Short abstract
Matrix metalloproteinases (MMPs) have been suggested to contribute to long-term potentiation, behavioral learning, and memory. In the dorsal horn of spinal cord, MMPs were reported to contribute to injury-related changes, and inhibitors of MMPs have been proposed as potential analgesics. However, it is unclear whether MMP inhibitors produce these effects by inhibiting the function of N-methyl-D-aspartate receptor (NMDAR), a key receptor for the induction of long-term potentiation. In this study, we wanted to examine if MMP inhibitors affect NMDAR-mediated excitatory postsynaptic currents in the anterior cingulate cortex of adult mice. Among different subtype inhibitors we used, we found that MMP-9 and MMP-2/9 inhibitors did not change NMDAR-mediated excitatory postsynaptic currents. However, MMP-3 and broad-spectrum MMP inhibitors reduced the NMDAR-mediated excitatory postsynaptic currents. Consistently, MMP-9 and MMP-2/9 inhibitors had no effect on NMDAR-dependent long-term potentiation, but MMP-3 and broad-spectrum MMP inhibitors inhibited the induction of long-term potentiation. Our results suggest that MMP inhibitors may produce their effects by inhibiting NMDAR functions in central synapses.
Keywords: Matrix metalloproteinase, anterior cingulate cortex, long-term potentiation, N-methyl-D-aspartate receptor
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc endopeptidases, which regulate the rigidity of the extracellular matrix. MMPs have been reported to contribute to behavioral sensitization in animal models of nerve injury.1 Although MMP inhibitors such as MMP-2 and 9 inhibitors produced analgesic effects in animal models,1 the side effects of these inhibitors prevent them to be used for the treatment of chronic pain.2 Fewer studies have been performed for possible synaptic mechanisms of their analgesic effects. Recent studies show that some MMP subtypes are associated with neuronal plasticity in the amygdala and hippocampus.3–7 In particular, it has been reported that MMP-9 plays a role in the modification of dendritic spine morphology and brain development/neurogenesis.8 For MMP-3 (including the gelatinase class), it has been shown that MMP-3 may affect synaptic plasticity through cleaving chondroitin sulfate proteoglycans.9
Long-term potentiation (LTP) is a key form of synaptic plasticity for learning and memory.10,11 Previous studies have shown that inhibitors of MMP-3 decreased LTP in the hippocampus.4,12 Furthermore, MMP-9 and MMP-2/9 inhibitors also decreased hippocampal LTP.4,13 One possible mechanism of MMP inhibitors inhibiting hippocampal LTP is affecting N-methyl-D-aspartate receptors (NMDARs).4,12 The anterior cingulate cortex (ACC) is a key cortical region that plays important roles in pain perception and emotional responses.14–17 Postsynaptic form of LTP induced by a pairing protocol in the ACC synapses depends on the activation of NMDARs.18,19 In this study, we investigated the roles of different subtype inhibitors of MMP on the NMDAR-mediated excitatory postsynaptic current (EPSCs) and postsynaptic LTP (induced by a pairing protocol) performing in whole-cell patch-clamp recordings on pyramidal neurons in the ACC of adult mice.
Materials and methods
Animals
Adult C57BL/6 male mice were purchased from Charles River (7–12 weeks old). All mice were maintained on a 12-h light/dark cycle with food and water provided ad libitum. The mouse protocols were approved by the Animal Care and Use Committee of the Xi’an Jiaotong University.
Slice preparation
Coronal brain slices including the ACC were prepared using our previous methods.17,20 Briefly, mice were anesthetized with isoflurane and then sacrificed by decapitation. The brains were quickly removed and placed in cold oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM NaHCO3, and 10 mM glucose. For making coronal brain slices (300 μm), the brains were glued to the cutting staged tissue slicer (Leica, VT1200S). Slices were transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) ACSF at room temperature for at least 1 h.
In vitro whole-cell patch-clamp recording
Experiments were performed in a recording chamber by using an Olympus BX51W1 microscope with infrared differential interference contrast (DIC) optics for the visualization of whole-cell patch clamp recording. In this study, evoked EPSCs (eEPSCs) were recorded from the layer II/III neurons of the ACC with an Axopatch 200B amplifier (Molecular Devices, CA), and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in the layer V/VI of the ACC slices. Control test pulses were given every 30 s. The amplitudes of eEPSCs were adjusted between 50 and 100 pA to obtain a baseline. Stable baseline recordings of eEPSCs were obtained for 10 min before the induction of the LTP. The recording pipettes (3–6 MΩ) were filled with a solution containing 145 mM K-gluconate, 5 mM NaCl, 1 mM MgCl2, 0.2 mM EGTA, 10 mM HEPES, 2 mM Mg-ATP, and 0.1 mM Na3-GTP (adjusted to pH 7.2 with KOH). The membrane potential was held at −60 mV throughout the experiment. We also isolated the NMDAR-mediated component of EPSCs pharmacologically in Mg2+-free ACSF containing 20 µM CNQX, 1 µM glycine, and 100 µM picrotoxin (PTX). NMDAR-mediated EPSCs were induced at 0.05 Hz, and neurons were voltage clamped at −30 mV. Access resistance was 15 to 30 MΩ and monitored throughout the experiment. Data were discarded if the access resistance changed >15% during the experiment. Data were filtered at 1 kHz and digitized at 10 kHz.
LTP induction protocols
For the induction of post-LTP, a pairing LTP protocol (also called the pairing protocol) was used by pairing 80 presynaptic pulses at 2 Hz with postsynaptic depolarization (holding at +30 mV) after obtaining stable EPSCs for 10 min.18 LTP was induced with the pairing protocol within 12 min after establishing the whole-cell configuration to avoid washout of intracellular contents that are critical for the establishment of synaptic plasticity.
Drug application
PTX, glycine, CNQX, and MMP-9 inhibitor I were obtained from Sigma Aldrich (Canada). AP5, GM 6001, UK 356618, and SB 3CT were obtained from Tocris Cookson (Bristol, UK). Glycine and AP5 were dissolved in distilled water. CNQX, GM 6001, UK 356618, MMP-9 inhibitor I, and SB 3CT were dissolved in dimethyl sulfoxide. PTX was dissolved in ethanol. All of these drugs were diluted from the stock solutions to the final desired concentration in the ACSF before immediate using. The dimethyl sulfoxide diluted in ACSF had no effect on basal synaptic transmission and plasticity.
Data analysis
Data were collected and analyzed with Clampex 10.2 and Clampfit 10.2 (Molecular Devices). For comparisons between two groups, we used the unpaired Student’s t-test or paired t-test. For comparison among three groups, we used one-way analysis of variance (ANOVA) or two-way ANOVA. Significance between groups was tested with a Bonferroni tests to adjust for multiple comparisons. Data were presented as the means ± standard error of the mean. In all cases, p < 0.05 was considered statistically significant.
Results
Effect of MMP-subtype inhibitors on NMDAR-mediated EPSCs
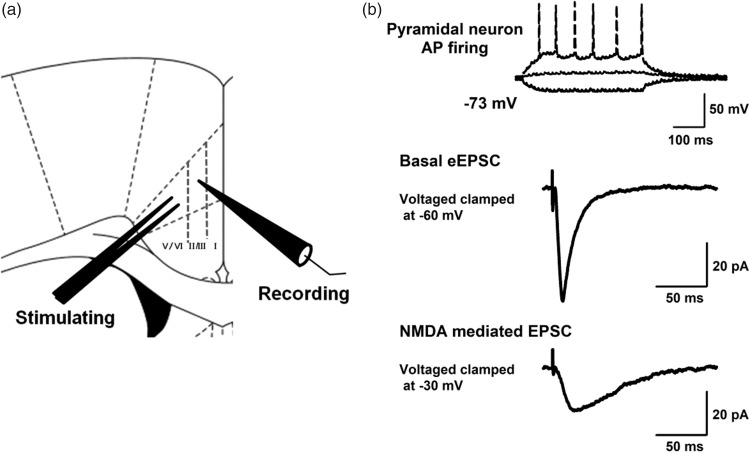
We performed whole-cell patch-clamp recordings on pyramidal neurons in layer ІІ/ІІІ of the ACC (Figure 1(a)). The pyramidal neurons were identified based on their morphological properties and action potential (AP) firing pattern (Figure 1(b)). PTX (100 µM), glycine (1 µM), and CNQX (20 µM) were applied in the bath for recording NMDAR-mediated EPSCs (Figure 1(b)). To examine whether broad-spectrum MMP, MMP-3, MMP-9, and MMP-2/9 inhibitors affect NMDAR-mediated responses, broad-spectrum MMP inhibitor GM 6001, MMP-3 inhibitor UK 356618, MMP-9 inhibitor I, or MMP-2/9 inhibitor SB 3CT were applied at different concentrations after 10 min of baseline recording. Finally, MMP inhibitors were washed out.
Figure 1.
Electrophysiological response of pyramidal neurons in layer ІІ/ІІІ of the ACC using whole-cell patch-clamp recordings. (a) Schematic drawing of a coronal ACC slice. The stimulation electrode was placed in layer V/VI of the ACC and the layer II/III pyramidal neurons were recorded. (b) Top: example AP firing pattern of a pyramidal neuron in current-clamp mode by injection of currents (−50, 0, and +50 pA) for 400 ms. Middle: example traces of basal eEPSCs with a stimulation artifact (voltage clamped at −60 mV). Bottom: example traces of NMDAR-mediated eEPSCs with a stimulation artifact (voltage clamped at −30 mV). AP: action potential; NMDA: N-methyl-D-aspartate; EPSC: excitatory postsynaptic current.
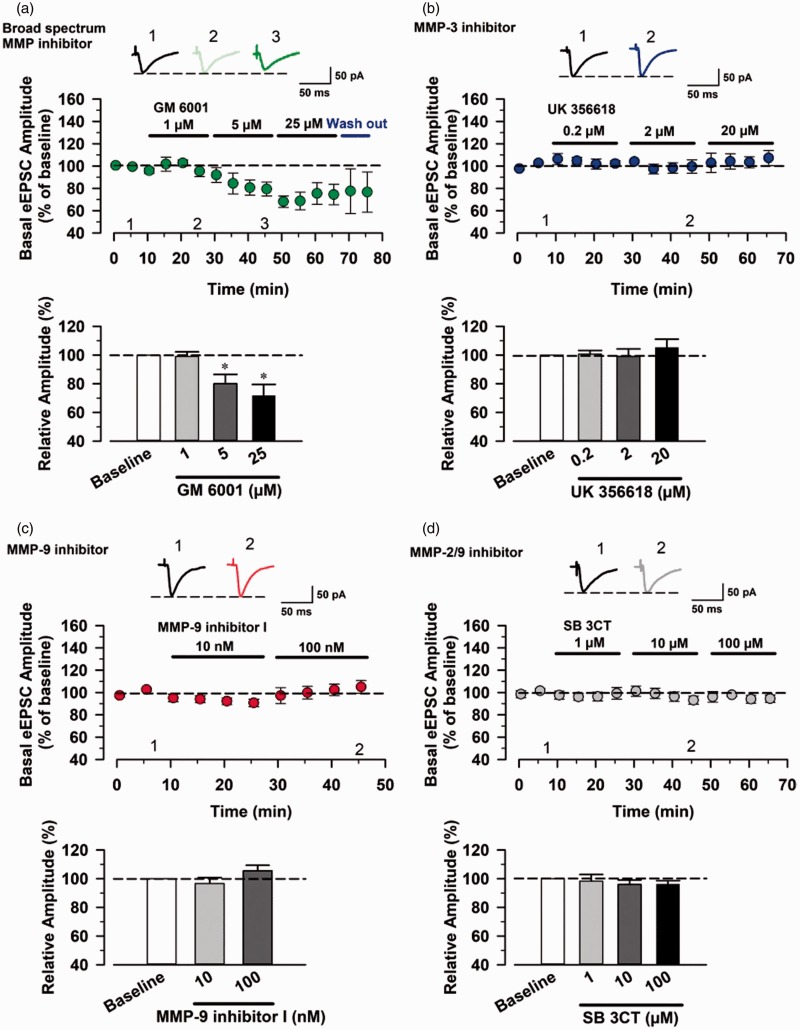
We found that NMDAR-mediated EPSCs were not significantly affected after application of UK 356618 (0.2 µM, n = 5 neurons/5 mice, Figure 2(b)), MMP-9 inhibitor I (10 nM and 100 nM, n = 5 neurons/5 mice, Figure 2(c)), and SB 3CT (1 µM, 10 µM, and 100 µM, n = 5 neurons/5 mice, Figure 2(d)). However, bath application of GM 6001 (1, 5, and 25 µM) produced dose-dependent inhibition of NMDAR-mediated responses (1 µM: 82.69 ± 11.70% of baseline, p = 0.030; 5 µM: 72.85 ± 7.83% of baseline, p = 0.0015; 25 µM: 64.80 ± 12.48% of baseline, p = 0.0032) (paired t-test, n = 5 neurons/5 mice, Figure 2(a)). Similarly, MMP-3 inhibitor UK 356618 (2 and 20 µM) also produced significant reduction (2 µM: 88.48 ± 4.25% of baseline, p = 0.0037; 20 µM: 77.95 ± 5.09% of baseline, p = 0.00064) (paired t-test, n = 5 neurons/5 mice, Figure 2(b)). In addition, the reduction did not return to the baseline level after the washout.
Figure 2.
Broad-spectrum MMP and MMP-3 inhibitors affect NMDAR-mediated EPSCs in ACC neurons. (a) Effect of bath application of GM 6001 (1, 5, and 25 µM) on NMDAR-mediated EPSCs. Top: example traces of the NMDAR-mediated eEPSCs before (1) and after (2) GM 6001 application. Middle: a time course plot of pooled data for the eEPSCs with bath application of different concentrations of GM 6001. Bottom: summary data of the different doses of GM 6001 on the NMDAR-mediated EPSCs in the ACC (n = 5 neurons/5 mice). (b) Effect of bath application of UK 356618 (0.2, 2, and 20 µM) on NMDAR-mediated EPSCs (n = 5 neurons/5 mice). (c) Effect of bath application of MMP-9 inhibitor I (10, and 100 µM) on NMDAR-mediated EPSCs (n = 5 neurons/5 mice). (d) Effect of bath application of SB 3CT (1, 10, and 100 µM) on NMDAR-mediated EPSCs (n = 5 neurons/5 mice). *P < 0.05, **P < 0.01, and ***P < 0.001. eEPSC: evoked excitatory postsynaptic current; MMP: matrix metalloproteinase.
MMP-subtype inhibitors on basal synaptic transmission
To examine whether these inhibitors affect basal synaptic transmission in the ACC, we checked whether the basal eEPSCs could be affected by application of GM 6001, UK 356618, MMP-9 inhibitor I, and SB 3CT (Figure 3). We found that none of these inhibitors except 5 µM and 25 µM GM 6001 produced significant effects on baseline synaptic responses (paired t-test, n = 5 neurons/5 mice in each group, Figure 3(a) to (d)). For GM 6001, bath application of 5 µM and 25 µM GM 6001 significantly reduced the amplitudes of eEPSCs (5 µM: 80.05 ± 14.42% of baseline, p = 0.036, 25 µM: 71.73 ± 17.38% of baseline, p = 0.022; paired t-test, n = 5 neurons/5 mice, Figure 3(a)). The inhibitory effects persisted during the washout period.
Figure 3.
MMP-3, MMP-9, and MMP-2/9 inhibitors do not affect basal EPSCs in the ACC neurons. (a) Effect of bath application of GM 6001 (1, 5, and 25 µM) on the basal eEPSCs. Top: example traces of the basal eEPSCs before (1) and after (2 and 3) bath application of GM 6001. Middle: a time course plot of pooled data for the eEPSCs with bath application of different concentrations GM 6001. Bottom: summary data of the different doses of GM 6001 on the basal eEPSCs in the ACC (n = 5 neurons/5 mice). (b) Effect of bath application of UK 356618 (0.2, 2, and 20 µM) on basal eEPSCs. (n = 5 neurons/5 mice). (c) Effect of bath application of MMP-9 inhibitor I (10, and 100 nM) on basal eEPSCs. (n = 5 neurons/5 mice). (d) Effect of bath application of SB 3CT (1, 10, and 100 µM) on basal eEPSCs. (n = 5 neurons/5 mice). *P < 0.05. eEPSC: evoked excitatory postsynaptic current; MMP: matrix metalloproteinase.
MMP inhibitors on the induction of postsynaptic ACC LTP
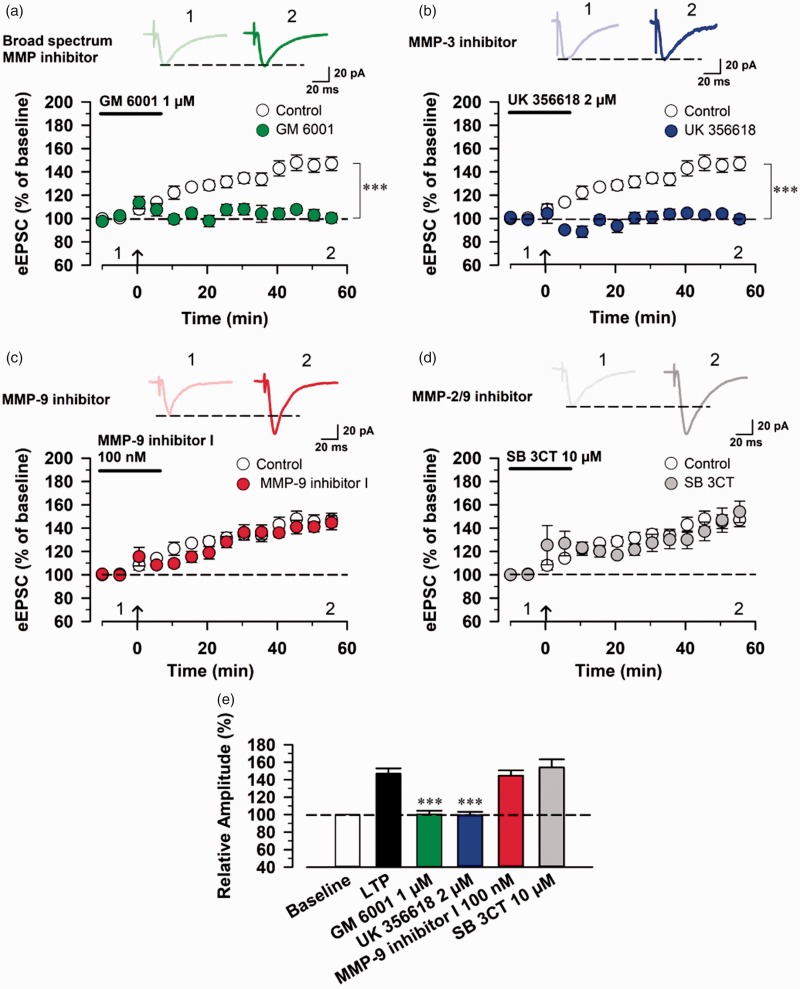
Previous studies have demonstrated that postsynaptic-LTP is a synaptic mechanism for chronic pain in the ACC.18,20 Thus, we investigated whether the perfusion of MMP inhibitors affects postsynaptic LTP in the ACC neurons. We first examined the effect of MMP inhibitors on the induction of postsynaptic LTP. Interestingly, we found that bath application of 1 µM GM 6001 blocked the induction of LTP (control group: 147.11 ± 14.30% of baseline, n = 6 neurons/6 mice; GM 6001 group: 100.42 ± 9.03% of baseline, n = 5 neurons/5 mice; one-way ANOVA, F1,9 = 39.65, p = 0.00014, Figure 4(a) and (e)). Similarly, bath application of 2 µM UK 356618 also blocked the induction of LTP (UK 356618 group: 99.43 ± 8.44% of baseline, n = 5 neurons/5 mice, one-way ANOVA, F1.9 = 42.63, p = 0.00011, Figure 4(b) and (e)). By contrast, bath application of 100 nM MMP-9 inhibitor I and 10 µM SB 3CT did not affect the induction of postsynaptic LTP in the ACC (MMP-9 inhibitor I group, n = 5 neurons/5 mice; SB 3CT 1 group, n = 5 neurons/5 mice, Figure 4(c) to (e)).
Figure 4.
Broad-spectrum MMP and MMP-3 inhibitors block the induction of postsynaptic LTP in the ACC. (a) Effect of bath application of 1 µM GM 6001 on the induction of LTP. Top: example traces of eEPSCs before (1) and after (2) LTP induction. Bottom: a time course plot of pooled data for the eEPSCs in the ACC with bath application of GM 6001 (control group: n = 6 neurons/6 mice, GM 6001 group: n = 5 neurons/5 mice). (b) Effect of bath application of 2 µM UK 356618 on the induction of LTP (control group: n = 6 neurons/6 mice, UK 356618 group: n = 5 neurons/5 mice). (c) Effect of bath application of 100 nM MMP-9 inhibitor I on the induction of LTP (control group: n = 6 neurons/6 mice, MMP-9 inhibitor I group: n = 5 neurons/5 mice). (d) Effect of bath application of 10 µM SB 3CT on the induction of LTP (control group: n = 6 neurons/6 mice, SB 3CT group: n = 5 neurons/5 mice). (e) Summary of the effects of MMP inhibitors on the induction of LTP in the ACC. The mean amplitudes of eEPSCs were determined at 55–60 min after the induction of LTP. ***P < 0.001. eEPSC: evoked excitatory postsynaptic current; MMP: matrix metalloproteinase.
Effects on the expression of postsynaptic LTP
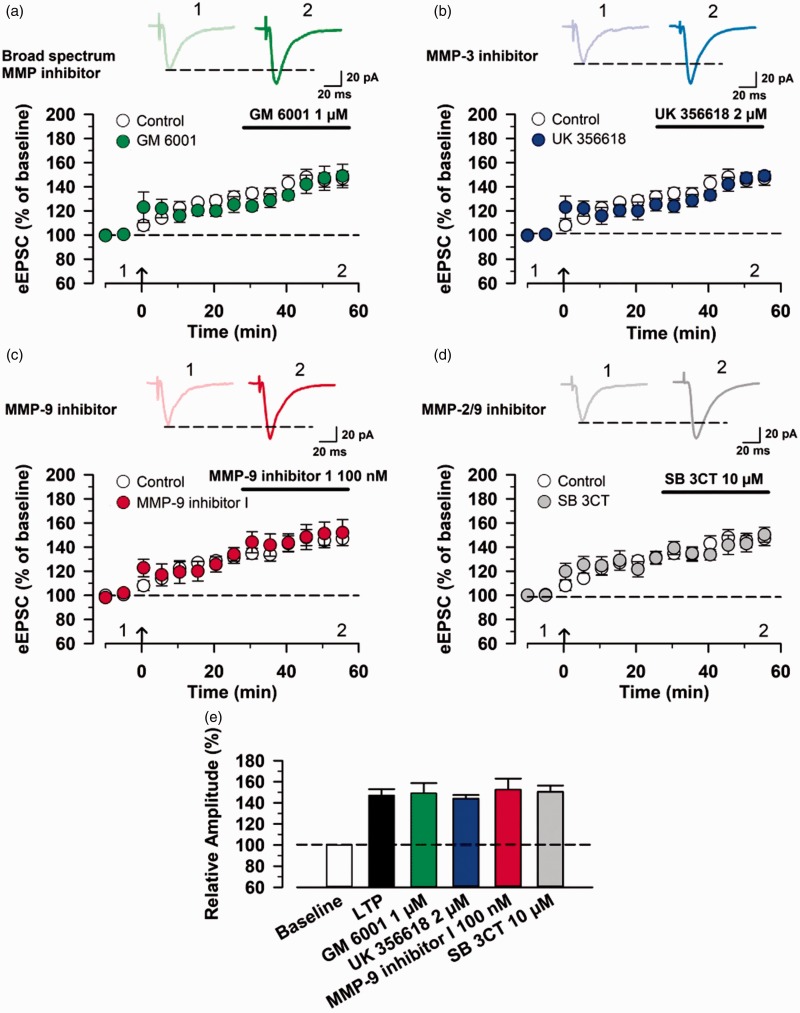
Finally, we wanted to examine the effects of MMP inhibitors on the maintenance of postsynaptic LTP. After achieving a stable baseline recording in response to single-pulse stimulation for at least 10 min at a holding potential of −60 mV, we applied the pairing protocol stimulation. MMP inhibitors were then applied in the bath 30 min after induction of postsynaptic LTP. We found that none of these inhibitors affected the expression of postsynaptic LTP in the ACC (control group, n = 6 neurons/6 mice; GM 6001 group: n = 5 neurons/5 mice; UK 356618 group, n = 5 neurons/5 mice; MMP-9 inhibitor I group, n = 5 neurons/5 mice; SB 3CT group, n = 5 neurons/5 mice, Figure 5(a) to (e)).
Figure 5.
MMP inhibitors do not affect the maintenance of postsynaptic LTP in the ACC. (a) Effect of bath application of 1 µM GM 6001 on the maintenance of LTP. Top: example traces of eEPSCs before (1) and after (2) GM6001 application. Bottom: a time course plot of pooled data for the eEPSCs in the ACC with bath application of GM 6001 (control group: n = 6 neurons/6 mice, GM 6001 group: n = 5 neurons/5 mice). (b) Effect of bath application of 2 µM UK 356618 on the maintenance of LTP (control group: n = 6 neurons/6 mice, UK 356618 group: n = 5 neurons/5 mice). (c) Effect of bath application of 100 nM MMP-9 inhibitor I on the maintenance of LTP (control group: n = 6 neurons/6 mice, MMP-9 inhibitor I group: n = 5 neurons/5 mice). (d) Effect of bath application of 10 µM SB 3CT on the maintenance of LTP (control group: n = 6 neurons/6 mice, SB 3CT group: n = 5 neurons/5 mice). (e) Summary of the effects of MMP inhibitors on the induction of LTP in the ACC. The mean amplitudes of eEPSCs were determined at 55–60 min after the induction of LTP. eEPSC: evoked excitatory postsynaptic current; MMP: matrix metalloproteinase.
Discussion
In this study, we investigated the effects of subtypes of MMP inhibitors on NMDAR-mediated responses and pairing protocol-induced post-LTP in the ACC. We showed that broad-spectrum MMP inhibitor and MMP-3 inhibitor reduced the NMDAR-mediated EPSCs, but MMP-9 and MMP-2/9 inhibitors did not affect NMDAR-mediated EPSCs. In addition, broad-spectrum MMP inhibitor and the MMP-3 inhibitor inhibited induction of post-LTP but had no effect on the maintenance of post-LTP. Our results indicate that broad-spectrum MMP and MMP-3 inhibitor-mediated reduction in LTP depends on NMDAR in ACC synapses.
MMPs and chronic pain
MMPs received some attentions, since they have been reported to be upregulated after nerve injury.1 Both MMP-2 and MMP-9 were increased at the dorsal root ganglion sensory neurons or spinal astrocytes after nerve injury. It has been also reported that MMP-9 inhibitor attenuates mechanical allodynia of complete freund Adjuvant (CFA) rats.21 Recently, a novel MMP-2/9 inhibitor AQU-118 has been reported to produce analgesic effects in an animal model of neuropathic pain.22 In this study, we found that MMP-9 and MMP-2/9 inhibitors did not affect cortical LTP, suggesting that these inhibitors may produce behavioral inhibition through other mechanisms, such as IL-1β and cytokines.23 We found that broad-spectrum MMP inhibitor and an MMP-3 inhibitor reduced both NMDAR-mediated responses and cortical LTP, suggesting that they may be effective in reducing injury triggered plasticity in the cortex.
MMPs, LTP, and memory
Previous studies showed that some MMP subtypes play a role in plasticity and learning.3–5 It has been reported that MMP-9 plays an important role in the modification of dendritic spine morphology.24 On the other hand, MMP-3 has been reported to potentially cleave all brain chondroitin sulfate proteoglycans9 and NMDARs in vitro.25 Using an MMP inhibitor that inhibits both MMP-3 and MMP-9, Meighan et al. have reported that spatial memory acquisition and storage were affected.26 These findings indicate that inhibiting MMPs including MMP-9 and MMP-3 may produce cognitive impairment. In addition, it has been reported that inhibition of MMP activities blocked hippocampal or amygdala LTP, which is critical for certain forms of learning and memory.4,5,26,27
MMPs and NMDAR functions
NMDARs are important for synaptic plasticity and memory in many brain regions.11 In the ACC, LTP induced by a pairing protocol depends on NMDARs.18 NMDARs are particularly important for the induction of LTP.28 Our data show that broad-spectrum MMP inhibitor and MMP-3 inhibitor inhibited the induction of LTP in the ACC but did not affect maintenance LTP. The finding of MMP-3 inhibitor’s effect on NMDAR is similar to a previous report in the hippocampus.12 We also examined whether these MMP-2, MMP-2/9, and MMP-3 inhibitors affected basal eEPSCs in the ACC neurons. We found that MMP-2, MMP-2/9, and MMP-3 inhibitors did not affect basal eEPSCs at different concentrations. Thus, it is likely that MMP inhibitors produce their effect through NMDARs. Indeed, MMP-9 and MMP-2/9 inhibitors did not change the induction of LTP and also did not affect NMDAR-mediated currents in the ACC. The exact mechanism for how MMPs may affect NMDAR function remains to be investigated. It has been reported that MMP-7 calves the NR1 NMDAR subunit and thus modifies NMDAR function in the hippocampus.29
It is known that LTP induced by a pairing protocol in the ACC is related to chronic pain and anxiety.17 Therefore, the reduction of LTP in the ACC by MMP-3 inhibitor may reduce chronic pain. Previous studies have shown that MMP-3 may be upregulated as a response to synovitis in rheumatoid arthritis patients who have chronic pain.30 Due to their important roles in learning and memory, it is also critical to investigate if these inhibitors may interfere with vital brain functions such as memory and emotion.
Authors’ contributions
TM, XHL, and CT performed the electrophysiological experiments. TM and XHL drafted the manuscript. TM, XHL, and MZ designed the project and finished the final vision of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MZ was in part supported by the Canadian Institute for Health Research Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, and project grant (PJT-148648), Azrieli Neurodevelopmental Research Program and Brain Canada. This work is also in part supported by JSPS KAKENHI Grant (JP 18H06268) and Nature Science Foundation of China Grant (NSFC 91732105).
References
- 1.Kawasaki Y, Xu Z-Z, Wang X, Park JY, Zhuang Z-Y, Tan P-H, Gao Y-J, Roy K, Corfas G, Lo EH, Ji R-R. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 2008; 14: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov 2006; 5: 785–799. [DOI] [PubMed] [Google Scholar]
- 3.Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 2006; 26: 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiera G, Nowak D, van Hove I, Dziegiel P, Moons L, Mozrzymas JW. Mechanisms of NMDA receptor- and voltage-gated L-type calcium channel-dependent hippocampal LTP critically rely on proteolysis that is mediated by distinct metalloproteinases. J Neurosci 2017; 37: 1240–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorkiewicz T, Balcerzyk M, Kaczmarek L, Knapska E. Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front Cell Neurosci 2015; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem 2006; 96: 1227–1241. [DOI] [PubMed] [Google Scholar]
- 7.Wojtowicz T, Mozrzymas JW. Matrix metalloprotease activity shapes the magnitude of EPSPs and spike plasticity within the hippocampal CA3 network. Hippocampus 2014; 24: 135–153. [DOI] [PubMed] [Google Scholar]
- 8.Wang X-b, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A 2008; 105: 19520–19525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J Neurochem 2012; 123: 203–216. [DOI] [PubMed] [Google Scholar]
- 10.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993; 361: 31–39. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain 2013; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzdak P, Wlodarczyk J, Mozrzymas JW. and Wojtowicz T. Matrix metalloprotease 3 activity supports hippocampal EPSP-to-spike plasticity following patterned neuronal activity via the regulation of NMDAR function and calcium flux. Mol Neurobiol 2017; 54: 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziembowska M, Wlodarczyk J. MMP9: a novel function in synaptic plasticity. Int J Biochem Cell Biol 2012; 44: 709–713. [DOI] [PubMed] [Google Scholar]
- 14.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond, B, Biol Sci 2014; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 17.Koga K, Descalzi G, Chen T, Ko H-G, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao M-G, Kaang B-K, Collingridge GL, Zhuo M. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 86: 1109. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M-G, Toyoda H, Lee Y-S, Wu L-J, Ko SW, Zhang X-H, Jia Y, Shum F, Xu H, Li B-M, Kaang B-K, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 19.Li X-H, Song Q, Chen T, Zhuo M. Characterization of postsynaptic calcium signals in the pyramidal neurons of anterior cingulate cortex. Mol Pain 2017; 13: 174480691771984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X-Y, Ko H-G, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park S-W, Shim J, Lee K, Collingridge GL, Kaang B-K, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 21.Kular L, Rivat C, Lelongt B. Calmel C, Laurent M, Pohl M, Kitabgi P, Melik-Parsadaniantz S and Martinerie C. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases-2 and -9. J Neuroinflammation 2012; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry MA, Fairchild DD, Patil MJ. Hanania T, Hain HS, Davis SF, Malekiani SA, Hu A, Sucholeiki R, Nix D and Sucholeiki I. Effect of a novel, orally active matrix metalloproteinase-2 and -9 inhibitor in spinal and trigeminal rat models of neuropathic pain. J Oral Facial Pain Headache 2015; 29: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji R-R, Xu Z-Z, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci 2009; 30: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci 2014; 34: 9867–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauly T, Ratliff M, Pietrowski E, Neugebauer R, Schlicksupp A, Kirsch J, Kuhse J. Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLoS One 2008; 3: e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem 2007; 102: 2085–2096. [DOI] [PubMed] [Google Scholar]
- 27.Wojtowicz T, Mozrzymas JW. Late phase of long-term potentiation in the mossy fiber-CA3 hippocampal pathway is critically dependent on metalloproteinases activity. Hippocampus 2010; 20: 917–921. [DOI] [PubMed] [Google Scholar]
- 28.Li XH, Miao HH, Zhuo M. NMDA receptor dependent long-term potentiation in chronic pain. Neurochem Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szklarczyk A, Ewaleifoh O, Beique J-C, Wang Y, Knorr D, Haughey N, Malpica T, Mattson MP, Huganir R, Conant K. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. Faseb J 2008; 22: 3757–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanaka H, Matsuda Y, Tanaka M, Sendo W, Nakajima H, Taniguchi A, Kamatani N. Serum matrix metalloproteinase 3 as a predictor of the degree of joint destruction during the six months after measurement, in patients with early rheumatoid arthritis. Arthritis Rheum 2000; 43: 852–858. [DOI] [PubMed] [Google Scholar]