Abstract
Intensive care unit–acquired weakness (ICU-AW) is the most common neuromuscular impairment in critically ill patients. We discuss critical aspects of ICU-AW that have not been completely defined or that are still under discussion. Critical illness polyneuropathy, myopathy, and muscle atrophy contribute in various proportions to ICU-AW. Diagnosis of ICU-AW is clinical and is based on Medical Research Council sum score and handgrip dynamometry for limb weakness and recognition of a patient’s ventilator dependency or difficult weaning from artificial ventilation for diaphragmatic weakness (DW). ICU-AW can be caused by a critical illness polyneuropathy, a critical illness myopathy, or muscle disuse atrophy, alone or in combination. Its diagnosis requires both clinical assessment of muscle strength and complete electrophysiological evaluation of peripheral nerves and muscles. The peroneal nerve test (PENT) is a quick simplified electrophysiological test with high sensitivity and good specificity that can be used instead of complete electrophysiological evaluation as a screening test in non-cooperative patients. DW, assessed by bilateral phrenic nerve magnetic stimulation or diaphragm ultrasound, can be an isolated event without concurrent limb muscle involvement. Therefore, it remains uncertain whether DW and limb weakness are different manifestations of the same syndrome or are two distinct entities. Delirium is often associated with ICU-AW but a clear correlation between these two entities requires further studies. Artificial nutrition may have an impact on ICU-AW, but no study has assessed the impact of nutrition on ICU-AW as the primary outcome. Early mobilization improves activity limitation at hospital discharge if it is started early in the ICU, but beneficial long-term effects are not established. Determinants of ICU-AW can be many and can interact with each other. Therefore, future studies assessing early mobilization should consider a holistic patient approach with consideration of all components that may lead to muscle weakness.
Keywords: Muscle weakness, ICU-acquired weakness, Critical Illness Polyneuropathy, Critical Illness Myopathy, muscle atrophy CRIMYNE, Critical Illness Polyneuromyopathy
Introduction
Intensive care unit–acquired weakness (ICU-AW), defined as “clinically detected weakness in critically ill patients in whom there is no plausible etiology other than critical illness” 1, is the most common neuromuscular impairment and it affects the clinical course and outcomes of ICU patients 2. ICU-AW is detected in 30 to 50% of patients and the incidence is even higher (up to 67%) in critically ill patients with sepsis 3. ICU-AW is associated with difficulty in weaning from the ventilator, prolonged ICU stay, and higher hospitalization charges and increases long-term morbidity and mortality 4, 5.
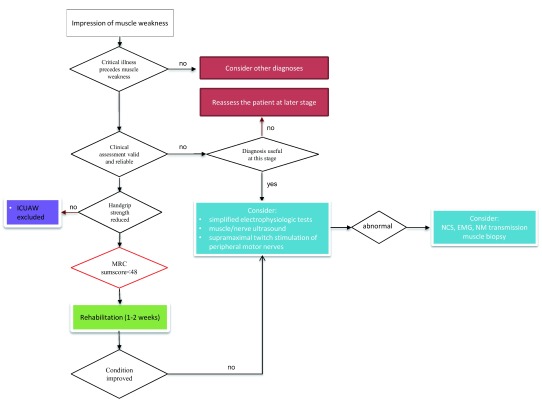
The term ICU-AW does not describe the condition accurately since muscle weakness is not limited to patients admitted to the ICU; indeed, it likely represents “the extreme end of a spectrum of weakness that begins with any serious illness regardless of care location” 6. By definition, ICU-AW is diagnosed after the onset of critical illness, which represents an important criterion to differentiate ICU-AW from Guillain–Barré syndrome or other acute neuromuscular disorders that may cause respiratory failure and ICU admission ( Figure 1 and Table 1) 7, 8. Weakness is symmetrical and affects all four limbs and the respiratory muscles with sparing of the facial muscles. The muscle tone is almost invariably reduced, but deep tendon reflexes can be either reduced or normal. The diaphragm is often involved, leading to prolonged mechanical ventilation and difficult weaning. ICU-AW can be ascribed to a critical illness polyneuropathy (CIP), a critical illness myopathy (CIM), or severe muscle disuse atrophy. These three conditions often coexist, and the combination of CIP and CIM – indicated as critical illness myopathy and neuropathy (CRIMYNE) or critical illness polyneuromyopathy (CIPNM) – is the most common overlap syndrome 2.
Figure 1. Diagnostic approach to patients developing intensive care unit–acquired weakness.
EMG, electromyography; ICU-AW, intensive care unit–acquired weakness; MRC, Medical Research Council; NCS, nerve conduction study; NM, neuromuscular. Modified from Latronico and Bolton 2.
Table 1. Definition and diagnostic criteria of intensive care unit–acquired weakness, diaphragmatic weakness, critical illness polyneuropathy, critical illness myopathy, and combined critical illness polyneuropathy and myopathy.
| Condition | Definition | Diagnosis |
|---|---|---|
| Intensive care unit–acquired
weakness (ICU-AW) 1, 2 |
Clinically detected, diffuse, symmetric
weakness involving all extremities and respiratory muscles arising after the onset of critical illness |
c) Medical Research Council (MRC) sum score of less
than 48/60 or mean MRC score of 4 in all testable muscle groups d) Dominant-hand handgrip dynamometry scores of less than 11 kg (interquartile range (IQR) 10–40) in males and less than 7 kg (IQR 0–7.3) in females |
| Diaphragmatic weakness (DW) 9 | Reduced pressure-generating capacity
of the diaphragm and a decreased diaphragm thickness and thickening fraction after initiation of mechanical ventilation |
d) Endotracheal tube pressures less than 11 cm H
2O
after bilateral phrenic nerve magnetic stimulation during airway occlusion e) Diaphragm excursion at muscle ultrasound less than 11 mm during tidal breathing f) Diaphragm thickening fraction at muscle ultrasound less than 20% |
| Critical illness polyneuropathy
(CIP) 1 |
An axonal, sensory-motor
polyneuropathy with reduced nerve excitability and loss of axons with preserved myelin sheet |
Reduced amplitude of compound muscle action
potentials and sensory nerve action potentials with normal or mildly reduced nerve conduction velocity on electroneurography |
| Critical illness myopathy (CIM) 1 | A primary acute myopathy with reduced
muscle membrane excitability and loss of myosin filaments, fiber atrophy, and necrosis |
Reduced amplitude of compound muscle action
potentials and normal sensory nerve action potentials on electroneurography and reduced muscle excitability on direct muscle stimulation and myopathic motor unit potentials on needle electromyography |
| Combined critical illness
polyneuropathy and myopathy (CRIMYNE) 1 |
Combined CIP and CIM | Reduced amplitude of compound muscle action
potentials and sensory nerve action potentials combined with myopathic features on needle electromyography |
CIP is a sensory-motor axonal polyneuropathy. Electrophysiological studies show a reduction in the amplitudes of compound muscle action potentials (CMAPs) and sensory nerve action potentials (SNAPs), with normal or near-normal nerve conduction velocity ( Table 1) 2. The histological counterpart is a primary distal axonal degeneration of motor and sensory fibers, which may cause muscle denervation and atrophy 2. CIM is an acute primary myopathy (that is, not related to denervation) with distinctive electrophysiological (low-amplitude motor unit potentials with early or normal full recruitment, with or without fibrillation potentials and increased CMAP duration with normal SNAPs) and morphological (loss of thick myosin filaments, muscle fiber atrophy, and necrosis) findings ( Table 1) 2. Muscle atrophy is the consequence of muscle unloading/inactivity, which promotes muscle catabolism that exceeds the loss in muscle cell size, resulting in decreased myocyte-specific force 10. Mechanical silencing – that is, the complete loss of mechanical stimuli in ICU patients who are mechanically ventilated or deeply sedated or receiving neuromuscular blocking agents or who are undergoing a combination of these – causes even more severe muscle wasting 11.
Although ICU-AW was defined years ago 1, many aspects concerning its diagnosis and its correlation with diaphragm weakness, delirium, nutritional status, and early mobilization in the ICU remain poorly understood. This review discusses these open issues that have not been completely defined and that should be addressed in future studies.
Diagnosis of ICU-AW
ICU-AW is a clinical diagnosis ( Table 1). The Medical Research Council sum score (MRC-SS) and handgrip dynamometry constitute the gold standard for diagnosis. With MRC-SS, muscle strength is assessed in 12 muscle groups and then individual scores are combined into a sum score, which yields an overall estimation of motor function. Summed scores below 48 out of 60 and below 36 out of 60 indicate significant 1 and severe 12 weakness, respectively. With handgrip dynamometry, the isometric muscle strength of the dominant hand is measured. Cutoff scores for ICU-AW are less than 11 kg (interquartile range (IQR) 10–40) in males and less than 7 kg (IQR 0–7.3) in females 8. Handgrip dynamometry and MRC-SS can be used serially, and dynamometry serves as a quick screening test that, if normal, excludes ICU-AW 13. If abnormal, the MRC-SS is necessary to specifically identify the typical ICU-AW distribution of muscle weakness. Both tests are volitional tests that require the patient to be alert, cooperative, and motivated. As such, because of delirium, coma, pain, and the use of sedative drugs, they often cannot be used in the ICU 14. In these cases, non-volitional tests can provide useful clues to the diagnosis.
Simplified electrophysiological tests are non-volitional tests that have long been shown to be able to predict long-term disability in survivors of critical illness. In 1995, Leijten 15 first demonstrated that patients with abnormal electromyography in the ICU had persistent disability at 1 year. The simplified peroneal nerve test (PENT) has been validated in two multicenter prospective studies in Italy – the CRIMYNE-1 16 and CRIMYNE-2 17 studies – as a high-sensitivity test with good specificity (100% and 85%, respectively) and can be used as a screening test to identify CIP or CIM ( Figure 1). Recently 18, PENT was confirmed to have high sensitivity (94%, with only one false-negative result out of 72 patients examined) and excellent specificity (91%). Combined unilateral peroneal (motor) and sural (sensory) nerve assessment also has 100% sensitivity; moreover, abnormally reduced sensory and motor nerve amplitudes are associated with increased hospital mortality 19 and severe physical dysfunction at hospital discharge 20. In a large sub-study of 730 patients in the EPaNIC (Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients) trial, an abnormal motor nerve action potential amplitude measured at 8 days after ICU admission was independently associated with increased 1-year mortality 21.
Non-volitional methods with supramaximal electrical or magnetic twitch stimulation of peripheral motor nerves – that is, ulnar nerve stimulation for the adductor pollicis muscle, femoral nerve for quadriceps muscle, peroneal nerve stimulation for ankle dorsiflexor muscles, or phrenic nerve stimulation for diaphragm 22 – can be used to provoke muscle contraction, providing a measure of muscle function regardless of whether the patient is awake and cooperative. With transcutaneous neuromuscular electrical stimulation in healthy subjects 23, ramp stimulations starting from low frequencies (1–2 Hz) up to tetanic stimulation (30–50 Hz) provide a force-frequency relationship, which is a recognized method to assess the contractile properties of skeletal muscles without the need for voluntary muscle activation. Muscle ultrasound is rapidly gaining popularity among ICU physicians as a non-invasive method to assess changes in limb muscle mass as well as structural muscle alteration such as myofiber necrosis, fatty muscle infiltration, or fasciitis 4, 24. Abnormal echogenicity may be associated with a reduced likelihood of discharge to home, fewer ICU-free days, and increased ICU mortality 18. Muscle ultrasound, however, does not discriminate between patients with and those without ICU-AW at the time the patient awakens 25, 26. Nerve ultrasound has been shown to be a reproducible tool for diagnostics in routine clinical practice in patients with chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, or chronic idiopathic axonal polyneuropathy, but its use in ICU patients has not been systematically assessed 27.
Diaphragmatic weakness and ICU-AW: different clinical entities or two sides of the same coin?
Diaphragmatic dysfunction or weakness (DW), defined as a decrease of diaphragm strength after initiation of mechanical ventilation ( Table 1), is common in ICU patients and, with modern technology, is easily documented 28. The inactivity of the diaphragm rather than mechanical ventilation per se seems to be the critical determinant of DW 9. Historically, concurrent limb muscle weakness or paralysis and respiratory muscle weakness causing failure to wean the patient from the ventilator have been considered pathognomonic presentations of the syndrome 29. However, recent studies show that DW is poorly correlated with ICU-AW 26 and that DW is twice as frequent as ICU-AW 30, favoring the hypothesis that weakness of the diaphragm and limbs might represent two distinct entities.
Several techniques are available for assessing diaphragm muscle function and these are reviewed elsewhere 31. When the level of endotracheal tube pressure (Pet,tw) induced by bilateral phrenic nerve magnetic stimulation during airway occlusion is used ( Table 1), DW is established if the Pet,tw falls below 11 cm H 2O. With this criterion, DW is described in up to 64% of patients within 24 hours after intubation 28. DW is documented in 63 to 80% of patients at the time of weaning and in about 80% of patients requiring prolonged mechanical ventilation 28. When ultrasound definitions are used ( Table 1), DW is identified if the diaphragm excursion is less than 11 mm or the diaphragm thickening fraction is less than 20%. With these criteria, the prevalence of DF is lower, ranging between 29% in patients submitted to the first spontaneous breathing trial and 36% at extubation 28, 31, 32.
Pathophysiological mechanisms of DW are usually classified as infection/inflammation-related or ventilator-induced mechanisms 33, 34. Infection causes cytokine release, which in turn induces mitochondrial free radical production 35, 36, contributing to the reduction in muscle endurance and strength 36. Histopathological findings include injury of the muscle fibers with disrupted sarcomeres 37, 38. Controlled mechanical ventilation with complete diaphragm unloading causes marked atrophy of human diaphragm myofibers within hours 38– 40. Conversely, excessive diaphragm loading is associated with high levels of inspiratory effort with increased diaphragm myofiber inflammation, edema, and injury 32. Although disuse atrophy and muscle fiber injury are probably linked, they represent two different insults to the diaphragm and the latter seems to be an earlier phenomenon 37.
Pathophysiology and risk factors such as immobility and inflammation are common to both ICU-AW and DW. Histopathological features are also similar, although muscle necrosis is highly prevalent in ICU-AW 41, 42 but not in DW. Regardless of the pathogenesis, DW is a marker of severity of critical illness and portends a poor prognosis. If diagnosed at an early stage of acute disease, DW is associated with increased mortality 28. With a later onset, it is strongly associated with weaning failure 32, a high risk of hospital readmission in patients with chronic respiratory failure 43, and increased 1-year mortality 44.
Different strategies can be implemented to prevent DW. First, it is helpful to implement diaphragm-protective mechanical ventilation by maintaining inspiratory efforts throughout a spontaneous breathing trial 31 unless high respiratory drive is required. Inspiratory muscle training – via isocapnic and normocapnic hyperpnea, inspiratory resistive training, threshold pressure training, or adjustment of ventilator pressure trigger sensitivity—has been shown to have a positive impact on (1) improving inspiratory muscle strength, (2) increasing success of weaning, and (3) reducing hospital and ICU length of stay (LOS) 45. Muscle training applied to patients after a successful spontaneous breathing trial may increase inspiratory muscle strength and quality of life 46. Several drug investigations of, for example, drugs that inhibit proteolytic pathways or enhance protein synthesis 33 or inhibit the phosphodiesterase PDE3 and PDE4 (theophylline, 1,3-dimethyl- xanthine) 28 are under way. In mice, β-hydroxy-β-methylbutyrate (HMB), a leucine metabolism product that reduces muscle protein breakdown, completely prevents the dramatic decrease in diaphragm force generation caused by sepsis at dosages comparable to those used to reduce protein breakdown in human studies 47. In a small randomized clinical trial of 30 healthy subjects, levosimendan, a calcium sensitizer that improves cardiac contractility in patients with acute heart failure, prevented the loss of twitch diaphragm contractility after loaded breathing compared with placebo 48.
Delirium, drugs, and ICU-AW
Delirium is defined as a disturbance of attention, awareness, and cognition which develops over a short period of time (hours to days) and fluctuates over time 49. Delirium is a severe complication in critically ill patients as it represents a decompensation of cerebral function – an “acute brain failure” – in response to one or more pathophysiological stressors.
With a prevalence rate ranging between 20 and 40%, delirium, particularly hypoactive delirium, is associated with deleterious clinical outcomes, including prolonged mechanical ventilation, increased ICU and hospital LOS, increased mortality, and impaired cognitive function for up to 12 months after discharge 49– 51.
Although they are clearly distinct entities, delirium and ICU-AW are possibly related and may even interact negatively with each other 4. Both are influenced by the severity of illness, are aggravated by the treatment adopted in the ICU, and may share some predisposing and trigger factors ( Supplementary Table 1). Disease severity at ICU admission assessed with APACHE II (Acute Physiology and Chronic Health Evaluation II) score is a predisposing factor for both conditions 52, 53. Benzodiazepines are strongly associated with delirium 53 and, by causing immobility, may increase the risk of ICU-AW 4. Propofol and benzodiazepines, the commonest sedative drugs used in the ICU, also directly decrease muscle excitability, worsening the effect of bed rest. Barbiturates and ketamine interact with N-methyl-D-aspartate receptors 54, which have an important role in maintaining muscle trophism 55.
A clear association between delirium and ICU-AW has not been established. The MOSAIC (Measuring Outcomes of Activity in Intensive Care) study (ClinicalTrials.gov Identifier: NCT03115840) is a prospective cohort study designed to assess the relationship between activity and long-term disability. When concluded (the expected date is 2020, Nathan E. Brummel, personal communication), this study will help clarify the relationship between physical activity, delirium, and cognitive dysfunction in survivors of critical illness. No study of patients with hypoactive delirium has explored the causes of reduced or absent mobility, whether it is the consequence of central nervous system or of central and peripheral nervous system and muscle dysfunction.
Muscle metabolism, nutrition, and ICU-AW
Nutritional status is associated with weakness: starvation in healthy volunteers causes loss of muscle mass, strength, and function 4. Critical illness is characterized by severe skeletal muscle loss in the early stage of the ICU stay (when measured by ultrasound rectus femoris cross-sectional area) 42 as well as hyperglycemia and low circulating amino-acid levels 56, 57. The hallmark of critical illness–associated muscle wasting is the catabolic state associated with depressed anabolism 42. Caloric and protein supplementations do not improve the catabolic state during the early phase of critical illness, as macronutrient deficit is well tolerated compared with early caloric parenteral substitution 58. Protein synthesis remains refractory to increased protein delivery 42. Although supplementation of high doses of amino acids is safe and can be well tolerated even in patients with renal failure 59, results from randomized controlled trials comparing high versus low protein supplementation have yielded inconsistent results 60– 62.
Recent insight into the glucagon pathophysiology suggests that an elevated level of this hormone during critical illness increases hepatic amino-acid catabolism 63, inducing hypo-aminoacidemia. Interestingly, infusion of amino acids by raising the level of glucagon increases amino-acid breakdown in the liver, aggravating rather than reversing catabolism 63. Moreover, skeletal muscle wasting in critical care is directly related to impaired lipid oxidation and reduced ATP, creatine, and phosphocreatine availability induced by muscle inflammation 64. Mitochondrial dysfunction and ATP depletion are observed also in nerve axons and may represent a generalized phenomenon during critical illness 65. No study has assessed the impact of various nutritional strategies and regimens on ICU-AW as the primary outcome. In a sub-study of the EPaNIC trial 58, weakness assessed at an early stage of disease was significantly more common in patients receiving early parenteral nutrition compared with those receiving late parenteral nutrition but this effect was of short duration and difference was no longer significant at a later stage. Thus, the interactions between nutrition and ICU-AW remain incompletely understood 66.
Early mobilization and ICU-AW
Early mobilization in the ICU has been advocated as a therapeutic strategy to prevent ICU-AW, reducing the negative effects of immobility on muscles and other organ systems 67. Mobilization in the ICU is feasible and safe provided that consensus guidelines are followed 68, 69. The incidence of potential safety events is low – cumulative incidence 2.6%, hemodynamic events 3.8, 95% confidence interval (CI) 1.3 – 11.4 and desaturation 1.9, 95% CI 0.9 – 4.3 per 1,000 mobilization/rehabilitation sessions – and medical consequences are rare (0.6% of 14,398 mobilization/rehabilitation sessions) 70. Evidence of efficacy, particularly long-term efficacy, remains uncertain. A recent meta-analysis of 14 randomized clinical trials enrolling 1753 patients showed no impact of active mobilization and rehabilitation in the ICU on short-term and long-term mortality, patient functional status, quality of life, ICU or hospital LOS, duration of mechanical ventilation, or discharge disposition 71. Patients receiving active mobilization and rehabilitation in the ICU had improved muscle strength at ICU discharge and improved walking ability without assistance at hospital discharge and more days alive and out of hospital at 6 months. In a subgroup analysis 71, patients receiving high-dose rehabilitation had improved quality of life in the role physical and role emotional domains compared with those receiving low-dose rehabilitation. In the recent EPICC (Extra Physiotherapy in Critical Care) trial 66, a 90-minute physical rehabilitation per day did improve physical outcomes at 6 months compared with 30 minutes per day, but rehabilitation started on about day 8 and the difference in terms of physical therapy actually received by the two groups was negligible (10 minutes) 72. Late initiation 73 may reduce the efficacy of mobilization as the beneficial effects of physical therapy have been found in studies in which the treatment was started early after ICU admission 72; however, definition of “earliness” remains undefined 67. In a recent randomized clinical trial, very early initiation of in-bed leg cycling and electrical quadriceps stimulation within a median of 30 hours of ICU admission did not improve global muscle strength (MRC score) at ICU discharge 74. The efficacy of active rehabilitation in the general ward after ICU discharge is also uncertain 73, 75– 77.
In stroke patients, early mobilization was demonstrated to reduce the odds of a favorable outcome at 3 months 78; however, the adoption of optimized session frequencies with increased daily frequency of mobilization sessions may be associated with improved outcome 79. Moreover, data in stroke patients may not apply to critically ill neurological patients admitted to the ICU; indeed, early mobilization is safe in this setting and might be beneficial 80, 81 because immobility is a common consequence of neurological impairments. A recent post-hoc analysis of a randomized controlled trial also showed that early, goal-directed mobilization is not harmful in patients with impaired consciousness and might be effective in achieving higher mobility levels and better functional status at hospital discharge 82.
Conclusions and Future directions
ICU-AW is a common complication in ICU patients and has a clinically relevant impact on short- and long-term outcomes. Several important questions remain unanswered concerning the optimal method for diagnosis and the relationship between ICU-AW and DW, delirium, muscle metabolism, and nutrition. The roles of early mobilization and rehabilitation in the ICU also remain to be elucidated. Future longitudinal studies should confirm the predictive ability of early abnormalities of electrophysiological tests of peripheral nerves and muscles, muscle ultrasound imaging, and non-volitional muscle strength measurements on long-term physical dysfunction. Future efficacy nutrition trials should consider ICU-AW a clinically relevant outcome measure. Individualized timing of protein administration 83 should also be considered in future research studies aiming at assessing the impact of specialized nutritional strategies or regimens on ICU-AW 84. The overall impact of ICU mobilization and rehabilitation needs to be assessed with standardization of the optimal timing, dosage, progression of exercise, and intensity and duration of physical therapy using a core set of long-term outcome measures collected at consistent times 4, 85. Inclusion of mobilization and rehabilitation programs into a coordinated series of interventions such as the ABCDEF bundle with optimal pain treatment, minimal sedation, and daily spontaneous breathing trial would also be important to consider in future efficacy studies 53, 86.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Marc Moss, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado, Denver School of Medicine, Denver, CO, USA
Catherine Hough, Division of Pulmonary and Critical Care Medicine, Harborview Medical Center, University of Washington, Seattle, WA, USA
Linda Denehy, Department of Physiotherapy, School of Health Sciences, The University of Melbourne, Parkville, Melbourne, Australia
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 3 approved]
Supplementary material
Possible risk factors for delirium and Intensive care unit–acquired weakness (ICU-AW).
References
- 1. Stevens RD, Marshall SA, Cornblath DR, et al. : A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S299–308. 10.1097/CCM.0b013e3181b6ef67 [DOI] [PubMed] [Google Scholar]
- 2. Latronico N, Bolton CF: Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–41. 10.1016/S1474-4422(11)70178-8 [DOI] [PubMed] [Google Scholar]
- 3. Fan E, Cheek F, Chlan L, et al. : An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–46. 10.1164/rccm.201411-2011ST [DOI] [PubMed] [Google Scholar]
- 4. Latronico N, Herridge M, Hopkins RO, et al. : The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017;43(9):1270–81. 10.1007/s00134-017-4757-5 [DOI] [PubMed] [Google Scholar]
- 5. Kelmenson DA, Held N, Allen RR, et al. : Outcomes of ICU Patients With a Discharge Diagnosis of Critical Illness Polyneuromyopathy: A Propensity-Matched Analysis. Crit Care Med. 2017;45(12):2055–60. 10.1097/CCM.0000000000002763 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Latronico N, Rasulo FA: Presentation and management of ICU myopathy and neuropathy. Curr Opin Crit Care. 2010;16(2):123–7. 10.1097/MCC.0b013e328336a229 [DOI] [PubMed] [Google Scholar]
- 7. Sharshar T, Citerio G, Andrews PJ, et al. : Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive Care Med. 2014;40(4):484–95. 10.1007/s00134-014-3214-y [DOI] [PubMed] [Google Scholar]
- 8. Latronico N, Gosselink R: A guided approach to diagnose severe muscle weakness in the intensive care unit. Rev Bras Ter Intensiva. 2015;27(3):199–201. 10.5935/0103-507X.20150036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dres M, Goligher EC, Heunks LMA, et al. : Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43(10):1441–52. 10.1007/s00134-017-4928-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Batt J, Herridge M, Dos Santos C: Mechanism of ICU-acquired weakness: skeletal muscle loss in critical illness. Intensive Care Med. 2017;43(12):1844–6. 10.1007/s00134-017-4758-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Renaud G, Llano-Diez M, Ravara B, et al. : Sparing of muscle mass and function by passive loading in an experimental intensive care unit model. J Physiol. 2013;591(5):1385–402. 10.1113/jphysiol.2012.248724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermans G, Clerckx B, Vanhullebusch T, et al. : Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45(1):18–25. 10.1002/mus.22219 [DOI] [PubMed] [Google Scholar]
- 13. Parry SM, Berney S, Granger CL, et al. : A new two-tier strength assessment approach to the diagnosis of weakness in intensive care: an observational study. Crit Care. 2015;19:52. 10.1186/s13054-015-0780-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Hough CL, Lieu BK, Caldwell ES: Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15(1):R43. 10.1186/cc10005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Leijten FS, Harinck-de Weerd JE, Poortvliet DC, et al. : The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274(15):1221–5. 10.1001/jama.1995.03530150045032 [DOI] [PubMed] [Google Scholar]
- 16. Latronico N, Bertolini G, Guarneri B, et al. : Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Crit Care. 2007;11(1):R11. 10.1186/cc5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Latronico N, Nattino G, Guarneri B, et al. : Validation of the peroneal nerve test to diagnose critical illness polyneuropathy and myopathy in the intensive care unit: the multicentre Italian CRIMYNE-2 diagnostic accuracy study [version 1; peer review: awaiting peer review]. F1000Res. 2014;3:127. 10.12688/f1000research.3933.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelmenson DA, Quan D, Moss M: What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: a prospective cohort study. Crit Care. 2018;22(1):342. 10.1186/s13054-018-2281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Moss M, Yang M, Macht M, et al. : Screening for critical illness polyneuromyopathy with single nerve conduction studies. Intensive Care Med. 2014;40(5):683–90. 10.1007/s00134-014-3251-6 [DOI] [PubMed] [Google Scholar]
- 20. Kelmenson DA, Quan D, Nordon-Craft A, et al. : Electrophysiological abnormalities can differentiate pre-hospital discharge functional status in critically ill patients with normal strength. Intensive Care Med. 2016;42(9):1504–5. 10.1007/s00134-016-4425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Hermans G, Van Mechelen H, Bruyninckx F, et al. : Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med. 2015;41(12):2138–48. 10.1007/s00134-015-3979-7 [DOI] [PubMed] [Google Scholar]
- 22. Rafferty G, Moxham J: Assessment of peripheral and respiratory muscle strength in ICU. In Textbook of Post-ICU Medicine: The Legacy of Critical Care(eds. Stevens RD., Hart N. & Herridge MS.) (Oxford University Press).2014;530–547. 10.1093/med/9780199653461.003.0047 [DOI] [Google Scholar]
- 23. Latronico N, Fagoni N, Gobbo M: Neuromuscular Electrical Stimulation in Critically Ill Patients. Essentials of Neuroanesthesia2017;771–781. 10.1016/B978-0-12-805299-0.00046-4 [DOI] [Google Scholar]
- 24. Mourtzakis M, Parry S, Connolly B, et al. : Skeletal Muscle Ultrasound in Critical Care: A Tool in Need of Translation. Ann Am Thorac Soc. 2017;14(10):1495–503. 10.1513/AnnalsATS.201612-967PS [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Witteveen E, Sommers J, Wieske L, et al. : Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: a cross-sectional observational study. Ann Intensive Care. 2017;7(1):40. 10.1186/s13613-017-0263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Jung B, Moury PH, Mahul M, et al. : Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42(5):853–61. 10.1007/s00134-015-4125-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Telleman JA, Stellingwerff MD, Brekelmans GJ, et al. : Nerve ultrasound: A useful screening tool for peripheral nerve sheath tumors in NF1? Neurology. 2017;88(17):1615–22. 10.1212/WNL.0000000000003870 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Demoule A, Jung B, Prodanovic H, et al. : Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–9. 10.1164/rccm.201209-1668OC [DOI] [PubMed] [Google Scholar]
- 29. Bolton CF, Gilbert JJ, Hahn AF, et al. : Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–31. 10.1136/jnnp.47.11.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dres M, Dubé BP, Mayaux J, et al. : Coexistence and Impact of Limb Muscle and Diaphragm Weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit Patients. Am J Respir Crit Care Med. 2017;195(1):57–66. 10.1164/rccm.201602-0367OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Schreiber A, Bertoni M, Goligher EC: Avoiding Respiratory and Peripheral Muscle Injury During Mechanical Ventilation: Diaphragm-Protective Ventilation and Early Mobilization. Crit Care Clin. 2018;34(3):357–81. 10.1016/j.ccc.2018.03.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Goligher EC, Fan E, Herridge MS, et al. : Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am J Respir Crit Care Med. 2015;192(9):1080–8. 10.1164/rccm.201503-0620OC [DOI] [PubMed] [Google Scholar]
- 33. Supinski GS, Morris PE, Dhar S, et al. : Diaphragm Dysfunction in Critical Illness. Chest. 2018;153(4):1040–51. 10.1016/j.chest.2017.08.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Petrof BJ: Diaphragm Weakness in the Critically Ill: Basic Mechanisms Reveal Therapeutic Opportunities. Chest. 2018;154(6):1395–403. 10.1016/j.chest.2018.08.1028 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Supinski GS, Alimov AP, Wang L, et al. : Calcium-dependent phospholipase A 2 modulates infection-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. 2016;310(10):L975–84. 10.1152/ajplung.00312.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Callahan LA, Nethery D, Stofan D, et al. : Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24(2):210–7. 10.1165/ajrcmb.24.2.4075 [DOI] [PubMed] [Google Scholar]
- 37. Jaber S, Petrof BJ, Jung B, et al. : Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–71. 10.1164/rccm.201004-0670OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Levine S, Nguyen T, Taylor N, et al. : Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35. 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Picard M, Jung B, Liang F, et al. : Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med. 2012;186(11):1140–9. 10.1164/rccm.201206-0982OC [DOI] [PubMed] [Google Scholar]
- 40. Hussain SN, Cornachione AS, Guichon C, et al. : Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax. 2016;71(5):436–45. 10.1136/thoraxjnl-2015-207559 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Latronico N, Fenzi F, Recupero D, et al. : Critical illness myopathy and neuropathy. Lancet. 1996;347(9015):1579–82. 10.1016/S0140-6736(96)91074-0 [DOI] [PubMed] [Google Scholar]
- 42. Puthucheary ZA, Rawal J, McPhail M, et al. : Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Zambon M, Greco M, Bocchino S, et al. : Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43(1):29–38. 10.1007/s00134-016-4524-z [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Medrinal C, Prieur G, Frenoy É, et al. : Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit Care. 2016;20(1):231. 10.1186/s13054-016-1418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elkins M, Dentice R: Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: a systematic review. J Physiother. 2015;61(3):125–34. 10.1016/j.jphys.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 46. Bissett BM, Leditschke IA, Neeman T, et al. : Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax. 2016;71(9):812–9. 10.1136/thoraxjnl-2016-208279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Supinski GS, Callahan LA: β-Hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respir Physiol Neurobiol. 2014;196:63–8. 10.1016/j.resp.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Doorduin J, Sinderby CA, Beck J, et al. : The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med. 2012;185(1):90–5. 10.1164/rccm.201107-1268OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Pandharipande PP, Ely EW, Arora RC, et al. : The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med. 2017;43(9):1329–39. 10.1007/s00134-017-4860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Salluh JIF, Latronico N: Does this critically ill patient with delirium require any drug treatment? Intensive Care Med. 2019;45(4):501–504. 10.1007/s00134-018-5310-x [DOI] [PubMed] [Google Scholar]
- 51. Pandharipande PP, Girard TD, Jackson JC, et al. : Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Yang T, Li Z, Jiang L, et al. : Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol Scand. 2018;138(2):104–14. 10.1111/ane.12964 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Urazaev AK, Magsumov ST, Poletayev GI, et al. : Muscle NMDA receptors regulate the resting membrane potential through NO-synthase. Physiol Res. 1995;44(3):205–8. [PubMed] [Google Scholar]
- 55. Malomouzh AI, Nurullin LF, Arkhipova SS, et al. : NMDA receptors at the endplate of rat skeletal muscles: precise postsynaptic localization. Muscle Nerve. 2011;44(6):987–9. 10.1002/mus.22250 [DOI] [PubMed] [Google Scholar]
- 56. Druml W, Heinzel G, Kleinberger G: Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73(5):908–13. 10.1093/ajcn/73.5.908 [DOI] [PubMed] [Google Scholar]
- 57. Fagoni N, Piva S, Marino R, et al. : The IN-PANCIA Study: Clinical Evaluation of Gastrointestinal Dysfunction and Failure, Multiple Organ Failure, and Levels of Citrulline in Critically Ill Patients. J Intensive Care Med. 2017; 885066617742594. 10.1177/0885066617742594 [DOI] [PubMed] [Google Scholar]
- 58. Hermans G, Casaer MP, Clerckx B, et al. : Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–9. 10.1016/S2213-2600(13)70183-8 [DOI] [PubMed] [Google Scholar]
- 59. Doig GS, Simpson F, Bellomo R, et al. : Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med. 2015;41(7):1197–208. 10.1007/s00134-015-3827-9 [DOI] [PubMed] [Google Scholar]
- 60. Clifton GL, Robertson CS, Contant CF: Enteral hyperalimentation in head injury. J Neurosurg. 1985;62(2):186–93. 10.3171/jns.1985.62.2.0186 [DOI] [PubMed] [Google Scholar]
- 61. Rugeles S, Villarraga-Angulo LG, Ariza-Gutiérrez A, et al. : High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: A randomized clinical trial. J Crit Care. 2016;35:110–4. 10.1016/j.jcrc.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 62. Allingstrup MJ, Kondrup J, Wiis J, et al. : Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43(11):1637–47. 10.1007/s00134-017-4880-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Thiessen SE, Derde S, Derese I, et al. : Role of Glucagon in Catabolism and Muscle Wasting of Critical Illness and Modulation by Nutrition. Am J Respir Crit Care Med. 2017;196(9):1131–43. 10.1164/rccm.201702-0354OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Puthucheary ZA, Astin R, Mcphail MJW, et al. : Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–35. 10.1136/thoraxjnl-2017-211073 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Latronico N, Friedrich O: Electrophysiological investigations of peripheral nerves and muscles: a method for looking at cell dysfunction in the critically ill patients. Crit Care. 2019;23(1):33. 10.1186/s13054-019-2331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wright SE, Thomas K, Watson G, et al. : Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax. 2018;73(3):213–21. 10.1136/thoraxjnl-2016-209858 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Denehy L, Lanphere J, Needham DM: Ten reasons why ICU patients should be mobilized early. Intensive Care Med. 2017;43(1):86–90. 10.1007/s00134-016-4513-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Hodgson CL, Stiller K, Needham DM, et al. : Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18(6):658. 10.1186/s13054-014-0658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gosselink R, Bott J, Johnson M, et al. : Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008;34(7):1188–99. 10.1007/s00134-008-1026-7 [DOI] [PubMed] [Google Scholar]
- 70. Nydahl P, Sricharoenchai T, Chandra S, et al. : Safety of Patient Mobilization and Rehabilitation in the Intensive Care Unit. Systematic Review with Meta-Analysis. Ann Am Thorac Soc. 2017;14(5):766–77. 10.1513/AnnalsATS.201611-843SR [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Tipping CJ, Harrold M, Holland A, et al. : The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–83. 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Schaller S, Nydahl P, Blobner M, et al. : What does the EPICC trial really tell us? Thorax. 2018. Reference Source [Google Scholar]
- 73. Moss M, Nordon-Craft A, Malone D, et al. : A Randomized Trial of an Intensive Physical Therapy Program for Patients with Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;193(10):1101–10. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fossat G, Baudin F, Courtes L, et al. : Effect of In-Bed Leg Cycling and Electrical Stimulation of the Quadriceps on Global Muscle Strength in Critically Ill Adults: A Randomized Clinical Trial. JAMA. 2018;320(4):368–78. 10.1001/jama.2018.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Walsh TS, Salisbury LG, Merriweather JL, et al. : Increased Hospital-Based Physical Rehabilitation and Information Provision After Intensive Care Unit Discharge: The RECOVER Randomized Clinical Trial. JAMA Intern Med. 2015;175(6):901–10. 10.1001/jamainternmed.2015.0822 [DOI] [PubMed] [Google Scholar]
- 76. Morris PE, Berry MJ, Files DC, et al. : Standardized Rehabilitation and Hospital Length of Stay Among Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA. 2016;315(24):2694–702. 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gruther W, Pieber K, Steiner I, et al. : Can Early Rehabilitation on the General Ward After an Intensive Care Unit Stay Reduce Hospital Length of Stay in Survivors of Critical Illness?: A Randomized Controlled Trial. Am J Phys Med Rehabil. 2017;96(9):607–15. 10.1097/PHM.0000000000000718 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. AVERT Trial Collaboration group: Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386(9988):46–55. 10.1016/S0140-6736(15)60690-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Bernhardt J, Churilov L, Ellery F, et al. : Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology. 2016;86(23):2138–45. 10.1212/WNL.0000000000002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Titsworth WL, Hester J, Correia T, et al. : The effect of increased mobility on morbidity in the neurointensive care unit. J Neurosurg. 2012;116(6):1379–88. 10.3171/2012.2.JNS111881 [DOI] [PubMed] [Google Scholar]
- 81. Piva S, Dora G, Minelli C, et al. : The Surgical Optimal Mobility Score predicts mortality and length of stay in an Italian population of medical, surgical, and neurologic intensive care unit patients. J Crit Care. 2015;30(6):1251–7. 10.1016/j.jcrc.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 82. Schaller SJ, Scheffenbichler FT, Bose S, et al. : Influence of the initial level of consciousness on early, goal-directed mobilization: a post hoc analysis. Intensive Care Med. 2019;45(2):201–10. 10.1007/s00134-019-05528-x [DOI] [PubMed] [Google Scholar]
- 83. Compher C, Chittams J, Sammarco T, et al. : Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med. 2017;45(2):156–63. 10.1097/CCM.0000000000002083 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Arabi YM, Casaer MP, Chapman M, et al. : The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med. 2017;43(9):1239–56. 10.1007/s00134-017-4711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Needham DM, Sepulveda KA, Dinglas VD, et al. : Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196(9):1122–30. 10.1164/rccm.201702-0372OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2019;47(1):3–14. 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.