2a monoclonal antibody that specifically binds to human RANKL and inhibits its interaction with RANK, thereby suppressing bone resorption. In the pivotal Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study, denosumab treatment for 3 years significantly and continuously increased BMD and reduced the risks of vertebral, non-vertebral, and hip fractures 27. Denosumab was effective in treating not only osteoporosis but other pathologic conditions such as bone cancer diseases and giant cell tumor of bone 28.
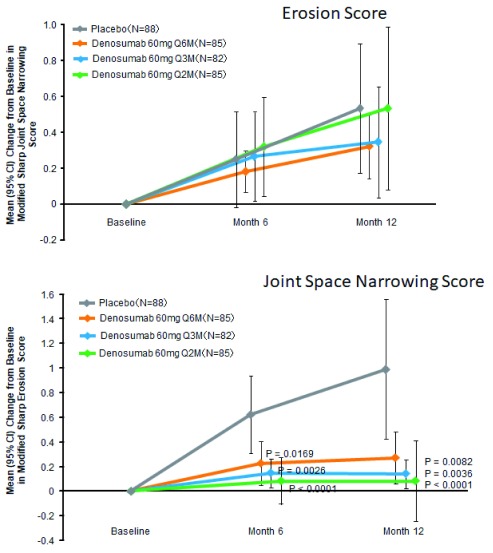
The effects of denosumab in patients with RA have been examined in several clinical trials. Cohen et al. 29 reported that the progression in the erosion score at 6 months on magnetic resonance imaging was lower in the denosumab group compared with the placebo group. In contrast, denosumab had no protective effect on the progression of joint-space narrowing or RA disease activity, probably because it cannot ameliorate the synovial inflammation in RA. The effect of denosumab in Japanese patients with RA was more recently reported 30. Patients with RA were randomly assigned to subcutaneous injection of placebo or denosumab 60 mg every 6 months (Q6M), Q3M, or Q2M. Compared with placebo, denosumab at all doses significantly inhibited the progression of bone erosion at 12 months as determined by the modified Sharp erosion score but had no obvious effect on joint-space narrowing ( Figure 2). Notably, no apparent difference in the safety profiles of denosumab and placebo was reported. These results give strong evidence that, in the early stage of RA, denosumab can prevent the progression of bone erosion but has little or no effect on cartilage deterioration or disease activity. Moreover, Hasegawa et al. 31 reported that, compared with biological agent treatment alone, the concurrent use of denosumab with biological agents was more efficacious in inhibiting structural damage in RA patients without increasing adverse events. Interestingly, Ebina et al. 32 reported that, compared with continuing oral bisphosphonates or switching to teriparatide, switching oral bisphosphonates to denosumab significantly reduced radiographic joint destruction at 12 months. Based on these results, denosumab obtained approval for “inhibition of the progression of bone erosion associated with RA” in Japan 33.
Figure 2. Denosumab significantly suppresses the bone erosion score but has no effect on the joint-space narrowing score determined by modified Sharp scores.
( a) Modified Sharp erosion score. ( b) Modified Sharp joint-space narrowing score. CI, confidence interval; Q2M, every 2 months; Q3M, every 3 months; Q6M, every 6 months. Modified from a figure in an article by Takeuchi et al. 30.
Conclusions
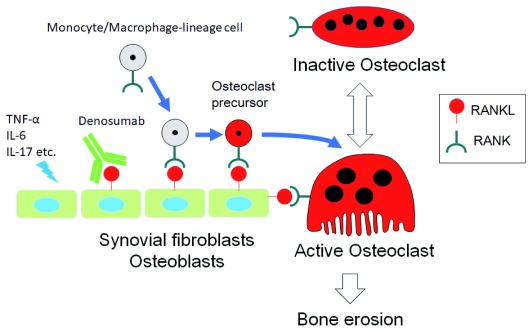
We have proposed a novel therapeutic approach to prevent bone destruction in RA by targeting RANKL 34 ( Figure 3). Denosumab effectively prevents bone destruction but has no effect on joint inflammation or cartilage destruction in RA. Therefore, denosumab should be used together with other therapeutic agents, like MTX and biologics, for the treatment of RA. However, several clinical questions remain to be addressed. These questions include whether denosumab should be started immediately after diagnosis of RA and whether it can be stopped. The latter question is important because multiple vertebral fractures were reported to be observed after discontinuation of denosumab in patients with osteoporosis 35. Further clinical and basic studies are required to address these questions and to establish the appropriate role of denosumab in treatment strategies for RA.
Figure 3. Schematic representation of the mechanism for osteoclast development and denosumab action in rheumatoid arthritis.
Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-17 directly or indirectly induce receptor activator of nuclear factor-kappa B ligand (RANKL) expression in synovial fibroblasts or osteoblasts or both. RANKL stimulates osteoclast differentiation from monocyte/macrophage-lineage precursor cells, leading to bone erosion in rheumatoid arthritis. Denosumab specifically binds to RANKL and suppresses osteoclast differentiation.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Naoyuki Takahashi, Institute for Oral Science, Matsumoto Dental University, Nagano, 399-0781, Japan
Seoung Hoon Lee, Department of Oral Microbiology and Immunology, College of Dentistry, Wonkwang University, Jeonbuk, 54538, South Korea
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Firestein GS: Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. 10.1038/nature01661 [DOI] [PubMed] [Google Scholar]
- 2. Schett G: Autoimmunity as a trigger for structural bone damage in rheumatoid arthritis. Mod Rheumatol. 2017;27(2):193–7. 10.1080/14397595.2016.1265907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Wassenberg S, Rau R: Radiographic healing with sustained clinical remission in a patient with rheumatoid arthritis receiving methotrexate monotherapy. Arthritis Rheum. 2002;46(10):2804–7. 10.1002/art.10568 [DOI] [PubMed] [Google Scholar]
- 4. Tanaka S, Nakamura K, Takahasi N, et al. : Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. 10.1111/j.0105-2896.2005.00327.x [DOI] [PubMed] [Google Scholar]
- 5. Bromley M, Woolley DE: Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27(9):968–75. 10.1002/art.1780270902 [DOI] [PubMed] [Google Scholar]
- 6. Gravallese EM, Harada Y, Wang JT, et al. : Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152(4):943–51. [PMC free article] [PubMed] [Google Scholar]
- 7. Takayanagi H, Oda H, Yamamoto S, et al. : A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun. 1997;240(2):279–86. 10.1006/bbrc.1997.7404 [DOI] [PubMed] [Google Scholar]
- 8. Sobacchi C, Schulz A, Coxon FP, et al. : Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–36. 10.1038/nrendo.2013.137 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Kadono Y, Tanaka S, Nishino J, et al. : Rheumatoid arthritis associated with osteopetrosis. Mod Rheumatol. 2014;19(6):687–90. 10.1007/s10165-009-0208-7 [DOI] [PubMed] [Google Scholar]
- 10. Anderson DM, Maraskovsky E, Billingsley WL, et al. : A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–9. 10.1038/36593 [DOI] [PubMed] [Google Scholar]
- 11. Wong BR, Josien R, Lee SY, et al. : TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186(12):2075–80. 10.1084/jem.186.12.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suda T, Takahashi N, Udagawa N, et al. : Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–57. 10.1210/edrv.20.3.0367 [DOI] [PubMed] [Google Scholar]
- 13. Lacey DL, Boyle WJ, Simonet WS, et al. : Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–19. 10.1038/nrd3705 [DOI] [PubMed] [Google Scholar]
- 14. Crockett JC, Mellis DJ, Scott DI, et al. : New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporos Int. 2011;22(1):1–20. 10.1007/s00198-010-1272-8 [DOI] [PubMed] [Google Scholar]
- 15. Ralston SH, Hacking L, Willocks L, et al. : Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. Ann Rheum Dis. 1989;48(5):396–9. 10.1136/ard.48.5.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggelmeijer F, Papapoulos SE, van Paassen HC, et al. : Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: a double blind placebo controlled study. J Rheumatol. 1994;21(11):2016–20. [PubMed] [Google Scholar]
- 17. Herrak P, Görtz B, Hayer S, et al. : Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004;50(7):2327–37. 10.1002/art.20384 [DOI] [PubMed] [Google Scholar]
- 18. Sims NA, Green JR, Glatt M, et al. : Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004;50(7):2338–46. 10.1002/art.20382 [DOI] [PubMed] [Google Scholar]
- 19. Jarrett SJ, Conaghan PG, Sloan VS, et al. : Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1410–4. 10.1002/art.21824 [DOI] [PubMed] [Google Scholar]
- 20. Gravallese EM, Manning C, Tsay A, et al. : Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43(2):250–8. [DOI] [PubMed] [Google Scholar]
- 21. Shigeyama Y, Pap T, Kunzler P, et al. : Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2523–30. [DOI] [PubMed] [Google Scholar]
- 22. Takayanagi H, Iizuka H, Juji T, et al. : Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43(2):259–69. [DOI] [PubMed] [Google Scholar]
- 23. Hashizume M, Hayakawa N, Mihara M: IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford). 2008;47(11):1635–40. 10.1093/rheumatology/ken363 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Kong YY, Feige U, Sarosi I, et al. : Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. 10.1038/46303 [DOI] [PubMed] [Google Scholar]
- 25. Pettit AR, Ji H, von Stechow D, et al. : TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. 10.1016/S0002-9440(10)63016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redlich K, Hayer S, Ricci R, et al. : Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110(10):1419–27. 10.1172/JCI15582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cummings SR, San Martin J, McClung MR, et al. : Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Brown JE, Coleman RE: Denosumab in patients with cancer—a surgical strike against the osteoclast. Nat Rev Clin Oncol. 2012;9(2):110–8. 10.1038/nrclinonc.2011.197 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Cohen SB, Dore RK, Lane NE, et al. : Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: A twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58(5):1299–309. 10.1002/art.23417 [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi T, Tanaka Y, Ishiguro N, et al. : Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2016;75(6):983–90. 10.1136/annrheumdis-2015-208052 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Hasegawa T, Kaneko Y, Izumi K, et al. : Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine. 2017;84(3):379–80. 10.1016/j.jbspin.2016.05.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Ebina K, Hirao M, Hashimoto J, et al. : Impact of switching oral bisphosphonates to denosumab or daily teriparatide on the progression of radiographic joint destruction in patients with biologic-naïve rheumatoid arthritis. Osteoporos Int. 2018;29(7):1627–36. 10.1007/s00198-018-4492-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Takeuchi T, Tanaka Y, Soen S, et al. : Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying anti-rheumatic drugs (DESIRABLE study): a randomized, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis. 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka S, Tanaka Y, Ishiguro N, et al. : RANKL: A therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol. 2018;28(1):9–16. 10.1080/14397595.2017.1369491 [DOI] [PubMed] [Google Scholar]
- 35. Cummings SR, Ferrari S, Eastell R, et al. : Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res. 2018;33(2):190–8. 10.1002/jbmr.3337 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation