Abstract
Hyperglycemia fluctuation is associated with diabetes mellitus (DM) complications when compared to persistent hyperglycemia. Previous studies have shown that paeoniflorin (PF), through its antiapoptosis, anti-inflammation, and antithrombotic properties, effectively protects against cardiovascular and cerebrovascular disease. However, the mechanism underlying the protection from PF against vascular injuries induced by hyperglycemia fluctuations remains poorly understood. Herein, we investigated the potential protective role of PF on human umbilical vein endothelial cells (HUVECs) subjected to intermittent glucose levels in vitro and in DM rats with fluctuating hyperglycemia in vivo. A remarkable increased apoptosis associated with elevated inflammation, increased oxidative stress, and high protein level of PKCβ1 was induced in HUVECs by intermittently changing glucose for 8 days, and PF recovered those detrimental changes. LY333531, a potent PKCβ1 inhibitor, and metformin manifested similar effects. Additionally, in DM rats with fluctuating hyperglycemia, PF protected against vascular damage as what has been observed in vitro. Taken together, PF attenuates the vascular injury induced by fluctuant hyperglycemia through oxidative stress inhibition, inflammatory reaction reduction, and PKCβ1 protein level repression, suggesting its perspective clinical usage.
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder, the fifth leading cause of death worldwide, accounting for 5.2% of all deaths [1]. Recent studies have shown that both increased glucose levels and glucose level fluctuations are closely related to the occurrence and development of DM complications [2]. Compared to chronic persistent hyperglycemia, fluctuant hyperglycemia can easily trigger endothelial cell dysfunction and vascular complications [3], but the exact underlying mechanisms are still unknown. Accumulated evidence indicates that increased vascular oxidative stress and inflammation are associated with vascular dysfunction in DM [4], whereas fluctuant hyperglycemia is more effective than constant hyperglycemia in inducing the apoptosis of human umbilical vein endothelial cells (HUVECs) [5] and the oxidative stress [6] and inflammation [7] in human coronary artery endothelial cells [8]. The high intracellular glucose levels in hyperglycemia cause overactivation of various biochemical pathways [9]. Of them, the protein kinase C (PKC) activation pathway, one of the biochemical mechanisms for the progression of diabetes-associated complications [10] is involved in vascular permeability, extracellular matrix synthesis, cell growth, cytokine activation, angiogenesis, ROS production, and vascular smooth muscle contractility [9, 11]. PKCβ, an isozyme of PKC, appears to play a critical role in the pathogenesis of microvascular complications [12, 13]. The microarray results from our previous study identified that PKCβ1 was one of the genes expressed in peripheral leukocytes with higher expression in coronary heart disease (CHD) patients compared to the healthy control [14]. Since CHD is the leading cause of morbidity and mortality in patients with DM [15], we assume that high levels of PKCβ1 might be involved in glucose fluctuation.
Paeoniflorin (PF, Figure 1) is an important component of Paeonia lactiflora Pall that shows various protective effects on the cardio-cerebral vascular system, mediated by antiapoptosis [16], anti-inflammation [17], and antioxidantation [18]. Furthermore, PF is reported to play an essential protective role in both DM-associated macrovascular complications, such as myocardial infarction via the transient receptor potential vanilloid 1/calcitonin gene-related peptide pathway [19], and DM-associated microvascular complications, such as diabetic nephropathy with renal protective effects by prevention of TLR2/4-mediated inflammation [20] or inhibition of the JAK2/STAT3 signaling pathway [21]. However, the effects and mechanisms of PF on glucose fluctuation-induced damages remain to be elucidated. Metformin is a potent antihyperglycemic agent which is widely used in the management of type 2 diabetes by suppressing gluconeogenesis and improving glucose uptake and insulin sensitivity [22]. Moreover, previous studies have shown that metformin improves vascular functions and dramatically reduces the incidence of vascular complications through improving glycemic control, insulin resistance, lipid profile, fibrinolitic activity, oxidative stress, and endothelial function [23]. However, some adverse effects of metformin such as digestive tract symptoms including diarrhea, flatulence, and abdominal discomfort limited its application in clinics [22]. Thus, additional effects should be made in developing new drugs for treatment of DM. Therefore, metformin was used as a positive control to assess the effect of PF on vascular endothelial injury, inflammation, and oxidative stress under intermittent hyperglycemia.
Figure 1.
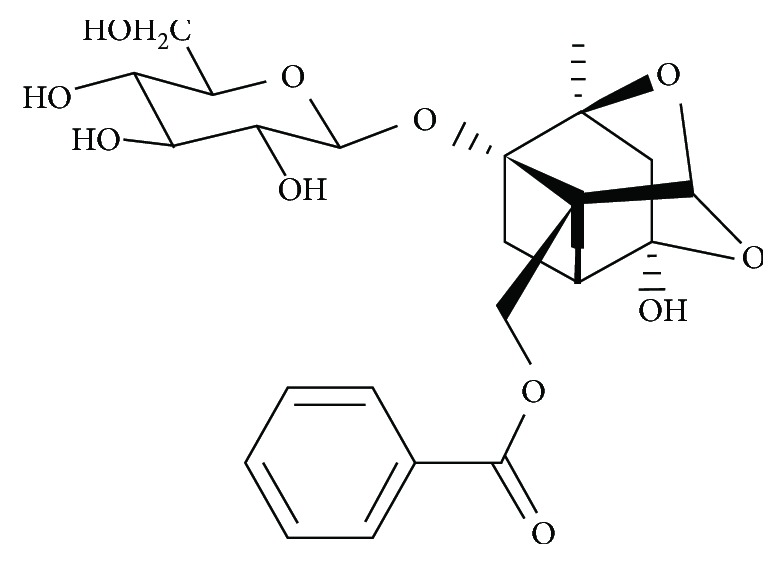
Chemical structure of PF.
This study is aimed at unveiling the potential protective role of PF in intermittent glucose-induced vascular endothelial injuries using HUVECs and a rat model of hyperglycemia fluctuation under different glycemic conditions. Inflammatory markers, oxidative stress indexes, and PKCβ1 protein levels were measured by ELISA, flow cytometry, and western blot. Our data demonstrated the important role of PF in protecting from hyperglycemia fluctuation-induced vascular injuries by minimizing inflammation and oxidative stress release and repression of the PKCβ1 level.
2. Materials and Methods
2.1. HUVEC Culture
HUVECs were obtained from the healthy human umbilical cords as described in Baudin et al. [24]. The cells were incubated for 8 days under one of the following seven glycemic conditions as previously described [8]: (1) constant normal glucose medium (5.56 mmol/L), (2) constant high-glucose medium (25 mmol/L), (3) constant high-glucose medium with 1 h pretreatment with LY333531 (PKCβ1 inhibitor), (4) alternating normal and high-glucose medium (5.56/25 mmol/L) every 24 h, (5) alternating normal and high-glucose medium (5.56/25 mmol/L) every 24 h with 1 h pretreatment with LY333531, (6) alternating normal and high-glucose medium every 24 h with daily PF (100 μM, Dalian Meilun Biotechnology, China) treatment, and (7) alternating normal and high-glucose medium every 24 h with daily metformin hydrochloride (MH) (1 mM) treatment.
2.2. Establishment of a Rat Model of Intermittently High Glucose
Sprague Dawley (SD) male rats weighing 170-190 g were obtained from the National Institutes for Food and Drug Control (SCXK 2009-0017) and housed in an animal facility at Xiyuan Hospital, China Academy of Chinese Medical Sciences, according to the guidelines for laboratory animals approved by the Beijing Experimental Animal Management Center. After 2 weeks of adaptation, the normal diet was switched to a high-fat and high-glucose diet containing 20% fat from lard with 1.25% (wt/wt) cholesterol and 10% (wt/wt) sucrose purchased from Huafukang Feed Company, Beijing, China. Four weeks later, rats were injected intraperitoneally with streptozotocin (STZ) at 30 mg/kg. An Ascensia BRIO blood glucose monitoring system (Bayer company, Germany) was used to measure plasma glucose levels, including the fasting state and 2 h postprandial blood glucose levels 3 days post-STZ injection. Rats with marked hyperglycemia (fasted blood glucose level greater than 11.1 mmol/L and postprandial blood glucose level > 33.3 mmol/L) after 3 days of administration of STZ were confirmed to have DM [25]. Fifty-two DM rats were divided into four groups according to blood glucose levels (the mean amplitude of glycemic excursion (MAGE) and the largest amplitude of glycemic excursion (LAGE)): (1) stable high blood glucose (SHG, n = 11) group, fed with low glycemic diet (Beijing Nuokangyuan Biotechnology) by gavage, (2) intermittent high blood glucose (IHG, n = 11) group, fed with high glycemic index diet (Beijing Nuokangyuan Biotechnology) by gavage, (3) PF-treated (PF, n = 10) group, fed with high glycemic index diet and received the treatment of PF (Dalian Meilun Biotechnology, China; 0.01 g/kg) by gavage, and (4) MH-treated (n = 10) group, fed with high glycemic index diet and received the treatment of MH (Dalian Meilun Biotechnology, China; 0.15 g/kg) by gavage. Ten untreated SD rats fed with normal diet, which only received PBS treatment by gavage, were used as a control group. Rats in these five groups were fed three times per day for 1 h and maintained for six weeks.
Establishment of the intermittently high glucose rat model was evaluated by measuring fasting blood glucose, triglyceride levels, fasting insulin levels, insulin resistance, and glucose variability using MAGE and LAGE, and the morphology of aortic roots with hematoxylin and eosin (H&E) staining. Fasting blood glucose (FBG) and postprandial 2 h blood glucose levels were determined for 5 days after feeding on low or high glycemic index forage for four weeks. Insulin resistance was evaluated using the homeostasis model assessment estimate of insulin resistance (HOMA-IR) [26, 27] with the following formula: fasting insulin level (μU/mL) × fasting blood glucose (mmol/L)/22.5. MAGE used for assessing the intraday glucose variability was calculated by measuring the arithmetic mean of the differences in consecutive peaks and nadirs, which were taken into consideration only if they exceeded the standard deviation from the mean. LAGE was defined as the maximal sensor glucose levels minus the minimal daily sensor glucose levels [28].
2.3. Cell Apoptosis Assay Using Flow Cytometry
To unveil the impact of fluctuant hyperglycemia on cell injury, HUVECs were subjected to different glycemic conditions followed by apoptosis analysis using flow cytometry. Annexin V-FITC/PI staining was performed to measure apoptosis using flow cytometry according to the manufacturer's protocol. Briefly, 1 × 106 HUVECs were collected, washed with PBS, and resuspended in 100 μL 1× binding buffer, followed by addition of 5 μL Annexin V-FITC. After incubation for 15 min at 37°C, the buffer was removed by centrifugation, and the cells were resuspended in a reaction buffer containing 5 μL propidium iodide (PI), followed by flow cytometry analysis. All reagents were purchased from BD Biosciences.
2.4. ELISA Assay and Western Blotting Analysis
To further verify the potential role of intermittent high glucose in the inflammatory response and oxidative stress, we determined the contents of tumor necrosis factor-α (TNF-α), platelet endothelial cell adhesion molecule (PECAM-1), advanced oxidation protein products (AOPPs), and total antioxidant capacity (T-AOC) in the supernatant from HUVEC culture and in sera of DM rats using ELISA kits (Westang Bio-Tech, Shanghai, China) according to the manufacturer's protocols. Serum von Willebrand factor (VWF) and soluble E-selectin (sE-selectin) levels were also determined as the endothelial injury indexes in rats by ELISA. The protein levels of PKCβ1 in HUVECs and rat aorta were determined using western blotting analysis according to the standard procedure as previously described [29]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
2.5. Statistical Analyses
SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were analyzed using one-way ANOVA with multiple comparison tests and presented as mean ± standard deviation (SD). The normality was checked using the Shapiro-Wilk test. The difference between groups was tested by least significant difference (LSD), and the Levene method was used for homogeneity test of variance. P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Hyperglycemia Fluctuation on the Apoptosis of HUVECs
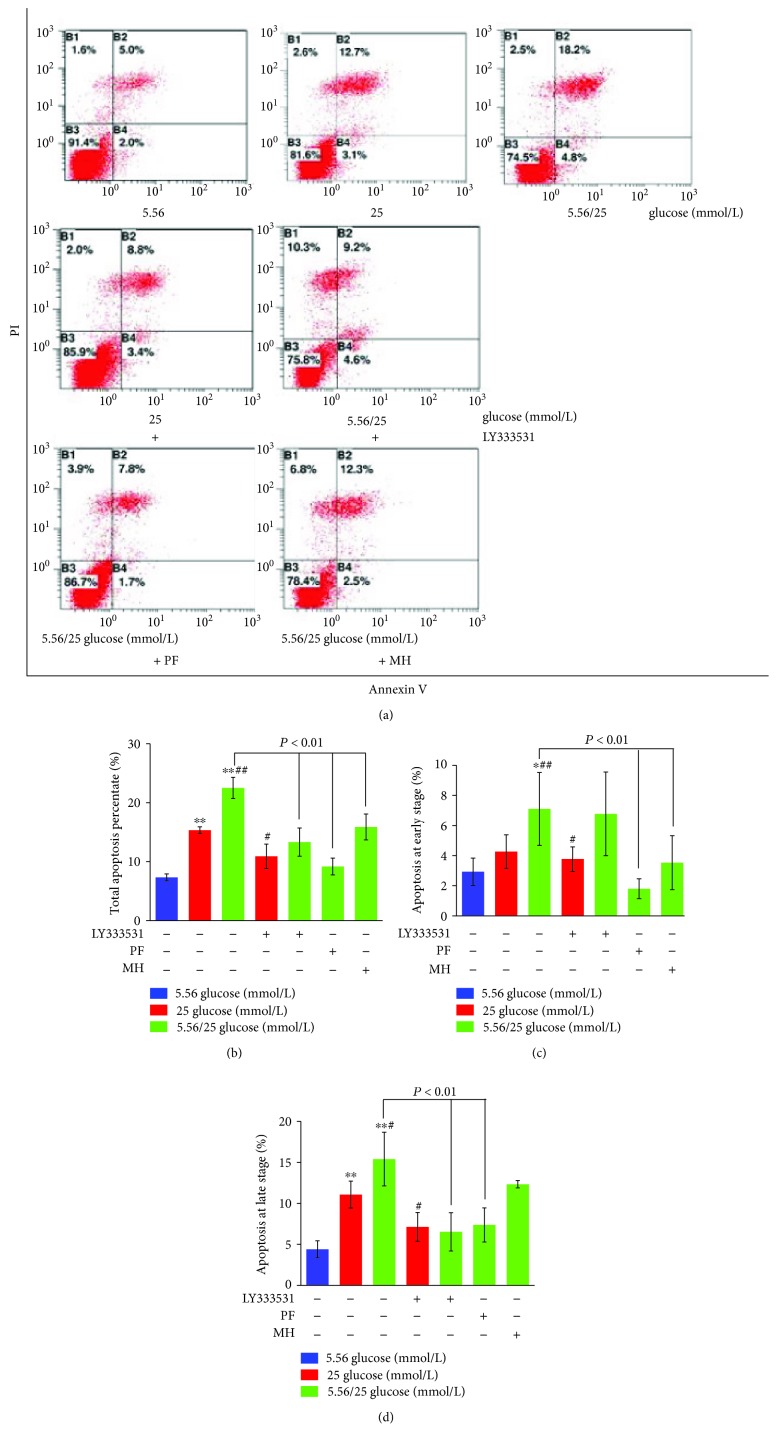
As shown in Figure 2, after 8 days, a large number of apoptotic cells were observed when cultured with fluctuating glucose levels. Stable high glucose levels significantly increased the total apoptosis rate during the whole period (Figures 2(a) and 2(b); P < 0.01) and at late stage (Figure 2(d); P < 0.01), but only an increasing tendency at early stage (Figure 2(c)), when compared with normal glucose concentration (5.56 mmol/L glucose). Surprisingly, intermittent high glucose levels significantly enhanced this apoptotic process (Figures 2(b)–2(d); P < 0.05 or 0.01). LY333531 treatment decreased the apoptosis rates both at the late stage and at the whole stage, but not at the early stage, induced by the stable and intermittent high glucose (Figures 2(d) and 2(a); P < 0.05 or 0.01). As expected, PF treatment significantly inhibited intermittent high glucose-induced cell apoptosis at the early, late, and whole stages (P < 0.01 vs. 5.56/25 mmol/L glucose).
Figure 2.
Effects of paeoniflorin (PF) on the apoptosis rate of human umbilical vein endothelial cells (HUVECs) cultured with different concentrations of glucose for 8 days. HUVECs were cultured in the presence of normal (5.56 mmol/L), high (25 mmol/L), or alternating normal/high concentrations as described in Materials and Methods and treated with LY333531, PF, or metformin hydrochloride (MH). Representative flow cytometry scatter plots of the total percentage of apoptosis are shown in (a). The total percentage of apoptosis was reflected by its fluorescent intensity (b). Early (c) and late (d) apoptosis was determined in the various groups. Data are presented as mean ± standard deviation (n = 6). ∗P < 0.05 and ∗∗P < 0.01 vs. glucose 5.56 mmol/L; #P < 0.05 and ##P < 0.01 vs. 25 mmol/L.
3.2. Inflammation, Oxidative Stress, and PKCβ1 Protein Levels in Hyperglycemia Fluctuation of HUVECs
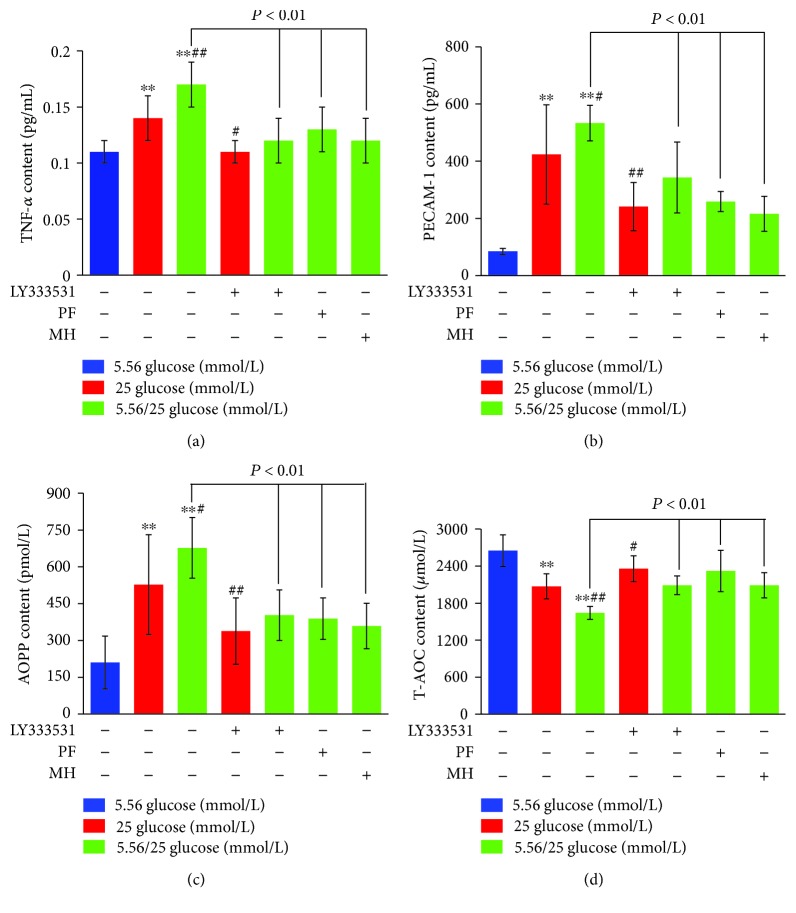
As shown in Figure 3(a), TNF-α levels increased under stable high glucose and intermittent high glucose compared to the normal glucose group (P < 0.01). The level of TNF-α under intermittent high glucose further significantly increased compared to the stable high glucose group (P < 0.01). In contrast, LY333531 pretreatment reduced TNF-α levels by 21.43% under stable high glucose and 29.41% under alternating 5.56/25 mmol/L glucose, respectively, as compared to nontreated cells under the corresponding conditions (Figure 3(a)). PF and MH pretreatment decreased the levels of TNF-α by 23.53% and 29.41%, respectively, in HUVECs cultured with alternating 5.56/25 mmol/L glucose (Figure 3(a)). Similarity, we observed the increased protein levels of PECAM-1 and AOPPs in stable high glucose (>5-fold and >2-fold, P < 0.01) and intermittent high glucose (>6-fold and >3-fold, P < 0.01), compared to the normal glucose group, while the levels of PECAM-1 and AOPPs in stable high glucose (75.62% and 56.11%, P < 0.01) and intermittent high glucose (55.44% and 68.10%, P < 0.01) with inhibition of PKCβ1 were decreased compared to those of the corresponding conditions (Figures 3(b) and 3(c)). T-AOC level was decreased in stable high glucose by 27.94% (P < 0.01) and intermittent high glucose by 61.40% (P < 0.01) when compared to the normal glucose group, with further significant decrease in intermittent high glucose (Figure 3(d)). The effect of PKCβ1 inhibition by LY333531 and PF on inflammation and oxidative stress was similar to the HUVECs treated with MH (P < 0.01 vs. 5.56/25 mmol/L glucose).
Figure 3.
Effects of paeoniflorin (PF) on inflammatory factor and oxidative stress levels in human umbilical vein endothelial cells (HUVECs) under different concentrations of glucose for 8 days. HUVECs were cultured in the presence of normal (5.56 mmol/L), high (25 mmol/L), or alternating normal/high concentrations, as described in Materials and Methods. The levels of TNF-α (a), PECAM-1 (b), AOPPs (c), and T-AOC (d) in HUVECs induced by different pretreatments were determined in the various groups. Data are presented as mean ± standard deviation. ∗P < 0.05 and ∗∗P < 0.01 vs. glucose 5.56 mmol/L; #P < 0.05 and ##P < 0.01 vs. 25 mmol/L. MH: metformin hydrochloride.
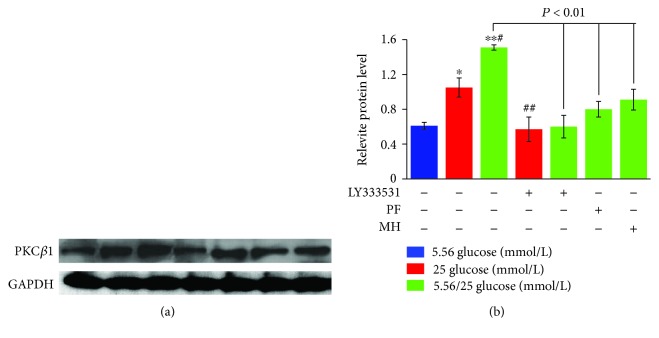
As shown in Figure 4, PKCβ1 protein levels in HUVECs under both stable and alternating high glucose conditions increased significantly as compared to the normal glucose condition (P < 0.05 and 0.01, respectively). In addition, the protein level of PKCβ1 in alternating high glucose condition was significantly increased by 43.81% (P < 0.05) compared to those of the stable high glucose. In contrast, pretreatment with LY333531, PF, and MH blocked the increase in PKCβ1 protein levels under the stable or alternating high glucose conditions.
Figure 4.
Western blot analysis of PKCβ1 in the supernatant of human umbilical vein endothelial cells under different glucose concentrations. The band intensity was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (a), and the average band intensity after normalization is presented as a bar graph (b). ∗P < 0.05 and ∗∗P < 0.01 vs. glucose 5.56 mmol/L; #P < 0.05 and ##P < 0.01 vs. 25 mmol/L. PF: paeoniflorin; MH: metformin hydrochloride.
3.3. Establishment of a Rat Model of Hyperglycemia Fluctuation
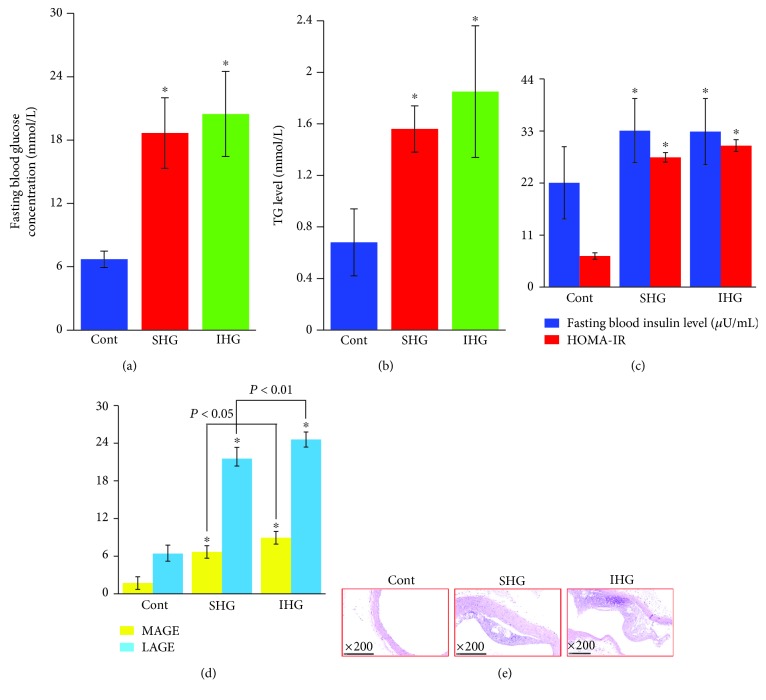
FBG (Figure 5(a)), TG (Figure 5(b)), fasting insulin, and HOMA-IR (Figure 5(c)) levels increased in rats fed on low or high glycemic index diet compared to rats fed on a normal diet (P < 0.01), indicating the successful establishment of the DM model of rats with insulin resistance. As shown in Figure 5(d), MAGE and LAGE markedly increased in rats fed on low or high glycemic index diet compared to rats fed a normal diet (P < 0.01). Furthermore, these two indices in rats fed on high glycemic index diet increased significantly compared to those in rats fed on low glycemic index diet (P < 0.05 and 0.01, respectively). As a result, a thickened aortic intimal layer and atherosclerosis plaque formation were observed in rats fed on low or high glycemic index diet (Figure 5(e)).
Figure 5.
Establishment of a blood glucose fluctuation rat model. (a) Fasting blood glucose concentration, (b) triglyceride (TG) levels, (c) fasting blood insulin level and homeostasis model assessment estimate of insulin resistance (HOMA-IR) index, and (d) mean amplitude glycemic excursions (MAGE) and largest amplitude glycemic excursions (LAGE). ∗P < 0.01 compared with control rats. (e) Representative images of histological examination of aorta in rats by H&E staining with 200x magnification. Cont: control; SHG: stable high blood glucose; IHG: intermittent high blood glucose.
3.4. Endothelial Function, Inflammatory Cytokines, Oxidative Stress, and PKCβ1 Protein Levels in DM Rats with Hyperglycemia Fluctuation
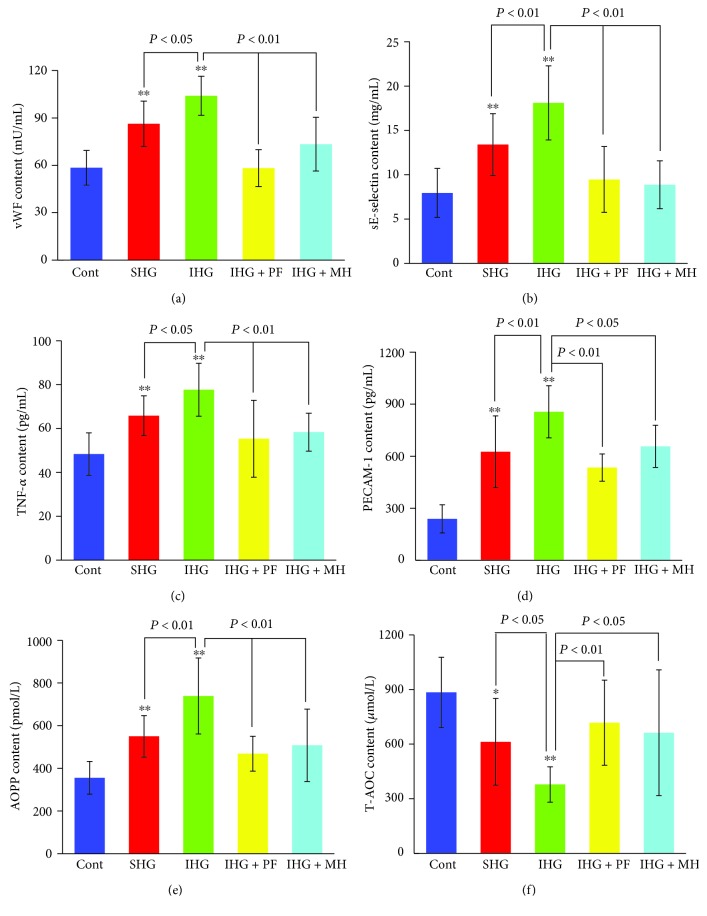
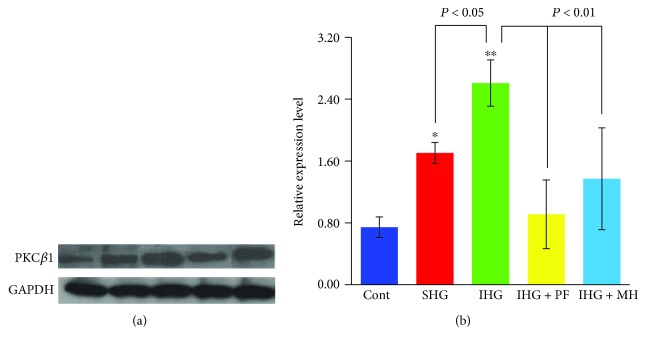
As shown in Figure 6, the serum levels of VWF, sE-selectin, TNF-α, PECAM-1, and AOPPs significantly increased, whereas T-AOC levels significantly decreased in SHG and particularly in IHG groups compared to the control (P < 0.05 and P < 0.01, respectively). Pretreatment with both PF and MH attenuated such changes (Figure 6), consistent with the in vitro observations (Figure 3). Similarly, as shown in Figure 7, PKCβ1 protein levels in the aorta increased in rats with low or high glycemic diet compared to control rats (P < 0.05 and P < 0.01, respectively), and pretreatment with PF and MH blocked the increase in PKCβ1 protein levels induced in SHG and IHG groups.
Figure 6.
Effects of paeoniflorin (PF) on endothelial injury, inflammatory factors, and oxidative stress levels in rats fed with different diets for 4 weeks. Rats in the SHG and IHG groups were fed with low and high glycemic diet, respectively. von Willebrand factor (VWF) (a), sE-selectin (b), TNF-α (c), PECAM-1 (d), AOPPs (e), and T-AOC (f) levels were determined in the various groups. Data are presented as mean ± standard deviation. ∗P < 0.05 and ∗∗P < 0.01 compared with the control rats. Cont: control; SHG: stable high blood glucose; IHG: intermittent high blood glucose; MH: metformin hydrochloride.
Figure 7.
Western blot analysis of PKCβ1 levels in rats fed with different diets for 4 weeks. Rats in the SHG and IHG groups were fed with a low and high glycemic diet, respectively. Signal intensity was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (a), and the average signal intensities after normalization are shown as a bar graph (b). ∗P < 0.05 and ∗∗P < 0.01 compared with the control rats. Cont: control; SHG: stable high blood glucose; IHG: intermittent high blood glucose; PF: paeoniflorin; MH: metformin hydrochloride.
4. Discussion
Vascular disorders, especially cardiovascular disorders, are major causes of morbidity and mortality in diabetic patients [30]. Vascular endothelial cell injury and dysfunction greatly contribute to the occurrence and development of DM complications [31]. Compared to persistent hyperglycemia, fluctuant hyperglycemia has more capacity to increase microvascular lesions and the risk of cardiovascular death [32], even though the underlying molecular mechanisms are still elusive. To figure out the mechanisms of endothelial dysfunction induced by fluctuant hyperglycemia, HUVECs with exposure to intermittent glucose and DM rat models with fluctuating hyperglycemia were used in our study.
A deleterious effect of glucose fluctuations for HUVECs has been reported. Periodic high glucose concentration increases apoptosis of HUVECs in terms of cell cycle analysis, DNA fragmentation evaluation, and the levels of Bcl-2 and Bax measurements in comparison with stable high glucose concentration [5]. Consistently, our data also found that hyperglycemia fluctuation led to exacerbation of the apoptotic effect on HUVECs. Oxidative stress generation [33], inflammation [34], and PKC activation [35] have been proposed as the factors for the damaging effect of hyperglycemia. Piconi et al. suggested that both stable and oscillating hyperglycemia increased HUVEC apoptosis through ROS overproduction at the mitochondrial electron transport chain [36]. In our study, levels of TNF-α and PECAM-1 were measured as inflammation cytokines, while AOPPs and T-AOC were used as oxidative stress indicators. Our findings demonstrated that high glucose induced an increase in TNF-α, PECAM-1, and AOPPs in HUVECs, which was accompanied by a decrease in T-AOC. These effects were amplified during glucose fluctuations. Previous study has demonstrated that PKC activation and its induced HAD(P)H oxidase participate in apoptosis of HUVECs exposed to glucose fluctuations [37]. To verify the role of PKCβ1 involved in endothelial dysfunction caused by glucose fluctuations, a PKCβ isoform selective inhibitor (LY333531) was used in our study. LY333531, or ruboxistaurin, is reported to treat diabetic retinopathy and nephropathy [38]. Upregulation of PKCβ1 protein was shown in HUVECs cultured with both stable and intermittent high glucose concentration, which was more pronounced in intermittent high glucose. However, a remarkably decreased expression of PKCβ1 protein induced by LY333531 pretreatment was associated with a significantly decreased apoptosis and an inhibited expression of TNF-α, PECAM-1, and AOPPs, but with a simultaneous increase in T-AOC. Thus, cumulating in vitro evidence suggested that glucose fluctuation was more deleterious to HUVECs than constant high glucose by intensifying apoptosis, aggravating inflammation activation, increasing oxidative stress indicators, and elevating PKCβ1 expression.
Previous studies have focused on in vitro experiments and rarely on in vivo. In our study, the DM rat model with fluctuating hyperglycemia treatment was used to further verify the findings in HUVECs. VWF and sE-selectin were used as biomarkers of endothelial injury. High glucose caused increased levels of VWF, sE-selectin, TNF-α, PECAM-1, and AOPPs in sera and high PKCβ1 protein levels in the aorta, which was accompanied by a declined serum level of T-AOC.
Paeoniflorin, a monoterpene glucoside, is the main active ingredient of the root of P. lactiflora and has multiple biological activities such as antioxidation, cognition-enhancing, anticoagulation, anticonvulsant, vasodilation, mild sedation, and potent analgesia [39, 40]. Till now, the protective roles of PF refer to amelioration of DM or its complications, such as diabetic nephropathy [21, 41], and cardiovascular events [19], nothing about PF and hyperglycemia fluctuation. In this study, it demonstrated that PF could alleviate vascular injuries induced by hyperglycemia fluctuations in terms of the release of TNF-α, PECAM-1, oxidative stress, and the expression of PKCβ1 in HUVECs subjected to intermittent glucose levels and in DM rats with fluctuating hyperglycemia. Metformin is verified to have beneficial effects on diabetes patients, especially for diabetes-initiated vascular disease, by a reduction in both oxidative stress and chronic inflammation [23]. Since PF showed a similar role as metformin, we demonstrated that PF exhibited a protective role during hyperglycemia fluctuation. Whether PF in combination with metformin may have better clinical efficacy, or PF can be better to alleviate the side effect of metformin, awaits further investigation.
In summary, our study suggests that fluctuant high glucose induces vascular endothelial injury by elevating inflammatory cytokines and aggravating oxidative stress. In addition, PKCβ1 might play a vital role in these intermittent hyperglycemia state-induced damages. Our data show that paeoniflorin ameliorates vascular endothelial injuries through antioxidative and anti-inflammatory effects and decreasing the PKCβ1 protein level.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81573803 and 81703862).
Abbreviations
- PF:
Paeoniflorin
- DM:
Diabetes mellitus
- HUVECs:
Human umbilical vein endothelial cells
- PKCβ1:
Protein kinase C beta 1
- SHG:
Stable high glucose
- IHG:
Intermittent high glucose
- MH:
Metformin hydrochloride
- MAGE:
Mean amplitude glycemic excursion
- LAGE:
Largest amplitude glycemic excursion
- GI:
Glucose index.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
The following are the authors' contributions to this study: conceptualization and funding acquisition, J.-S.W. and Y. H.; methodology, L. Z. and Y.-H. Z.; data analysis, D.-D. L. and Y.-W. S.; writing of the original draft, Y. H.; writing, review, & editing, S.-P. Z.; and supervision, H.-J.Y. All authors read and approved the final manuscript.
References
- 1.American diabetes association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Supplement 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolli G. B. Glucose variability and complications. Diabetes Care. 2006;29(7):1707–1709. doi: 10.2337/dc06-0716. [DOI] [PubMed] [Google Scholar]
- 3.Jung H. S. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinology and Metabolism. 2015;30(2):167–174. doi: 10.3803/EnM.2015.30.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domingueti C. P., Dusse L. M. S.'. A., Carvalho M. G., de Sousa L. P., Gomes K. B., Fernandes A. P. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and its Complications. 2016;30(4):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Risso A., Mercuri F., Quagliaro L., Damante G., Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. American Journal of Physiology. Endocrinology and Metabolism. 2001;281(5):E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 6.Liu T., Pei Y., Peng Y., Chen J., Jiang S., Gon J. Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. Journal of Endocrinological Investigation. 2014;37(7):645–651. doi: 10.1007/s40618-014-0086-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu T. S., Gong J. B., Chen Y. T., Jiang S. S. Periodic vs. constant high glucose in inducing pro-inflammatory cytokine expression in human coronary artery endothelial cells. Inflammation Research. 2013;62(7):697–701. doi: 10.1007/s00011-013-0623-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang J. S., Yin H. J., Huang Y., et al. Panax quinquefolius saponin of stem and leaf attenuates intermittent high glucose-induced oxidative stress injury in cultured human umbilical vein endothelial cells via PI3K/Akt/GSK-3β pathway. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/196283.196283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das Evcimen N., King G. L. The role pf protein kinase C activation and the vascular complications of diabetes. Pharmacological Research. 2007;55(6):498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Kizub I. V., Klymenko K. I., Soloviev A. I. Protein kinase C in enhanced vascular tone in diabetes mellitus. International Journal of Cardiology. 2014;174(2):230–242. doi: 10.1016/j.ijcard.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch I. B. Glycemic variability: it’s not just about A1C anymore! Diabetes Technology & Therapeutics. 2005;7(5):780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 13.Geraldes P., King G. L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation Research. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin H. J., Ma X. J., Jiang Y. R., Shi D. Z., Chen K. J. Investigation of gene expression profiles in coronary heart disease and functional analysis of target gene. Chinese Science Bulletin. 2009;54(5):759–765. doi: 10.1007/s11434-009-0118-2. [DOI] [Google Scholar]
- 15.Newman J. D., Rockman C. B., Kosiborod M., et al. Diabetes mellitus is a coronary heart disease risk equivalent for peripheral vascular disease. American Heart Journal. 2017;184:114–120. doi: 10.1016/j.ahj.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizamutdinova I. T., Jin Y. C., Kim J. S., et al. Paeonol and paeoniflorin, the main active principles of Paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Medica. 2008;74(1):14–18. doi: 10.1055/s-2007-993775. [DOI] [PubMed] [Google Scholar]
- 17.Chen C., Du P., Wang J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Molecular Medicine Reports. 2015;12(3):3937–3943. doi: 10.3892/mmr.2015.3870. [DOI] [PubMed] [Google Scholar]
- 18.Li P., Li Z. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Experimental and Therapeutic Medicine. 2015;9(5):1768–1772. doi: 10.3892/etm.2015.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han F., Zhou D., Yin X., et al. Paeoniflorin protects diabetic mice against myocardial ischemic injury via the transient receptor potential vanilloid 1/calcitonin gene-related peptide pathway. Cell & Bioscience. 2016;6(1):p. 37. doi: 10.1186/s13578-016-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T., Zhu Q., Shao Y., Wang K., Wu Y. Paeoniflorin prevents TLR2/4-mediated inflammation in type 2 diabetic nephropathy. Bioscience Trends. 2017;11(3):308–318. doi: 10.5582/bst.2017.01104. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Wang Y., Wang K., Wu Y. Renal protective effect of Paeoniflorin by inhibition of JAK2/STAT3 signaling pathway in diabetic mice. Bioscience Trends. 2018;12(2):168–176. doi: 10.5582/bst.2018.01009. [DOI] [PubMed] [Google Scholar]
- 22.Correia S., Carvalho C., Santos M., Seica R., Oliveira C., Moreira P. Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Reviews in Medicinal Chemistry. 2008;8(13):1343–1354. doi: 10.2174/138955708786369546. [DOI] [PubMed] [Google Scholar]
- 23.Triggle C. R., Ding H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiologica. 2017;219(1):138–151. doi: 10.1111/apha.12644. [DOI] [PubMed] [Google Scholar]
- 24.Baudin B., Bruneel A., Bosselut N., Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nature Protocols. 2007;2(3):481–485. doi: 10.1038/nprot.2007.54. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R., Pate D. K., Prasad S. K., Sairam K., Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced type 2 diabetic rats. Asian Pacific Journal of Tropical Medicine. 2011;4(11):904–909. doi: 10.1016/s1995-7645(11)60216-2. [DOI] [PubMed] [Google Scholar]
- 26.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Haffner S. M., Greenberg A. S., Weston W. M., Chen H., Williams K., Freed M. I. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106(6):679–684. doi: 10.1161/01.CIR.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 28.DeVries J. H. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62(5):1405–1408. doi: 10.2337/db12-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koya D., King G. L. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 30.Ali M. K., Narayan K. M. V., Tandon N. Diabetes & coronary heart disease: current perspectives. The Indian Journal of Medical Research. 2010;132(11):584–597. [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y., Vanhoutte P. M. Macro- and microvascular endothelial dysfunction in diabetes. Journal of Diabetes. 2017;9(5):434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 32.Monnier L., Mas E., Ginet C., et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa T., Edelstein D., du X. L., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Lontchi-Yimagou E., Sobngwi E., Matsha T. E., Kengne A. P. Diabetes mellitus and inflammation. Current Diabetes Reports. 2013;13(3):435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 35.Booth G., Stalker T. J., Lefer A. M., Scalia R. Mechanisms of amelioration of glucose-induced endothelial dysfunction following inhibition of protein kinase C in vivo. Diabetes. 2002;51(5):1556–1564. doi: 10.2337/diabetes.51.5.1556. [DOI] [PubMed] [Google Scholar]
- 36.Piconi L., Quagliaro L., Assaloni R., et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes/Metabolism Research and Reviews. 2006;22(3):198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 37.Quagliaro L., Piconi L., Assaloni R., Martinelli L., Motz E., Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 38.Mehta N. N., Sheetz M., Price K., et al. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovascular Drugs and Therapy. 2009;23(1):17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L., Wang Y.-Z., Liu J., Luo X.-T., Ye Y., Zhu X.-Z. Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sciences. 2005;78(4):413–420. doi: 10.1016/j.lfs.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 40.Liu T. P., Liu I. M., Tsai C. C., Lai T. Y., Hsu F. L., Cheng J. T. Stimulatory effect of paeoniflorin on the release of noradrenaline from ileal synaptosomes of guinea-pig in-vitro. The Journal of Pharmacy and Pharmacology. 2002;54(5):681–688. doi: 10.1211/0022357021778835. [DOI] [PubMed] [Google Scholar]
- 41.Shao Y. X., Xu X. X., Wang K., Qi X. M., Wu Y. G. Paeoniflorin attenuates incipient diabetic nephropathy in streptozotocin-induced mice by the suppression of the toll-like receptor-2 signaling pathway. Drug Design Development and Therapy. 2017;11:3221–3233. doi: 10.2147/DDDT.S149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.