Abstract
Background. Lactococcus garvieae (LG) is a gram-positive coccus known to be a major pathogen in aqua farming, which is responsible for severe outbreaks. Its incidence in humans is extremely rare. Prior to 1985, all bacteria in the genus Lactococcus were included in the Streptococcus genus. The first human infection was documented in 1991, and since then, the relevance and clinical significance in humans has increased. Case Description. We present the clinical course of an LG endocarditis in a 78-year-old man who had a history of exertional dyspnea. The patient’s blood tests showed increased inflammation values, and a transesophageal ultrasound (TEE) showed a stenosis of the prosthetic aortic valve. Blood cultures were positive for LG, leading to a diagnosis of infective endocarditis. After 6 weeks of intravenous antibiotics and a prosthetic aortic valve replacement, the patient made a good recovery. Review of the Literature. After the first documented case in 1991 to 2018, 25 cases of LG endocarditis have been described in PubMed and MEDLINE. We reviewed all reported cases of LG endocarditis, commenting on predisposing risk factors, the course and outcome of the disease. Conclusion. LG endocarditis is a rare disease. Consumption of raw fish, abnormalities of the digestive tract, immune deficiency, and underlying cardiac conditions appear to be risk factors for an infective endocarditis due to LG. Improved determination techniques are likely to lead to a better and faster identification of the bacterium. This identification allows a faster and individualized therapy, which in turn affects the outcome.
Keywords: Lactococcus garvieae infections, infective endocarditis, heart valve prosthesis
Introduction
Lactococcus garvieae is a catalase-negative, facultative anaerobic, nonhemolytic, gram-positive chain cocci1 present throughout the world. It is also known as Enterococcus seriolicida.2
Lactococcus garvieae was first described in 1983.3 Since then, it has been described frequently in the literature as a highly opportunistic pathogen in aqua farming, and outbreaks of L garvieae infections in fish colonies significantly affect production.4
Lactococcus garvieae proliferation peaks during summer, when the water temperature rises, and this also coincides with a rise in the number of L garvieae infections in fish.4
The first case of human infection with L garvieae was reported in 1991.5 Since then, various types of infections have been described, including lumbar osteomyelitis, meningitis, hepatic abscess, and infective endocarditis (IE).6
The relevance of L garvieae has increased in recent decades, as has its clinical significance in humans.7 However, meager information is currently available about the process of transmission to humans. We assume the infection follows the consumption of raw fish, especially in patients with digestive disorders, which was previously described in other cases of L garvieae IE.8 More recently, studies carried out on dairy products obtained from raw milk suggest another possible ecological niche as an origin of L garvieae.2
It is difficult to distinguish L garvieae from enterococci,9 and misidentifications of L garvieae as Enterococcus species are common in small clinical microbiology laboratories.7 Laboratories in tertiary hospitals are more easily able to identify L garvieae species.
Different molecular methods, such as API strips, BD Phoenix, the VITEK system, and MicroScan, have been examined to identify L garvieae from clinical specimens.1,10-14 L garvieae can also be obtained by specific polymerase chain reaction (PCR) amplification.15 True identification is now possible due to the use of mass spectrometry, especially matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF). MALDI-TOF mass spectrometry has emerged in recent years as an alternative to conventional identification techniques for the accurate, rapid, and inexpensive identification of a broad spectrum of pathogenic bacteria in clinical microbiology laboratories.16
The treatment is not yet standardized because no test has been established to determine the exact criteria of susceptibility screening.17 Due to the similarity between L garvieae and streptococcal infections, treatment plans are often the same or comparable. These treatments usually require high doses of β-lactams, including ampicillin, amoxicillin, or ceftriaxone, administered alone or in combination with aminoglycosides such as netilmicin, tobramycin, or gentamicin. In cases where no β-lactams are possible, aminoglycosides could be paired with glycopeptides such as vancomycin or teicoplanin.18,19 Other combinations, with the risk of more side effects, contain the application of ceftriaxone/levofloxacin or vancomycin/gentamicin.1,20
The low prevalence of L garvieae infections in humans, despite physicians increasing awareness of the clinical relevance of L garvieae as a human pathogen, can be explained by incorrect interpretation of other streptococcal species and, in the past, by a lack of sufficient laboratory equipment. Only 25 cases of IE due to L garvieae have been described since 1991,5 when it first was identified as the cause of an IE. We will describe L garvieae IE in a patient who has an aortic prosthetic valve replacement.
Case Presentation
We present a case of a 78-year-old male with extensive cardiac history, including paroxysmal atrial fibrillation, essential hypertension, chronical renal dysfunction (III-IV, conservative therapy), stenosis of the right external carotid artery, and stenting of the right coronary artery. The patient underwent cardiac surgery including aortic valve replacement (Medtronic Hancock II, 23 mm) and coronary artery bypass grafting (LIMA-LAD, LIMA-PLA T-Graft) 1½ years prior this presentation.
He was admitted to the hospital with a 5-day history of exertional dyspnea NYHA III (New York Heart Association Class III) and expectoration. On admission, he had a tympanic temperature of 38.5°C, a heart rate of 71 beats/min, blood pressure of 148/68 mm Hg, and oxygen saturation of 97% of room air. During his physical examination, there was an aortic systolic murmur (4/6) on auscultation. His medications included mucolytic, aspirin, β-blocker, statin, diuretic, pantoprazole, and rivaroxaban. He was admitted for progressive heart failure with a fever of 38.5°C and chills.
Laboratory Findings
Admission laboratory results were significant for a white blood cell count of 20.1 × 109/L, a C-reactive protein level of 209 mg/L, and an interleukin-6 level of 24.93 pg/mL (<6.4 pg/mL). Four blood cultures were drawn, and it was then started to treat the patient with empirical antibiotics (vancomycin, gentamicin, and rifampicin) due to concern for prosthetic valve IE.
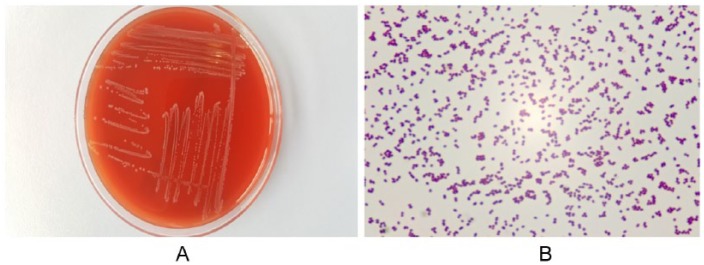
Three out of 4 blood cultures were positive for gram-positive chain cocci, and 4 more blood cultures were drawn. After 48 hours, L garvieae had grown in all 3 positive blood cultures. L garvieae was also found in the repeated blood cultures (Figure 1).
Figure 1.
(A) Growth of colonies of Lactococcus garvieae on a blood agar plate after 48 hours of incubation in aerobic conditions. (B) Gram stain of Lactococcus garvieae with clusters and short chains of gram-positive cocci.
Lactococcus garvieae was susceptible to ampicillin, vancomycin, cefotaxime, ceftriaxone, penicillin, and gentamicin (high level). It was resistant to clindamycin, which has previously been described in other cases. Empiric antibiotic treatment was completed for 6 weeks with 4 million units of penicillin given every 4 hours. During the first 2 weeks, gentamicin was also given, initially 320 mg given every 24 hours, then later reduced to 240 mg every 24 hours.
A transesophageal ultrasound (TEE) was performed and we obtained the results described below. Left atrial appendage without detection of thrombotic material with good flow velocity (>0.5 m/s). Atrial septum without detection of atrial septal defect or patent foramen ovale. Aortic valve replacement (Hancock II, 23 mm) morphologically with degenerative stenosis (Vmax 5.42 m/s, Pmax 117 mm Hg, and Pmean 71 mm Hg) and visually impaired pocket valve separation (AVAI [indexed aortic valve area] = 0.6 cm2) and pedunculated echogenic material of 10-mm diameter on the prosthetic aortic valve, no paravalvular leak. No vegetation was seen on pacemaker leads. Mitral valve with degenerative change emphasizes posterior mitral leaflet/anulus with central mitral valve insufficiency I°/II°. Tricuspid valve was inconspicuous as far as visible. No tricuspid valve insufficiency. Aorta with evidence of arteriosclerotic changes. An abdominal ultrasound showed shrunken kidneys and an enlarged spleen.
Operational Approach and Findings
Five days after admission, the patient underwent surgery for the repeated procedure. After careful preparation, the heart valve was removed, and the vitreous deposits were extracted. Intraoperative findings showed thrombotic/fibrinous flat deposits, which were attached to the valve leaflets. After the explanation of the prosthetic aortic valve and the removal of the infected tissue, an aorta ascendens enlargement plastic (Manugian) was needed and performed. A Medtronic Hancock II Porcine, 21 mm, was inserted into the aortic valve position. The intraoperative TEE showed no evidence of paravalvular leaks or prosthetic heart valve insufficiency. The following intensive care stay was uneventful, as well as the rest of the patient’s hospitalization.
Microbiological Findings and Genome Detection of Lactococcus garvieae
Microscopic examination of the prosthetic aortic valve showed sclerotic tissue, and microbiological testing detected L garvieae bacterial as well as inflammatory cells, in the form of granulocytes. Microbiological testing of the blood culture detected L garvieae by using MALDI-TOF mass spectrometry. The final diagnosis was IE caused by L garvieae.
The patient was discharged on postoperative day 8, after 11 days of intravenous antibiotics, which were continued during the patient’s post-hospitalization rehabilitation. The patient underwent a total course of 6 weeks of penicillin and 2 weeks of gentamicin.
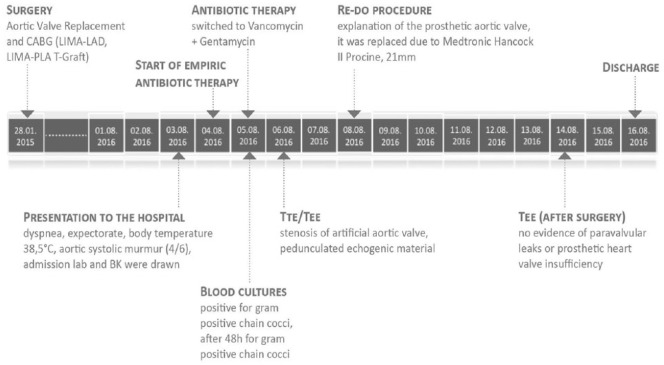
During the 6-month follow-up period, the patient was free of L garvieae IE (Figure 2).
Figure 2.
Timeline of patient’s medical history.
Discussion
Infective endocarditis is a common disease, especially in patients with heart valve dysfunction or after a prosthetic valve replacement. IE is most commonly caused by Streptococcus viridans and staphylococci group.
Lactococcus garvieae is an unusual human pathogen and seems to behave like an opportunistic agent with low virulence.21 Prior to the case we describe above, only 25 cases (Table 1) of IE due to L garvieae have been described since its first description in 1991. L garvieae has been related to a variety of different opportunistic infections.10,11,14,20,22-24
Table 1.
Table of LG endocarditis cases described in the literature (incidence, demographics, type, risk factors, antibiotics, surgery, outcome).
| Reference | Acquired Infection Location | Sex/Age | Type | Risk Factors | Antibiotics | Surgery | Outcome |
|---|---|---|---|---|---|---|---|
| Navas et al13 | USA | Male/64 | NAV | VAN | Yes | Survival | |
| Ortiz et al25 | Spain | Female/70 | NMV | AMX + GEN | Yes | Survival | |
| Ortiz et al25 | Spain | Female/77 | NMV/NAV | GD | AMX + GEN | No | Death |
| Backes et al26 | Holland | Female/68 | NAV | GD | No | Survival | |
| Zuily et al8 | France | Female/64 | PMV | RF/GD | AMX + GEN | No | Survival |
| Heras et al27 | Spain | Male/68 | NMV | GEN + AMP + CTX | Yes | Death | |
| Watanabe et al17 | Japan | Female/55 | NMV | PEN + GEN, CTX + GEN | No | Survival | |
| Li et al12 | China | Male/41 | NMV | PEN + GEN | Yes | Survival | |
| Clavero et al28 | Chile | Female/72 | NMV | RF | AMP + CLX, VAN + GEN | No | Death |
| Rasmussen et al29 | Sweden | Male/81 | PAV/NMV | GD | PEN + TOB | No | Survival |
| Bazemore et al30 | USA | Male/45 | PAV | GD | VAN + Pip/Taz, CTX + GEN | Yes | Survival |
| James et al22 | UK | Female/56 | PAV | VAN | No | Survival | |
| Tsur et al31 | Israel | Male/76 | PAV | GD | CTX + GEN | No | Survival |
| Russo et al1 | Italy | Male/63 | PAV/NMV | GD | VAN + GEN, AMP + GEN | No | Survival |
| Hirakawa et al32 | Brazil | Female/58 | PMV | RF | VAN | No | Survival |
| Suh et al33 | Korea | Female/75 | PMV | RF | CTX + GEN + RI, TEI + CTX | Yes | Survival |
| Wilbring et al34 | Germany | Male/55 | PTV | VAN + GEN, AMX + LEV | No | Survival | |
| Landeloos et al35 | Belgium | Female/82 | PMV | GD | AMX, PEN + GEN | No | Survival |
| Lim and Jenkins36 | UK | Male/57 | NMV | GD | AMX + GEN | Yes | Survival |
| Wang et al37 | China | Male/72 | NMV | RF/GD | PEN + GEN | No | Survival |
| Fleming et al18 | Korea | Male/68 | PAV/NMV | RF/GD | VAN | No | Death |
| Fihman et al21 | France | Female/86 | PAV | GD | AMX + GEN | No | Survival |
| Vinh et al6 | Canada | Male/80 | NAV | GD | AMP | Yes | Survival |
| Yiu et al38 | China | Male/67 | NMV | AMP | Yes | Survival | |
| Fefer et al39 | USA | Female/84 | NMV/PAV | CTX | Yes | Death |
Abbreviations: NAV, native aortic valve; VAN, vancomycin; NMV, native mitral valve; AMX, amoxicillin; GEN, gentamicin; GD, gastrointestinal disorder; PMV, prosthetic mitral valve; RF, raw fish; AMP, ampicillin; CTX, ceftriaxone; CLX, cloxacillin; PAV, prosthetic aortic valve; PEN, penicillin; TOB, tobramycin; Pip/Taz, piperacillin/tazobactam; RI, rifampicin; TEI, teicoplanin; PTV, prosthetic tricuspid valve; LEV, levofloxacin.1,6,8,12,13,17,18,21,22,25-39
Including our case, a total of 26 cases of IE caused by L garvieae have been reported in the literature. The infection has occurred in the following areas: 15.4% in North America, 7.7% in Latin America, 23% in Asia, and 50% in Europe. One infection (3.9%) occurred in the Middle East (Israel). Fourteen patients were men (53.8%), and 12 were women (46.2%). The mean age was 68 years (67.8 years), and 18 patients were 60 years of age or older (69.2%).
Eleven patients underwent cardiac surgery, and 4 of the 11 were repeated procedures. Seventeen cases (65.4%) involved a native valve. Prior the case we describe above, the previous 25 cases included 5 patients (20%) with multivalve IE, for a total of 31 affected valves. Among these were 4 native aortic valves (12.9%), 13 native mitral valves (41.9%), 8 prosthetic aortic valves (25.8%), prosthetic mitral valves, and 1 prosthetic tricuspid valve (3.2%).
Of these 25 cases, 5 patients (19.2%) died due to multi-organ failure, heart failure, or cerebral hemorrhage.18,25,27,28,39
The exact mechanism leading to transmission to humans is not yet established. Previous case reports assume that L garvieae infection occurred due to a barrier gap in the digestive tract (portal of entry) while consuming contaminated food, especially raw fish or fermented milk products. This fits with the observation that most of the L garvieae infections are described during summer and in patients who have a history of consuming raw fish.4 The number of L garvieae infections in fish also peak in the summer months (June, July, and August) when the water temperature is warmest.
This study shows that out of the 26 cases, 6 cases (23%) had a history of prior raw fish consumption. Gastrointestinal disorders (potentially leading to barrier gaps) were present in 13 cases (53.8%; 6 colonic polyps, 3 colonic/rectal diverticulosis, 1 gastric ulcer, 1 duodenal ulcer, 2 colorectal cancer).
Our patient was immunocompromised and had a postoperative ileus 1½ years ago and colorectal cancer, which could have facilitated the infection, together with the predisposing factor of a biological prosthetic valve. However, he denied eating raw fish in the last couple of weeks prior to admission to the hospital.
Even with only 26 cases described in the world, an infection due to L garvieae requires specific care and therapy. IE may be higher than reported in the literature and might be explained due to the fact that it is often misdiagnosed. Lactococcus is difficult to distinguish from Enterococcus,9 and some microbiological laboratories do not have the optimal equipment and capabilities to differentiate this bacterium. Although L garvieae is considered opportunistic, there is more need for research in this area.
In conclusion, the best way to identify L garvieae is through 2 or 3 sets of blood cultures and, in case of surgery, by analyzing a sample of the removed valve. With this material, it is possible to distinguish L garvieae by MALDI-TOF mass spectrometry and the 16s rRNA gene PCR, in addition to finding a susceptibility profile. In order to determine the final diagnosis of IE, the clinical symptoms, the microbiological findings, and the imaging procedures (transthoracic echocardiogram, TEE) must be evaluated using the Duke criteria.
The presented case fulfills the modified Dukes criteria of IE40 with 1 major criterion (TEE: vegetation on the prosthetic valve) and 3 minor criteria (fever >38°C, prosthetic heart valve, positive blood cultures—not listed as typical pathogen).
All 26 patients were treated with antibiotic therapy, mainly β-lactam antibiotics. The identified organism in this case appeared in the antimicrobial susceptibility test (Etest method) and showed a susceptibility to ampicillin, vancomycin, cefotaxime, ceftriaxone, penicillin, and gentamicin (high level). It was resistant to clindamycin, similar to other described cases of L garvieae (Table 2).
Table 2.
Microbiological Findings: Susceptibility Testing of Lactococcus garvieae.
| Antibiotic | MIC (mg/L) | MIC Interpretationa |
|---|---|---|
| Penicillin | 0.25 | S |
| Ampicillin | 0.5 | S |
| Ceftriaxone | 0.25 | S |
| Cefotaxime | 0.25 | S |
| Gentamicin | 6 | S |
| Clindamycin | >1.0 | R |
| Vancomycin | 1.0 | S |
Abbreviations: MIC, minimal inhibitory concentration; S, sensitive; R, resistant.
Because of the lack of standardized susceptibility testing, the MIC interpretation is of orienting character only.
The treatment for L garvieae IE is not yet standardized because the exact criteria of the susceptibility test have not been established. In the case described above, the patient received a 6-week course of intravenous penicillin, 4 million units every 4 hours, and a 2-week course of gentamicin, 320 mg every 24 hours, later reduced to 240 mg every 24 hours.
Conclusion
Lactococcus garvieae endocarditis is a rare disease in humans. Including our case, a total of 26 cases have been reported in the world.1
Prior to 1985, Lactococcus bacteria were included in the Streptococcus genus. The first human infection with L garvieae was documented in 1991, and since then, the relevance and clinical significance in humans has increased. Improved determination techniques, such as MALDI-TOF mass spectrometry and the 16s rRNA gene PCR, are likely to lead to a better and faster identification of the bacterium.
As observed in other cases of L garvieae endocarditis, consumption of raw fish, abnormalities in the digestive tract, and underlining cardiac conditions (prosthetic valve) seem to be risk factors for an IE due to L garvieae. This bacterium seems to mainly affect elderly and immunocompromised individuals with underlying cardiac conditions or those with anatomical or physiological alterations of their gastrointestinal tract. However, there have also been infections described in young and healthy people.
On the basis of existing literature of L garvieae endocarditis cases, it is assumed that L garvieae exposure can lead to infections in immunosuppressed humans via raw fish consumption and/or with underlying gastrointestinal disorders.
In the future, consideration should be given to examining patients with L garvieae endocarditis for changes in their gastrointestinal tract as a potential port of entry for infection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- 1. Russo G, Iannetta M, D’Abramo A, et al. Lactococcus garvieae endocarditis in a patient with colonic diverticulosis: first case report in Italy and review of the literature. New Microbiol. 2012;35:495-501. [PubMed] [Google Scholar]
- 2. Ferrario C, Ricci G, Milani C, et al. Lactococcus garvieae: where is it from? A first approach to explore the evolutionary history of this emerging pathogen. PLoS One. 2013;8:e84796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins MD, Farrow JA, Phillips BA, Kandler O. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J Gen Microbiol. 1983;129:3427-3431. [DOI] [PubMed] [Google Scholar]
- 4. Vendrell D, Balcázar JL, Ruiz-Zarzuela I, de Blas I, Gironés O, Múzquiz JL. Lactococcus garvieae in fish: a review. Comp Immunol Microbiol Infect Dis. 2006;29:177-198. [DOI] [PubMed] [Google Scholar]
- 5. Elliott JA, Collins MD, Pigott NE, Facklam RR. Differentiation of Lactococcus lactis and Lactococcus garvieae from humans by comparison of whole-cell protein patterns. J Clin Microbiol. 1991;29:2731-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vinh DC, Nichol KA, Rand F, Embil JM. Native-valve bacterial endocarditis caused by Lactococcus garvieae. Diagn Microbiol Infect Dis. 2006;56:91-94. [DOI] [PubMed] [Google Scholar]
- 7. Gibello A, Galán-Sánchez F, Blanco MM, Rodríguez-Iglesias M, Domínguez L, Fernández-Garayzábal JF. The zoonotic potential of Lactococcus garvieae: an overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Res Vet Sci. 2016;109:59-70. [DOI] [PubMed] [Google Scholar]
- 8. Zuily S, Mami Z, Meune C. Lactococcus garvieae endocarditis. Arch Cardiovasc Dis. 2011;104:138-139. [DOI] [PubMed] [Google Scholar]
- 9. Facklam R, Elliott JA. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev. 1995;8:479-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dylewski J. Urinary tract sepsis caused by Lactococcus garvieae. Clin Microbiol Newsl. 2014;36:30-31. [Google Scholar]
- 11. Kim JH, Go J, Cho CR, Kim JI, Lee MS, Park SC. First report of human acute acalculous cholecystitis caused by the fish pathogen Lactococcus garvieae. J Clin Microbiol. 2013;51:712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li WK, Chen YS, Wann SR, Liu YC, Tsai HC. Lactococcus garvieae endocarditis with initial presentation of acute cerebral infarction in a healthy immunocompetent man. Intern Med. 2008;47:1143-1146. [DOI] [PubMed] [Google Scholar]
- 13. Navas ME, Hall G, El Bejjani D. A case of endocarditis caused by Lactococcus garvieae and suggested methods for identification. J Clin Microbiol. 2013;51:1990-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nadrah K, Cerar T, Papst L, et al. Lactococcus garvieae septicaemia in a patient with artificial heart valves. Wien Klin Wochenschr. 2011;123:677-679. [DOI] [PubMed] [Google Scholar]
- 15. Zlotkin A, Eldar A, Ghittino C, Bercovier H. Identification of Lactococcus garvieae by PCR. J Clin Microbiol. 1998;36:983-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeMarco ML, Ford BA. Beyond identification: emerging and future uses for MALDI-TOF mass spectrometry in the clinical microbiology laboratory. Clin Lab Med. 2013;33:611-628. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe Y, Naito T, Kikuchi K, et al. Infective endocarditis with Lactococcus garvieae in Japan: a case report. J Med Case Rep. 2011;5:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleming H, Fowler SV, Nguyen L, Hofinger DM. Lactococcus garvieae multi-valve infective endocarditis in a traveler returning from South Korea. Travel Med Infect Dis. 2012;10:101-104. [DOI] [PubMed] [Google Scholar]
- 19. Fog-Møller T, Andersen J. Serious infection with Lactococcus garvieae [in Danish]. Ugeskr Laeger. 2012;174:1096-1097. [PubMed] [Google Scholar]
- 20. Aubin GG, Bémer P, Guillouzouic A, et al. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger. J Clin Microbiol. 2011;49:2074-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fihman V, Raskine L, Barrou Z, et al. Lactococcus garvieae endocarditis: identification by 16S rRNA and sodA sequence analysis. J Infect. 2006;52:e3-e6. [DOI] [PubMed] [Google Scholar]
- 22. James PR, Hardman SM, Patterson DL. Osteomyelitis and possible endocarditis secondary to Lactococcus garvieae: a first case report. Postgrad Med J. 2000;76:301-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee JY, Seo MY, Yang J, et al. Polymicrobial peritonitis with Lactococcus lactis in a peritoneal dialysis patient. Chonnam Med J. 2014;50:67-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mofredj A, Baraka D, Kloeti G, Dumont JL. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am J Med. 2000;109:513-514. [DOI] [PubMed] [Google Scholar]
- 25. Ortiz C, López J, Del Amo E, Sevilla T, García PE, San Román JA. Lactococcus garvieae infective endocarditis: report of 2 cases and review of the literature. Rev Esp Cardiol (Engl Ed). 2014;67:776-778. [DOI] [PubMed] [Google Scholar]
- 26. Backes Y, Gruteke P, Branger J. Lactococcus garvieae endocarditis [in Dutch]. Ned Tijdschr Geneeskd. 2015;159:A8738. [PubMed] [Google Scholar]
- 27. Cañas VH, Ramirez MDP, Jiménez FB, et al. Lactococcus garvieae endocarditis in a native valve identified by MALDI-TOF MS and PCR-based 16s rRNA in Spain: a case report. New Microbes New Infect. 2015;5:13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clavero R., Escobar J, Ramos-Avasola S, Merello L, Álvarez F. Lactococcus garvieae endocarditis in a patient undergoing chronic hemodialysis. First case report in Chile and review of the literature [in Spanish]. Rev Chilena Infectol. 2017;34:397-403. [DOI] [PubMed] [Google Scholar]
- 29. Rasmussen M, Björk Werner J, Dolk M, Christensson B. Lactococcus garvieae endocarditis presenting with subdural haematoma. BMC Cardiovasc Disord. 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bazemore TC, Maskarinec SA, Zietlow K, Hendershot EF, Perfect JR. Familial adenomatous polyposis manifesting as Lactococcus endocarditis: a case report and review of the association of Lactococcus with underlying gastrointestinal disease. Case Rep Infect Dis. 2016;2016:5805326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsur A, Slutzki T, Flusser D. Lactococcus garvieae endocarditis on a prosthetic biological aortic valve. Zoonoses Public Health. 2015;62:435-437. [DOI] [PubMed] [Google Scholar]
- 32. Hirakawa TF, Costa FA, Vilela MC, Rigon M, Abensur H, Araújo MR. Lactococcus garvieae endocarditis: first case report in Latin America. Arq Bras Cardiol. 2011;97:e108-e110. [DOI] [PubMed] [Google Scholar]
- 33. Suh Y, Kim MJ, Jung JS, et al. Afebrile multi-valve infective endocarditis caused by Lactococcus garvieae: a case report and literature review. Intern Med. 2016;55:1011-1015. [DOI] [PubMed] [Google Scholar]
- 34. Wilbring M, Alexiou K, Reichenspurner H, Matschke K, Tugtekin SM. Lactococcus garvieae causing zoonotic prosthetic valve endocarditis. Clin Res Cardiol. 2011;100:545-546. [DOI] [PubMed] [Google Scholar]
- 35. Landeloos ER, Berkow EL, Chow N, Welsh RM. Lactococcus garvieae prosthetic mitral valve endocarditis: a case report and literature review. Clin Microbiol Newsl. 2017;39:99-103.29503491 [Google Scholar]
- 36. Lim FH, Jenkins DR. Native valve endocarditis caused by Lactococcus garvieae: an emerging human pathogen. BMJ Case Rep. 2017;2017:bcr-2017-220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang CY, Shie HS, Chen SC, et al. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int J Clin Pract. 2007;61:68-73. [DOI] [PubMed] [Google Scholar]
- 38. Yiu KH, Siu CW, To KK, et al. A rare cause of infective endocarditis; Lactococcus garvieae. Int J Cardiol. 2007;114:286-287. [DOI] [PubMed] [Google Scholar]
- 39. Fefer JJ, Ratzan KR, Sharp SE, Saiz E. Lactococcus garvieae endocarditis: report of a case and review of the literature. Diagn Microbiol Infect Dis. 1998;32:127-130. [DOI] [PubMed] [Google Scholar]
- 40. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200-209. [DOI] [PubMed] [Google Scholar]