Abstract
Background: Schizophrenia, a severe psychological disorder, shows symptoms such as hallucinations and delusions. In addition, patients with schizophrenia often exhibit a deficit in working memory which adversely impacts the attentiveness and the behavioral characteristics of a person. Although several clinical efforts have already been made to study working memory deficit in schizophrenia, in this paper, we investigate the applicability of a machine learning approach for identification of the brain regions that get affected by schizophrenia leading to the dysfunction of the working memory.
Methods: We propose a novel scheme for identification of the affected brain regions from functional magnetic resonance imaging data by deploying group independent component analysis in conjunction with feature extraction based on statistical measures, followed by sequential forward feature selection. The features that show highest accuracy during the classification between healthy and schizophrenia subjects are selected.
Results: This study reveals several brain regions like cerebellum, inferior temporal gyrus, superior temporal gyrus, superior frontal gyrus, insula, and amygdala that have been reported in the existing literature, thus validating the proposed approach. We are also able to identify some functional changes in the brain regions, such as Heschl gyrus and the vermian area, which have not been reported in the literature involving working memory studies amongst schizophrenia patients.
Conclusions: As our study confirms the results obtained in earlier studies, in addition to pointing out some brain regions not reported in earlier studies, the findings are likely to serve as a cue for clinical investigation, leading to better medical intervention.
Keywords: functional Magnetic Resonance Imaging, fMRI, Schizophrenia, Working Memory, Group Independent Component Analysis, Classification, Computer-aided Diagnosis
Introduction
Schizophrenia is a psychological disorder that involves auditory and visual hallucinations and delusions. A schizophrenia patient often shows symptoms such as disorganized thinking, difficulty in speech, and abnormal motor behavior. Structural and functional changes occur in the brain due to various chemical alterations in the schizophrenic patient. These changes adversely impact behavioral, emotional and cognitive capabilities of a patient. Schizophrenia patients often experience deterioration or impairment in working memory (WM) 1. WM is a short-term memory of a person for perceiving things that relate to immediate consciousness that helps in language processing, decision making and reasoning 2, 3. It is an active and readily accessible mental state that maintains information and processes the information selectively 4.
The use of functional magnetic resonance imaging (fMRI) has facilitated the diagnosis and treatment of neurological and psychological disorders and enhanced our understanding of the brain. The blood oxygenation level-dependent (BOLD) technique has been widely used in fMRI studies; it relies on the effect of magnetic susceptibility of deoxyhaemoglobin. When a brain region is activated by a task, it demands an increased inflow of oxygenated blood and a net increase in signal intensity is observed. Various paradigms such as visual task and auditory oddball task have been designed to find the pattern of functioning of the brain during different cognitive processes. As the Sternberg item recognition paradigm (SIRP) task is a popular working memory task 5, 6, we have used the fMRI data of the subjects performing this task.
As fMRI data involves 3-D scans of the whole brain volume across time, it is inherently high dimensional. Independent component analysis (ICA) 7 is a popular method that can be applied on fMRI data to produce the temporally coherent brain networks. ICA is a data-driven approach that generates independent components without making any assumptions about the characteristics of the task and time courses. Group-ICA (GICA) is an extension of ICA that helps to analyze group fMRI studies. In this study, ICA is employed to find such independent networks that have significant differences in the regions between healthy subjects and schizophrenic patients affecting the working memory of a person.
In this study, we aim to identify the brain regions, potentially responsible for the working memory dysfunction, using fMRI data involving SIRP task. Towards this end, we have developed a decision model to differentiate between schizophrenia patients and healthy subjects (controls). We have applied group ICA to find the functionally connected components. We have demarcated the brain regions based on Automated Anatomical Labeling (AAL) atlas. In order to carry out the feature extraction, statistical measures are used to evaluate the significance of different regions. Finally, classification guided feature selection is done using support vector machine (SVM) and 1-NN classifiers.
Related Work
Several psychological, neurological, and computational studies 1, 6, 8– 12 have been conducted to identify the pattern of brain activation for different mental tasks in the schizophrenia patient. Park and Holzman 1 found that schizophrenia patients suffer a loss in representational processing, leading to working memory deficit. Impairment of performance in working memory tasks such as the Wisconsin Card-Sorting Test (WCST) is an important evidence of the dysfunction of frontal lobe amongst the schizophrenia patients. Gold et al. 11 studied the effect of schizophrenia on working memory dysfunction by performing WCST and letter-number (LN) span test on a group of 36 patients with schizophrenia and 30 healthy controls. They found that patients with schizophrenia showed poor performance on the WCST and LN span test, indicating the failure of working memory, typically attributed to frontal lobe dysfunction. Bertilino et al. 8 performed WCST on a population of 13 patients with schizophrenia and an equal number of healthy subjects to identify the relationship between neuronal pathology of the dorsolateral prefrontal cortex (DLPFC) and activation of working memory network in the cortical region. They found that the rate of N-acetylaspartate level in the DLPFC was firmly linked with the activation of the working memory cortical network during the working memory tasks in schizophrenia patients.
Some researchers experimented with other visual tasks like Sternberg Item Recognition Paradigm (SIRP) to evaluate the impact of schizophrenia on the working memory. Manoach et al. 6 performed the SIRP task on 12 schizophrenic and 10 healthy subjects. Using SIRP task in fMRI, they compared the activation of DLPFC between the patients and the healthy subjects. A high working memory load condition was compared with non-working memory condition as well as with low working memory load condition. They found that schizophrenia patients performed poorly in comparison to the healthy subjects under different load conditions. They also noted increased DLPFC activation in schizophrenics in comparison to healthy subjects during WM task. In another study, Manoach et al. 13 examined the participation of brain regions in WM performance by analyzing region-wise brain activations in fMRI data from nine schizophrenic subjects and an equal number of healthy subjects while performing a modified version of the SIRP task, which included a cash reward for correct responses. Again, they compared the high and the low working memory load conditions to each other, keeping the non-working memory condition as a baseline. It was seen that schizophrenic patients showed weak working memory performance along with activation in basal ganglia and thalamus. These regions were found to be activated only in the schizophrenia group. In an fMRI study involving 106 schizophrenic subjects and 111 healthy matched controls, Potkin et al. 12 examined the BOLD signal change in the DLPFC in a working memory study using SIRP task. They found significantly greater DLPFC activation in patients with schizophrenia. The activation was found to vary with variation in working memory load. The mean BOLD signal was also found to be higher during intermediate memory loads in schizophrenic subjects as compared to the healthy controls. Wible et al. 14 examined auditory hallucinations while performing the SIRP task in a group of 74 schizophrenic patients, subdivided into non-hallucinating and hallucinating groups. They found that the patients having auditory hallucinations showed decreased functional activity during the probe condition in working memory task mainly in the inferior parietal regions and superior temporal regions in comparison to those not having hallucinations.
ICA treats fMRI data as a linear combination of spatially independent components. These components derived from the fMRI data suggest the functional connectivity between brain regions (also called brain networks). Some of the fMRI studies 9 used general linear model (GLM) approach to convert 4D time-series data into a 3D statistical parametric map. Pearson’s correlation coefficient 15 and regional homogeneity 16 were also applied in fMRI study to extract information from temporal data. Kim et al. 17 used ICA to trace the temporally coherent networks in fMRI activity using a working memory task. Using the fMRI dataset for 115 patients with chronic schizophrenia and 130 healthy controls performing the SIRP task, they identified six components mainly showing disease-relevant brain networks. These components showed the regions that exhibited significant differences in the functioning of WM networks between schizophrenic patients and healthy controls. Two out of the six networks showed regions covering working memory areas such as bilateral DLPFC, inferior parietal lobules and cerebellum. They observed dysfunction in default mode network (DMN) in schizophrenia which exists across multiple subnetworks in the region. Correa et al. 10 also explored the role of ICA in the analysis of fMRI data. They compared the performance of different ICA algorithms and performed an analysis of fMRI data having visual-motor task and estimated activations using Infomax, FastICA, eigenvalue decomposition (EVD) and joint approximate diagonalization of eigen matrices (JADE). The authors concluded that the infomax performed quite well on the fMRI data and showed the highest t-values and successfully estimated maximally independent components.
Methods
Dataset
The fMRI data used in this study were downloaded from the Functional BIRN Data Repository ( http://fbirnbdr.birncommunity.org:8080/BDR/) 12. A detailed description of the data is available at the repository. In brief, all the acquisitions were carried out using 1.5T scanners keeping all other parameters same for all the subjects across the datasets. In this study, we have considered SIRP task fMRI data available at site 0009 and site 0010 of the FBIRN repository. All the three runs of each subject’s scan are used in our experiments. All subjects had regular hearing levels and sufficient eyesight to perform the SIRP task. They were able to perform the cognitive task. Healthy subjects were excluded if they had a current or past history of head injury and major medical illness. All the healthy subjects were free from any antipsychotic exposure and they had no recent history of medication effect. fMRI data from the patients with schizophrenia and schizoaffective disorder meeting the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria were included in this study. FBIRN had determined the symptom scores by using the Schedule for the Assessment of Positive Symptoms (SAPS) and Negative Symptoms assessment measures 5. Table 1 summarizes the database details.
Table 1. Demographic details of the dataset.
| Subject | No. of
subjects |
Age group,
years * |
Sex ratio,
male/female |
|---|---|---|---|
| Healthy | 34 | 40.4 ±12.29 | 24/10 |
| Schizophrenia | 34 | 40.3 ±10.89 | 17/17 |
*Data given as mean ± standard deviation.
Imaging parameters
The functional scans were acquired using T2*-weighted gradient echo planar imaging (EPI) sequences and were parameterized by Orientation: anterior commissure-posterior commissure line; the number of slices: 27; slice thickness: 4 mm; TR: 2 seconds; time to echo: 40 ms; matrix: 64 × 64; field of view: 22 cm; and flip angle: 90° 12.
Task details
In this paper, we have considered the Sternberg item recognition paradigm (SIRP) task 18, 19. The SIRP is a block design task that assesses the maintenance and scanning components of WM 4, 19. Each phase began with the presentation of a memory set composed of one, three, or five digits, constituting three levels of WM load (low 1L, medium 3L, high 5L). This encode phase was followed by the presentation of 14 probe digits. Participants responded to each probe using a button box to indicate whether the probe digit was in the memory set. Each of the three runs contained two blocks of each of the three load phases, presented in a pseudorandom order with the blocks of each phase alternating with fixation epochs (a baseline resting period). Each run lasted for 6 minutes.
Data preprocessing
For preprocessing the raw fMRI datasets taken from FBIRN repository, we have used the Statistical Parametric Mapping version 8 (SPM8, Wellcome Trust Centre for Neuroimaging, University College London, UK) 20 toolbox in Matlab. The preprocessing steps are as follows. Realignment and reslicing were performed on each of the images using the default parameters. Slice timing correction was applied to correct possible errors introduced by temporal variations during the acquisition of fMRI data. Subsequently, the fMRI scans were spatially normalized into the standard Montreal Neurological Institute (MNI) space using an EPI template. Thus, the volume of each voxel in raw fMRI scans changed from 3.4 × 3.4 × 4 mm 3 to 3 × 3 × 3 mm 3. This resulted in a brain volume of 53 × 63 × 46 voxels. Finally, spatial smoothing was done using a 9 × 9 × 9 mm 3 full width at half-maximum (FWHM) Gaussian kernel on the normalized volumes to get the smoothed volumes.
Proposed approach
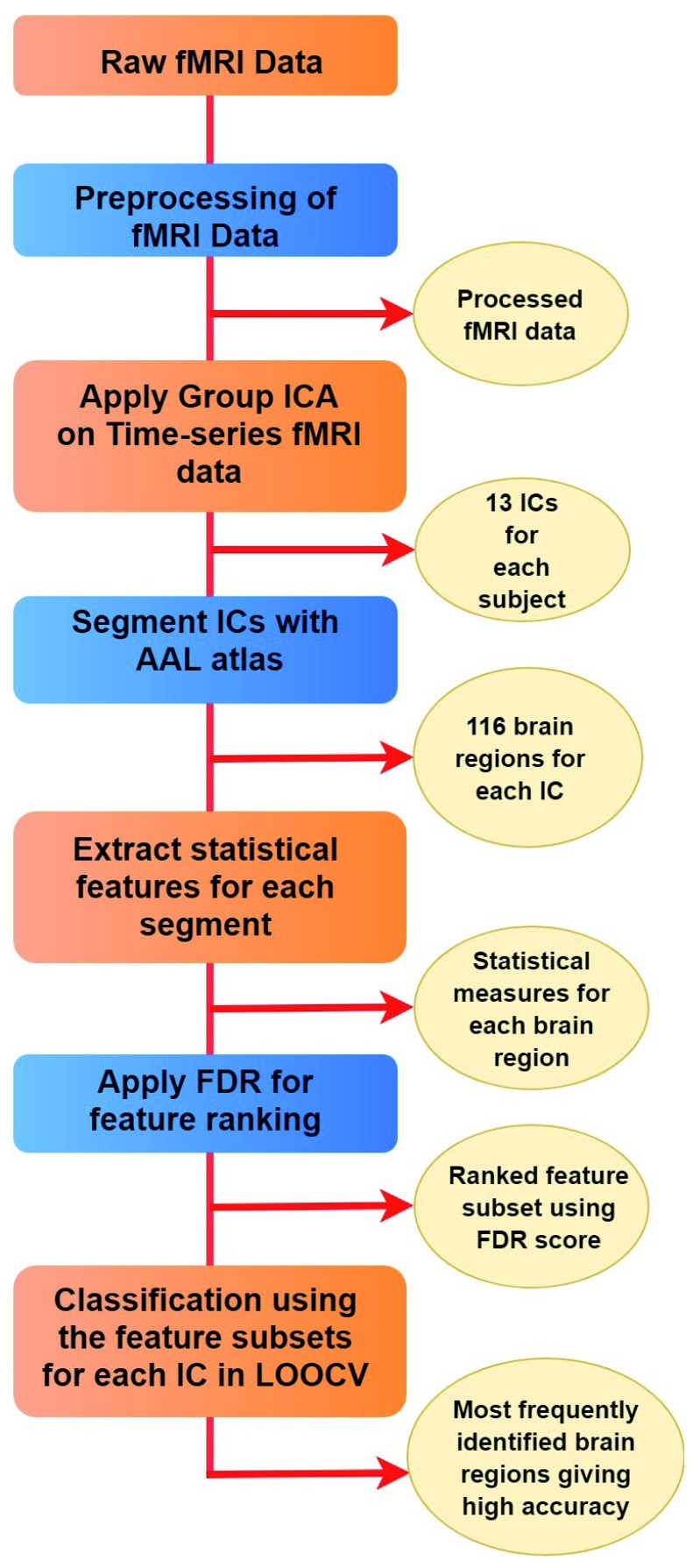
The proposed approach is divided into the following phases: (i) application of group ICA; (ii) statistical feature extraction; (iii) classification guided feature selection; and (iv) visualization. These phases are described in the following sub-sections. The proposed approach is applied to individual ICs. The stepwise description of the proposed approach is outlined in Algorithm 1. Figure 1 shows the overall workflow of the study.
Algorithm 1. The proposed approach.
1. Application of group ICA:
-
(a)
Apply GICA on the pre-processed fMRI data, where, the modified MDL criteria is used to identify the number of IC.
2. Feature Extraction:
-
(a)
Segment each IC for each subject in 116 regions using AAL atlas.
-
(b)
Extract five statistical features namely, mean, standard deviation, kurtosis, skewness and entropy from each region for every subject on the basis of voxel values of that particular region. Thus a subject is represented as 580 features (=116 x 5). The dataset is represented as
where is the i th feature.
3. Feature Selection:
-
(a)
Carry out feature selection in LOOCV manner. In i th fold of LOOCV, all, but i th sample is used for training.
-
(b)
Compute FDR score for each feature using equation mentioned in Section 4.2.4.
-
(c)
Rank the features on the basis of FDR score (the feature with highest FDR score is assigned rank 1).
-
(d)
Build decision model (DM) incrementally (forward feature selection) using SVM classifier. Begin by building the DM with the first ranked feature and add the FDR ranked features, one by one, to obtain the high classification accuracy.
4. Visualization:
-
(a)
Identify the set of features having maximum classification accuracy.
-
(b)
Backtrack the features to the MNI brain space to locate the affected brain regions.
Figure 1. Stepwise representation of the proposed approach.
Application of ICA
The BOLD fMRI technique acquires 3-D brain volumes across time. Each voxel in the whole brain volume contains a value that corresponds to the change of signal intensity of the voxel across time. To identify the connected brain networks that are activated while performing a task, we applied group ICA (GICA) using the GIFT toolbox v.4.0b 21 in MATLAB. There are three main stages in GICA: data compression (also called data reduction), ICA, and back reconstruction. In the data compression step, principal components analysis (PCA) is used to reduce the size of the data. Group PCA is applied to all subjects. Then, ICA is used to find the independent components (ICs) and the spatial maps. Although several ICA algorithms, such as Infomax, FastICA, Jade, and AMUSE, are available in the GIFT toolbox, we use the most widely used Informax algorithm to find the ICs. The Infomax algorithm 22 uses a non-linear function to maximize the information transfer from the input layer to the output layer of a network. The components resulting from ICA represent the brain networks activated during the task. The back-reconstruction step produces the ICs with the most accurate spatial maps and time courses for each subject.
In our experiments, the number of ICs was estimated using the modified minimum description length (MDL) 23 criteria, which generated 13 ICs. We have used the average ICs spatial map for each subject corresponding to the three runs. In the proposed approach, we have analyzed 13 ICs independently for each subject.
Segmentation of ICs
In the first phase, we have segmented each of the 13 ICs for each subject using Automated Anatomical Labeling (AAL) 24 atlas. AAL atlas segments the whole brain volume into 116 brain regions. Thereafter, subject-wise features were extracted from each of these 116 regions for each IC.
Feature extraction
For dimensionality reduction, we have extracted five statistical features for each brain region of each subject, namely, mean, standard deviation (std), skewness, kurtosis, and entropy. If V r = [ v 1, v 2, v 3, ..., v N] is the voxel set having N voxels for r th region, then these statistical measures are defined as follows:
Thus, for each subject, we extracted 580 (= 116 × 5) features. To identify the features relevant for identifying affected brain regions in schizophrenia, we carried out feature selection.
Classification guided feature selection
Feature selection is a process of selecting a relevant subset of the feature set. In this paper, we have incorporated the classification guided sequential forward feature selection method in a leave-one-out cross-validation (LOOCV) manner. In the sequential forward selection, one adds the best features in every iteration, until the best classification accuracy is achieved. We have used Fisher’s discriminant ratio (FDR) score for ranking each feature. FDR score was computed using the formula,
where
x = vector containing the feature x values corresponding to all subjects,
mean h = mean of feature x corresponding to healthy patients;
mean s = mean of feature x corresponding to schizophrenic patients;
var h = variance of feature x corresponding to healthy patients;
var s = variance of feature x corresponding to schizophrenic patients.
After scoring all the features, the scores were arranged in descending order. The high value of FDR indicates that the within-class scatter is low, while between-class scatter/variance is high. Forward feature selection approach is employed to identify the feature set generating high classification accuracy.
Classification
Several works 9, 25– 27 have attempted to identify the affected brain regions using a decision model to classify schizophrenia patients and healthy controls. In this paper, classification task is performed on the combined data involving the healthy and schizophrenic subjects using linear SVM and k - nearest neighbors (k-NN) classifiers. Classification is done in LOOCV manner i.e., training is done on all the subjects excluding one subject, which is used for testing. The classification model is built incrementally. Finally, the feature subset yielding high classification accuracy for a given test sample is chosen.
Each sample is an input vector X i ( i = 1, 2, 3, ..., n) having features selected from the statistical measure of a particular region (set of voxels) and is associated with one of the two classes Y i = +1 or Y i = -1 (binary class). The class labels +1 and -1 refers to the positive class (schizophrenia) and the negative class respectively. For classification using SVM, the libsvm version 3.23 28 package in Matlab-2014b is used that uses C-SVC. Besides setting all the training parameters as default, we experiment with the different values of cost parameter ( C), varying C in the range of 0.01 to 1000 in powers of 10. For k-NN classifier, we take the value of k as 1 and use Euclidean distance as the distance metric.
Visualization
The set of selected features, obtained after all the iterations of LOOCV approach for each IC, were backtracked to brain space to identify the affected brain regions. In order to find the most relevant regions that may contribute to the dysfunction of the working memory in the schizophrenia patients, the brain regions identified by the proposed approach, marked by different independent components, were coalesced. The frequently occurring regions were plotted on a mask using WFU PickAtlas. The mask was then overlaid onto a standard T1-weighted MRI using MANGO version 4.0.1 toolbox 29. These identified brain regions were overlaid onto a standard T-1 weighted image and visualized MANGO toolbox.
Results
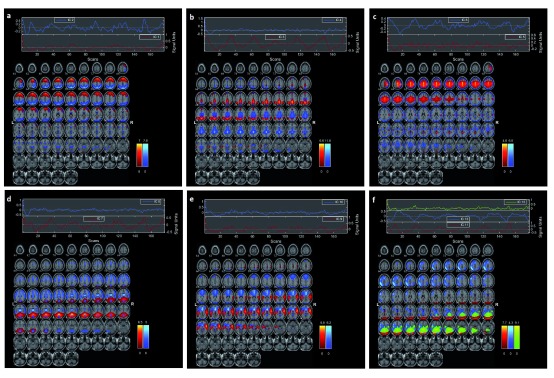
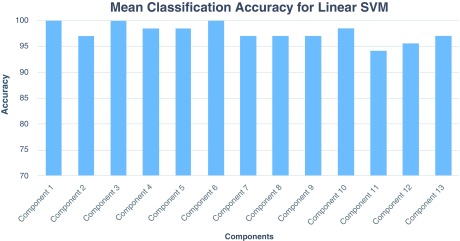
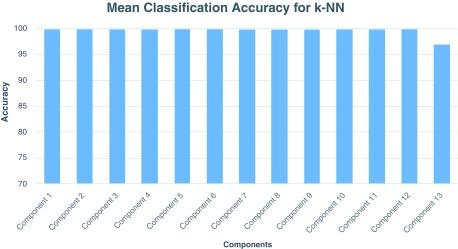
The first phase of the proposed method resulted in 13 spatial ICs (see Figure 2). Figure 2a–f shows the composite view of multiple independent components showing functionally connected brain regions involved during the task. The figure highlights task-related components with functional differences across healthy and schizophrenia subjects. We have used the forward feature selection method in the second phase. The average classification accuracy using LOOCV scheme for SVM and k-NN classifiers for each IC is shown in Figure 3 and Figure 4, respectively. Overall, the linear SVM classifier ( C=1.09) yielded classification accuracy in the range 94% to 100%. Similarly, the use of k-NN classifier resulted in classification accuracy in the range of 96–100% (see Figure 4 for linear SVM and Figure 5 for 1-NN classifier). Finally, the affected brain regions identified from the visualization phase are mentioned in Table 2 for each spatial IC. Table 2 shows the regions marked by increased activation in case of schizophrenia patients when compared to the healthy controls. Figure 5 (a–c)) shows the identified regions such as the cerebellum, temporal and frontal gyrus, insula, amygdala, cuneus, putamen, Heschl gyrus, and vermis.
Figure 2. Composite images showing the 13 independent components across all the subjects.
The connected brain networks identified by ( A) independent component (IC) 1–IC 2, ( B) IC 3–IC 4, ( C) IC 5–IC 6, ( D) IC 7–IC 8, ( E) IC 9–IC 10, and ( F) IC 11–IC 13, are shown.
Figure 3. Mean classification accuracy with linear SVM classifier for each independent component across 10 different runs.
Figure 4. Mean classification accuracy with k-NN classifier (k=1) for each independent component across 10 different runs.
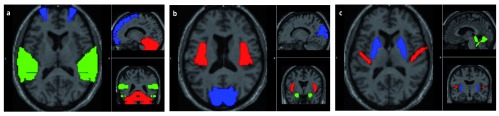
Figure 5. The most distinct regions identified in brain responsible for dysfunction in schizophrenia are shown.
( A) shows the cerebellum (red), inferior and superior temporal gyrus (green), and superior frontal gyrus (blue); ( B) shows the insula (red), amygdala (green), and cuneus (blue); ( C) shows the Heschl gyrus (red), vermis (green) and putamen (blue).
Table 2. Brain regions identified by the proposed approach for each independent component (IC).
| IC 1 | IC 2 | IC 3 | IC 4 | IC 5 | IC 6 | IC 7 | IC 8 | IC 9 | IC 10 | IC 11 | IC 12 | IC 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala_L | Amygdala_L | Amygdala_L | Amygdala_R | Amygdala_L | Amygdala_L’ | Amygdala_L | Amygdala_L | Amygdala_R | Amygdala_R | Amygdala_R | Amygdala_L | Amygdala_L’ |
| Amygdala_R | Angular_L | Angular_L | Angular_R | Angular_L | Angular_L | Angular_L | Angular_R | Angular_R | Angular_R | Calcarine_L | Angular_R | Angular_L |
| Angular_L | Calcarine_L | Calcarine_R | Calcarine_R | Calcarine_L | Caudate_R | Calcarine_R | Calcarine_R | Calcarine_R | Caudate_L | Caudate_R | Calcarine_R | Cerebelum_10_L |
| Cerebelum_
10_L |
Caudate_L | Cerebelum_10_R | Cerebelum_10_L | Caudate_R | Cerebelum_10_R | Caudate_R | Caudate_L | Cerebelum_9_R | Cerebelum_10_R | Cerebelum_
10_L |
Caudate_R | Cerebelum_Crus1_L |
| Cerebelum_
10_R |
Cerebelum_10_L | Cerebelum_Crus1_L | Cerebelum_Crus1_L | Cerebelum_
10_L |
Cerebelum_
Crus1_R |
Cerebelum_10_L | Cerebelum_10_L | Cerebelum_
Crus2_R |
Cerebelum_Crus1_L | Cerebelum_6_R | Cerebelum_10_L | Frontal_Inf_
Oper_L |
| Cerebelum_6 | Cerebelum_Crus1_L | Cingulum_Ant_L | Cingulum_Ant_L | Cerebelum_
3_L |
Cingulum_Ant_R | Cerebelum_Crus1_R | Cerebelum_
Crus1_R |
Cingulum_Post_R | Cuneus_L | Cerebelum_
Crus1_L |
Cerebelum_6_R | Frontal_Med_Orb_L |
| Cerebelum_
6_R |
Cingulum_Ant_L | Cingulum_Mid_L | Cingulum_Post_R | Cingulum_Ant_L | Cuneus_R | Cuneus_L | Cingulum_
Ant_L |
Cuneus_R | Frontal_Inf_
Oper_L |
Cingulum_Ant_R | Cerebelum_
Crus1_L |
Frontal_Mid_R |
| Cerebelum_
9_L |
Cingulum_Mid_L | Cingulum_Post_L | Cuneus_L | Cuneus_R | Frontal_Inf_Orb_L | Frontal_Med_Orb_L | Cingulum_Post_L | Frontal_Inf_Tri_L | Frontal_Inf_Tri_R | Cuneus_R | Cingulum_Ant_R | Frontal_Sup_
Medial_R |
| Cerebelum_
Crus1 |
Cingulum_Post_L | Cuneus_R | Frontal_Inf_Oper_L | Frontal_Inf_
Oper_L |
Frontal_Mid_L | Frontal_Mid_Orb_L | Cingulum_Post_R | Frontal_Mid_
Orb_L |
Frontal_Mid_Orb_R | Frontal_Inf_Oper_L | Frontal_Inf_Orb_R | Hippocampus_L |
| Cuneus_R | Cuneus_L | Frontal_Inf_Oper_L | Frontal_Med_Orb_R | Frontal_
Med_Orb_L |
Frontal_Sup_
Medial_L |
Frontal_Sup_L | Cuneus_R | Frontal_Sup_R | Fusiform_L | Frontal_Med_Orb_R | Frontal_Med_Orb_L | Insula_L |
| Frontal_
Med_Orb_L |
Frontal_Inf_Oper_L | Frontal_Med_Orb_L | Frontal_Sup_R | Frontal_Mid_
Orb_L |
Heschl_R | Fusiform_R | Frontal_Inf_
Oper_L |
Fusiform_R | Heschl_L | Frontal_Sup_L | Frontal_Mid_Orb_L | Lingual_L |
| Frontal_Sup_
Medial_R |
Frontal_Inf_Orb_L | Frontal_Mid_L | Fusiform_L | Fusiform_L | Hippocampus_R | Heschl_R | Frontal_Inf_Tri_R | Heschl_L | Heschl_R | Fusiform_R | Frontal_Sup_L | Occipital_Inf_L |
| Frontal_
Sup_R |
Frontal_Med_Orb_L | Frontal_Sup_L | Heschl_R | Heschl_L | Occipital_Mid_R | Lingual_R | Frontal_Med_
Orb_R |
Hippocampus_R | Hippocampus_R | Heschl_R | Fusiform_R | Occipital_Sup_R |
| Fusiform_R | Frontal_Mid_L | Fusiform_L | Hippocampus_R | Insula_R | Olfactory_L | Occipital_Mid_R | Frontal_Sup_L | Insula_R | Lingual_R | Hippocampus_R | Heschl_R | Olfactory_L |
| Heschl_L | Frontal_Sup_L | Heschl_R | Insula_R | Olfactory_L | Parietal_Sup_R | ParaHippocampal_R | Fusiform_R | Occipital_Inf_R | Occipital_Inf_L | Insula_L | Hippocampus_R | Pallidum_R |
| Insula_L | Fusiform_L | Hippocampus_R | Occipital_Inf_L | Pallidum_L | Precuneus_R | Parietal_Inf_L | Heschl_L | Occipital_Sup_R | Occipital_Sup_R | Occipital_Inf_R | Insula_L | Paracentral_
Lobule_R |
| Occipital_
Inf_R |
Heschl_L | Insula_L | Pallidum_R | Paracentral_
Lobule_R |
Rectus_R | Precuneus_R | Hippocampus_R | Pallidum_R | Paracentral_
Lobule_R |
Pallidum_L | Occipital_Inf_L | Parietal_Sup_R |
| Occipital_
Sup_R |
Hippocampus_R | Lingual_L | Paracentral_Lobule_R | Postcentral_L | Temporal_Inf_L | Putamen_R | Insula_L | Paracentral_
Lobule_R |
ParaHippocampal_R | Paracentral_
Lobule_L |
Occipital_Sup_R | Precuneus_L |
| Precuneus_L | Insula_L | Occipital_Inf_R | ParaHippocampal_L | Putamen_R | Temporal_Mid_R | Supp_Motor_Area_R | Insula_R | Parietal_Sup_L | Parietal_Inf_L | Parietal_Inf_R | Olfactory_L | Putamen_R |
| Temporal_
Inf_R |
Lingual_L | Occipital_Mid_L | Parietal_Sup_L | Rectus_L | Thalamus_L | SupraMarginal_R | Occipital_Inf_L | Postcentral_L | Postcentral_R | Precuneus_R | Paracentral
_Lobule_L |
Rectus_L |
| Temporal_
Pole_Mid_R |
Occipital_Inf_L | Occipital_Sup_L | Putamen_L | Rectus_R | Vermis_1_2 | Temporal_
Pole_Mid_L |
Occipital_Sup_R | Putamen_R | Precuneus_R | Putamen_R | ParaHippocampal_L | Supp_Motor
_Area_L |
| Vermis_1_2 | Occipital_Mid_L | Olfactory_R | Supp_Motor_Area_R | Temporal_
Inf_L |
Thalamus_L | Pallidum_R | Rolandic_Oper_L | Putamen_L | Rectus_R | Parietal_Sup_R | SupraMarginal_R | |
| Vermis_6 | Occipital_Sup_R | Pallidum_R | Temporal_Inf_L | Thalamus_R | Vermis_3 | Paracentral_
Lobule_L |
Supp_Motor_
Area_R |
Rectus_L | SupraMarginal_L | Postcentral_L | Temporal_Inf_L | |
| Olfactory_L | Paracentral_
Lobule_R |
Thalamus_L | Vermis_1_2 | Parietal_Inf_L | SupraMarginal_R | Rolandic_Oper_R | Temporal_Mid_R | Precuneus_L | Temporal_Mid_L | |||
| Pallidum_L | ParaHippocampal_R | Vermis_3 | Precentral_L | Temporal_Mid_L | SupraMarginal_L | Temporal_Sup_R | Putamen_R | Temporal_Sup_R | ||||
| Paracentral_Lobule_L | Parietal_Inf_R | Precentral_R | Temporal_Sup_R | Temporal_Inf_L | Thalamus_L | Rectus_L | Thalamus_L | |||||
| ParaHippocampal_L | Parietal_Sup_R | Supp_Motor_Area_R | Thalamus_R | Temporal_Pole_Mid_L | Vermis_1_2 | Supp_Motor_Area_R | Vermis_1_2 | |||||
| Parietal_Inf_L | Precentral_L | SupraMarginal_L | Vermis_1_2 | Temporal_Sup_L | Vermis_6 | SupraMarginal_L | Vermis_3 | |||||
| Parietal_Sup_L | Precuneus_L | Temporal_Inf_R | Vermis_10 | Vermis_1_2 | Temporal_Mid_R | Vermis_6 | ||||||
| Postcentral_L | Putamen_L | Temporal_Sup_R | Vermis_6 | Vermis_6 | Temporal_Sup_L | |||||||
| Precentral_L | Rolandic_Oper_L | Thalamus_L | Thalamus_L | |||||||||
| Precuneus_R | Supp_Motor_Area_R | Vermis_3 | Vermis_1_2 | |||||||||
| Putamen_L | SupraMarginal_R | Vermis_6 | Vermis_6 | |||||||||
| Rolandic_Oper_L | Temporal_Inf_L | |||||||||||
| Supp_Motor_Area_L | Temporal_Mid_R | |||||||||||
| SupraMarginal_L | Thalamus_R | |||||||||||
| Temporal_Inf_L | Vermis_1_2 | |||||||||||
| Temporal_Mid_L | ||||||||||||
| Thalamus_L | ||||||||||||
| Vermis_1_2 |
Discussion
This study aimed to identify affected brain regions in the working memory of schizophrenia patients. To achieve this, we proposed a model wherein we utilized the GICA to obtain spatial ICs, extracted statistical features from 116 brain regions, selected features using a classifier-guided forward feature selection approach, and visualization of affected brain regions. Using the proposed approach, we marked the differences in the functional activation of the following brain regions in most of the ICs (the cerebellum, inferior temporal gyrus, superior temporal gyrus, superior frontal gyrus, Heschl gyrus, insula, amygdala, vermis, thalamus, calcarine, occipital lobe and hypocampus) in schizophrenia patients in comparison to healthy controls. It may be noted that the brain regions identified in this study are largely in conformity with the previous studies 30– 34. Further, the connected brain regions discovered by the different spatial ICs, largely confirm to each other.
Our results show the functional changes in the cerebellum region. Previous studies 32, 33 also suggest some changes in cortical cerebellar regions and its functional connectivity in working memory performance in schizophrenia patients. We found functional changes in the inferior temporal gyrus, superior temporal gyrus and superior frontal gyrus. While earlier studies 32, 35, 36 suggest changes in activation and abnormal functional connectivity in temporal and frontal gyri in schizophrenia patients compared to healthy subjects, our results show changes in the Heschl gyrus region. In support of our finding, we may note that in a study by Hirayasu et al. 30, they found structural volume reduction in Heschl gyri in schizophrenia patients. Grey matter atrophy in Heschl gyri was also found in a study by Kasai et al. 31. In this regard, we may say that the study of relationships between the functional activations in the relating to working memory dysfunction and structural brain changes in Heschl gyrus could be an important direction of research. We find significant changes in the insula region of the schizophrenic brain similar to the result of other previous studies 37, 38. Our results also show functional changes in the amygdala region. While performing working memory tasks, evidence of the dysfunction or abnormalities in the amygdala in schizophrenia patients were found in several previous studies 39– 42. We also found some changes in the functional activation in the vermian area, specifically in cerebellar vermis. Although, some literature 43, 44 reports structural changes in the vermis region, our study may be a cue for further research based on ROI study to trace the changes in this region associated with schizophrenia and working memory task.
In addition, we obtained a high classification accuracy (>95%) to distinguish healthy subjects and schizophrenic. The high classification accuracy obtained by applying the proposed approach proves its efficacy in comparison to the other fMRI studies 17, 45, 46. Overall, the proposed approach found to be effective and efficient in the identification of affected brain regions responsible for working memory dysfunction in schizophrenia.
Conclusion
In this fMRI study, based on working memory task, a feature selection scheme has been proposed to identify the brain regions affected amongst the schizophrenia patients. This study helps in the identification of brain regions responsible for impairment of working memory in schizophrenia patients. While many connected brain regions identified in our study confirm the findings of the previous studies, the results reveal some new regions in the brain which have not been reported till date in the working memory literature for schizophrenia. These regions may play role in dysfunction of working memory in the patients and could be the subject of further studies.
Data availability
The SIRP task fMRI data from the FBIRN phase II repository can be downloaded from http://schizconnect.org/queries/new, querying 1.5T fMRI data for healthy and schizophrenia subjects available at site 0009 and 0010. The list of subjects chosen for this study is mentioned in the ‘dataset_SubjectID_list.txt’ file available with the codes. Users are required to sign-up to SchizConnect to download data; conditions of use are as written in the data use agreement of the FBIRN project.
To download the data used in this study, the user has to select the project as ‘Study: fBIRNPhaseII__0010’, add ‘AND’, and select MRI as ‘Field Strength: 1.5’.
Software availability
The complete source code is archived at: https://doi.org/10.5281/zenodo.2528773 47.
License: Creative Commons Zero v1.0 Universal.
Acknowledgements
Data used in this work are taken from the Functional Biomedical Informatics Research Networks (FBIRN) data repository, under the following support: for function data, U24-RR021992, Function BIRN, and U24 GM104203, Bio-Informatics Research Network Coordinating Centre (BIRN-CC). The data were obtained from the Function BIRN Data Repository, Project Accession Number 2007-BDR-6UHZ1.
Funding Statement
This work was supported by the research fellowship of Indranath Chatterjee from Council of Scientific and Industrial Research (CSIR), India having grant number 09/045(1323)/2014-EMR-I.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49(12):975–982. 10.1001/archpsyc.1992.01820120063009 [DOI] [PubMed] [Google Scholar]
- 2. Conway A, Jarrold C, Kane M: Variation in working memory. Oxford University Press,2008. 10.1093/acprof:oso/9780195168648.001.0001 [DOI] [Google Scholar]
- 3. Miller GA, Galanter E, Pribram KH: Plans and the structure of behavior. Adams Bannister Cox,1986. Reference Source [Google Scholar]
- 4. Baddeley A: Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–39. 10.1038/nrn1201 [DOI] [PubMed] [Google Scholar]
- 5. Kim DI, Mathalon DH, Ford JM, et al. : Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35(1):67–81. 10.1093/schbul/sbn133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manoach DS, Press DZ, Thangaraj V, et al. : Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45(9):1128–1137. 10.1016/S0006-3223(98)00318-7 [DOI] [PubMed] [Google Scholar]
- 7. Common P: Independent component analysis, a new concept? Signal processing. 1994;36(3):287–314. 10.1016/0165-1684(94)90029-9 [DOI] [Google Scholar]
- 8. Bertolino A, Esposito G, Callicott JH, et al. : Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry. 2000;157(1):26–33. 10.1176/ajp.157.1.26 [DOI] [PubMed] [Google Scholar]
- 9. Chatterjee I, Agarwal M, Rana B, et al. : Bi-objective approach for computer-aided diagnosis of schizophrenia patients using fMRI data. Multimed Tools Appl. 2018;77(20):26991–27015. 10.1007/s11042-018-5901-0 [DOI] [Google Scholar]
- 10. Correa N, Adali T, Calhoun VD: Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magn Reson Imaging. 2007;25(5):684–694. 10.1016/j.mri.2006.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold JM, Carpenter C, Randolph C, et al. : Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54(2):159–165. 10.1001/archpsyc.1997.01830140071013 [DOI] [PubMed] [Google Scholar]
- 12. Potkin SG, Turner JA, Brown GG, et al. : Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35(1):19–31. 10.1093/schbul/sbn162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manoach DS, Gollub RL, Benson ES, et al. : Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. 10.1016/S0006-3223(00)00227-4 [DOI] [PubMed] [Google Scholar]
- 14. Wible CG, Lee K, Molina I, et al. : fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophr Bull. 2009;35(1):47–57. 10.1093/schbul/sbn142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chyzhyk D, Savio A, Graña M: Computer aided diagnosis of schizophrenia on resting state fMRI data by ensembles of ELM. Neural Netw. 2015;68:23–33. 10.1016/j.neunet.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 16. Zang Y, Jiang T, Lu Y, et al. : Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- 17. Kim DI, Manoach DS, Mathalon DH, et al. : Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30(11):3795–3811. 10.1002/hbm.20807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown GG, McCarthy G, Bischoff-Grethe A, et al. : Brain-performance correlates of working memory retrieval in schizophrenia: a cognitive modeling approach. Schizophr Bull. 2008;35(1):32–46. 10.1093/schbul/sbn149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sternberg S: Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci. 1969;57(4):421–457. [PubMed] [Google Scholar]
- 20. Penny WD, Friston KJ, Ashburner JT, et al. : Statistical parametric mapping: the analysis of functional brain images. Elsevier,2011. 10.1016/B978-0-12-372560-8.X5000-1 [DOI] [Google Scholar]
- 21. Rachakonda S, Egolf E, Correa N, et al. : Group ica of fmri toolbox (gift) manual. Dostupn´e z [cit 2011-11-5];2007. Reference Source [Google Scholar]
- 22. Bell AJ, Sejnowski TJ: The "independent components" of natural scenes are edge filters. Vision Res. 1997;37(23):3327–3338. 10.1016/S0042-6989(97)00121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li YO, Adalı T, Calhoun VD: Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28(11):1251–1266. 10.1002/hbm.20359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 25. Caprihan A, Pearlson GD, Calhoun VD: Application of principal component analysis to distinguish patients with schizophrenia from healthy controls based on fractional anisotropy measurements. NeuroImage. 2008;42(2):675–682. 10.1016/j.neuroimage.2008.04.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du W, Calhoun VD, Li H, et al. : High classification accuracy for schizophrenia with rest and task FMRI data. Front Hum Neurosci. 2012;6:145. 10.3389/fnhum.2012.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viviani R, Grön G, Spitzer M: Functional principal component analysis of fMRI data. Hum Brain Mapp. 2005;24(2):109–29. 10.1002/hbm.20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang CC, Lin CJ: LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems and Technology. 2011;2(3):27 10.1145/1961189.1961199 [DOI] [Google Scholar]
- 29. Lancaster JL, Laird AR, Eickhoff SB, et al. : Automated regional behavioral analysis for human brain images. Front Neuroinform. 2012;6:23. 10.3389/fninf.2012.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirayasu Y, McCarley RW, Salisbury DF, et al. : Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57(7):692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kasai K, Shenton ME, Salisbury DF, et al. : Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. 10.1001/archpsyc.60.8.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer-Lindenberg A, Poline JB, Kohn PD, et al. : Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. 10.1176/appi.ajp.158.11.1809 [DOI] [PubMed] [Google Scholar]
- 33. Schlösser R, Gesierich T, Kaufmann B, et al. : Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. NeuroImage. 2003;19(3):751-63. 10.1016/S1053-8119(03)00106-X [DOI] [PubMed] [Google Scholar]
- 34. Chatterjee I: Mean deviation based identification of activated voxels from time-series fMRI data of schizophrenia patients [version 2; referees: 2 approved]. F1000Res. 2018, 2018;7:1615 10.12688/f1000research.16405.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrity AG, Pearlson GD, McKiernan K, et al. : Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. 10.1176/ajp.2007.164.3.450 [DOI] [PubMed] [Google Scholar]
- 36. Stevens AA, Goldman-Rakic PS, Gore JC, et al. : Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry. 1998;55(12):1097–1103. 10.1001/archpsyc.55.12.1097 [DOI] [PubMed] [Google Scholar]
- 37. Glahn DC, Ragland JD, Abramoff A, et al. : Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–9. 10.1002/hbm.20138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan HY, Choo WC, Fones CS, et al. : fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162(10):1849–1858. 10.1176/appi.ajp.162.10.1849 [DOI] [PubMed] [Google Scholar]
- 39. Anticevic A, Repovs G, Corlett PR, et al. : Negative and nonemotional interference with visual working memory in schizophrenia. Biol Psychiatry. 2011;70(12):1159–1168. 10.1016/j.biopsych.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 40. Gruber O, Tost H, Henseler I, et al. : Pathological amygdala activation during working memory performance: Evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp. 2010;31(1):115–125. 10.1002/hbm.20849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meda SA, Stevens MC, Folley BS, et al. : Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS One. 2009;4(11):e7911. 10.1371/journal.pone.0007911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seidman LJ, Thermenos HW, Poldrack RA, et al. : Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res. 2006;85(1–3):58–72. 10.1016/j.schres.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 43. Levitt JJ, McCarley RW, Nestor PG, et al. : Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156(7):1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tran KD, Smutzer GS, Doty RL, et al. : Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155(9):1288–1290. 10.1176/ajp.155.9.1288 [DOI] [PubMed] [Google Scholar]
- 45. Arbabshirani MR, Kiehl KA, Pearlson GD, et al. : Classification of schizophrenia patients based on resting-state functional network connectivity. Front Neurosci. 2013;7:133. 10.3389/fnins.2013.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang H, Liu J, Sui J, et al. : A Hybrid Machine Learning Method for Fusing fMRI and Genetic Data: Combining both Improves Classification of Schizophrenia. Front Hum Neurosci. 2010;4:192. 10.3389/fnhum.2010.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chatterjee I: GICA supported region-based feature selection technique for fMRI data. (Version v1.0). Zenodo. 2018. 10.5281/zenodo.2528773 [DOI] [Google Scholar]