Abstract
Cytokines play a crucial role in mediating immune responses to tuberculosis (TB). The aim of this study was to evaluate the levels of cytokines in patients with different forms of pulmonary tuberculosis (PTB) and identify valuable cytokine biomarkers for the diagnosis of PTB. We measured the levels of six cytokines (interleukin (IL-2, IL-4, IL-6, and IL-10), tumor necrosis factor (TNF-α), and interferon-γ (IFN-γ)) in the serum of healthy donors (n = 30). Patients with active PTB (n = 46) and those with latent tuberculosis infection (LTBI, n = 38) were examined using cytometric bead arrays. The levels of the six cytokines in the serum samples were measured promptly, sensitively, and simultaneously. The levels of IL-2, IL-6, IL-10, and IFN-γ were significantly higher in the PTB group compared with those reported in the healthy donors (P < 0.01 or P < 0.05). In addition, significantly higher levels of IL-2, IL-6, IL-10, and IFN-γ were found in the active PTB group compared with those observed in the LTBI group (P < 0.01 or P < 0.05). However, the levels of IL-4 and TNF-α in the sera of patients from the PTB group did not show a significant correlation with those measured in the healthy donor group. Our data demonstrated that IL-2, IL-6, IL-10, and IFN-γ may be useful in the auxiliary diagnosis of tuberculosis and as biomarkers for distinguishing LTBI from TB.
Keywords: cytokine, cytometric bead array, interleukin, latent infection, Mycobacterium tuberculosis, pulmonary tuberculosis
Introduction
Tuberculosis (TB) is an air-borne chronic infection caused by Mycobacterium tuberculosis (MTB).1 In humans, the lung is the portal of MTB infection. Subsequently, MTB causes a localized infection in the lung through bacterial inhalation and deposition, resulting in pulmonary tuberculosis (PTB). PTB can be classified into the following two categories based on clinical symptoms: (1) active PTB—defined as both bacteriologically confirmed cases and clinically diagnosed cases based on the chest radiograph or skin test; and (2) latent tuberculosis infections (LTBIs)—defined as cases in which the lung is infected with MTB bacteria and maintains a state of persistent immune response to MTB without any clinically manifested symptom.2 This means that the infected individuals are asymptomatic, show negative results in acid-fast bacilli tests, and exhibit a normal radiological appearance.3 On average, 5%–15% of those infected will develop active TB during their lifetime, typically within the first 2–5 years after initial infection. Therefore, the identification and effective treatment of individuals with LTBI who do not exhibit signs and symptoms of TB disease will be key in achieving the 2030 and 2035 targets of the End TB Strategy.3 At present, the increased expression of interferon-γ (IFN-γ) by CD4 T-cells following stimulation with MTB-specific antigens is the biomarker used in the Interferon gamma release assay (IGRA). However, this test possesses limited ability in distinguishing between LTBI and active disease, and is unable to effectively identify the subset of patients with TB infection who are likely to develop active TB. Obviously, cytokines play an irreplaceable role in cell-mediated immune responses against MTB infection. Currently, cytokines specifically expressed in different stages of TB infection remain to be determined. Therefore, the discovery of new biomarkers for TB is urgently required.4
In this study, the cytometric bead array (CBA) system was used to identify potential biomarkers for the diagnosis of TB. This method is based on the enzyme-linked immunosorbent assay for the detection of cytokines and other biomolecules. We used CBA to investigate differences in the concentrations of specific cytokines in the serum among patients with different clinical forms confirmed. The aim of this study was to identify additional TB-related cytokines—besides IFN-γ—and assess their value as biomarkers to distinctly diagnose active PTB from latent PTB.
Materials and methods
Study population
This research study was performed in accordance with the tenets of the Declaration of Helsinki and its later amendments, and approved by the Ethics Committee of Kunming Medical University, Kunming, Yunnan, China. Written informed consent was provided by all patients.
Serum samples were collected from 84 patients treated at the Third People’s Hospital of Kunming, Kunming, Yunnan, China, and 30 healthy uninfected individuals (controls). The participants were categorized into the following three groups based on conventional clinical, roentgenological, and bacteriological criteria: active PTB group (n = 46, including 31 male and 15 female participants; age range = 9–70 years), LTBI group (n = 38, including 30 male and 8 female participants; age range = 21–82 years), and healthy donors (n = 30, including 13 male and 17 female participants; age range = 19–58 years). Healthy uninfected individuals with negative tuberculin skin test (TST) and T-SPOT.TB test (Oxford Immunotec, Abingdon, UK), normal chest computed tomography, and absence of clinical evidence of any disease were enrolled. TB patients were classified according to their clinical presentation being consistent with TB infection (i.e. a positive MTB culture and a positive smear). Patients with a previous history of TB and those who received anti-TB therapy prior to enrollment were excluded from the study. Patients with latent infection were determined based on a positive TST and IGRA using the T-SPOT.TB, with normal chest computed tomography, without clinical symptoms/evidence of active TB or other non-TB respiratory infections. The TST/IGRA two-step strategy was used because confirmatory IGRA markedly reduces the rate of false positivity due to Bacillus Calmette–Guérin vaccination or nontuberculous mycobacterial infection in the initial TST.
Sample collection and measurement
A total of 114 blood samples were collected and centrifuged at 4000 r/min for 10 min. The samples were immediately examined on the day of collection, or stored at −20°C for next-day detection. The CBA Flex Set kit (BD Biosciences, San Jose, CA, USA) was used for the simultaneous detection of six cytokines (i.e. interleukin (IL-2, IL-4, IL-6, and IL-10), tumor necrosis factor (TNF-α), and IFN-γ) in the blood. The CBA technique was performed according to the instructions provided by the manufacturer. Data were analyzed using the CellQuest Software (BD Biosciences) and BD Pharmingen (BD Biosciences).
Statistical analysis
The levels of the six aforementioned cytokines in each sample were recorded after CBA analysis. In addition, the concentrations of cytokines were compared among the different groups. Statistical analysis was performed using the GraphPad Prism software 7.0 (GraphPad Software, San Diego, CA, USA). The Kruskal–Wallis test was applied for group differences. A P < 0.05 denoted statistical significance.
Results
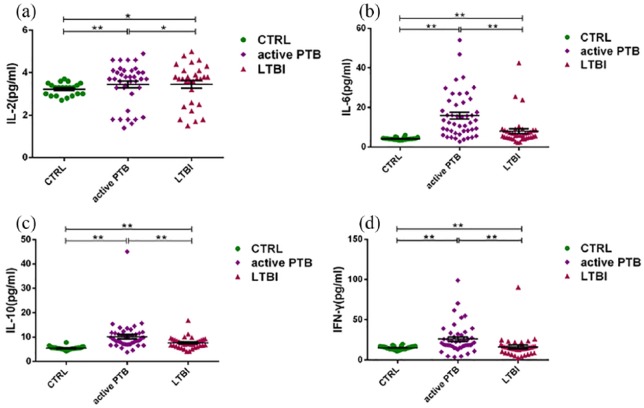
The levels of IL-2, IL-6, IL-10, and IFN-γ in the sera of patients classified in the active PTB and latent PTB (LTBI) groups were significantly higher than those reported in the healthy donor group (CTRL). Moreover, these levels were significantly higher in the active PTB group compared with the latent PTB group (Figure 1).
Figure 1.
Comparisons of the levels of cytokines (i.e. (a) IL-2, (b) IL-6, (c) IL-10, and (d) IFN-γ) in the serum using the cytometric bead array. The levels of IL-2, IL-6, IL-10, and IFN-γ in the serum were significantly increased in the active PTB group compared with those observed in the LTBI group. Simultaneously, the levels of cytokines were significantly higher in patients with pulmonary tuberculosis than in healthy donors (*P < 0.05, **P < 0.01).
IL: interleukin; IFN-γ: interferon-γ; active PTB: patients with active pulmonary tuberculosis; LTBI: patients with latent tuberculosis infections.
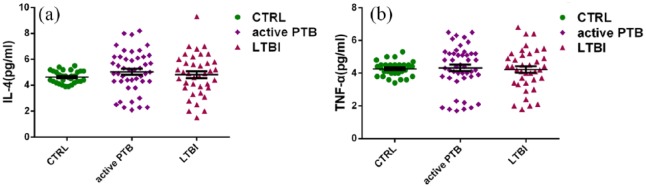
Welch’s t-test was used in an approximate variance analysis to determine the levels of IL-4 and TNF-α. The results showed that the levels of IL-4 and TNF-α did not increase in patients with PTB, and there were no significant differences with those measured in the healthy donor group (CTRL) (Figure 2).
Figure 2.
Comparisons of the levels of cytokines (i.e. (a) IL-4 and (b) TNF-α) in the serum using the cytometric bead array. There were no significant differences in the levels of IL-4 and TNF-α between patients with pulmonary tuberculosis and healthy donors.
IL-4: interleukin-4; TNF-α: tumor necrosis factor-α.
Discussion
IFN-γ is an excellent biomarker for the diagnosis of MTB infection, enhancing antigen presentation and leading to the recruitment of helper T-lymphocytes and/or cytotoxic T-lymphocytes. IL-2 responses to MTB-specific antigens are significantly higher in active TB patients. Thus, IL-2 may be useful as an infection biomarker in distinguishing active TB patients, latently infected individuals, and healthy donors. The IL-2 release assay improves the ability of IGRA to identify individuals with LTBI. In addition, IFN-γ and IL-2 can be used to monitor individuals infected with MTB.5,6 YG Hur et al. reported that the production of IL-10 in ESAT-6-induced LTBI groups was significantly higher than that observed in TB cases. The expression of IL-4 and IL-10 downregulates IFN responses and protective Th1 responses.7 Moreover, in PTB patients, IL-4 induces nitric oxide synthase, toll-like receptor 2, and macrophage activation. These effects may determine the outcome of the MTB infection and are important in maintaining a balanced situation for anti-MTB activity.8,9 IL-6 stimulates the secretion of IFN-γ for the activation of macrophages, required in the initial protective response during MTB infection. Thus, IL-6 may be used as a biomarker for the identification of high-risk individuals and to determine the effectiveness of therapy.10 During MTB infection, TNF-α activates immune responses (i.e. innate and T-cell-mediated), and participates in the formation and maintenance of the granuloma.9
Our study showed that the levels of TNF-α expression did not increase in patients with TB compared with those observed in healthy donors. This finding may be attributed to the inhibition of macrophages by IL-6 to reduce the production of TNF-α, indicating that the immune system in patients with lung TB is not severely damaged and the protective immune mechanism remains stable. A similar study had shown that the gene expression of IL-10 and IFN-γ was significantly increased from the time of infection to the development of clinical TB. In contrast, the gene expression of TNF-α was significantly and progressively decreased with the progression of clinical TB.11 The aberrant expression of IL-2, IL-6, and IL-10 indicates impaired T-cell function in TB patients.12 The dynamic balance of the Th1 and Th2 cytokine levels in TB infection is a key factor affecting the development of the condition. The initial infection with MTB is frequently unrecognized. Therefore, monitoring the balance of these cytokines is important for the detection of PTB activity. According to a report published by the World Health Organization, the major gaps that remain in the diagnostic approach to TB infection include the tests, which would accurately predict the progression from LTBI to active TB disease. This study identified new cytokines which may assist physicians in improving the diagnosis of progression from LTBI to active TB. In conclusion, the combination of IL-2, IL-6, IL-10, and IFN-γ may be valuable as potential biomarkers for the differentiation of LTBI from TB.
Acknowledgments
The Yunnan Province Key Laboratory for Tropical Infectious Diseases in Universities, Kunming, China; Yunnan Province Integrative Innovation Center for Public Health, Diseases Prevention and Control, Kunming Medical University, Kunming, China; and Yunnan Demonstration Base of International Science and Technology Cooperation for Tropical Diseases, Kunming, China supported this study. The authors thank the reviewers for their helpful comments.
Footnotes
Author contributions: F.B. and A.L. conceived the study, participated in its design and coordination, and revised the manuscript. L.T., R.B., B.L., X.D., Z.J., M.J., M.M., and Y.P. participated in the design of the study, performed the study and primary data analysis, and drafted the manuscript. All authors read and approved the final version of the manuscript. R.B., L.T. and B.L. contributed equally to the study.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (nos 81560596, 31560051, and 81860644) and the Natural Foundation of Yunnan Province (2017FE467-001 and 2014FA011). The funding institutions had no role in the study design or review of the manuscript.
ORCID iD: Fukai Bao  https://orcid.org/0000-0003-2652-6660
https://orcid.org/0000-0003-2652-6660
References
- 1. You E, Kim MH, Lee WI, et al. (2016) Evaluation of IL-2, IL-10, IL-17 and IP-10 as potent discriminative markers for active tuberculosis among pulmonary tuberculosis suspects. Tuberculosis 99: 100–108. [DOI] [PubMed] [Google Scholar]
- 2. Lyon SM, Rossman MD. (2017) Pulmonary tuberculosis. Microbiology Spectrum 5: 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (2016) Global Tuberculosis Report 2016. Geneva: World Health Organization. [Google Scholar]
- 4. Hur YG, Crampin AC, Chisambo C, et al. (2014) Identification of immunological biomarkers which may differentiate latent tuberculosis from exposure to environmental nontuberculous mycobacteria in children. Clinical and Vaccine Immunology 21(2): 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mamishi S, Pourakbari B, Teymuri M, et al. (2014) Diagnostic accuracy of IL-2 for the diagnosis of latent tuberculosis: A systematic review and meta-analysis. European Journal of Clinical Microbiology 33(12): 2111–2119. [DOI] [PubMed] [Google Scholar]
- 6. Biselli R, Mariotti S, Sargentini V, et al. (2010) Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clinical Microbiology and Infection 16(8): 1282–1284. [DOI] [PubMed] [Google Scholar]
- 7. Hur YG, Gorak-Stolinska P, Ben-Smith A, et al. (2013) Combination of cytokine responses indicative of latent TB and active TB in Malawian adults. PLoS ONE 8(11): e79742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redford PS, Murray PJ, O’Garra A. (2011) The role of IL-10 in immune regulation during M. tuberculosisinfection. Mucosal Immunology 4(3): 261–270. [DOI] [PubMed] [Google Scholar]
- 9. Lioté H, Lioté F. (2011) Role for interferon-gamma release assays in latent tuberculosis screening before TNF-α antagonist therapy. Joint Bone Spine 78: 656–657. [DOI] [PubMed] [Google Scholar]
- 10. Chowdhury IH, Ahmed AM, Choudhuri S, et al. (2014) Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Molecular Immunology 62(1): 159–168. [DOI] [PubMed] [Google Scholar]
- 11. Abebe F, Mustafa T, Nerland AH, et al. (2006) Cytokine profile during latent and slowly progressive primary tuberculosis: A possible role for interleukin-15 in mediating clinical disease. Clinical and Experimental Immunology 143(1): 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harling K, Adankwah E, Guler A, et al. (2018) Constitutive STAT3 phosphorylation and IL-6/IL-10 co-expression are associated with impaired t-cell function in tuberculosis patients. Cellular & Molecular Immunology 16: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]